Abstract
Background
Liver transplantation (LT) is one of the most effective surgical treatment for patients with end-stage liver disease. Steatosis is a contributor for inferior graft quality. But its impact and safety on transplantation was less assessed in Chinese patients.
Methods
Graft steatosis and related information involved in recipients, donors and surgical procedures were retrospectively collected from 239 patients.
Results
Donor macrosteatosis (MaS) caused about 2.14 and 2.80 folds of increment on patient and graft mortality. Dose-response analysis revealed prominent risk of grafts on overall patient/organ mortality when MaS content exceeded 10% (P<0.05). Noteworthy, deaths were only observed in MaS group when concurrent with extremely higher post-transplant alanine aminotransferase (ALT, 64%). However, microsteatosis (MiS) grafts didn’t affect outcomes after LT. In a cohort of Chinese patients, MaS had comprehensive effects on post-transplant outcomes with relatively lower safety threshold at 10%. Mortality gap caused by MaS grafts was observed in patients with severer ischemia reperfusion injury.
Conclusions
Our study revealled the graft MaS affected the post-transplant outcomes in lower risk cutoff in Chinese patients. Further study is worthy to validate these results and investigate inner mechanism under the phenomenon.
Keywords: Steatosis, prognosis, liver transplantation (LT), dose-response analysis
Introduction
Until now, liver transplantation (LT) is still one of the most effective therapeutic strategy for most end-stage liver disease. Pathologic steatosis is one of the major causes for declined organ utilization. More than half of allografts were discarded for severer steatosis in some specific regions (1). Fatty infiltration decreased the tolerance of graft hepatic cells on ischemic-reperfusion injuries in many pathological aspects, which might finally cause primary non-function (PNF) and patient death (2-5). While, many prior clinical trials revealed that the use of grafts with mild to moderate steatosis can be relatively safe with comparable risk of post-transplant mortality and complications (6-10). [Reference: (I) the use of fatty liver grafts in modern allocation systems risk assessment by the Balance of Risk (BAR) score; (II) the biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment]. Hence, clinicians are trying to expand the donor pool by maximizing the utilization of marginal grafts like steatotic donor liver on the premise of acceptable post-transplant prognosis. And achievements were observed on decrement of waitlist mortality for this endeavor (11-13). Otherwise, strategies were employed from machine perfusion to pharmacological intervention as approaches to salvage the graft would be discarded before (14,15).
Graft steatosis is a quantitative covariate, which can’t be simply used to define the boundary between “absolute” acceptance or unacceptance for LT. “Dose-response” risk-effect model seemed more reasonable to present the interrelationship between donor steatosis and post-transplant outcomes (16). However, the continuous risk of graft steatosis on post-transplant prognosis was less discussed in a fixed cohort before.
LT is a complex systematic engineering. And its quality has comprehensive associations with donor, recipient, graft, surgical characteristics and their interaction (17,18). Therefore, it is important to evaluate the continuous effects of steatosis on post-transplant outcomes in predicative model with uniform adjustment of risk index in a fixed cohort.
Previously, less cohort study was performed in Chinese patients to systematically evaluate the impact of graft steatosis on post-transplant complications and graft/patient mortality. In the context of discrepancy between limited organ supply and increasing demands for LT, it is urgent to have accurate assessment on continuous risk trend of donor steatosis on post-transplant prognosis based on the clinical model from Chinese cohort with adjustment of comprehensive risk profiles.
In current cohort-based study, we separately assessed the impacts of risk cofactors from donor, recipient, graft and surgical aspects on post-transplant complications, graft failure and patient survival. Continuous risks of steatosis on post-transplant outcomes were also evaluated based on clinical model constructed before. Interaction of donor steatosis with other risk covariates in the complex network was also assessed. Predictive nomogram was plotted as reference for marginal graft donation. Our study might help clinicians to better deal with the dilemma between utilization and cancellation of steatotic allografts for LT.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/hbsn.2019.12.02).
Methods
Definition for several concept of complications
Diagnosis of early allograft dysfunction (EAD) was defined according to the updated criteria designed by Nicolau‐Raducu et al. before (19). To be specific, patients who met the following items simultaneously within the first week after liver transplantation including: (I) ALT >3,000 IU/mL or AST >6,000 IU/mL; (II) total bilirubin (TB) ≥10 mg/dL; (III) international normalized ratio (INR) ≥1.6 was diagnosed as EAD (19). And diagnostic criteria of PNF was defined as non-recoverable liver function necessitating emergency re-transplantation within the first 72 hours after LT (20).
Enrollment criteria and study population
This study retrospectively enrolled participants who received LT in Shulan (Hangzhou) Hospital in the period from July 1, 2017 to September 30, 2018. Exclusion criteria were listed as follows: (I) adolescent recipients (aged <18 years); (II) donor grafts with absence of histological information; (III) multi-organ transplantation (n≥2); (IV) ABO-incompatible transplant cases; (V) Living donor LT.
Informed consents were obtained from each enrolled participant. This study was performed in accordance with the Declaration of Helsinki and approved by the ethical board of Shulan Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College (2019-IIT-237).
Data collection and biochemical assay
Indicators which might affect the post-transplant outcomes were collected for further analysis. And the data collected for evaluating the quality of LT can be categorized into following aspects including donor, recipient, graft and surgery related risk factors.
To be specific, donor/recipient demographic data (age, gender, height, weight); recipient pre-operative viral infection, tumor biomarkers, liver function tests (LFTs) including TB, alanine aminotransferase (ALT), and aspartate aminotransferase (AST), prothrombin time (PT), INR in the first 2 weeks after LT.
Blood loss and transfusion of packed red blood cells (pRBC), prothrombin complex concentrate (PCC), fresh frozen plasma (FFP); length of operation, cold ischemia time (CIT), warm ischemia time (WIT) were collected respectively from medical record system of International Hospital of Zhejiang University.
Measurement of LFTs, tumor biomarker (AFP) and electrolyte were assayed by and was assayed by automatic biochemistry analyzer (Abbott i2000). The primary disease of recipients and cause of donor death was diagnosed according to ICD-10 criteria (21). Most information can be obtained from medical record system. Covariates with impact on quality of LT including donor, patient, graft and surgery factors was mainly obtained from medical records of Shulan (Hangzhou) hospital.
Liver biopsy and histological analysis
Wedge liver biopsy was routinely assayed in all grafts after reperfusion. To be specific, liver samples were infiltrated in 10% of formalin solution and proceeded to hematoxylin and eosin (H&E) stained sections followed with standardized protocol. Pathological features and presence of hepatocytes with macro-, micro- or mixed steatosis were evaluated according to criteria defined before (22). And steatotic severity was assessed based on percentage of hepatocytes involved with corresponded features by two experienced pathologists under microscopic observation in double-blinded manner. According to the distribution of cases using steatotic allografts, patients were classified into five groups according to the steatosis degree of allografts (≤10%, 10–20%, ≥20%) in categorical analysis.
Risk scoring system and post-transplant complications
As major predictive system on post-operative risk for patients with chronic liver disease, model for end-stage liver disease (MELD) score was calculated by formula provided in previous study (23). And the Child-Pugh score was counted based on clinical measures including TB, ALB, PT, ascites and encephalopathy interpreted before (24).
With regard to post-transplant outcomes, graft function was assessed by the peak values of hepatic enzyme in first two weeks after LT. And the short-and long-term prognosis of recipients was assessed by occurrence of EAD and PNF, overall survival and rate of graft failure.
Statistic analysis
Descriptive data in normal distribution was presented as mean ± standard deviation (SD) and compared by one-way ANOVA; and data in abnormal distribution was presented as median [inter-quartile range (IQR)] and compared by Mann-Whitney U test. Comparison on distribution in different groups was performed by chi-square test. Multi-variable analysis was performed on dichotomous covariates by binary logistic regression model. Hazard ratio (HR) of factors on patient/graft survival was compared by Cox regression model. And analysis was performed by SPSS software (V20.0).
Analysis on dose-response relationship was conducted by two-stage random effects model (25). Risk curves fitting and linearity test was evaluated by STATA software (release 14) according to method described before (16).
Nomogram was formulated based on the risk from multicovariate analysis to quantitatively predict the integrative risk on post-transplant outcomes. Reliability of nomogram was measured by internal validation test. To be specific, calibration curves were plotted to show consistence between actual probability and predicted outcomes from nomogram. Discriminative performance of nomogram was quantitatively assessed by Harrell’s C-index (26). Nomogram and related validation test was conducted by rms package in R software (version 3.6.1) (27).
Results
Demographic and clinical characteristics of enrolled participants
Totally, 245 transplant cases in 239 patients (6 recipients received re-transplantation) were enrolled into final analysis after excluding 11 cases who didn’t meet the selection criteria (nine cases for absence of information on allograft steatosis and two cases for LT for adolescent patient). All patients received cadaveric organ donation in Hepatobiliary and Pancreatic Surgery Department (HBPD) of Zhejiang University International Hospital in the period from July 1, 2017 to September 30, 2018. All transplant cases were mainly performed by one surgeon (SSZ). And prognosis of patients was followed until March 31, 2019, with median follow-up duration on 314 days [interquartile (IQD) time: 192–402 days].
