Abstract
LGP2 and MDA5 cooperate to detect viral RNA in the cytoplasm of Picornavirus-infected cells and activate innate immune responses. To further define regulatory components of RNA recognition by LGP2/MDA5, a yeast two-hybrid screen was used to identify LGP2-interacting proteins. The screening has identified the TAR-RNA binding protein (TRBP), which is known to be an essential factor for RNA interference (RNAi). Immuno-precipitation experiments demonstrated that TRBP interacted specifically with LGP2 but not with related RIG-I-like receptors, RIG-I or MDA5. siRNA knockdown experiments indicate that TRBP is important for Cardiovirus-triggered interferon responses, but TRBP is not involved in Sendai virus-triggered interferon response that is mediated mainly by RIG-I. To support functional interaction with LGP2, overexpressed TRBP increased Cardiovirus-triggered interferon promoter activity only when LGP2 and MDA5 are co-expressed but not MDA5 alone.
Together, our findings illustrate a possible connection between an RNAi-regulatory factor and antiviral RNA recognition that is specifically required for a branch of the virus induced innate immune response.
Keywords: LGP2, TRBP, MDA5, Type-I interferon, RIG-I-like receptors, Picornavirus
1. Introduction
Innate immune responses to invading pathogens are mediated by pattern recognition receptors [1,2]. For RNA virus infections, RNAs that accumulate during infection can be detected by cytosolic sensors in the RIG-I-like receptor (RLR) family to initiate antiviral signaling and type-I interferon (IFN) production that is essential for anti-viral immunity [3]. The RLR proteins, RIG-I (retinoic acid-inducible gene-I), MDA5 (melanoma differentiation-associated gene 5) and LGP2 (Laboratory of Genetics and Physiology 2) are DExD/H-box RNA helicases with intrinsic double stranded RNA (dsRNA) binding and ATP hydrolysis ability. In addition to RNA binding regions, RIG-I and MDA5 have tandem N-terminal caspase activation and recruitment domain (CARD) motifs that are essential for transducing signals to the downstream mitochondrial adaptor, MAVS (Mitochondrial antiviral signaling) [4–7], but LGP2 lacks the signaling module CARDs entirely [8–12]. Analysis of RLR specificity has revealed that RIG-I is responsible for recognition of viral RNAs that harbor 5′ tri or di phosphorylated ends within a double stranded region [13–17], including influenza virus, Newcastle disease virus, and vesicular stomatitis virus. MDA5 can detect dsRNAs without any known 5′ end preferences, including the Picornavi-ruses encephalomyocarditis virus (EMCV), and Theiler’s murine encephalitis virus (TMEV) [18,19]. RNA ligands for LGP2 have been poorly characterized, but its strong dsRNA binding activity has been linked to enhancement of RNA recognition by MDA5, and LGP2 has been shown to contribute to the recognition of Picornavirus infections [20,21]. For example, LGP2 is thought to bind to an RNA derived from the negative strand of the Leader region (L1) of EMCV, and present this RNA for MDA5 interaction and subsequent activation of IFN signaling [22]. It has been demonstrated that LGP2 increases the rate of MDA5-dsRNA interaction to facilitate MDA5 filament assembly on dsRNA that generates greater type-I IFN signaling [23]. These findings have made it clear that LGP2 and MDA5 have a synergistic relationship for dsRNA recognition and IFN signaling, and suggest that co-activators of RNA recognition are important components of antiviral signal transduction.
To further define regulatory components of RNA recognition by LGP2/MDA5, a yeast two-hybrid screen was used to identify LGP2 interacting proteins. One of these was identified as the TAR RNA binding protein (TRBP), which is known to be an essential factor in RNA interference (RNAi) and HIV-1 replication [24–27] as well as known as a cellular inhibitor of dsRNA-dependent protein kinase (PKR) [28,29]. Results shown in this paper indicate that TRBP is important for Cardiovirus-triggered IFN responses mediated by the LGP2/MDA5 RNA recognition system, but is not involved in Sendai virus-triggered IFN response mediated mainly by RIG-I.
2. Materials and methods
2.1. Plasmids
FLAG-tagged LGP2, RIG-I, MDA5, GFP, His-tagged LGP2, HA-tagged LGP2 plasmids are described elsewhere [8]. FLAG-tagged Dicer was generous gifts from Dr. V. N. Kim (Seoul National University). mIFNβ-luc plasmid was generous gift from Dr. J. Marques (Universidade Federal de Minas Gerais). IMAGE clone of TRBP (IMAGE 3162979; GenBank: BC002419.2) is purchased from Thermo Scientific. The open reading frame of TRBP was PCR amplified with restriction enzyme sites and ligated to multiple cloning sites of pB42AD (Clontech), pLexA (Clontech), p3 × FLAG CMV10 (Sigma-Aldrich), pcDNA3 HA (Thermo Fisher Scientific, Waltham, MA) vectors. The coding region of LGP2 is PCR amplified with restriction enzyme sites and ligated to pLexA (Clontech) and pB42AD (Clontech).
2.2. Yeast two-hybrid system
The matchmaker LexA two-hybrid system (Clontech) was used for screening of HeLa cells library with pLexA-LGP2 as bait. To confirm interaction of LGP2 and TRBP, pLexA-TRBP and p42AD-LGP2 were co-introduced into yeast strain EGY48 (p8op-lacZ) and transformants were grown on Trp-, His-, and Ura-deficient plates. These colonies were subjected to β-galactosidase assay on an SD plate containing 5-bromo-4-chloro-3-indolyl-β-D-(−)-galactopyr-anoside (X-gal) according to the manufacturer’s instructions.
