Significance
We report on structure-function studies of the conserved 3′-to-5′ exoribonuclease Nibbler (Nbr) toward understanding its role in 3′-end trimming of small regulatory RNAs. Our studies of the Nbr N-terminal domain provide insights into its role as a double-strand RNA-binding recruitment platform that is required for Nbr-directed microRNA trimming and that experimentally can be bypassed through an Argonaute-interacting peptide. Structural studies on Nbr’s exonucleolytic domain suggest a substrate selectivity of the Mn2+-coordinated DEDDy catalytic domain toward non–2′-O-methylated small RNAs. Our findings provide conceptual insights into how Nbr participates in small RNA biogenesis.
Keywords: Nibbler, exoribonuclease, microRNA trimming, HEAT repeats
Abstract
Nibbler (Nbr) is a 3′-to-5′ exoribonuclease whose catalytic 3′-end trimming activity impacts microRNA (miRNA) and PIWI-interacting RNA (piRNA) biogenesis. Here, we report on structural and functional studies to decipher the contributions of Nbr’s N-terminal domain (NTD) and exonucleolytic domain (EXO) in miRNA 3′-end trimming. We have solved the crystal structures of the NTD core and EXO domains of Nbr, both in the apo-state. The NTD-core domain of Aedes aegypti Nbr adopts a HEAT-like repeat scaffold with basic patches constituting an RNA-binding surface exhibiting a preference for binding double-strand RNA (dsRNA) over single-strand RNA (ssRNA). Structure-guided functional assays in Drosophila S2 cells confirmed a principal role of the NTD in exonucleolytic miRNA trimming, which depends on basic surface patches. Gain-of-function experiments revealed a potential role of the NTD in recruiting Nbr to Argonaute-bound small RNA substrates. The EXO domain of A. aegypti and Drosophila melanogaster Nbr adopt a mixed α/β-scaffold with a deep pocket lined by a DEDDy catalytic cleavage motif. We demonstrate that Nbr’s EXO domain exhibits Mn2+-dependent ssRNA-specific 3′-to-5′ exoribonuclease activity. Modeling of a 3′ terminal Uridine into the catalytic pocket of Nbr EXO indicates that 2′-O-methylation of the 3′-U would result in a steric clash with a tryptophan side chain, suggesting that 2′-O-methylation protects small RNAs from Nbr-mediated trimming. Overall, our data establish that Nbr requires its NTD as a substrate recruitment platform to execute exonucleolytic miRNA maturation, catalyzed by the ribonuclease EXO domain.
Small regulatory RNAs, including microRNAs (miRNAs), small interfering RNAs (siRNAs), and PIWI-interacting RNAs (piRNAs), play essential roles in eukaryotic gene silencing by guiding diverse Argonaute-family proteins within their respective RNA-induced silencing complexes to target transcripts (1–5). Both miRNAs and siRNAs interact with Ago-clade Argonaute proteins to repress gene expression involving a wide variety of biological processes in most organisms (6–10). By contrast, piRNAs guide PIWI-clade Argonaute proteins to silence transposable elements and thus maintain genome integrity in animal gonads (11–13).
The biogenesis of the distinct small RNA classes shows notable differences. As for miRNAs, long transcripts containing hairpins and flanking sequences are processed into 21- to 23-mer small RNA duplexes by Drosha and Dicer (14–20). After loading of the sRNA duplex into an Argonaute, one strand (passenger) is discarded to leave the single-stranded guide strand bound to the Argonaute protein. The 3′-ends of a subset of miRNAs in Drosophila melanogaster undergo an extra processing step after loading into Argonaute, where the 3′-to-5′ exonuclease Nibbler (Nbr) shapes the 3′-ends of miRNAs by trimming the 3′-overhang to their final length (21–23). As for piRNAs, the long and single-stranded precursors, transcribed from piRNA clusters and exported to the cytoplasm, are first processed through one of two different cleavage mechanisms involving the mitochondria-anchored endoribonuclease Zucchini (24, 25) or the Argonaute proteins Aub/Ago3 (26, 27) to generate piRNA 5′-ends. In Drosophila, two distinct pathways subsequently define piRNA 3′-ends: While Zucchini can directly cleave piRNA precursors to the correct 3′-ends with a phasing signature (28, 29), a subset of piRNAs requires Nbr to trim prepiRNAs with extending 3′ nucleotides (3′ nt) generated by Aub/Ago3-mediated slicing (30, 31) to their mature length. piRNA biogenesis concludes by 2′-O-methylation of piRNA 3′-ends by the Hen1 methyltransferase (32, 33).
As an important player in the maturation process of both miRNAs and piRNAs, D. melanogaster Nibbler (DmNbr, UniProt: Q9VIF1) is composed of an N-terminal domain (NTD), the structure and function of which are unknown, and a C-terminal 3′-to-5′ exonuclease domain (EXO) containing four conserved acidic amino acids (DEDD) in the catalytic pocket (34) (Fig. 1A). However, the precise mechanism by which DmNbr identifies and trims its small RNA substrates has remained elusive. Nbr belongs to the Mut-7 family of exoribonucleases (35, 36), which is conserved from sponges to mammals and shares domain architectures consisting of an NTD of unknown function and a catalytic EXO domain (many family members, but not flies, also have an additional C-terminal extension). No structure-based mechanistic insight into the function of any Mut-7 family members exists at this time.
Fig. 1.
Nbr NTD is required for miRNA trimming and contains a scaffold of HEAT repeats. (A) Schematic drawing of DmNbr and AaNbr proteins, showing NTD domain in green and EXO domain in orange. Numbers above the sequence indicate the domain boundaries. AaNbr NTD core used for crystallization is indicated. (B and C) Side (left) and bottom (right) views of the crystal structure of AaNbr NTD core. α-Helices 1 to 16 are labeled, with each heat repeat composed of a pair of adjacent α-helices. The structures are shown as cartoons and colored green, in which HEATs 1 to 5 and C-cap regions are indicated. (D) The indicated FM-tagged Nbr variants were stably expressed in S2 cells depleted of Nbr by CRISPR/Cas9 engineering (S2-nbrko), and miR-34–5p trimming was assessed by Northern hybridization. Wild-type S2 cells (S2WT) served as control. Trimmed and untrimmed miR-34–5p isoforms are indicated. 2S rRNA served as loading control. n/a, not applicable. (E) Mean +/− SD of at least three independent replicates of experiment shown in D. Individual data points are indicated. P values were determined by Student’s t test.
Here, we report on the structure-based identification and characterization of an unanticipated RNA-binding activity of the NTD from Aedes aegypti Nbr. Structure-based mutagenesis on the NTD pinpoints the requirement of conserved, positively charged residues that line the RNA-binding cleft for trimming activity, which might be involved in recognizing small-RNA–processing intermediates. We also report on the structures of EXO domains from DmNbr and AaNbr, which, together with biochemical studies, reveal that Nbr’s EXO domain exhibits Mn2+-dependent single-strand–specific exoribonuclease activity. Furthermore, a conserved tryptophan within the active site likely contributes to the protection of mature 2′-O-methylated RNA 3′-ends from extensive trimming. Our findings provide important clues toward understanding how Nbr acts in small RNA biogenesis pathways.
Results
Structure Determination of AaNbr NTD Core.
The NTD of DmNbr is predicted to have well-defined secondary structural elements but does not show apparent sequence homology with other proteins. Aggregation of recombinantly purified DmNbr NTD hampered crystallization trials. We therefore turned to A. aegypti Nbr (AaNbr, UniProt: Q179T2), which contains 719 residues (Fig. 1A) and shares 32% identity and 52% similarity with DmNbr (SI Appendix, Fig. S1). The function of AaNbr in shaping 3ʹ-ends of small RNAs has been confirmed recently (37), establishing it as a functional ortholog of DmNbr. Recombinant AaNbr NTD (residues 26 to 405) formed a monodisperse peak on a size-exclusion column but failed to yield crystals. Limited proteolysis of AaNbr NTD by trypsin established the presence of a protease-resistant core domain (NTD core) with a molecular weight of about 30 kDa (SI Appendix, Fig. S2A). We then successfully grew crystals and determined the 3.05-Å resolution crystal structure of this NTD core of AaNbr (Fig. 1 B and C) by single-wavelength anomalous diffraction (SAD), using crystals derivatized with selenomethionine, and refined the structure to Rfree of 26.9% and Rwork of 23.6% with good stereochemistry (X-ray statistics in SI Appendix, Table S1). The crystals belong to space group P62 and contain three independent copies of the NTD core in the asymmetric unit (SI Appendix, Fig. S2B), with individual copies virtually identical to each other. The final structure of the NTD core includes residues 72 to 315, and all residues could be traced in the electron density map.
