Significance
To adapt to the changing environment, bacteria must quickly transduce extracellular information into appropriate cellular response pathways. Two-component systems consisting of a membrane-embedded histidine kinase (HK) and a cytoplasmic response regulator often perform this function. In this work, we present structural and functional data showing that HptRSA, a G6P sensor from Staphylococcus aureus, undergoes conformational changes after G6P binding with HptA that causes it to switch its interface with the periplasmic domain of HptS. As a result, a rotation and closure occur in the periplasmic side of the HK. This previously unreported mode of activation not only expands our understanding of HK signal perception in kinase activation but also provides a framework for designing new antimicrobial drugs.
Keywords: two-component system, HptRSA, G6P sensing, cross-membrane signaling
Abstract
Two-component systems (TCS), which typically consist of a membrane-embedded histidine kinase and a cytoplasmic response regulator, are the dominant signaling proteins for transduction of environmental stimuli into cellular response pathways in prokaryotic cells. HptRSA is a recently identified TCS consisting of the G6P-associated sensor protein (HptA), transmembrane histidine kinase (HptS), and cytoplasmic effector (HptR). HptRSA mediates glucose-6-phosphate (G6P) uptake to support Staphylococcus aureus growth and multiplication within various host cells. How the mechanism by which HptRSA perceives G6P and triggers a downstream response has remained elusive. Here, we solved the HptA structures in apo and G6P-bound states. G6P binding in the cleft between two HptA domains caused a conformational closing movement. The solved structures of HptA in complex with the periplasmic domain of HptS showed that HptA interacts with HptS through both constitutive and switchable interfaces. The G6P-free form of HptA binds to the membrane-distal side of the HptS periplasmic domain (HptSp), resulting in a parallel conformation of the HptSp protomer pair. However, once HptA associates with G6P, its intramolecular domain closure switches the HptA-HptSp contact region into the membrane-proximal domain, which causes rotation and closure of the C termini of each HptSp protomer. Through biochemical and growth assays of HptA and HptS mutant variants, we proposed a distinct mechanism of interface switch-mediated signaling transduction. Our results provide mechanistic insights into bacterial nutrient sensing and expand our understanding of the activation modes by which TCS communicates external signals.
Two-component systems (TCSs), widely distributed across prokaryotic taxa, are key proteins in the transduction of environmental stimuli into cellular response pathways (1, 2). Tens to hundreds of TCS systems may be present in a given bacterium (3, 4), enabling the perception of a variety of extracellular stimuli such as light, temperature, nutrients, antimicrobial chemicals, and so on (5–11). A typical TCS consists of a membrane-embedded histidine kinase (HK) and its cognate cytoplasmic response regulator (RR). A canonical HK contains four major domains: a modular periplasmic domain to sense the signal; two transmembrane (TM) helices; an intracellular signal-relay HAMP (domain found in Histidine kinases, Adenyl cyclases, Methyl-accepting proteins, and Phosphatases); and a conserved intracellular kinase domain that catalyzes autophosphorylation and phosphotransfer to the RR (11–15). Once phosphorylated at a conserved aspartate, the RR typically regulates transcription of the cognate genes to trigger an appropriate cellular response. The HK usually exists as a preformed dimer with four discrete TM domain helices spanning the membrane (11). A conformational change in the sensor domain induced by binding or recognition of the relevant signal then rearranges the four-helix bundle, subsequently activating the cytoplasmic phosphorelay (11, 13, 14, 16). In order to adapt to various environmental cues, microorganisms have accumulated thousands of different HKs (3, 4). Accordingly, various sensor domains with divergent sequences have evolved to respond to specific stimuli. Although the primary amino acid sequences lack significant similarity, common folds like the combined α-β fold and α-helical fold were predominantly adopted by the HK sensor domain. Among those, the PDC (PhoQ-DcuS-CitA) domain, which has an α-β fold, has been repeatedly observed in small-molecule sensing HKs such as CitA and DcuS (8, 17). In contrast, the ligand-binding pocket of each PDC is typically divergent, accommodating various, specific (or multiple) ligands. Notably, the precise mechanism of activation also varies with the nature of the receptor and input signal (18). Structural investigations have led to several proposed modes of sensor activation. For example, the contraction of CitA upon citrate binding induces a piston-like displacement in the TM region to activate the kinase activity (19). In contrast, a scissor-like closing movement in the disaccharide-bound sensor domain of Bacteroides BT4663 was shown to drive signal transduction (20). A state change caused by ligand-responsive oligomerization was demonstrated to activate XylFII-LytS in d-xylose sensing (21). The symmetry-to-asymmetry switch in HK or vice versa, performed by LuxPQ (22) and TorT-TorS (23), respectively, were shown to be directly linked to the activation state (22, 23). While these models were proposed for each of these respective HK systems, they are not actually mutually exclusive. This point was clearly revealed by structural study of NarX, wherein piston-like, scissoring, as well as symmetry rearrangement were all observed simultaneously (24).
Recently, the HptRSA TCS from Staphylococcus aureus was identified as responsible for carbon uptake when the only available sugar was limited to glucose-6-phosphate (G6P) (25, 26). Notably, S. aureus is a major human pathogen, causing a wide spectrum of nosocomial and community-acquired diseases (27). In the past decades, its prevalence and increasing antibiotic resistance has resulted in S. aureus infection becoming a leading cause of mortality among infectious diseases (28). New treatment strategies against S. aureus are thus urgently needed that use detailed understanding of relevant metabolic and physiological processes, such as the mechanisms of nutrient uptake. To this end, we investigated the HptRSA system, which is comprised of three components: HptA, the G6P sensor; HptS, an HK; and HptR, an intracellular effector (25, 26). Once phosphorylated, HptR initiates the expression of UhpT, which is a G6P transporter (25, 26, 29). The HptRSA system thus plays a critical role in S. aureus multiplication within the various types of host cells where G6P is the predominant sugar (25, 30). However, the underlying mechanism by which HptA specifically perceives G6P, and transmits this signal to HptS, remains largely unknown.