2.3. Antibodies
Anti-LGP2, FLAG (M2), HA (12CA5), His6, and TRBP antibodies were purchased from Protein Tech, Sigma-Aldrich, Roche Diagnostics, Sigma-Aldrich and Protein Tech, respectively and phospho-STAT1 (Y701) were purchased from Cell Signaling Technology.
2.4. Immunoprecipitation assay for overexpressed proteins
HEK293T cells on a 6-well plate were transfected with total 5 mg of indicated plasmids using Lipofectamine 3000. Twenty four to thirty six hours after transfection, cells were lysed with 250 μl of whole cell extract buffer (50 mM Tris-HCl [pH 8.0]-280 mM NaCl-0.5% NP-40–0.2 mM EGTA-2 mM EDTA-10% glycerol-1 mM dithiothreitol-1 mM NaF-1 mM sodium orthovanadate) and anti-HA-agarose beads (Sigma-Aldrich) or anti-FLAG M2 beads (Sigma-Aldrich) were added to 200 μl (500–600 μg of protein) of the pre-cleared lysate. After incubation for 2 h, the beads were washed 5 times with whole cell extract buffer and the beads were eluted with SDS-sample buffer followed by Western blot analysis using indicated antibodies.
2.5. Immunoprecipitation assay for endogenous proteins
L929 cells (2 × 107) were mock infected or EMCV-infected (MOI = 1) for 15 h then lysed with whole cell extract buffer. Fifty microliter of Dynabeads protein G (Thermo Fisher Scientific) bound with 2.5 μg rabbit IgG (Santa Cruz) or anti-TRBP antibody (Protein Tech) were added to the cells lysate (2 × 107 cells) and incubated for 4 h. Immunoprecipitated proteins were analyzed by western blot using LGP2 antibody (Protein Tech) and TRBP antibody (Protein Tech).
2.6. Cells
L929 (CCL-1), HEK293T (CRL-3216), Vero cells (CRL-1587) were purchased from ATCC. BHK cells were kindly provided by Drs. Yoshiro Ohara (Kanazawa Medical University) and Toshiki Himeda (Kanazawa Medical University).
HEK293T, L929, Vero cells and 293FT cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) supplemented with 10% fetal calf serum (Hyclone) and penicillin/streptomycin. BHK cells were cultured with DMEM (Sigma-Aldrich) supplemented with 5% fetal calf serum (Hyclone) and penicillin/streptomycin.
2.7. Virus infection
Theiler’s mouse encephalitis virus (TMEV, DA strain) and Sendai virus (SeV, Z strain) were generous gifts from Drs. Yoshiro Ohara (Kanazawa Medical University) and Toshiki Himeda (Kanazawa Medical University), and Dr. Atsushi Kato (National Institute of Infectious Disease), respectively. Encephalomyocarditis virus (ATCC, VR-129B) was purchased from ATCC. Viral infection was performed with multiplicity of infection 1 to 10 in DMEM supplemented with 1–2% fetal calf serum for 2 h followed by replacing with complete media.
2.8. RNAs and RNA transfection
ON-TARGETplus Non-targeting siRNA#1, SMARTpool ON-TARGETplus mouse Tarbp2 siRNA and Set of 4 Upgrade ON-TARGETplus mouse Tarbp2 siRNA were purchased from Dharmacon-GE lifescience. Target sequences of Non-targeting siRNA#1, and ON-TARGETplus mouse Tarbp2 siRNA (siTRBP#1, 2, 3, 4) were 5′-UGGUUUACAUGUCGACUAA-3′, 5′-GGGCAAGAAUAU-CAUUAUA-3′, 5′-ACGUAGAGCUCAGAGAAUU-3′, 5′-GCAAGCAUCU-CUAGUUAAG-3′, 5′-CUACAAUGAUGCGCUAUUU-3′, respectively. For siRNA knockdown, L929 cells (3 × 104) were seeded onto a 48-well dish and transfected with 200 nM siRNA with Lipofectamine 2000 (Thermo Fisher Scientific) for 48 h.
2.9. RNA analysis and cytokine assay
Total RNA was purified using RNeasy kit (QIAGEN) and DNase-treated RNA was reverse transcribed with PrimeScript RT reagent kit (Takara-Bio) for quantitative real-time PCR using Thermal Cycler Dice Real Time System (Takara-Bio). The relative abundance of mRNA was obtained by the ΔΔCT method, using GAPDH for normalization. Culture media from infected cells or control cells were analyzed for cytokine production using mouse IL-6 Quanti-kine ELISA kit (R&D systems) and mouse IFNμ ELISA kit (PBL Assay Science).
2.10. Reporter gene assays
L929 cells were transfected with p3xFLAG-LGP2 (100 ng), p3xFLAG-TRBP (25, 50, or 100) along with mIFNβ luc (200 ng) and pRL-SV40 (100 ng) (Promega) using Lipofectamine 3000. HEK293T cells were transfected with p3xFLAG-CMV10 empty vector or p3xFLAG-LGP2 (20 ng), together with pEFBOS -FLAG-MDA5 (20 ng) or pEFBOSFLAG-RIG-I (20 ng) and p3xFLAG-TRBP (20–200 ng) along with IFNβ−110luc (125 ng) and pRLnull (25 ng) (Promega) using Lipofectamine 3000. The Dual-Luciferase Assay System (Promega) was used for luciferase assays. As an internal control, the Renilla luciferase construct was used to normalize luciferase activity.
