Due to the abundance of and potential exposure to crystalline and amorphous silica dusts (Fig. 1A), silica toxicity has been investigated for over 100 y, and currently the International Agency for Research on Cancer classifies cristobalite and quartz as group 1 (carcinogenic to humans) (1, 2). In contrast, amorphous silica is classified as having inadequate evidence for carcinogenicity (group 3) and is considered as generally recognized as safe for use in food additives and packaging by the Food and Drug Administration (2, 3). However, all amorphous silica is not created equal, and there is mounting evidence that pyrolytic or so-called fumed silica is significantly more toxic than colloidal amorphous silica (e.g., precipitated, Stöber, or mesoporous silica) (4). The synthesis processes of these two classes of amorphous silicas are significantly different and may account for the observed differences in toxicity. Pyrolytic silica is synthesized via flame pyrolysis of SiCl4 at high temperature (ca. 1,300 °C) according to the net reaction
followed by rapid thermal quenching, whereas colloidal amorphous silica is formed by the condensation of soluble silicates under low-temperature aqueous conditions according to the net reaction
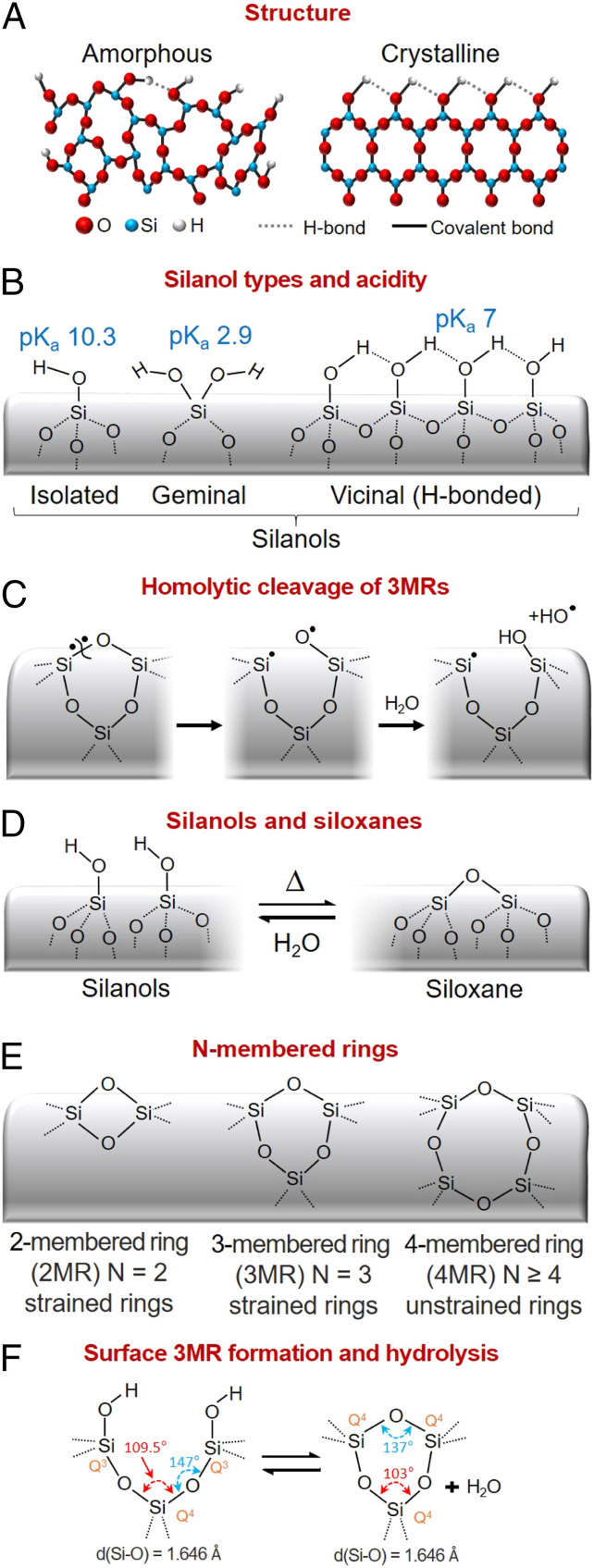
The most common toxicity pathway identified for both crystalline and amorphous silica particles is inflammasome activation. The Nalp3–inflammasome activation pathway, which underlies asbestosis and silicosis (5), is a cellular defense mechanism requiring two signals. Signal 1 (priming) results from toll-like receptor 4 activation causing nuclear factor κB activation and pro-interleukin 1β (pro-IL-1β) production. Signal 2 (activation) is provided by a plethora of stimuli, such as pore-forming toxins, viral RNA, and particulate matter and activates the NLRP3 inflammasome, resulting in generation of the proinflammatory cytokine IL-1β. Exogenous silica particles can serve as primers through cellular membrane disruption arising from attractive, noncovalent hydrogen bonding and electrostatic interactions between various silanol species (Fig. 1B) having different negative logarithms of acid dissociation constants (pKas) (and correspondingly different electrostatic interactions) with membrane-bound cellular components. Silica particles serve as activators through various reactive oxygen species (ROS)-generating mechanisms such as hemolytic cleavage of three-membered rings (3MRs) followed by hydrolysis to generate hydroxyl radicals (Fig.1C), triggering ROS generation by mitochondria (6), activation of membrane NADPH oxidase complexes (7), and lysosome disruption leading to K+ efflux and cathepsin B release. The physicochemical properties of the silica surface, namely the abundance, type, and extent of hydrogen bonding of surface silanols and ability to generate ROS, which depend upon processing pathway and environmental exposure, establish its hemolytic potential and cytotoxicity (8–11). In PNAS, Pavan et al. employ infrared spectroscopy and density functional theory to identify the structure of a unique subfamily of silanols (Fig.1 B and D) referred to as nearly free silanols (NFS), which they claim to be the major structural determinant of crystalline and amorphous silica particle toxicity due to their potential to perturb/disrupt membranes (12).
