Significance
Greater autonomy afforded to women in matrilineal societies has been hypothesized to benefit women’s health. Among the Mosuo, a society with both matrilineal and patrilineal subpopulations, we found that gender disparities in chronic disease are not only ameliorated but reversed in matriliny compared with patriliny. Gender disparities in health and chronic disease can thus be tied directly to cultural influences on health, including inequalities in autonomy and resource access between men and women.
Keywords: gender norms, health, chronic disease, matrilineal societies
Abstract
Women experience higher morbidity than men, despite living longer. This is often attributed to biological differences between the sexes; however, the majority of societies in which these disparities are observed exhibit gender norms that favor men. We tested the hypothesis that female-biased gender norms ameliorate gender disparities in health by comparing gender differences in inflammation and hypertension among the matrilineal and patrilineal Mosuo of China. Widely reported gender disparities in health were reversed among matrilineal Mosuo compared with patrilineal Mosuo, due to substantial improvements in women’s health, with no concomitant detrimental effects on men. These findings offer evidence that gender norms limiting women’s autonomy and biasing inheritance toward men adversely affect the health of women, increasing women’s risk for chronic diseases with tremendous global health impact.
It has long been a puzzle why women, who outlive men in most societies, nonetheless experience worse health over their lifetimes (1) (see also refs. 2 and 3). Many explanations center on inherent biological differences that contribute to high morbidity in women (e.g., ref. 4). Yet, health is shaped extensively by social and cultural factors, including gender norms that impact autonomy, resource control, and social support (2, 5). Kinship systems, which structure gender norms in human societies, serve as useful proxies for examining the effects of gender norms on gendered health disparities. In patrilineal kinship systems—where resources are preferentially passed via the male line—women may be disadvantaged by lower social status and limited control over resources (6), compromising their health (7). Conversely, in matrilineal systems—where resources are preferentially passed through the female line—women may experience better health because they have greater control over resources and more kin support (8–10). Men’s health is less likely to be affected by differences in kinship, as men often retain access to resources and positions of authority in matrilineal systems (11, 12).
Broad ecological and cultural differences between most matrilineal and patrilineal societies make it difficult to isolate the effects of their contrasting gender norms on health (13). Here, we leverage a unique natural experiment offered by the matrilineal and patrilineal Mosuo of China, who live in similar environments and share broad cultural norms, allowing us to isolate the effect of gender norms on disparities in health. We investigated the hypothesis that gender norms contribute to gender disparities in health by testing the prediction that the disparity typically observed in chronic disease (poorer health among women) would be present in patrilineal Mosuo communities but ameliorated in matrilineal Mosuo communities.
Matrilineal and patrilineal Mosuo identify as a single ethnic group but differ in predominant gender norms (13). Matrilineal families are organized around maternal kin; resources are transmitted along the female line, women are actively involved in household decision making, and women have higher status than men in many domains (14). Spouses often live apart, remaining instead in their natal homesteads (i.e., residence is natalocal), and children are raised in their mother’s household as a member of her lineage (14). Among patrilineal Mosuo, men maintain household authority, and sons inherit household resources (15). Monogamous marriage and patrilocality prevail, producing nuclear or stem families. Given broad similarities in cultural and ecological context, the matrilineal and patrilineal Mosuo thus provide a rare opportunity to test whether gender disparities in health are influenced by societal gender norms.
To investigate the effects of variation in gender norms on health, we analyzed inflammation and hypertension. These are important indicators of long-term health and have been linked with social factors that may differ between men and women depending on societal gender norms (16, 17). As a biomarker of systemic inflammation, we used C-reactive protein (CRP), which, when elevated (3 mg/L), is strongly predictive of risk for many chronic diseases, including cardiovascular disease and type 2 diabetes (18, 19). Hypertension, defined as elevated arterial blood pressures [systolic 130 mm Hg or diastolic 80 mm Hg (20)], is also a risk factor for numerous chronic diseases (21) and is a leading cause of premature death worldwide (22). In Western countries and developed parts of China, women tend to experience greater inflammation than men and blood pressures that are lower than men’s at reproductive ages but higher than men’s at postreproductive ages (2, 23–28).
