Abstract
BACKGROUND
Rituximab added to chemotherapy prolongs survival among adults with B-cell cancer. Data on its efficacy and safety in children with high-grade, mature B-cell non-Hodgkin’s lymphoma are limited.
METHODS
We conducted an open-label, international, randomized, phase 3 trial involving patients younger than 18 years of age with high-risk, mature B-cell non-Hodgkin’s lymphoma (stage III with an elevated lactate dehydrogenase level or stage IV) or acute leukemia to compare the addition of six doses of rituximab to standard lymphomes malins B (LMB) chemotherapy with standard LMB chemotherapy alone. The primary end point was event-free survival. Overall survival and toxic effects were also assessed.
RESULTS
Analyses were based on 328 patients who underwent randomization (164 patients per group); 85.7% of the patients had Burkitt’s lymphoma. The median follow-up was 39.9 months. Events were observed in 10 patients in the rituximab–chemotherapy group and in 28 in the chemotherapy group. Event-free survival at 3 years was 93.9% (95% confidence interval [CI], 89.1 to 96.7) in the rituximab–chemotherapy group and 82.3% (95% CI, 75.7 to 87.5) in the chemotherapy group (hazard ratio for primary refractory disease or first occurrence of progression, relapse after response, death from any cause, or second cancer, 0.32; 95% CI, 0.15 to 0.66; one-sided P = 0.00096, which reached the significance level required for this analysis). Eight patients in the rituximab–chemotherapy group died (4 deaths were disease-related, 3 were treatment-related, and 1 was from a second cancer), as did 20 in the chemotherapy group (17 deaths were disease-related, and 3 were treatment-related) (hazard ratio, 0.36; 95% CI, 0.16 to 0.82). The incidence of acute adverse events of grade 4 or higher after prephase treatment was 33.3% in the rituximab–chemotherapy group and 24.2% in the chemotherapy group (P = 0.07); events were related mainly to febrile neutropenia and infection. Approximately twice as many patients in the rituximab–chemotherapy group as in the chemotherapy group had a low IgG level 1 year after trial inclusion.
CONCLUSIONS
Rituximab added to standard LMB chemotherapy markedly prolonged event-free survival and overall survival among children and adolescents with high-grade, high-risk, mature B-cell non-Hodgkin’s lymphoma and was associated with a higher incidence of hypogammaglobulinemia and, potentially, more episodes of infection. (Funded by the Clinical Research Hospital Program of the French Ministry of Health and others; ClinicalTrials.gov number, NCT01516580.)
CURE RATES AMONG CHILDREN AND ADolescents with high-grade, mature B-cell non-Hodgkin’s lymphoma (mainly Burkitt’s lymphoma but also diffuse large B-cell lymphoma) have dramatically improved over the past 30 years, with trials showing survival of approximately 90%.1–4 However, well-known prognostic factors such as higher stage, elevated lactate dehydrogenase (LDH) level, leukemic bone marrow, and central nervous system (CNS) involvement and treatment-related factors such as lack of early or complete response can identify patients at high risk for treatment failure.
Rituximab has shown efficacy in adults with B-cell cancers, including diffuse large B-cell lymphoma and Burkitt’s lymphoma, and is considered to be the standard of care in addition to chemotherapy in most patients with high-grade B-cell non-Hodgkin’s lymphoma. The outcome in children and adolescents with B-cell non-Hodgkin’s lymphoma receiving chemotherapy alone is superior to that in adults; therefore, the potential benefits of rituximab must be balanced against potential unexpected and severe toxic effects. Furthermore, subtypes of mature B-cell non-Hodgkin’s lymphoma differ between adults and children in terms of molecular anomalies that may confer different prognoses and sensitivities to treatments.5,6
A phase 2 trial involving children showed that rituximab was active as a single-agent therapy for high-grade, high-risk, mature B-cell non-Hodgkin’s lymphoma and could be safely added to the lymphomes malins B (LMB) chemotherapy regimen.7 Therefore, we conducted an international, randomized, phase 3 trial (Inter-B-NHL ritux 2010) to establish whether the addition of rituximab to LMB chemotherapy could improve event-free survival among children and adolescents with high-grade, high-risk, mature B-cell non-Hodgkin’s lymphoma or leukemia.
METHODS
TRIAL OVERSIGHT AND DESIGN
In this trial, we investigated the efficacy and safety of adding rituximab to a modified LMB chemotherapy regimen. This academic trial involved two international cooperative groups — the European Intergroup for Childhood Non-Hodgkin Lymphoma (EICNHL) and the Children’s Oncology Group (COG) — spanning 12 countries (see the Supplementary Appendix, available with the full text of this article at NEJM.org). The trial sponsors were Gustave Roussy (for countries in the EICNHL) and COG (for Australia, Canada, and the United States) and included a partnership with F. Hoffmann–La Roche–Genentech, which provided partial funding and provided rituximab at no cost but had no role in the design or conduct of the trial nor in the preparation of the manuscript. The manuscript was written by the authors, who vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol, available at NEJM.org.
Parents and patients (if age appropriate) signed informed consent and assent forms before enrollment. The research protocol was approved in each country by the necessary ethics and regulatory committees. An international, independent data and safety monitoring committee, which included two pediatric oncologists, one adult oncologist, and one statistician, monitored progress and interim analysis reports.
Randomization was performed separately for patients at the EICNHL and COG sites. For patients at the EICHNL sites, randomization was performed centrally at Gustave Roussy with the use of a minimization algorithm that accounted for histologic features (large-cell or non–large-cell), therapeutic group (B, C1, C3; defined in the next subsection), and the national group. To avoid predictability, a probability of 0.80 to assign the treatment that minimized imbalance was used. For patients at the COG sites, randomization was performed centrally at the COG data center, with the use of block randomization stratified according to histologic features and therapeutic group. Physicians and patients were aware of the treatment-group assignments.