Characteristics of recipients categorized by allograft steatotic status were listed in Table 1. According to allograft steatosis, patients were categorized into non-steatosis, MaS, MiS and mixed steatosis group. Around half (50.2%) of donor allograft suffered the steatosis in different severity. The upper limit for fat content used for LT was 35% for donor with MaS or MiS, and 50% for grafts with mixed steatosis, respectively. Patients across each group was comparable on distribution of gender, blood type, primary liver disease, MELD score, pre-operative body mass index (BMI), α-fetoprotein (AFP), operational time, hepatitis B virus (HBV) infection and status of type 2 diabetes (P>0.05). The mean age of recipients using MiS steatotic allografts was 5.1 years older than patients in non-MiS group (54.8 vs. 49.7 years, P<0.05). Based on the LFTs results, most patients reached their peak value on liver enzyme (85.3% for ALT and 92.2% for AST), TB (79.5%), PT (82.8%) and INR (83.3%) in the first or second day after LT (Figure S1). Overall, no difference was observed on the peak values of abovementioned indicators across different post-LT days, except slight decrement on TB value occurred in second post-LT day (P<0.05). Compared to non-MaS group, the peak value for ALT level was much higher in patients received grafts with MaS (1,860 vs. 1,101 U/L, P<0.05). And the peak value for AST level in non-steatosis group was about 55% lower than the correspond value in MaS group (2,178 vs. 4,839 U/L, P<0.05). According to criteria (19) defined before, EAD was occurred in 41 patients (accounting for 16.7% of whole patients). Compared to patients in steatosis group, EAD occurrence in non-steatosis group was much lower than steatosis group (10.7% vs. 22.8%, P<0.05). Four cases (1.6%) were developed into PNF and allocated for re-transplantation in 3 days after first transplantation. And PNF was occurred only in steatotic groups (2 in MaS and 2 in MiS group, respectively). Cases using MaS allografts had more blood loss (1,500 vs. 1,000 mL) and RBC transfusion (4 vs. 2 U) than non-MaS group (both P<0.05). Although relatively shorter length of patient and organ survival was observed in MaS group, insignificant inter-group difference was presented on follow-up duration across patients received donors with non-steatosis, MaS, MiS and mixed steatosis (P>0.05).
Table 1. Clinical characteristics of recipients and operational factors classified by graft histological features.
| Variables | Non-steatosis group | MiS group | MaS group | Mixed steatosis group | P valuea |
|---|---|---|---|---|---|
| Number (%) | 122 (49.8) | 55 (22.4) | 48 (19.6) | 20 (8.1) | NA |
| Steatosis degree | 0 | 14.9±9.4 | 12.7±7.7 | 14.8±12.5 | 0.28 |
| Recipient variables | |||||
| Age (years) | 50.3±10.7 | 54.8±8.3* | 48.7±10.9 | 47.9±9.1 | <0.05 |
| Gender (M/F) | 98/24 | 45/10 | 44/4 | 17/3 | 0.35 |
| BMI (kg/m2) | 23.8±3.6 | 23.3±2.7 | 23.4±3.6 | 23.1±3.0 | 0.67 |
| Blood type (A/B/O/AB) | 44/37/32/9 | 19/12/18/6 | 16/9/22/1 | 7/5/5/3 | 0.28 |
| Indication for liver transplantation, n (%) | |||||
| HBV/HCV related cirrhosis | 29 (23.8) | 12 (21.8) | 17 (35.4) | 8 (40.0) | 0.22 |
| Alcohol related cirrhosis | 5 (4.1) | 0 | 1 (2.1) | 2 (10.0) | |
| Alcohol + virus related cirrhosis | 3 (2.5) | 1 (1.8) | 0 | 0 | |
| PBC/PSC | 3 (2.5) | 1 (1.8) | 0 | 0 | |
| Liver cancer | 78 (63.9) | 38 (69.1) | 27 (56.3) | 9 (45.0) | |
| Others | 4 (3.3) | 3 (5.5) | 3 (6.3) | 1 (5.0) | |
| Type 2 diabetes, n (%) | 108 (88.5) | 49 (89.1) | 45 (93.8) | 18 (90.0) | 0.79 |
| Pre-operative AFP (ng/mL) | 23.9 (3.3–335.6) | 13.6 (2.3–137.8) | 7.9 (2.1–89.9) | 7.3 (3.1–237.5) | 0.35 |
| HBV infectors, n (%) | 87 (71.3) | 39 (70.9) | 35 (72.9) | 16 (80.0) | 0.87 |
| Post-LT peak TB level (U/L) | 189±19.2 | 181±30.9 | 248±32.9 | 206±36.2 | 0.49 |
| Post-LT peak ALT level (U/L) | 995±76.8 | 1,166±113.7 | 1,860±299.1* | 1,567±299.4 | <0.05 |
| Post-LT peak AST level (mg/dL) | 2,178±197.5* | 3,099±407.9 | 4,839±736.3 | 4,825±1,425.7 | <0.05 |
| MELD score | 22.3±5.1 | 24.0±3.2 | 22.6±3.3 | 21.2±4.2 | 0.36 |
| EAD occurrence, n (%) | 13 (10.7)* | 10 (18.2) | 10 (20.8) | 8 (40.0) | <0.05 |
| PNF occurrence, n (%) | 0 | 1 (1.8) | 3 (6.3)* | 0 | <0.05 |
| Patients’ survival time after LT (days) | 324±154 | 312±173 | 274±177 | 324±154 | 0.72 |
| Grafts’ survival time after LT (days) | 324±154 | 301±176 | 266±185 | 324±154 | 0.46 |
| Operational variables | |||||
| Blood transfusion | |||||
| pRBC (U) | 2 (0–6.5) | 2 (0–4.5) | 4 (2–8)* | 2 (0.5–6.1) | <0.05 |
| FFP (mL) | 830 (710–1,140) | 800 (620–990) | 865 (600–1,230) | 825 (757–990) | 0.24 |
| PCC (U) | 2 (0–5) | 3 (0–5) | 4.4 (0.5–7.8) | 3 (0–8) | 0.76 |
| Blood loss (mL) | 1,000 (800–1,800) | 1,000 (800–1,500) | 1,500 (1,000–2,500)* | 1,000 (800–1,200) | <0.05 |
| Surgical duration (mins) | 301±65.5 | 299±78.1 | 313±79.4 | 274±47.8 | 0.27 |
| Steatosis degree (%) | 0 | 14.9±9.4 | 12.7±7.7 | 14.8±12.5 | 0.28 |
Data was presented in mean ± SD for data in normal distribution and median (IQR) for data in abnormal distribution. One-way ANOVA test was assayed for normal distributed data; Mann-Whitney U test was assayed for abnormal distributed data; and chi-square test was assayed for patient distribution. * represents the significant difference compared to other groups. a, comparison was performed in groups using grafts with MiS, MaS, and mixed steatosis. AFP, alpha fatoprotein; ALT, alanine aminotransferase; AST, aspertate aminotransferase; BMI, body mass index; EAD, early allograft dysfunction; F, female; FFP, fresh frozen plasma; HBV, hepatitis b virus; HCV, hepatitis C virus; ICU, intensive care unit; LT, liver transplantation; M, male; MELD, Model for End-Stage Liver Disease; NA, not available; TB, total bilirubin; PBC, primary biliary cholangitis; PNF, primary non-function; pRBC, packed red blood cells; PCC, prothrombin complex concentrate; PSC, primary sclerosing cholangitis.
Matched information was available in 140 donors (accounting for 57.1% of all LT cases). Categorized by allograft steatosis, donors seemed to be younger in MiS group (P<0.05). Except that, most indicators that related to graft quality and donor features was comparable in groups categorized by allograft fat content (P>0.05, Table 2).
Table 2. Clinical features of donors and grafts quality classified by donor pathological steatosis severity.
| Variables | Absent | MiS group | MaS group | Mixed steatosis group | P valuea |
|---|---|---|---|---|---|
| Number (%) | 71 (50.7) | 31 (22.1) | 24 (17.1) | 14 (9.4) | NA |
| Donor variables | |||||
| Age (year) | 44.8±16.1 | 35.4±15.3* | 51.1±8.0 | 51.8±10.9 | <0.05 |
| Gender (M/F) | 56/15 | 24/7 | 20/4 | 11/3 | 0.78 |
| BMI (kg/m2) | 21.9±3.7 | 23.0±2.7 | 22.4±3.0 | 23.1±1.9 | 0.56 |
| Pre-donate blood test | |||||
| Potasium (mmol/L) | 4.2±0.8 | 3.8±1.3 | 3.9±0.9 | 3.7±1.1 | 0.21 |
| Sodium (mmol/L) | 144±9.1 | 139±29.4 | 147±9.0 | 145±9.1 | 0.34 |
| ALT (U/L) | 64.5±9.9 | 58.3±11.4 | 43.6±11.9 | 83.8±31.9 | 0.48 |
| FBG (mmol/L) | 9.5±8.7 | 9.7±3.6 | 9.0±3.6 | 12.1±5.6 | 0.72 |
| CR (μmol/L) | 110±26.6 | 113±17.6 | 117±32.1 | 143±31.7 | 0.95 |
| BUN (mmol/L) | 10.4±2.0 | 8.1±1.0 | 7.0±0.9 | 10.5±1.6 | 0.54 |
| Graft variables | |||||
| CIT (min) | 672±191 | 698±176 | 671±130 | 535±176 | 0.26 |
| WIT (min) | 15.2±7.9 | 15.5±9.3 | 15.6±6.3 | 17.1±10.9 | 0.96 |
Information was extracted from donors matched to enrolled recipients. Data was presented in mean ± SD for data in normal distribution and median (IQR) for data in abnormal distribution. a, one-way ANOVA test was assayed for normal distributed data; Mann-Whitney U test was assayed for abnormal distributed data; chi-square test was assayed for patient distribution. * represents the significant difference compared to other groups. M, male; F, female; BMI, body mass index; ALT, alanine aminotransferase; FBG, fasting blood glucose; CR, creatinine; BUN, blood urea nitrogen; CIT, cold ischemia time; WIT, warm ischemia time.