3. Results
3.1. LGP2 associates with TRBP
A yeast two-hybrid screen was used to identify LGP2 interacting proteins. Multiple positive interactions were identified as the TAR RNA binding protein (TRBP), an essential component of RNAi machinery as well as known as a cellular factor utilized for human immunodeficiency virus 1 (HIV-1) replication [25,26]. Further characterization demonstrated that LGP2 interacted with the full-length TRBP protein and was confirmed bidirectionally. In order to determine the region of TRBP involved in LGP2 association, several TRBP variants were tested. TRBP has two double stranded RNA binding domains (dsRBD 1, and 2; see Fig. 1A) and the endonuclease, Dicer, is known to interact with the C-terminal region [30]. LGP2 was found to interact with both dsRBD 1 and dsRBD 2, individually or in tandem, but not with Dicer binding region (Fig. 1 B, C).
Fig. 1. LGP2 associates with TRBP in a yeast two-hybrid system.
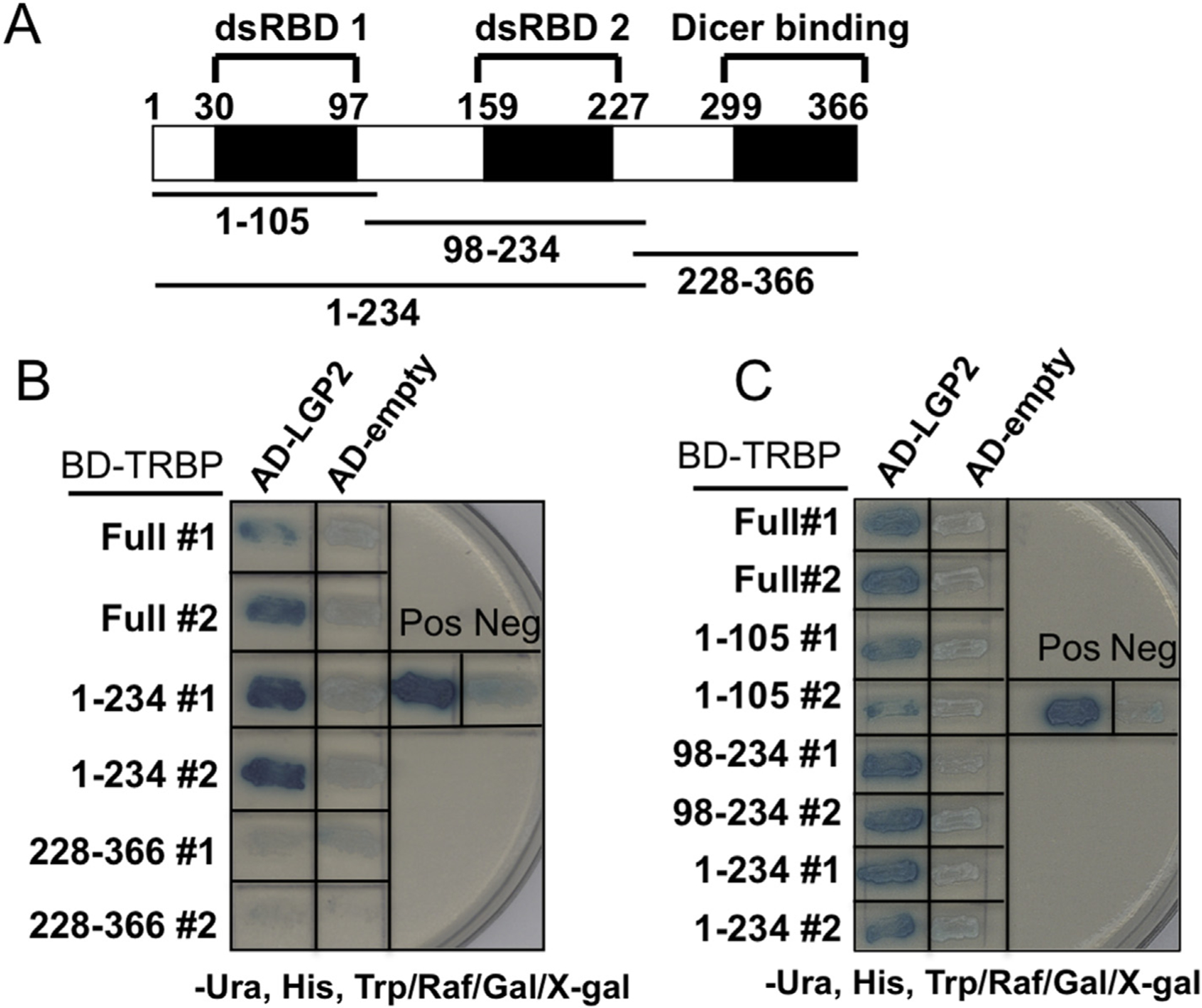
Schematic representation of TRBP structure. dsRBDs: Double stranded RNA binding domains (A). LexA activation domain fused to LGP2 and LexA DNA binding domain fused with TRBP domain (Full length, 1–234, or 228–366) were co-expressed in yeast and subjected to the beta-galactosidase assay. As a negative control, activation domain only was included (B, C). As in B, other combinations with TRBP domain (Full length, 1–105, 98–234, or 1–234) were also tested (C). Pos: LexA DNA binding domain fused with p53 (72–390) and activation domain fused with SV40 large T-antigen (87–708) were co-expressed; Neg: LexA DNA binding fused with Lamin C (66e230) and activation domain fused with SV40 large T-antigen (87–708) were co-expressed.
3.2. LGP2 co-precipitates with TRBP in mammalian cells
To verify the two-hybrid results and to further assess interaction between LGP2 and TRBP, immunoprecipitation experiments were performed with lysates of transfected HEK293T cells. TRBP was co-expressed with LGP2 as well as several control proteins, including RIG-I, MDA5 and TRBP-interacting protein Dicer. Expressed TRBP was found to co-precipitate LGP2, but not RIG-I and MDA5 indicating a specific interaction for LGP2, as neither MDA5 nor RIG-I were found to coprecipitate with TRBP (Fig. 2A). Co-precipitation of TRBP and Dicer was confirmed as reported [25].