Here, in this work, we present the structures of HptA complexed with the periplasmic domain of HptS in both G6P-bound and apo forms. We show first that HptA is highly specific to G6P and that G6P binds in the cleft between two HptA domains. G6P binding induces a conformational change in HptA in which its intramolecular domain switches the HptA-HptSp contact region to the membrane-proximal domain, thereby causing a rotation and closure of the C termini of each HptSp protomer to activate the HptS. Our results provide mechanistic insights into bacterial nutrient sensing and expand our understanding of the activation modes by which TCS communicate external signals.
Results
Ligand Specificity of HptA.
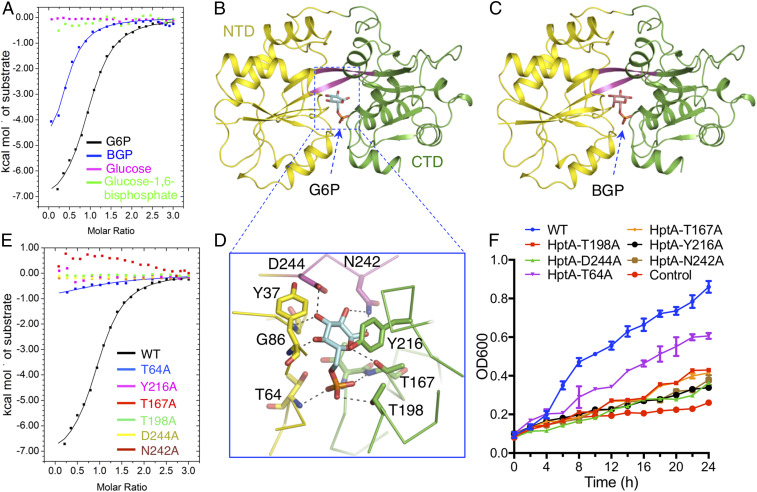
To further explore the ligand perception range of HptA, we conducted isothermal titration calorimetry (ITC) assays to screen for binding of HptA to common sugar phosphates. HptA binds to G6P as reported (26) but cannot tolerate replacement of a carbon atom or phosphate group in G6P (Fig. 1A and SI Appendix, Fig. S1A and Table S1). The only other ligand to which it displayed comparable affinity was galactose-6-phosphate (BGP), which shares high structural similarity with G6P (Fig. 1A and SI Appendix, Fig. S1A and Table S1). These results suggest that the sugar ring and phosphate group jointly contribute to determining the specificity in ligand sensing. To decipher the structural details recognized by HptA, we cocrystallized HptA with G6P and BGP and solved the structures at 1.5-Å and 2.0-Å resolution, respectively (SI Appendix, Table S2). HptA possesses two globular α/β domains (termed the N-terminal domain, NTD, and C-terminal domain, CTD, respectively) that are connected by a dual-β-strand hinge (Fig. 1B). The two structures of HptA share an almost identical conformation, with both ligands bound at the cleft of the two domains (rmsd 0.1 Å) (Fig. 1 B and C).
Fig. 1.
The ligand specificity of HptA. (A) ITC assays demonstrate that HptA interacts specifically with G6P and BGP. Structures of HptA in complex with G6P (B) and BGP (C). The HptA NTD, linker β-strand, and CTD are indicated by yellow, magenta, and green, respectively. G6P and BGP are shown in cyan and orange sticks, respectively. (D) The contacts between G6P and HptA residues in the ligand-binding site. (E) The G6P binding affinity of HptA mutants measured by ITC. (F) The growth kinetics in G6P-limited medium of ΔhptRSA S. aureus strains carrying HptRSA mutant constructs with the indicated single amino acid conversions in HptA. “Control” and “WT” represent the ΔhptRSA S. aureus strain transformed with the empty vector or plasmid containing a WT hptRSA gene cassette, respectively.
Residues from both the NTD and CTD as well as the hinge β-stand comprise the ligand-binding pocket (Fig. 1D and SI Appendix, Fig. S1 B and C). In the HptA-G6P structure, the sugar ring buried deep in the pocket is mainly coordinated by the hydrogen bonds with N242, D244, T167, and G86 (Fig. 1D). In addition, the sugar ring also forms van der Waals interactions with the aromatic ring of Y216 (Fig. 1D), which ostensibly fixes the G6P in the specified orientation. Consistent with the structural observations, ITC assays revealed that mutation of these residues abolished the G6P binding affinity (Fig. 1E). The phosphate group sits more outside and interacts with the side chains of T64, T167, and T198 as well as the main chain amino groups from T166 and T167 (Fig. 1D). The corresponding mutations this set of residues also removed or largely impaired the HptA binding affinity for G6P (Fig. 1E), thus highlighting the essential role of the phosphate group in ligand discrimination.
To confirm the structural observations, the respective mutant was introduced into the hptRSA knockout S. aureus strain to check the influence on G6P consumption. As expected, only the WT hptRSA gene but not those with mutations successfully rescued the growth defect in G6P medium (Fig. 1F). Interestingly, all of the mutants still lead to a growth slightly over the baseline of control, especially the T64A mutant, consistent with the observation that HptAT64A retains weak G6P binding capability (Fig. 1E). Therefore, to support the growth, a very low HptA-G6P affinity, even undetectable with ITC, may be compensated by the high concentration of G6P used in the medium. BGP overlaps well with G6P in the ligand-binding site with the exception of C4 hydroxyl group (SI Appendix, Fig. S1A). The hydrogen bond between C4 hydroxyl group and HptA Y37 found in HptA-G6P disappears in the HptA-BGP (SI Appendix, Fig. S1A), which may result in the slightly lower affinity of BGP.