Risk of donor steatosis on major clinical outcomes
EAD occurrence and peak levels of ALT/AST were chosen as indicators to evaluate the perioperative effects of LT. Post-operative peak for ALT level was closely correlated to respective peak AST level in whole cohort (pearson coefficient =0.758, P<0.05).
In univariate analysis, patients using MaS grafts had significantly higher post-transplant liver enzyme compared to recipients using non-steatotic allografts (2,072 vs. 844 U/L for ALT and 4,839 vs. 2,177 U/L for AST level, both P<0.05, Figure S2). Although it seems higher for post-operative peak bilirubin level, donor organ steatosis didn’t affect the clinical biochemical indicators including the peak values on TB, PT and INR (P>0.05, Figure S2).
In subsequent stratified analysis, recipient height, allograft with mixed steatosis and transfusion of PCC were potential risk factors for EAD occurrence (P<0.05, Table 3). EAD prevalence was higher in group using grafts with mixed steatosis (OR =3.88, 95% CI: 1.47–10.2, Figure 1A and Table 3). EAD in G3 MaS group was significantly higher with about two folds risk than non-MaS group (OR =1.91, 95% CI: 1.23–2.97, P<0.05, Figure 1B and Table 3). And this effect was still prominent even after adjustment of potential confounders (P<0.05, Table 3). Mixed steatosis might contribute to higher post-transplant AST level (P<0.05, Table S1). And risk of post-transplant AST elevation was higher in G2 and G3 MaS groups, but this trend was overwhelmed after adjustment of post-transplant ALT level (Table S1). Post-transplant ALT level was not associated with stratified donor MaS/MiS severity (P>0.05, Table S2, Table S3).
Table 3. Impact of steatosis on early allograft dysfunction occurrence after liver transplantation.
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Mixed steatosis (yes/noa) | 3.88 (1.47–10.2) | 0.01 | 3.53 (1.13–11.0) | 0.03 | |
| MiS (yes/noa) | 1.14 (0.52–2.50) | 0.74 | |||
| MaS (yes/noa) | 1.41 (0.64–3.12) | 0.40 | 1.37 (0.56–3.36) | 0.49 | |
| Age (years) | 0.96 (0.93–0.99) | 0.02 | 0.97 (0.94–1.01) | 0.10 | |
| Blood type (A/B/O/AB) | 1.28 (0.99–1.67) | 0.06 | |||
| Gender (F/M) | 0.83 (0.32–2.12) | 0.69 | |||
| BMI (kg/m2) | 1.04 (0.94–1.15) | 0.44 | |||
| AFP (ng/mL)/100 | 0.95 (0.89–1.02) | 0.18 | |||
| MELD score | 0.99 (0.95–1.03) | 0.70 | |||
| Operation time (hours) | 0.79 (0.57–1.10) | 0.17 | |||
| ALT (U/L)/1,000 | 1.51 (1.18–1.94) | <0.01 | |||
| AST (U/L)/1,000 | 1.25 (1.13–1.38) | <0.01 | |||
| TB (μmol/L)/100 | 1.49 (1.28–1.74) | <0.01 | |||
| PT (s) | 1.02 (1.01–1.04) | <0.01 | |||
| INR | 1.01 (0.99–1.02) | 0.24 | |||
| Blood loss (mL)/1,000 | 1.04 (0.80–1.35) | 0.80 | |||
| pRBC (U) | 1.04 (0.99–1.09) | 0.09 | |||
| FFP (mL)/1,000 | 0.91 (0.47–1.76) | 0.78 | |||
| PCC (U) | 1.10 (1.03–1.18) | <0.01 | 1.11 (1.04–1.19) | <0.01 | |
| Height (cm) | 1.06 (1.00–1.20) | 0.04 | 1.08 (1.00–1.17) | 0.04 | |
| Weight (kg) | 1.03 (0.99–1.06) | 0.08 | |||
| MiS degree | |||||
| MiS G1 (yes/noa) | 1.80 (0.63–5.15) | 0.28 | 2.46 (0.78–7.74) | 0.12 | |
| MiS G2 (yes/noa) | 1.14 (0.51–2.52) | 0.75 | 1.09 (0.46–2.56) | 0.85 | |
| MiS G3 (yes/noa) | 1.61 (0.89–2.93) | 0.12 | 1.57 (0.82–2.98) | 0.17 | |
| MaS degree | |||||
| MaS G1 (yes/noa) | 1.68 (0.50–5.67) | 0.41 | 1.66 (0.49–5.61) | 0.42 | |
| MaS G2 (yes/noa) | 0.84 (0.29–2.41) | 0.74 | 0.82 (0.28–2.38) | 0.72 | |
| MaS G3 (yes/noa) | 1.91 (1.23–2.97) | <0.01 | 1.93 (1.17–3.19) | 0.01 | |
Logistic regression was tested for univariate and multivariate analysis. Factors significantly associated with EAD occurrence were enrolled into multi-covariate logistic regression analysis. a, “no” represented the patients received allografts with absence of steatosis (non-steatosis group). G1/G2/G3 respectively represented the steatosis degree (0–10%], (10–20%), and [20%, upper limit]. AFP, alpha fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; F, female; INR, international normalized ratio; FFP, fresh frozen plasma; M, male; MaS, macrosteatosis; MELD, model for end-stage liver disease; MiS, microsteatosis; PCC, prothrombin complex concentrate; pRBC, packed red blood cells; PT, prothrombin time; TB, total bilirubin.
Figure 1.
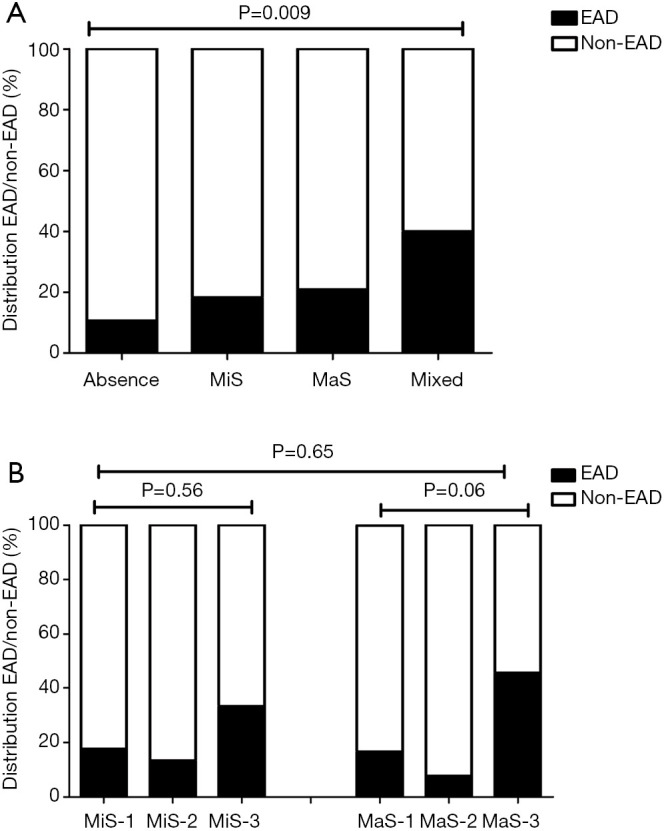
EAD prevalence in patients categorized by steatosis type. (A) EAD prevalence in patients categorized by steatosis type (non-steatosis, MaS, MiS and mixed steatosis); (B) EAD prevalence in patients categorized by steatotic severity. MiS/MaS-1/2/3 respectively represented the steatosis degree (0–10%], (10–20%), and [20%, upper limit]. Comparison was performed by chi-square test. EAD, early allograft dysfunction; MaS, macrosteatosis; MiS, microsteatosis.
Risk of allograft steatosis on recipients’ prognosis
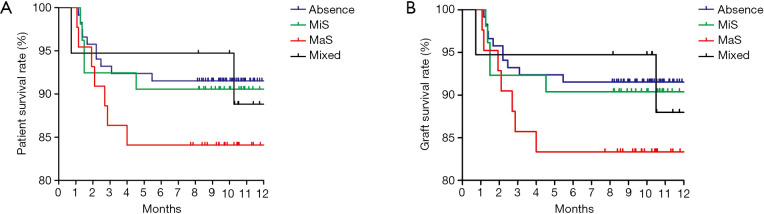
The 3-, 6-month and overall patient/organ survival were selected to present the prognosis of patients after LT. The patients were categorized into three groups according to MaS/MiS contents (0< G1 ≤10%, 10%< G2 ≤20%, G3 >20%). Risk profiles of donor steatosis on post-transplant prognosis was analyzed by combination with other prominent clinical indicators. Compared to non-MaS group, donor MaS affected the patient survival at borderline significance (P=0.07, Table 4). While this effect became significant on graft survival (P<0.05, Table 5). Allograft MaS exerted its positive impacts on the whole profiles of patient mortality and organ failure (P<0.05). To be specific, two to three-folds higher patient death (OR =2.14, 95% CI: 1.06–4.30, Figure 2A) and graft failure (OR =2.80, 95% CI: 1.51–5.18, Figure 2B) was observed in patients using MaS donors, even after adjusting potential susceptible factors in respective circumstance (Table 4 and Table 5, all P<0.05).
