Abstract
Background:
Activation of hematopoietic stem cells [HSCs, lineage(lin)−stem cell growth factor receptor (c-kit)+stem cell antigen-1(Sca-1)+, or LKS cells in mice] is critical for initiating the granulopoietic response. This study determined the effect of alcohol exposure on sonic hedgehog (SHH) signaling in the regulation of HSC activation during bacteremia.
Methods:
Acute alcohol intoxication was induced in mice by intraperitoneal (i.p.) injection of 20% alcohol (5 g alcohol/kg body weight). Control mice received i.p. saline. Thirty minutes later, mice were intravenously (i.v.) injected with Escherichia coli (E. coli, 1 to 5 × 107 CFUs/mouse) or saline.
Results:
SHH expression by lineage-negative bone marrow cells (BMCs) was significantly increased 24 hours after E. coli infection. Extracellular signal-regulated kinase 1/2 (ERK1/2)-specificity protein 1 (Sp1) signaling promotes SHH expression. ERK1/2 was markedly activated in BMCs 8 hours following E. coli infection. Alcohol suppressed both the activation of ERK1/2 and up-regulation of SHH expression following E. coli infection. E. coli infection up-regulated GLI family zinc finger 1 (Gli1) gene expression by BMCs and increased Gli1 protein content in LKS cells. The extent of Gli1 expression was correlated with the activity of proliferation in LKS cells. Alcohol inhibited up-regulation of Gli1 expression and activation of LKS cells in response to E. coli infection. Alcohol also interrupted the granulopoietic response to bacteremia.
Conclusion:
These data show that alcohol disrupts SHH-Gli1 signaling and HSC activation in the early stage of the granulopoietic response, which may serve as an important mechanism underlying the impairment of immune defense against bacterial infection in host excessively consuming alcohol.
Keywords: Alcohol, Sonic Hedgehog, Hematopoietic Stem Cells, Host Response, Bacteremia
THE GRANULOPOIETIC RESPONSE is critically important in immune defense against serious infections, particularly those caused by bacterial pathogens (Manz and Boettcher, 2014; Shi et al., 2019). In adult humans, granulocytes (neutrophilic granulocytes, neutrophils, polymorphonuclear leukocyte, or PMNs) are derived from hematopoietic stem cells (HSCs) in the bone marrow (Yamamoto et al., 2018). Since mature granulocytes in the circulation are terminally differentiated with a relatively short life span, bone marrow tightly regulates its daily generation of these phagocytes in order to maintain the homeostasis of granulocyte population in the body (Strydom and Rankin, 2013). During bacterial infection, the host requirement of granulocytes increases. Concomitantly, the bone marrow initiates the granulopoietic response to promote production of granulocytes through enhancing granulocyte lineage development at the expense of generating cells from other lineages (Ueda et al., 2005). Our recent investigations have demonstrated that the rapid activation of HSC proliferation along with reprogramming their commitment to granulocyte lineage differentiation is a critical step in the early stage of the granulopoietic response (Shi et al., 2013, 2017, 2018; Zhang et al, 2008b, 2009). In the process of the granulopoietic response, the bone marrow accelerates granulocyte differentiation, shortens their marrow retention, and increases the release of these phagocytes into the systemic circulation (Sato et al., 1998; Shahbazian et al., 2004; Terashima et al., 1996).
Alcohol is the most frequently abused substance (Merikangas and McClair, 2012). Excessive consumption of alcohol injures the bone marrow and causes pathological changes in hematopoietic precursor cells (Shi et al., 2019). Alcohol also inhibits the granulopoietic response, particularly HSC activation during the early stage of host defense response to serious infections (Melvan et al., 2011, 2012; Siggins et al., 2011; Zhang et al., 2009). In the clinic, it has long been observed that alcohol abusers are susceptible to developing serious bacterial infections, particularly septicemia (Zhang et al., 2002, 2008a). Septicemia in alcohol abusers can develop from a broad profile of origins including bacterial translocation of gut flora, peritonitis, pneumonia, urinary tract infection, biliary infection, wound infection, and colonization of intravenous catheters (Cook, 1998; Macgregor and Loubia, 1997). Gram-negative bacilli are frequent pathogens (Macgregor and Loubia, 1997). Alcoholic patients with septic infection often present with granulocytopenia, which is a predictor for fatal consequences (Perlino and Rimland, 1985).
Signaling from the hedgehog (HH) pathway regulates stem cell activity during embryogenesis (Dyer et al., 2001; Gering and Patient, 2005) and in adulthood (Bhardwaj et al., 2001; Trowbridge et al., 2006). Our recent studies have also shown that the sonic hedgehog (SHH)-GLI family zinc finger 1 (glioma-associated oncogene homolog 1 or Gli1) signal transduction system is involved in the regulation of HSC activation during the granulopoietic response (Shi et al., 2018). We postulated that excessive alcohol exposure might interrupt SHH-Gli1 signaling in the regulation of primitive hematopoietic precursor cell activities. In the current study, we determined the effect of acute alcohol intoxication on SHH-Gli1 signaling in mediating the early activation of hematopoietic stem/progenitor cells in the bone marrow during the granulopoietic response to systemic Escherichia coli (E. coli) infection.
MATERIALS AND METHODS
Animals
Male mice (BALB/c strain, 6 to 8 weeks old, Charles River Laboratories, Wilmington, MA) were housed in specific pathogen-free facilities on the cycle of 12-hour light/dark schedule. All procedures were approved by the Institutional Animal Care and Use Committees of Northeast Ohio Medical University and Michigan State University in accordance with the recommendations of the National Institutes of Health guidelines.
Animals were acutely intoxicated with alcohol through intraperitoneal (i.p.) injection of 20% alcohol in normal saline at a dose of 5 g alcohol/kg. Alcohol levels in the blood are 106.3 to 132.8, 87.7 to 122.4, and 48.4 to 61.4 mM, respectively, at 90 minutes, 3, and 6 hours postadministration of alcohol (Melvan et al., 2011; Zhang et al., 2009). Control mice were i.p. injected with an equal volume of saline. Thirty minutes thereafter, systemic infection was initiated by intravenous injection (i.v., via penile vein) of live Escherichia coli (E11775 from the American Type Culture Collection, Rockville, MD; 1 to 5 × 107 CFUs in 100 μl of saline/mouse) under isoflurane anesthesia. Controls received i.v. saline. In a subgroup of mice, an i.v. dose of 5-bromo-2-deoxyuridine [BrdU, BD Biosciences, San Diego, CA; 1 mg in 100 μl of phosphate-buffered saline (PBS)/mouse] was administered simultaneously. Mice were sacrificed at 24 hours thereafter. Upon sacrifice, a heparinized blood sample was obtained by cardiac puncture. White blood cells were quantified under a light microscope with a hemocytometer. Femurs and tibias were obtained to collect bone marrow cells (BMCs) by flushing out bone marrow from the bones with PBS (Life Technologies, Grand Island, NY) containing 1% bovine serum albumin (BSA, HyClone Laboratories, Logan, UT). After filtering BMCs through a 70-μm nylon mesh (Sefar America INC. Kansas City, MO), lysis of erythrocytes in BMC samples was conducted using RBC lysis solution (Qiagen Sciences, MD). Nucleated BMCs were washed with PBS containing 1 % BSA and then quantified by counting cells under a light microscope with a hemocytometer.