Both inflammation and hypertension are associated with a range of social factors, including poverty, stress, early-life trauma, and lack of social support (17, 29–32), that are experienced differently by men and women depending on cultural context. If gender disparities in long-term health are influenced primarily by biological sex differences, then we expect women to exhibit greater inflammation and lower rates of hypertension than men in both matrilineal and patrilineal contexts. If, however, inflammation and hypertension are affected by gender norms, we hypothesize that matriliny will ameliorate gender disparities in both outcomes.
We estimated CRP in 369 Mosuo participants and identified chronic inflammation as CRP 3 to 5 mg/L (33). Pregnant women were excluded, as were individuals with CRP 5 mg/L (to avoid misinterpreting acute inflammatory episodes produced by transient infection). In patrilineal communities the prevalence of chronic inflammation in women (8.4%) was more than double that for men (3.2%), but this pattern was reversed in matrilineal communities (6.5% for men; 3.6% for women). Blood pressures from 993 individuals reveal the same pattern for hypertension: women exhibited a higher prevalence (37.4%) than men (29.7%) in patrilineal communities, while the reverse was true in matrilineal communities (35.2% for men; 31.2% for women).
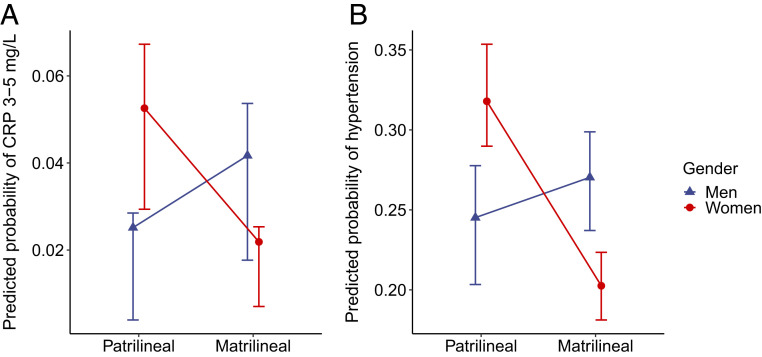
Using Bayesian logistic regression, we modeled an interaction between gender and kinship to test whether men and women experience differences in chronic inflammation and hypertension in matrilineal and patrilineal communities, controlling for age (Table 1). Fig. 1 shows the predicted probabilities of elevated CRP and hypertension resulting from this model. We find reversed gender disparities in both inflammation and hypertension under matriliny, driven primarily by reduced probabilities of both chronic inflammation (0.02) and hypertension (0.20) for women in matriliny compared with patriliny (inflammation: 0.05, Δ = 0.03; hypertension: 0.32, Δ = 0.13). These effects were robust to controls for both age and body mass index (SI Appendix, Table S1).
Table 1.
Bayesian logistic regression models predicting chronic inflammation (CRP 3–5 mg/L) and hypertension (systolic 130 mm Hg or diastolic 80 mm Hg)
| Inflammation | Hypertension | ||||||||
| Base model ( = 369) | Head of household model ( = 324) | Base model ( = 993) | Head of household model ( = 945) | ||||||
| Estimate (SD) | Odds ratio | Estimate (SD) | Odds ratio | Estimate (SD) | Odds ratio | Estimate (SD) | Odds ratio | ||
| (Intercept) | −5.93 (1.15) | 0.00 | −5.59 (1.17) | 0.00 | −3.92 (0.32) | 0.02 | −4.05 (0.34) | 0.02 | |
| Age | 0.07*** (0.02) | 1.07 | 0.06*** (0.02) | 1.06 | 0.07*** (0.01) | 1.07 | 0.07*** (0.01) | 1.07 | |
| Men† | −0.93 (0.81) | 0.39 | −0.62 (0.84) | 0.54 | −0.36* (0.24) | 0.70 | −0.47** (0.25) | 0.63 | |
| Matriliny‡ | −1.00** (0.59) | 0.37 | −0.61 (0.67) | 0.54 | −0.61*** (0.20) | 0.54 | −0.71*** (0.20) | 0.49 | |
| Men matriliny†‡ | 1.66* (1.08) | 5.26 | 1.46* (1.13) | 4.31 | 0.74** (0.31) | 2.10 | 0.81*** (0.33) | 2.25 | |
| Head of household | — | — | −1.03* (0.68) | 0.36 | — | — | 0.16 (0.16) | 1.17 | |
The probability that a positive coefficient is less than zero (or a negative coefficient is greater than zero) is * 0.10–0.05, ** 0.05–0.01, *** <0.01.
