Abstract
Objectives:
Interactions between mesenchymal stem cells (MSCs) and native vocal fold fibroblasts (VFFs) have not been described in spite of promising preliminary data regarding the effects of MSCs on vocal fold repair in vivo. The current study employed a conditioned media (CM) model to investigate the paracrine effects of bone marrow-derived mesenchymal stem cells (BMSCs) on VFFs.
Methods:
Human VFFs were treated with transforming growth factor-β1 (TGF-β1; 10 ng/mL), CM from human BMSCs following 48 hours of TGF-β1 stimulation, or CM+TGF-β1. Proliferation, immunocytochemistry for alpha smooth muscle actin (αSMA), migration, and collagen gel contraction were quantified as well as transcription of components of the TGF-β signaling pathway.
Results:
Transforming growth factor-β1 accelerated proliferation and induced αSMA in VFFs; these effects were suppressed with CM (P = .009, P < .001, respectively). The CM+TGF-β1 condition increased cell migration (P = .02) and decreased gel contraction; CM+TGF-β1 also inhibited TGF-β signaling via significant upregulation of NR4A1 as well as downregulation of SMAD3 and TGF-β1 relative to TGF-β1 stimulation in the absence of CM (P = .002, P < .001, and P = .005, respectively).
Conclusions:
Conditioned media affected many profibrotic cell activities in TGF-β1-stimulated VFFs, likely related to altered TGF-β signaling. These data provide preliminary insight regarding the antifibrotic effects of MSCs and further support their progression to clinical utility.
Keywords: vocal fold, fibrosis, bone marrow-derived mesenchymal stem cells, transforming growth factor-β, conditioned media
Introduction
Following injury, an exuberant inflammatory environment disrupts extracellular matrix (ECM) metabolism, ultimately yielding scar formation and increased tissue stiffness. In the vocal folds, this process frequently leads to intractable loss of tissue viscoelasticity critical for vocal fold vibration. Treatment of vocal fold fibrosis remains challenging due to difficulty in restoring the native architecture; standardized and optimal strategies for the management of vocal fold fibrosis have not yet been established. In that regard, stem cell therapy has emerged as a promising therapeutic strategy for vocal fold fibrosis. Recent studies described successful approaches employing mesenchymal stem cells (MSCs), fibroblasts, induced pluripotent stem (iPS) cells, and embryonic stem (ES) cells.1
Mesenchymal stem cells have long been identified for their unique characteristics, including self-renewal and multidirectional differentiation into a variety of cell lineages (eg, bone, fat, and cartilage),2 as well as ease of harvest and isolation (eg, bone marrow,3 adipose tissue,4 placenta,5 peripheral blood,6 etc). Several investigators have demonstrated that MSCs transplanted into injured vocal folds yield impressive histological and functional outcomes in rat,7,8 rabbit,9,10 and canine11,12 models of vocal fold injury. In spite of these encouraging preliminary data, few studies have investigated the mechanism(s) associated with these outcomes. We hypothesize that this mechanism is likely complex; in addition to directly altering the wound environment, MSCs likely interact with native cells in the wound milieu to alter the wound healing phenotype. We sought to investigate these interactions with the overarching goal of providing further foundational insight into the relevant variables underlying the therapeutic efficacy of MSCs to improve wound healing in the vocal folds.
Wound healing is a complex, multistage process with the goal of recapitulation of the epithelium and lamina propria layers of the vocal folds following injury. Vocal fold fibroblasts (VFFs) play a central role in the wound healing process by migrating into the wound bed to synthesize new ECM. A subpopulation of fibroblasts differentiate to become α-smooth muscle actin (αSMA)-expressing myofibroblasts.13 Myofibroblasts are the main effector cells in tissue fibrosis that synthesize ECM in the wound bed, particularly type I collagen. Transforming growth factor-β1 (TGF-β1) is a key factor involved in scar formation and has been shown to promote myofibroblast differentiation.14 Transforming growth factor-β1 signaling is primarily mediated via the Smad family of signaling proteins, which transduce extracellular cues from cell-surface transmembrane receptors to the nucleus. Smad2 and Smad3 are receptor-activated or pathway-restricted proteins directly activated by TGF-β1 binding to the receptor, leading to heterodimerization with Smad4. This complex is then translocated into the nucleus to regulate transcription. Smad3 in particular is thought to be mainly related to fibrosis, and knockdown of SMAD3 has been shown to suppress collagen gene expression and contractile features in human VFFs.15,16
Smad7 is a competitive inhibitor of Smad activation and therefore thought to be an endogenous antifibrotic agent. In addition, recent data suggest Nr4a1 is also likely a potent attenuator of TGF-β signaling. Nr4a1 deficiency has been linked to skin and lung fibrosis, and Nr4a1 agonists ameliorated fibrosis in various fibrotic models.17 Nr4a1 belongs to the nuclear receptor subfamily 4 group (NR4A), which act as transcription factors to regulate expression of genes by targeting specific response elements.18 Nr4a1 is a key transcriptional regulator and has been implicated in glucose and lipid homeostasis, adipogenesis, inflammation, and vascular remodeling.19 The role of Nr4a1 in vocal fold homeostasis and fibrosis is unknown. Furthermore, the effects of MSCs on Smad3 and Nr4a1 expression in VFFs have not been described. These data are critical to our understanding of the development of fibrosis as well as the development of targeted, physiologically relevant therapeutics for this challenging population.