Preparation of Bacteria
Live E. coli suspension in saline for i.v. injection in each experiment was freshly prepared as described previously (Shi et al., 2013, 2017,2018).
Fluorochrome Conjugation of Antibody
The DyLight 405 Microscale Antibody Labeling Kit (Thermo Fisher Scientific, Waltham, MA) was used to label anti-human/antimouse Gli1 antibody (Clone #388516, R&D Systems, Minneapolis, MN) and the matched isotype control antibody (Clone # 54447, R&D Systems) using the protocol provided by the manufacturer.
Flow Cytometric Analysis
Flow cytometric analysis of cell phenotype, expression of SHH and Gli1, and incorporation of BrdU were performed as previously described (Shi et al., 2013, 2017, 2018; Zhang et al., 2008b). Reagents used for labeling cells included a panel of biotinylated antibodies against mouse lineage markers [CD3e (clone 145-2C11), CD45R/B220 (clone RA3-6B2), CD11b (Mac-1, clone M1/70), TER-119 (clone TER-119), with or without Gr1 (granulocyte differentiation antigen 1, Ly-6G/Ly-6C, clone RB6-8C5) (BD Biosciences)]; biotinylated isotype control antibodies (clones A19-3, R35-95, and A95-1; BD Biosciences); fluorochrome-conjugated streptavidin (BD Biosciences); fluorochrome-conjugated antibodies against mouse c-kit (stem cell growth factor receptor or CD117, clone 2B8), Sca-1 (stem cell antigen-1 or Ly-6A/E, clone D7), Gr1 (Ly-6G, clone 1A8), CD34 (clone RAM34) (BD Biosciences), and F4/80 (clone BM8) (eBioscience, San Diego, CA); anti-human/mouse SHH (Clone E1, Santa Cruz Biotechnology, Inc., Dallas, TX); fluorochrome-conjugated polyclonal goat anti-mouse IgG (H + L; Life Technologies, Eugene, OR); fluorochrome-conjugated anti-human/anti-mouse Gli1 (Clone #388516, R&D Systems) and the isotype control antibody (Clone # 54447, R&D Systems); and BD BrdU Flow Kit (BD Biosciences). Flow cytometry was conducted on a FACSAria Fusion flow cytometer with FACSDiva software (Becton Dickinson, San Jose, CA). The number of cells acquired in each sample was in the range of 5,000 to 300,000.
Western Blot Analysis
Phosphorylated extracellular signal-regulated kinase 1/2 (phospho-ERK1/2) and total ERK1/2 in cells were assessed with Western blot analysis as reported previously (Shi et al., 2017, 2018).
Real-Time RT-PCR Determination
Gli1 mRNA expression by cells was measured with real-time RT-PCR as previously described (Shi et al., 2018).
Statistical Analysis
Data are reported as mean ± SEM. The sample size is presented in each figure legend. One-way ANOVA followed by Student–Newman–Keuls test was used for comparisons among multiple groups. To examine the relationship between Gli1 expression and proliferative activity in lin−c-kit+Sca-1+ (LKS) cells, linear regression and correlation analysis was performed. Statistical significance of difference is considered at p < 0.05.
RESULTS
Alcohol Intoxication Suppressed Up-regulation of SHH Expression
In comparison with mice receiving i.v. saline, intravenous challenge with E. coli for 24 hours caused a significant increase in SHH expression by marrow lineage− (lin−) cells as reflected by the increase in mean channel fluorescence (MCF) of SHH in this cell population (p < 0.05, Fig. 1). Similarly, the lin−c-kit+ subset of lin− cells also showed a significant up-regulation of SHH expression following systemic E. coli infection (p < 0.05). The baseline expression of SHH by all examined subpopulations of BMCs was not affected by acute alcohol intoxication in animals receiving i.v. saline. However, alcohol intoxication suppressed the increase in SHH expression by both lin− and lin−c-kit+ cell types in the bone marrow in response to bacteremia (p < 0.05). SHH expression by bone marrow lineage-positive (lin+) cells tended to increase following systemic E. coli infection, but this tendency did not reach statistical significance (p > 0.05). Mice with acute alcohol intoxication did not show any elevation in SHH expression by lin+ cells following E. coli infection as compared to the baseline level of SHH expression by lin+ cells in animals receiving i.v. saline.
Fig. 1.
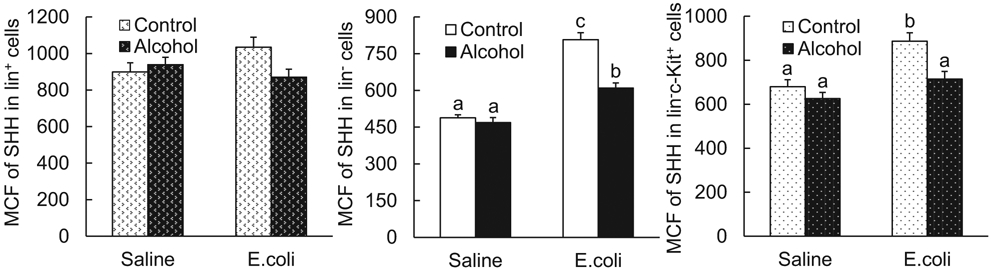
Acute alcohol intoxication suppressed up-regulation of SHH expression by bone marrow cells 24 hours following i.v. challenge with E. coli. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli: i.v. E. coli. Values are mean ± SEM. N = 4 to 5 in each group. Bars with different letters in each panel are statistically different (p < 0.05).
Alcohol Intoxication Impaired Activation of the ERK1/2 Pathway
ERK1/2-specificity protein 1 (SP1) signaling plays an important role in promoting expression of SHH (Shi et al., 2018). To understand the signaling mechanism underlying alcohol-induced impairment of SHH up-regulation, we determined the effect of acute alcohol intoxication on ERK1/2 activation in BMCs following systemic E. coli infection. As shown in Fig. 2, at 8 hours post-E. coli challenge, the level of phospho-ERK1/2 in BMCs was markedly increased in comparison with its baseline level in BMCs from mice receiving i.v. saline (p < 0.05). Density quantification of phospho-ERK1/2 bands against the reference loading bands of either total ERK1/2 (Fig. 2B) or β-actin (Fig. 2C) in BMCs on Western blot images showed marked increases in the ratios of both phospho-ERK1/2 versus total ERK and phospho-ERK1/2 versus β-actin 8 hours following systemic E. coli infection. Acute alcohol intoxication did not change the baseline level of phospho-ERK1/2 in BMCs from mice receiving i.v. saline. However, the elevation of ERK1/2 phosphorylation in BMCs in response to systemic E. coli infection was profoundly inhibited by acute alcohol intoxication (p < 0.05).
Fig. 2.