PATIENTS
Eligible patients were 6 months to 18 years of age and had newly diagnosed high-grade, mature B-cell neoplasms (Burkitt’s lymphoma; diffuse large B-cell lymphoma; or high-grade, mature B-cell non-Hodgkin’s lymphoma not otherwise specified) and advanced St. Jude stage disease (stage III with an LDH level that was more than twice the institutional upper limit of the normal range or any stage IV or leukemia presentation). Patients with primary mediastinal (thymic) large B-cell lymphoma were not eligible. Pathological slides were centrally reviewed at the national level, but centralized review was not mandatory before enrollment. Detailed inclusion and exclusion criteria and initial workup data are provided in the Supplementary Appendix.
Therapeutic groups were defined as in the French–American–British (FAB)/LMB96 international study for all patients except those with blasts in cerebrospinal fluid (CSF), who were treated with high-dose methotrexate that was administered as a 24-hour infusion (group C3).3,4 The rationale for this modification was a retrospective review of previous LMB studies that showed poorer survival among these patients than among other patients in group C.8 In the FAB/LMB96 study, outcomes in patients in group B who had stage I, II, or III disease with an LDH level that was equal to or less than twice the upper limit of the normal range were excellent (event-free survival, 95%). Therefore, with regard to group B, only patients who had stage III disease and an LDH level that was more than twice the upper limit of the normal range or non-CNS stage IV disease with bone marrow involvement of less than 25% were eligible for this trial. Patients in group C1 had CSF-negative stage IV disease with bone marrow involvement of at least 25% (leukemia presentation), CNS-positive disease, or both. Group C3 included patients with CSF-positive stage IV disease.
TREATMENT
Chemotherapy was administered according to the FAB/LMB96-based protocol, with some minor modifications (Fig. S1 in the Supplementary Appendix).3,4 All the patients received prephase treatment with low-dose cyclophosphamide, vincristine, and prednisone (COP regimen). Patients in group B received four cycles of chemotherapy, which was similar to the therapy used in group B4 in the FAB/LMB96 study. For all the patients in group C, the maintenance chemotherapy was reduced to two courses, in contrast to the four maintenance cycles that were used in the FAB/LMB96 study. Information about monitoring the risk of jeopardizing efficacy with this reduction in therapy is presented in the Supplementary Appendix. Patients in group C1 received high-dose methotrexate (at a dose of 8 g per square meter of body-surface area) over a period of 4 hours, as in the FAB/LMB96 study, whereas patients in group C3 (whose disease was CSF-positive) received high-dose methotrexate (at a dose of 8 g per square meter) over a period of 24 hours. Consecutive courses were administered as soon as blood counts recovered and the patient’s condition allowed, except for the maintenance courses, which were administered at 28-day intervals.
Rituximab was administered as an intravenous infusion (at a dose of 375 mg per square meter) on day 2 before (i.e., day −2) and day 1 of each of the two induction chemotherapy courses and on day 1 of each of the two consolidation courses, for a total of six doses.7 When a course had to be postponed, rituximab was administered at the planned time in order to ensure the intensity of the therapy.
An initial response evaluation was performed on day 7 after the receipt of the COP regimen. Patients in groups B and C1 who had a reduction in the tumor size of less than 20% were switched to a more intensive therapeutic group (i.e., from group B to C1 or from group C1 to C3). Remission assessment was performed after receipt of the first consolidation course in group B and after receipt of the second consolidation course in group C. In patients with a residual mass as assessed by radiographic evaluation, an excision or biopsy for pathological review was recommended. If a biopsy was not performed, the patients were to continue receiving the assigned treatment. For patients in group B, if viable tumor cells were identified, the therapy was switched to the more intensive regimen that was given to patients in group C1. Patients in group C1 or C3 with biopsy-proven viable tumor cells after the second consolidation course (continuous infusion and high-dose cytarabine and etoposide, with or without rituximab [depending on the randomly assigned treatment group]) were considered to have primary refractory disease and were considered to have had an event; such patients discontinued the protocol therapy. No treatment decisions were to be based on the results of 18F-fluorodeoxyglucose positron-emission tomography–computed tomography.
END POINTS
The primary end point was event-free survival, which was defined as the minimum time between randomization and the detection of residual viable tumor cells after receipt of the second consolidation course of therapy (i.e., primary refractory disease), relapse, progressive disease, second cancer, or death from any cause, or the date of the last follow-up for patients who did not have any event. All events were validated by the steering committee. Details of the secondary end points (overall survival, complete remission at assessment time, toxic effects, and immune reconstitution as assessed by IgG levels) are presented in the Supplementary Appendix.
STATISTICAL ANALYSIS
We expected the 3-year event-free survival to be 84% on the basis of data from the FAB/LMB96 study.3,4 Because this trial was designed to determine the efficacy of adding rituximab to standard treatment, a one-sided test was used. At a one-sided 5% level of statistical significance and assuming randomization in a 1:1 ratio, we calculated that 72 events would need to be observed in order for the trial to have 90% power to detect a hazard ratio of 0.50 (on the basis of an expected 3-year event-free survival of 84% in the chemotherapy group and 92% in the rituximab–chemotherapy group). It was estimated that 600 patients would need to undergo randomization in order for 72 events to be observed.
Interim analyses for efficacy with the use of the Lan–DeMets alpha-spending function approach applied to an O’Brien–Fleming boundary, truncated at 3 SD, were planned.9 The first interim analysis was planned to take place when one third of the total expected events had occurred; the subsequent interim analyses were expected to occur yearly after that.
Event-free survival and overall survival were estimated by means of the Kaplan–Meier method, and 95% confidence intervals of yearly rates were estimated by the Rothman method. Hazard ratios with adjustment for therapeutic group, histologic type, and national group were estimated by the Cox model. Subgroup analyses according to three baseline characteristics (age, histologic features, and therapeutic group) were prespecified.
RESULTS
INTERIM ANALYSES
The first interim analysis was based on 27 events in 310 patients, which corresponded to 37.5% of the expected events and a nominal alpha error of 0.00137. The median follow-up was 11.5 months. Event-free survival at 1 year was 94.2% (95% confidence interval [CI], 88.5 to 97.2) in the rituximab–chemotherapy group and 81.5% (95% CI, 73.0 to 87.8) in the chemotherapy group (hazard ratio for event [defined as primary refractory disease or first occurrence of progression, relapse after response, death from any cause, or second cancer], 0.33; 95% CI, 0.14 to 0.79).10 The full results of this interim analysis are presented in the Supplementary Appendix.