In that regard, we recently reported that MSCs altered ECM metabolism in VFFs employing a co-culture model.20 Mesenchymal stem cells had favorable effects on VFFs across multiple fibrotic processes. However, it is unclear if direct cell-to-cell interactions, paracrine signaling, or a combination of the two mediated these outcomes. To address this experimental question, we employed a conditioned media (CM) model. It is well known that MSCs facilitate tissue repair and mitigate excessive inflammation via the paracrine actions of various tropic factors and cytokines. In this regard, it is essential to eliminate direct cell-to-cell interactions to quantify the regulatory effects of MSCs via paracrine signaling. Conditioned media models are ideal to investigate these issues. These data are fundamental to both increase our collective insight into the biochemical processes underlying vocal fold injury and repair but also improve the platform for enhanced pre-clinical and clinical investigation.
Materials and Methods
Preparation of Conditioned Media
Human bone marrow-derived mesenchymal stem cells (BMSCs; Thermo Fisher Scientific, Waltham, Massachusetts, USA) at passage 3 to 5 were employed for all experimentation. Bone marrow-derived mesenchymal stem cells at 70% to 80% confluency were treated with serum-free Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with recombinant human TGF-β1 (10 ng/mL; Life Technologies, Grand Island, New York, USA) following 12-hour serum starvation (DMEM+0.1% Bovine Serum Albumin). After 48 hours, the media was collected, centrifuged at 1000 rpm for 5 minutes, and filtered through a .22 μm syringe filter. This CM was employed immediately for all experimentation.
Cell Culture Model
The immortalized human vocal fold fibroblast cell line created in our laboratory, referred to as HVOX,21 was employed for all experimentation. This line has been shown to remain stable through multiple population doublings. HVOX were seeded and cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and antibiotic/antimycotic for 24 hours under standard cell culture conditions. After 12 hours of serum starvation, the cells were washed with phosphate-buffered saline (PBS) and exposed to the following experimental conditions: serum-free media (control), TGF-β1 (10 ng/mL), CM, or CM+TGF-β1 (10 ng/mL).
Cell Proliferation
The CellTiter 96 Assay (Promega, Madison, Wisconsin, USA), an assay of metabolic activity and viability, was employed as described previously.21 Briefly, 5.0 × 103 cells were seeded in each well of a 96-well plate and cultured under each experimental condition for 72 hours. At the experimental endpoint, 20 μL of dye solution regent was added to each well and absorbance was measured in triplicate via Synergy H1 microplate reader (BioTek, Winooski, Vermont, USA) at 490 nm.
Immunocytochemistry
HVOX (1.0 × 105) were seeded on chamber slides and grown for 72 hours under each experimental condition. Cells were then fixed in 4% paraformaldehyde for 10 minutes and then permeabilized and blocked with a PBS solution containing .1% Triton-X and 5% normal goat serum. The slides were incubated at 4°C overnight with primary mouse monoclonal antibodies against αSMA (1:200; Sigma-Aldrich, St. Louis, Missouri, USA) and then the corresponding Alexa-Fluor 555 goat anti-mouse IgG (1:500; Invitrogen, Carlsbad, California, USA) secondary antibody. For negative controls, primary antibodies were omitted. Digital images were captured with a Nikon Eclipse Ni-U fluorescence microscope (Nikon Inc, Tokyo, Japan), and ImageJ Software (National Institutes of Health; v1.41) was used to count αSMA-positive cells and nuclei. Analyses were performed on 10 randomly chosen, 3.0 mm2 fields for each condition under 10× magnification.