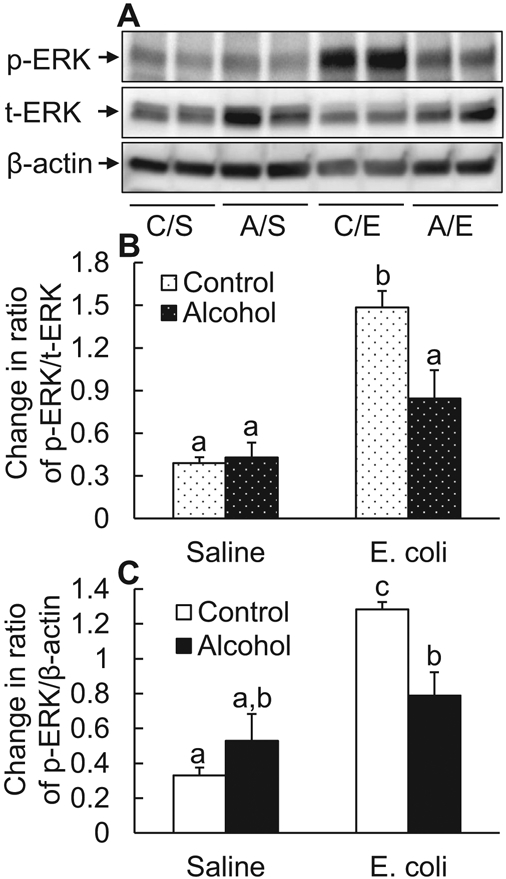
Acute alcohol intoxication suppressed ERK1/2 activation in nucleated bone marrow cells 8 hours following i.v. challenge with E. coli. (A) Representative Western blot image. C/S: i.p. saline plus i.v. saline; A/S: i.p. alcohol plus i.v. saline; C/E: i.p. saline plus i.v. E. coli; A/E: i.p. alcohol plus i.v. E. coli. (B and C) Quantitative analysis of Western blot images. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli: i.v. E. coli. Values are mean ± SEM. N = 4 in each group. Bars with different letters in each panel of (B) and (C) are statistically different (p < 0.05).
Alcohol Intoxication Inhibited Increase in Gli1 Expression
SHH ligand engagement with its receptors patched (PTCH) 1 and 2 activates transcription of the downstream target genes, particularly Gli1 (Irvine and Copland, 2012; Villavicencio et al., 2000). Gli1 is a master transcriptional activator promoting expression of functional gene products for cell survival, proliferation, and differentiation (Merchant et al., 2010; Villavicencio et al., 2000). Accompanied by the increase in SHH expression, a significant up-regulation of Gli1 mRNA expression by BMCs was detected 24 hours after E. coli infection (p < 0.05; Fig. 3A). Acute alcohol intoxication did not alter the baseline level of Gli1 expression by BMCs in mice receiving i.v. saline, but inhibited the up-regulation of Gli1 expression by BMCs in response to E. coli bacteremia (p < 0.05). Further, the level of Gli1 protein expression in marrow LKS cell pool (with enrichment of HSCs) as reflected by the integrated MCF (IMCF) of Gli1 was substantially increased 24 hours following E. coli bacteremia (p < 0.05, Fig. 3B). Alcohol intoxication suppressed bacteremia-induced increase in Gli1 expression by LKS cells. The baseline expression of Gli1 by LKS cells in mice receiving i.v. saline was not affected by alcohol exposure. Figure 3C shows the representative plots of changes in Gli1 expression by bone marrow LKS cells measured with flow cytometry.
Fig. 3.
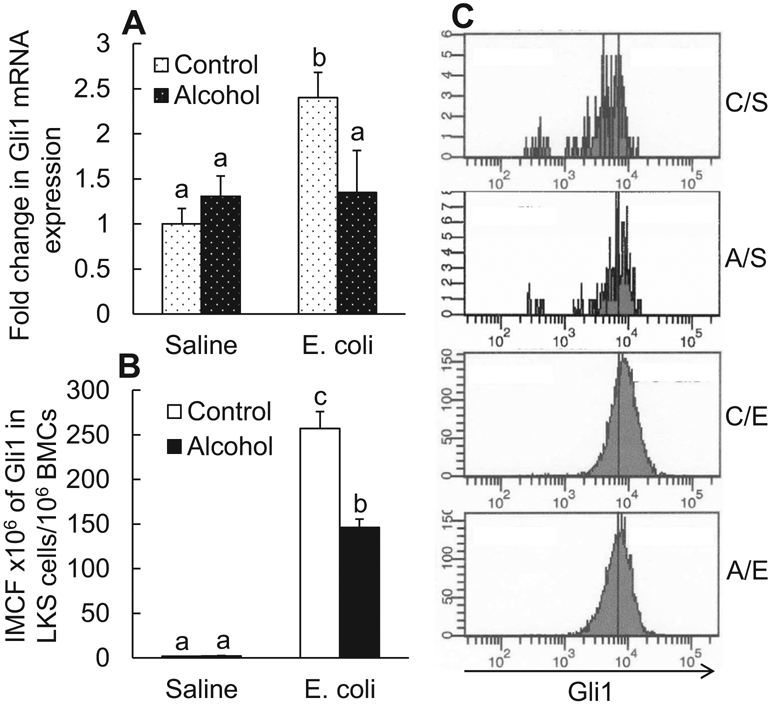
(A) Changes in Gli1 mRNA expression by bone marrow cells; (B) changes in integrated mean channel fluorescence (IMCF) of Gli1 protein expression by bone marrow LKS cells 24 hours following i.v. challenge with E. coli. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli. i.v. E. coli. Values are mean ± SEM. N = 4 to 5 in each group. Bars with different letters in each panel of (A) and (B) are statistically different (p < 0.05). (C) Representative histograms of flow cytometry about changes in Gli1 protein expression by bone marrow LKS cells 24 hours following i.v. challenges. C/S: i.p. saline plus i.v. saline; A/S: i.p. alcohol plus i.v. saline; C/E: i.p. saline plus i.v. E. coli; A/E: i.p. alcohol plus i.v. E. coli.
Change in LKS Cell Activity of Proliferation
Using the in vivo BrdU incorporation technique, we determined the proliferative activity of LKS cells in relation to their expression of Gli1. As shown in Fig. 4A, systemic E. coli infection caused a substantial increase in the number of BrdU+ (proliferating) Gli1-expressing LKS cells as compared with the baseline level of these cells in animals administered with i.v. saline (p < 0.05). Interestingly, this bacteremia-induced enhancement of proliferation was much weaker in Gli1-negative LKS cells (Fig. 4B) than that in Gli1-positive LKS cells. Alcohol intoxication did not change the baseline extent of BrdU incorporation into LKS cells regardless of their status of Gli1 expression. However, the bacteremia-induced enhancement of BrdU incorporation into LKS cells with or without expression of Gli1 was suppressed by acute alcohol intoxication (p < 0.05). Since both Gli1 expression and BrdU incorporation in marrow LKS cells were significantly elevated following systemic E. coli infection and acute alcohol intoxication suppressed both of these increases, we further determined the relationship between changes in Gli1 expression and BrdU incorporation in marrow LKS cells. As shown in Fig. 4C, linear regression and correlation analysis showed that the extent of BrdU incorporation was positively correlated with the level of Gli1 expression in LKS cells in the bone marrow (r = 0.987, p = 0.000).