After the first interim analysis, randomization was stopped for efficacy in November 2015 on the recommendation of the independent data and safety monitoring committee. An additional 120 patients were subsequently enrolled and treated with rituximab and chemotherapy (single-group cohort) for prespecified secondary aims; these patients are not included in the analyses presented here. The current analyses are based on 38 events, corresponding to 52.8% of the expected events and a nominal alpha error of 0.00562. The cumulative alpha error is 0.00699.
ANALYSIS SETS
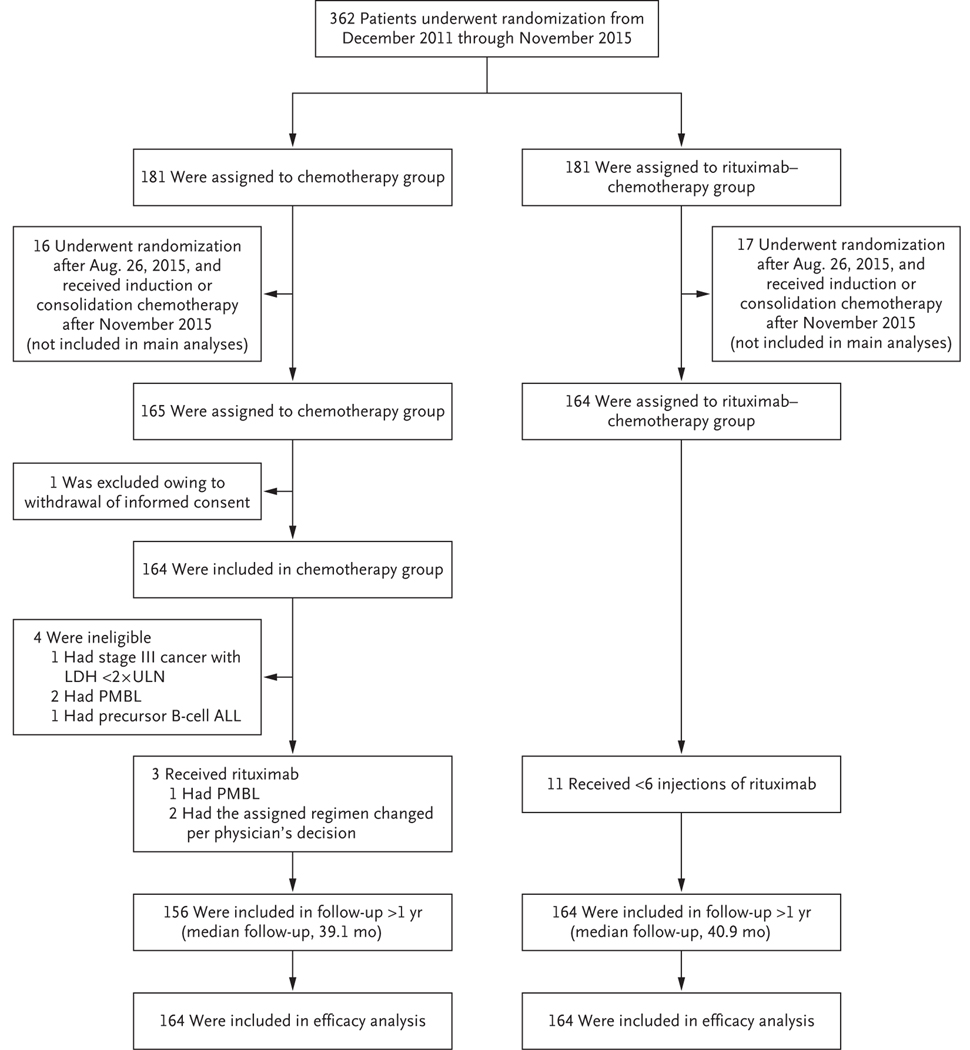
From December 2011 through November 2015, a total of 362 patients were enrolled at 176 centers. Data from 1 patient were removed owing to withdrawal of consent. The 33 patients who were still receiving induction or consolidation chemotherapy after the closure of randomization in November 2015 were recommended to receive rituximab and chemotherapy regardless of the randomly assigned group. These patients were not included in the primary analysis but were included in the sensitivity analysis. Thus, the main analyses included 328 patients who had undergone randomization until August 26, 2015 (Fig. 1), including 4 patients who were declared to be ineligible: 3 patients after central pathological review (1 patient had precursor B-cell acute lymphoblastic leukemia and 2 had primary mediastinal B-cell lymphoma) and 1 patient who had stage III disease and an LDH level that was less than two times the upper limit of the normal range.
Figure 1. Randomization, Treatment, and Follow-up of the Patients.
The 33 patients who were still receiving induction or consolidation chemotherapy after the closure of randomization in November 2015 were recommended to receive rituximab and chemotherapy regardless of the randomly assigned group; these patients were not included in the main analyses. ALL denotes acute lymphoblastic leukemia, LDH lactate dehydrogenase, PMBL primary mediastinal B-cell lymphoma, and ULN upper limit of the normal range.
PATIENTS AND TREATMENT
The characteristics of the patients at baseline were well balanced between the two treatment groups (Table 1). A total of 85.7% of the patients had Burkitt’s lymphoma. Three patients in the chemotherapy group received rituximab (2 owing to physicians’ decisions and 1 because of a diagnosis of primary mediastinal B-cell lymphoma). All the patients in the rituximab–chemotherapy group received rituximab, with 11 patients (7%) receiving fewer than six doses (Table S1).