Cell Migration/Scratch Assay
HVOX migration was assessed as previously described by our group.16,21 Briefly, cells were grown in 6-well plates until 100% confluence. Following serum starvation, a gentle scratch was performed along the longitudinal axis of each well using a small pipette tip, followed by a PBS wash to remove detached cells. The plates were then incubated for 8 hours under each media condition. Digital images were captured at 0 and 8 hours using a Nikon Eclipse TS100 microscope (Nikon Inc) with an attached C-mount Morrell HDMI-02 camera (Morrell Instrument Company, Melville, New York, USA) at 4× magnification. Transverse markings on the bottom of each well served as landmarks to ensure the location of imaging was consistent across time points. The denuded area was quantified in pixels using ImageJ Software. The following formula was employed to quantify migration: initial area – final area = Δarea/8 h.
Collagen Contraction
Contraction was assayed as previously reported by our group.16,22 HVOX were gently pipetted onto gels at a concentration of 3.0 × 104 cells in 200 μL and incubated for 2 hours. The gels were then submerged in each media condition supplemented with 5% FBS. Gels were imaged at 0 and 24 hours using a digital 8-megapixel camera (Apple Inc, Cupertino, California, USA). Contraction was quantified by measuring the gel area in pixels using ImageJ Software using the following formula to evaluate percent contraction: initial area – final area = Δarea/8 h.
Quantitative Real-time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to quantify changes in components of the TGF-β signaling pathway. HVOX at ~80% confluence in 6-well plates were cultured for 3 hours under each experimental condition. Total RNA was extracted from the cells using the RNeasy Mini Kit (Qiagen, Valencia, California, USA). The quantity and quality of mRNA was evaluated via 260/280 ratio. RNA was reverse transcribed with a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, California, USA). The qRT-PCR employed the TaqMan Gene Expression Kit (Life Technologies) and StepOne Plus (Applied Biosystems). The TaqMan primer probes employed were: NR4A1 (Hs00374226_m1), SMAD2 (Hs00183425_m1), SMAD3 (Hs00969210_m1), SMAD7 (Hs00998193_m1), TGF-β1 (Hs00998133_m1), and GAPDH (Hs02758991_g1). The ΔΔCt method was employed with GAPDH as the housekeeping gene for the determination of relative expression levels.