Fig. 1.
Silica framework and surface structure. (A) Crystalline and amorphous silica frameworks are constructed of siloxane rings which terminate in silanol groups at the silica surface. (B) Silanol species include isolated and geminal, which are not hydrogen-bonded, and vicinal, each having different acidities (pKas) (18). (C) Strained rings are susceptible to homolytic cleavage followed by hydrolysis to produce hydroxyl radicals. (D) Siloxane bonds form reversibly by condensation and hydrolysis. (E) While quartz is composed of six-membered rings, amorphous silica has variable ring size and, accordingly, ring strain. (F) Surface 3MRs form by thermally promoted condensation and preferentially hydrolyze upon water exposure (15). Adapted from ref. 4.
Pavan et al. (12) identified NFS by emergence of an SiO-D stretching band at 2,758 cm−1 following grinding of crystalline quartz and positively correlated its concentration (varied by thermal annealing) with hemolysis potential, a measure of membrane perturbation, and IL-1β generation, indicative of inflammasome activation. The overall idea is that grinding disrupts/amorphizes the pristine quartz surface, which is terminated with fully hydrogen-bonded silanols and is relatively inert (Fig. 1A), creating silanols with varying hydrogen-bonding interactions and pKas (Fig. 1B), and accordingly hydrogen-bonding and electrostatic interactions with cellular membranes. NFS are separated by O..H and O..O dimensions that exceed the optimized O…H distance in a hydrogen bond, meaning that rather than mutual H bonding they can interact/hydrogen-bond readily with other chemical entities. Using an empirical force field calculation, the authors demonstrate that a pair of NFS could interact in a bidentate fashion with phosphatidylcholine, a model membrane lipid, and therefore result in greater membrane perturbation and hemolytic potential than isolated/free silanols that interact in a monodentate fashion or fully hydrogen-bonded silanols that are relatively inert. Their density functional theory calculations of an increasing SiO-D vibrational mode frequency with increasing O…O distances of surface silanols (weaker H-bonding interactions) are consistent with existing calculations (13, 14) and with the frequency of infrared absorption peaks revealed by spectral subtraction (difference spectra). For fractured quartz, fractured mineral quartz, and pyrogenic silica nanoparticles (NPs), they found that NFS concentrations increase with heating to 450 °C and then decrease with further heating to 800 °C. Similar trends were observed for hemolysis and IL-1β generation.