Table 4. Impact of steatosis on patients’ survival after liver transplantation.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | OR (95% CI) | P value | ||
| Mixed steatosis (yes/noa) | 0.89 (0.28–2.88) | 0.85 | |||
| MiS (yes/noa) | 0.82 (0.38–1.78) | 0.62 | |||
| MaS (yes/noa) | 2.34 (1.23–4.48) | 0.01 | 2.14 (1.06–4.30) | 0.03 | |
| Age (years) | 1.03 (1.00–1.06) | 0.10 | 1.05 (1.01–1.09) | 0.01 | |
| Gender (F/M) | 0.98 (0.44–2.22) | 0.97 | |||
| Blood type (A/B/O/AB) | 1.17 (0.85–1.59) | 0.34 | |||
| BMI (kg/m2) | 1.01 (0.92–1.11) | 0.91 | |||
| AFP (ng/mL)/100 | 1.00 (0.98–1.03) | 0.79 | |||
| MELD score | 1.01 (0.97–1.05) | 0.72 | |||
| Operation time (hours) | 1.26 (0.98–1.63) | 0.08 | |||
| ALT (U/L)/1,000 | 1.33 (1.17–1.52) | <0.01 | 1.53 (1.11–2.12) | 0.01 | |
| AST (U/L)/1,000 | 1.10 (1.05–1.17) | <0.01 | |||
| TB (μmol/L)/100 | 1.20 (1.08–1.17) | <0.01 | 1.22 (1.07–1.40) | <0.01 | |
| PT (s) | 1.00 (0.99–1.33) | 0.76 | |||
| INR | 1.01 (1.00–1.02) | 0.16 | |||
| Blood loss (mL)/1,000 | 1.41 (1.22–1.64) | <0.01 | |||
| pRBC (U) | 1.05 (1.02–1.08) | <0.01 | |||
| FFP (mL)/1,000 | 1.94 (1.23–3.08) | 0.01 | |||
| PCC (U) | 1.15 (1.09–1.21) | <0.01 | 1.11 (1.03–1.20) | <0.01 | |
| Height (cm) | 1.01 (0.97–1.06) | 0.58 | |||
| Weight (kg) | 1.01 (0.98–1.04) | 0.63 | |||
| MiS degree | |||||
| MiS G1 (yes/noa) | 0.92 (0.31–2.78) | 0.89 | 1.01 (0.32–3.16) | 0.98 | |
| MiS G2 (yes/noa) | 1.29 (0.69–2.39) | 0.42 | 0.94 (0.48–1.85) | 0.86 | |
| MiS G3 (yes/noa) | 1.10 (0.56–2.16) | 0.78 | 1.16 (0.58–2.30) | 0.67 | |
| MaS degree | |||||
| MaS G1 (yes/noa) | 2.03 (0.79–5.19) | 0.14 | 1.89 (0.68–5.21) | 0.22 | |
| MaS G2 (yes/noa) | 2.05 (1.28–3.29) | <0.01 | 2.27 (1.21–4.25) | 0.01 | |
| MaS G3 (yes/noa) | 1.16 (0.71–1.89) | 0.55 | 1.34 (0.80–2.24) | 0.26 | |
Cox regression model was used for univariate and multivariate analysis. Factors significantly associated with patients’ survival (plus age) were enrolled into multi-covariate cox regression analysis. a, “no” represented the patients received allografts with absence of steatosis (non-steatosis group). G1/G2/G3 respectively represented the steatosis degree (0–10%], (10–20%), and [20%, upper limit]. AFP, alpha fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; F, female; INR, international normalized ratio; FFP, fresh frozen plasma; M, male; MaS, macrosteatosis; MELD, model for end-stage liver disease; MiS, microsteatosis; PCC, prothrombin complex concentrate; pRBC, packed red blood cells; PT, prothrombin time; TB, total bilirubin.
Table 5. Impact of steatosis on grafts’ failure after liver transplantation.
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | OR (95% CI) | P value | ||
| Mixed steatosis (yes/noa) | 0.80 (0.25–2.57) | 0.70 | |||
| MiS (yes/noa) | 0.86 (0.42–1.79) | 0.69 | |||
| MaS (yes/noa) | 2.82 (1.04–5.15) | <0.01 | 2.80 (1.51–5.18) | <0.01 | |
| Age (years) | 1.02 (0.99–1.05) | 0.16 | 1.04 (1.00–1.07) | 0.03 | |
| Gender (F/M) | 1.06 (0.49–2.28) | 0.88 | |||
| Blood type (A/B/O/AB) | 1.16 (0.86–1.56) | 0.32 | |||
| BMI (kg/m2) | 0.99 (0.91–1.09) | 0.87 | |||
| AFP (ng/mL)/100 | 1.00 (0.98–1.02) | 0.92 | |||
| MELD score | 1.01 (0.97–1.05) | 0.62 | |||
| Operation time (hours) | 1.24 (0.98–1.58) | 0.08 | |||
| ALT (U/L)/1,000 | 1.35 (1.20–1.52) | <0.01 | |||
| AST (U/L)/1,000 | 1.13 (1.07–1.18) | <0.01 | |||
| TB (μmol/L)/100 | 1.21 (1.10–1.33) | <0.01 | 1.22 (1.09–1.37) | <0.01 | |
| PT (s) | 1.00 (0.99–1.01) | 0.76 | |||
| INR | 1.01 (1.00–1.02) | 0.20 | |||
| Blood loss (mL)/1,000 | 1.40 (1.21–1.61) | <0.01 | |||
| pRBC (U) | 1.05 (1.02–1.07) | <0.01 | |||
| FFP (mL)/1,000 | 1.95 (1.26–3.01) | <0.01 | |||
| PCC (U) | 1.15 (1.09–1.21) | <0.01 | 1.11 (1.03–1.18) | <0.01 | |
| Height (cm) | 1.02 (0.97–1.06) | 0.50 | |||
| Weight (kg) | 1.01 (0.98–1.03) | 0.73 | |||
| MiS degree | |||||
| MiS G1 (yes/noa) | 0.92 (0.31–2.78) | 0.89 | 1.01 (0.32–3.15) | 0.99 | |
| MiS G2 (yes/noa) | 1.55 (0.89–2.67) | 0.12 | 1.15 (0.63–2.10) | 0.64 | |
| MiS G3 (yes/noa) | 1.10 (0.56–2.16) | 0.78 | 1.18 (0.60–2.34) | 0.64 | |
| MaS degree | |||||
| MaS G1 (yes/noa) | 2.39 (0.98–5.80) | 0.06 | 2.51 (0.97–6.49) | 0.06 | |
| MaS G2 (yes/noa) | 2.30 (1.47–3.59) | <0.01 | 1.69 (1.04–2.76) | 0.03 | |
| MaS G3 (yes/noa) | 1.36 (0.90–2.06) | 0.14 | 1.83 (1.14–2.94) | 0.01 | |
Cox regression model was used for univariate and multivariate analysis. Factors significantly associated with graft failure (plus age) were enrolled into multi-covariate cox regression analysis. a, “no” represented the patients received allografts with absence of steatosis (non-steatosis group). G1/G2/G3 respectively represented the steatosis degree (0–10%], (10–20%), and [20%, upper limit]. AFP, alpha fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; F, female; INR, international normalized ratio; FFP, fresh frozen plasma; M, male; MaS, macrosteatosis; MELD, model for end-stage liver disease; MiS, microsteatosis; PCC, prothrombin complex concentrate; pRBC, packed red blood cells; PT, prothrombin time; TB, total bilirubin.
Figure 2.
Patient/graft survival rate in patients categorized by graft steatosis type/severity. (A) Patient survival rate categorized by steatosis type; (B) graft survival categorized by steatosis type. Comparison was performed by cox regression model. MaS, macrosteatosis; MiS, microsteatosis.
While in stratified analysis, this effect seemed inconsistent followed with increasing MaS severity. Compared to non-steatosis group, G2 MaS induced inferior patient mortality (OR =2.14, 95% CI: 1.06–4.30, Table 4, Figure 2A) and graft failure (Table 5, Figure 2B). While, this positive effect was only presented in G3 MaS group on overall organ failure (OR =1.83, 95% CI: 1.14–2.94, P<0.05, Figure 2B, Table 4). With respect to specific survival time, the risk of G2 MaS was ranged between 1.69 and 2.55 folds on 90-d, and 180-d patient/organ mortality, respectively (all P<0.05). But G3 MaS lost its significance on risk of patient/organ mortality in respective time points (Tables S4,S5,S6,S7).
As expected, donor MiS or mixed steatosis showed insignificant impact on post-transplant prognosis in multi-covariate model (P>0.05, Table 4, Table 5, and Figure 2). Otherwise, older age, higher post-transplant TB and prothrombin complex transfusion stably affected the overall organ and patient survival by interactive association with donor MaS in multi-covariate analysis (all P<0.05, Tables 4,5).