To date, the only other reported periplasmic G6P binding protein is Actinobacillus pleuropneumoniae AfuA, a component of AfuABC (31), which was identified as a bona fide sugar phosphate-specific, binding protein-dependent transporter. In contrast to the strict ligand specificity of HptA, AfuA exhibits a broader range of ligand binding, exemplified by its interaction with G6P, mannose-6-phosphate, fructose-6-phosphate, and others (31). Moreover, AfuA possesses a much higher (∼300-fold) affinity for G6P (31). Structural comparison revealed that the ligand-binding site in HptA and AfuA is completely different: The key sugar-phosphate binding motif (H205, D206, and E229) in AfuA is replaced with Y216, N242, and D244 residues in HptA. Most interestingly, the G6P is captured in a different conformation by HptA (SI Appendix, Fig. S1D). That is, the aromatic side chain of HptA Y216 pushes a 90-degree rotation of the sugar ring with respect to that in AfuA, resulting in a distorted configuration of G6P (SI Appendix, Fig. S1D). These variations in the ligand binding site may account for the difference in affinity and lead to the extreme selectivity for G6P by HptA.
G6P Binding Induces Intramolecular Domain Closure in HptA.
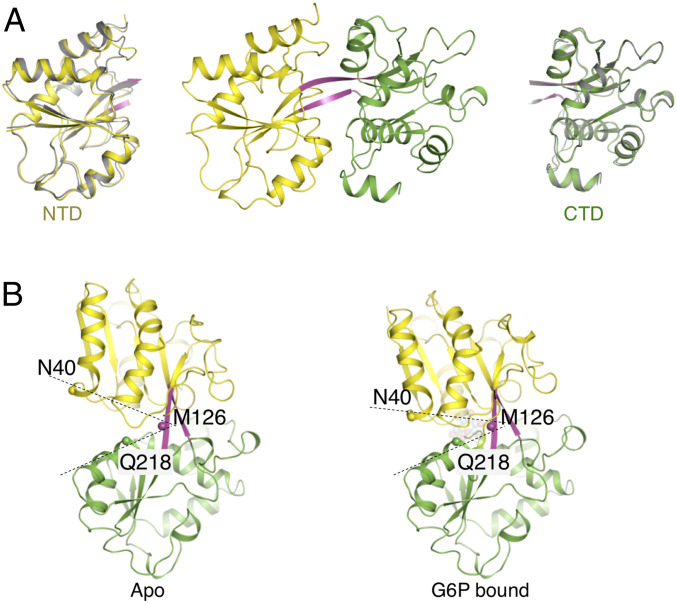
To track the potential conformational changes induced by G6P binding, we next crystallized HptA in the absence of G6P. Two nearly identical (rmsd = 0.271Å for 267 Cα) HptA monomers occupy the asymmetric unit (Fig. 2A). As expected, no electron density could be assigned as a G6P molecule in the previously identified site (SI Appendix, Fig. S2A). However, a positive density at the position corresponding to the G6P phosphate group was observed in both molecules, which was later assigned as malic acid due to its presence in the crystallized state (SI Appendix, Fig. S2B).
Fig. 2.
Conformational changes induced by G6P binding. (A) HptA structure in apo form. Superimposition of the NTD (Left) and CTD (Right) from the apo (gray) and G6P-bound structures. (B) Conformational change induced by G6P binding. The CTD from apo and G6P-bound structure were superimposed to show the 15-degree closing movement of the G6P bound structure. The Cα atom of N40, M126, and Q218 were used as a reference.
To investigate whether malic acid is also a ligand of HptA, we examined its ability to bind HptA and subsequently impact growth of S. aureus. ITC results showed that malic acid did not interact with HptA (SI Appendix, Fig. S2C) nor inhibit G6P binding with HptA, even at very high concentrations (SI Appendix, Fig. S2D). Moreover, malic acid can neither support the growth of S. aureus in minimal medium, nor can it block G6P-mediated growth (SI Appendix, Fig. S2E). Malic acid is thus unlikely to serve as a natural ligand of HptA, and the HptA structure solved here may potentially represent its inactive state. Supporting this possibility, this open conformation of HptA was retained in the G6P-free structure of the HptA–HptS complex (see the next section below). Moreover, the open conformation of HptA was demonstrated to form a specialized interface with HptS to maintain the inactive state of HK (see the next two sections below). Although NTD and CTD of HptA were preserved in a rigid conformation in the inactive state, their relative orientations were rearranged compared with that of the G6P-bound structure. Superposition of the CTD revealed a 15-degree closing rotation of the NTD through the dual β-strand linker in the HptA-G6P (Fig. 2B). The structural rearrangement induced by ligand binding is the common trigger for transmembrane signal transduction in TCS (18, 19, 22). Here, the observed G6P binding-coupled intramolecular domain reorientation may represent a sensing mechanism in the HptRSA system.
HptA Forms a Tetramer with HptSp.
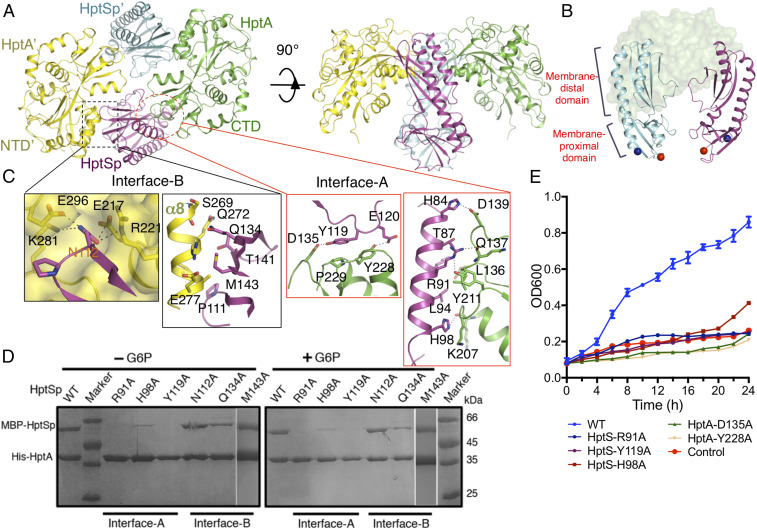
To facilitate crystallographic study, HptS residues 1–44, containing the first transmembrane region (TM1), and 216–518, containing the second transmembrane region (TM2) were truncated (SI Appendix, Fig. S3A). The periplasmic domain consisting of residues 45–215 (HptSp) are the predicted binding region with HptA (SI Appendix, Fig. S3A). The purified HptSp interacted with HptA in both conditions, i.e., with or without G6P (SI Appendix, Fig. S3B), thus indicating constitutive binding between HptA and HptSp. To clarify how HptA interacts with HptSp, we first crystallized the HptA–HptSp complex without G6P. The solved structure revealed that two protomers of HptA bind to two protomers of HptSp to form a compact tetramer with an intrinsic twofold symmetry (Fig. 3A and SI Appendix, Table S2). Each HptA protomer simultaneously contacts with two HptSp molecules through two distinct interfaces and vice versa for HptSp (Fig. 3B). No direct interaction of HptA-HptA′ or HptSp-HptSp′ was observed. The HptA in complex adopted an open conformation, that is, the apo form of HptA alone (SI Appendix, Fig. S3C), and this complex structure could be representative of HptA-HptSp state in the absence of the ligand.