Fig. 2. LGP2 but not RIG-I or MDA5 co-precipitates with TRBP in HEK293T cells.
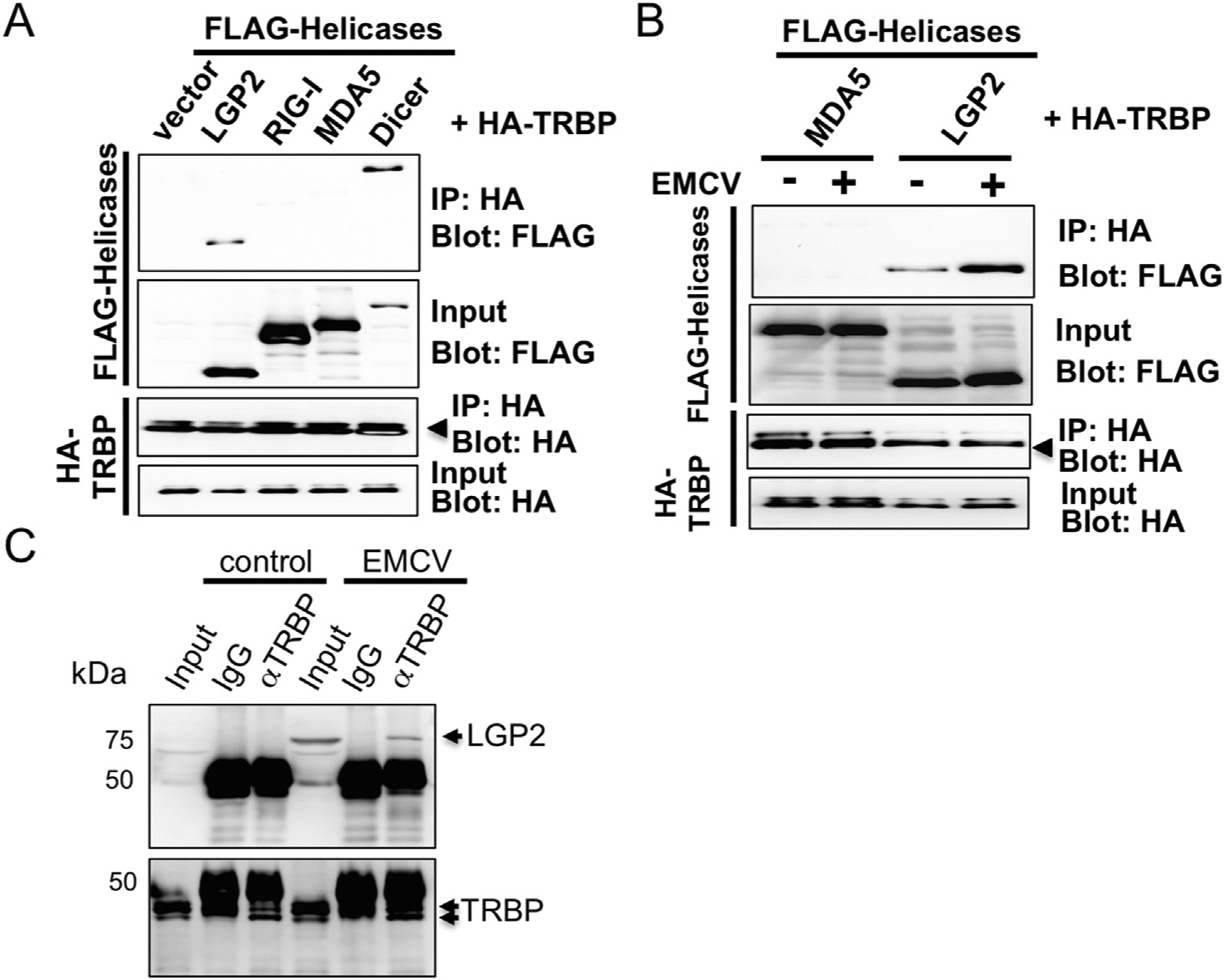
HA-tagged TRBP plasmid was co-introduced with FLAG-tagged LGP2, -RIG-I, MDA5 or Dicer plasmid in HEK293T cells and the lysate was subjected to immuno-precipitation with HA beads to co-purity FLAG-tagged proteins for Western blot (A). HA-tagged TRBP plasmid was co-introduced with FLAG-tagged LGP2 or MDA5 plasmid in HEK293T cells and cells were infected or mock infected with EMCV (MOI = 1) for 9.5 h. The lysate was subjected to immunoprecipitation as in A (B). The intensity was determined by densitometric analysis using Quantity One software (Bio-Rad). L929 cells were mock infected or infected with EMCV (MOI = 1) and the lysate was subjected to immunoprecipitation with anti-TRBP antibody or control rabbit IgG to co-purity endogenous LGP2 protein. The precipitant was probed with anti-LGP2 antibody for Western blot analysis (C).
Next, we asked whether viral infection enhances the association between LGP2 and TRBP, since TRBP is also an RNA binding protein. The immunoprecipitation experiments indicated that the percentage of co-precipitated LGP2 from total input in the lysate of EMCV infected cells was about 3-fold increased compared to the non-infected cells (Fig. 2B). Next, we tested immuno-precipitation with endogenous proteins. LGP2 has been known as an IFN-inducible protein. Endogenous LGP2 was detectable in EMCV-infected cells but not control cells as reported [12]. Endogenous TRBP also co-precipitated EMCV-induced endogenous LGP2 (Fig. 2C) as seen in overexpressed proteins.