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Chemotherapy Group (N = 164) | Rituximab–Chemotherapy Group (N = 164) |
|---|---|---|
| Male sex — no. (%) | 137 (83.5) | 135 (82.3) |
| Age | ||
| Mean — yr | 8.6±4.4 | 9.2±4.0 |
| Range — yr | 2–17 | 2–17 |
| Distribution — no. (%) | ||
| <6 yr | 56 (34.1) | 44 (26.8) |
| 6 to <12 yr | 67 (40.9) | 73 (44.5) |
| 12 to <15 yr | 22 (13.4) | 30 (18.3) |
| ≥15 yr | 19 (11.6) | 17 (10.4) |
| Pathological diagnosis† | ||
| Burkitt’s lymphoma | 142 (86.6) | 139 (84.8) |
| Diffuse large B-cell lymphoma | 12 (7.3) | 19 (11.6) |
| High-grade B-cell lymphoma, not otherwise specified | 7 (4.3) | 6 (3.7) |
| Primary mediastinal B-cell lymphoma‡ | 2 (1.2) | 0 |
| Precursor B-cell acute lymphoblastic leukemia‡ | 1 (0.6) | 0 |
| Prognosis group | ||
| Group B, low risk‡ | 1 (0.6) | 0 |
| Group B, high risk | 82 (50.0) | 81 (49.4) |
| Group C1, without CSF blasts | 63 (38.4) | 65 (39.6) |
| Group C3, with CSF blasts | 16 (9.8) | 18 (11.0) |
| Primary mediastinal B-cell lymphoma‡ | 2 (1.2) | 0 |
| Murphy stage | ||
| Stage III | 73 (44.5) | 70 (42.7) |
| Stage IV | 32 (19.5) | 32 (19.5) |
| Leukemic disease, mature B-cell acute leukemia | 57 (34.8) | 62 (37.8) |
| Primary mediastinal B-cell lymphoma‡ | 2 (1.2) | 0 |
| Central nervous system involvement | ||
| No | 120 (73.2) | 119 (72.6) |
| Yes | 44 (26.8) | 45 (27.4) |
| Lactate dehydrogenase | ||
| ≤2× ULN | 18 (11.0) | 17 (10.4) |
| >2× ULN | 146 (89.0) | 147 (89.6) |
| Primary site or type | ||
| Thorax | 6 (3.7) | 6 (3.7) |
| Abdomen or retroperitoneum | 95 (57.9) | 96 (58.5) |
| Head and neck, except skin and nodes | 9 (5.5) | 20 (12.2) |
| Peripheral lymph node | 6 (3.7) | 2 (1.2) |
| Cerebral lymphoma | 3 (1.8) | 2 (1.2) |
| Other tumor site | 11 (6.7) | 8 (4.9) |
| Clinical presentation of leukemia disease | 34 (20.7) | 30 (18.3) |
Percentages may not total 100 because of rounding. CSF denotes cerebrospinal fluid, and ULN upper limit of the normal range (according to institutional values).
These data were obtained from national pathological review in 235 patients (72%) or from local pathological diagnosis.
Patients with these conditions were not eligible for the trial. On central review, these patients were found to be ineligible, but they were included in the intention-to-treat analyses.
EFFICACY
The median follow-up was 39.9 months overall, with follow-up of 40.9 months (interquartile range, 35.1 to 48.9) in the rituximab–chemotherapy group and 39.1 months (interquartile range, 34.3 to 49.0) in the chemotherapy group. Data regarding the response after the administration of the COP regimen and remission at the time of assessment are shown in Table S2. In the primary analysis, there were 38 events: 10 in the rituximab–chemotherapy group and 28 in the chemotherapy group (Table 2). Six patients (three in each group) died from toxic events. Four patients (two in each group) had primary refractory disease. The major between-group difference in events was observed in relapse or progression, which occurred in 3 patients in the rituximab–chemotherapy group and in 23 in the chemotherapy group; all events of relapse or progression occurred within 9 months after randomization. A total of 18 of these patients subsequently died: all 3 of the patients in the rituximab–chemotherapy group and 15 of the 23 patients in the chemotherapy group. A second cancer developed in 2 patients in the rituximab–chemotherapy group: 1 patient had melanoma, and 1 had fatal histiocytic sarcoma with a t(8;14) IGH–MYC anomaly identical to the primary lymphoma. In total, 8 patients in the rituximab–chemotherapy group died (4 deaths were disease-related, 3 were treatment-related, and 1 was from a second cancer), as did 20 in the chemotherapy group (17 deaths were disease-related, and 3 were treatment-related).
Table 2.
Number and Type of Events and Number of Deaths.*
| Variable | Chemotherapy Group | Rituximab–Chemotherapy Group | ||||
|---|---|---|---|---|---|---|
| No. of Patients | No. of Patients with Event | No. of Deaths | No. of Patients | No. of Patients with Event | No. of Deaths | |
| All patients | 164 | 164 | ||||
| Death from toxic event | 3 | 3 | 3 | 3 | ||
| Primary refractory disease, with viable cells after CYVE2 therapy | 2 | 2 | 2 | 1 | ||
| Relapse or progression | 23 | 15 | 3 | 3 | ||
| Second cancer | 0 | 0 | 2 | 1 | ||
| Total no. of events | 28 | — | 10 | — | ||
| Total no. of deaths | — | 20 | — | 8 | ||
| Group B | 84† | 81 | ||||
| Death from toxic event | 2 | 2‡ | 0 | 0 | ||
| Primary refractory disease, with viable cells after CYVE2 therapy | 1 | 1 | 1 | 0 | ||
| Relapse or progression | 11 | 6 | 3 | 3 | ||
| Second cancer | 0 | 0 | 1§ | 0 | ||
| Total no. of events | 14 | — | 5 | — | ||
| Total no. of deaths | — | 9 | — | 3 | ||
| Group C1 | 64¶ | 65 | ||||
| Death from toxic event | 1 | 1 | 3 | 3‖ | ||
| Primary refractory disease, with viable cells after CYVE2 therapy | 1 | 1 | 1 | 1 | ||
| Relapse or progression | 8 | 5 | 0 | 0 | ||
| Second cancer | 0 | 0 | 0 | 0 | ||
| Total no. of events | 10 | — | 4 | — | ||
| Total no. of deaths | — | 7 | — | 4 | ||
| Group C3 | 16 | 18 | ||||
| Death from toxic event | 0 | 0 | 0 | 0 | ||
| Primary refractory disease, with viable cells after CYVE2 therapy | 0 | 0 | 0 | 0 | ||
| Relapse or progression | 4 | 4 | 0 | 0 | ||
| Second cancer | 0 | 0 | 1 | 1** | ||
| Total no. of events | 4 | — | 1 | — | ||
| Total no. of deaths | — | 4 | — | 1 | ||
CYVE2 denotes the second course of continuous infusion and high-dose cytarabine and etoposide.