Statistical Analyses
Four samples were assayed from each group run in triplicate. For all analyses, the dependent variable of interest was subjected to a one-way analysis of variance. If the main effect was significant at P < .05, post hoc comparisons were performed via the Scheffé method. Statistical significance was defined as P < .05 using StatView 5.0 (SAS Institute, Berkeley, California, USA). All data are expressed as mean ± standard error.
Results
Conditioned Media Altered Cell Proliferation and Myofibroblast Differentiation
TGF-β1 and CM alone induced HVOX proliferation (Figure 1A). This effect was blunted by CM+TGF-β1; proliferation was significantly decreased compared to TGF-β1 (P = .009). The αSMA-positivity increased in response to TGF-β1 (Figure 1B; a, c) and decreased in response to CM+TGF-β1 as well as CM alone (Figure 1C; b, d). Quantitatively, a significant difference in αSMA positive cells was observed between CM+TGF-β1 and TGF-β1 (P < .001; Figure 1C).
Figure 1.
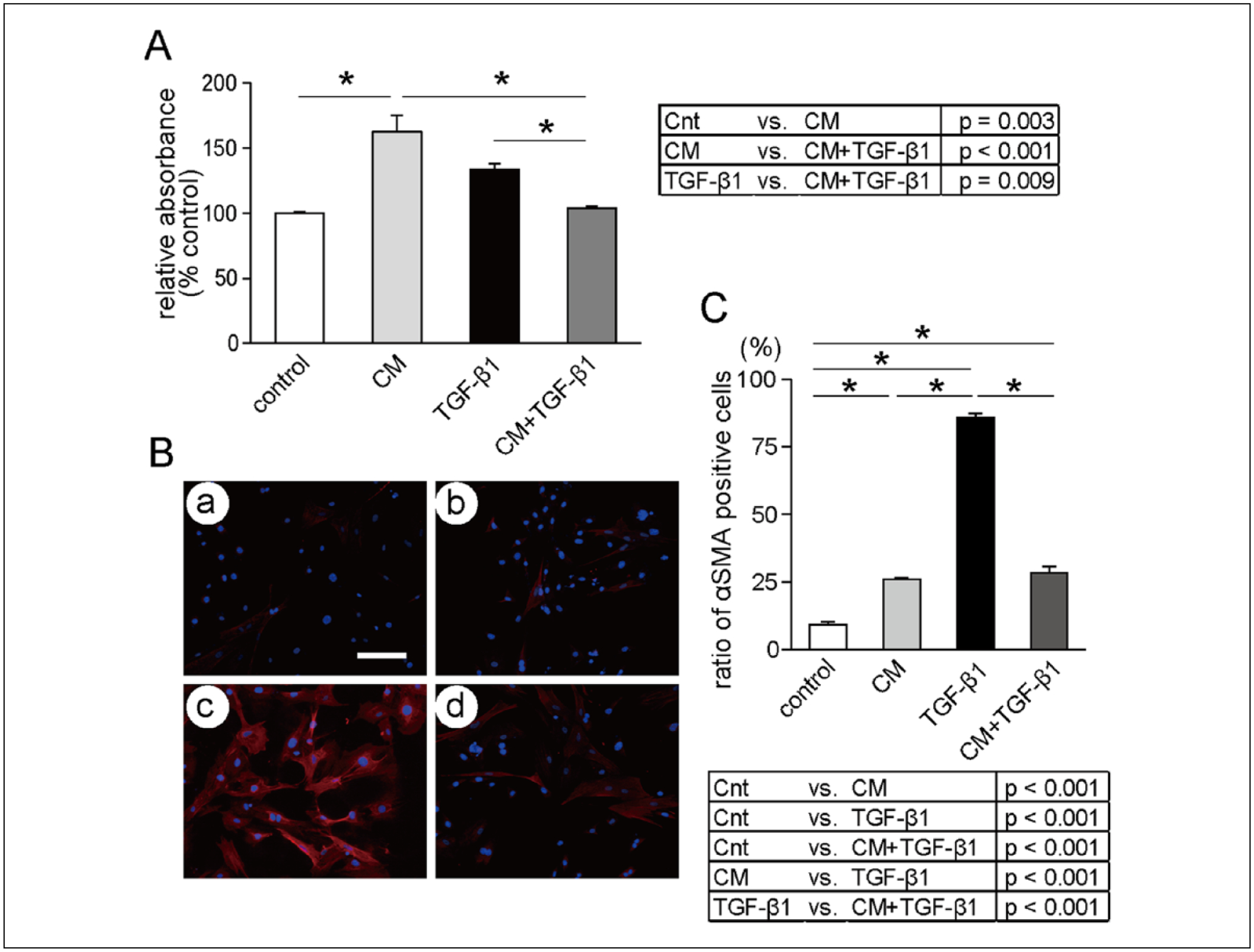
Effects of (TGF-β1) and conditioned media on HVOX proliferation. (A) Cell viability is presented as percent control. Data presented as mean ± SEM of assays run in triplicate (n = 4; *P < .05). Effects of TGF-β1 and MSCs conditioned media on cell phenotypic change into expressing αSMA; representative immunofluorescent images of HVOX cultured for 3 days under (B, a-control) serum-free media, (b) 10 ng/mL TGF-β1 (c) CM, and (d) CM with 10 ng/mL TGF-β1. Blue, DAPI; red αSMA. Scale bar: 100 μm. (C) Ratio of αSMA-positive cells was calculated from 10 randomly chosen fields of 4 samples in each media group. Data presented as mean ± SEM (*P < .05).
Abbreviations: αSMA, alpha smooth muscle actin; CM, conditioned media; Cnt, control; MSC, mesenchymal stem cells; TGF-β1, transforming growth factor-β1.
Conditioned Media Increased Vocal Fold Fibroblast Migratory Rate
As shown in Figure 2A and 2B, TGF-β1, CM, and CM+TGF-β1 induced higher migratory rates relative to control. Conditioned media had an additive effect on TGF-β1-mediated migration, although the difference between CM+TGF-β1 and TGF-β1 did not achieve statistical significance (P = .55).
Figure 2.