We analyzed the oligomeric state of AaNbr NTD (residues 26 to 405) in solution by size-exclusion chromatography coupled with in-line multiangle light-scattering analysis (SEC-MALS). The measured molecular mass (46.2 kDa) is close to the theoretical molecular mass of the monomer (44.9 kDa), indicating that AaNbr NTD exists in the monomeric state in solution (SI Appendix, Fig. S2C).
AaNbr NTD Core Consists of a Scaffold Composed of HEAT-Like Repeats.
The AaNbr NTD core is composed almost entirely of α-helices (labeled α1 to α16), forming an elongated scaffold of the approximate dimensions 75 × 25 × 20 Å (Fig. 1 B and C). The first 10 helices (residues 81 to 251) adopt a regular HEAT-like repeat topology, with each HEAT-like repeat composed of two adjacent α-helices labeled A and B. In addition, we identified several long insertions, either as loops between successive HEAT-like repeats (interrepeats) or as loops between the A and B helices of the same repeat (intrarepeat). The unusual intrarepeat insertions involved the long loop between the two helices of HEAT2 (α3 and α4), while HEAT3 (α5 and α6) and HEAT4 (α8 and α9) each contain a large interrepeat insertion whereby the loop ends in an extended helix α7 that twists the first helix of HEAT4. In addition, the loop at the N terminus of HEAT-like repeats wraps around the surface of HEAT1 (α1 and α2) and HEAT2 (α3 and α4) that presents a virtually identical conformation in all three copies of the NTD core. Capping the C terminus of the HEAT-like repeats is another subdomain of the NTD core, which mainly contains four α-helices (α13 to α16), referred to as the C-cap subdomain (Fig. 1C). Within this subdomain, a long loop with an additional short helix (α15) connects the α14 and α16 helices. The HEAT-like repeats and C-cap subdomain pack tightly against each other to form a protease-resistant globular folded topology (Fig. 1 B and C).
NTD Is Required for Nbr-Directed miRNA Trimming.
To perform structure-function analyses of Nbr-mediated miRNA trimming, we performed rescue experiments in Drosophila S2 cells lacking Nbr due to CRISPR/Cas9-induced frameshift mutations (S2-nbrko) (23). In the absence of Nbr, miR-34–5p, which is predominantly produced as a 24-mer by Dicer-1 fails to be converted into its mature ∼21 to 22-mer form after Ago1 loading when assessed by high-resolution Northern hybridization (22) (Fig. 1 D and E). This miR-34–5p trimming phenotype is fully rescued upon stably expressing FLAG-Myc (FM)-tagged wild-type (FLWT) but not catalytically inactive Nbr (FLCM encoding the mutations D435A and E437A; Fig. 1 D and E and SI Appendix, Fig. S3). To test Nbr domain dependencies, we determined the length distribution of miR-34–5p in S2-nbrko cells stably expressing truncated FM-Nbr constructs (Fig. 1 D and E). Neither the expression of the C-terminal domain (CTD)—harboring the predicted EXO domain—in the presence (FLCM) or absence (FLWT) of a predicted catalytic site mutation, nor the expression of the NTD alone had any statistically significant impact on miR-34–5p trimming when compared to the knockout alone (each P > 0.13, Fig. 1 D and E and SI Appendix, Fig. S3). These data indicate that Nbr requires its NTD for exonucleolytic miRNA maturation, which is catalyzed by the CTD ribonuclease domain. Coexpression of NTD and CTD as separate polypeptides did not rescue miR-34–5p trimming when compared to Nbr-FLWT (P > 0.13, Fig. 1 D and E), suggesting that a covalent linkage between NTD and EXO domains is required to promote miRNA maturation by Nbr.
EXO-Domain Recruitment to Ago1 Promotes miRNA Trimming.
We hypothesized that Nbr’s NTD serves as a recruitment domain to bring the EXO-domain–encoded exonucleolytic site in close proximity to Ago1-bound miRNAs (Fig. 2A). This model is challenged by the fact that we previously failed to detect a stable interaction between Nbr and Ago proteins in coimmunoprecipitation experiments (22), perhaps because of the transient nature of this interaction. To independently evaluate such a substrate recruitment scenario, we replaced the NTD by a direct Ago1-binding module to recruit the Nbr-EXO domain to Ago1. To this end, we fused the EXO domain to the N-terminal GW-repeat motif of GW182/TNRC6 (GWpep) that directly interacts with miRNA-bound Ago proteins (38, 39). Upon stable expression in S2-nbrko cells (SI Appendix, Fig. S4A), a Nbr-EXO domain fusion to D. melanogaster (dm) GW182-GWpep (C399-625-GWpepdm) or Mus musculus (mm) TNRC6-GWpep (C399-625-GWpepmm) resulted in rescue of miR-34–5p trimming (Fig. 2 B and C). Small RNA sequencing confirmed this observation for a broader set of miRNAs (Fig. 2 D and E): When compared to untreated S2-nbrko, the weighted average length of previously established Nbr substrates decreased significantly upon expression of Nbr-CTD fused to GW182-GWpep (C399-625-GWpepdm; Mann–Whitney U test P = 0.002) as well as full-length Nbr (FLWT; P = 0.03), while expression of Nbr-CTD alone did not significantly alter Nbr substrate length (C399-625; P = 0.49, Fig. 2D and SI Appendix, Fig. S4B) (23). In contrast, none of the constructs significantly influenced the weighted average length of non-Nbr substrates (Whitney-Mann test, P > 0.29; Fig. 2D). Finally, the extent of trimming among Nbr substrates significantly correlated between Nbr-C399-625-GWpepdm and Nbr-FLWT (Pearson correlation coefficient rP = 0.82, P < 10−4; Fig. 2E). We conclude that Nbr’s EXO domain is active in isolation and can promote miRNA trimming upon recruitment to Ago1, providing indirect support to the hypothesis that the NTD of Nbr serves as a substrate recruitment domain. Notably, GWpep fused to the N terminus of Nbr-EXO did not rescue miRNA trimming, indicating the requirement for specific positioning of the EXO domain relative to Ago1-bound miRNAs to execute exonucleolytic miRNA maturation (SI Appendix, Fig. S4 C and D).
Fig. 2.
Constitutive recruitment of Nbr-CTD to Ago1 is sufficient to promote miRNA trimming. (A) Exonucleolytic miRNA maturation requires the exonucleolytic activity encoded in Nbr’s C-terminal domain (EXO) that may be recruited to Ago1 via Nbr’s NTD. Upon loading, Ago1-bound miRNAs mediate mRNA repression by recruiting GW182/TNRC6, which directly interacts with Ago1 through its N-terminal GW-repeat motif (GWpep). (B) The indicated FM-tagged Nbr variants were stably expressed in S2 cells depleted of Nbr by CRISPR/Cas9 engineering (S2-nbrko), and miR-34–5p trimming was assessed by Northern hybridization. Wild-type S2 cells (S2WT) served as control. Trimmed and untrimmed miR-34–5p isoforms are indicated. 2S rRNA served as control. n/a, not applicable. (C) Quantification of experiment shown in B. (D) Tukey boxplots show weighted average length of miRNAs classified as Nbr substrates (n = 24; Left) or non-Nbr substrates (n = 16; Right) before (w/o) and after expression of FM-tagged full-length Nbr (FLWT), Nbr-CTD (C399-625), or Nbr-CTD fused to D. melanogaster (dm) GWpep of GW182 (C399-625-GWpepdm) in S2-nbrko, as determined by high-throughput sequencing of small RNAs. Outliers are not shown. P values (Mann–Whitney U test) are indicated. (E) Change in average length of miRNAs classified as Nbr substrates (yellow) or non-Nbr substrates (gray) in S2-nbrko cells upon expression of full-length Nbr (FLWT) or Nbr-CTD fused to D. melanogaster (dm) GWpep of GW182 (C399-625-GWpepdm). Select miRNAs are annotated. Pearson correlation coefficient (rP) and associated P value is indicated.