Does-response relationship between graft steatosis and post-transplant outcomes
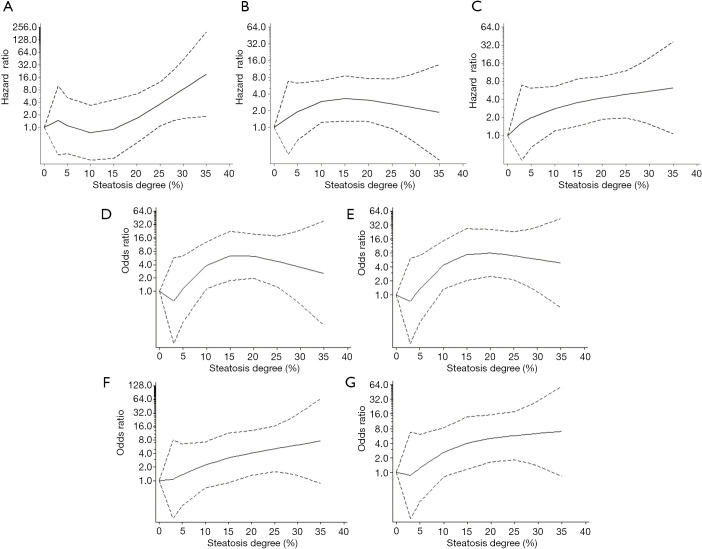
In addition, steatosis degree as a continuous risk on post-transplant outcomes was further quantified by combinative adjustment of prominent clinical factors in prior multi-covariate analysis. And risk curve of steatosis on post-transplant outcomes was plotted by instant OR/HR derived in each point (Figure 3 and Figure S3).
Figure 3.
Dose-response risk of increasing MaS degree on post-transplant outcomes. (A) Dose-response risk of increasing MaS degree on EAD occurrence; (B) dose-response risk of increasing MaS degree on patients’ mortality; (C) dose-response risk of increasing MaS degree on graft failure; (D) dose-response risk of increasing MaS degree on 90-day patients’ mortality; (E) dose-response risk of increasing MaS degree on 90-day graft failure; (F) dose-response risk of increasing MaS degree on 180-day patients’ mortality; (G) dose-response risk of increasing MaS degree on 180-day graft failure. The black solid and dashed curves represented instant ORs and their respective 95% CIs for post-transplant outcomes compared to subgroup using allografts without steatosis based on the restricted cubic splines model. CI, confidence interval; EAD, early-allograft dysfunction; MaS, macrovesicular steatosis; OR, odds ratio.
Severer MaS caused significant EAD increment in linear pattern (Figure 3A). Compared to non-steatotic cases, about 3.64 folds of higher risk was observed in patients received grafts with 30% of MaS content (OR =13.7, 95% CI: 1.74–118.7, P<0.05). While, the risk trend of organ/patient mortality was not increased accordingly followed with EAD increment (Figure 3). Gentle increment on organ failure was observed in linear manner (P for non-linearity >0.05). And the threshold for MaS content was approximate 10%, 10% and 15% for overall, 90-d, and 180-d mortality (Figure 3, Table S8).
Interestingly, MaS affected 90-d and overall patient survival in non-linear pattern (P for non-linearity test <0.05, Figure 3, Table S8). Prominent MaS related risk came into plateau within content between 10% and 25%, and lost significance in higher MaS group (>25%). Linearity was recovered on risk of 180-d patient mortality. And the safety threshold of MaS was below 15% for 180-d patient survival (Figure 3, Table S8).
MiS affected the EAD occurrence and patient/organ mortality in linear pattern. But the trend was insignificant followed with increasing MiS content (all P>0.05, Figure S3, Table S8).
Clear risk stratification in cases using steatotic grafts with simultaneous serious post-transplant liver damage
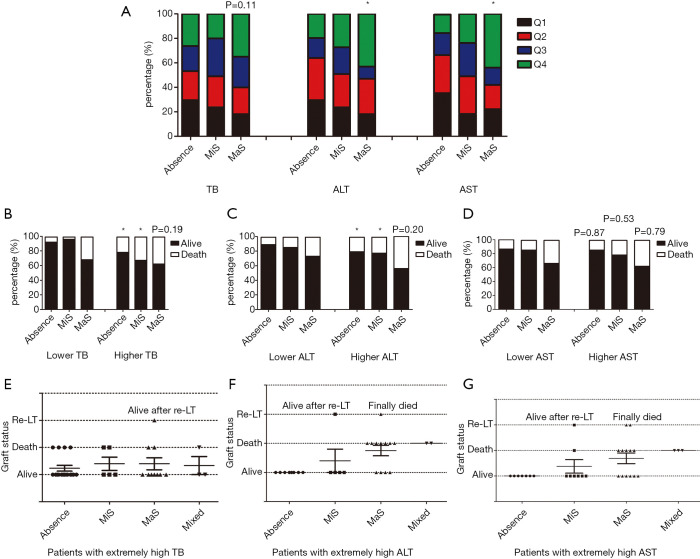
Evaluation was performed on inter-relationship across donor steatosis, post-transplant liver function and prognosis. Compared to absent group, patients received MaS donor tended to have severer liver damage (OR for Q4 ALT =3.33, 95% CI: 1.30–8.54; and OR for Q4 AST =4.12, 95% CI: 1.65–10.2, P<0.05, Figure 4A). While, this trend was unobvious on TB level (OR for Q4 TB =2.13, 95% CI: 0.83–5.43, P=0.11, Figure 4A). Interestingly, significant increment on peak TB and ALT related mortality was only observed in patients received MiS or non-steatotic grafts (P<0.05, Figure 4). But MaS related mortality was stable and consistent, with independence of TB or ALT classification (P>0.05, Figure 4).
Figure 4.
Impact of steatosis on stratification of post-transplant LFTs indicators and their integrative effects on prognosis after LT. (A) Stratification of TB, ALT and AST level by quartiles in subgroups categorized by stetosis types, percentage of patients with Q4 ALT/AST was higher in MaS group; (B) mortality stratification in MaS, MiS and absence subgroups categorized by TB level, elevated mortality was presented in MiS and absence group caused by higher TB level; (C) mortality stratification in MaS MiS and absence subgroups categorized by ALT level, elevated mortality was presented in MiS and absence group caused by higher ALT level; (D) mortality stratification in MaS MiS and absence subgroups categorized by AST level, elevated mortality was presented in MiS and absence group caused by higher AST level; (E) stratification of graft status categorized by steatosis type in patients with extremely higher TB value; (F) stratification of graft status categorized by steatosis type in patients with extremely higher ALT value; (G) stratification of graft status categorized by steatosis type in patients with extremely higher ALT value. * represented significant difference in corresponded comparisons (P<0.05). P value above the bars represented the significance in corresponded comparisons. ALT, alanine aminotransferase; AST, aspartate aminotransferase; LFTs, liver function tests; LT, liver transplantation; MaS, macro-steatosis; MiS, micro-steatosis; TB, total bilirubin.
Effects of steatosis were evaluated separately in patients with extremely high ALT (>2,500 U/L), AST (>7,000 U/L) or TB (>400 µmol/L) value. Interestingly, death was only occurred in MaS or mixed group when patients were confined in extremely high ALT level (Figure 4). Much less death was observed in MiS or absent group with extremely high AST level [1 (12.5%) in MiS group vs. 5 (38.5%) in MaS group]. However, this trend was not obvious in patients with extremely high peak TB value. Compared to MiS or non-steatotic group, no more death/graft failure was observed in patients using MaS allografts (P for distribution >0.05, Figure 4).
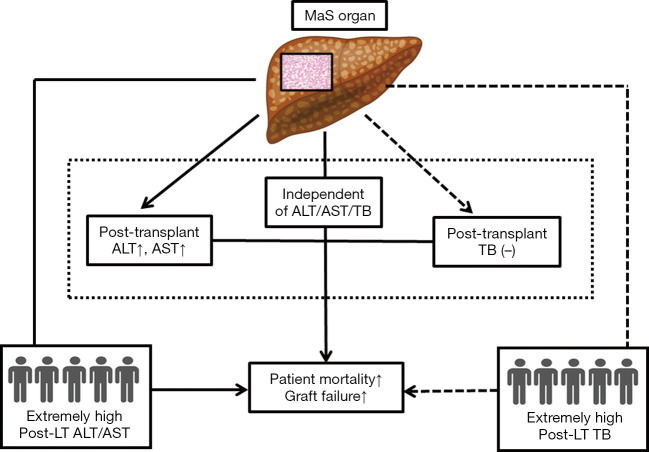
In addition, death was still frequent (62.5%) even in patients without EAD occurrence. MaS effect on post-transplant prognosis was independent of EAD prevalence in subgroup with extremely high ALT (P>0.05 for distribution, Table S9). Therefore, MaS allografts seemed to have significant impacts on post-transplant aminotransferase but not bilirubin levels. Meanwhile, donor MaS significantly worsen the post-transplant survival in patients experienced severe hepatic damage (presented by extremely high ALT or AST). And the inter-relationship amongst donor MaS, LFTs, and post-transplant outcomes were summarized in Figure 5.
Figure 5.
Schematic diagram revealing the complex inter-relationship amongst MaS and post-transplant LFTs and prognosis. Solid arrowed line was represented as positive connection between two covariates (e.g., MaS and post-transplant ALT level); dashed arrowed line was represented as negative connection between two covariates (e.g., MaS and post-transplant TB level). ALT, alanine aminotransferase; AST, aspartate aminotransferase; LT, liver transplantation; MaS, macrosteatosis; TB, total bilirubin.