3.3. TRBP is required for normal antiviral responses to Cardiovirus infection
LGP2 has been reported for important roles in immune responses against Picornavirus infection [20,21]. We also confirmed requirement of LGP2 in TMEV - triggered immune responses (Supplementary Fig. 1). Next, in order to explore involvement of TRBP in the role of LGP2 for Picornavirus infection, we performed knockdown experiments using TRBP siRNA. TMEV infection induced the expression of IFNβ mRNA in murine L929 cells, but was significantly reduced by silencing of TRBP expression with siRNA compared to non-targeting siRNA control (Fig. 3A; Supplementary Fig. 3). IFNβ mRNA induction with another Cardiovirus, EMCV infection was also impaired by silencing of TRBP severely (Fig. 3B; Supplementary Fig. 3). To further examine the effect of TRBP on TMEV responses, we measured cytokine levels secreted from TMEV or EMCV infected cells with or without TRBP knockdown. TRBP knockdown with both pooled siRNAs (Fig. 3D) or with individual siRNAs (Supplementary Fig. 2A and B) was found to significantly impair production of IFNβ. Accordingly, phosphorylation of STAT1 that is mediated by type-I IFN secretion was also abolished in TRBP-silenced cells after TMEV infection (Fig. 3E). Importantly, silencing of TRBP did not affect SeV-induced IFNβ induction, confirming the specificity of TRBP for the LGP2/MDA5 pathway and not the RIG-I pathway (Fig. 3C; Supplementary Fig. 3).
Fig. 3. Knock down of TRBP reduces induction of IFNβ mediated by TMEV and EMCV infection but not SeV infection.
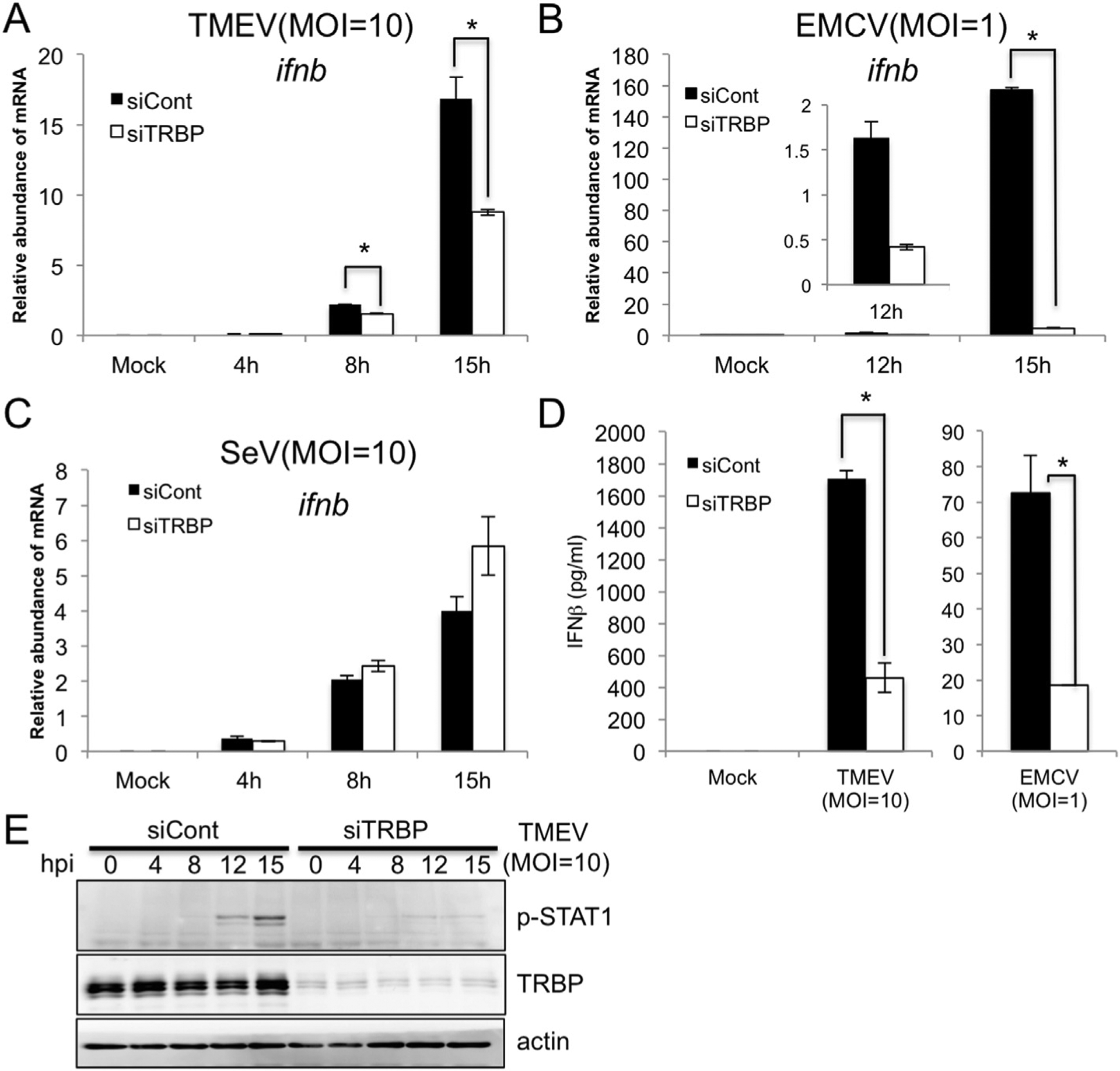
L929 cells were transfected with pooled TRBP siRNAs and infected with TMEV (A, E), EMCV (B) or SeV (C) at indicated MOI. Cells were harvested for quantitative PCR for IFNβ and TRBP mRNA and for Western blot (E). IFNβ secreted in the media from L929 cells infected with TMEV or EMCV for 24 h are measured by ELISA (D). Data are representative of three independent experiments with similar result and bars indicate the average (n = 2) ± the standard deviation. Student’s t-test was performed: *(p < 0.05).