NTD Acts as the RNA-Binding Platform of Nbr.
The top matching structures to the AaNbr NTD core from the DALI server (40) include RNA-binding protein exportin-T that serves to export transfer RNA (tRNA) to the cytoplasm (41), together with other HEAT-like repeat-containing proteins, such as pre-messenger RNA (pre-mRNA)–splicing factor 8 and TSC1 (42, 43). Mapping the electrostatic potential on the molecular surface of AaNbr’s NTD core revealed extended patches of positively charged residues, namely K97, K133, K211, and K240 within the HEAT-like repeat subdomains and K296, K300, R304, and K307 in the C-cap subdomain (Fig. 3A). Of note, the distribution of the positively charged residues lining NTD core surfaces mirrors the distribution of evolutionarily conserved residues (Fig. 3B), supporting their functional importance in serving as potential RNA-binding interfaces.
Fig. 3.
The RNA-binding activity of AaNbr NTD as identified from structural and FP analysis. (A) Electrostatic surface potentials of AaNbr NTD core viewed from the same direction as in Fig. 1C. Electrostatic surface potentials were calculated by Adaptive Poisson-Boltzmann Solver (APBS) and contoured from –4 kT/e (red) to +4 kT/e (blue). (B) Surface properties of AaNbr NTD core according to conservation, viewed from the same direction as in A. The gradient from green to red indicates increasingly conserved residues. Conservation scores were calculated using ConSurf. Conserved residues in potential RNA-binding surface of AaNTD are indicated. The proteins sequences are listed in SI Appendix, Table S4. (C and D) RNA-binding affinities of AaNTD determined by FP assays. A constant amount of 5′-FAM–labeled 20-mer ssRNA, 40-mer ssRNA, or 20-bp dsRNA was incubated with increasing concentrations of AaNTD proteins at 150 mM NaCl (C) or 50 mM NaCl (D). (E) FP assays results showed no detectable RNA-binding activity of the DmNbr EXO D435A mutant in low salt buffer.
To investigate the RNA-binding potential of Nbr NTD, we used fluorescence polarization (FP) assays. We incubated purified AaNbr NTD with three 5′-6-fluorescein amidite (5′-FAM)-labeled RNA samples at physiological salt concentrations (150 mM NaCl). As shown in Fig. 3C, the affinity of AaNbr NTD for 20-bp double-strand RNA (dsRNA) was in the low micromolar range (Kd = 0.60 µM). We observed a roughly 8-fold weaker affinity (Kd = 4.54 µM) for a 40-mer single-stranded RNA (ssRNA) and a roughly 20-fold weaker affinity (Kd = 13.5 µM) for a 20-mer ssRNA. When performing the binding assays at a lower salt concentration (50 mM NaCl), AaNbr NTD showed an affinity of 0.16 µM to a 20-bp dsRNA, a roughly two-fold weaker affinity (Kd = 0.29 µM) to a 40-mer ssRNA and a six-fold weaker affinity (Kd = 0.93 µM) to a 20-mer ssRNA (Fig. 3D). The RNA-binding affinities of AaNbr NTD for all three substrates were significantly higher under low salt conditions compared to physiological salt concentrations. These findings support a critical contribution of electrostatic protein-RNA interactions to the binding. We conclude that AaNbr NTD shows binding activities to different RNA molecules, but that it has a preference to bind dsRNA compared to ssRNA.
We next tested the RNA-binding affinities of the purified, nuclease-dead EXO domains of DmNbr (D435A) by FP assays. The DmNbr EXO domains showed no detectable binding to 5-FAM–labeled 20-mer ssRNA or 20-bp dsRNA at physiological or at low salt concentrations (Fig. 3E). Together, our data indicate that the NTD rather than the EXO domain is Nbr’s RNA-binding platform and that the NTD is critical for the recognition and loading of RNA substrates.
Structure-Guided Mutations Affect RNA-Binding Ability of the NTD.
To assess the contributions of residues within the predicted RNA-binding patches of the NTD, we generated several single- and two double-alanine–substituted sumo-tagged AaNbr NTD mutants along the basic interface in the crystal structure of AaNbr NTD (Fig. 3A) and measured their RNA-binding affinity. When incubated with 5′-FAM–labeled 20-bp dsRNA in physiological buffer, wild-type sumo-tagged AaNbr NTD showed an affinity of Kd = 0.40 µM (Fig. 4 A and B), compared to an affinity of Kd = 0.60 µM for the untagged wild-type AaNbr NTD (Fig. 3C). All single alanine mutants within the HEAT-like repeat subdomain (K97A, K133A, K211A, and K240A), and within the C-cap subdomain (K296A, K300A, R304A, and K307A) showed a pronounced reduction (from about 3-fold to 40-fold) of the dsRNA-binding affinity, while the double mutant (K296A/K300A and R304A/K307A) essentially abolished dsRNA-binding activity (Fig. 4 A and C). We also tested the effect of mutations in binding 5′-FAM–labeled 40-mer ssRNA under low salt (50 mM NaCl) conditions. Interestingly, all mutants showed reduced ssRNA binding, but the K211A and K240A mutants that caused strong reduction in binding to dsRNA appeared to have only slightly impaired binding to ssRNA (Fig. 4 B and C). The double mutants (K296A/K300A and R304A/K307A) caused the strongest reduction in ssRNA-binding activity (Fig. 4 B and C). These results support the critical role of the multiple conserved positively charged residues for the dsRNA-binding ability of Nbr’s NTD.
Fig. 4.
Impact of the Nbr NTD mutations on RNA binding and trimming. (A and B) RNA-binding affinity of AaNTD mutants determined by FP assays. The 5′-FAM–labeled 20-bp dsRNA (A) or 40-mer ssRNA (B) was incubated with increasing concentrations of AaNTD mutants at 150 mM NaCl (A) or 50 mM NaCl (B), respectively. The FP data are fitted to the Hill equation to obtain the Kd. Data represent three independent experiments with mean ± SD. (C) Kd values obtained from experiments in A and B. (D) The indicated FM-tagged Nbr variants were stably expressed in S2 cells depleted of Nbr by CRISPR/Cas9 engineering (S2-nbrko), and miR-34–5p trimming was assessed by Northern hybridization. Wild-type S2 cells served as control (S2WT). Trimmed and untrimmed miR-34–5p isoforms are indicated. 2S rRNA served as control. n/a, not applicable. (E) Change in miR-34–5p trimming relative to WT-Nbr rescue. Mean +/− SD of at least three independent replicates of experiment shown in D. Individual data points are indicated. P values were determined by Student’s t test.
To test whether Nbr’s dsRNA-binding activity harbored in the NTD is required for miRNA maturation, we performed miR-34–5p trimming assays in S2-nbrko cells upon expression of full-length DmNbr with single-, double-, and quadruple-alanine substitutions along the basic interface of NTD (SI Appendix, Fig. S5). While single-alanine mutations, as well as the double-mutant R280A/K284A (counterpart of AaNbr R304A/K307A), did not significantly affect miRNA trimming (P > 0.18, Fig. 4 D and E), the double mutant R272A/K276A (counterpart of AaNbr K296A/K300A) resulted in a measurable 1.3-fold decrease and the quadruple mutant in a 2.7-fold decrease in miR-34–5p trimming when compared to wild-type Nbr (Fig. 4 D and E). We conclude that a basic interface along Nbr’s NTD, most likely constituting an RNA-binding module, is required for efficient miRNA trimming.
Structural Insights into the Nbr EXO Domain.