Disease outcome of re-transplanted case series and their association with allograft steatosis
Features of cases received re-transplantation were listed respectively in Table 6. Six patients had re-transplantation in 1–15 days after first operation for the failure of transplanted grafts. PNF was defined in 4 of these cases (1.6%) for shorter interval (<72 hours) across two LT.
Table 6. Clinical features of patients received liver re-transplantation.
| Case no. | Recipient general information | Graft & surgery | Post-transplant outcome | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Gender | Primitive disease | HBV infection | Blood type | BMI (kg/m2) | Type | Degree (%) | LT-time | PCC use (U) | Operation length (min) | EAD | PNF | Treatment | Graft survival (day) | Death cause | |||
| 1 | 50 | M | HCC | Y | O | 25.5 | MaS | 35 | 1st | 10 | 337 | Y | Y | Re-transplanted | 1 | |||
| MiS | 30 | 2nd | 10 | 228 | Y | N | Dead | 136 | MODS | |||||||||
| 2 | 54 | M | HCC | Y | O | 21.9 | MiS | 10 | 1st | 0 | 319 | Y | Y | Re-transplanted | 3 | |||
| MiS | 10 | 2nd | 0 | 330 | Y | N | Alive | 559 | ||||||||||
| 3 | 64 | M | HCC | Y | O | 22.4 | MaS | 10 | 1st | 15 | 399 | Y | N | Re-transplanted | 14 | |||
| MaS | 10 | 2nd | 14 | 240 | Y | N | Dead | 4 | Graft failure | |||||||||
| 4 | 30 | M | Liver failure | N | A | 23.4 | MaS | 20 | 1st | 8 | 278 | N | Y | Re-transplanted | 3 | |||
| MaS | 20 | 2nd | 8 | 237 | N | N | Alive | 288 | ||||||||||
| 5 | 39 | M | HCC | Y | O | 22.6 | no | 0 | 1st | 0 | 329 | N | N | Re-transplanted | 15 | |||
| no | 0 | 2nd | 0 | 222 | N | N | Alive | 422 | ||||||||||
| 6 | 52 | F | Cholestatic cirrhosis | N | B | 18.0 | MiS | 20 | 1st | 0 | 239 | Y | Y | Re-transplanted | 3 | |||
| no | 0 | 2nd | 0 | 191 | Y | N | Alive | 465 | ||||||||||
BMI, body mass index; EAD, early allograft dysfunction; F, female; HCC, hepatocellular carcinoma; LT, liver transplantation; M, male; MaS, macrosteatosis; MiS, microsteatosis; MODS, multi-organ dysfunction syndrome; N, no; PCC, prothrombin complex concentrate; PNF, primary non-function; Y, yes.
Donor MaS seemed to be the major contributor of PNF and related mortality. In our cohort, two of four PNF patients received MaS grafts for first LT. Otherwise, both two death cases after re-transplantation received MaS organs in the first LT. The only one survivor (case 4) received MaS related LT might be benefited from his relative younger age (30 years) and less burden of primitive liver disease (non-cancer, absence of HBV infection). MaS donor seemed not the absolute contradiction for further transplantation, but the recipient should be highly selective in less serious patients for better prognosis. Unlike the MaS donors, it seemed to be safer for LT with the use of MiS grafts. Two MiS related PNF cases had favorable prognosis after re-transplantation. And no death was observed in the end of follow-up duration (Table 6).
Correspondingly, trend of LFTs indicators and PT value was presented respectively in peri-operative period from 3 days before LT to 2 weeks after LT. As shown in Figure S4, post-transplant liver transaminase seemed much higher in death cases. The peak AST level was higher in both death cases (>15,000 U/L) than the remaining alive cases (<8,000 U/L). However, post-transplant PT and TB value was comparable across individuals, regardless of difference on post-transplant prognosis.
Individualized risk nomogram of post-transplant outcomes and internal validation test
Nomograms for prediction of post-transplant prognosis and complications was plotted by incorporation of graft steatosis and other independent clinical factor which was validated in aforementioned risk evaluation. MaS caused severer post-transplant outcomes followed with increasing severity by gradient. Severer MaS (G3) caused significant decrease on post-transplant patient/graft survival. To be specific, the 180-day patient/graft survival was decreased to less than 85% and around 80% in patients using severer MaS organs (Figure 6). MiS or mixed allograft steatosis can also predict parts of EAD occurrence, but these effects were limited and inconsistent (Figure 6). C-index for nomogram on EAD occurrence, patient and graft survival was 0.75 (0.73–0.77), 0.73 (0.71–0.75), and 0.73 (0.71–0.75), respectively. And calibration plot revealed consistent trend between actual and nomogram predicted post-transplant outcomes (Figure S5).
Figure 6.
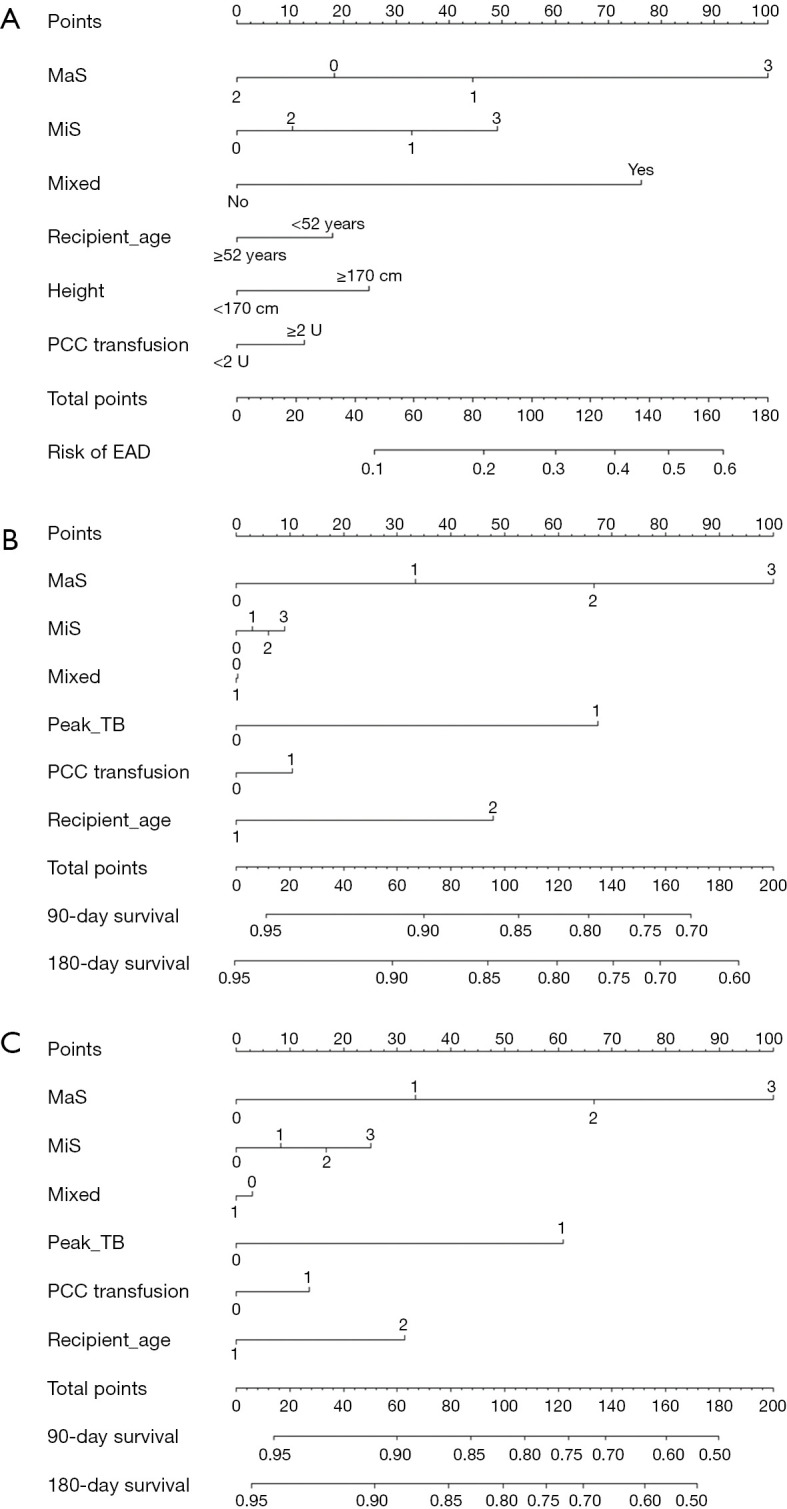
Nomogram for prediction of outcomes in patients after liver transplantation. Risk of incidence is located on the point in each point in each variable axis, and line drawn upwards can determine the points received for each variable. Cumulative risk of individual factors is located on total point axis, and the line drawn downwards can determine the risk of each post-transplant outcomes. (A) Nomogram for prediction of post-transplant EAD occurrence; (B) nomogram for prediction of 90-, 180-day patients’ survival after LT; (C) nomogram for prediction of 90-, 180-day grafts’ survival after LT. MaS, macrosteatosis; MiS, microsteatosis; PCC, prothrombin complex concentrate; TB, total bilirubin.