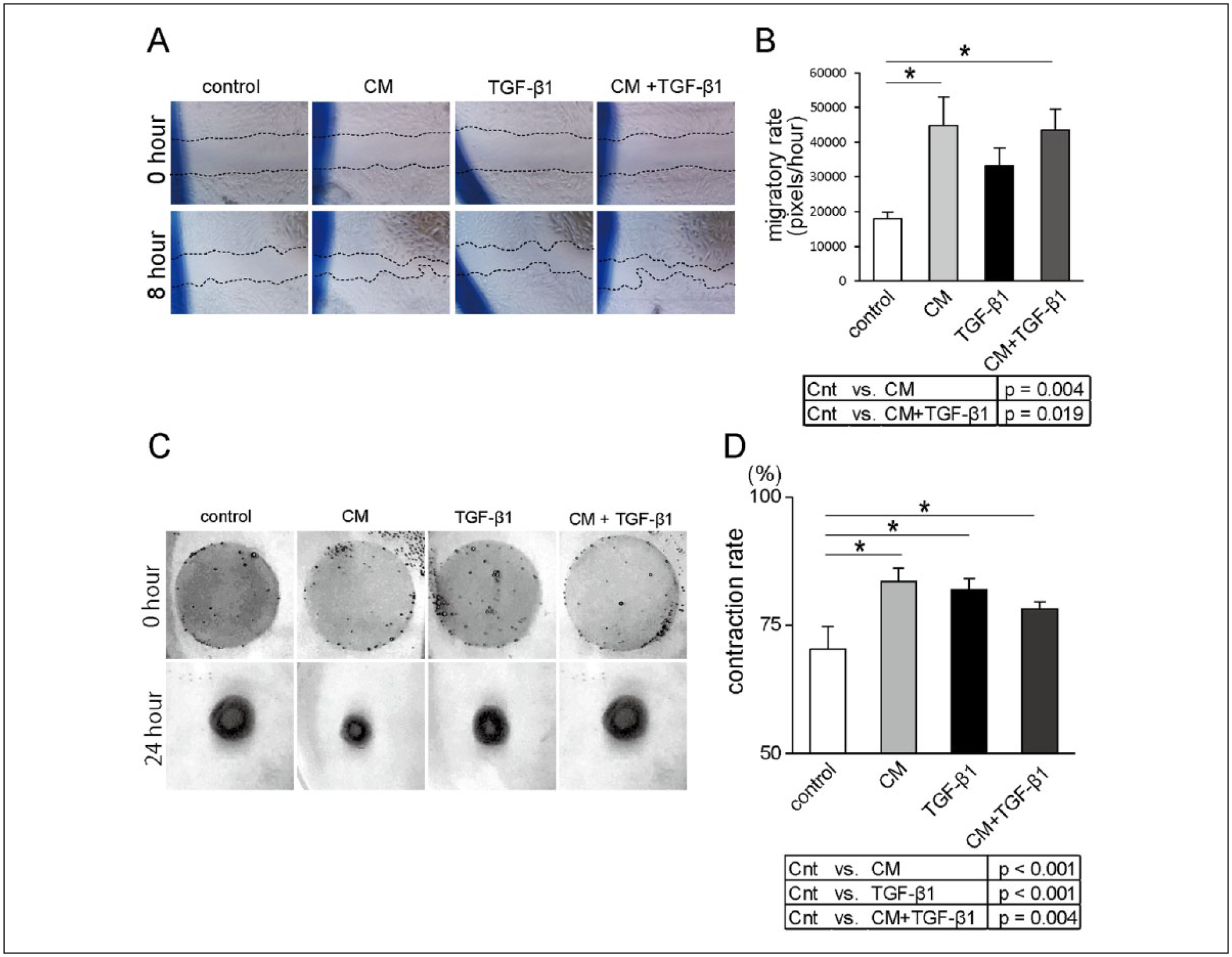
Effects of TGF-β1 and MSCs conditioned media on cell migration using scratch assay. (A) Representative bright-field images under each culture media condition directly after or 24 hours after scratching (original magnification 4×). (B) Conditioned media–facilitated cell migration, although there was no significant difference between TGF-β1 and CM+TGF-β1 (n = 4; *P < .05). Effects of TGF-β1 and MSCs CM on 3-dimensional collagen gel contraction. Representative gels under each culture media condition directly after or 24 hours after cell seeding (C). Data presented as mean ± SEM of assays run in triplicate (D; n = 4; *P < .05).
Abbreviations: CM, conditioned media; Cnt, control; MSC, mesenchymal stem cells; TGF-β1, transforming growth factor-β1.
Condition Media Attenuated Collagen Gel Contraction
TGF-β1 stimulated HVOX contraction (Figure 2C and 2D). Although CM and CM+TGF-β1 increased contraction compared to control, this response was less robust in response to CM+TGF-β1; this difference did not achieve significance (P = .23).
Conditioned Media Regulated Transforming Growth Factor-β Signaling Expression
Expression of NR4A1 was upregulated in response to CM compared to control. Upregulation of NR4A1 in response to CM+TGF-β1 was significant when compared to TGF-β1 in the absence of CM (P = .002; Figure 3A). Although TGF-β1 stimulation did not alter SMAD2 expression, CM+TGF-β1 decreased SMAD3 expression compared to TGF-β1 stimulation (P < .001; Figure 3B, 3C). SMAD7 was also upregulated in response to both TGF-β1 and CM stimulation; however, no additive effect of CM was observed (Figure 3D). Endogenous TGF-β1 was upregulated in response to TGF-β1 stimulation and significantly down-regulated in response to CM+TGF-β1 compared to TGF-β1 alone (P = .005; Figure 3E).