To gain mechanistic insights into Nbr’s enzymatic activity, we determined the crystal structure of the DmNbr EXO domain (residues 396 to 625, Fig. 1A) containing a catalytically inactivating D435A mutation at 1.5-Å resolution. The initial model of DmNbr EXO was constructed using SAD on crystals of the protein derivatized with selenomethionine. The crystals belong to the space group C2122 and contain one molecule in the asymmetric unit. The structure was refined to Rfree of 23.2% and Rwork of 20.2% with good stereochemistry (X-ray statistics in SI Appendix, Table S1). The electron density is continuously traceable along the entire length of the protein. The structure of DmNbr EXO is composed of a six-stranded twisted β-sheet bracketed mainly by seven α-helices (Fig. 5A). The order of β-strands is β1β4β3β2β5β6, with β-strand 3 aligned antiparallel to the remaining β-strands. Helices α2, α3, and α4 cover the convex face of the twisted β-sheet, while the remaining α-helices are positioned along the opposite face. Mapping of the electrostatic potential on the molecular surface of DmNbr EXO revealed an active site pocket (Fig. 5B, yellow box) that exhibits the DEDDy motif (Fig. 5C) that is conserved in other DEDDy family nucleases. We also solved the 1.5-Å resolution structure of AaNbr’s EXO domain (residues 425 to 652, D462A mutant, Fig. 5 D and E) by molecular replacement using the structure of the DmNbr EXO domain as a model (X-ray statistics in SI Appendix, Table S1). The DmNbr and AaNbr EXO structures (sequence alignment in SI Appendix, Fig. S6) are virtually identical (superposing with an rmsd of ∼0.86 Å over all Cα atoms) (Fig. 5F), indicating that Nbr EXO adopts a structurally conserved fold.
Fig. 5.
Crystal structures of DmNbr and AaNbr EXO domains. (A) Overall structure of DmNbr EXO (DmEXO). The structure is shown as cartoon and colored salmon. (B) Electrostatic surface potentials of DmEXO. Electrostatic surface potentials were calculated by APBS and contoured from −4 kT/e (red) to +4 kT/e (blue). The catalytic pocket is highlighted by yellow box. (C) Close-up view of the DEDD-Y motif in the catalytic pocket of DmEXO. The catalytic residues with D435A mutation are shown as sticks and colored magenta. (D) Overall structure of AaNbr EXO (AaEXO). The structure is shown as cartoon and colored cyan. (E) Electrostatic surface potentials of AaEXO. (F) Structural alignment of DmEXO and AaEXO. Structures are shown as ribbons and colored salmon and cyan, respectively.
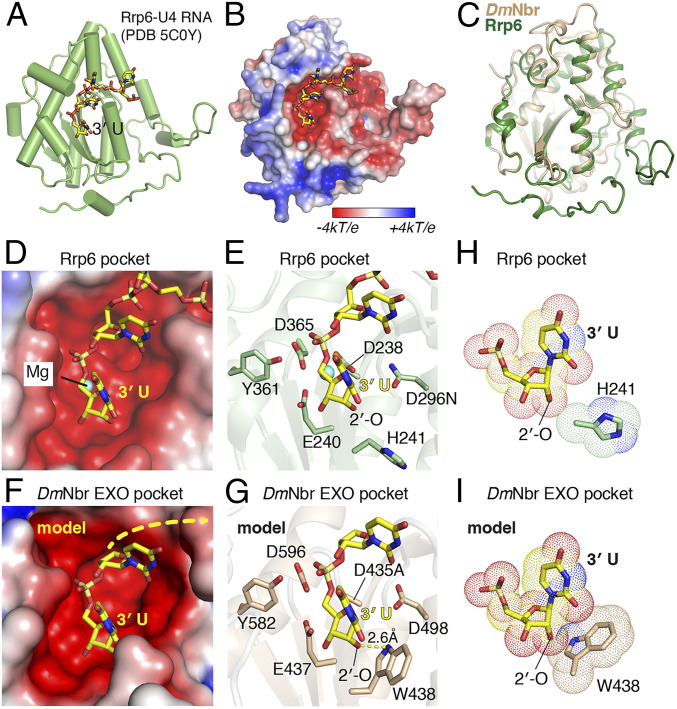
Sequence-based homology searches using the HHpred server showed that yeast Rrp6 (44), a component of the exosome complex, represented the top hit that is structurally related to DmNbr EXO. This result is further confirmed by our structure-based data searches on the DALI server. Notably, Rrp6 is also a ssRNA-binding 3′-to-5′ exoribonuclease containing the DEDDy motif (44). According to the published 2.1-Å structure of U4 RNA bound to the D296N mutant of Rrp6 (Protein Data Bank [PDB] code 5C0Y, Fig. 6 A and B), the EXO domains of Nbr and Rrp6 superpose well with an rmsd of 0.94 Å (Fig. 6C). Based on the positioning of the terminal U of U4 within its DEDDy catalytic pocket of Rrp6 (Fig. 6 D and E), we modeled a 3′-U bound in the DmNbr EXO catalytic pocket (Fig. 6 F and G). This indicated that Nbr most likely cleaves a substrate phosphodiester linkage through a similar catalytic mechanism as Rrp6, but also revealed a notable difference adjacent to the 2′-O position of bound terminal 3′-U of U4 in the Rrp6 complex (Fig. 6H) compared to modeled 3′-U in the DmNbr EXO complex (Fig. 6I). For Nbr, the indole nitrogen of Trp438 has the potential to form a hydrogen bond with 2′-O of modeled bound 3′-U (Fig. 6 G and I). By contrast, 2′-O-methylation appears to not only disrupt this hydrogen bond but also may cause a clash with the side chain of the Trp438 (Fig. 6I). For Rrp6, Trp438 is replaced by the smaller side chain of His241 (Fig. 6 E and H), thereby likely preventing a clash with 2′-O-methylation (Fig. 6H). Interestingly, Trp438 is conserved among all Nibbler or mut-7 homologs from flies to humans.
Fig. 6.
Structural comparison of DmNbr EXO and yeast Rrp6 EXO. (A) Overall structure of yeast Rrp6 EXO-U4 RNA complex (PDB code 5C0Y). The protein is shown as cartoon and colored green. The U4 RNA is shown in a stick representation and colored yellow. (B) Electrostatic surface potentials of yeast Rrp6 EXO domain. Electrostatic surface potentials were calculated by APBS and contoured from −4 kT/e (red) to +4 kT/e (blue). The U4 RNA is shown in a stick representation and colored yellow. (C) Superposition of the protein structures of apo-DrNbr (in wheat) and U4-bound Rrp6 (in green) EXO domains, which exhibit an rmsd of 0.935 Å. (D and E) Close-up views of the catalytic pocket of yeast Rrp6. The 3′ rUMP of U4 RNA is shown in a stick representation and colored yellow. (F and G) The catalytic pocket of DmNbr EXO (D435A) with modeled UMP based on the structure of the yeast Rrp6-RNA complex. (H) Positioning of 3′ UMP of U4 RNA relative to His241 of Rrp6. (I) The 2′-O-methylation of modeled UMP potentially causes a clash with the bulky side chain of the W438.
Nbr EXO Domain Exhibits Mn2+-Dependent ssRNA-Specific 3′-to-5′ Exoribonuclease Activity.
To test the exoribonuclease activity of DmNbr’s EXO domain, single-stranded 20-mer RNA oligonucleotides labeled with 5′-FAM at the 5′-end were incubated with purified DmNbr EXO protein for 30 min, and reaction products were detected by fluorescence in 15% TBE-Urea polyacrylamide gel electrophoresis (PAGE). As shown in Fig. 7A, DmNbr EXO displayed metal-specific activity with strong 3′-to-5′ exoribonuclease activity in the presence of Mn2+, minimal activity in the presence of Mg2+, and no exonuclease activity with other divalent metal ions such as Zn2+ and Ca2+. Dose-dependent assays also confirmed that DmNbr EXO exhibited enzymatic activity against RNA substrates in the presence of both Mn2+ and Mg2+ ions with the activity being significantly stronger with Mn2+ ions. Additionally, DmNbr EXO exhibited no enzymatic activity against single-stranded 20-mer DNA substrates labeled with Cy3 at the 5′-end (Fig. 7B) under conditions similar to those used for RNA substrates. From these results, we conclude that DmNbr EXO is a Mn2+-dependent exoribonuclease targeting RNA substrates.
Fig. 7.