Discussion
Currently, allograft steatosis is one of the most prominent risk cofactor on inferior post-transplant survival (28). Emerging evidence has revealed that steatosis (especially for MaS) was the major reason for potential allograft being discarded for transplantations, affecting 40–60% of cancelled organ donation (1,29). While, expansion of donor pool is urgently needed by optimizing the utilization of steatotic allografts to meet the demands from increasing patients on waitlist for LT. Meanwhile, concurrence of steatosis with other risk factor was also observed before (28). Therefore, continuous risk profiles of donor steatosis in complex context of specific population should be assessed to keep benefits from liver transplantation on the premise of acceptable post-operational prognosis. In a Chinese cohort from a newly-established transplant center, we found: (I) steatosis was highly prevalent in Chinese donation for LT, affecting around half (48.6%) of allografts; (II) MaS (but not MiS) amplified the risk of inferior post-transplant prognosis; (III) more complications and inferior prognosis were presented followed with increasing MaS (but not MiS) severity in dose-response manner; (IV) Chinese recipients seemed more susceptive to MaS grafts and the safety threshold of MaS was lowered to 10%; (V) positive impacts of MaS on post-transplant complications were stable and consistent with independence of peak bilirubin or transaminase level; (VI) MaS graft application in first LT was the main cause of death in patients received re-transplantation; (VII) mortality gap was clearly stratified by MaS classification, in patients with serious liver damage.
To the extent of our knowledge, this is a retrospective study with enrollment of largest Chinese patients with focus on building connection between allograft steatosis and post-transplant outcomes. We systematically reviewed the published data (16) and found only one literature (30) assessed the continuous risk of MaS on post-transplant outcomes in Chinese recipients. In the small sized cohort (70 LT pairs), Li et al. found insignificant impact of MaS on patients’ prognosis, but the conclusion seemed inaccurate for inappropriate classification in comparison. Patients using non-steatotic grafts weren’t categorized as an independent group which might cause overestimation of baseline data. In our study, obvious risk was presented on overall patient/graft mortality. Categorical comparison revealed insignificant risk for severe MaS organ (>20%) on patients’ survival (Table 4). However, “severity-response” analysis found the risk for MaS was stable and consistent on patient/organ mortality when MaS exceeded 10% (Table S8). Interestingly, risk plateau was observed between moderate and severe MaS group, and patient mortality didn’t increase linearly followed with MaS severity (Table S8). However, the risk of MaS grafts still exists on post-LT prognosis for higher organ failure followed with increasing MaS severity in linear pattern (Figure 3, Table S8). Unlike MaS, there seems no obvious connection between MiS allograft and inferior prognosis in categorical comparison and continuous regression model (Figure 3, Table S9) conformed to majority of other studies (6,31-33).
Conformed to prior study (34), severe MaS also increased the post-transplant EAD occurrence in our patient cohort. However, the risk cut-off of MaS on EAD occurrence was around 25%, much higher than expected safety threshold of MaS patient/organ mortality (10%, Table 3). Otherwise, similar transaminase level was observed in patients grouped by MaS severity. These results indicated MaS organs might have inferior effects even in similar post-transplant recovery. More concerns are needed on MaS organ recipients. Consistent with previous studies (35,36), retransplantation was mainly contributor for PNF/DNF, and MaS was the major culprit for death of retransplanted patients in our study (Table 6). Two re-LT patients received MaS allografts had inferior prognosis, regardless of the quality of second implants, but another relatively younger patient with non-malignant disease was still alive until the end of follow-up duration. And this discrepancy implied the importance of recipient selection in utilization of steatotic organ on further LT (37). However, less patients with re-LT in this study and more cases are needed for further validation.
ALT is a sensitive biomarker in detecting and screening liver damage (38). But its clinical meaning and interactions with allograft MaS on prognosis was still unclear. Peak ALT level was considered as a quantitative biomarker to evaluate the extend of ischemia–reperfusion injury (IRI) (39). Knowledge from animal experiment found hepatic steatosis per se might cause severer injury in the same circumstance of ischemia and reperfusion treatment (5,40). In our study, donor MaS seemed to have complex association by interaction with peak liver enzyme and post-transplant prognosis. Organ MaS caused more IRI presented as higher peak ALT in patients after LT. But on the other side, MaS induced inferior prognosis independent of its effects on ALT elevation. In addition, clear mortality gap was observed in subgroup with severer IRI pressure. In patients with extremely higher post-transplant ALT level, disproportionately higher mortality was observed in patients received MaS organ (64% vs. 0% for MaS vs. MiS, Figure 4). Similar effect was also observed in group with extremely high AST for its correlation with ALT. Noteworthy, graft loss was highly prevalent in MaS patients, regardless of EAD occurrence after LT. These data revealed MaS (but not MiS) organ might be more fragile under the exposure of severer IRI. And this effect can last longer than peri-operative period. Coincident with our results, scholars found the quality of MaS donor could be guaranteed on the premise of limited CIT (8). Hence, MaS organ should be treated with more cautions or avoided to be used in patients with high-risk of severer post-transplant IRI.
Except MaS, clinical factors including the intra-operative PCC transfusion and post-transplant peak TB value also affected the prognosis of patients after LT (Table 4 and Table 5). Increased blood product transfusion is usually connected to adverse post-transplant outcomes after LT (41). PCC administration was considered as safe and effective strategy for improved haemostasis in LT (42). But our results found higher patient/organ mortality followed in dose-response manner with unclear reasons. More concerns should be raised on management of PCC utilization in further clinical practice. In consistence with previous study (42), elevated post-transplant TB value was another stronger indictor on prediction of severer adverse outcomes after LT. However, we didn’t find donor MaS had interactive effects on intra-operative PCC transfusion or post-transplant TB value. After adjustment of these potential confounders, MaS was still a stable and independent predictor on inferior post-transplant outcomes.
MaS but not MiS might affect recipient post-LT prognosis in our study. The MaS organ seemed to have lower resistance under the same ischemia-reperfusion exposure. However, the reason is unclear and worthy for elucidation (14). Croome et al. summarized potential mechanism with connection between MaS and liver damage, including impaired sinusoidal blood flow, abnormal mitochondria function, increased neutrophil aggregation and cytokine release (37). Meanwhile, cumulative evidence revealed genetic factors might be involved in graft steatosis and LT (43,44). In addition, machine perfusion (MP) is considered as an effective strategy for recondition of steatotic grafts for LT (29). Integrated multi-omic analysis might help to find out the main culprits covered under the complex network and potential targets for improving the organ quality (45).
In our study, all cases were collected from a newly established LT center, and finished by a senior experienced surgeon (SSZ). Unified standard was adopted on donor/recipient selection, surgical procedure and perioperative management, which can avoid potential confounders as guarantee and prerequisite for further comparison. Limitations of this study should also be noted as well. First, relatively shorter follow-up in enrolled patients. Second, results of this study need validation from external cohort. Third, potential bias was inevitable for disequilibrium on age distribution and missing information in donors. Forth, previous study found possible stratification in MaS group for varied Liver texture between large-droplet and small-droplet MaS donors (10). This internal bias might also cause inaccurate results into risk assessment. Fifth, precise MaS cut-off for LT should also be assessed by comparison with similar patients who chose non-transplant therapies, which we didn’t perform. But regardless of these limitations, results are still worthy to be reported for its novelty and revelation on further mechanistic investigation.
Conclusions
In conclusion, we found donor MaS, but MiS positively affected post-transplant prognosis and complication with relatively lower safety threshold (around 10%) for LT in Chinese patient cohort. Stable effects were exerted by MaS on patient death with independence of post-transplant liver damage. MaS related mortality gap was much more prominent in patients with extremely high post-transplant transaminase levels. Allograft MaS was the major cause of PNF occurrence and patients’ death after re-transplantation. Further external validation in larger cohort and mechanistic investigation are needed to validate these findings.
Acknowledgments
Funding: This study is supported by Innovative Research Groups of National Natural Science Foundation of China (81721091), Major program of National Natural Science Foundation of China (91542205), National S&T Major Project (2017ZX10203205), National Natural Science Foundation of China (81902813), Zhejiang International Science and Technology Cooperation Project (2016C04003), Zhejiang Provincial Natural Science Foundation of China (LY18H030002), Zhejiang Medical Association (2019ZYC-A81), International Youth Exchange Programme by China Association for Science and Technology (2019), Tianqing Liver Diseases Research Fund (TQGB20200114), and Open Fund of Key laboratory of High-Incidence-Tumor Prevention & Treatment (Guangxi Medical University), Ministry of Education.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consents were obtained from each enrolled participant. This study was performed in accordance with the Declaration of Helsinki and approved by the ethical board of Shulan Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College (2019-IIT-237).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/hbsn.2019.12.02
Data Sharing Statement: Available at http://dx.doi.org/10.21037/hbsn.2019.12.02
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/hbsn.2019.12.02). The authors have no conflicts of interest to declare.