Fig. 3.
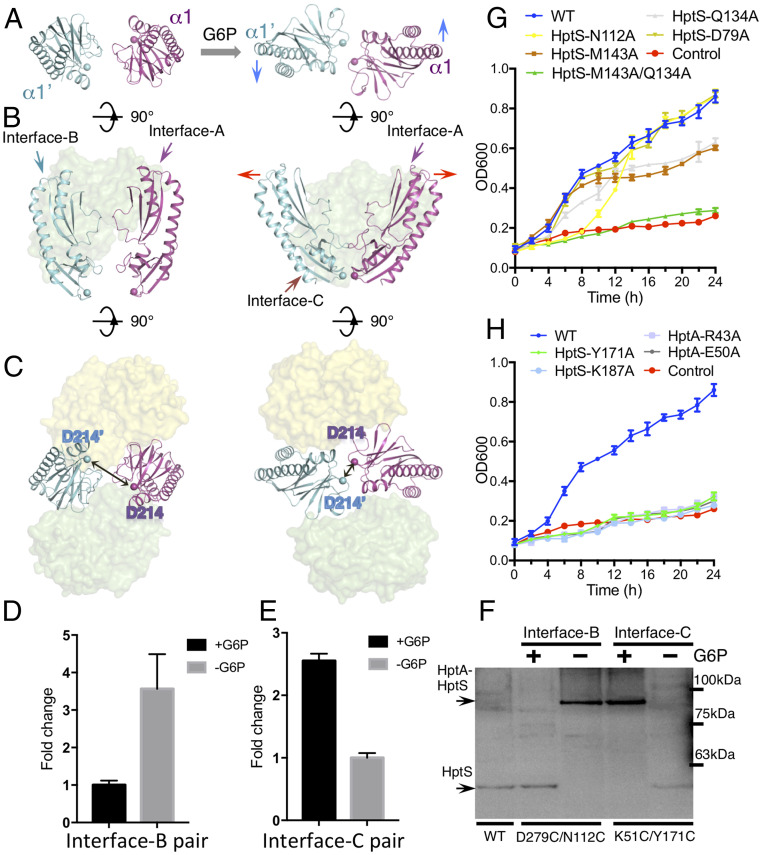
Structures of the HptA-HptSp tetramer without the ligand. (A) Overall structure of the HptA–HptSp complex. The HptA and HptA’ protomers are shown in green and yellow, respectively. The HptSp and HptSp’ protomers are shown in magenta and cyan, respectively. (B) Each HptA binds to the two parallel HptSp protomers. The N- and C-terminal Cα atoms (T45 and D214) of HptS are shown in blue and red spheres, respectively. HptA’ is omitted for clarity. (C) The HptA-HptSp interface in the apo structure. The close-up views of Interface-B (Left) and Interface-A (Right). Residues involved in the interactions are highlighted as sticks. (D) Pull-down assay between HptA and HptSp. The WT HptA was used to pull down the HptSp variant. Both conditions, the presence and absence of G6P, are included. (E) The growth kinetics of ΔhptRSA S. aureus strain transformed with Interface-A mutant variants. “WT” and “Control” are same as in Fig. 1.
Although HptS is a predicted histidine kinase (HK), the primary sequence of its periplasmic domain lacks similarity with those of known HK structures. However, the HptS structure determined in this study showed that it has a tandem PDC (PhoQ/DcuS/CitA) fold featuring the α1 helix packed against the combined α/β folded units (SI Appendix, Fig. S3D), in agreement with previous observations of sequence divergence among PDC domains (32). In contrast to the typical five-stranded β-sheet core of the membrane-distal domain, HptSp possesses only four strands and the canonical ligand-binding site degenerates into a flat surface to accommodate a helix from HptA (SI Appendix, Fig. S3D). The membrane-proximal domain of HptSp displays more similarity to that of the typical tandem PDC, which is characterized by four antiparallel β-strands.
Although TM1 and TM2 were not included in the crystallization construct, the N and C terminus of each HptSp protomer remained in close proximity and kept an appropriate orientation for the TM1 and TM2 (SI Appendix, Fig. S3D), suggesting a plausible, natural conformation for these structures. In all previously reported PDC or tandem PDC structures, the α1/α1′ and α2/α2′ helices from the two protomers constitute a four-helix bundle, which thus generates a “back-to-back” dimer (32, 33) (SI Appendix, Fig. S3E). In contrast, the two HptSp protomers observed in this work were positioned perpendicular to the membrane plane, adopting an opposite, “face-to-face” configuration (Fig. 3B). Moreover, the two HptSp protomers run roughly parallel with each other, and minimal direct contact was observed between them (Fig. 3B). As a result, the TM2 and TM2′ following the membrane-proximal domain can be located relatively far away in the unliganded complex, a conformation that suggests the inactive state.