One patient in the low-risk group B (who did not have an event) was counted in group B.
One patient who was receiving group C1 chemotherapy died after receipt of the first course of therapy with cyclophosphamide, vincristine, prednisone, doxorubicin, and methotrexate.
One patient, a 14-year-old girl, received a diagnosis of melanoma that had developed on a congenital skin nevus 5 months after the diagnosis of Burkitt’s lymphoma.
One patient with primary mediastinal B-cell lymphoma (who did not have an event) was counted in group B, and one (who had disease progression) was counted in group C1.
One patient switched to group C3 after receipt of the COP regimen; the death occurred after receipt of the second course of group C3 chemotherapy (cyclophosphamide, vincristine, prednisone, doxorubicin, and methotrexate).
One patient, a 6-year-old boy, had fatal histiocytic sarcoma with an identical t(8;14) IGH–MYC anomaly, which had been diagnosed when he was 16.6 months of age.
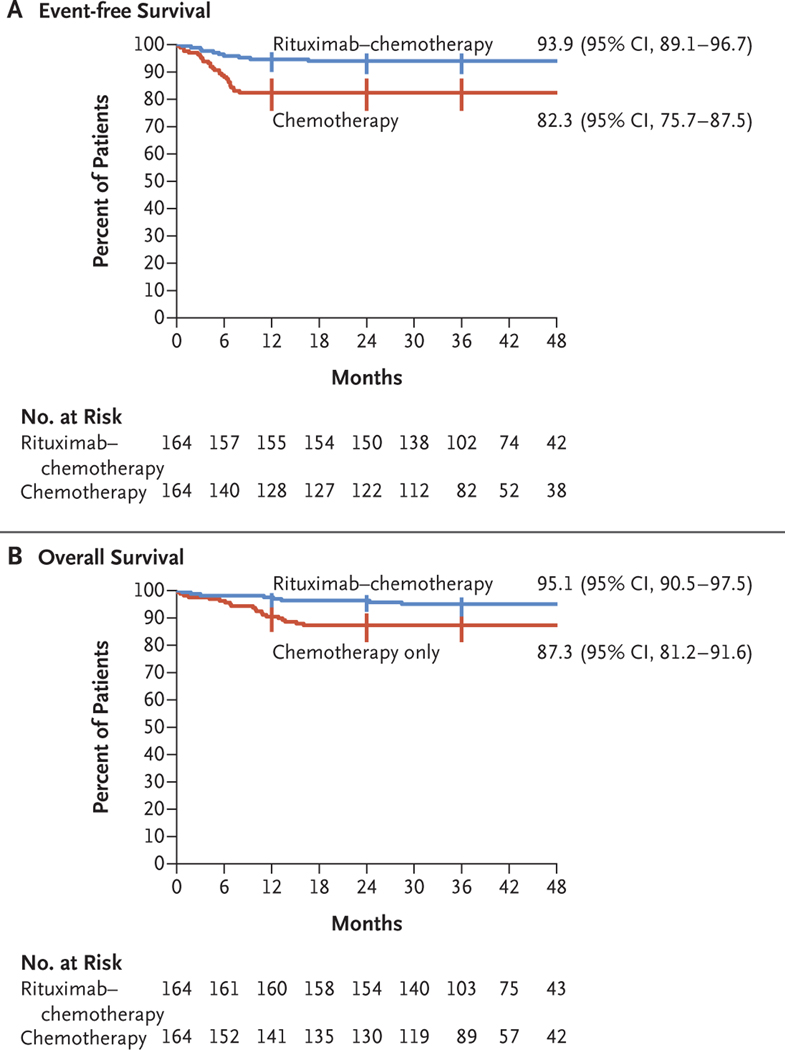
Event-free survival at 3 years was 93.9% (95% CI, 89.1 to 96.7) in the rituximab–chemotherapy group and 82.3% (95% CI, 75.7 to 87.5) in the chemotherapy group (Fig. 2A). In the analysis of event-free survival, the hazard ratio for an event was 0.32 (95% CI, 0.15 to 0.66; one-sided P = 0.00096, which reached the significance level required for this second analysis). Overall survival at 3 years was 95.1% (95% CI, 90.5 to 97.5) in the rituximab–chemotherapy group and 87.3% (95% CI, 81.2 to 91.6) in the chemotherapy group (hazard ratio for death, 0.36; 95% CI, 0.16 to 0.82) (Fig. 2B).
Figure 2. Event-free Survival and Overall Survival, According to Treatment Group.
Shown are Kaplan–Meier estimates of event-free survival (i.e., freedom from primary refractory disease or first occurrence of progression, relapse after response, death from any cause, or second cancer) and of overall survival. Vertical bars represent the Rothman 95% confidence intervals; point estimates of 36-month event-free survival and overall survival with 95% confidence intervals are shown.
The sensitivity analysis, which involved 361 patients (including those in the period between August 26, 2015, and November 2015, who were recommended to receive rituximab and chemotherapy regardless of the randomly assigned group), showed similar results. Event-free survival at 3 years was 92.8% in the rituximab–chemotherapy group and 83.4% in the chemotherapy group (hazard ratio for an event, 0.40; 95% CI, 0.21 to 0.78). Overall survival at 3 years was 94.3% in the rituximab–chemotherapy group and 88.5% in the chemotherapy group (hazard ratio for death, 0.45; 95% CI, 0.21 to 0.97). The benefit of rituximab was similar across subgroups defined according to age, histologic features, and therapeutic group (Fig. S2).
ADVERSE EVENTS
The incidence of rituximab infusion–related reactions was 33.3% during the first infusion (with 4.3% of the patients having a grade 3 event); the incidence decreased to 3.7 to 9.6% during subsequent infusions (with 1.0 to 2.1% of the patients having a grade 3 event). No immediate infusion-related grade 4 toxic effect occurred. Treatment-related toxic effects of grade 4 or higher were observed in 35.2% of the patients overall, including in 28.9% of the patients after prephase treatment with the COP regimen (Table 3). The most frequent adverse events after prephase treatment were febrile neutropenia (in 91.7% of the patients), infection (in 54.0%), and stomatitis (in 77.5%).
