Significance
Transmembrane 16A (TMEM16A, anoctamin1), a Cl− channel activated by intracellular Ca2+ and membrane voltage, displays several distinctive biophysical properties based on differential splicing of alternative exons. Recently, this channel was reported to be regulated by the membrane phospholipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. Here, we report that two splice variants of TMEM16A show different PI(4,5)P2 sensitivity and the difference is related with the presence or absence of intracellular ATP. We reveal the mechanisms underlying differential PI(4,5)P2 sensitivity of the alternatively spliced TMEM16A channels by identifying potential PI(4,5)P2-binding and phosphorylation sites. Through the simulation of predicted structures, our data demonstrate an allosteric regulation of TMEM16A by membrane PI(4,5)P2 and channel phosphorylation.
Keywords: Ca2+-activated Cl− channel; TMEM16A; PI(4,5)P2; intracellular ATP; splice variants
Abstract
Transmembrane 16A (TMEM16A, anoctamin1), 1 of 10 TMEM16 family proteins, is a Cl− channel activated by intracellular Ca2+ and membrane voltage. This channel is also regulated by the membrane phospholipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. We find that two splice variants of TMEM16A show different sensitivity to endogenous PI(4,5)P2 degradation, where TMEM16A(ac) displays higher channel activity and more current inhibition by PI(4,5)P2 depletion than TMEM16A(a). These two channel isoforms differ in the alternative splicing of the c-segment (exon 13). The current amplitude and PI(4,5)P2 sensitivity of both TMEM16A(ac) and (a) are significantly strengthened by decreased free cytosolic ATP and by conditions that decrease phosphorylation by Ca2+/calmodulin-dependent protein kinase II (CaMKII). Noise analysis suggests that the augmentation of currents is due to a rise of single-channel current (i), but not of channel number (N) or open probability (PO). Mutagenesis points to arginine 486 in the first intracellular loop as a putative binding site for PI(4,5)P2, and to serine 673 in the third intracellular loop as a site for regulatory channel phosphorylation that modulates the action of PI(4,5)P2. In silico simulation suggests how phosphorylation of S673 allosterically and differently changes the structure of the distant PI(4,5)P2-binding site between channel splice variants with and without the c-segment exon. In sum, our study reveals the following: differential regulation of alternatively spliced TMEM16A(ac) and (a) by plasma membrane PI(4,5)P2, modification of these effects by channel phosphorylation, identification of the molecular sites, and mechanistic explanation by in silico simulation.
TMEM16A (anoctamin1) plays a wide range of physiological roles in diverse cell types, including contraction of smooth muscle and gastrointestinal motility, secretion of Cl− in epithelial cells, detection of noxious heat in nociceptive neurons, modulation of neuronal excitability, and regulation of cell volume (1). TMEM16A channels, from a family of 10 anoctamin proteins (TMEM16A–K), continuously monitor the concentration of intracellular Ca2+ and function as Ca2+-activated Cl− channels (2–4). Several splice variants of TMEM16A generated by combinatorial inclusion or exclusion of four exon segments, a, b, c, and d (5–7), display unique electrophysiological properties in tissues. Segments a and b lie in the N terminus, and segments c and d lie in the first intracellular loop of TMEM16A. Among the four segments, it is known that b and c help regulate the cytosolic Ca2+ sensitivity and voltage dependence of channel gating. For example, inclusion of the b-segment results in decreased channel sensitivity to intracellular Ca2+ rise, whereas skipping of the c-segment reduces channel activity and also impairs Ca2+ sensitivity (5, 8, 9). In addition to inclusion or skipping of each segment, calmodulin (10–13), phosphorylation (14–16), protons (17–19), and lipids (20–27) also impact on the gating of TMEM16A channels.
Phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] is a key signaling phospholipid in the inner leaflet of the plasma membrane. It acts as a cofactor that regulates many types of ion channels and receptors (28–30), and thus depletion of membrane PI(4,5)P2 by the activation of either phospholipase C (PLC) or phosphoinositide 5-phosphatases leads to decreases or increases in gating activity of ion channels. Of the TMEM16 family, TMEM16A, TMEM16B, and TMEM16F are ion channels best known to be modulated by PI(4,5)P2 (21–27, 31). Several studies showed that PI(4,5)P2 is required for sustained TMEM16A channel activity and stabilizes the Ca2+-bound open state of the channels (23, 24, 32). Further work located a PI(4,5)P2 regulatory region and demonstrated how PI(4,5)P2 interacts with TMEM16A to regulate channel gating by performing computational simulation. Le et al. (25) proposed that channel activation and desensitization are mediated by two distinct structural modules; one is a PI(4,5)P2-binding module formed by putative PI(4,5)P2-binding residues of TMs 3–5 located near the cytoplasmic membrane interface and another is a Ca2+-binding module of TMs 6–8 involved in the primary opening of the channel pore by Ca2+. Yu et al. (26) identified three key binding sites involved in TMEM16A–PI(4,5)P2 interaction. When PI(4,5)P2 interacts with these binding residues, which form networks with each other, it affects TMEM16A channel gating as a result of the conformational change of TM6.
In our study, using exogenous lipid phosphatase tools and mutagenesis, we found that PI(4,5)P2 differentially regulates channel activity depending on the TMEM16A splice variant. In addition, we found that the presence or absence of intracellular ATP is a key determinant of the PI(4,5)P2 sensitivity of TMEM16A. Through structural analysis partly based on a recent cryogenic electron microscopy (cryo-EM) structure of TMEM16A, we also confirmed that phosphorylation of serine 673 by CaMKII allosterically regulates the structure of a PI(4,5)P2 interaction site in the RDR domain of TMEM16A(ac) near to transmembrane segment 3 (TM3). Together, our data reveal a molecular mechanism of TMEM16A channel regulation by PI(4,5)P2, demonstrating that PI(4,5)P2-dependent TMEM16A channel activation can be allosterically modulated by phosphorylation and alternative splicing.
Results
Differential Regulation of TMEM16A(a) and TMEM16A(ac) Channel Gating by Reduction of Plasma Membrane PI(4,5)P2.
TMEM16A has four alternatively spliced exon segments a, b, c, and d. Here, we compared the TMEM16A(a) and TMEM16A(ac) forms, in which the channel activity is determined by the presence or absence of the c-segment (SI Appendix, Fig. S1). We first examined the subcellular distribution and Ca2+-dependent gating properties of TMEM16A(a) and TMEM16A(ac) in human embryonic kidney 293T (HEK293T) cells. In confocal microscopy, both variants overlapped highly with the plasma membrane marker Lyn-mCherry, but not with the endoplasmic reticulum marker mCherry-Cb5, suggesting that they are expressed at the cell surface without appreciable differences (SI Appendix, Fig. S2 A–C). In the absence of intracellular free Ca2+, cells expressing TMEM16A(a) or TMEM16A(ac) showed no currents. However, graded channel activity appeared as the intracellular free Ca2+ was increased to 115 or 455 nM (SI Appendix, Fig. S2D). As previous studies have shown (8), these currents were voltage dependent at positive potentials showing an outwardly rectifying current–voltage relationship (SI Appendix, Fig. S2E). Since TMEM16A(a) channels lack the c-segment that augments the Ca2+ sensitivity of gating (5), they showed lower current density than TMEM16A(ac).
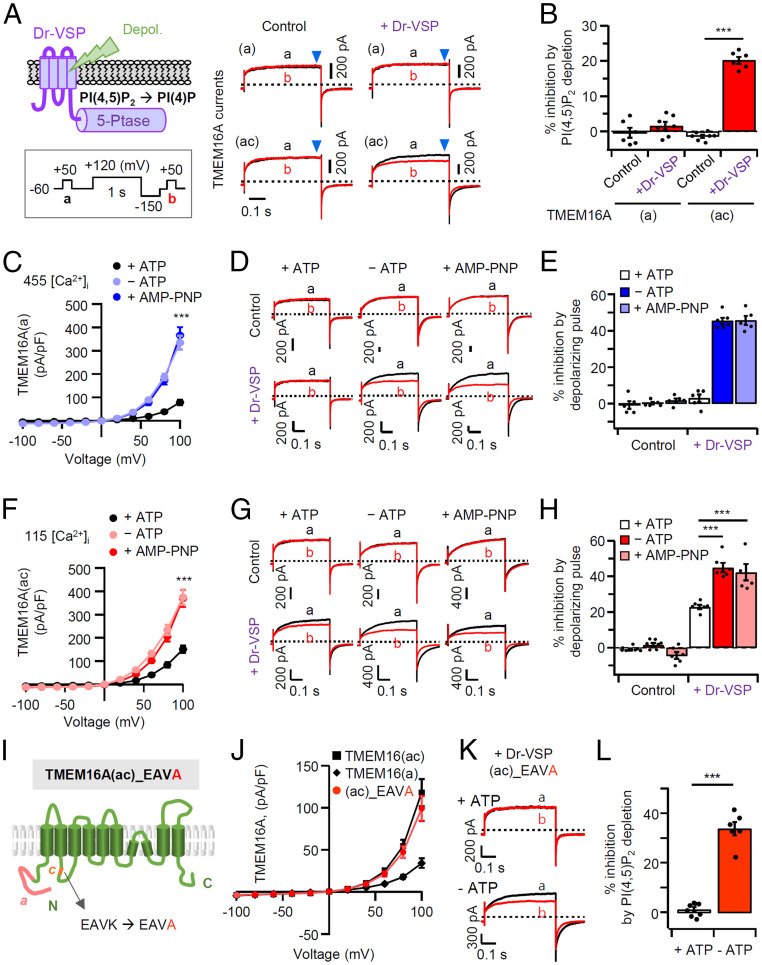
We examined the PI(4,5)P2 regulation of the TMEM16A channel using the zebrafish voltage-sensing phosphatase (Dr-VSP). This lipid phosphatase quickly removes the 5-phosphate from PI(4,5)P2 when the membrane potential is strongly depolarized (33, 34). In control cells without Dr-VSP expression, TMEM16A(a) and TMEM16A(ac) currents were almost the same before (trace a) and after (trace b) a 1-s depolarization to +120 mV (Fig. 1 A, Left). In cells expressing Dr-VSP, however, the strong depolarizing pulse attenuated subsequent TMEM16A(ac) current by 20 ± 2% (Fig. 1 A and B). In contrast, activation of Dr-VSP did not attenuate TMEM16A(a) current. This reveals a differential sensitivity to PI(4,5)P2 depletion between the two splice variants.