Figure 3.

Expression of TGF-β1 signaling components under each condition. All genes were standardized to GAPDH expression and expressed as fold expression relative to control. Data presented as mean ± SEM of assays run in triplicate (n = 4; *P < .05).
Abbreviations: CM, conditioned media; Cnt, control; MSC, mesenchymal stem cells; TGF-β1, transforming growth factor-β1.
Discussion
Under normal wound healing conditions, a subpopulation of fibroblasts differentiate into myofibroblasts and actively synthesize and deposit neo-matrix, often leading to aberrant biomechanical tissue properties and subsequent dysphonia. Typically during the remodeling phase of healing, myofibroblast presence decreases via either apoptosis or dedifferentiation into fibroblasts. However, pathological and persistent myofibroblast accumulation in the wound bed is associated with excessive collagen deposition and tissue stiffness.14,23 A favorable balance between fibroblasts and myofibroblasts is critical to tissue homeostasis, and furthermore, the prevention of excessive or prolonged myofibroblast differentiation is critical to both avoid and/or treat scar.
Vocal fold fibrosis remains of significant interest in both clinical practice and research laboratories. As stem cell-based therapies increase in popularity and appear to be nearing clinical practice, clear insight is required to fully understand the mechanism(s) underlying these therapeutic approaches to ensure they are ideally employed to optimize in vivo outcomes. In addition, basic investigation regarding the key biochemical switches underlying the development of fibrosis is warranted. In that regard, the current investigation focused on suppression of TGF-β1–mediated fibroplasia in vitro. Following vocal fold injury, TGF-β transcription is upregulated as early as 8 hours24 and peaks approximately 3 to 7 days following injury. Sustained TGF-β expression has been described well into the remodeling and chronic healing phases.25,26 Our laboratory and others have hypothesized that TGF-β is likely a master regulator of vocal fold fibrosis. As such, in the current study, vocal fold fibroblasts were stimulated with TGF-β1 to elicit profibrotic features. In addition, MSCs were also stimulated with TGF-β1 to create the CM model. Admittedly, CM models are a bit contrived and oversimplified as there are likely many soluble mediators of healing that could alter the wound healing phenotype. However, this model allows for us to eliminate direct cell-to-cell interactions.
Interestingly, CM combined with TGF-β1 did not increase cell proliferation in the context of suppressed myofibroblast differentiation. Mesenchymal stem cells have been previously shown to reduce myofibroblast persistence via apoptosis.27 This apoptotic effect may be exerted on VFF under TGF-β1 stimulation, leading to contrary findings. TGF-β1-mediated VFF proliferation and increased myofibroblast differentiation has been reported previously.28 However, MSC-mediated paracrine signaling stimulated cell proliferation and suppressed myofibroblast differentiation in skin fibroblasts, keratinocytes, hepatic stellate cells, and glomerular fibroblasts.29–32 These previous reports concur with data from the current study.
Fibroblast migration and contraction are critical cell activities related to wound healing and tissue homeostasis. However, aberrant or excessive migration and contraction are thought to be related to the development of fibrosis. Our data suggest that MSC conditioned media had similar effects compared to TGF-β1 stimulation alone. Li et al30 reported similar findings in that CM derived from MSCs enhanced rat keratinocyte migration under disease-specific conditions. In contrast, CM combined with TGF-β1 significantly reduced VFF contraction compared to TGF-β1 stimulation alone. This reduced contractile phenotype was greater in the CM+TGF-β1 condition when compared to CM alone. These data may suggest that TGF-β1 is not only a master regulator of fibrosis but also key component of the negative feedback loop to initiate antifibrotic activities.
To further clarify the mechanisms underlying suppressed myofibroblast differentiation as described via immunocytochemistry and gel contraction assays, key components the TGF-β signaling machinery were investigated. Significant upregulation of NR4A1 as well as downregulation of SMAD3 and endogenous TGF-β1 were observed in response to CM+TGF-β1 compared to TGF-β1 stimulation alone. Smad3 is emerging as a central target for regulating TGF-β1 signaling, and knockdown of SMAD3 has been shown to have antifibrotic effects in VFFs under TGF-β1 stimulation.16 Nr4a1 is a newly detected nuclear receptor and molecular target to alter TGF-β signaling. Cytosporone B, a Nr4a1 transcriptional agonist, has been shown to be antifibrotic in animal models of fibrotic disease.17 Our data indicate that MSC-mediated paracrine signaling modulated TGF-β1 signaling processes via both SMAD3 and NR4A1. Cumulatively, these data suggest that these transcriptional changes likely encourage decreased myofibroblast differentiation and contraction. Zhang et al33 previously recently reported that CM derived from MSCs inhibited αSMA expression and reduced Smad2/3 and Smad4 expression and increased Smad7 expression in TGF-β1-stimulated hepatic stellate cells. Our data concur with these previous reports and further confirm the inhibitory effects of CM on myofibroblast differentiation and TGF-β signaling.
