Abstract
Hyperinsulinaemic hypoglycaemia (HH) is a biochemical finding of low blood glucose levels due to the dysregulation of insulin secretion from pancreatic β-cells. Under normal physiological conditions, glucose metabolism is coupled to β-cell insulin secretion so that blood glucose levels are maintained within the physiological range of 3.5–5.5 mmol/L. However, in HH this coupling of glucose metabolism to insulin secretion is perturbed so that insulin secretion becomes unregulated. HH typically occurs in the neonatal, infancy and childhood periods and can be due to many different causes. Adults can also present with HH but the causes in adults tend to be different. Somatostatin (SST) is a peptide hormone that is released by the delta cells (δ-cells) in the pancreas. It binds to G protein-coupled SST receptors to regulate a variety of location-specific and selective functions such as hormone inhibition, neurotransmission and cell proliferation. SST plays a potent role in the regulation of both insulin and glucagon secretion in response to changes in glucose levels by negative feedback mechanism. The half-life of SST is only 1–3 min due to quick degradation by peptidases in plasma and tissues. Thus, a direct continuous intravenous or subcutaneous infusion is required to achieve the therapeutic effect. These limitations prompted the discovery of SST analogues such as octreotide and lanreotide, which have longer half-lives and therefore can be administered as injections. SST analogues are used to treat different forms of HH in children and adults and therapeutic effect is achieved by suppressing insulin secretion from pancreatic β-cells by complex mechanisms. These treatments are associated with several side effects, especially in the newborn period, with necrotizing enterocolitis being the most serious side effect and hence SS analogues should be used with extreme caution in this age group.
Keywords: hyperinsulinaemic hypoglycaemia, insulinoma, lanreotide, octreotide, somatostatin
Introduction
Hypoglycaemia is a common biochemical finding in the newborn, infancy, childhood and adulthood periods. In children hypoglycaemia may be due to many different causes.1 Adults can also present with hypoglycaemia but the underlying causes tend to be different. The most severe form of hypoglycaemia, in children and adults, is hyperinsulinaemic hypoglycaemia (HH). In HH the secretion of insulin from the pancreatic β-cell becomes unregulated and uncoupled from glucose metabolism, so that insulin secretion becomes inappropriate for the blood glucose level, leading to persistent and recurrent hypoglycaemia. In children the congenital forms of HH are more common than in adults and the clinical presentation in newborns and infants is typically with severe and persistent hypoglycaemia.2 The medical management of newborns and infants with severe forms of HH is complex and some patients are treated with somatostatin analogues, both short and long acting (such as octreotide and lanreotide respectively) to suppress insulin secretion, so that blood glucose levels can be maintained within the normal range.3 Somatostatin (SST) is a peptide hormone that is released by the delta cells (δ-cells) of the pancreas and has powerful effects on insulin and glucagon secretion from the pancreatic β-cell and α-cell respectively. Although SST analogues are widely used in the treatment of HH both in children and in adults, to our knowledge, no reviews have been published on this subject previously. In this review we will first give the clinical background of the different types of HH; then, we discuss the physiology and pharmacology of SS; outline the use of SS analogues for treating different types of HH; review the literature on the use of SS analogues for treating HH and finally we discuss the side effects related to the use of SS analogues for HH.
Background to hyperinsulinaemic hypoglycaemia
HH is a biochemical finding of low blood glucose levels in the presence of dysregulated insulin secretion. It typically presents with severe and persistent hypoglycaemia.2 Under normal physiological conditions, glucose metabolism is coupled to β-cell insulin secretion so that blood glucose levels are maintained between 3.5 mmol/L and 5.5 mmol/L. However, in HH this coupling of glucose metabolism to insulin secretion is perturbed so that insulin secretion becomes unregulated.4
HH is a major cause of severe and persistent hypoglycaemia in children, especially in the newborn period.5 The congenital forms of HH (shown in Table 1) are more common in children than in adults. In adults an insulinoma is the commonest cause of endogenous HH. HH is also observed in patients who have undergone gastric by-pass surgery for obesity and the secondary cause is due to the side effects of medications such as sulphonylureas. Clinically it is important to make the diagnosis of HH as quickly as possible and stabilize the blood glucose levels as the episodes of hypoglycaemia can lead to brain damage, especially in the newborn. Table 1 lists all the causes of HH.
Table 1.