Fig. 1.
Enhanced PI(4,5)P2 sensitivity of TMEM16A(a) and TMEM16A(ac) in the absence of intracellular ATP. (A, Left) Cartoon of Dr-VSP–mediated PI(4,5)P2 depletion in the plasma membrane. Inset shows the voltage protocol with a large depolarization to activate Dr-VSP. (Right) Comparison of inhibition of TMEM16A(a) and TMEM16A(ac) currents by membrane depolarization in control and Dr-VSP–expressing cells. The currents before (a, black) and after (b, red) 1-s depolarizing pulses to +120 mV are superimposed. The pipette solution contains 3 mM ATP and 455 nM [TMEM16A(a)] or 115 nM [TMEM16A(ac)] [Ca2+]i. (B) Summary of percent current inhibition by Dr-VSP–mediated PI(4,5)P2 depletion in cells expressing TMEM16A(a) (n = 6–7) or TMEM16A(ac) (n = 6–7). Each dot indicates a single cell. (C and F) Current–voltage relationships for TMEM16A(a) and TMEM16A(ac) in whole-cell recording. The pipette solution contains 3 mM ATP or 3 mM AMP–PNP, or no ATP. (D and G) Representative current traces of TMEM16A(a) and TMEM16A(ac) before (A) and after (B) membrane depolarization in control and in Dr-VSP–expressing cells intracellularly perfused with or without ATP, or with AMP–PNP instead of ATP. (E and H) Summary of TMEM16A current inhibition (in percentage) by the depolarizing pulse in control and in Dr-VSP–expressing cells (n = 5–7). The dots indicate individual data points for each cell, and the bars show mean ± SEM. ***P < 0.001, two-way ANOVA followed by Dunnett’s post hoc test. (I) Schematic diagram showing the c-segment mutant of TMEM16A(ac) where the lysine (K) residue of EAVK is replaced by alanine (A). (J) Whole-cell current–density–voltage relationships for TMEM16A(ac), TMEM16A(a), or TMEM16A(ac)_EAVA (n = 5–10). (K) Representative current traces for TMEM16A(ac)_EAVA before (A) and after (B) Dr-VSP activation in the presence (Top) or absence (Bottom) of ATP. (L) Summary of current inhibition (in percentage) by PI(4,5)P2 depletion in cells expressing TMEM16A(ac)_EAVA with or without ATP (n = 6–8). The dots indicate the individual data points for each cell. ***P < 0.001, Student’s two-tailed unpaired t test.
Effects of Intracellular ATP on PI(4,5)P2 Sensitivity of TMEM16A(a) and TMEM16A(ac).
Previous studies had shown that the Ca2+-activated Cl− current could be increased by removing intracellular ATP and suggested reversal of an inhibitory channel phosphorylation (35, 36). To further investigate this effect, we measured whole-cell currents of TMEM16A(a) and TMEM16A(ac) without ATP in the pipette solution. In all experiments, cells were equilibrated for 10 min with a 0 mM ATP pipette solution. Lowering ATP in the cytosol augmented current densities of TMEM16A(a) and TMEM16A(ac) by ∼4.5- and ∼2.5-fold, respectively (Fig. 1 C and F). In addition, current inhibition by PI(4,5)P2 depletion now became apparent in TMEM16(a) and became stronger in TMEM16(ac) (Fig. 1 D and G). The current amplitude in control cells without Dr-VSP showed no significant change resulting from a strong depolarization with or without ATP. In contrast, in cells cotransfected with Dr-VSP, the TMEM16A(a) and TMEM16A(ac) currents were attenuated by 45 ± 2% and 45 ± 3%, respectively, after a strong depolarization without ATP (Fig. 1 E and H). Replacement of ATP with AMP–PNP in the pipette solution had the same effects as removal of ATP (Fig. 1 D, E, G, and H). Apparently, the presence of intracellular ATP reduces the amplitude of current and its suppression by PI(4,5)P2 depletion in both splice variants of TMEM16A.
A Role for Lysine (K451) in the c-Segment of the TMEM16A(ac) Channel.
Why do TMEM16A(a) and TMEM16A(ac) channels differ in sensitivity to PI(4,5)P2 depletion in the presence of ATP? The difference should lie in the c-segment (EAVK), which is known to regulate the Ca2+ sensitivity of the channels. We mutated EAVK to EAVA, replacing the final K451 in the c-segment with alanine (Fig. 1I). TMEM16A(ac) with this mutation displayed a current density similar to wild type, but no PI(4,5)P2 sensitivity in the presence of ATP (Fig. 1 I–L), suggesting that K451 is needed for PI(4,5)P2 regulation of TMEM16A(ac) in normally energized cells. We also tested the other residues in the c-segment by replacing EAVK with AAAA (SI Appendix, Fig. S3A). TMEM16A(ac)_AAAA displayed lower channel activity and dramatically reduced PI(4,5)P2 sensitivity; it had become not very different from TMEM16A(a) (SI Appendix, Fig. S3 B and C). Evidently, the specific conserved c-segment sequence is critical for imparting the characteristic regulatory properties to TMEM16A(ac).
The First Intracellular Loop Could Be a Site for Association with PI(4,5)P2.
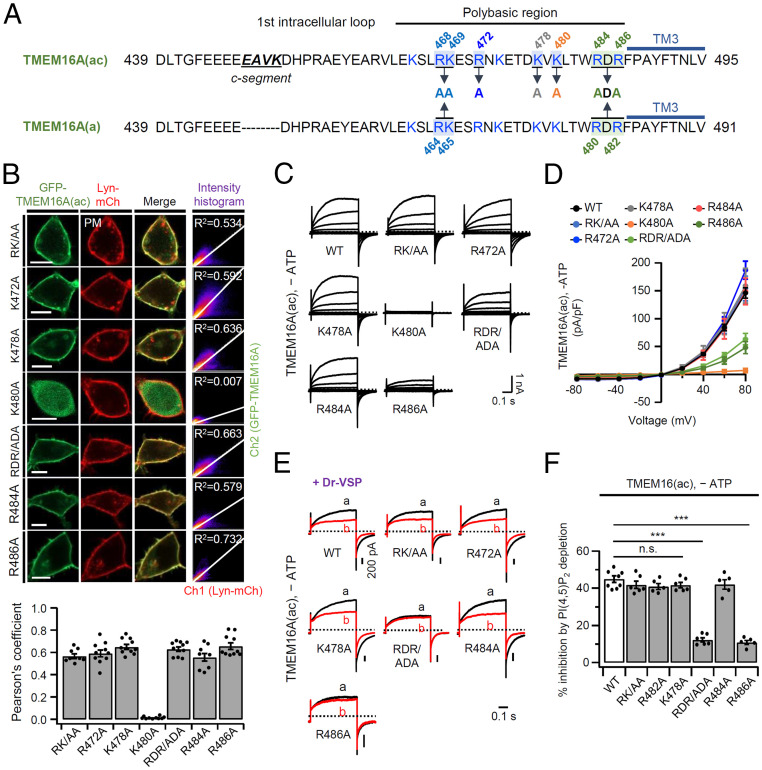
PI(4,5)P2 is highly negatively charged with a net charge of about −4. As might be expected, the inositol head group of PI(4,5)P2 binds to positively charged arginine (R) and lysine (K) residues in known ion channel structures (37). We found that, in the presence of intracellular ATP, the PI(4,5)P2 sensitivities of TMEM16A(a) and TMEM16A(ac) are quite different from each other (Fig. 1B). The difference arises from simple deletion or insertion of the c-segment (448EAVK451) in the first intracellular loop. However, we have shown that a single mutation of K451 to alanine does not entirely eliminate PI(4,5)P2 sensitivity (Fig. 1 I–L), so we explored the hypothesis that, rather than interacting directly with PI(4,5)P2 itself, the included c-segment induces a propagated conformational change in the loop. This first intracellular loop contains several basic amino acids downstream of the c-segment (Fig. 2A). To evaluate their possible contributions, we substituted alanine for each basic residue mostly located in the TM3 transmembrane-proximal region of the first loop: RK/AA, R472A, K478A, K480A, RDR/ADA, R484A, and R486A. Confocal microscopy confirmed that six of the TMEM16A(ac) mutant forms, RK/AA, R472A, K478A, RDR/ADA, R484A, and R486A, were well expressed in the plasma membrane and colocalized with the plasma membrane marker Lyn-mCh. On the other hand, the K480A mutant was not trafficked to the cell surface (Fig. 2B). Then, we investigated the channel activity of the mutants in the absence of intracellular ATP. As shown in Fig. 2 C and D, four of the TMEM16A(ac) mutants, RK/AA, R472A, K478A, and R484A, displayed almost the same channel current as wild-type TMEM16A(ac). However, overexpression of the RDR/ADA and R486A mutants gave reduced current density and the nontrafficked K480A mutant did not show any current (Fig. 2 C and D). We also examined PI(4,5)P2 sensitivity. As shown in Fig. 2 E and F, four mutants, RK/AA, R472A, K478A, and R484A, had unchanged PI(4,5)P2 sensitivity compared to wild type, whereas the mutants RDR/ADA and R486A showed dramatically decreased inhibition by PI(4,5)P2 depletion (Fig. 2 E and F). These results suggest that R486 might contribute to the interaction with the negative head group of the PI(4,5)P2 lipid.
Fig. 2.
Effects of site-directed mutagenesis of basic residues in the first intracellular loop on channel activity and PI(4,5)P2 sensitivity of TMEM16A(ac). (A) Sequence of the first intracellular loop of TMEM16A(ac) and TMEM16A(a). The blue line indicates the third transmembrane helix (TM3) domain. Positively charged residues indicated in blue were selectively mutated to alanine (A). (B, Top) Representative confocal images of HEK293T cells expressing R468A-K469A (RK/AA), R472A, K478A, K480A, R484A-R486A (RDR/ADA), R484A, or R486A TEM1M6A(ac) mutants with the plasma membrane marker Lyn-mCh. (Scale bars: 10 μm.) The scatter plot shows a 2D intensity histogram of the red (Lyn-mCh) and green (TMEM16A) pixels in the confocal image. The text indicates the Pearson’s correlation coefficient (R2) values. (Bottom) R2 values for TMEM16A(ac) mutants and Lyn-mCh (n = 8–10). The dots indicate the individual data points for each cell. The bars are mean ± SEM. (C) Families of whole-cell currents elicited by voltage steps from –80 to +80 mV in 20-mV intervals in cells expressing wild-type and mutant TMEM16A(ac) channels in the absence of ATP. Holding potential is –60 mV, and the dashed line is zero current. (D) Current density versus voltage relationships for wild-type and mutant TMEM16A(ac) channels in the absence of intracellular ATP (n = 5–8 for each point). (E) PI(4,5)P2 sensitivity of mutant TMEM16A(ac) forms in the absence of ATP. Currents before (A) and after (B) the depolarizing pulse are superimposed. (F) Summary of current inhibition (in percentage) by Dr-VSP–induced PI(4,5)P2 depletion in cells expressing wild-type, RK/AA, R472A, K478A, RDR/ADA, R484A, or R486A TMEM16A(ac) without ATP (n = 5–8). Data are mean ± SEM. ***P < 0.001; n.s., not significant, one-way ANOVA followed by Dunnett’s post hoc test.