Qualitatively, their correlation of increasing NFS concentrations with increasing hemolytic potential and IL-1β generation is consistent with our general understanding of silica-induced toxicity, but quantitatively it cannot seemingly explain the enormous difference in toxicity between fumed silica NPs synthesized at high temperatures, having high toxicity, and colloidal silica NPs formed under aqueous conditions at low temperature, having low toxicity. A distinguishing structural difference between amorphous silicas formed at high temperature (fumed or vitreous silica) and low temperature (colloidal silica) is the population of strained 3MRs (Fig. 1 E and F). The 3MRs are readily identified by their symmetric oxygen ring-breathing vibration at ca. 600 cm−1 observed by Raman spectroscopy and form during the high-temperature synthesis of vitreous or fumed silica and through thermal dehydration of amorphous silica surfaces (Fig. 1F) (15), where their concentrations are maximized at intermediate temperatures (400 to 600 °C) and largely disappear at high temperature due to thermal annealing. The 3MRs are highly strained (Si-O-Si bond angle is reduced from 147° to 137°) (Fig. 1F), causing them to be susceptible to homolytic cleavage followed by water adsorption generating hydroxyl radicals (Fig. 1C) identified by electron paramagnetic resonance spectroscopy (8). Further, ring strain causes the bridging oxygen to become more basic and the Si more acidic, increasing the rate of hydrolysis compared to unstrained siloxane bonds to form nonhydrogen-bonded hydroxyl groups (or conceivably NFS) upon exposure to water vapor (Fig. 1F).
The enormous difference in toxicity between fumed silica NPs and colloidal silica NPs was revealed in a study comparing structure/toxicity relationships of colloidal Stöber silica NPs and fumed silica NPs (Cab-O-Sil) with identical particle sizes. Stöber silica NPs had no 3MRs (because strained rings preferentially hydrolyze) and accordingly had a low potential to generate hydroxyl radicals (8). Stöber silica NP surfaces were fully hydroxylated (4.5 OH/nm2) and the silanols were completely hydrogen-bonded (Fig. 1B). By comparison, Cab-O-Sil had a high concentration of 3MRs and a high potential to generate hydroxyl radicals. Cab-O-Sil possessed a lower overall silanol concentration, ca. 3 OH/nm2, comprising both isolated and hydrogen-bonded silanols. Dose-dependent hemolysis and cytotoxicity assays showed fumed silica to have overall toxicity comparable to or exceeding Min-U-Sil, a crystalline silica used as a positive control, whereas Stöber silica had negligible hemolytic potential and a minimal cytotoxic effect at doses up to 200 µg/mL. Heat treatments to 800 °C reduced the overall and hydrogen-bonded silanol concentrations and increased the isolated silanol concentration. These treatments reduced the toxicity, whereas rehydration increased the toxicity to that of its as-prepared state. A follow-up study showed that Ti and Al doping of fumed silica reduced silanol density and 3MR concentrations, resulting in a dose-dependent reduction in hydroxyl radical generation, membrane perturbation, potassium efflux, NLRP3 inflammasome activation, and cytotoxicity. Ti and Al doping also reduced acute pulmonary inflammation, demonstrating a safer fumed silica design (16).
The concept of NFS as a unifying structural determinant of silica particle toxicity must be considered in light of all of the experimental findings. That NFS exist seems unequivocal based on spectroscopic evidence and the fact that they would form naturally upon progressive thermal dehydroxylation of the silica surface, as remaining silanols become on average more distant. Their existence on ground quartz, whose immediate surface should be considered amorphous (17), and fumed silica suggests they might be present on all amorphous silicas depending on silanol surface coverage and spacing. This ubiquity of NFS does not explain why fumed silica would be so much more toxic than colloidal silica. Further, although ground quartz surfaces must be amorphous, allowing for a spectrum of hydrogen-bonded, isolated, and NFS silanols, Pavan et al. (12) do not consider the evolving siloxane ring structures of the silica surfaces examined, and, despite overwhelming evidence to the contrary, they claim that the silica particles under investigation do not produce ROS. To convincingly establish the role of NFS in silica particle toxicity, we recommend future investigations comparing NFS concentrations in colloidal and pyrolytic silicas as a function of heating and rehydration to answer the outstanding question as to why these two classes of amorphous silica have such different toxicities. Given the observed thermal dependence of NFS, which are maximized at intermediate temperature and disappear at high temperature, we also suggest evaluation of nonbridging silanols formed by hydrolysis of surface 3MRs (Fig. 1F) as the structural origin of NFS.