Reference is women;
Reference is patriliny.
Fig. 1.
Predicted probabilities of chronic inflammation (A) and hypertension (B) by gender and community. Means and high-probability density intervals were calculated for 1,000 predictions drawn from posterior distributions produced by the Bayesian logistic regressions reported in Table 1 (base model); = 371 (A) and 958 (B). The effect of gender on inflammation and hypertension depends on prevailing gender norms. In patrilineal communities, women have higher probabilities of both elevated CRP and hypertension than men, but in matrilineal communities, these gender disparities are reversed.
It is remarkable that widely reported gender disparities in inflammation and hypertension are not only attenuated but reversed in matrilineal Mosuo communities. We do not have direct evidence of the pathways that produce this reversal. Research in other populations points to relatively high autonomy, greater social and material support, and enhanced parental investment in daughters as potential mechanisms buffering against stress or adversity (9, 34).
Our data provide partial support for the hypothesis that the effect of matriliny on women’s health is associated with increased autonomy and resource control. Specifically, we find that being head of household is inversely associated with elevated CRP, suggesting that autonomy in the household is protective against chronic inflammation in both communities (Table 1). Hypertension is increased among heads of household; however, the protective effect of matriliny among women remains. Household autonomy is thus an important predictor of embodied gender norms, even if specific outcomes vary in direction of association.
We find no significant effect of matriliny on inflammation or hypertension among men (Fig. 1). This result strengthens the inference that gender norms are important drivers of health, as men’s experiences in matriliny are distinct from women’s experiences in patriliny among the Mosuo, in that men retain a large degree of autonomy and resource access in matrilineal communities (12). Men in these communities are likely further buffered by natalocal residence, which preserves networks of social support among kin (35).
Comparing matrilineal and patrilineal Mosuo illustrates that societal gender norms can have profound effects on health, particularly for women. The public health implications of patrilineal gender norms have been underappreciated. While patriliny has been linked to reduced autonomy and resource access for women, we demonstrate that these inequalities can have tangible biological effects that contribute to gender disparities in health.
Materials and Methods
Data Collection and Laboratory Analysis.
We focus here on inflammation and hypertension because they are some of the most common manifestations of chronic disease processes, as well as those most relevant to the hypothesized causal pathway between gender norms and health. Household surveys were conducted between January and August of 2017 in six matrilineal and six patrilineal villages in Yunnan Province, China; every household in each village was recruited, with nearly universal participation. Systolic and diastolic blood pressures were estimated for each adult household member (at least 16 y of age) present at the time of survey using Omron HEM-BP785 N; up to two estimates were averaged for each individual. Blood specimen collection occurred during a subsequent phase of data collection in a convenience sample of eight villages that participated in the initial survey. For these reasons, the sample size available for inflammation analyses differs from the sample size available for hypertension analyses. This study was approved by the University of New Mexico’s Institutional Review Board and by the ethical review committee at Fudan University; informed consent was obtained from all study participants.
Dried blood spots (DBSs) were collected by pricking each participant’s finger with a sterile safety lancet and allowing at least two 50-L drops of whole capillary blood to fall freely onto Whatman #903 filter paper specimen collection cards (36). DBSs were then allowed to dry for up to 24 h and frozen until they could be transported to the Ministry of Education (MOE) Key Laboratory for Contemporary Anthropology at Fudan University. Samples were evaluated for CRP using a commercially available kit (BioCheck BC-1119) modified for use with DBS specimens. One 1/8-inch disc of DBS specimen was removed with a hole punch, combined with the specimen dilution buffer provided with each kit, and allowed to soak overnight at C. The resulting eluent was assayed without further dilution; dilution was calculated as 1.525 L serum equivalent per volume of dilution buffer.
Statistical Analysis.