With TMEM16(a) channels, which lack the c-segment, the results were similar. The mutant forms RK/AA, RDR/ADA, R480A, and R482A were well expressed in the plasma membrane (SI Appendix, Fig. S4 A and B); and regardless of intracellular ATP, the mutants RK/AA and R480A displayed almost the same channel current as wild-type TMEM16A(a), whereas the mutants RDR/ADA and R482A showed reduced current density (SI Appendix, Fig. S4C). We again found that the mutant RK/AA and R480A had unchanged PI(4,5)P2 sensitivity, whereas the mutant RDR/ADA and R482A showed dramatically decreased inhibition by PI(4,5)P2 depletion in the absence of ATP (SI Appendix, Fig. S4 D and E). Therefore, we concluded that the TM3-proximal RDR region in the first intracellular loop may form a PI(4,5)P2-binding site and could be responsible for the PI(4,5)P2 regulation of TMEM16A(a) and TMEM16A(ac) in the absence of ATP.
Dephosphorylation Elevates PI(4,5)P2 Sensitivity of TMEM16A.
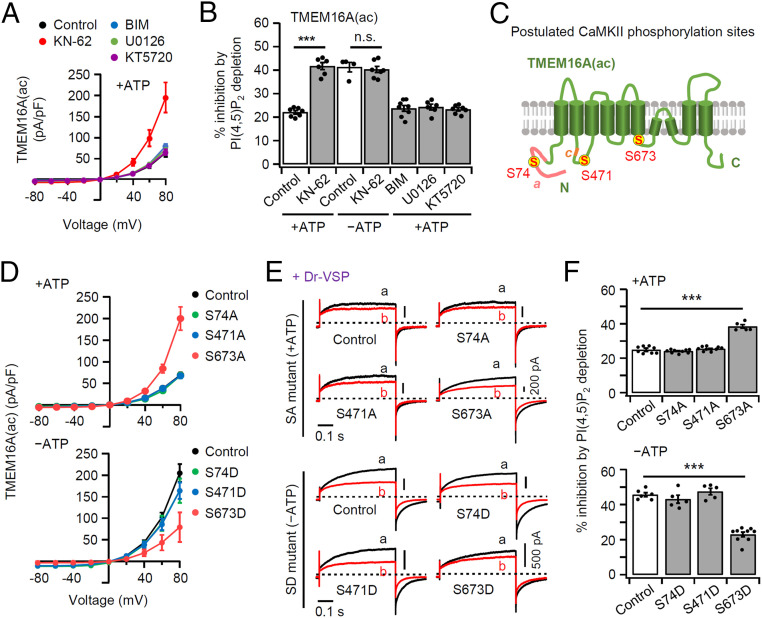
What are the functional roles of intracellular ATP in regulating PI(4,5)P2 sensitivity of TMEM16A channel? It is well known that ATP is used for the phosphorylation of intracellular proteins by protein kinases. Phosphorylation of ion channels has been reported to change their open probability, voltage dependence, and PI(4,5)P2 sensitivity, and may lead to conformational changes of the protein (38). The N and C termini of TMEM16A possess several potential phosphorylation sites that could be responsive to protein kinases including protein kinase C (PKC), protein kinase A (PKA), casein kinase II (CKII), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and extracellular regulated protein kinase 1, 2 (ERK 1,2) (39). We tested whether selective inhibition of those protein kinases might mimic the changes seen with ATP-free intracellular solution. We tried selective kinase inhibitors: for CaMKII (KN-62), PKC (BIM), ERK 1,2 (U0126), and PKA (KT5720) (12). Application of the CaMKII inhibitor KN-62 resulted in elevation of TMEM16A(ac) current density (Fig. 3A and SI Appendix, Fig. S5A), consistent with results reported for pulmonary arterial smooth muscle cells (35, 36). On the other hand, the application of BIM, U0126, or KT5720 showed no significant changes in current density. KN-62 did not change the current density in the absence of ATP (SI Appendix, Fig. S5 B and C). Similarly, application of KN-62, but not the others, also elevated TMEM16A(a) current density (SI Appendix, Fig. S6A).
Fig. 3.
Serine phosphorylation by CaMKII regulates channel activity and PI(4,5)P2 sensitivity of TMEM16A(ac). (A) Current–voltage relationship in the presence of 3 mM ATP for cells preincubated with KN-62 (10 μM; inhibitor of CaMKII) or BIM (1 μM; inhibitor of PKC) or U0126 (1 μM; inhibitor of Erk) or KT5720 (1 μM; inhibitor of PKA) in TMEM16A(ac). (B) Percent inhibition of TMEM16A(ac) currents by Dr-VSP–induced PI(4,5)P2 depletion in control and cells treated with KN-62, BIM, U0126, or KT5720 (n = 4–8). The dots indicate the individual data points for each cell. ***P < 0.001, one-way ANOVA followed by Dunnett’s post hoc test and two-way ANOVA followed by Sidak’s multiple-comparisons test. (C) Schematic diagram of TMEM16A(ac) splice variant showing the postulated CaMKII phosphorylation sites; S74, S471, and S673 (yellow circles). (D) Summary current–voltage (I–V) relationship for the SA/SD mutant TMEM16A(ac) in the presence (Top) or absence (Bottom) of ATP: wild type (control), black; S74A/S74D, green; S471A/S471D, blue; S673A/S673D, red. n = 3–5. (E) Representative current traces for wild-type (control) and mutant TMEM16A(ac) before (A) and after (B) Dr-VSP–induced PI(4,5)P2 depletion with (Top) or without (Bottom) intracellular ATP. (F) Summary of current inhibition (in percentage) by PI(4,5)P2 depletion in cells expressing wild-type (control) and SA/SD mutant TMEM16A(ac) (n = 5–9). The bars are mean ± SEM. ***P < 0.001, one-way ANOVA followed by Dunnett’s post hoc test.
Then, we tested whether the inhibition of protein kinases also affected the PI(4,5)P2 sensitivity. In the presence of ATP, application of KN-62 augmented the TMEM16A inhibition by PI(4,5)P2 depletion up to the level of current inhibition seen in ATP-free conditions, whereas the other kinase inhibitors had no effects on regulation by PI(4,5)P2 (Fig. 3B and see SI Appendix, Fig. S5D for current traces). In ATP-free conditions, adding CaMKII inhibitor made no further change in PI(4,5)P2 sensitivity (Fig. 3B and SI Appendix, Fig. S5E). Similarly, application of KN-62, but not the others, elevated the PI(4,5)P2 sensitivity of TMEM16A(a) current in the presence of ATP, but was without effect in the absence of ATP (SI Appendix, Fig. S6 A and B). These results suggest that the PI(4,5)P2 sensitivity of TMEM16A(a) and TMEM16A(ac) is down-regulated solely by CaMKII-mediated phosphorylation.
Based on these results, we used the GPS3.0 program to suggest potential phosphorylation sites in TMEM16A that could be targets of CaMKII. The program identified three potential sites S74, S471, and S673 in TMEM16A(ac) and S74, S467, and S669 in TMEM16A(a), so we constructed mutant forms by replacing serine (S) with alanine (A) or aspartic acid (D) to either prevent or mimic putative channel phosphorylation (Fig. 3C and SI Appendix, Fig. S6C). We first measured the current densities of the mutant forms. As shown in Fig. 3D and SI Appendix, Fig. S6D, the currents of nonphosphorylatable TMEM16A(ac)_S673A and TMEM16A(a)_S669A, but not the others, remained large in the presence of ATP. In contrast, the currents of phosphomimetic TMEM16A(ac)_S673D and TMEM16A(a)_S669D remained small in the absence of ATP. Thus, the current amplitudes of these four modified forms [TMEM16A(ac)_S673A and D and TMEM16A(a)_S669A and D] had become insensitive to changes of ATP (SI Appendix, Fig. S7), suggesting that the ATP-induced effect on TMEM16A current regulation is mainly mediated through phosphorylation. Current amplitude differences between TMEM16A(ac)_S673A and TMEM16A(ac)_S673D were not due to a difference in expression level because the fluorescence intensity of TMEM16A(ac)_S673A and TMEM16A(ac)_S673D relative to the membrane intensity value of the plasma membrane marker Lyn-mCh appeared almost the same (SI Appendix, Fig. S8 A and B). In Western blot analysis, expression levels of TMEM16A(ac)_S673A and TMEM16A(ac)_S673D in the total protein fraction and in the plasma membrane protein fraction were not different (40) (SI Appendix, Fig. S8 C and D). Finally, we investigated the effects of S673 and S669 on current regulation by PI(4,5)P2 depletion using S/A- or S/D-mutated TMEM16A(ac) and TMEM16A(a) with or without intracellular ATP. The TMEM16A(ac)_S673A and TMEM16A(a)_S669A mutants retained a prominent elevated PI(4,5)P2 sensitivity when ATP was present, and the TMEM16(ac)_S673D and TMEM16(a)_S669D mutants retained a diminished PI(4,5)P2 sensitivity when ATP was absent (Fig. 3 E and F and SI Appendix, Fig. S6 E and F). Thus, the sensitivity of TMEM16A(ac)_S673A and TMEM16A(ac)_S673D to PI(4,5)P2 depletion was not significantly affected by changing the pipette ATP (SI Appendix, Fig. S9), again confirming that phosphorylation on S673 is key for TMEM16A current regulation by ATP. In summary, the phosphorylation state of S673 onTMEM16A(ac) and S669 on TMEM16A(a) is critical for regulation.
Augmentation of Current Density by KN-62 Treatment Is due to an Increase of Single-Channel Current.