Fig. 4.
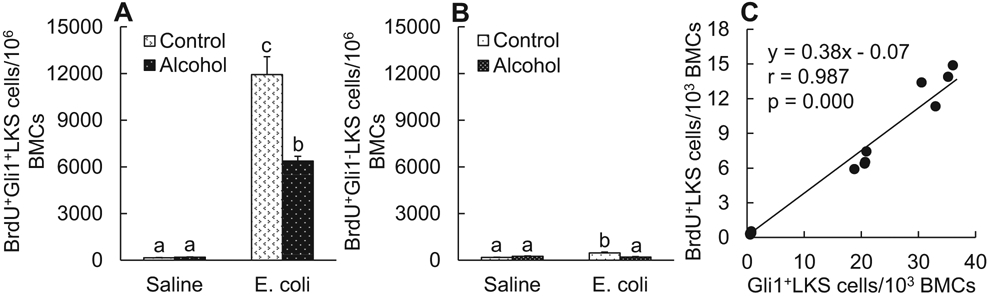
(A) and (B) BrdU incorporation into bone marrow LKS cells 24 hours following i.v. challenge with E. coli. Values are mean ± SEM. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli. i.v. E. coli. Values are mean ± SEM. N = 4 in each group. Bars with different letters in each panel are statistically different (p < 0.05). (C) Correlation between BrdU incorporation and Gli1 expression in LKS cell population 24 hours following i.v. challenge with E. coli or saline in the absence and presence of acute alcohol intoxication.
Change in Bone Marrow Pools of Gli1-Expressing LKS Cells and Total LKS Cells
Enhanced proliferation of LKS cells contributes to increase in marrow LKS cell pool during the granulopoietic response to serious infection (Shi et al., 2013; Zhang et al., 2008b, 2009). Since expression of Gli1 was positively correlated with the proliferation of LKS cells in the bone marrow, we determined the change in marrow pools of Gli1-expressing LKS cells and total LKS cells following systemic E. coli infection as well as how alcohol intoxication would affect it. As shown in Fig. 5A, the marrow pool of Gli1-expressing LKS cells expanded substantially at 24 hours post-systemic E. coli infection as compared to its baseline size in mice receiving i.v. saline (p < 0.05). Acute alcohol intoxication inhibited this increase in marrow pool of Gli1-expressing LKS cells in response to systemic E. coli infection (p < 0.05). The substantial increase in Gli1-expressing LKS cell subset apparently linked to the change in the number of total LKS cells in the bone marrow. The marrow pool of total LKS cells was also markedly expanded following E. coli infection in comparison with its baseline level (p < 0.05. Fig. 5B). Similarly, this bacteremia-induced expansion of marrow total LKS cell population was inhibited in mice with acute alcohol intoxication (p < 0.05). Alcohol intoxication did not show any effect on the baseline level of both Gli1-expressing LKS cell and total LKS cell populations in the bone marrow of mice receiving i.v. saline (Fig. 5A,B). Figure 5C shows representative plots of flow cytometry for changes in marrow total LKS cell population following systemic E. coli infection in the absence and presence of acute alcohol intoxication.
Fig. 5.
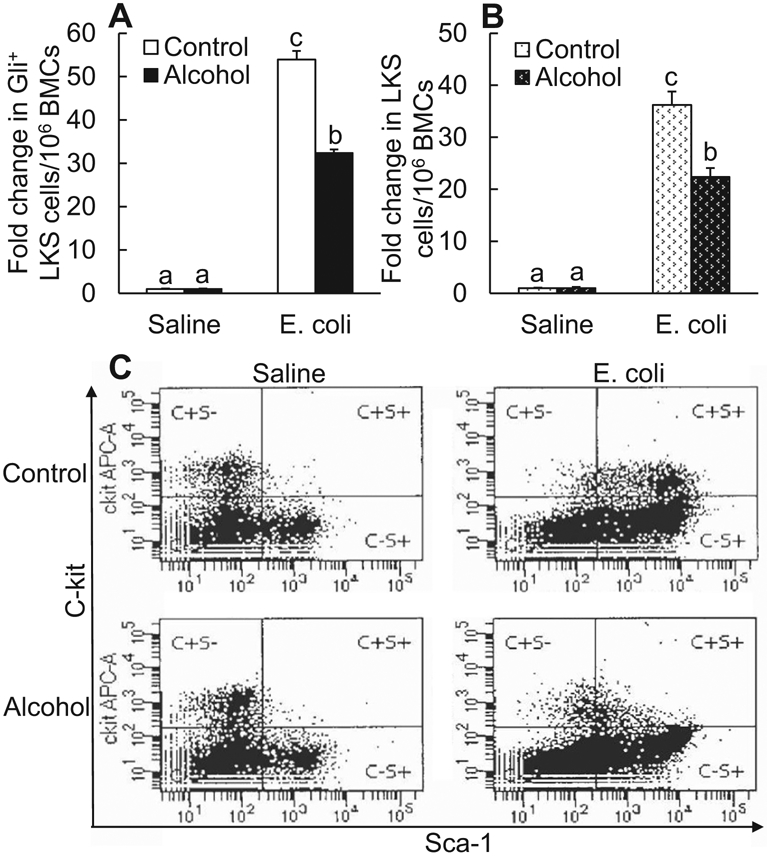
Acute alcohol intoxication suppressed increase in the number of Gli1+ LKS cells (A N = 4 in each group) and total LKS cells (B N = 10 in each group) in the bone marrow 24 hours following i.v. challenge with E. coli. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli: i.v. E. coli. Values are mean ± SEM. Bars with different letters in each panel of (A) and (B) are statistically different (p < 0.05). (C) Representative plots of flow cytometry about changes in bone marrow LKS cells.
Alcohol Intoxication Diminished Marrow Reserve of Granulocytes Following E. coli Infection
Bone marrow houses a giant compartment for storing the majority of granulocytes in the body (Furze and Rankin, 2008; Strydom and Rankin, 2013; Summers et al., 2010). In response to serious bacterial infection, granulocyte release from the marrow storage compartment into the systemic circulation is substantially increased in order to reinforce host defense against invading pathogens (Furze and Rankin, 2008; Strydom and Rankin, 2013). Activation of LKS cells and increase in the LKS cell population occur in concert with programming and/or reprogramming of these primitive hematopoietic precursors to enhance their commitment to granulocyte lineage development in the bone marrow (Shi et al., 2018; Zhang et al., 2008b, 2009), which secures producing adequate amount of granulocytes for defending the infected host. As shown in Fig. 6A, the total amount of granulocytes baring Gr1 surface marker in the bone marrow remained stable 24 hours following systemic E. coli infection. Acute alcohol intoxication did not affect the capacity of marrow storage of granulocytes in mice receiving i.v. saline. However, a marked decrease in the marrow reserve of granulocytes occurred 24 hours following systemic E. coli infection in mice with acute alcohol intoxication (p < 0.05).
Fig. 6.