3.4. TRBP activates LGP2/MDA5-dependent IFNβ promoter activity induced by Cardiovirus infection
siRNA Knockdown experiments demonstrated requirement of TRBP in Cardiovirus-induced IFN responses (Fig. 3). Next, in order to assess whether TRBP activates Cardiovirus-induced IFN responses via association with LGP2, we performed a luciferase assay. The effect of TRBP overexpression on TMEV-induced IFNβ promoter activity was tested in L929 cells in the presence or absence of LGP2 expression. Increasing amounts of TRBP plasmid enhanced the TMEV-mediated IFNβ promoter activity in dose dependent manner when co-expressed with LGP2 (Fig. 4A). Interestingly, the TRBP dose dependency was not observed when LGP2 is not co-expressed (Fig. 4A). To further confirm it, the similar experiments were performed using HEK293T cells that do not respond well against RLR ligands for EMCV triggered-IFN induction [22]. The promoter activity was not stimulated by EMCV infection as expected, but the cells responded well with expression of MDA5 and further expression of LGP2 synergistically enhanced the activity (Fig. 4B). Similarly, increasing amount of TRBP plasmid enhanced the promoter activity in dose-dependent manner only when LGP2 is present with MDA5 (Fig. 4B) but not MDA5 alone. These results suggest that TRBP cooperates with LGP2 for TMEV or EMCV-triggered IFN responses reflecting the importance of the interaction between LGP2 and TRBP. As a control experiment, we also tested the effect of TRBP on the RIG-I pathway using SeV and RIG-I expression plasmid. HEK293T cells do not respond well but RIG-I expression recovered the IFN promoter activity. LGP2 did not have synergistic effect on the SeV-triggered signaling and TRBP expression did not enhance the promoter activity regardless of LGP2 expression (Fig. 4C) suggesting that TRBP is not involved in the RIG-I pathway as demonstrated in the TRBP siRNA experiments (Fig. 3).
Fig. 4. TRBP activates LGP2/MDA5-dependent IFNβ promoter activity induced by Cardiovirus infection.
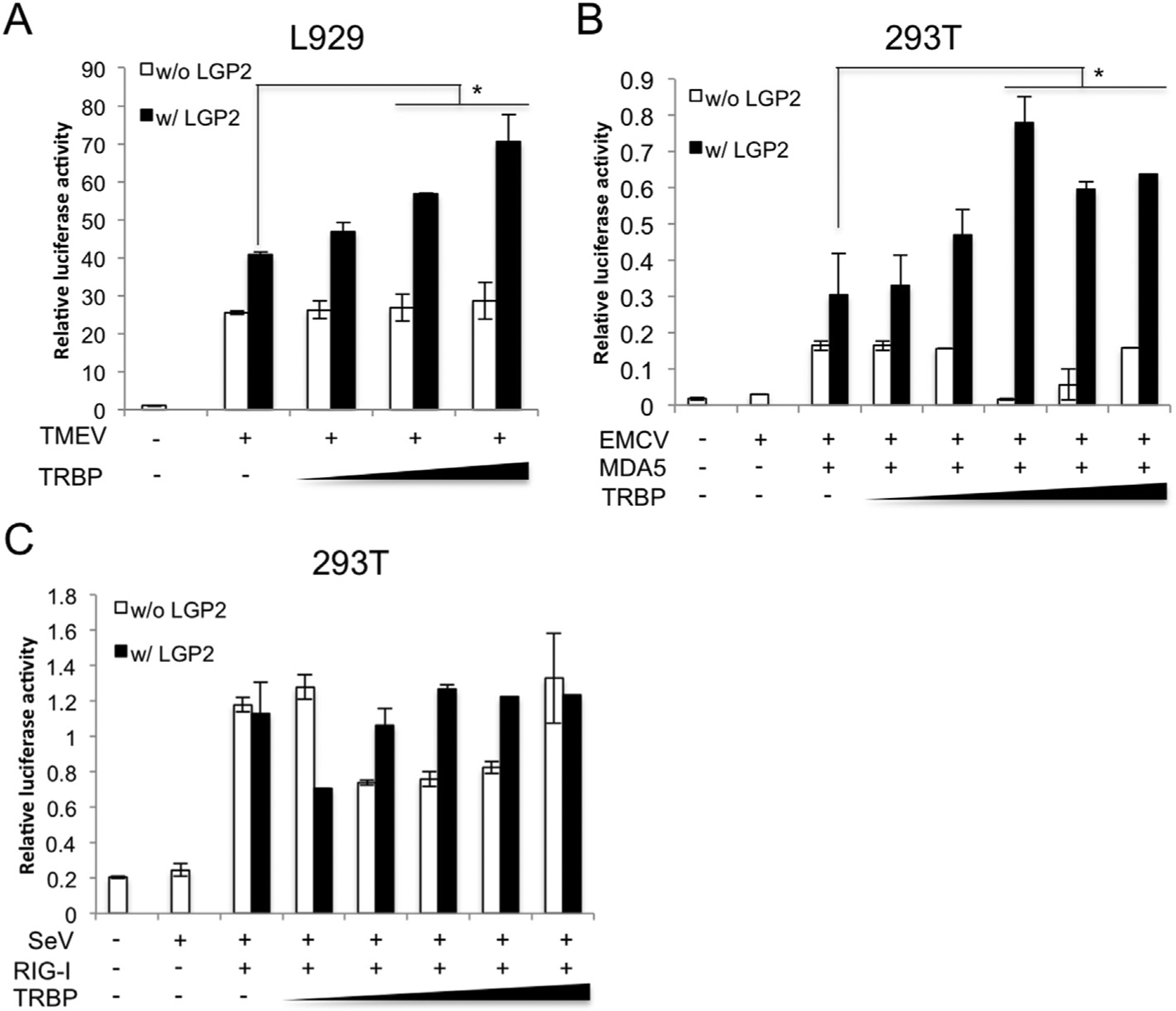
L929 cells transfected with (w/LGP2) or without (w/o LGP2) LGP2 vector and TRBP vector (25, 50, 100 ng) along with mouse IFNβ promoter vector and pRL-SV40 were infected with TMEV (MOI = 10) for 12 h (A). HEK293T cells transfected with empty vector (vector), MDA5 (B) or RIG-I vector (C) together with (w/LGP2) or without (w/o LGP2) LGP2 expression vector and TRBP expression vector (B) (20, 50, 100, 150, 200 ng) along with −110 luc IFNβ promoter vector and pRL-SV40 were infected with EMCV (MOI = 1) (B) or SeV (MOI = 10) (C) for 17 h. Lysates were subjected to luciferase assays. The data are representative of three independent experiments with similar result and bars indicate the average (n = 2). Asterisks indicate P < 0.05.