Interfaces A and B Mediate HptA–HptSp Interaction in the Apo Structure.
Two HptA protomers bind each HptSp at a distinct region in the membrane-distal domain through the NTD and CTD (Fig. 3C). Binding at the first interface, termed as “Interface-A,” is mainly mediated by the α2 helix from HptSp and the CTD of HptA (Fig. 3C and SI Appendix, Fig. S3F). Hydrogen bonds, salt bridges, and hydrophobic interactions constitute a buried surface of 563 Å2. The HptSp Y119 residue stacks against the protruding loops of HptA CTD and forms hydrophobic interactions with HptA Y228 and P229 (Fig. 3C). In addition, Y119 also interacts with HptA D135 through hydrogen bonding (Fig. 3C). HptSp R91 from the middle region of the α2 helix creates two hydrogen bonds with the carbonyl oxygen from HptA L136 and Q137. H84 and H98 from the HptS α2 helix also form hydrogen bonds with HptA residues (Fig. 3C). Three representative residues, R91, H98, and Y119, were mutated in order to validate the structural observations. As expected, mutation of these residues either abolished or markedly impaired the interaction between HptSp and HptA in both the presence and absence of G6P (Fig. 3D), which indicated that Interface-A is constant and dominates the HptA–HptSp interaction. Consistent with this finding, Interface-A mutants lost the capacity for G6P uptake from medium (Fig. 3E). However, similar to the G6P binding site mutations, the weakened HptA–HptSpH98A interaction (Fig. 3D) still supported some growth (Fig. 3E).
The second interface, “Interface-B,” also has a buried surface of 596 Å2 and is composed of the HptA′ NTD packed against the β-sheet core from HptSp. At the same time, the HptSp N112 residue inserted its side chain into a hydrophilic pocket at the junction of NTD and CTD of HptA′ (Fig. 3C). Furthermore, HptSp P111, Q134, T141, and M143 form a complementary surface with the α8 helix of HptA′ through hydrogen bonding and hydrophobic interactions (Fig. 3C). However, the single mutation of N112A, Q134A, or M143A produced no obvious effect on the HptA–HptSp interaction (Fig. 3D), possibly due to the dominant role of Interface-A, which alone may support complex formation. Given the determinative role of Interface-A in HptA-HptS tetramer formation, we subsequently proposed that Interface-B may function to lock the HptA-HptS in the inactivate state.
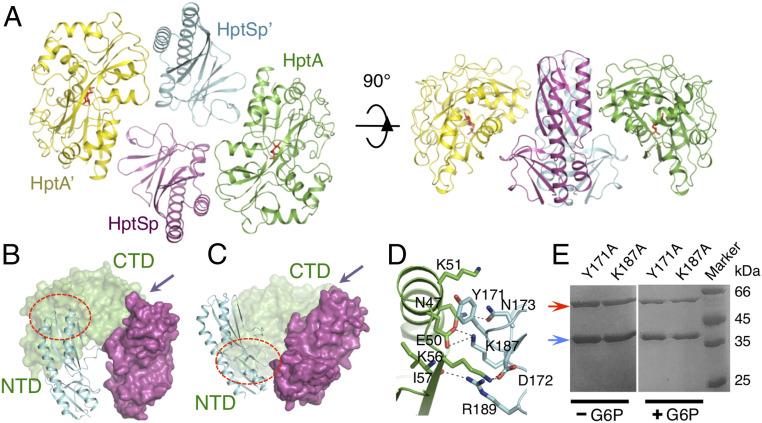
Interfaces A and C Mediate HptA–HptSp Interaction in the G6P Bound State.
HptA–HptSp in complex with G6P showed a similar solution behavior as the apo complex (SI Appendix, Fig. S4A) and crystallized in two forms, belonging to space group P21 and C2, respectively (SI Appendix, Table S2). Although crystallized in different lattices, both structures present a 2:2 stoichiometry of the HptA–HptSp complex in the same conformation (Fig. 4A and SI Appendix, Fig. S4 B and C), suggesting that G6P bound-state complex conformation is unlikely to be an artifact of crystal packing. The structure with the higher resolution in the P21 space group was used for analysis. As expected, G6P was bound at the previously identified ligand-binding site and HptA adopted a closed conformation as that of G6P-HptA (SI Appendix, Fig. S4D). In contrast, no intramolecular conformational change was observed for each HptSp monomer with respect to that in the apo HptA–HptSp complex, except for a loop in the proximal domain and a turn where N112 is located (SI Appendix, Fig. S4 E and F). Most strikingly, while Interface-A is maintained in the G6P-bound HptA–HptSp complex (Fig. 4 B and C), the α8 helix from NTD moves away from the membrane-distal domain of HptSp, while Interface-B disappears from the structure (Fig. 4 B and C). Concurrently, a new interface, “Interface-C,” was generated through the interaction between the α1 helix of the HptA NTD and the membrane-proximal domain of HptSp (Fig. 4 B and C). Interface-C has a buried surface of 312 Å2 and is highly hydrophilic, exhibiting very specific HptA–HptSp interactions: HptSp D172 and K187 form two salt bridges with K56 and E50 from HptA, respectively; HptSp Y171 and R189 undergo hydrogen bonding with HptA (Fig. 4D). Again, mutation in Interface-C resulted in no obvious impact on HptA–HptSp interaction (Fig. 4E). Thus, Interface-C, which is formed in response to G6P binding, may function to activate HptS.
Fig. 4.