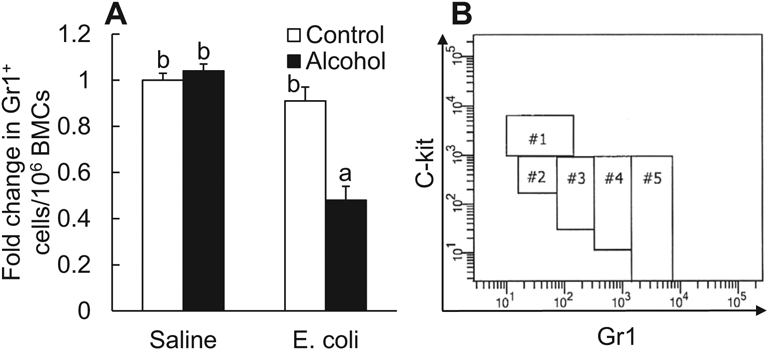
(A) Acute alcohol intoxication decreased the number of total Gr1+ cells in the bone marrow 24 hours following i.v. challenge with E. coli. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli: i.v. E. coli. Values are mean ± SEM. N = 6 in each group. Bars with different letters in the panel are statistically different (p < 0.05). (B) Gating strategy of flow cytometry for analyzing cell subsets along granulocyte lineage development.
Alcohol Intoxication Impaired Granulocyte Development in the Bone Marrow Following E. coli Infection
In the adult bone marrow, granulocyte development from primitive hematopoietic precursors passes through multiple steps of differentiation and maturation, during which cell surface expression of Gr1 increases with a gradual loss of c-kit expression (Satake et al., 2012). Based on these characteristic changes, a flow cytometric gating strategy has been developed for analyzing cells at different stages along the granulocyte lineage development. BMC subpopulations without the potential to give rise to granulocytes were removed from consideration by serial dump-gating processes. The remaining cells along the granulocyte lineage were plotted with their surface level of Gr1 against that of c-kit. Accordingly, the cells were divided into 5 subsets (#1 to #5) as reported previously (Satake et al., 2012). In Fig. 6B, cells included in subsets #1, 2, and 5 were gated, respectively, with their surface marker features of c-kithigh Gr1−, c-kitint Gr1−, and c-kitlow Gr1high. The remaining cells between #2 and #5 subsets were divided into #3 and #4 subsets depending on the level of their expression of Gr1.
Figure 7A contains representative plots of flow cytometry for changes in marrow granulocyte lineage cell subsets (#1 to #5) following systemic E. coli infection in the absence and presence of acute alcohol intoxication. Quantification analysis showed that the number of cells in #1 subset did not alter among groups with different treatments(p > 0.05). Systemic E. coli infection caused a significant increase in the number of cells belonging to #2 subset compared to the baseline level in mice receiving i.v. saline (p < 0.05). Alcohol intoxication did not affect the change in #2 subset following E. coli infection (p > 0.05). The number of cells in #3 subset increased markedly following systemic E. coli infection (p < 0.05). Alcohol intoxication further increased the quantity of #3 subset cells following E. coli infection (p < 0.05). Systemic E. coli infection also caused a substantial increase in the number of cells belonging to the #4 subset (p < 0.05). However, alcohol intoxication inhibited the increase in the number of #4 subset cells following E. coli infection (p < 0.05). In contrast to changes in the quantity of cells belonging to #2 to #4 subsets, the number of cells in #5 subset reduced significantly following systemic infection with E. coli (p < 0.05). This reduction of #5 subset cells following E. coli infection was much more profound in mice with acute alcohol intoxication (p < 0.05). Acute alcohol intoxication did not alter baseline levels of cell quantity in #2 to #5 subsets in mice receiving i.v. saline (p > 0.05). Figure 7G shows changes in blood Gr1+ cell counts. Blood Gr1+ cell counts increased significantly in mice with systemic E. coli infection in comparison with the baseline level in mice receiving i.v. saline (p < 0.05). Alcohol intoxication impaired this increase in blood Gr1+ cell counts in response to E. coli infection.
Fig. 7.
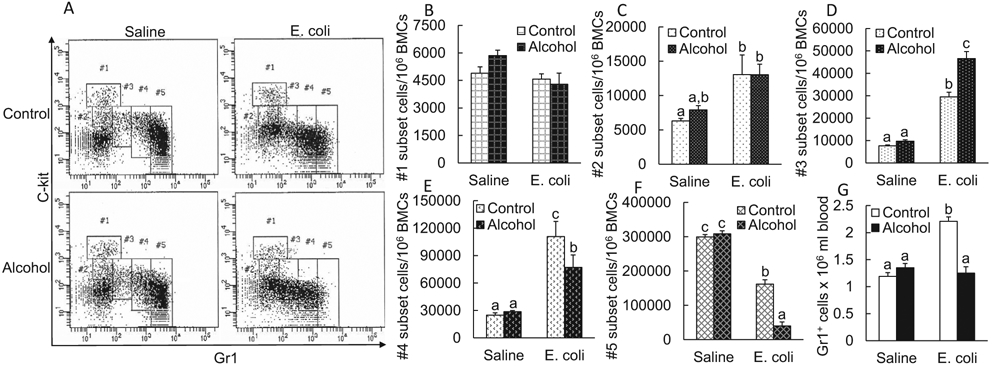
A. Representative plots of flow cytometry about change in cell subsets along granulocyte lineage development in the bone marrow 24 hours following i.v. challenge with E. coli in the absence and presence of acute alcohol intoxication. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli: i.v. E. coli. B-F Changes in cell subsets along granulocyte lineage development in the bone marrow 24 hours following i.v. challenge with E. coli in the absence and presence of acute alcohol intoxication. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli i.v. E. coli. N = 6 in each group. Values are mean ± SEM. Bars with different letters in each panel are statistically different (p < 0.05). (G) Changes in blood Gr1+ cell counts 24 hours following i.v. challenge with E. coli in the absence and presence of acute alcohol intoxication. Values are mean ± SEM. Control: i.p. saline; alcohol: i.p. alcohol; saline: i.v. saline; E. coli: i.v. E. coli. N = 6 to 8 in each group. Bars with different letters are statistically different (p < 0.05).