Table 3.
Acute Adverse Events.*
| Event | All Patients (N = 315) | Chemotherapy Group (N = 153) | Rituximab–Chemotherapy Group (N = 162) | P Value |
|---|---|---|---|---|
| no. of patients (%) | ||||
| During all therapy | ||||
| ≥1 Adverse event | 306 (97.1) | 148 (96.7) | 158 (97.5) | |
| ≥1 Adverse event of grade ≥4 | 111 (35.2) | 50 (32.7) | 61 (37.7) | 0.36 |
| During COP prephase treatment | ||||
| ≥1 Adverse event | 63 (20.0) | 31 (20.3) | 32 (19.8) | |
| ≥1 Adverse event of grade ≥4 | 27 (8.6) | 17 (11.1) | 10 (6.2) | 0.12 |
| After COP prephase treatment | ||||
| ≥1 Adverse event | 303 (96.2) | 147 (96.1) | 156 (96.3) | |
| ≥1 Adverse event of grade ≥4 | 91 (28.9) | 37 (24.2) | 54 (33.3) | 0.07 |
| Most frequent adverse events after COP prephase treatment | ||||
| Febrile neutropenia | 289 (91.7) | 139 (90.8) | 150 (92.6) | |
| Grade 3 | 260 (82.5) | 129 (84.3) | 131 (80.9) | |
| Grade ≥4 | 29 (9.2) | 10 (6.5) | 19 (11.7) | 0.11 |
| Stomatitis | 244 (77.5) | 115 (75.2) | 129 (79.6) | |
| Grade 3 | 224 (71.1) | 108 (70.6) | 116 (71.6) | |
| Grade ≥4 | 20 (6.3) | 7 (4.6) | 13 (8.0) | 0.21 |
| Enteritis | 63 (20.0) | 24 (15.7) | 39 (24.1) | |
| Grade 3 | 62 (19.7) | 24 (15.7) | 38 (23.5) | |
| Grade ≥4 | 1 (0.3) | 0 | 1 (0.6) | 1.00 |
| Infection | 170 (54.0) | 75 (49.0) | 95 (58.6) | |
| Grade 3 | 123 (39.0) | 58 (37.9) | 65 (40.1) | |
| Grade ≥4 | 47 (14.9) | 17 (11.1) | 30 (18.5) | 0.07 |
| Main types of infection | ||||
| Sepsis | 45 (14.3) | 17 (11.1) | 28 (17.3) | |
| Central venous catheter–related infection | 38 (12.1) | 17 (11.1) | 21 (13.0) | |
| Lung infection | 32 (10.2) | 13 (8.5) | 19 (11.7) | |
| Enterocolitis infection | 32 (10.2) | 18 (11.8) | 14 (8.6) | |
| Biologic adverse events | ||||
| Alanine aminotransferase increased | 41 (13.0) | 18 (11.8) | 23 (14.2) | |
| Grade 3 | 25 (7.9) | 12 (7.8) | 13 (8.0) | |
| Grade ≥4 | 16 (5.1) | 6 (3.9) | 10 (6.2) | 0.36 |
| Hypokalemia | 36 (11.4) | 15 (9.8) | 21 (13.0) | |
| Grade 3 | 28 (8.9) | 11 (7.2) | 17 (10.5) | |
| Grade ≥4 | 8 (2.5) | 4 (2.6) | 4 (2.5) | 1.00 |
Shown are the numbers and percentages of patients who had at least one adverse event, those who had at least one adverse event of grade 4 or higher, and those who had the most frequent acute adverse events (i.e., events occurring in >10% of the patients overall). A total of 13 patients who did not have data on treatment or adverse events or who received only prephase treatment (a regimen of cyclophosphamide, vincristine, and prednisone [COP]) were not included in this analysis. Only nonhematologic adverse events of grade 3 or higher or cardiac adverse events of grade 2 or higher were recorded. P values are shown for events of grade 4 or higher.
Six patients (1.8% of the patients overall; three patients in each group) died from toxic effects. Five of the deaths, four of which were from infection, occurred in patients receiving group C chemotherapy after the receipt of the first course of therapy with cyclophosphamide, vincristine, prednisone, doxorubicin, and methotrexate, with or without rituximab depending on treatment group (in three patients); after the receipt of the second course of therapy with cyclophosphamide, vincristine, prednisone, doxorubicin, and methotrexate, with rituximab (in one patient); or after the receipt of a continuous infusion and high-dose cytarabine and etoposide, with rituximab (in one patient). One death from toxic effects occurred in a patient receiving group B chemotherapy who had undergone hemicolectomy at diagnosis and died from small-bowel obstruction and septic shock after the receipt of COPADM2.
The incidence of adverse events of grade 4 or higher was 37.7% in the rituximab–chemotherapy group and 32.7% in the chemotherapy group (P = 0.36). In an analysis that was focused on the period after the receipt of COP prephase treatment, the incidence of adverse events of grade 4 or higher was 33.3% in the rituximab–chemotherapy group and 24.2% in the chemotherapy group (P = 0.07). After prephase treatment, the incidence of febrile neutropenia of grade 4 or higher was 11.7% in the rituximab–chemotherapy group and 6.5% in the chemotherapy group (P = 0.11), and the incidence of infection of grade 4 or higher was 18.5% and 11.1%, respectively (P = 0.07). No other acute unexpected toxic effects were reported in either group. The most frequent adverse events according to treatment course and all adverse events of grade 4 or higher are presented in Tables S3 and S4, respectively.
IMMUNOGLOBULIN STATUS
The percentage of patients with a low IgG level (less than the lower limit of the normal range) was significantly higher in the rituximab–chemotherapy group than in the chemotherapy group at the end of therapy (70.3% vs. 46.8%, P = 0.002) and at 1 year after inclusion (55.9% vs. 25.4%, P<0.001), respectively (Table S5). Approximately twice as many patients in the rituximab–chemotherapy group as in the chemotherapy group received intravenous immune globulin (15.8% vs. 7.0%). The primary reason for immune globulin replacement was a low immunoglobulin level without infection (Table S6). At 1 year after enrollment, seven patients were still receiving intravenous immune globulin in the rituximab–chemotherapy group and three in the chemotherapy group. Complete and longer follow-up with regard to immune status and late infections have not been evaluated.