All these data suggested that TRBP cooperates with LGP2 for Cardiovirus-induced IFNβ promoter activity consistent with a positive regulatory function observed in the siRNA experiments (Fig. 3).
4. Discussion
In this study, we identified TRBP, a component of the RNA interference machinery, as a binding partner of the innate immune RNA sensor, LGP2, and reveal a role for TRBP as a mediator of the antiviral innate immune response triggered by the Cardiovirus, TMEV and EMCV. Results demonstrate that TRBP binds to LGP2 but not the closely related RNA sensor proteins, RIG-I or MDA5, consistent with the observation that LGP2 is a positive regulator of antiviral responses to the Cardiovirus. In addition, the interaction between LGP2 and TRBP is enhanced by virus infection (Fig. 2B). Reduction of TRBP expression by siRNA impaired production of IFNβ by TMEV and EMCV infection suggesting that TRBP has a positive regulatory role in the LGP2/MDA5 pathway but not in the Sendai virus-mediated RIG-I pathway (Fig. 3). Expression of TRBP stimulated TMEV or EMCV-induced IFNβ promoter activity (Fig. 4) only when LGP2 is expressed together with MDA5 but not MDA5 alone. The data supports involvement of TRBP in the LGP2/MDA5 pathway through functional interaction with LGP2. Importantly, the role of TRBP is specific in this sensing pathway, as no TRBP-mediated stimulation was seen in the RIG-I pathway triggered by SeV infection (Figs. 3 and 4).
Another protein involved in Dicer-mediated RNA biogenesis, PACT, functions as a cellular activator of RIG-I [31]. PACT directly associates with RIG-I and stimulates RIG-I-dependent signal transduction induced by SeV or poly I:C. By analogy, we speculate that TRBP acts as an LGP2/MDA5 version of PACT, stimulating Cardiovirus-triggered IFN induction. This study has provided a new addition of RNAi accessory factor to anti-viral innate immune sensing.
One of the interesting differences between RIG-I and LGP2/MDA5 RNA sensing is that RIG-I recognizes viral genomic RNAs but LGP2/MDA5 often requires viral replication [22]. TRBP might participate in the initial recognition of replicating viral RNAs together with LGP2 by modifying viral RNA as indicated in HIV-1 transactivating response RNA (TAR) unwinding [32] or TRBP/LGP2 might unwind viral RNAs by utilizing ATPase activity for displacing viral proteins from RNAs as indicated in RIG-I and MDA5 [33]. However, how TRBP contributes to Cardiovirus-triggered immune responses via association with LGP2 remains unknown at this stage and more intense investigations are needed to clarify the mechanisms of viral recognition by the TRBP/LGP2/MDA5 system.
Supplementary Material
Acknowledgments
We thank Drs. Yoshiro Ohara and Toshiki Himeda for providing TMEV, Dr. Atsushi Kato for providing SeV, Dr. V. Narry Kim for Dicer plasmid, Dr. Joao Marques for mIFNβ-luc plasmid, and Daiki Ishima, Mamoru Kodama, Tsubasa Naito and Hiroto Iguchi for technical assistance. Curt Horvath and Glen Barber are recipients of the NIH research grant R01-GM111652 and R01-AI-079336-07, respectively.
Footnotes
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2016.10.023.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbrc.2016.10.023.