DISCUSSION
In the HH signaling system, 3 ligand proteins, including Sonic (SHH), Indian (IHH), and Desert (DHH) HH encoded by their genes (shh, ihh, and dhh, respectively), have been identified in mammals (Hammerschmidt et al., 1997; Perrimon, 1995). Cell expression of HH ligand is a multiple-step process. Initially, a 45-kDa precursor polypeptide is synthesized, from which, the active HH ligand composed of the 19-Kda N-terminal fragment is generated through the cholesterol-dependent autocatalytic cleavage (Beachy et al., 1997; Lee et al., 1994; Porter et al., 1995; Ryan and Chiang, 2012). This 19-Kda N-terminal fragment is then further modified by the addition of cholesterol and palmitoyl groups at its C- and N-termini, respectively (Lee et al., 2016; Murone et al., 1999; Ramsbottom and Pownall, 2016). Among 3 types of HH ligands, the best studied one is SHH (VChoy and Cheng, 2012). HH ligands are membrane-tethered before being released from HH-producing cells, as monomers and multimers or even in association with lipoproteins as well as exosomes, into the extracellular space (Lee et al., 2016; Ortmann et al., 2015; Ramsbottom and Pownall, 2016; Ryan and Chiang, 2012). SHH ligand binds to its receptor expressed on target cells to incite SHH signaling. Two vertebrate PTCH homologues, PTCH1 and PTCH2, have been identified (Lee et al., 2016; Murone et al., 1999) Engagement of ligand SHH to its receptor PTCH stops PTCH-exerted repression of Smoothened (SMO; Irvine and Copland, 2012; Murone et al., 1999; Ryan and Chiang, 2012). Activation of SMO promotes transcription of SHH target genes through the Gli transcription factor family. In the Gli family, Gli2 exists in both a full-length active form and a truncated repressor form (Hui and Angers, 2011; Sasaki et al., 1999). Activated SMO obstructs the truncating process of full-length Gli2, which empowers nuclear translocation of Gli2 to activate the transcription of downstream genes, particularly the transcription of Gli1 (Hui and Angers, 2011; Pan et al., 2009; Wang et al., 2010). Gli1 is a master transcriptional activator for expression of genes required for survival and proliferation of cells. Further, Gli1 can activate expression of Gli1 and PTCH (1 and 2) genes to provide both positive and negative feedbacks to SHH signaling, respectively (Irvine and Copland, 2012; Ryan and Chiang, 2012). Previous studies have revealed that SHH signaling is involved in the regulation of primitive hematopoietic precursor cell activity, such as promoting HSC proliferation and their myeloid differentiation (Bhardwaj et al., 2001; Merchant et al., 2010). Investigations from our group have shown that SHH signaling participates in the regulation of hematopoietic stem/progenitor cell activation during the granulopoietic response to systemic bacterial infection (Shi et al., 2018).
In this study, we observed early activation of SHH signaling in marrow hematopoietic cells during systemic infection with E. coli. This observation is in agreement with our previous investigations (Shi et al., 2018). Acute alcohol intoxication impaired the early increase in SHH expression. SP1 has been shown as a key transcription factor activating shh gene transcription (Shi et al., 2018). Activation of the Toll-like receptor 4 (TLR4)-ERK1/2 signal transduction pathway mediates expression of SP1 (Chanteux et al., 2007). Lipopolysaccharide (LPS) derived from gram-negative bacteria is the natural ligand to TLR4 for the activation of TLR4 signaling. LPS is a potent stimulator for up-regulation of SHH expression by BMCs (Shi et al., 2018). Results of our current study showed that systemic bacterial infection caused a rapid activation of ERK1/2 in BMCs. Acute alcohol intoxication exerted a profound inhibition of this rapid increase in the level of phospho-ERK1/2 in BMCs following the systemic bacterial infection. In previous studies, we have observed that excessive alcohol exposure impairs the activation of ERK1/2 signaling in marrow hematopoietic cells in murine models of bacterial infection (Melvan et al., 2012; Shi et al., 2017). Investigations conducted by other researchers have also demonstrated that acute alcohol intoxication impairs activation of ERK1/2 signaling in cells in response to injurious challenges (Li et al., 2009; Mandrekar et al., 2009). These data suggest that impaired activation of ERK1/2 signaling may serve as a mechanism underlying the inhibited up-regulation of SHH expression by marrow hematopoietic cells during systemic E. coli infection in mice acutely intoxicated with alcohol. In addition to SP1, studies have shown that other transcription factors including NF-κB and STAT6 may participate in the regulation of SHH expression (Duan et al., 2015; Wang et al., 2020). During infection and inflammation, cytokines and other endogenous mediators can signal through these transcription factors. Likewise, cytokines such as interleukin (IL)-4/IL-13, IL-1b, and IFN-γ have been reported being involved in the regulation of SHH expression in different pathological circumstances (Sherman and Zavros, 2011; Wang et al., 2020). Further investigations are warranted for clarifying the potential role of endogenous mediators in the regulation of SHH expression during systemic bacterial infection.
Bacteremia-induced increase in the generation of SHH ligands in the bone marrow would activate SHH signal transduction and Gli1 expression in SHH-responding cells. In the current study, Gli1 mRNA expression by BMCs was significantly up-regulated 24 hours following systemic E. coli infection, indicating the activation of SHH signaling in these cells. Acute alcohol intoxication suppressed the up-regulation of Gli1 mRNA expression by BMCs following systemic E. coli infection, which was consistent with the negative effect of alcohol on bacteremia-induced increase in the generation of SHH ligands in the bone marrow. Studies have shown that SHH-Gli1 signaling is active in upstream hematopoietic precursor cells (Bhardwaj et al., 2001; Shi et al., 2018). The activity of this signaling pathway reduces with differentiation of cells into the mature stage. In this study, marrow LKS cells (a cell subtype enriched with HSCs) substantially increased Gli1 expression following i.v. challenge with E. coli. It implies that these primitive hematopoietic precursor cells actively respond to the stimulation of increased SHH ligands in the marrow niche environment following systemic bacterial infection. Acute alcohol intoxication inhibited the increase in Gli1 protein expression by LKS cells following E. coli infection, indicating the impaired activation of SHH-Gli1 signaling in these upstream precursor cells in the presence of alcohol intoxication.
Activation of SHH-Gli1 signaling enhances HSC proliferation and promotes expansion of primitive hematopoietic precursor cell pool in the bone marrow (Bhardwaj et al., 2001; Trowbridge et al., 2006). One mechanism underlying the enhancement of hematopoietic precursor cell proliferation may link to the role of SHH signaling in inducing expression of D and E cyclins in SHH-responding cells (Duman-Scheel et al., 2002). D and E cyclins promote S-phase entry and progression in cell cycles. In addition, studies have shown that bone morphogenetic protein-4 (BMP-4) is involved in SHH signaling for the regulation of primitive hematopoietic cell activities (Bhardwaj et al., 2001). BMP-4 stimulates primitive hematopoietic precursor cell proliferation. Noggin is a potent inhibitor of BMP-4. SHH signaling represses cell expression of Noggin to facilitate BMP-4-mediated cell proliferation. In this study, we analyzed activation of SHH-Gli1 signaling in relation to the change in proliferative activity in primitive hematopoietic precursor cells. The number of proliferating LKS cells, as reflected by their positive BrdU incorporation, markedly increased in the bone marrow following systemic E. coli infection. The majority proliferating LKS cells are those expressing Gli1. Acute alcohol intoxication inhibited this early activation of LKS cell proliferation. Linear regression and correlation analysis showed that BrdU incorporation into LKS cells was positively correlated with the expression of Gli1 in these primitive hematopoietic precursor cells. These data suggest that alcohol intoxication inhibits the SHH-Gli1 signal transduction pathway in marrow LKS cells, which may serve as a mechanism by which alcohol impairs early proliferative activation of these primitive precursors in response to serious bacterial infection.
Enhancement of LKS cell proliferation contributes to rapid increase in the marrow pool of primitive hematopoietic precursor cells during the granulopoietic response (Zhang et al., 2008b, 2009). In our current study, sizes of both the Gli1-expressing LKS cell subpopulation and total LKS cell population in the bone marrow were substantially increased following systemic E. coli infection. This rapid expansion of marrow primitive hematopoietic precursor pool was significantly inhibited in animals with acute alcohol intoxication, which reflects the adverse effect that alcohol exerted on the granulopoietic response at the initial stage of HSC activation.