Acknowledgments
C.J.B. acknowledges support by the NIH under grant 1R01CA226537-01. S.H.G. acknowledges support from the NSF Environmental Chemical Science Program, Chemistry, grant 1609044. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International Inc., for the US Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the US Department of Energy or the US Government.
Footnotes
The authors declare no competing interest.
See companion article, “Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles,” 10.1073/pnas.2008006117.
References
- 1.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans , Arsenic, Metals, Fibres and Dusts (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans No. 100C, International Agency for Research on Cancer, 2012), pp 355–397. [PMC free article] [PubMed] [Google Scholar]
- 2.Napierska D., Thomassen L. C. J., Lison D., Martens J. A., Hoet P. H., The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 7, 39 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans , Silica, Some Silicates, Coal Dust and Para-Aramid Fibrils (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans No. 68, International Agency for Research on Cancer, 1997), pp 1–475. [PMC free article] [PubMed] [Google Scholar]
- 4.Croissant J. G., Butler K. S., Zink J. I., Brinker C. J., Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater., 10.1038/s41578-020-0230-0 (2020). [DOI] [Google Scholar]
- 5.Dostert C., et al. , Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo C., et al. , Silica nanoparticles induced endothelial apoptosis via endoplasmic reticulum stress-mitochondrial apoptotic signaling pathway. Chemosphere 210, 183–192 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Yang B., Chen Y., Shi J., Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 119, 4881–4985 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Zhang H., et al. , Processing pathway dependence of amorphous silica nanoparticle toxicity: Colloidal vs pyrolytic. J. Am. Chem. Soc. 134, 15790–15804 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavan C., Fubini B., Unveiling the variability of “quartz hazard” in light of recent toxicological findings. Chem. Res. Toxicol. 30, 469–485 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Pavan C., et al. , Ζ potential evidences silanol heterogeneity induced by metal contaminants at the quartz surface: Implications in membrane damage. Colloids Surf. B Biointerfaces 157, 449–455 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Murugadoss S., et al. , Toxicology of silica nanoparticles: An update. Arch. Toxicol. 91, 2967–3010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavan C., et al. , Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc. Natl. Acad. Sci. U.S.A. 117, 27836–27846 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gierada M., De Proft F., Sulpizi M., Tielens F., Understanding the acidic properties of the amorphous hydroxylated silica surface. J. Phys. Chem. C 123, 17343–17352 (2019). [Google Scholar]
- 14.Lentz J., Garofalini S. H., Structural aspects of the topological model of the hydrogen bond in water on auto-dissociation via proton transfer. Phys. Chem. Chem. Phys. 20, 16414–16427 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Brinker C. J., Brow R. K., Tallant D. R., Kirkpatrick R. J., Surface structure and chemistry of high surface area silica gels. J. Non-Cryst. Solids 120, 26–33 (1990). [Google Scholar]
- 16.Sun B., et al. , Reduction of acute inflammatory effects of fumed silica nanoparticles in the lung by adjusting silanol display through calcination and metal doping. ACS Nano 9, 9357–9372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray R. C., The effect of long grinding on quartz (silver sand). Proc. R. Soc. Lond. A 102, 640–642 (1923). [Google Scholar]
- 18.Pfeiffer-Laplaud M., Costa D., Tielens F., Gaigeot M., Sulpizi M., Bimodal acidity at the amorphous silica/water interface. J. Phys. Chem. C 119, 27354–27362 (2015). [Google Scholar]