For regression analyses, we defined the presence of chronic inflammation as CRP 3 mg/L (16, 37, 38). At very high levels of inflammation, it is difficult to disentangle effects of acute infectious disease and chronic, longer-term processes. Since our focus here was only on chronic processes, we excluded individuals with CRP 5 mg/L. Pregnant women were also excluded. Blood pressure estimates were characterized as normal (systolic 120 mm Hg and diastolic 80 mm Hg), elevated (120 mm Hg systolic 130 mm Hg and diastolic 80 mm Hg), stage 1 hypertension (130 mm Hg systolic 140 mm Hg or 80 mm Hg diastolic 90 mm Hg), and stage 2 hypertension (systolic 140 mm Hg or diastolic 90 mm Hg) and grouped into binary outcomes for logistic regression (stage 1 and stage 2 hypertensive vs. normal and elevated blood pressures) (20).
To model the effect of matriliny on elevated inflammation and hypertension, we estimated the logit function using a binomial prior. Predictor variables were given weakly regularizing priors ( = 0, = 10), which make the model more skeptical of nonzero parameter estimates than the flat priors assumed by a frequentist approach. Patrilineal women were used as the reference category for all models. Age was included as a control in all models because the data show a monotonically increasing prevalence of elevated inflammation and hypertension with age for all groups. The models were specified as follows:
Because Bayesian models produce entire posterior probability densities (rather than point estimates) for coefficients, predicted effects and their uncertainty can be captured by the distribution’s mean and continuous interval of highest probability density; note that posterior probability densities are not constrained to symmetrical distributions, so high-probability density intervals may not always be symmetrical. To assess the possibility that the model has yielded a false relationship, we calculated the proportion of the posterior distribution with the sign opposite that of the mean parameter estimate.
To test household autonomy and resource control as pathways through which kinship may impact inflammation and hypertension, we used only cases for which head of household status was known, resulting in a sample size of 324 for the inflammation model and 945 for the hypertension model. The head of household parameter was also assigned a weakly regularizing prior ( = 0, = 10). Analyses were conducted in R (39) using the rethinking package (40). All data used in analyses are available in Dataset S1. Code is available on the GitHub repository of A.Z.R., which is accessible at https://doi.org/10.5281/zenodo.3998722.
Supplementary Material
Acknowledgments
We thank our participants and field assistants for their time and support of our research. Funding was provided by NSF Grants BCS 1461514 and BCS 1738978 and the Ministry of Education (MOE) Key Laboratory of Contemporary Anthropology at Fudan University. This material is based upon work supported by (while serving at) the National Science Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014403117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
Change History
September 7, 2021: The text of this article, Figure 1, Table 1, and the SI files have been updated; please see accompanying Correction for details.
References
- 1.Oksuzyan A., Juel K., Vaupel J. W., Christensen K., Men: Good health and high mortality. Sex differences in health and aging. Aging Clin. Exp. Res. 20, 91–102 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crimmins E. M., Shim H., Zhang Y. S., Kim J. K., Differences between men and women in mortality and the health dimensions of the morbidity process. Clin. Chem. 65, 135–145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruger D. J., Nesse R. M., An evolutionary life-history framework for understanding sex differences in human mortality rates. Hum. Nat. 17, 74–97 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Austad S. N., Why women live longer than men: Sex differences in longevity. Gend. Med. 3, 79–92 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Weber A. M., et al. , Gender norms and health: Insights from global survey data. Lancet 393, 2455–2468 (2019). [DOI] [PubMed] [Google Scholar]
- 6.BenYishay A., Grosjean P., Vecci J., The fish is the friend of matriliny: Reef density and matrilineal inheritance. J. Dev. Econ. 127, 234–249 (2017). [Google Scholar]
- 7.Thomas D., Like father, like son; like mother, like daughter: Parental resources and child height. J. Hum. Resour. 29, 950–988 (1994). [Google Scholar]
- 8.Dyson T., Moore M., On kinship structure, female autonomy, and demographic behavior in India. Popul. Dev. Rev. 9, 35–60 (1983). [Google Scholar]
- 9.Leonetti D., Nath D., Hemam N., In-law conflict: Women’s reproductive lives and the roles of their mothers and husbands among the matrilineal Khasi. Curr. Anthropol. 48, 861–890 (2007). [Google Scholar]
- 10.Carranza E., Soil endowments, female labor force participation, and the demographic deficit of women in India. Am. Econ. J. Appl. Econ. 6, 197–225 (2014). [Google Scholar]