RNA-cleavage activity of DmNbr EXO domain. (A and B) Metal cation-dependent 3′-to-5′ exoribonuclease activity of DmEXO. Wild-type DmEXO proteins (0.5 µM) were incubated with 1 µM 5′-FAM–labeled 20-mer ssRNA (A) or Cy3-labeled 20-mer ssDNA (B) for 0.5 h at room temperature with 5 mM metal ions (MnCl2, MgCl2, ZnCl2, or CaCl2) or without (w/o) metal. TBE-Urea PAGE indicated that DmEXO selectively cleave 5′-FAM–labeled ssRNA in the presence of MnCl2. (C) Dose dependency and substrate specificities of DmEXO. Wild-type DmEXO proteins (0.125 to 2 µM) were incubated with 1 µM 5′-FAM–labeled 20-mer ssRNA, 20-bp dsRNA, or 2′-O-methylated ssRNA in the presence of 1 mM MnCl2 for 0.5 h at room temperature. The samples were detected by TBE-Urea PAGE. (D) Quantification of cleavage experiments in C with mean faction (bars), SD (black lines), and fraction values obtained for each measurement (black dots). Data are representative of three independent experiments. *P < 0.05; **P < 0.01 by two-tailed paired t test.
Nbr EXO possesses a narrow and deep active pocket (Fig. 5B) that can apparently accommodate ssRNA but not dsRNA. This active-site architecture is due to three long loops of Nbr surrounding the pocket: the long loop that connects α7 and α8 helices, the loop between β2 and β3 strands, and the C-terminal loop following the α9 helix (Fig. 5A). To examine whether Nbr EXO has ssRNA or dsRNA substrate specificity, we performed dose-dependent enzymatic activity assays using 5′-FAM–labeled 20-mer ssRNA or 20-bp dsRNA as the substrates in the buffer containing Mn2+ ions (Fig. 7). DmNbr EXO exhibited significantly stronger enzymatic activity against ssRNA compared to dsRNA substrates (Fig. 7 C and D), indicating that Nbr EXO is predominantly a single-strand–specific 3′-to-5′ exoribonuclease.
The 2′-O-methylation at the 3′-end is a hallmark of mature piRNAs (45–47). Nbr and Hen1, the methyltransferase involved in 2′-O-methylation at the 3′ terminal nucleotides of piRNAs, have been shown to play antagonistic roles in modulating piRNA 3′-ends in Drosophila (32). The proposed model of 3′-U bound DmNbr EXO (Fig. 6 F and G) also implies that 2′-O-methylation may cause a clash within the active pocket to impair Nbr’s trimming activity (Fig. 6 G and I). We therefore investigated the enzymatic activity of DmNbr EXO toward 5′-FAM–labeled 20-mer ssRNA with a terminal 2′-O-methylation (2-OMe ssRNA). The results showed that processing of the 2-OMe ssRNA by DmNbr’s EXO was significantly impaired compared to that of unmethylated ssRNA (Fig. 7 C and D). Together, these biochemical data reveal that Nbr’s EXO domain is a Mn2+-dependent ssRNA-specific 3′-to-5′ exoribonuclease.
Crystallization Trials for AaNbr NTD Core Bound to dsRNA and AaNbr/DmNbr EXO Bound to ssRNA.
Our attempt to express and purify full-length AaNbr was not successful, and hence our structural studies were limited to the individual NTD and EXO domains of Nbr.
Our attempts to grow diffraction quality crystals of the AaNbr NTD-core domain bound to dsRNA (12, 14, 17, and 20 bp) were unsuccessful. In addition, our attempts at crystallization of AaNbr EXO (individual D435A and D435N mutants to prevent cleavage) and DmNbr EXO (individual D462A and D462N mutants to prevent cleavage) domains bound to ssRNA (5, 7, 9, and 15 nt) were also unsuccessful. Hence, we were unable to obtain details of protein-RNA intermolecular contacts for both dsRNA-bound Nbr NTD and ssRNA-bound Nbr EXO.
Discussion
The 3′-to-5′ exoribonuclease Nibbler trims subpopulations of >60% of Drosophila miRNAs at their 3′-termini after their loading into Argonaute1 (Ago1) (22, 23). Nbr is also required for the 3′ trimming of pre-piRNAs generated by the Aub/Ago3 ping-pong processing pathway (31). miRNAs and piRNAs use fundamentally different biogenesis pathways and are loaded into very diverse Argonaute-family proteins. The participation of Nbr in the 3′-end maturation of both small RNA classes suggests a basic principle of how small RNA 3′-ends associated with an Argonaute protein are recognized and trimmed. In this paper, we focused on the miRNA biogenesis pathway and used structural characterization of Nbr’s NTD and EXO domains as well as structure-guided functional studies to determine mechanistic insights into miRNA trimming. Our findings are most likely also applicable to other members of the conserved Mut-7/Nbr family that share the conserved NTD and catalytic domains.
Structure-Based Mechanistic Insights into Nbr NTD Domain Function.
The AaNbr NTD core consists mainly of a HEAT-like repeat scaffold with high structural similarity to exportin-T (41). The AaNbr NTD domain bound dsRNA more tightly than ssRNA, with the binding affinities being dependent on the salt concentration and the length of the ssRNA (Fig. 3 C and D). We observed a conserved, positively charged patch on one face of the AaNbr NTD (Fig. 3A). Targeted mutations of basic lysine residues lining this surface impacted on the NTD’s ability to target both dsRNA and ssRNA (Fig. 4 A and B). It is therefore very likely that this basic surface of the Nbr NTD domain is targeted by both dsRNA and ssRNA through direct and extensive nonsequence-specific electrostatic interactions. In the absence of structural information on NTD-RNA complexes, it is currently not possible to explain the preference of Nbr’s NTD for binding to dsRNA over ssRNA (Fig. 3 C and D). Double mutations of conserved, basic surface residues in DmNbr’s NTD in Drosophila S2 cells resulted in partial miRNA trimming defects (Fig. 4 D and E). The severity of the phenotype, however, did not directly scale with the observed reduction in RNA binding in vitro (Fig. 4C). This may relate to the fact that Ago1-bound miRNAs lack extensive protrusions that exceed 2 to 3 nt before trimming and that substrate recruitment involves additional protein-protein interactions between Nibbler and Argonaute. It remains to be tested if trimming of piRNA precursors, which frequently encompass a size of ∼60 nt, exhibits a stronger dependence on the RNA-binding ability of Nbr’s NTD.
A Role of Nbr-NTD in Substrate Recruitment.
While multiple lines of evidence indicate that Nbr trims its substrates after assembly into Argonaute (22, 23), the mode of Nbr recruitment remains enigmatic. Based on rescue experiments in Drosophila S2 cells mutant for endogenous Nbr, we show that the NTD is required for exonucleolytic maturation of miRNAs (Fig. 1 D and E). Notably, this requirement can be overcome by fusing Nbr’s EXO domain to the N-terminal GW-repeat motif of GW182, which directly interacts with Ago1, implying a possible role of NTD in the recruitment of Nbr-EXO to Ago-bound small RNA precursors (Fig. 2). Future studies will test if Nbr’s NTD can serve as a protein-protein interaction platform, a hypothesis that is supported by the observed tendency for aggregation in solution. In fact, during purification, DmNbr NTD formed aggregates, and AaNbr NTD—although monomeric in solution—formed precipitates under most crystallization conditions. We hypothesize that Nbr could potentially use the NTD for cooperative recruitment of RNA-bound Ago proteins, perhaps involving target mRNA as a supportive scaffold (Fig. 2A). In such a case, our inability to detect a stable complex of Nbr and Ago proteins following coimmunoprecipitation of Nbr and Ago1 most likely reflects the transient nature of such an interaction (22).
Structure-Based Mechanistic Insights into Nbr EXO Domain Function.
The structures of DmEXO (Fig. 5 A and B) and AaEXO (Fig. 5 D and E) adopt a mixed α/β topology, similar to that reported for the 3′-to-5′-exonuclease Rrp6 (44). The apo structures of the Nbr EXO domain contain a deep catalytic pocket (Fig. 5 B and E) composed of an acidic DEDDy residue cluster (Fig. 5C). We did not observe a divalent cation in the catalytic pocket of either apo-Nbr EXO structure, which could reflect the incorporation of an Asp-to-Ala mutant for Nbr under crystallization conditions to prevent RNA cleavage. Exonuclease assays demonstrated that the EXO domain exhibits a strong preference for ssRNA over dsRNA substrates (Fig. 7 C and D) and requires divalent cations for catalysis with a strong preference for Mn2+ (Fig. 7A).