DISCUSSION
In this randomized, international, phase 3 trial, we found that among children and adolescents with high-grade, high-risk, mature B-cell lymphoma, the addition of six doses of rituximab to LMB therapy led to significantly better event-free survival outcomes (hazard ratio for an event, 0.32; 95% CI, 0.15 to 0.66; 3-year event-free survival, 93.9% in the rituximab–chemotherapy group vs. 82.3% in the chemotherapy group). The benefit was similar across the various therapeutic groups, including the group of patients with CNS disease. Higher 3-year overall survival was also observed (95.1% in the rituximab–chemotherapy group vs. 87.3% in the chemotherapy group; hazard ratio for death, 0.36; 95% CI, 0.16 to 0.82). Overall, the acute toxic effects that were associated with the addition of rituximab mainly involved a higher incidence of myelotoxic effects, but further follow-up is needed to provide information on long-term safety because rituximab therapy induced more hypogammaglobulinemia than chemotherapy alone.
The benefit of rituximab therapy in this trial appeared to be similar to that observed in trials involving adult patients. In a randomized French trial that compared the addition of four doses of rituximab to adult-adapted LMB chemotherapy with chemotherapy alone in 257 patients with Burkitt’s lymphoma (61% of the patients were ≥40 years of age), superior 3-year event-free survival (75% in the rituximab–chemotherapy group vs. 62% in the chemotherapy group; hazard ratio for event, 0.59; P = 0.02) and overall survival (83% vs. 70%; hazard ratio for death, 0.51; P = 0.01) were observed.11 A direct comparison of outcomes in adults and children is difficult, however, because the outcomes in children who are treated with chemotherapy are superior to those in adults, and molecular analyses suggest that there is age-related biologic heterogeneity in Burkitt’s lymphomagenesis.5,6
The data from this trial suggest that the addition of rituximab may result in a higher incidence of severe (grade ≥4) acute adverse events (primarily febrile neutropenia and infection) after prephase treatment. Although specific viral infections have been reported in approximately 2% of patients treated with rituximab, we did not observe such infections in the rituximab–chemotherapy group (which included 164 patients), but longer follow-up is needed.12,13 Two patients (1%) in the rituximab–chemotherapy group had second cancers: one patient had melanoma within a congenital skin nevus, and one patient had a clonally related histiocytic sarcoma suggesting transdifferentiation of the Burkitt’s lymphoma clone. Some reports have suggested that among adult patients with lymphoma, the introduction of rituximab may increase the risk of a second cancer (especially acute myeloid leukemia, thyroid cancer, and melanoma),14 but a meta-analysis did not support this finding.15 Finally, approximately twice as many patients in the rituximab–chemotherapy group as in the chemotherapy group had a low IgG level 1 year after trial inclusion, although very few patients received immune globulin replacement for infections. An assessment of the long-term effects of combining rituximab with this chemotherapy regimen in children with non-Hodgkin’s lymphoma, including data on immune status, will be useful.
A previous phase 2 window study involving children with newly diagnosed high-grade, mature B-cell non-Hodgkin’s lymphoma showed that 21% of the patients had a complete or partial response during a 5-day window after receiving a single dose of rituximab16; another study is currently comparing one dose of rituximab with six doses in children with advanced-stage B-cell non-Hodgkin’s lymphoma (ClinicalTrials.gov number, NCT03206671). With respect to pharmacokinetics, rituximab clearance appears to be related to total tumor burden; exposure to rituximab decreases as tumor metabolic volume increases.
Higher rituximab exposure is associated with a higher percentage of patients with a response and longer progression-free survival among patients with diffuse large B-cell lymphoma.17 A small cohort study involving children and adolescents with high-grade, mature B-cell non-Hodgkin’s lymphoma showed some age dependency with regard to rituximab pharmacokinetics.18 Thus, we are conducting additional pharmacokinetic analyses, including examination of the effects of age and tumor burden on rituximab disposition.
Although the results of the Inter-B-NHL ritux 2010 trial showed a benefit with the addition of rituximab in the treatment of children with high-grade, high-risk, mature B-cell non-Hodgkin’s lymphoma, the use of rituximab in children with standard-risk B-cell non-Hodgkin’s lymphoma (approximately 40% of patients) is still questionable, because their survival after treatment with chemotherapy alone is very high (97 to 98%).19 It is conceivable that the addition of rituximab could allow for a reduction of cytotoxic chemotherapy; however, given the very poor outcome in patients with refractory or relapsed disease,20 it would be ethically challenging to conduct such a study. Furthermore, although current chemotherapy is associated with substantial acute toxic effects, deaths from toxic effects are rare and the risks of clinically significant long-term sequelae are relatively modest with the use of this chemotherapy backbone.21 To help inform such a study, more data are necessary in order to evaluate the long-term safety of rituximab administered with chemotherapy in children with non-Hodgkin’s lymphoma.
This trial showed that the addition of rituximab to chemotherapy was effective therapy in children and adolescents with high-risk, high-grade, mature B-cell non-Hodgkin’s lymphoma and resulted in long-term complete remission in 95% of the patients. In addition, we found an effective global framework for academic-based, collaborative pediatric groups that leveraged both public and private sector support to conduct clinical trials involving children with a rare cancer.
Supplementary Material
Acknowledgments
Supported by a grant (PHRC2010) from the Clinical Research Hospital Program of the French Ministry of Health, by Cancer Research UK, the National Institute for Health Research Clinical Research Network, and Children’s Cancer Foundation Hong Kong, by grants (U10CA180886 and U10CA180899) from the U.S. National Cancer Institute, and by F. Hoffmann–La Roche–Genentech.