Structure of the G6P-bound HptA–HptSp complex. (A) Overall structure of the G6P-bound HptA–HptSp complex. The HptA’ and HptA protomers are shown in yellow and green, respectively. The HptSp’ and HptSp protomers are shown in cyan and magenta, respectively. G6P is shown as red sticks. (B) Interface-B in the apo structure. HptA’ is omitted for clarity. The red circle and arrow indicate the position of Interface-B and Interface-A, respectively. (C) The Interface-C in the G6P bound HptA-HptSp structure. One HptA molecule is omitted for clarity. The red circle and arrow indicate the position of Interface-C and Interface-A, respectively. (D) The contact details of the Interface-C. Residues making interactions are highlighted as sticks. Residues from HptA and HptSp are colored green and cyan, respectively. (E) Pull-down assay of the Interface-C mutants. The red arrow indicates the MBP-HptSp mutant, and the blue arrow indicates WT HptA.
Conformational Changes Induced by G6P Binding.
As mentioned above, HptA undergoes domain closure, while no intramolecular conformational change happens to either HptSp monomer; however, the relative orientation of the two HptSp protomers alters significantly. Along with the interface switch from Interface-B to Interface-C, two kinds of conformational change occur in the HptSp pair. First, the twisted HptSp pair in the apo structure is pushed back. As a result, the HptSp pair sits almost in the same plane when viewed with the axis of the α1 helix as a reference (Fig. 5A). Second, the roughly parallel HptSp pair tilts, with the two membrane-proximal domains moving closer to each other (Fig. 5B). Together, these two movements bring the D214/D214′ residues closer, from 23 Å to 9 Å apart, where they are at their closest point to TM2/TM2′ (Fig. 5C), which as a result, could potentially reorient the TM2 pair to activate HptS.
Fig. 5.
Conformational changes in HptA-HptSp induced by G6P binding. (A) Top view of the relative orientation of the two HptSp protomers. The blue arrow indicates the twisting movement upon G6P binding. HptA is omitted for clarity. (B) Side view of the two HptSp protomers in the complex. The red arrow indicates the rotational opening in the membrane-distal end. HptA’ is omitted for clarity. (C) Bottom view of the C termini of the HptS pair. The Cα of D214 is represented as a sphere. Their distance changes from 23 Å in the apo form to 9 Å in the G6P-bound structure. The FRET state of the HptA–HptSp complex using fluorophore labeled proteins around Interface-B (D) and Interface-C (E). Influence of G6P binding is indicated by fold change in fluorescence intensity between G6P-absent and G6P-present conditions. (F) Cysteine-directed cross-linking of HptA/HptS in bacterial cells. The cross-linked and uncross-linked bands were visualized by Western blotting with C-myc–tagged HptS. Full-length HptA and HptS have predicted molecular masses of 35 and 61 kDa, respectively. The representative image is from one of the four replicates. (G and H) The growth kinetics of ΔhptRSA S. aureus strain transformed with Interface-B mutant variants (G) and Interface-C mutant variants (H). The variant with HptS D79A sitting away from the Interface-B is included as a control. WT and control are the same as in Fig. 1.
To validate the structural observations of G6P-induced molecular rearrangement, we employed Förster resonance energy transfer (FRET) to probe the conformational changes. Two residues positioned nearby Interface-B, HptA S291 and HptSp T81, were converted to cysteines and labeled with Alexa Fluor 555 (as donor) and Alexa Fluor 647 (as acceptor) (SI Appendix, Fig. S5A), respectively. Their distance would allow high FRET in the apo structure but low FRET in the G6P bound form (SI Appendix, Fig. S5A). These mutations did not cause any detectable functional disruption to the HptA–HptS complex, as shown by cell growth assays and solution behavior of the labeled proteins (SI Appendix, Fig. S5 B–D).
As expected, a high FRET state under G6P-free conditions was changed into a low FRET state when G6P was added (Fig. 5D), indicating that the apo conformation was disrupted by G6P binding. Subsequently, when the donor and acceptor fluorophores were labeled at residues around Interface-C, HptA V99 and HptSp G194, respectively (SI Appendix, Fig. S5 B–E), the reverse phenomenon was observed (Fig. 5E), thereby indicating that G6P binding initiated the formation of Interface-C. Although other reasons, such as the changes in fluorophore orientation, could affect the FRET state, here the FRET change induced by G6P binding most likely reflected the conformational change in the HptA-HptSp. Next, we carried out site-directed disulfide-bridge cross-linking in the context of full-length HptA/HptS to determine whether the observed interface switch occurs in the native environment. HptA D279/HptS N112 and HptA K51/HptS Y171 were converted into cysteine pairs, as they were only located in close proximity as pairs in Interface-B and Interface-C, respectively (SI Appendix, Fig. S5F). Similar to the FRET results, the HptAD279C/HptSN112C pair cross-linked when G6P was absent (Fig. 5F). In contrast, the HptAK51C/HptSY171C pair only cross-linked in the presence of G6P (Fig. 5F). Collectively, these results confirmed that G6P binding drives the interaction switch from Interface-B to Interface-C.
To further validate the biological functions of Interface-B and Interface-C in HptA-HptS–mediated uptake of G6P, we introduced mutations to each interface and checked their impact on cell growth in G6P medium. As shown above, mutations to Interface-A caused growth arrest in G6P medium (Fig. 3E). While individual mutations of Interface-B residues HptS N112A, Q134A, and M143A only led to mild growth defects, double mutations such as HptSQ134A/M143A caused severe growth defects (Fig. 5G). In contrast, all of the Interface-C residue conversions, including HptS Y171A, K187A, HptA, R43A, and E50A, caused complete growth failure (Fig. 5H). These results, together with our structural observations that Interface-B does not overlap with Interface-C, suggest that Interface-B maintains the inactive state of HptA-HptS, and that G6P binding drives the transition to Interface-C, potentially activating HptA-HptS.