- 11.Schneider D. M., “The distinctive features of matrilineal descent groups” in Matrilineal Kinship, Schneider D. M., Gough K., Eds. (University of California Press, Berkeley, CA, 1961), pp. 1–29. [Google Scholar]
- 12.Nongbri T., Khasi women and matriliny: Transformations in gender relations. Gend. Technol. Dev. 4, 359–395 (2000). [Google Scholar]
- 13.Mattison S. M., Beheim B., Chak B., Buston P., Offspring sex preferences among patrilineal and matrilineal Mosuo in Southwest China revealed by differences in parity progression. R. Soc. Open Sci. 3, 160526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih C. K., Quest for Harmony: The Moso Traditions of Sexual Union and Family Life (Stanford University Press, Stanford, CA, 2010). [Google Scholar]
- 15.Mathieu C., “Lost kingdoms and forgotten tribes: Myths, mysteries and mother-right in the history of the Naxi nationality and the Mosuo People of Southwest China,” PhD dissertation, Murdoch University, Murdoch, Australia: (1996). [Google Scholar]
- 16.Finch C. E., Crimmins E. M., Inflammatory exposure and historical changes in human life-spans. Science 305, 1736–1739 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Liu M. Y., Li N., Li W. A., Khan H., Association between psychosocial stress and hypertension: A systematic review and meta-analysis. Neurol. Res. 39, 573–580 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Ridker P. M., Buring J. E., Shih J., Matias M., Hennekens C. H., Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 98, 731–733 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Pradhan A. D., C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J. Am. Med. Assoc. 286, 327–334 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Whelton P., et al. , High blood pressure clinical practice guideline. Hypertension 71, 1269–1324 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Hage F. G., C-reactive protein and hypertension. J. Hum. Hypertens. 28, 410–415 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Beevers D. G., Lip G. Y. H., O’Brien E. T., ABC of Hypertension (John Wiley & Sons, ed. 6, 2014). [Google Scholar]
- 23.Mills K. T., Stefanescu A., He J., The global epidemiology of hypertension. Nat. Rev. Nephrol. 16, 223–237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khera A., et al. , Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol. 46, 464–469 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Lakoski S. G., et al. , Gender and C-reactive protein: Data from the multiethnic study of atherosclerosis (MESA) cohort. Am. Heart J. 152, 593–598 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Gao Y., et al. , Prevalence of hypertension in China: A cross-sectional study. PLoS One 8, e65938 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye X., et al. , Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J. Am. Coll. Cardiol. 49, 1798–1805 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Gu H., et al. , Hypertension prevalence, awareness, treatment and control among Han and four ethnic minorities (Uygur, Hui, Mongolian and Dai) in China. J. Hum. Hypertens. 29, 555–560 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Danese A., Pariante C. M., Caspi A., Taylor A., Poulton R., Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U.S.A. 104, 1319–1324 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y. C., Schorpp K., Harris K. M., Social support, social strain and inflammation: Evidence from a national longitudinal study of U.S. adults. Soc. Sci. Med. 107, 124–135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroope S., Seclusion, decision-making power, and gender disparities in adult health: Examining hypertension in India. Soc. Sci. Res. 53, 288–299 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Cohen S., et al. , Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. U.S.A. 109, 5995–5999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson T. A., et al. , Markers of inflammation and cardiovascular disease. Circulation 107, 499–511 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Das Gupta M., Life course perspectives on women’s autonomy and health outcomes. Am. Anthropol. 97, 481–491 (1995). [Google Scholar]
- 35.Mattison S. M., Evolutionary contributions to solving the “matrilineal puzzle.” Hum. Nat. 22, 64–88 (2011). [DOI] [PubMed] [Google Scholar]
- 36.McDade T. W., Williams S. A., Snodgrass J. J., What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 44, 899–925 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Okin D., Medzhitov R., Evolution of inflammatory diseases. Curr. Biol. 22, R733–R740 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDade T. W., Early environments and the ecology of inflammation. Proc. Natl. Acad. Sci. U.S.A. 109, 17281–17288 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing. Vienna, 2020). [Google Scholar]
- 40.McElreath R., Statistical Rethinking: A Bayesian Course with Examples in R and STAN (CRC Press, Boca Raton, FL, ed. 2, 2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.