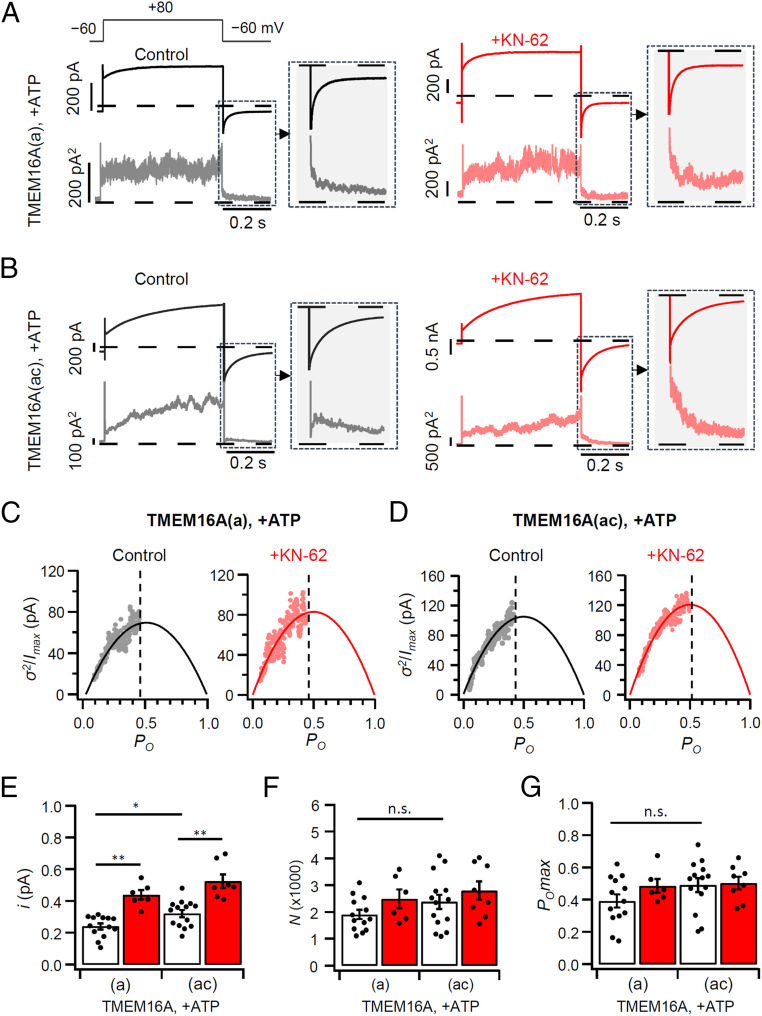
In the present study, we found that the currents of the two TMEM16A isoforms were increased in the absence of ATP as well as after treatment with KN-62. To investigate the biophysical basis for the current enhancement, we used nonstationary noise analysis (NSNA). As mentioned in Fig. 1, when both channel forms were stimulated by 115 nM intracellular Ca2+ solution, the current density of TMEM16A(ac) was greater than for TMEM16A(a) in control and in KN-62–treated cells (Fig. 4 A and B). NSNA extracted properties of the underlying channels. We analyzed the deactivating currents following a depolarizing pulse to +80 mV and plotted the normalized current variance (σ2/Imax) against the normalized current (I/Imax = open probability, Po). This was done for both channel isoforms without and with KN-62 (Fig. 4 C and D). Single-channel currents (i) of TMEM16A(a) and TMEM16A(ac) were 0.24 ± 0.02 and 0.32 ± 0.02 pA, respectively. The analysis showed that the current density of TMEM16A(ac) is greater than for TMEM16A(a) because of an increase in single-channel current (i), without any significant changes in channel number (N) or maximal open probability (POmax) (Fig. 4 E–G and SI Appendix, Table S1, open bars). This result accords with our confocal imaging, which detected no difference in the channel trafficking to the plasma membrane between the two channel isoforms. Similarly, the NSNA showed that the augmentation of current density after KN-62 treatment was due to an increase in single-channel current and not channel number or maximal open probability (Fig. 4 E–G and SI Appendix, Table S1, red bars).
Fig. 4.
NSNA during deactivation of TMEM16A currents. (A and B) Representative mean current (top trace) and variance time courses (bottom trace) obtained during control (black) and +KN-62 (red) conditions in cells expressing TMEM16A(a) (A) and TMEM16A(ac) (B). Currents were elicited using a 200-ms voltage step to −60 mV after a 500-ms pulse to +80 mV. (C and D) Normalized variance (σ2) for the maximal current (Imax) plotted against open probability (PO) for traces recorded in control cells or treated with KN-62. The solid lines are the best fit of the data to the parabolic equation. The dashed lines indicate the POmax. (E–G) Bar graphs showing the single-channel current i (E), the number of channels N (F), and the open probability PO (G) of TMEM16A(a) and TMEM16A(ac) obtained from control (open bars) and +KN-62 (red bars) conditions (n = 6–14). All experiments were conducted at 115 nM [Ca2+]i and in the presence of ATP. The dots are the individual data points for each cell. The bars are mean ± SEM. n.s., not significant. *P < 0.05, **P < 0.01, one-way ANOVA followed by Sidak’s post hoc test.
Allosteric Regulation of the PI(4,5)P2-Interacting Site by Phosphorylation at S673 of TMEM16A(ac).
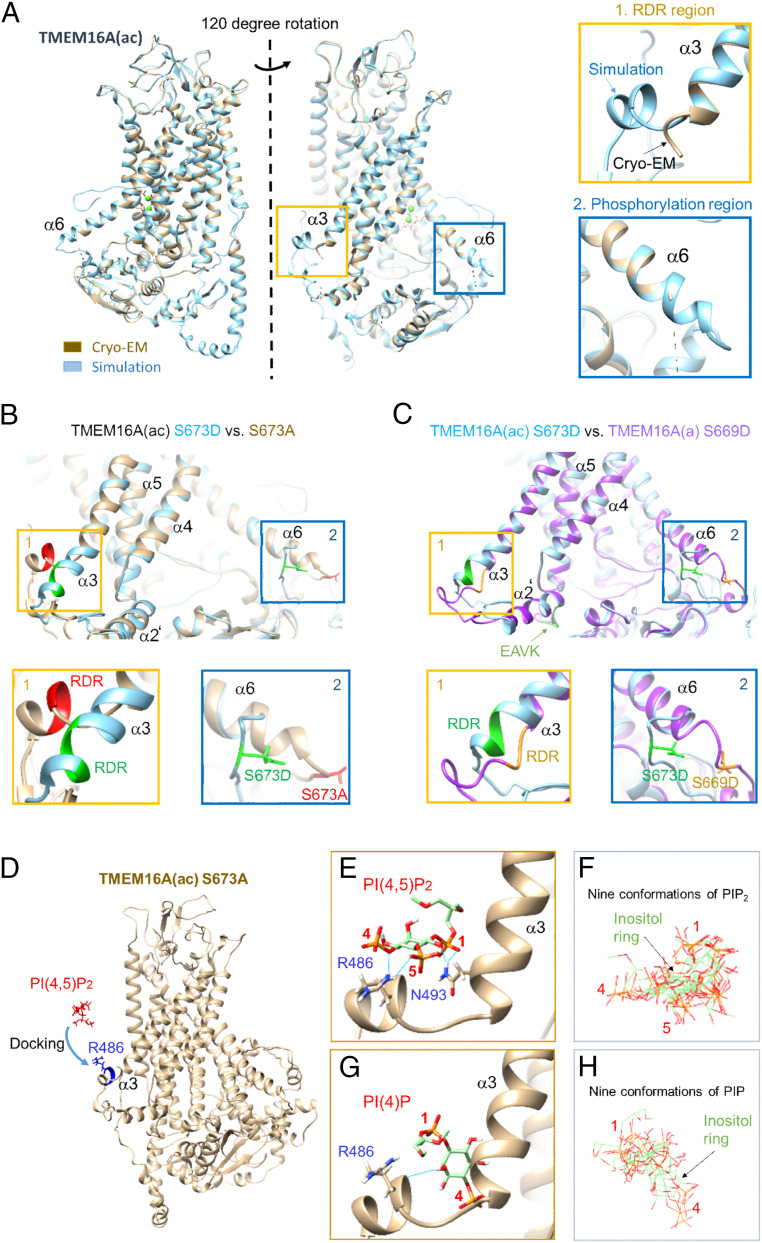
We wanted to understand the molecular impact of the RDR region of intracellular loop 3 on channel regulation by phosphorylation and PI(4,5)P2. Based on our experiments, we formulated the following hypotheses: First, the phosphorylated form of TMEM16A(a) does not bind PI(4,5)P2 with its cytoplasmic loop, but a significant cytoplasmic conformational change induced by the c-segment permits TMEM16A(ac) to bind moderately; and second, dephosphorylation changes the cytoplasmic conformation so that both channel splice forms can bind PI(4,5)P2 well. These hypotheses were evaluated by developing atomic models of the various TMEM16A forms, looking for any rearrangements predicted to occur as a consequence of phosphorylation, and attempting to dock PI(4,5)P2. The available cryo-EM structure of TMEM16A(ac) (41) is missing the two most relevant regions, RDR for PI(4,5)P2 binding and S673 for phosphorylation (Fig. 5A), because they are conformationally flexible. Therefore, we generated new full structures by ab initio simulation using the I-TASSER (iterative threading assembly refinement) server (Materials and Methods). The resulting structures were validated by the close similarity of their Ramachandran plots (SI Appendix, Fig. S10) and of their atomic models (SI Appendix, Fig. S11A) to those of the known parts of the Ca2+-bound cryo-EM structure of TMEM16A(ac) (41), as well as by their C scores (confidence score) and TM scores (template modeling score) provided by the I-TASSER program (SI Appendix, Table S2). The prediction included the missing relevant regions, RDR and S673, and the annotations in the Ramachandran plots showed that they too have consensus conformational angles. Comparing the predicted structures of the phosphomimetic mutant (S673D) with that of the nonphosphorylatable mutant (S673A) revealed a major rearrangement of the cytoplasmic domain [Fig. 5B and SI Appendix, Table S3; also see SI Appendix, Fig. S12 for TMEM16A(a)]. There was a partial unwinding of the TM6 (α6) helix, which induced a significant change of intracellular loops and the RDR region. The predicted structure of RDR at TM3 (α3), the potential PI(4,5)P2 binding site, was significantly rearranged by including the phosphomimetic mutant S673D in α6. The partial α3 including the RDR region of the S673A form became a more rigid helix with S673D. Interestingly, in the model another putative PI(4,5)P2 binding region (KRK residues or “site2”) proposed by Yu et al. (26) also had a flexible α-helix that potentially could engage in PI(4,5)P2 binding depending on phosphorylation of the S673 residue (SI Appendix, Fig. S13). In our modeling, when the KRK region near by the phosphorylation site (residues 682–684) was mutated to AAA, the RDR region was also conformationally changed by allosteric modulation (SI Appendix, Fig. S14). Without the c-segment (EAVK), the predicted conformations of both the RDR and the phosphorylation site in the phosphomimetic mutant form were significantly changed (Fig. 5C and SI Appendix, Fig. S11 B–D) within the confidence limits of the simulation (SI Appendix, Table S3). Thus, the c-segment is predicted to be key for conformational sensitivity. Root-mean-squared deviation estimates for different regions of interest from simulated TMEM16A structures support these statements (SI Appendix, Table S3). Finally, an allosteric modulation of the TMEM16A(ac) channel by phosphorylation was also confirmed with Rosetta comparative modeling (SI Appendix, Fig. S15).