Discussion
The recently identified HptRSA system mediates G6P uptake to support S. aureus growth and multiplication within various host epithelial cells (25). The structural and functional data presented in this study elucidate the mechanism for G6P sensing by a TCS. Although HptA-HptSp was only crystallized with the periplasmic domain, the subunits still formed a tetramer with plausible structural conformations in both the apo and G6P-bound states. Moreover, the biological relevance of the observed interfaces was further confirmed in the context of full-length proteins through cysteine cross-linking and growth assays (Fig. 5). As a result, the structures described here provide insights into the G6P sensing process in bacteria. Similar to the reported G6P sensor AfuA, HptA utilizes a two-domain structure to recognize G6P, although the ligand-binding site of HptA bears almost no resemblance to that of AfuA. The presence of the aromatic residue Y216 in HptA results in a narrow cavity that does not accommodate any alterations to the sugar-phosphate ligand. This extreme selectivity of HptA may reflect a precise bacterial response to environmental stimuli: For example, only in the presence of abundant G6P, such as in the cytosol of the host cells, is the downstream transporter expressed to initiate nutrient uptake.
Tandem PDC domains have been identified in several TCS proteins, and those that dimerize all do so as four-helix bundles (32), including LuxPQ with its periplasmic sensor protein (22). Here, HptS was found as a dimer-like pair, in which each protomer shared binding with HptA. However, in this complex, HptS monomers adopt an opposite orientation to that in other TCSs, with the combined α/β folding units facing each other. Whether this mode represents a unique evolutionary event remains unclear. Regardless of its distribution in nature, this conformation is functionally convergent with other TCS systems in that it transmits an external signal to the cytoplasm. While the membrane-distal domain of the tandem PDC was found to be involved in ligand or associated periplasmic sensor binding, the function of the membrane-proximal domain has remained elusive (13, 32). Here, in HptS, we provide a description of its role in forming an interface for the ligand-bound periplasmic sensor, through which HptS was transformed into the active state. The salt bridges and hydrogen bonds in the interface formed a kind of “ionic lock,” which has been commonly reported as the functional switch in G protein-coupled receptors and GRK interactions (34). Thus, Interface-C may perform a similar role in response to the ligand perception. Taken together, these structural data provide a framework for precise drug design targeting G6P uptake. For instance, small molecules targeting the unique G6P binding site that prevent the intramolecular closing of HptA could be designed to inhibit the growth of S. aureus. Alternatively, blocking the formation of Interface-C with an antibody, which is currently being investigated in this laboratory, may also work effectively to prevent bacterial growth.
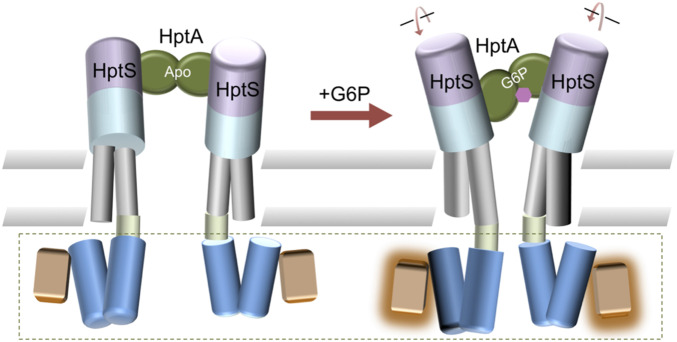
Although it is well known that TCSs employ conformational changes in the sensor to initiate transmembrane signal transduction, it remains unclear how the conformational change on the extracellular side is transmitted into the cytosol. Piston-like, scissor closing, and helical rotation conformational changes have been previously identified as common mechanisms (18). In this study, we proposed an activation mode, namely an interface switch-mediated rotation and intramolecular closure induced by ligand binding (Fig. 6). In the absence of G6P, HptA interacts with HptS on the membrane-distal side and the TM2 of each HptS protomer are twisted and separated. As a result, the adjacent HAMP domain may form a helix bundle that is unable to activate the signaling helix and kinase. However, once G6P binds to HptA, HptA switches the contact site to the membrane-proximal domain, which causes untwisting and tilting movement in the HptS.
Fig. 6.
The proposed activation model of the HptA-HptS system. In the apo state, HptA (green; HptA’ is omitted for clarity) binds HptS at the membrane-distal side (light magenta). G6P binding switches the contact region to the membrane-proximal domain (cyan), which causes untwisting and tilting movement in the TM2 region of HptS (gray). This conformational change is then transmitted to the HAMP domain (blue), which eventfully conveys the binding state information to activate the kinase. The kinase activation (green dashed box) is inferred based on sequence similarity between HptS and other histidine kinases.
The conformational change in the periplasmic domain can then rotate the HptS TM2, allowing a scissor-like movement to occur. The control cable of TM2 then transmits the signals further to the HAMP domain. As a result, the stability of HAMP helix bundle may change (15, 35) or conformational rearrangement such as scissoring may happen (15, 36). The periplasmic signal will eventually activate the kinase activity through this concerted structural movement. In summary, our results provide mechanistic insights into the activation of TCS and provides rational insight into the future design of antimicrobial agents.
Materials and Methods
All experiments are described in detail in the SI Appendix, Materials and Methods. Briefly, the HptA protein and HptA–HptSp protein complex were expressed in Escherichia coli and purified to homogeneity for crystallization. All diffraction data were collected and processed with HKL3000 (37). The structures were determined using CCP4i (38) and PHENIX (39), and structural models were built with PHENIX and Coot (40). The ligand-binding affinity of HptA and the HptA–HptSp complex were measured by ITC assays. Pulldown and surface plasmon resonance (SPR) assays were performed to investigate the interaction between HptA and HptSp. Cell growth assays were carried out using a ∆hptRSA mutant S. aureus strain.
Supplementary Material
Acknowledgments
We thank staff members at Shanghai Synchrotron Radiation Facility for assistance in data collection. This work was supported by National Key Research and Development Program of China Grants 2018YFA0902700 (to Y.T.) and 2017YFA0503600 (to M.T.) and Chinese National Natural Science Foundation Grants U1732114 (to X.L.), 31770788 (to M.T.), and 31770895 (to Y.T.). Y.T. is also supported by the “Thousand Young Talent program.” We thank Professor Baolin Sun for his kindly assistance in providing technical support in S. aureus growth assay.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912080117/-/DCSupplemental.