Mesenchymal stem cells have diverse immunomodulatory effects via suppression of host T cell proliferation.34 Admittedly, alternative mechanisms are feasible with regard to the antifibrotic effects of MSCs. For example, MSCs have been shown to stimulate angiogenesis via secretion of several pro-angiogenic factors, most notably, vascular endothelial growth factor or via pre-differentiation into angiogenic precursors.35 It is likely that multiple mechanisms contribute to favorable wound healing outcomes, and advanced understanding of these processes is likely to enhance the development of novel, targeted therapeutics.
The current study, however, is not without limitation. As noted previously, the in vitro environment is highly contrived and artificially simplistic. From a methodological perspective, CM was extracted from TGF-β1-stimulated MSCs. Following serum starvation, MSC secretion of trophic factors decreased substantially, and the cells became largely senescent. Clearly, these conditions do not precisely correspond to the in vivo milieu, but provide some preliminary insight regarding relevant cell signaling underlying the inherent tissue response to injury. Exogenous TGF-β1 was employed as a stimulator; MSCs are likely to be transplanted into the scarred vocal fold and exposed to TGF-β1 stimulation associated with the inherent tissue response to injury. This model, although physiologically relevant, is quite simple as many factors contribute wound healing. Regardless, the current study provides marked insight into potential mechanisms to exploit to enhance wound healing outcomes in the vocal folds. In that regard, investigation regarding more clinically-based issues including safety, timing of injection, and/or cell number is warranted in the preclinical setting prior to clinical application. Data from the current study contribute to the expanding platform for this progression.
Conclusions
Mesenchymal stem cell transplantation has emerged as a promising strategy for the treatment of vocal fold fibrosis, and further insight into the mechanisms of the purported efficacy of MSCs is warranted. In addition to cell-to-cell interactions, MSCs likely exert antifibrotic effects on vocal fold fibroblasts via paracrine mechanisms. In the current study, MSC CM modulated chemotactic features in vocal fold fibroblasts, including proliferation and migration as well as decreased myofibroblast differentiation. In addition, CM reduced the contractile features in these cells via altered TGF-β signaling. These data provide a foundation for future clinical/therapeutic use of MSCs for vocal fold fibrosis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this work was provided by the National Institutes of Health/National Institute on Deafness of and Other Communication Disorders (RO1 DC013277, PI-Branski).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kumai Y, Kobler JB, Herrera VL, Zeitels SM. Perspectives on adipose-derived stem/stromal cells as potential treatment for scarred vocal folds: opportunity and challenges. Curr Stem Cell Res Ther. 2010;5:175–181. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 3.Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249–258. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. [DOI] [PubMed] [Google Scholar]
- 6.Kassis I, Zangi L, Rivkin R, et al. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967–976. [DOI] [PubMed] [Google Scholar]
- 7.Kanemaru S, Nakamura T, Yamashita M, et al. Destiny of autologous bone marrow-derived stromal cells implanted in the vocal fold. Ann Otol Rhinol Laryngol. 2005;114:907–912. [DOI] [PubMed] [Google Scholar]
- 8.Hiwatashi N, Hirano S, Mizuta M, et al. Adipose-derived stem cells versus bone marrow-derived stem cells for vocal fold regeneration. Laryngoscope. 2014;124(12):E461–E469. [DOI] [PubMed] [Google Scholar]
- 9.Hertegard S, Cedervall J, Svensson B, et al. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope. 2006;116: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Hu R, Fan E, Han D. Adipose-derived mesenchymal stem cells in collagen-hyaluronic acid gel composite scaffolds for vocal fold regeneration. Ann Otol Rhinol Laryngol. 2011;120:123–130. [DOI] [PubMed] [Google Scholar]
- 11.Kanemaru S, Nakamura T, Omori K, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112:915–920. [DOI] [PubMed] [Google Scholar]
- 12.Hiwatashi N, Hirano S, Suzuki R, et al. Comparison of ASCs and BMSCs combined with atelocollagen for vocal fold scar regeneration. Laryngoscope. 2016;126:1143–1150. [DOI] [PubMed] [Google Scholar]