Fig. 5.
Comparison of cryo-EM and simulated TMEM16A structures and PI(4,5)P2 docking. (A, Left) TMEM16A(ac)-Ca2+ bound cryo-EM (gray) and simulated structure (sky blue). When the structures were rotated by about 120° counterclockwise, the RDR region becomes visible. The Insets (orange and blue boxes) indicate the regions that are poorly defined in the cryo-EM structure presumably due to flexibility of the region and technical limitations (41). The ab initio I-TASSER molecular simulation provides the previously unresolved regions, which have potential important roles for regulation of the channel. (Right) Simulated structures of the RDR region (box 1) and of the unphosphorylated phosphorylation region containing serine residue 673 (S673) (box 2) in the TMEM16A(ac) channel. (B) TMEM16A(ac) S673A (gray) versus S673D (sky blue). The S673A mutant is the nonphosphorylatable form. The S673D mutant mimics phosphorylation of the serine residue. (C) TMEM16A(ac) S673D (sky blue) versus TMEMA(a) S669D (purple). TMEM16A(a) is the splice variant that lacks the EAVK region (c-segment) in TM2 prime (light green, bottom of structure) connected to the RDR coil between TM2 and TM3. Without EAVK, the partial helix including the RDR region in TM3 (highlighted by a yellow box) was completely disrupted. (D) Preparation for docking between R486 in the RDR region of simulated TMEM16A(ac) S673A and PI(4,5)P2 or PI(4)P, showing the initial orientation of the ion channel and PI(4,5)P2 before docking. (E) Autodock Vina was used in UCSF Chimera for docking of GIRK PI(4,5)P2 to the simulated structure. (F) Superposition of nine conformations of PI(4,5)P2. (G) Docking of PI(4)P to the RDR region. None of the hydrogen bonds interacts with R486. The lowest energy level was –5.5 kcal/mol. (H) Superposition of nine conformations of PI(4)P.
Next, we turned to docking the lipid. Using in silico simulated TMEM16A(ac) channel structures, we attempted to dock PI(4,5)P2 into the mutated and wild-type structures (Fig. 5D and Materials and Methods). To validate our docking method, we first extracted the partially resolved, bound PI(4,5)P2 molecule from the known X-ray structure of a GIRK channel (acyl chains were missing; Protein Data Bank [PDB] 3SYA) (37), and then redocked it back into the same GIRK channel. As shown in SI Appendix, Fig. S16 A–E, the calculation nicely reproduced the native conformation of the inositol group of the PI(4,5)P2 in the GIRK channel. Second, to test with a different PI(4,5)P2 starting configuration, we tried to dock a partial PI(4,5)P2 extracted from the X-ray structure of the Kir2.2 channel into the GIRK channel. This PI(4,5)P2 structure included three acyl carbons and five acyl carbons, respectively, attached to the glycerol backbone, which meant that we could see the flexibility of the fatty acid chains as well as the conformation of the inositol ring. As expected, we obtained almost the same configuration for the inositol ring compared to the GIRK PI(4,5)P2 in the structure (root-mean-square distance: 2.5 Å) (SI Appendix, Fig. S16B). There were no clear interactions between the short flexible fatty acid chains and amino acids in the binding pocket region.
With this validation, we assessed PI(4,5)P2 binding to the RDR region of TMEM16A(ac) focusing on the R486 residue. First, we used the PI(4,5)P2 extracted from GIRK. After the docking steps, the structure reached a docked state with four hydrogen bonds having a lowest energy level of –4.5 kcal/mol. Two hydrogen bonds from R486 interacted with oxygens on the 5-phosphate and the 4-phosphate of PI(4,5)P2 (Fig. 5 E and F) and two more between N493 and the oxygens of the 1-phosphate of PI(4,5)P2. The PI(4,5)P2 extracted from Kir2.2 showed similar binding (SI Appendix, Fig. S16 F–J). Consistent with our finding that PI(4)P does not substitute for PI(4,5)P2 in functional studies, PI(4)P did not make a clear interaction with R486 in docking simulations although PI(4)P could make one hydrogen bond to the protein backbone (Fig. 5 G and H). In agreement with experiment, the KRK in site2 to AAA mutation in the S673A channel, which in simulations had produced a conformational change in the RDR region, did not provide good PI(4,5)P2 binding (SI Appendix, Fig. S17). There were only the two hydrogen bonds between the oxygen on the 4-phosphate of PI(4,5)P2 and the nitrogen of R486, and none with the oxygen on the 5-phosphate. Strikingly, when tested in our in silico simulation, the phosphomimetic ion channel mutant (S673D) formed only two hydrogen bonds with the GIRK PI(4,5)P2, possibly accounting for its smaller PI(4,5)P2-dependent chloride current (SI Appendix, Fig. S18).
Our I-TASSER and docking simulations implicated hydrogen bonds to N493 for PI(4,5)P2-dependent current regulation. We tested that prediction with a new mutation, N493A (SI Appendix, Fig. S19). The mutation significantly decreased the current amplitude and PI(4,5)P2 sensitivity of the TMEM16A(ac)_S673A channel as predicted. In the wild-type TMEM16A(ac) channel, the N493 residue is buried by other transmembrane domains (SI Appendix, Fig. S20 A and B). We found that instead of the N493 residue in α3, multiple other residues in the wild-type N terminus and α5 are involved in PI(4,5)P2 binding together with R486 of the RDR region (SI Appendix, Fig. S20 C–E). In total, five hydrogen bonds from four residues [H18 (N terminus), R486 (α3), Y490 (α3), R579 (α5)] contributed to PI(4,5)P2. R486 had two hydrogen bonds with the oxygen of the 5-phosphate. In summary, several hydrogen bonds can form between phosphate oxygens of PI(4,5)P2 and R486 that could contribute to positive regulation of TMEM16A(ac) channel activity. We repeated these simulations for the TMEM16A(a) channel sequence in SI Appendix, Fig. S21. PI(4,5)P2 docked well to the nonphosphorylated S669A form and PI(4)P docked less well. PI(4,5)P2 also docked less well to the phosphomimetic S669D form.
Discussion
We have demonstrated that phosphorylation and alternative splicing modulate PI(4,5)P2-dependent augmentation of TMEM16A channel current. In our experiments with HEK293T cells, PI(4,5)P2 depletion by activation of the voltage-sensing phosphatase Dr-VSP selectively reduced Ca2+-activated currents in one splice variant, TMEM16A(ac), but not in another, TMEM16A(a). However, in the absence of ATP, both isoforms were inhibited by PI(4,5)P2 depletion. We conclude that the actions of ATP on pore activity and on the PI(4,5)P2 sensitivity of TMEM16A reflect CaMKII-mediated phosphorylation of serine S673 on the channel protein. This key phosphorylation site is in the third intracellular loop close to the pore-forming transmembrane helix TM6, whereas the putative PI(4,5)P2-binding site is some distance away in the first intracellular loop near TM3. Molecular simulation predicts that phosphorylation at S673 induces a considerable allosteric structural change in the putative PI(4,5)P2-binding site despite their separation.
The actions of PI(4,5)P2 in our experiments agree with other studies that PI(4,5)P2 plays a significant role in TMEM16A activity. A previous work reported that activation of Dr-VSP suppresses TMEM16A(ac) currents, as we observed, whereas it stimulates TMEM16B isoform A currents (22). Others discovered a role for PI(4,5)P2 by observing a rundown of TMEM16A currents, where TMEM16A(a) and (ac) currents decrease with time in the presence of high intracellular Ca2+ without ATP; this rundown can be attenuated by inclusion of exogenous water-soluble diC8-PI(4,5)P2 in the cytosolic solution (24–26). Our study further show that the (ac) and (a) isotypes of TMEM16A present different PI(4,5)P2 sensitivity and that a single mutation of a lysine (K) residue in the c-segment suffices to remove the PI(4,5)P2-mediated TMEM16A(ac) regulation in the presence of ATP. Similar differential phosphoinositide regulation dependent on a channel’s alternative splicing was previously reported in cyclic nucleotide-gated (CNG) channels, where inclusion of CNGA3 exon3 endows outstandingly potentiated sensitivity to PI(4,5)P2 and PI(3,4,5)P3 (42). Because the alternative exons of TMEM16A are spliced in a tissue-specific manner (5), the sensitivity to PI(4,5)P2 should differ in different tissues. In addition, the splice variants, TMEM16A(a, ab, ac, and ad) and the TMEM16A(ac) long form containing a new start codon, exon 0, are reported to have differing pharmacological properties (43). Further study will be necessary to learn the PI(4,5)P2 sensitivity of these other isoforms.