Data Availability.
All coordinates and structure factors are freely available for download from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB) (PDB ID codes 6LKG–6LKL).
References
- 1.Stock A. M., Robinson V. L., Goudreau P. N., Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Parkinson J. S., Kofoid E. C., Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26, 71–112 (1992). [DOI] [PubMed] [Google Scholar]
- 3.Ulrich L. E., Zhulin I. B., The MiST2 database: A comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res. 38, D401–D407 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortet P., Whitworth D. E., Santaella C., Achouak W., Barakat M., P2CS: Updates of the prokaryotic two-component systems database. Nucleic Acids Res. 43, D536–D541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabin R. S., Stewart V., Either of two functionally redundant sensor proteins, NarX and NarQ, is sufficient for nitrate regulation in Escherichia coli K-12. Proc. Natl. Acad. Sci. U.S.A. 89, 8419–8423 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguilar P. S., Hernandez-Arriaga A. M., Cybulski L. E., Erazo A. C., de Mendoza D., Molecular basis of thermosensing: A two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 20, 1681–1691 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Möglich A., Ayers R. A., Moffat K., Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 385, 1433–1444 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sevvana M., et al. , A ligand-induced switch in the periplasmic domain of sensor histidine kinase CitA. J. Mol. Biol. 377, 512–523 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Bader M. W., et al. , Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122, 461–472 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Miller S. I., Kukral A. M., Mekalanos J. J., A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. U.S.A. 86, 5054–5058 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob-Dubuisson F., Mechaly A., Betton J. M., Antoine R., Structural insights into the signalling mechanisms of two-component systems. Nat. Rev. Microbiol. 16, 585–593 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Bhate M. P., Molnar K. S., Goulian M., DeGrado W. F., Signal transduction in histidine kinases: Insights from new structures. Structure 23, 981–994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zschiedrich C. P., Keidel V., Szurmant H., Molecular mechanisms of two-component signal transduction. J. Mol. Biol. 428, 3752–3775 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao R., Stock A. M., Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63, 133–154 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkinson J. S., Signaling mechanisms of HAMP domains in chemoreceptors and sensor kinases. Annu. Rev. Microbiol. 64, 101–122 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Casino P., Rubio V., Marina A., The mechanism of signal transduction by two-component systems. Curr. Opin. Struct. Biol. 20, 763–771 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Moore J. O., Hendrickson W. A., Structural analysis of sensor domains from the TMAO-responsive histidine kinase receptor TorS. Structure 17, 1195–1204 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Gushchin I., Gordeliy V., Transmembrane signal transduction in two-component systems: Piston, scissoring, or helical rotation? Bioessays 40, 1700197 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Salvi M., et al. , Sensory domain contraction in histidine kinase CitA triggers transmembrane signaling in the membrane-bound sensor. Proc. Natl. Acad. Sci. U.S.A. 114, 3115–3120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe E. C., Baslé A., Czjzek M., Firbank S. J., Bolam D. N., A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proc. Natl. Acad. Sci. U.S.A. 109, 7298–7303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., et al. , Molecular mechanism of environmental d-xylose perception by a XylFII-LytS complex in bacteria. Proc. Natl. Acad. Sci. U.S.A. 114, 8235–8240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neiditch M. B., et al. , Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell 126, 1095–1108 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore J. O., Hendrickson W. A., An asymmetry-to-symmetry switch in signal transmission by the histidine kinase receptor for TMAO. Structure 20, 729–741 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gushchin I., et al. , Mechanism of transmembrane signaling by sensor histidine kinases. Science 356, eaah6345 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Park J. Y., et al. , Characterization of a novel two-component regulatory system, HptRS, the regulator for the hexose phosphate transport system in Staphylococcus aureus. Infect. Immun. 83, 1620–1628 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y., et al. , Regulatory mechanism of the three-component system HptRSA in glucose-6-phosphate uptake in Staphylococcus aureus. Med. Microbiol. Immunol. (Berl.) 205, 241–253 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Lowy F. D., Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Klevens R. M. et al.; Active Bacterial Core surveillance (ABCs) MRSA Investigators , Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Verhamme D. T., Arents J. C., Postma P. W., Crielaard W., Hellingwerf K. J., Glucose-6-phosphate-dependent phosphoryl flow through the Uhp two-component regulatory system. Microbiology (Reading) 147, 3345–3352 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Fraunholz M., Sinha B., Intracellular Staphylococcus aureus: Live-in and let die. Front. Cell. Infect. Microbiol. 2, 43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sit B., et al. , Active transport of phosphorylated carbohydrates promotes intestinal colonization and transmission of a bacterial pathogen. PLoS Pathog. 11, e1005107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z., Hendrickson W. A., Structural characterization of the predominant family of histidine kinase sensor domains. J. Mol. Biol. 400, 335–353 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y. C., Machuca M. A., Beckham S. A., Gunzburg M. J., Roujeinikova A., Structural basis for amino-acid recognition and transmembrane signalling by tandem Per-Arnt-Sim (tandem PAS) chemoreceptor sensory domains. Acta Crystallogr. D Biol. Crystallogr. 71, 2127–2136 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Komolov K. E., et al. , Structural and functional analysis of a beta2-adrenergic receptor complex with GRK5. Cell 169, 407–421.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Q., Ames P., Parkinson J. S., Mutational analyses of HAMP helices suggest a dynamic bundle model of input-output signalling in chemoreceptors. Mol. Microbiol. 73, 801–814 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Airola M. V., Watts K. J., Bilwes A. M., Crane B. R., Structure of concatenated HAMP domains provides a mechanism for signal transduction. Structure 18, 436–448 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minor W., Cymborowski M., Otwinowski Z., Chruszcz M., HKL-3000: The integration of data reduction and structure solution–From diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Collaborative Computational Project, Number 4 , The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Adams P. D., et al. , PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All coordinates and structure factors are freely available for download from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB) (PDB ID codes 6LKG–6LKL).