References
- [1].Takeuchi O, Akira S, Pattern recognition receptors and inflammation, Cell 140 (2010) 805–820. [DOI] [PubMed] [Google Scholar]
- [2].Goubau D, Deddouche S, Reis e Sousa C, Cytosolic sensing of viruses, Immunity 38 (2013) 855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T, The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses, Nat. Immunol 5 (2004) 730–737. [DOI] [PubMed] [Google Scholar]
- [4].Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB, VISA is an adapter protein required for virus-triggered IFN-beta signaling, Mol. Cell 19 (2005) 727–740. [DOI] [PubMed] [Google Scholar]
- [5].Seth RB, Sun L, Ea CK, Chen ZJ, Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3, Cell 122 (2005) 669–682. [DOI] [PubMed] [Google Scholar]
- [6].Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J, Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus, Nature 437 (2005) 1167–1172. [DOI] [PubMed] [Google Scholar]
- [7].Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S, IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction, Nat. Immunol 6 (2005) 981–988. [DOI] [PubMed] [Google Scholar]
- [8].Komuro A, Horvath CM, RNA- and virus-independent inhibition of antiviral signaling by RNA helicase LGP2, J. Virol 80 (2006) 12332–12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rothenfusser S, Goutagny N, DiPerna G, Gong M, Monks BG, Schoenemeyer A, Yamamoto M, Akira S, Fitzgerald KA, The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I, J. Immunol. Baltim. Md. 1950 175 (2005) 5260–5268. [DOI] [PubMed] [Google Scholar]
- [10].Saito T, Hirai R, Loo YM, Owen D, Johnson CL, Sinha SC, Akira S, Fujita T, Gale M Jr., Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Suthar MS, Ramos HJ, Brassil MM, Netland J, Chappell CP, Blahnik G, McMillan A, Diamond MS, Clark EA, Bevan MJ, Gale M Jr., The RIG-I-like receptor LGP2 controls CD8(+) T cell survival and fitness, Immunity 37 (2012) 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M Jr., Akira S, Yonehara S, Kato A, Fujita T, Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity, J. Immunol. Baltim. Md. 1950 175 (2005) 2851–2858. [DOI] [PubMed] [Google Scholar]
- [13].Goubau D, Schlee M, Deddouche S, Pruijssers AJ, Zillinger T, Goldeck M, Schuberth C, Van der Veen AG, Fujimura T, Rehwinkel J, Iskarpatyoti JA, Barchet W, Ludwig J, Dermody TS, Hartmann G, Reis e Sousa C, Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates, Nature 514 (2014) 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G, 5′-Triphosphate RNA is the ligand for RIG-I, Sci. (New York, N.Y.) 314 (2006) 994–997. [DOI] [PubMed] [Google Scholar]
- [15].Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C, RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates, Sci. (New York, N.Y.) 314 (2006) 997–1001. [DOI] [PubMed] [Google Scholar]
- [16].Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, Juranek S, Kato H, Kawai T, Poeck H, Fitzgerald KA, Takeuchi O, Akira S, Tuschl T, Latz E, Ludwig J, Hartmann G, Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus, Immunity 31 (2009) 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schmidt A, Schwerd T, Hamm W, Hellmuth JC, Cui S, Wenzel M, Hoffmann FS, Michallet MC, Besch R, Hopfner KP, Endres S, Rothenfusser S, 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 12067–12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Feng Q, Hato Stanleyson V., Langereis Martijn A., Zoll J, Virgen-Slane R, Peisley A, Hur S, Semler Bert L., van Rij Ronald P., van Kuppeveld Frank J.M., MDA5 detects the double-stranded RNA replicative form in picornavirus-infected cells, Cell Rep. 2 (2012) 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S, Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses, Nature 441 (2006) 101–105. [DOI] [PubMed] [Google Scholar]
- [20].Satoh T, Kato H, Kumagai Y, Yoneyama M, Sato S, Matsushita K, Tsujimura T, Fujita T, Akira S, Takeuchi O, LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Venkataraman T, Valdes M, Elsby R, Kakuta S, Caceres G, Saijo S, Iwakura Y, Barber GN, Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses, J. Immunol. Baltim. Md. 1950 178 (2007) 6444–6455. [DOI] [PubMed] [Google Scholar]
- [22].Deddouche S, Goubau D, Rehwinkel J, Chakravarty P, Begum S, Maillard PV, Borg A, Matthews N, Feng Q, van Kuppeveld FJ, Reis e Sousa C, Identification of an LGP2-associated MDA5 agonist in picornavirus-infected cells, eLife 3 (2014) e01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bruns Annie M., Leser George P., Lamb Robert A., Horvath Curt M., The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly, Mol. Cell 55 (2014) 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].MacRae IJ, Ma E, Zhou M, Robinson CV, Doudna JA, In vitro reconstitution of the human RISC-loading complex, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, Filipowicz W, TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing, EMBO Rep. 6 (2005) 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gatignol A, Buckler-White A, Berkhout B, Jeang KT, Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR, Sci. (New York, N.Y.) 251 (1991) 1597–1600. [DOI] [PubMed] [Google Scholar]
- [27].Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R, TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing, Nature 436 (2005) 740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sanghvi VR, Steel LF, The cellular TAR RNA binding protein, TRBP, promotes HIV-1 replication primarily by inhibiting the activation of double-stranded RNA-dependent kinase PKR, J. Virol 85 (2011) 12614–12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Park H, Davies MV, Langland JO, Chang HW, Nam YS, Tartaglia J, Paoletti E, Jacobs BL, Kaufman RJ, Venkatesan S, TAR RNA-binding protein is an inhibitor of the interferon-induced protein kinase PKR, Proc. Natl. Acad. Sci. U. S. A 91 (1994) 4713–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Laraki G, Clerzius G, Daher A, Melendez-Pena C, Daniels S, Gatignol A, Interactions between the double-stranded RNA-binding proteins TRBP and PACT define the Medipal domain that mediates protein-protein interactions, RNA Biol. 5 (2008) 92–103. [DOI] [PubMed] [Google Scholar]
- [31].Kok KH, Lui PY, Ng MH, Siu KL, Au SW, Jin DY, The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response, Cell Host Microbe 9 (2011) 299–309. [DOI] [PubMed] [Google Scholar]
- [32].Dorin D, Bonnet MC, Bannwarth S, Gatignol A, Meurs EF, Vaquero C, The TAR RNA-binding protein, TRBP, stimulates the expression of TAR-containing RNAs in vitro and in vivo independently of its ability to inhibit the dsRNA-dependent kinase PKR, J. Biol. Chem 278 (2003) 4440–4448. [DOI] [PubMed] [Google Scholar]
- [33].Yao H, Dittmann M, Peisley A, Hoffmann HH, Gilmore RH, Schmidt T, Schmid-Burgk JL, Hornung V, Rice CM, Hur S, ATP-dependent effector-like functions of RIG-I-like receptors, Mol. Cell 58 (2015) 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