Many studies show that PI(4,5)P2 interacts with basic lysine (K) and arginine (R) residues in other ion channels. The binding site is best characterized in some PI(4,5)P2-sensitive K+ channels where functional experiments, atomic structures, and structural simulations show these amino acids distributed around interacting membrane PI(4,5)P2 molecules (44–47). We discovered a potential PI(4,5)P2-binding site in the first intracellular loop near TM3 of TMEM16A through site-directed-mutagenesis. Although many basic residues are located in the first intracellular loop, we identify arginine 486 (R486) in the RDR region as a putative PI(4,5)P2-interacting residue. According to our docking simulation, oxygens of the 4- and 5-phosphates of PI(4,5)P2 form two obvious hydrogen bonds with R486. However, as shown experimentally in Fig. 2F, the RDR binding site contributes ∼70 to 80% rather than 100% to PI(4,5)P2 dependency, as if residual weaker effects are induced from other PI(4,5)P2 interaction sites. Two recent papers identified other residues participating in PI(4,5)P2-binding at TMEM16A. One paper reported that TMEM16A(a) isoforms with individual mutation at any of six separate basic amino acids (R451, K461, R482, K567, R575, and K579) show more fast channel desensitization and destabilization of the channel open state without ATP (25). Through molecular docking and atomistic simulation, they also showed that the six possible PI(4,5)P2-interacting residues have different features in PI(4,5)P2 coordination. Some residues can bind directly to PI(4,5)P2, whereas other residues can cause conformational alterations at the PI(4,5)P2-binding site by salt-bridge interaction with other amino acids, but not PI(4,5)P2. They indicated that TMEM16A(a) R482 corresponding to R486 in our TMEM16A(ac) channels plays an important role in PI(4,5)P2 regulation of the channel through direct interaction (25). By using patch-clamp electrophysiology with mutagenesis and molecular-dynamics simulations of TMEM16A(ac), another paper identified three critical regions located in the cytoplasmic membrane interface as PI(4,5)P2 regulatory sites that suppress the stimulatory effect of PI(4,5)P2 (26). According to our I-TASSER simulation, site2 (K682, R683, and K684) close to the S673 phosphorylation residue could act as an auxiliary PI(4,5)P2 interaction site inducing conformational changes of the RDR region in the allosteric manner.
It is structurally established that specific PI(4,5)P2 binding to the so-called PI(4,5)P2-binding module (25) promotes Ca2+-bound TMEM16A(ac) activation (48). TM6 bends toward TM4 to block the pore, and when two Ca2+ are bound in the binding site, the TM6 helix is stretched (41, 49). In multimicrosecond atomistic simulations and clustering analysis, opening of the TMEM16A(ac) pore occurs when Ca2+ and PI(4,5)P2 are bound. The authors propose allosteric coupling between a PI(4,5)P2-binding site located in cytosolic vicinity of TM3–5 and the neck region of TM4 and 6 interfaces. PI(4,5)P2 binding to TMEM16A(ac) results in pore opening owing primarily to movement of TM4, followed by small conformational changes of TM3 and 5. Since the straightening of TM6 by Ca2+ binding is not enough to open the channel pore, the specific binding of PI(4,5)P2, which leads to a hinge movement of TM4, is needed for full activation of the pore. In short, published molecular simulations predict that specific PI(4,5)P2 binding results in conformational changes in the neck region around the inner gate, further promoting TMEM16A(ac) activity.
According to our results in Fig. 3, application of the CaMKII blocker KN-62 increased the current density of TMEM16A. Our noise analysis indicated that current enhancement by CaMKII block is a result of increase in the single-channel current (i). We performed NSNA of the tail current at −60 mV. Ta et al. (50), using the same method at −70 mV, reported a single-channel current (i) for TMEM16A(ac) of ∼0.22 pA similar to our measurements of the single-channel currents of TMEM16A(a) (0.24 pA) and TMEM16A(ac) (0.32 pA). Our single-channel currents (i) correspond to single-channel conductances (γ) of 3.0 ± 0.2 pS for TMEM16A(a) and 3.9 ± 0.3 pS for TMEM16A(ac). A review (51) concludes that the single-channel conductance of TMEM16A in native tissues is ∼3 pS but that of recombinant TMEM16A is ∼3.5 pS by single-channel recording (52) and noise analysis (53). Our recombinant TMEM16A single-channel conductance therefore accords with the published recombinant value (53). We further showed that the values are increased by channel dephosphorylation induced by CaMKII inhibition or by intracellular ATP removal. In a similar way, the single-channel conductances of ligand-gated glycine receptors, glutamate receptors, and 5-HT3 receptors are modulated by phosphorylation (54–56).
Phosphorylation by protein kinases is known to regulate the PI(4,5)P2 sensitivity of other ion channels. For example, PKA modulates M1 muscarinic receptor-induced inhibition of Kir3.1/Kir3.4, KCNQ1/KCNE1, and TREK1 K+ channels through suggested phosphorylation sites on each channel (57). With H-89, a PKA inhibitor, the muscarinic inhibition is strengthened for Kir3.1/Kir3.4 and KCNQ1/KCNE1 channels and weakened for TREK1 channels. For KV7.2 channels, five serine residues are phosphorylated by CDK5, p38 MAPK, CaMKIIα, and PKA, and this phosphorylation promotes the PI(4,5)P2 sensitivity (58). The PI(4,5)P2 sensitivity was decreased by a channel dephosphomimetic mutation where serine was replaced by alanine, and similarly, muscarinic inhibition of KV7.2/7.3 currents in rat SCG neurons was decreased by kinase inhibitors. These observations indicate that resting phosphorylation of KV7.2 channels confers high PI(4,5)P2 sensitivity and enables tight channel regulation through G protein-coupled receptors. Similarly, Ca2+-activated SK channels are complexed with calmodulin (CaM) forming a PI(4,5)P2 binding interface, and channel inhibition during PI(4,5)P2 hydrolysis is promoted by phosphorylation of residue T79 of CaM (59). Both studies implicate putative phosphorylation sites near to a putative PI(4,5)P2-binding site.
We find that the channel activity and PI(4,5)P2 sensitivity of TMEM16A are increased by ATP-free pipette solutions (Fig. 1), an effect that is absent with a phosphomimetic mutant. We attribute the ATP-free effect to loss of protein phosphorylation and a conformational change. When CaMKII was blocked pharmacologically or with a nonphosphorylatable mutation of the channel, current inhibition by PI(4,5)P2 depletion increased significantly, so we concluded that dephosphorylation of the channel augments its PI(4,5)P2 sensitivity. The phosphorylation site we identified is identical to a CaMKII-dependent phosphorylation site reported by Lin et al. (15). As in other channels, phosphorylation of this site in TMEM16A is closely associated with PI(4,5)P2 sensitivity, although in this case it may not be close to the PI(4,5)P2 binding site. Recent cryo-EM structures suggest that transmembrane α-helices 3–8 contribute to the ion conductive pore (32, 41). We point to R486 in the proximal-pore region of the TM3 helix as part of a target for docking with PI(4,5)P2 and suggest that a specific hydrogen bonding interaction between R486 and the 5-phosphate of PI(4,5)P2 is more important to induce pore opening than the total energy level for the binding. When the channel is phosphorylated at S673 in TM6 by CaMKII, the flexible RDR region 4.4 nm away is significantly rearranged and PI(4,5)P2 becomes less effective. In channel splice variants lacking the c-segment, the PI(4,5)P2 modulation fails in the presence of CAMKII activity because the putative binding site for PI(4,5)P2 is disordered by phosphorylation. We suggest that S673 phosphorylation of TMEM16A(ac) diminishes PI(4,5)P2 sensitivity by weakening the interaction of the channel with PI(4,5)P2 and not the PI(4,5)P2-channel gating relationship.
In conclusion, by comparing the differential regulatory effects of PI(4,5)P2 degradation, intracellular ATP application, and protein kinases on the two alternative splice variants of TMEM16A channel and by modeling the channel structures and PI(4,5)P2 docking by in silico simulation, we provide mechanistic understanding of an interrelation of channel phosphorylation, membrane lipid binding, and splice variants in the regulation of TMEM16A channel activity. Our findings also suggest that the PI(4,5)P2 sensitivity of other TMEM16A isoforms may be controlled allosterically not only by the different combinatorial expression of exon segments in the channel protein but also by diverse receptor-mediated signaling pathways triggering protein kinase activation and channel phosphorylation. Mechanistic insight into this allosteric gating mechanism by two distally located regulatory sites will facilitate future study of the physiological functions of TMEM16A channels in the various tissues.
Materials and Methods
Cell Culture and Transfection.
HEK293T cells (large T-antigen transformed HEK293 cells) were cultured in DMEM (HyClone; Thermo Fisher Scientific) with 10% fetal bovine serum (HyClone; Thermo Fisher Scientific) and 0.2% penicillin/streptomycin (HyClone; Thermo Fisher Scientific) at 37 °C with 5% CO2. Cells were transiently transfected using Lipofectamine 2000 (Invitrogen) with various cDNAs. The plasmids and amounts were 500 ng of mouse GFP-TMEM16A, 50 ng of Lyn-mCh, and 1,000 ng of Dr-VSP per 35-mm plate at 50 to 60% confluency. Transfected cells were plated onto poly-l-lysine (0.1 mg/mL; Sigma)–coated chips and used for voltage-clamp recordings and imaging experiments 24 to 36 h after transfection.
Plasmids and Chemicals.
Mouse cDNA clones of TMEM16A(ac) (GenBank accession no. AAH_62959.1) were generously given by Frank H. Yu (University of Seoul, Seoul, Korea). The Dr-VSP was from Yasushi Okamura (Osaka University, Osaka, Japan). The following compounds were obtained: KN-62 (Tocris); BIM (Calbiochem); U-0126 (Enzo); KT5720 (Santa Cruz); AMP–PNP (Roche); and Na2ATP, CaCl2, and other chemicals (Sigma-Aldrich).
Molecular Cloning.
All mutant channel forms were constructed from wild-type mTMEM16A. The TMEM16A(a) isoform was made by deleting the c-segment (448EAVK451) from TMEM16A(ac). Deletion or single amino acid mutations were generated by the inverse PCR method using Pfu Turbo DNA polymerase (Agilent Technologies), plasmid DNA was digested by Dpn I (Agilent Technologies), the PCR product was 5′-phosphorylated by T4 polynucleotide kinase (Enzynomics), and then the PCR product was ligated by T4 DNA ligase (NEB). All of the mutations were verified by DNA sequencing (Macrogen).
Solutions.
The extracellular solution used for TMEM16A current recording and confocal imaging contained 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM Hepes, adjusted to pH 7.4 with NaOH. The pipette (intracellular) solution contained 130 mM CsCl, 1 mM MgCl2, 10 mM EGTA, and either 3 mM Na2ATP or 3 mM AMP-PNP or no added ATP. For the standard pipette solution, added CaCl2 was 5.83 mM to make 115 nM free Ca2+ and 8.47 mM to make 455 nΜ free Ca2+ (calculated with the Ca/Mg/ATP/EGTA calculator, version 2.2b, available at https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/webmaxc/webmaxcS.htm), adjusted to pH 7.35 with CsOH.
Current Recording.