The limited size dimensions of the catalytic cavity in the EXO domain (Fig. 5 B and E) are consistent with Nbr preferring ssRNA over dsRNA as a substrate. To gain further insight into Nbr-mediated catalysis, we drew on available structural information of U4 bound to the EXO catalytic pocket of Rrp6 (Fig. 6 A and B) (44). Based on the positioning of the terminal 3′-U of bound U4 in the catalytic pocket of Rrp6 (Fig. 6 D and E), we modeled 3′-U into the catalytic pocket of DmEXO (Fig. 6 F and G). Notably, the 2′-OH of the terminal 3′-U of U4 is positioned close to the side chain of His241 in the Rpr6 EXO catalytic pocket (Fig. 6H) while, in the Nbr EXO pocket, the modeled 3′-U would position close to the bulky Trp438 side chain (Fig. 6I), which is conserved throughout all Mut-7 family members from flies to humans. We speculate that RNA substrates containing 2′-O-methylation at their 3′-end represent suboptimal substrates for trimming by Nbr as they result in a steric clash with the Trp438 ring of Nbr.
Comparison of NTD Domains of Nbr and SDN1.
It is instructive to compare our structure-function studies on Nbr with those for the related DEDDh 3′-to-5′ exoribonuclease SDN1 (Small RNA Degrading Nuclease 1), which initiates the turnover of AGO1-bound miRNAs in Arabidopsis by trimming their 3′-ends (48). SDN1 contains NTD, EXO, and RNA recognition motif (RRM) domains (SI Appendix, Fig. S7A), with its NTD domain adopting a seven-α–helical bundle (SI Appendix, Fig. S7B) that is distinct from the HEAT-like repeat fold observed for the Nbr NTD (Fig. 1 B and C). The RNA-binding ability of SDN1 is restricted to its RRM domain given that its NTD does not bind RNA. Notably, miRNAs that are subjected to trimming by Nbr exhibit significantly shorter half-lives compared to untrimmed miRNAs (23). We expect that the presented structural data will guide future functional studies on the molecular principles underlying exonucleolytic maturation and turnover of small RNAs.
Materials and Methods
Protein Expression and Purification.
Condon-optimized DNA sequences of AaNbr NTD (residues 26 to 405), DmNbr EXO (residues 396 to 625), or AaNbr EXO (residues 425 to 652) were synthesized by Integrated DNA Technologies and cloned into a pRSFDuet-1 vector (Novagen) engineered with an N-terminal His-SUMO tag. All mutants were generated by site-directed mutagenesis with a QuikChange II XL kit (Agilent) according to the manufacturer’s instruction and confirmed by sequencing.
All proteins were expressed in Escherichia coli BL21-CodonPlus(DE3)-RIL (Stratagene). The bacteria were grown in Luria–Bertani medium supplemented with 50 mg/mL kanamycin at 37 °C to an OD600 of 0.6, induced with 0.3 mM isopropyl β-D-1-thiogalactopyranoside overnight at 18 °C. Cells were collected via centrifugation at 5,000 × g and lysed via sonication in Lysis Buffer (500 mM NaCl, 20 mM imidazole, and 20 mM Tris⋅HCl, pH 8.0) supplemented with 1 mM phenylmethylsulfonyl fluoride and 0.5% Triton X-100. Cellular debris was removed by centrifugation at 20,000 × g, and the supernatant was loaded onto 5 mL Nickel Sepharose 6 fast flow resins (GE Healthcare) in a gravity flow column. The target protein was eluted using Lysis Buffer supplemented with 500 mM imidazole. The eluted protein was incubated with ULP1 during dialysis at 4 °C overnight against a buffer containing 20 mM Tris⋅HCl, pH 7.5, 20 mM imidazole, 200 mM NaCl, and 5 mM β-mercaptoethanol. Then the sample was loaded onto the HisTrap FF column (GE Healthcare) to remove His-SUMO tag, and the flow-through was collected. The protein sample was diluted to a final NaCl concentration of 100 mM and purified through a HiTrap Heparin column (GE Healthcare). The target proteins were further purified by a Superdex200 10/300 gel filtration column (GE Healthcare) in Buffer S (20 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, and 1 mM dithiothreitol [DTT]). The fractions were detected by sodium dodecyl sulfate (SDS) PAGE and concentrated to around 5 mg/mL. The protein was flash-frozen in liquid nitrogen and stored at –80 °C. Selenomethionine (Se-Met) proteins were expressed in M9 medium (Sigma) supplied with L-Se-Met (Sigma). The expression and purification of Se-Met or mutant proteins were the same as that for native proteins.
For limited proteolysis of AaNbr NTD, purified AaNbr NTD protein (5 mg/mL) was incubated with trypsin (Sigma) in a molar ratio of 200:1 at room temperature for 50 min and further purified by Superdex200 10/300 column (GE Healthcare) in Buffer S. The fractions containing the AaNbr NTD core were detected by SDS/PAGE and concentrated to around 5 mg/mL.
Crystallization and Data Collection.
All crystallization conditions were determined with crystal screens (Qiagen) by sitting-drop vapor diffusion with a Mosquito crystallization robot (TTP Labtech). Crystallization conditions were optimized using the hanging-drop vapor diffusion method at 20 °C. The AaNbr NTD-core crystals were grown from drops mixed from 1.5 μL of AaNbr NTD-core solution (about 3 mg/mL protein) and 1.5 μL of reservoir solution (0.2 M sodium tartrate, 0.1 M Hepes, pH 7.0, 20% PEG3350). The DmNbr EXO (D435A) crystals were grown from drops mixed from 1.5 μL of protein solution (2 mg/mL) and 1.5 μL of reservoir solution (0.064 M sodium citrate 7.0, 0.1 M Hepes, pH 7.0, 10% PEG5000MME). The AaNbr EXO (D462A) crystals were grown from drops mixed from 1.5 μL of protein solution (4 mg/mL) and 1.5 μL of reservoir solution (0.2 M potassium formate, 20% PEG3350). Crystals of Se-Met–substituted AaNbr NTD core and DmNbr EXO (D435A) were grown under the same condition as that for native proteins.
For data collection, crystals were cryoprotected in reservoir solution supplemented with 30% glycerol and flash-frozen in liquid nitrogen. All of the diffraction datasets were collected on the 24-ID beamline at the Advanced Photon Source (APS) at the Argonne National Laboratory. All diffraction data were indexed, integrated, and scaled with the XDS package on the NECAT RAPD server.
Structure Determination.
The Se-Met AaNbr NTD core and Se-Met DmNbr EXO (D435A) structures were determined using single anomalous diffraction method with the AutoSol and AutoBuild programs embedded in the PHENIX suite (49–51). The native DmNbr EXO (D435A) structure was solved via molecular replacement with program PHASER of the PHENIX suite, using the Se-Met DmNbr EXO (D435A) structure as the search model. The structure of the native AaNbr EXO (D462A) was solved by molecular replacement with the program PHASER (52) of the PHENIX suite, using the DmNbr EXO (D435A) structure as the search model. Iterative manual model building was performed using the program COOT (53), and refinement with PHENIX.refine (54) produced the final models. The statistics of the data collection and refinement are shown in SI Appendix, Table S1. Structural figures were generated using PyMOL (https://pymol.org/2/), and the coordinates were deposited in the PDB under accession codes 7JW2, 7JW3, and 7JW6.
SEC-MALS Experiments.
For protein molar mass determination, purified AaNbr NTD (20 to 405) proteins were analyzed using an ÄKTA-MALS system. A miniDAWN TREOS multiangle light scattering detector (Wyatt Technology) and an Optilab TrEX refractometer (Wyatt Technology) were used in-line with Superdex200 10/300 gel filtration column (GE Healthcare) pre-equilibrated in Buffer S at a flow rate of 0.2 mL/min. Separation and ultraviolet light detection were performed by the ÄKTA Pure System (GE Healthcare), light scattering was monitored by the miniDAWN TREOS system, and concentration was measured by the Optilab TrEX differential refractometer. Molar masses of proteins were calculated using the Astra 6.1 program (Wyatt Technology) with a dn/dc value (refractive index increment) of 0.185 mL/g.