Dr. Minard-Colin reports receiving grant support, paid to her institution, from F. Hoffmann–La Roche; Dr. Aupérin, receiving grant support, paid to her institution, from F. Hoffmann–La Roche; Dr. Burke, receiving consulting fees from F. Hoffmann–La Roche, Janssen Pharmaceuticals, Merck, and Takeda Pharmaceutical; Dr. Adamson, receiving grant support, paid to Children’s Hospital of Philadelphia, from Roche Genentech, owning stock in Merck, and being employed by Sanofi and Genzyme US; Dr. Vassal, receiving grant support, paid to his institution, from F. Hoffmann–La Roche; and Dr. Patte, receiving grant support, paid to her institution, from F. Hoffmann–La Roche. No other potential conflict of interest relevant to this article was reported.
We thank the members of the national and international review panels (P. Dartigues, T. Molina, J. Bosq, S. Perkins, K. McCarthy, A. Ferrández, O. Balagué, F. Loong, J.C. So, and E.S.G. d’Amore); all the European Intergroup for Childhood Non-Hodgkin Lymphoma (EICNHL) and Children’s Oncology Group (COG) committee members and investigators who participated in the trial; F. Rotolo, of the Gustave Roussy Unit of Biostatistics and Epidemiology; the national data managers (R. Banusz, E. Carraro, A. Raxworthy Cooper, V. Femke, O. Hung, A. Michiels, M. Peiró, J. Vreijling, O. Wajsen, and T. Yu); G. Goma and A. Mangin for data management for the patients at the EICNHL sites; J. Anderson, A. Buxton, D. Hall, L. Saguilig, A. Miranda, T. Hlaing, and G. Galit for data management for the patients at the COG sites; A. Tulard, D. Vuillier, and J. Rubino, of the Gustave Roussy clinical research team; S. Laghouati for pharmacovigilance; the members of the independent data and safety monitoring board (R. Sposto, F. Pein, R. Pinkerton, and M. Robert); and the F. Hoffmann–La Roche–Genentech team. This study was initially proposed to the Paediatric Committee by the Marketing Authorisation Holder on May 13, 2009, as the sole measure in the MabThera PIP (EMEA-000308-PIP01-08). The initial MabThera PIP was submitted on June 28, 2008, and was agreed on in the European Medicines Agency decision adopted on July 14, 2009.
Footnotes
References
- 1.Minard-Colin V, Brugières L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol 2015; 33: 2963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood 2005; 105:9 48–58. [DOI] [PubMed] [Google Scholar]
- 3.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood 2007; 109:2 773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 2007; 109:2 736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havelange V, Pepermans X, Ameye G, et al. Genetic differences between paediatric and adult Burkitt lymphomas. Br J Haematol 2016; 173: 137–44. [DOI] [PubMed] [Google Scholar]
- 6.López C, Kleinheinz K, Aukema SM, et al. Genomic and transcriptomic changes complement each other in the pathogenesis of sporadic Burkitt lymphoma. Nat Commun 2019; 10: 1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman S, Smith L, Anderson JR, et al. Rituximab and FAB/LMB 96 chemotherapy in children with stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia 2013; 27: 1174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minard-Colin V, Auperin A, Leverger G. In childhood B-cell non Hodgkin’s lymphoma (B-NHL) and mature B-cell acute leukemia (B-AL) with CNS disease at diagnosis, patients with blasts in CSF are at higher risk of event. Ann Oncol 2011; 22: Suppl 4: iv112–iv114. [Google Scholar]
- 9.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med 1994; 13:1 341–52. [DOI] [PubMed] [Google Scholar]
- 10.Minard-Colin V, Auperin A, Pillon M, et al. Results of the randomized Intergroup trial Inter-B-NHL ritux 2010 for children and adolescents with high-risk B-cell non-Hodgkin lymphoma (B-NHL) and mature acute leukemia (B-AL): evaluation of rituximab (R) efficacy in addition to standard LMB chemotherapy (CT) regimen. J Clin Oncol 2016;3 4: Suppl:1 0507. abstract. [Google Scholar]
- 11.Ribrag V, Koscielny S, Bosq J, et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: a randomised, controlled, open-label, phase 3 trial. Lancet 2016; 387:2 402–11. [DOI] [PubMed] [Google Scholar]
- 12.Focosi D, Tuccori M, Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: what do we know after 20 years of rituximab. Rev Med Virol 2019; 29(6): e2077. [DOI] [PubMed] [Google Scholar]
- 13.Kusumoto S, Arcaini L, Hong X, et al. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood 2019; 133:1 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao L, Clarke CA, Rosenberg AS, et al. Subsequent primary malignancies after diffuse large B-cell lymphoma in the modern treatment era. Br J Haematol 2017; 178:7 2–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleury I, Chevret S, Pfreundschuh M, et al. Rituximab and risk of second primary malignancies in patients with non-Hodgkin lymphoma: a systematic review and meta-analysis. Ann Oncol 2016;2 7: 390–7. [DOI] [PubMed] [Google Scholar]
- 16.Meinhardt A, Burkhardt B, Zimmer-mann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol 2010; 28:3 115–21. [DOI] [PubMed] [Google Scholar]
- 17.Tout M, Casasnovas O, Meignan M, et al. Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: a Lymphoma Study Association report. Blood 2017;1 29: 2616–23. [DOI] [PubMed] [Google Scholar]
- 18.Barth MJ, Goldman S, Smith L, et al. Rituximab pharmacokinetics in children and adolescents with de novo intermediate and advanced mature B-cell lymphoma/leukaemia: a Children’s Oncology Group report. Br J Haematol 2013; 162: 678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patte C, Zimmerman M, Auperin A, Reiter A. Similar results are currently observed in the LMB and BFM studies for B-cell Non-Hodgkin’s lymphoma and B-AL allowing future common studies. Pediatr Blood Cancer 2010; 55: 795. abstract. [Google Scholar]
- 20.Pearson ADJ, Scobie N, Norga K, et al. ACCELERATE and European Medicine Agency Paediatric Strategy Forum for medicinal product development for mature B-cell malignancies in children. Eur J Cancer 2019; 110:7 4–85. [DOI] [PubMed] [Google Scholar]
- 21.Ehrhardt MJ, Chen Y, Sandlund JT, et al. Late health outcomes after contemporary Lymphome Malin de Burkitt therapy for mature B-cell non-Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2019; 37:2 556–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.