Patch-clamp recording using the whole-cell configuration was performed at room temperature (22 to 25 °C) using a HEKA EPC-10 amplifier with Pulse software (HEKA Elektronik) (60). Electrodes pulled from borosilicate glass micropipette capillaries (Sutter Instrument) using a Flaming/Brown micropipette puller (P-97; Sutter Instrument) had resistances of 2 to 5 MΩ, and series-resistance errors were compensated by >60%. Fast and slow capacitances were compensated before the application of test pulses. TMEM16A currents were recorded with a membrane holding potential of −60 mV and applying a 500-ms test pulse. During recording, the external solutions were delivered to a QuikChange chamber narrow slotted bath (RC-46SNLP; Warner Instruments) using a six-channel pinch valve controller system (VC-6; Warner Instruments). Complete solution exchange is achieved within a second. Dr-VSP experiments used application of step depolarizations to 120 mV for 1 s to activate Dr-VSP and to deplete PI(4,5)P2 in cells. For the acquisition and analysis of data, we used Pulse/Pulse Fit software in combination with an EPC-10 patch-clamp amplifier (HEKA Elektronik) and Igor Pro (WaveMetrics).
Confocal Imaging.
HEK293T cells were imaged 1 to 2 d after transfection on poly-l-lysine–coated chips with a Carl Zeiss LSM 700 or LSM 800 confocal microscope (Carl Zeiss) at room temperature. Cell images were scanned by using a 40× (water) objective lens at 1,024 × 1,024 pixels using digital zoom. Image processing was carried out using Zeiss ZEN 2.3 SP1 software. To analyze colocalization, we performed quantitative colocalization analysis using Fiji software (61) with the Colocalization Threshold plugin to determine the Pearson’s correlation coefficient (R2) (62). Coefficient R2 values of “1” and “0” correspond to perfect colocalization and random (no) colocalization, respectively. Pixel intensities are presented as two-dimensional (2D) intensity histograms with a linear regression line and as bar graphs with mean R2 values. All images were transferred from LSM4 to JPEG format. The raw data were processed with Excel 2016 (Microsoft) and Igor Pro (WaveMetrics).
NSNA.
NSNA was performed to estimate the single-channel current (i), the number of channels (N), and the open probability (PO) of TMEM16A (63–66). TMEM16A currents were activated by a 500-ms pulse to +80 mV, and then currents were sampled for 200 ms during channel deactivation at −60 mV. TMEM16A current traces were collected (20 to 60 repeats) after an initial 3- to 5-min wait to allow dialysis of a saturating concentration of Ca2+ into the cells. TMEM16A currents were low-pass filtered at 10 kHz and digitized with a sampling rate of 100 kHz. The NSNAs were calculated starting 10 ms after the voltage steps from +80 to −60 mV to minimize capacitive transient current. The mean current versus variance was binned into 200 bins, and data were fitted with following equation:
where σ2 is variance, I is mean current, i is single-channel current, N is total number of channels, and is background variance. The open probability, PO, of the peak current can be calculated from the following equation:
where <I>max is the maximum value of mean current. The nonzero X-intercept corresponds to the estimated maximal current (Imax) where the open probability is 1. For presenting plots of variance versus mean current, the background variance () is subtracted and each individual trace was divided by the estimated Imax to yield the open probability (65). The NSNAs were estimated by calculating variance and performing least-squares fitting of the parabolic curve in Igor Pro.
TMEM16A Structures Predicted with I-TASSER.
The I-TASSER server was developed by the Y. Zhang laboratory (University of Michigan, Ann Arbor, MI). In principle, I-TASSER predicts the structure of proteins of interest using an iterative threading assembly refinement (67, 68). I-TASSER modeling starts from LOMETS, which generates tens of thousands of template alignments. It selects “10 best templates” from LOMETS according to sequence similarity. It does not mean that I-TASSER is not dependent on (a large number of) templates, but at least each template is chosen by an unbiased algorithm de novo, an ab initio approach. Furthermore, I-TASSER adds energy minimization and molecular dynamics simulation to calculate an acceptable structure, including calculating flexible regions that are not well visualized using conventional approaches. At the end of modeling, simulated structures are compared with known structures to get root-mean-square deviation (RMSD) values related to the TM score that reflects topological information. In the last 10 y, many membrane proteins have been studied by X-ray crystallography or visualized with cryo-EM, which means that I-TASSER RMSD values have become correspondingly more reliable for membrane proteins. I-TASSER provides 10 different TM scores in comparison with 10 known structures and then lists the top scores to calculate the averaged TM scores. Finally, the I-TASSER server provides five different simulated structures. We selected the results that have the best TM, c, and RMSD values.
In this study, we used the I-TASSER server to simulate TMEM16A structures. With I-TASSER, the c score indicates the accuracy of the predictions and 90% accuracy of protein fold is expected for a c score greater than −1.5. Another important parameter is the TM score (0 to 1), which provides topological information. TM > 0.5 means a correct topology and TM score < 0.17 suggests a random structure. For example, the c scores for simulated TMEM16A(ac) structures were −0.12 for TEMEM16A(ac) S673A and −0.77 for TMEM16A(ac) S673D. TM scores were 0.7 for TMEM16A(ac) S673A and 0.62 for TMEM16A(ac) S673D. The c scores and TM scores are summarized in SI Appendix, TableS1.
The simulated results were rendered in University of California, San Francisco (UCSF) Chimera. Cryo-EM structures of TMEM16A(ac) have been reported (32, 41). The TMEM16A(a) splice variant structure was also generated by the I-TASSER server, although the experimental structure for the variant was not yet reported. Paulino et al. showed two different forms of the Ca2+-activated Cl− channels: Ca2+-bound and unbound forms. The simulated TMEM16A structure from the I-TASSER server was similar to the Ca2+-bound form. In a Ramachandran plot, most of the amino acids fell within the primary contour lines (a criterion of plausibility) and the cryo-EM structure did as well; thus, our simulation is a successful match with the Ca2+-bound cryo-EM structure produced by Paulino et al. (41). In their structure, the potential PI(4,5)P2 binding site was not resolved, but the I-TASSER server predicted both the structured and the unstructured regions. To examine the effect of a lipid membrane on the simulated I-TASSER structures, the membrane builder was used in the CHARMM-GUI server. Four hundred POPC lipid molecules were implanted for the inner and outer leaflets of lipid bilayer. In addition, normal PI(4,5)P2 (38:4) lipid molecules are introduced into the lipid bilayer. Thirty PI(4,5)P2 lipid molecules were added for the inner leaflet and five for the outer leaflet. The lipid membrane was translated up to 2.5 nm through the z axis of the protein to best align the putative PI(4,5)P2 binding site with the lipid membrane.
For comparative modeling with the Rosetta server, we submitted the desired full sequence of amino acids of the TMEM16A(ac) ion channel via their website (https://robetta.bakerlab.org/login.php?next_url=%2Fsubmit.php). We visualized and analyzed the output with UCSF chimera.
Molecular Docking in UCSF Chimera.
Autodock Vina was originally developed by the A. J. Olson laboratory (The Scripps Research Institute, La Jolla, CA) and Chimera incorporates it. Autodock Vina improves time and accuracy compared to previous Autodock versions (69). In order to rank docked states between ligand and receptor, it uses a score directly related to free-energy levels. To minimize the calculation time, we defined a box of small volume (3.2 × 3.2 × 3.9 nm) located around the potential PI(4,5)P2 docking region in the TMEM16A channel. A size of at least 2.5 nm is needed to allow ligand rotation in the simulation. The energy score sums many possible interactions, including hydrogen bonding, van der Waals interactions, steric effects, electrostatic interactions, hydrophobic interactions, and so on. Hydrogen bonding is important, but hydrophobic and van der Waals interactions and steric effects also contribute to ligand docking. Nine or 10 structures come from the simulation. For efficiency, we used a minimal PI(4,5)P2 structure including only glycerol and the inositol ring with five negative charges due to two phosphates in the 4 and 5 positions and one phosphate in the 1 position. For preparing the glyceryl-headgroup of PI(4,5)P2, we extracted the coordinates from the crystal structure of either the GIRK channel (PDB 3SYA) or the Kir2.2 channel (PDB 3SPI) complexed with diC8-PI(4,5)P2 in which the lipid chains were not fully resolved (37, 70). Using the “dock prep” step in Chimera, we added hydrogens to this extracted structure and to the simulated TMEM16A structure generating two “mol2” extension files to start Autodock Vina [i.e., PI(4,5)P2.mol2 and TMEM16A.mol2]. After fixing the box size and position, we could start the molecular docking on a personal computer.
Statistical Analysis.
All data were analyzed using Microsoft Office Excel 2016 (Microsoft), Igor Pro-6.0 (WaveMetrics), or GraphPad Prism 7.02 (GraphPad Software). Statistics in text or figures represent mean ± SEM. Statistical comparisons between two groups were analyzed using Student’s t test. The significance of observations among more than two groups was assessed by one-way ANOVA followed by Sidak’s and Dunnett’s post hoc test. Differences were considered significant at the *P < 0.05, **P < 0.01, and ***P < 0.001 levels.