Fluorescence Polarization.
All RNA oligonucleotides were synthesized by Integrated DNA Technologies and dissolved in diethyl pyrocarbonate water. As for single-stranded RNA (20- or 40-mer) in FP assays, RNA oligonucleotides were labeled with 6-FAM at their 5′-ends. To generate double-stranded 20-bp RNA, one 5′-6-FAM (5′-FAM)–labeled 20-mer RNA oligonucleotide and its complementary unlabeled 20-mer RNA oligonucleotide were annealed according to the manufacturer’s instructions. A total of 50 nM 5′-FAM–labeled RNA was incubated with different concentrations of purified AaNbr NTD protein samples at room temperature for 20 min. Protein samples, including wild-type apo AaNbr NTD, sumo-tagged wild-type AaNbr NTD, or sumo-tagged AaNbr NTD mutants, were two-fold diluted in the physiological buffer (20 mM Hepes 7.2, 150 mM NaCl) or in the low salt buffer (20 mM Hepes 7.2, 50 mM NaCl). The final reaction volume was 25 μL. Each titration point was measured three times, with the FP of each reaction quantified on the TECAN infinite M1000 plate reader using 470 nm excitation and 517 emission wavelengths. The FP data were analyzed by the nonlinear regression Hill equation with Prism 8 software (GraphPad).
In Vitro Enzymatic Activity Assays.
The preparation of RNA oligonucleotides was the same as that for the FP assays. The 5′-Cy3–labeled 20-mer DNA oligonucleotide was synthesized by Integrated DNA Technologies and dissolved in water. A total of 1 µM RNA or DNA substrates were incubated with different concentrations of recombinant wild-type DmNbr EXO proteins at room temperature for 30 min in reaction buffer (20 mM Hepes, pH 7.2, 50 mM KCl). As for metal cation-dependent activity assays, the reaction buffer also contained 5 mM different metal ions as indicated, respectively. As for substrate specificity assays, all reaction buffers also included 1 mM MnCl2. The final sample volume of each reaction was 10 μL. After reaction, 10 μL Novex TBE-Urea Sample Buffer (Invitrogen) was added. The reaction products were then resolved on 15% Novex TBE-Urea Gel (Invitrogen). Gels were scanned using the Typhoon FLA-9500 imager (GE healthcare) and visualized using FIJI/ImageJ and Prism 8 software (GraphPad).
Constructs for Expression of Nbr Variants in Drosophila S2 Cells.
Truncated or mutated D. melanogaster Nbr constructs for expression in Drosophila S2 cells were based on previously described pAFMW-Nbr except that the start codon of Nbr CDS was removed (22). Truncated and mutated Nbr versions were then generated by PCR amplification (for oligonucleotides, see SI Appendix, Table S2), directional TOPO cloning into pENTR, sequence verification by Sanger sequencing, and transfer to the destination vector pAFMW, following the instructions for Gateway cloning (ThermoFisher Scientific). Nbr-GWpep fusion constructs were generated by genomic PCR amplification of GWpep-encoding region (residues 2–100) of gw182 using genomic DNA extracted from Drosophila S2 cells (for oligonucleotides, see SI Appendix, Table S2). The homologous M. musculus TNRC6-derived peptide was derived as previously described (39). The PCR amplicons were introduced into pAFMW-Nbr(CTD) by Gibson cloning as previously described (55).
Drosophila S2 Cell-Line Culturing and Engineering.
Drosophila Schneider 2 (S2) cells were obtained from the P. D. Zamore Laboratory (University of Massachusetts Medical School, Worcester, MA) and cultured in Schneider’s Drosophila Medium containing 10% fetal bovine serum (FBS). Cells were maintained at 27 °C and passaged every 3 d. Cells have been validated (by genome, mRNA, and small RNA sequencing) and are of male origin. S2-NbrKO cell lines were derived as published described (23). S2-NbrKO cells were cotransfected with 400 ng of corresponding pAFMW-Nbr plasmid (full-length, truncated, or fused version of Nibbler; see SI Appendix, Table S2) or pAFMW-Nbr-CM (D435A, E437A) (10) and 100 ng pHygro plasmids. Briefly, the respective expression plasmids were added to 50 µL of FBS-free Schneider’s Drosophila media supplemented with 3.5 µL FuGENE HD Transfection Reagent (Promega). After incubating this for 5 min, the mix was added to 2 mL of a 106 cells/mL cell suspension and plated. The following day, 300 µg of hygromycin B was added per mL of Schneider’s Drosophila media containing 10% FBS. After 2 wk of hygromycin selection, cell populations were used for further experiments or expanded and frozen in aliquots (56).
Western Blotting.
S2 cells were pelleted and lysed on ice with lysis buffer (30 mM Hepes KOH, pH 7.4; 100 mM KOAc; 2 mM MgOAc; 5 mM DTT; 0.5% Nonidet P-40; 5% glycerol). Protein lysates were loaded on 4 to 15% gradient SDS/PAGE (BioRad), transferred to Immobilon-P polyvinylidene difluoride membrane (Millipore), and probed overnight at 4 °C. Antibodies were used at a dilution of 1:10,000 for anti-FLAG (M2-FLAG, monoclonal mouse, F3165-5MG, Sigma Aldrich), 1:1,000 for anti-Nibbler [mouse; produced in-house as described previously (31)] and 1:5,000 for anti-Actin (A2066, rabbit, Sigma-Aldrich) and detected by secondary HRP-antibody conjugates G21040 (Invitrogen; dilution 1:10,000). Images were acquired on a ChemiDoc MP Imaging System (BioRad) using ImageLab v5.1.1 (BioRad).
Northern Hybridization.
Northern hybridization experiments were performed as described previously (22). For Northern probes, see SI Appendix, Table S1. Northern blots were imaged using a Typhoon PhosphoImager (Amersham) and quantified using ImageQuant TL and Excel.
Small RNA Library Preparation and Data Analysis.
Small RNA libraries were prepared as previously described (57). In brief, total RNA was resolved on a 15% denaturing polyacrylamide gel, and 18- to 30-nt-long RNA was excised, gel-eluted, and subjected to 2S ribosomal RNA (rRNA) depletion as previously described (31). Size-selected and 2S rRNA-depleted RNA was subjected to 3ʹ and 5ʹ adaptor ligation. Both adaptors contain four random nucleotides at the ligation interfaces to reduce ligation biases. Ligation products were subjected to reverse transcription using SuperScript III (Invitrogen), according to the manufacturer’s instructions, PCR amplified, and sequenced on an Illumina HiSEq. 2500 on SR50 mode. Small RNA library analysis was performed as previously described (58). Only microRNAs with a steady-state abundance of >100 ppm in any library were considered. Classification of Nbr substrates and non-Nbr substrates were as previously described (23).
Supplementary Material
Acknowledgments
This project has received funding from the Mathers Foundation (D.J.P.) and the Memorial Sloan-Kettering Cancer Center Core Grant P30-CA016086. Support was also provided by the Austrian Academy of Science (S.L.A. and J.B.); the Austrian Science Fund (FWF, SFB F80-02); and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (ERC-CoG-866166, RiboTrace) (to S.L.A.). Small RNA sequencing was performed at the Vienna Biocenter Core Next Generation Sequencing Facility Unit (https://www. viennabiocenter.org/facilities/). This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by NIH National Institute of General Medical Sciences Grant P30 GM124165. The Pilatus 6M detector on 24‐ID‐C beamline is funded by NIH‐ORIP HEI Grant S10 RR029205. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE‐AC02‐06CH11357, and those of the Minnesota Supercomputing Institute.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018156117/-/DCSupplemental.
Data Availability.
The atomic coordinates have been deposited in the Protein Data Bank under the codes 7JW3 (AaNbr NTD), 7JW2 (DmNbr EXO), and 7JW6 (AaNbr EXO). Small-RNA–sequencing datasets have been deposited in the Gene Expression Omnibus database (GSE158054).