- 13.Desmouliere A Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995;19:471–476. [DOI] [PubMed] [Google Scholar]
- 14.Darby IA, Laverdet B, Bonte F, Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul BC, Rafii BY, Gandonu S, et al. Smad3: an emerging target for vocal fold fibrosis. Laryngoscope. 2014;124: 2327–2331. [DOI] [PubMed] [Google Scholar]
- 16.Branski RC, Bing R, Kraja I, Amin MR. The role of Smad3 in the fibrotic phenotype in human vocal fold fibroblasts. Laryngoscope. 2016;126(5):1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palumbo-Zerr K, Zerr P, Distler A, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med. 2015;21:150–158. [DOI] [PubMed] [Google Scholar]
- 18.Philips A, Lesage S, Gingras R, et al. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol. 1997;17:5946–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safe S, Jin UH, Morpurgo B, Abudayyeh A, Singh M, Tjalkens RB. Nuclear receptor 4A (NR4A) family—orphans no more. J Steroid Biochem Mol Biol. 2016;157:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiwatashi N, Bing R, Kraja I, Branski RC. Mesenchymal stem cells have antifibrotic effects on transforming growth factor-beta1-stimulated vocal fold fibroblasts. Laryngoscope. 2017;127(1):E35–E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branski RC, Barbieri SS, Weksler BB, et al. Effects of transforming growth factor-beta1 on human vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2009;118:218–226. [DOI] [PubMed] [Google Scholar]
- 22.Parekh A, Sandulache VC, Lieb AS, Dohar JE, Hebda PA. Differential regulation of free-floating collagen gel contraction by human fetal and adult dermal fibroblasts in response to prostaglandin E2 mediated by an EP2/cAMP-dependent mechanism. Wound Repair Regen. 2007;15:390–398. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Chen B, Liu T, Chen X. Reversal of myofibroblast differentiation: a review. Eur J Pharmacol. 2014;734:83–90. [DOI] [PubMed] [Google Scholar]
- 24.Lim X, Tateya I, Tateya T, Munoz-Del-Rio A, Bless DM. Immediate inflammatory response and scar formation in wounded vocal folds. Ann Otol Rhinol Laryngol. 2006;115:921–929. [DOI] [PubMed] [Google Scholar]
- 25.Ohno T, Hirano S, Rousseau B. Gene expression of transforming growth factor-beta1 and hepatocyte growth factor during wound healing of injured rat vocal fold. Laryngoscope. 2009;119:806–810. [DOI] [PubMed] [Google Scholar]
- 26.Chang Z, Kishimoto Y, Hasan A, Welham NV. TGF-beta3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis Model Mech. 2014;7:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunes de Carvalho S, da Cunha Lira D, Costa Cortez EA, et al. Bone marrow cell transplantation is associated with fibrogenic cells apoptosis during hepatic regeneration in cholestatic rats. Biochem Cell Biol. 2013;91:88–94. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Thibeault SL. Response of fibroblasts to transforming growth factor-beta1 on two-dimensional and in three-dimensional hyaluronan hydrogels. Tissue Eng Part A. 2012;18:2528–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Peng Y, Gao D, et al. Mesenchymal stem cells suppress fibroblast proliferation and reduce skin fibrosis through a TGF-beta3-dependent activation. Int J Low Extrem Wounds. 2015;14:50–62. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Zhao Y, Hao H, et al. Mesenchymal stem cell-conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int J Low Extrem Wounds. 2015;14:73–86. [DOI] [PubMed] [Google Scholar]
- 31.Sun XE, Zhang XQ, Liu MM. Effect of bone marrow mesenchymal stem cells on the TGF-beta1/Smad signaling pathway of hepatic stellate. Genet Mol Res. 2015;14:8744–8754. [DOI] [PubMed] [Google Scholar]
- 32.Lv S, Liu G, Sun A, et al. Mesenchymal stem cells ameliorate diabetic glomerular fibrosis in vivo and in vitro by inhibiting TGF-beta signalling via secretion of bone morphogenetic protein 7. Diab Vasc Dis Res. 2014;11:251–261. [DOI] [PubMed] [Google Scholar]
- 33.Zhang LT, Fang XQ, Chen QF, et al. Bone marrow-derived mesenchymal stem cells inhibit the proliferation of hepatic stellate cells by inhibiting the transforming growth factor beta pathway. Mol Med Rep. 2015;12:7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. [DOI] [PubMed] [Google Scholar]
- 35.Roura S, Bago JR, Soler-Botija C, et al. Human umbilical cord blood-derived mesenchymal stem cells promote vascular growth in vivo. PLoS One. 2012;7:e49447. [DOI] [PMC free article] [PubMed] [Google Scholar]