Supplementary Material
Acknowledgments
We thank Dr. William A. Catterall for helpful discussions and Lea M. Miller for technical assistance. We thank many laboratories for providing the plasmids. This work was supported by grants from the National Research Foundation of Korea grant funded by the Korea government (Ministry of Science, Information and Communications Technology, and Future Planning) (2019R1A2B5B01070546 to B.-C.S.), the Basic Science Research Program (2020R1A4A1019436 to B.-C.S.), the Korea Brain Research Institute basic research program funded by the Ministry of Science, Information and Communications Technology, and Future Planning (20-BR-04-01 to B.-C.S.), NIH Grant R37-NS08174 (B.H.), and the Wayne E. Crill Endowed Professorship (B.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014520117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Pedemonte N., Galietta L. J., Structure and function of TMEM16 proteins (anoctamins). Physiol. Rev. 94, 419–459 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Schroeder B. C., Cheng T., Jan Y. N., Jan L. Y., Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y. D., et al. , TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Caputo A., et al. , TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Ferrera L., et al. , Regulation of TMEM16A chloride channel properties by alternative splicing. J. Biol. Chem. 284, 33360–33368 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrera L., et al. , A minimal isoform of the TMEM16A protein associated with chloride channel activity. Biochim. Biophys. Acta 1808, 2214–2223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Driscoll K. E., Pipe R. A., Britton F. C., Increased complexity of Tmem16a/Anoctamin 1 transcript alternative splicing. BMC Mol. Biol. 12, 35 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Q., et al. , Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc. Natl. Acad. Sci. U.S.A. 108, 8891–8896 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strege P. R., et al. , EAVK segment “c” sequence confers Ca2+-dependent changes to the kinetics of full-length human Ano1. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G572–G579 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Y., et al. , Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 25, 1058–1068 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Jung J., et al. , Dynamic modulation of ANO1/TMEM16A HCO3− permeability by Ca2+/calmodulin. Proc. Natl. Acad. Sci. U.S.A. 110, 360–365 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vocke K., et al. , Calmodulin-dependent activation and inactivation of anoctamin calcium-gated chloride channels. J. Gen. Physiol. 142, 381–404 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang T., Hendrickson W. A., Colecraft H. M., Preassociated apocalmodulin mediates Ca2+-dependent sensitization of activation and inactivation of TMEM16A/16B Ca2+-gated Cl− channels. Proc. Natl. Acad. Sci. U.S.A. 111, 18213–18218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutta A. K., et al. , PKCα regulates TMEM16A-mediated Cl− secretion in human biliary cells. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G34–G42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C. X., et al. , Ca2+/calmodulin-dependent protein kinase II γ-dependent serine727 phosphorylation is required for TMEM16A Ca2+-activated Cl− channel regulation in cerebrovascular cells. Circ. J. 82, 903–913 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Ayon R. J., et al. , Molecular mechanism of TMEM16A regulation: Role of CaMKII and PP1/PP2A. Am. J. Physiol. Cell Physiol. 317, C1093–C1106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun H., et al. , Protons inhibit anoctamin 1 by competing with calcium. Cell Calcium 58, 431–441 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Cruz-Rangel S., et al. , Extracellular protons enable activation of the calcium-dependent chloride channel TMEM16A. J. Physiol. 595, 1515–1531 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segura-Covarrubias G., et al. , Voltage-dependent protonation of the calcium pocket enable activation of the calcium-activated chloride channel Anoctamin-1 (TMEM16A). Sci. Rep. 10, 6644 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sones W. R., Davis A. J., Leblanc N., Greenwood I. A., Cholesterol depletion alters amplitude and pharmacology of vascular calcium-activated chloride channels. Cardiovasc. Res. 87, 476–484 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard H. A. T., Leblanc N., Albert A. P., Greenwood I. A., Inhibitory role of phosphatidylinositol 4,5-bisphosphate on TMEM16A-encoded calcium-activated chloride channels in rat pulmonary artery. Br. J. Pharmacol. 171, 4311–4321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ta C. M., Acheson K. E., Rorsman N. J. G., Jongkind R. C., Tammaro P., Contrasting effects of phosphatidylinositol 4,5-bisphosphate on cloned TMEM16A and TMEM16B channels. Br. J. Pharmacol. 174, 2984–2999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Jesús-Pérez J. J., et al. , Phosphatidylinositol 4,5-bisphosphate, cholesterol, and fatty acids modulate the calcium-activated chloride channel TMEM16A (ANO1). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863, 299–312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tembo M., Wozniak K. L., Bainbridge R. E., Carlson A. E., Phosphatidylinositol 4,5-bisphosphate (PIP2) and Ca2+ are both required to open the Cl− channel TMEM16A. J. Biol. Chem. 294, 12556–12564 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le S. C., Jia Z., Chen J., Yang H., Molecular basis of PIP2-dependent regulation of the Ca2+-activated chloride channel TMEM16A. Nat. Commun. 10, 3769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu K., Jiang T., Cui Y., Tajkhorshid E., Hartzell H. C., A network of phosphatidylinositol 4,5-bisphosphate binding sites regulates gating of the Ca2+-activated Cl− channel ANO1 (TMEM16A). Proc. Natl. Acad. Sci. U.S.A. 116, 19952–19962 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arreola J., Hartzell H. C., Wasted TMEM16A channels are rescued by phosphatidylinositol 4,5-bisphosphate. Cell Calcium 84, 102103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh B. C., Hille B., Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 15, 370–378 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Suh B. C., Hille B., PIP2 is a necessary cofactor for ion channel function: How and why? Annu. Rev. Biophys. 37, 175–195 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hille B., Dickson E. J., Kruse M., Vivas O., Suh B. C., Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye W., et al. , Phosphatidylinositol-(4,5)-bisphosphate regulates calcium gating of small-conductance cation channel TMEM16F. Proc. Natl. Acad. Sci. U.S.A. 115, E1667–E1674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dang S., et al. , Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552, 426–429 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murata Y., Iwasaki H., Sasaki M., Inaba K., Okamura Y., Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435, 1239–1243 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Okamura Y., Murata Y., Iwasaki H., Voltage-sensing phosphatase: Actions and potentials. J. Physiol. 587, 513–520 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angermann J. E., Sanguinetti A. R., Kenyon J. L., Leblanc N., Greenwood I. A., Mechanism of the inhibition of Ca2+-activated Cl− currents by phosphorylation in pulmonary arterial smooth muscle cells. J. Gen. Physiol. 128, 73–87 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiwchar M., Ayon R., Greenwood I. A., Leblanc N., Phosphorylation alters the pharmacology of Ca2+-activated Cl channels in rabbit pulmonary arterial smooth muscle cells. Br. J. Pharmacol. 158, 1356–1365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whorton M. R., MacKinnon R., Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell 147, 199–208 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ismailov I. I., Benos D. J., Effects of phosphorylation on ion channel function. Kidney Int. 48, 1167–1179 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Kunzelmann K., et al. , Bestrophin and TMEM16-Ca2+ activated Cl− channels with different functions. Cell Calcium 46, 233–241 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Davis A. J., et al. , Expression profile and protein translation of TMEM16A in murine smooth muscle. Am. J. Physiol. Cell Physiol. 299, C948–C959 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulino C., Kalienkova V., Lam A. K. M., Neldner Y., Dutzler R., Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 552, 421–425 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Dai G., Sherpa T., Varnum M. D., Alternative splicing governs cone cyclic nucleotide-gated (CNG) channel sensitivity to regulation by phosphoinositides. J. Biol. Chem. 289, 13680–13690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung T. S., et al. , Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. Am. J. Physiol. Cell Physiol. 311, C437–C451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacin E., et al. , Dynamic role of the tether helix in PIP2-dependent gating of a G protein-gated potassium channel. J. Gen. Physiol. 149, 799–811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen S. B., Lipid agonism: The PIP2 paradigm of ligand-gated ion channels. Biochim. Biophys. Acta 1851, 620–628 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M., Meng X. Y., Zhang J. F., Cui M., Logothetis D. E., Molecular overlap in the regulation of SK channels by small molecules and phosphoinositides. Sci. Adv. 1, e1500008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasimova M. A., Tarek M., Shaytan A. K., Shaitan K. V., Delemotte L., Voltage-gated ion channel modulation by lipids: Insights from molecular dynamics simulations. Biochim. Biophys. Acta 1838, 1322–1331 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Jia Z., Chen J., Specific PIP2 binding promotes calcium activation of TMEM16A chloride channels. bioRxiv:2020.04.13.039743 (14 April 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters C. J., et al. , The sixth transmembrane segment is a major gating component of the TMEM16A calcium-activated chloride channel. Neuron 97, 1063–1077.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ta C. M., Adomaviciene A., Rorsman N. J. G., Garnett H., Tammaro P., Mechanism of allosteric activation of TMEM16A/ANO1 channels by a commonly used chloride channel blocker. Br. J. Pharmacol. 173, 511–528 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitlock J. M., Hartzell H. C., A pore idea: The ion conduction pathway of TMEM16/ANO proteins is composed partly of lipid. Pflugers Arch. 468, 455–473 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burris S. K., Wang Q., Bulley S., Neeb Z. P., Jaggar J. H., 9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels. Br. J. Pharmacol. 172, 2459–2468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adomaviciene A., Smith K. J., Garnett H., Tammaro P., Putative pore-loops of TMEM16/anoctamin channels affect channel density in cell membranes. J. Physiol. 591, 3487–3505 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moraga-Cid G., et al. , Modulation of glycine receptor single-channel conductance by intracellular phosphorylation. Sci. Rep. 10, 4804 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derkach V., Barria A., Soderling T. R., Ca2+/calmodulin-kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 96, 3269–3274 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Hooft J. A., Vijverberg H. P., Phosphorylation controls conductance of 5-HT3 receptor ligand-gated ion channels. Receptors Channels 3, 7–12 (1995). [PubMed] [Google Scholar]
- 57.Lopes C. M., et al. , Protein kinase A modulates PLC-dependent regulation and PIP2-sensitivity of K+ channels. Channels (Austin) 1, 124–134 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Salzer I., et al. , Phosphorylation regulates the sensitivity of voltage-gated Kv7.2 channels towards phosphatidylinositol-4,5-bisphosphate. J. Physiol. 595, 759–776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang M., et al. , Selective phosphorylation modulates the PIP2 sensitivity of the CaM-SK channel complex. Nat. Chem. Biol. 10, 753–759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keum D., Baek C., Kim D. I., Kweon H. J., Suh B. C., Voltage-dependent regulation of CaV2.2 channels by Gq-coupled receptor is facilitated by membrane-localized β subunit. J. Gen. Physiol. 144, 297–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schindelin J., et al. , Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunn K. W., Kamocka M. M., McDonald J. H., A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 300, C723–C742 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinemann S. H., Conti F., Nonstationary noise analysis and application to patch clamp recordings. Methods Enzymol. 207, 131–148 (1992). [DOI] [PubMed] [Google Scholar]
- 64.Alvarez O., Gonzalez C., Latorre R., Counting channels: A tutorial guide on ion channel fluctuation analysis. Adv. Physiol. Educ. 26, 327–341 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Hartveit E., Veruki M. L., Studying properties of neurotransmitter receptors by non-stationary noise analysis of spontaneous postsynaptic currents and agonist-evoked responses in outside-out patches. Nat. Protoc. 2, 434–448 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Lim N. K., Lam A. K. M., Dutzler R., Independent activation of ion conduction pores in the double-barreled calcium-activated chloride channel TMEM16A. J. Gen. Physiol. 148, 375–392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roy A., Kucukural A., Zhang Y., I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J., et al. , The I-TASSER suite: Protein structure and function prediction. Nat. Methods 12, 7–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trott O., Olson A. J., AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whorton M. R., MacKinnon R., X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature 498, 190–197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.