References
- 1.Ghildiyal M., Zamore P. D., Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 10, 94–108 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malone C. D., Hannon G. J., Small RNAs as guardians of the genome. Cell 136, 656–668 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutvagner G., Simard M. J., Argonaute proteins: Key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 9, 22–32 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., et al. , Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456, 921–926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Sheng G., Juranek S., Tuschl T., Patel D. J., Structure of the guide-strand-containing argonaute silencing complex. Nature 456, 209–213 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swarts D. C., et al. , The evolutionary journey of Argonaute proteins. Nat. Struct. Mol. Biol. 21, 743–753 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel D. P., Metazoan microRNAs. Cell 173, 20–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treiber T., Treiber N., Meister G., Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 20, 5–20 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Eulalio A., Huntzinger E., Izaurralde E., Getting to the root of miRNA-mediated gene silencing. Cell 132, 9–14 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Brennecke J., Hipfner D. R., Stark A., Russell R. B., Cohen S. M., Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki Y. W., Siomi M. C., Siomi H., PIWI-interacting RNA: Its biogenesis and functions. Annu. Rev. Biochem. 84, 405–433 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Ozata D. M., Gainetdinov I., Zoch A., O’Carroll D., Zamore P. D., PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 20, 89–108 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Brennecke J., et al. , Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Jin W., Wang J., Liu C.-P., Wang H.-W., Xu R.-M., Structural basis for pri-miRNA recognition by Drosha. Mol. Cell 78, 423–433.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Partin A. C., et al. , Cryo-EM structures of human Drosha and DGCR8 in complex with primary microRNA. Mol. Cell 78, 411–422.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim V. N., Han J., Siomi M. C., Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10, 126–139 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Song M.-S., Rossi J. J., Molecular mechanisms of dicer: Endonuclease and enzymatic activity. Biochem. J. 474, 1603–1618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon S. C., et al. , Structure of human DROSHA. Cell 164, 81–90 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Liu Z., et al. , Cryo-EM structure of human dicer and its complexes with a pre-miRNA substrate. Cell 173, 1191–1203.e12 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Ameres S. L., Zamore P. D., Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell Biol. 14, 475–488 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Liu N., et al. , The exoribonuclease Nibbler controls 3′ end processing of microRNAs in Drosophila. Curr. Biol. 21, 1888–1893 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han B. W., Hung J.-H., Weng Z., Zamore P. D., Ameres S. L., The 3′-to-5′ exoribonuclease Nibbler shapes the 3′ ends of microRNAs bound to Drosophila Argonaute1. Curr. Biol. 21, 1878–1887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichholf B., et al. , Time-resolved small RNA sequencing unravels the molecular principles of microRNA homeostasis. Mol. Cell 75, 756–768.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimasu H., et al. , Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature 491, 284–287 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Ipsaro J. J., Haase A. D., Knott S. R., Joshua-Tor L., Hannon G. J., The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature 491, 279–283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X., Fejes Tóth K., Aravin A. A., piRNA biogenesis in Drosophila melanogaster. Trends Genet. 33, 882–894 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czech B., Hannon G. J., One loop to rule them all: The ping-pong cycle and piRNA-guided silencing. Trends Biochem. Sci. 41, 324–337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohn F., Handler D., Brennecke J., PiRNA-guided slicing specifies transcripts for Zucchini-dependent, phased piRNA biogenesis. Science 348, 812–817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han B. W., Wang W., Li C., Weng Z., Zamore P. D., Noncoding RNA. piRNA- guided transposon cleavage initiates Zucchini-dependent, phased piRNA production. Science 348, 817–821 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feltzin V. L., et al. , The exonuclease Nibbler regulates age-associated traits and modulates piRNA length in Drosophila. Aging Cell 14, 443–452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi R., et al. , Genetic and mechanistic diversity of piRNA 3′-end formation. Nature 539, 588–592 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H., et al. , Antagonistic roles of Nibbler and Hen1 in modulating piRNA 3′ ends in Drosophila. Development 143, 530–539 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y., et al. , Structural insights into mechanisms of the small RNA methyltransferase HEN1. Nature 461, 823–827 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo Y., Deutscher M. P., Exoribonuclease superfamilies: Structural analysis and phylogenetic distribution. Nucleic Acids Res. 29, 1017–1026 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ketting R. F., Haverkamp T. H. A., van Luenen H. G. A. M., Plasterk R. H. A., Mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell 99, 133–141 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Bosher J. M., Labouesse M., RNA interference: Genetic wand and genetic watchdog. Nat. Cell Biol. 2, E31–E36 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Joosten J., Van Rij R. P., Miesen P., Slicing of viral RNA guided by endogenous piRNAs triggers the production of responder and trailer piRNAs in Aedes mosquitoes. bioRxiv: 10.1101/2020.07.08.193029 (8 July 2020). [DOI] [PMC free article] [PubMed]
- 38.Eulalio A., Huntzinger E., Izaurralde E., GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol. Biol. 15, 346–353 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Hauptmann J., et al. , Biochemical isolation of Argonaute protein complexes by Ago-APP. Proc. Natl. Acad. Sci. U.S.A. 112, 11841–11845 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holm L., “Using Dali for protein structure comparison BT” in Structural Bioinformatics: Methods and Protocols, Gáspári Z., Ed. (Springer, New York, 2020), pp. 29–42. [DOI] [PubMed] [Google Scholar]
- 41.Cook A. G., Fukuhara N., Jinek M., Conti E., Structures of the tRNA export factor in the nuclear and cytosolic states. Nature 461, 60–65 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Wan R., Yan C., Bai R., Huang G., Shi Y., Structure of a yeast catalytic step I spliceosome at 3.4 Å resolution. Science 353, 895–904 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Sun W., et al. , Crystal structure of the yeast TSC1 core domain and implications for tuberous sclerosis pathological mutations. Nat. Commun. 4, 2135 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Makino D. L., et al. , RNA degradation paths in a 12-subunit nuclear exosome complex. Nature 524, 54–58 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Tian Y., Simanshu D. K., Ma J.-B., Patel D. J., Structural basis for piRNA 2′-O-methylated 3′-end recognition by Piwi PAZ (Piwi/Argonaute/Zwille) domains. Proc. Natl. Acad. Sci. U.S.A. 108, 903–910 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schütz P., et al. , Crystal structure of the yeast eIF4A-eIF4G complex: An RNA-helicase controlled by protein-protein interactions. Proc. Natl. Acad. Sci. U.S.A. 105, 9564–9569 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazzaretti D., et al. , The bicoid mRNA localization factor Exuperantia is an RNA-binding pseudonuclease. Nat. Struct. Mol. Biol. 23, 705–713 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Chen J., et al. , Structural and biochemical insights into small RNA 3′ end trimming by Arabidopsis SDN1. Nat. Commun. 9, 3585 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams P. D., et al. , PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terwilliger T. C., et al. , Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr. D Biol. Crystallogr. 64, 61–69 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terwilliger T. C., et al. , Decision-making in structure solution using Bayesian estimates of map quality: The PHENIX AutoSol wizard. Acta Crystallogr. D Biol. Crystallogr. 65, 582–601 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCoy A. J., et al. , Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afonine P. V., et al. , Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibson D. G., Smith H. O., Hutchison C. A. III, Venter J. C., Merryman C., Chemical synthesis of the mouse mitochondrial genome. Nat. Methods 7, 901–903 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Reimão-Pinto M. M., et al. , Molecular basis for cytoplasmic RNA surveillance by uridylation-triggered decay in Drosophila. EMBO J. 35, 2417–2434 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jayaprakash A. D., Jabado O., Brown B. D., Sachidanandam R., Identification and remediation of biases in the activity of RNA ligases in small-RNA deep sequencing. Nucleic Acids Res. 39, e141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reimão-Pinto M. M., et al. , Uridylation of RNA hairpins by tailor confines the emergence of microRNAs in Drosophila. Mol. Cell 59, 203–216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates have been deposited in the Protein Data Bank under the codes 7JW3 (AaNbr NTD), 7JW2 (DmNbr EXO), and 7JW6 (AaNbr EXO). Small-RNA–sequencing datasets have been deposited in the Gene Expression Omnibus database (GSE158054).