In response to bacterial infection, bone marrow increases its release of granulocytes into the systemic circulation in order to reinforce host defense against invading pathogens (Shi et al., 2019). During the process, proliferative activation of HSCs and transcriptional reprogramming of these upstream precursors for enhancing their commitment to granulocyte lineage development are critical for empowering the bone marrow to produce sufficient granulocytes. It has been reported that SHH-Gli1 signaling not only mediates primitive hematopoietic precursor proliferation, but regulates their myeloid differentiation as well (Merchant et al., 2010). In this study, we observed that along with activation of SHH-Gli1 signaling and enhancement of LKS cell proliferation, the level of total granulocytes (Gr1+ cells) in the bone marrow was able to maintain stable 24 hours following systemic E.coli infection. However, the marrow pool of total granulocytes was markedly reduced following bacteremia in mice with acute alcohol intoxication. These results suggest that alcohol-induced disruption of SHH-Gli1 signaling and impairment of primitive hematopoietic precursor cell activation impede host ability to produce adequate amount of granulocytes through initiating a normal process of granulopoietic response.
We further measured alterations in committed cell subsets (#1 to #5 subsets) along the granulocyte lineage in the bone marrow based on their surface expression of c-kit versus Gr1 using flow cytometry as reported previously (Satake et al., 2012). Predominant cell compositions in these 5 subsets are myeloblasts in #1, promyelocytes in #2, myelocytes in #3, metamyelocytes in #4, and band-to-segmented granulocytes in #5, respectively, which reflects the stepwise progression of granulopoiesis (Satake et al., 2012). In this study, terminally differentiated granulocytes associated with #5 subset constituted the majority (87.2%) of total granulocyte lineage-committed cells in control mice receiving i.v. saline. This observation supports the substantial capacity of marrow storage pool of mature granulocytes in homeostatic condition (Furze and Rankin, 2008; Strydom and Rankin, 2013). Systemic E. coli infection caused a significant reduction of cells belonging to #5 subset (45.9%) in the bone marrow, which could be resulted from the enhanced release of terminally differentiated granulocytes from the bone marrow. Concomitantly, cells in #2, #3, and #4 subsets increased markedly, indicating the activation of granulopoietic activity in the bone marrow following E. coli infection. The number of myeloblasts associated with #1 subset was not altered following i.v. challenge with E.coli, which might represent a status of dynamic equilibrium of precursor cell traveling through this typical stage of granulopoiesis in this model of septic infection. The increase in commitment of primitive hematopoietic precursors to #1 subset was apparently balanced by the acceleration for myeloblasts to exit this subset and differentiate into cells belonging to the downstream subsets. In a recent study on emergency granulopoiesis in response to Gram-positive bacterial infection, significant increases in subpopulations #1, #3, and #4 with a decrease in subpopulation #5 in the bone marrow of mice with Streptococcus pneumoniae pneumonia-derived sepsis have been reported (Paudel et al., 2019). In our current study, acute alcohol intoxication did not affect bacteremia-induced increase in cells in #2 subset. The number of cells in #3 subset was further increased in mice with alcohol intoxication following systemic E. coli infection in comparison with that in infected animals without alcohol exposure. This increase in cells of #3 subset was seemingly due to an impediment of cell further differentiation, as evidenced by the significant inhibition of increase in cells in the downstream #4 subset following systemic E. coli infection in alcohol-intoxicated mice. Bone marrow analysis of alcoholic patients during their neutropenic stage has observed that nearly all of neutrophil precursors are trapped at the early developmental stage without further maturation (Ballard, 1997). The mechanism underlying this alcohol-induced accumulation of granulopoietic cells at the #3 subset (myelocyte) stage remains to be elucidated. Further investigations following this path may clarify if the accumulation of #3 subset cells has any relationship to the defective SHH-Gli1 signaling in alcohol-intoxicated animals. Along with the impaired SHH-Gli1 signaling in HSCs and disrupted process of granulocyte development during the granulopoietic response, the number of terminally differentiated granulocytes in #5 subset was diminished in alcohol-intoxicated mice following system E. coli infection. Similar to no disruption of baseline SHH-Gli1 signaling activity in primitive hematopoietic precursor cells following alcohol exposure, acute alcohol intoxication did not affect cell distribution in all subsets of granulocyte lineage-committed hematopoietic cells in control mice receiving i.v. saline. Supported by enhancement of granulopoiesis and release of mature granulocytes from the bone marrow into circulation, blood granulocyte (Gr1+) counts were increased significantly following systemic E. coli infection. Alcohol intoxication impaired this granulopoietic response, which would compromise host immune defense. Previous studies from our group have shown that alcohol intoxication increases mortality in mice with systemic E. coli infection (Zhang et al., 2009).
ACKNOWLEDGMENTS
The research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R01AA022816 and the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM132449. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by Watanakunakorn Chair Endowment Fund. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Ballard HS (1997) The hematological complications of alcoholism. Alcohol Health Res World 21:42–52. [PMC free article] [PubMed] [Google Scholar]
- Beachy PA, Cooper MK, Young KE, von Kessler DP, Park WJ, Hall TM, Leahy DJ, Porter JA (1997) Multiple roles of cholesterol in hedgehog protein biogenesis and signaling. Spring Harb Symp Quant Biol 62:191–204. [PubMed] [Google Scholar]
- Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M (2001) Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol 2:172–180. [DOI] [PubMed] [Google Scholar]
- Chanteux H, Guisset AC, Pilette C, Sibille Y (2007) LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir Res 8:71 10.1186/1465-9921-8-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R (1998) Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res 22:1927–1942. [PubMed] [Google Scholar]
- Duan Z, Wang H, Zhao D, Ji X, Song M, Yang X, Cui W (2015) Cooperatively transcriptional and epigenetic regulation of sonic hedgehog overexpression drives malignant potential of breast cancer. Cancer Sci 106:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel M, Weng L, Xin S, Du W (2002) Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature 417:299–304. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH (2001) Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neuroectodermal cell fate in the mouse embryo. Development 128:1717–1730. [DOI] [PubMed] [Google Scholar]
- Furze RC, Rankin SM (2008) Neutrophil mobilization and clearance in the bone marrow. Immunology 125:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gering M, Patient R (2005) Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell 8:389–400. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Brook A, McMahon AP (1997) The world according to hedgehog. Trends Genet 13:14–21. [DOI] [PubMed] [Google Scholar]
- Hui CC, Angers S (2011) Gli proteins in development and disease. Annu Rev Cell Dev Biol 27:513–537. [DOI] [PubMed] [Google Scholar]
- Irvine DA, Copland M (2012) Targeting hedgehog in hematologic malignancy. Blood 119:2196–2204. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA (1994) Autoproteolysis in hedgehog protein biogenesis. Science 266:1528–1537. [DOI] [PubMed] [Google Scholar]