References
- 1.Moosburner S, Gassner JM, Nösser M, et al. Prevalence of steatosis hepatis in the eurotransplant region: impact on graft acceptance rates. HPB Surg 2018;2018:6094936. 10.1155/2018/6094936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koneru B, Dikdan G. Hepatic steatosis and liver transplantation current clinical and experimental perspectives. Transplantation 2002;73:325-30. 10.1097/00007890-200202150-00001 [DOI] [PubMed] [Google Scholar]
- 3.Selzner N, Selzner M, Jochum W, et al. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol 2006;44:694-701. 10.1016/j.jhep.2005.07.032 [DOI] [PubMed] [Google Scholar]
- 4.Hatsugai K, Ohkohchi N, Fukumori T, et al. Mechanism of primary graft non-function in a rat model for fatty liver transplantation. Transpl Int 2000;13:S583-90. 10.1111/j.1432-2277.2000.tb02111.x [DOI] [PubMed] [Google Scholar]
- 5.Liss KH, McCommis KS, Chambers KT, et al. The impact of diet‐induced hepatic steatosis in a murine model of hepatic ischemia/reperfusion injury. Liver Transpl 2018;24:908-21. 10.1002/lt.25189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed EA, El-Badry AM, Mocchegiani F, et al. Impact of graft steatosis on postoperative complications after liver transplantation. Surg J (N Y) 2018;4:e188-96. 10.1055/s-0038-1675236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong TC, Fung JY, Chok KS, et al. Excellent outcomes of liver transplantation using severely steatotic grafts from brain‐dead donors. Liver Transpl 2016;22:226-36. 10.1002/lt.24335 [DOI] [PubMed] [Google Scholar]
- 8.Westerkamp AC, de Boer MT, van den Berg AP, et al. Similar outcome after transplantation of moderate macrovesicular steatotic and nonsteatotic livers when the cold ischemia time is kept very short. Transpl Int 2015;28:319-29. 10.1111/tri.12504 [DOI] [PubMed] [Google Scholar]
- 9.Dutkowski P, Schlegel A, Slankamenac K, et al. The use of fatty liver grafts in modern allocation systems: risk assessment by the balance of risk (BAR) score. Ann Surg 2012;256:861-8. 10.1097/SLA.0b013e318272dea2 [DOI] [PubMed] [Google Scholar]
- 10.Bidkhori G, Benfeitas R, Klevstig M, et al. Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proc Natl Acad Sci U S A 2018;115:E11874-83. 10.1073/pnas.1807305115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl 2003;9:651-63. 10.1053/jlts.2003.50105 [DOI] [PubMed] [Google Scholar]
- 12.Barshes NR, Horwitz I, Franzini L, et al. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant 2007;7:1265-70. 10.1111/j.1600-6143.2007.01758.x [DOI] [PubMed] [Google Scholar]
- 13.Tector AJ, Mangus RS, Chestovich P, et al. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg 2006;244:439-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Álvarez‐Mercado AI, Gulfo J, Romero Gómez M, et al. Use of steatotic grafts in liver transplantation: Current status. Liver Transpl 2019;25:771-86. 10.1002/lt.25430 [DOI] [PubMed] [Google Scholar]
- 15.Linares I, Hamar M, Selzner N, et al. Steatosis in Liver Transplantation: Current Limitations and Future Strategies. Transplantation 2019;103:78-90. 10.1097/TP.0000000000002466 [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Jia J, Ning H, et al. Systematic Evaluation of the Safety Threshold for Allograft Macrovesicular Steatosis in Cadaveric Liver Transplantation. Front Physiol 2019;10:429. 10.3389/fphys.2019.00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalisvaart M, de Haan JE, Polak WG, et al. Comparison of postoperative outcomes between donation after circulatory death and donation after brain death liver transplantation using the comprehensive complication index. Ann Surg 2017;266:772-8. 10.1097/SLA.0000000000002419 [DOI] [PubMed] [Google Scholar]
- 18.Rana A, Hardy M, Halazun K, et al. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant 2008;8:2537-46. 10.1111/j.1600-6143.2008.02400.x [DOI] [PubMed] [Google Scholar]
- 19.Nicolau‐Raducu R, Cohen AJ, Bokhari A, et al. Predictive model and risk factors associated with a revised definition of early allograft dysfunction in liver transplant recipients. Clin Transplant 2017;31:e13097. 10.1111/ctr.13097 [DOI] [PubMed] [Google Scholar]
- 20.Briceño J, Padillo J, Rufián S, et al. Assignment of steatotic livers by the Mayo model for end‐stage liver disease. Transpl Int 2005;18:577-83. 10.1111/j.1432-2277.2005.00091.x [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 22.de Graaf EL, Kench J, Dilworth P, et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol 2012;27:540-6. 10.1111/j.1440-1746.2011.06844.x [DOI] [PubMed] [Google Scholar]
- 23.Kamath PS, Kim WR, Advanced Liver Disease Study Group The model for end‐stage liver disease (MELD). Hepatology 2007;45:797-805. 10.1002/hep.21563 [DOI] [PubMed] [Google Scholar]
- 24.Cholongitas E, Papatheodoridis G, Vangeli M, et al. Systematic review: the model for end‐stage liver disease–should it replace Child‐Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther 2005;22:1079-89. 10.1111/j.1365-2036.2005.02691.x [DOI] [PubMed] [Google Scholar]
- 25.Orsini N, Bellocco R, Greenland SJ. Generalized least squares for trend estimation of summarized dose–response data. Stata Journal 2006;6:40-57. 10.1177/1536867X0600600103 [DOI] [Google Scholar]
- 26.Pencina MJ, D'Agostino RBJSim. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109-23. 10.1002/sim.1802 [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE Jr. rms: Regression modeling strategies. 2016;5.
- 28.Moosburner S, Sauer IM, Gassner JM, et al. Macrosteatosis is a huge problem in liver transplantation—however, not the only one we face. Am J Transplant 2019;19:2661-2. 10.1111/ajt.15418 [DOI] [PubMed] [Google Scholar]
- 29.Boteon YL, Boteon AP, Attard J, et al. Ex situ machine perfusion as a tool to recondition steatotic donor livers: Troublesome features of fatty livers and the role of defatting therapies. A systematic review. Am J Transplant 2018;18:2384-99. 10.1111/ajt.14992 [DOI] [PubMed] [Google Scholar]
- 30.Li J, Liu B, Yan LN, et al. Reversal of graft steatosis after liver transplantation: prospective study. In: Transplantation proceedings. Elsevier; 2009:3560-63. [DOI] [PubMed] [Google Scholar]
- 31.Han S, Ko JS, Kwon G, et al. Effect of pure microsteatosis on transplant outcomes after living donor liver transplantation: A matched case‐control study. Liver Transpl 2014;20:473-82. 10.1002/lt.23824 [DOI] [PubMed] [Google Scholar]
- 32.Han S, Ha SY, Park CK, et al. Microsteatosis may not interact with macrosteatosis in living donor liver transplantation. J Hepatol 2015;62:556-62. 10.1016/j.jhep.2014.10.027 [DOI] [PubMed] [Google Scholar]
- 33.Croome KP, Lee DD, Croome S, et al. Does donor allograft Microsteatosis Matter? comparison of outcomes in liver transplantation with a propensity matched cohort. Liver Transpl 2019;25:1533-40. 10.1002/lt.25583 [DOI] [PubMed] [Google Scholar]
- 34.Hoyer DP, Paul A, Gallinat A, et al. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int 2015;35:156-63. 10.1111/liv.12443 [DOI] [PubMed] [Google Scholar]
- 35.Zamboni F, Franchello A, David E, et al. Effect of macrovescicular steatosis and other donor and recipient characteristics on the outcome of liver transplantation. Clin Transplant 2001;15:53-7. 10.1034/j.1399-0012.2001.150109.x [DOI] [PubMed] [Google Scholar]
- 36.Kulik U, Lehner F, Klempnauer J, et al. Primary non‐function is frequently associated with fatty liver allografts and high mortality after re‐transplantation. Liver Int 2017;37:1219-28. 10.1111/liv.13404 [DOI] [PubMed] [Google Scholar]
- 37.Croome KP, Lee DD, Taner CB. The “Skinny” on Assessment and Utilization of Steatotic Liver Grafts: A Systematic Review. Liver Transpl 2019;25:488-99. 10.1002/lt.25408 [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Que S, Xu J, et al. Alanine aminotransferase-old biomarker and new concept: a review. Int J Med Sci 2014;11:925-35. 10.7150/ijms.8951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Dulk AC, Sebib Korkmaz K, de Rooij BJ, et al. High peak alanine aminotransferase determines extra risk for nonanastomotic biliary strictures after liver transplantation with donation after circulatory death. Transpl Int 2015;28:492-501. 10.1111/tri.12524 [DOI] [PubMed] [Google Scholar]
- 40.Gehrau RC, Mas VR, Dumur CI, et al. Donor hepatic steatosis induce exacerbated ischemia-reperfusion injury through activation of innate immune response molecular pathways. Transplantation 2015;99:2523-33. 10.1097/TP.0000000000000857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rana A, Petrowsky H, Hong JC, et al. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. J Am Coll Surg 2013;216:902-7. 10.1016/j.jamcollsurg.2012.12.047 [DOI] [PubMed] [Google Scholar]
- 42.Hartmann M, Szalai C, Saner FH. Hemostasis in liver transplantation: Pathophysiology, monitoring, and treatment. World J Gastroenterol 2016;22:1541-50. 10.3748/wjg.v22.i4.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Šeda O, Cahova M, Míková I, et al. Hepatic gene expression profiles differentiate steatotic and non-steatotic grafts in liver transplant recipients. Front Endocrinol (Lausanne) 2019;10:270. 10.3389/fendo.2019.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raza A, Dikdan G, Desai KK, et al. Global gene expression profiles of ischemic preconditioning in deceased donor liver transplantation. Liver Transpl 2010;16:588-99. 10.1002/lt.22049 [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Zhang C, Liu Z, et al. Network analyses identify liver‐specific targets for treating liver diseases. Mol Syst Biol 2017;13:938. 10.15252/msb.20177703 [DOI] [PMC free article] [PubMed] [Google Scholar]