- Lee RT, Zhao Z, Ingham PW (2016) Hedgehog signaling. Development 143:367–372. [DOI] [PubMed] [Google Scholar]
- Li X, Chaudry IH, Choudhry MA (2009) ERK and not p38 pathway is required for IL-12 restoration of T cell IL-2 and IFN-gamma in a rodent model of alcohol intoxication and burn injury. J Immunol 183:3955–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor RR, Loubia DB (1997) Alcohol and infection. Curr Clin Top Infect Dis 17:291–315. [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G (2009) The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol 183:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz MG, Boettcher S (2014) Emergency granulopoiesis. Nat Rev Immunol 14:302–314. [DOI] [PubMed] [Google Scholar]
- Melvan JN, Siggins RW, Bagby GJ, Stanford WL, Welsh DA, Nelson S, Zhang P (2011) Suppression of the stem cell antigen-1 response and granulocyte lineage expansion by alcohol during septicemia. Crit Care Med 39:2121–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvan JN, Siggins RW, Stanford WL, Porretta C, Nelson S, Bagby GJ, Zhang P (2012) Alcohol impairs the myeloid proliferative response to bacteremia in mice by inhibiting the stem cell antigen-1/ERK pathway. J Immunol 188:1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant A, Joseph G, Wang Q, Brennan S, Matsui W (2010) Gli1 regulates the proliferation and differentiation of HSCs and myeloid progenitors. Blood 115:2391–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, McClair VL (2012) Epidemiology of substance use disorders. Hum Genet 131:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murone M, Rosenthal A, de Sauvage FJ (1999) Hedgehog signal transduction: from flies to vertebrates. Exp Cell Res 253:25–33. [DOI] [PubMed] [Google Scholar]
- Ortmann C, Pickhinke U, Exner S, Ohlig S, Lawrence R, Jboor H, Dreier R, Grobe K (2015) Sonic hedgehog processing and release are regulated by glypican heparan sulfate proteoglycans. J Cell Sci 128:2374–2385. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wang C, Wang B (2009) Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol 326:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel S, Baral P, Ghimire L, Bergeron S, Jin L, DeCorte JA, Le JT, Cai S, Jeyaseelan S (2019) CXCL1 regulates neutrophil homeostasis in pneumonia-derived sepsis caused by Streptococcus pneumoniae serotype 3. Blood 133:1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlino CA, Rimland D (1985) Alcoholism, leukopenia, and pneumococcal sepsis. Am Rev Respir Dis 132:757–760. [DOI] [PubMed] [Google Scholar]
- Perrimon N (1995) Hedgehog and beyond. Cell 80:517–520. [DOI] [PubMed] [Google Scholar]
- Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, Beachy PA (1995) The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature 374:363–366. [DOI] [PubMed] [Google Scholar]
- Ramsbottom SA, Pownall ME (2016) Regulation of hedgehog signalling inside and outside the cell. J Dev Biol 4:23 10.3390/jdb4030023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KE, Chiang C (2012) Hedgehog secretion and signal transduction in vertebrates. J Biol Chem 287:17905–17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H (1999) Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126:3915–3924. [DOI] [PubMed] [Google Scholar]
- Satake S, Hirai H, Hayashi Y, Shime N, Tamura A, Yao H, Yoshioka S, Miura Y, Inaba T, Fujita N, Ashihara E, Imanishi J, Sawa T, Maekawa T (2012) C/EBPβ is involved in the amplification of early granulocyte precursors during candidemia-induced “emergency” granulopoiesis. J Immunol 189:4546–4555. [DOI] [PubMed] [Google Scholar]
- Sato Y, van Eeden SF, English D, Hogg JC (1998) Bacteremic pneumococcal pneumonia: bone marrow release and pulmonary sequestration of neutrophils. Crit Care Med 26:501–509. [DOI] [PubMed] [Google Scholar]
- Shahbazian LM, Quinton LJ, Bagby GJ, Nelson S, Wang G, Zhang P (2004) Escherichia coli pneumonia enhances granulopoiesis and the mobilization of myeloid progenitor cells into the systemic circulation. Crit Care Med 32:1740–1746. [DOI] [PubMed] [Google Scholar]
- Sherman AE, Zavros Y (2011) Role of Sonic Hedgehog signaling during progression from inflammation to cancer in the stomach. World J Gastroin-test Pathophysiol. 2:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, DeLucia AL, Bao J, Zhang P (2019) Alcohol abuse and disorder of granulopoiesis. Pharmacol Ther 198:206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Lin YP, Gao B, Zhang P (2017) Impairment of hematopoietic precursor cell activation during the granulopoietic response to bacteremia in mice with chronic-plus-binge alcohol administration. Infect Immun 85:e00369–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Siggins RW, Stanford WL, Melvan JN, Basson MD, Zhang P (2013) Toll-like receptor 4/stem cell antigen 1 signaling promotes hematopoietic precursor cell commitment to granulocyte development during the granulopoietic response to Escherichia coli bacteremia. Infect Immun 81:2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Wei S, Simms KJ, Cumpston DN, Ewing TJ, , Zhang P (2018) Sonic hedgehog signaling regulates hematopoietic stem/progenitor cell activation during the granulopoietic response to systemic bacterial infection. Front Immunol 9:349 10.3389/fimmu.2018.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins RW, Melvan JN, Welsh DA, Bagby GJ, Nelson S, Zhang P (2011) Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J Immunol 186:4306–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom N, Rankin SM (2013) Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun 5:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER (2010) Neutrophil kinetics in health and disease. Trends Immunol 31:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima T, Wiggs B, English D, Hogg JC, van Eeden SF (1996) Polymorphonuclear leukocyte transit times in bone marrow during streptococcal pneumonia. Am J Physiol 271:L587–L592. [DOI] [PubMed] [Google Scholar]
- Trowbridge JJ, Scott MP, Bhatia M (2006) Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci USA 103:14134–14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kondo M, Kelsoe G (2005) Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med 201:1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VChoy SW, Cheng SH (2012) Hedgehog signaling. Itam Horm 88:1–23. [DOI] [PubMed] [Google Scholar]
- Villavicencio EH, Walterhouse DO, Iannaccone PM (2000) The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet 67:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Pan Y, Wang B (2010) Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development 137:2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu C, Ji J, Cai Y, Shu Y, Chao Y, Wu X, Zou C, Wu X, Tang L (2020) IL-4/IL-13 upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling. Am J Physiol Lung Cell Mol Physiol 318: L888–L899. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Wilkinson AC, Nakauchi H (2018) Changing concepts in hematopoietic stem cells. Science 362:895–896. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, Raasch CE, Nelson S (2008a) Alcohol abuse, immunosuppression, and pulmonary infection. Curr Drug Abuse Rev 1:56–67. [DOI] [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Happel KI, Summer WR, Nelson S (2002) Pulmonary host defenses and alcohol. Front Biosci 7:d1314–d1330. [DOI] [PubMed] [Google Scholar]
- Zhang P, Nelson S, Bagby GJ, Siggins R, Shellito JE, Welsh DA (2008b) The lineage-c-kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells 26:1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Welsh DA, Siggins RW II, Bagby GJ, Raasch CE, Happel KI, Nelson S (2009) Acute alcohol intoxication inhibits the lineage- c-kit+ Sca-1+ cell response to Escherichia coli bacteremia. J Immunol 182:1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]