Causes of hyperinsulinaemic hypoglycaemia.
| Transient nature | |
| Infant born to diabetic mother (gestational or permanent diabetes) | |
| Intrauterine growth retardation | |
| Rhesus disease | |
| Perinatal asphyxia | |
| Erythroblastosis fetalis | |
| Childhood onset – persistent nature | |
| Congenital causes | Genetic mutations in: |
| ABCC8, KCNJ11, GDH, HADH, GCK, SLC16A1, GLUD1, UCP2, HNF4A, HNF1A, HK1, PGM1, PPM2 | |
| Syndromic causes | Beckwith–Wiedemann |
| Mosaic Turner | |
| Timothy | |
| Soto | |
| Simpson–Golabi–Behmel | |
| Kabuki | |
| Patau – Trisomy 13 | |
| Rubenstein Taybi | |
| Costello | |
| Congenital disorders of glycosylation (CDG types 1A, 1B and 1D) | |
| Congenital hypoventilation | |
| Poland | |
| Childhood/adult onset – persistent nature | |
| Tumours | IGF-2-oma |
| Benign and malignant insulinoma | |
| Non-islet cell tumour hypoglycaemia | |
| Drug-use related | Glinides |
| Insulin | |
| Sulphonylureas | |
| Factitious hypoglycaemia | Munchausen syndrome by proxy |
| Postprandial | Dumping syndrome |
| Post gastric by-pass surgery for morbid obesity Non-insulinoma pancreatogenous hypoglycaemia |
|
| Other causes | Auto-antibodies against insulin |
| Insulin resistance syndrome | |
HH in children
In children, HH can be transient (secondary to maternal diabetes, intrauterine growth retardation or perinatal asphyxia) or permanent. The transient forms of HH usually resolve spontaneously but occasionally may need treatment.6 The commonest cause of transient HH in the newborn period is secondary to maternal diabetes mellitus (gestational or pre-gestational) and in the vast majority of newborns this will resolve within a few days. Some newborns with intrauterine growth retardation or perinatal asphyxia may have a protracted form of HH which will require treatment.6
The permanent forms of HH are due to genetic causes that perturb the normal physiological mechanisms of regulated insulin secretion from the pancreatic β-cell. Currently, genetic mutations in 14 different genes lead to different forms of permanent HH, which lead to a spectrum of severity ranging from mild, moderate to severe HH.7 The most severe forms of HH are due to mutations in ABCC8/KCNJ11, which encode the SUR/KIR6.2 proteins, respectively, of the pancreatic β-cell KATP channels.8,9 These KATP channels play a pivotal role in linking the metabolism of glucose inside the β-cell to changes in the β-cell membrane potential, intracellular calcium homeostasis and insulin secretion.4 Mutations in the ABCC8/KCNJ11 lead to unregulated insulin secretion and in most cases to severe hypoglycaemia, which is usually medically unresponsive.
In the congenital forms of HH that occur in children, there are two main histological subtypes, namely diffuse and focal HH,10,11 and in a few patients, atypical forms have been described.11 In diffuse HH the whole of the pancreas is affected whereas in focal only a small region of the pancreas is involved in the abnormality. In terms of clinical management, children with focal lesions can now be identified pre-operatively and the focal lesion can be removed surgically, providing a cure for hypoglycaemia.12 In contrast, infants with the diffuse type of HH usually do not respond to medical therapy and will require a near-total pancreatectomy, which leads to the development of diabetes mellitus and pancreatic exocrine insufficiency.13 Therefore, given that infants with diffuse HH develop diabetes following near total pancreatectomy, if it is possible they should be managed aggressively medically.14 The use of SST analogues (both short-term and long-term) is one form of medical therapy where it might be possible to avoid the need for partial or subtotal pancreatectomy in these patients. Interestingly, in the atypical forms of HH, there is evidence to suggest that the δ-cells that secrete SST might be increased in numbers and may contribute to the pathophysiology.15
HH in adults
Insulinoma
Insulinomas are the most common functioning endocrine neoplasm of the pancreas, occurring most commonly in the fifth and sixth decades16 with an incidence of about 1–4 million.17,18 Presentation during childhood is extremely rare but both benign and malignant cases have been reported in children.19 The majority (90%) of insulinomas are benign, solitary, intrapancreatic and <2 cm in diameter. Classically, symptoms become evident in the fasting state or following exercise; however, it is now known that insulinoma can also present with postprandial symptoms.20 Somatostatin analogues have been used for the management of adult patients with both benign and malignant insulinomas.
Postprandial hyperinsulinaemic hypoglycaemia after gastric by-pass surgery
Roux-en-Y gastric by-pass is a surgical procedure used in the management of morbid obesity mostly in adults but now also being used in adolescents. Postprandial HH (PPHH) is a well-recognized complication of this procedure. It was first described in 2005 in six patients who had undergone Roux-en-Y gastric by-pass surgery.21 PPHH is relatively common after gastric by-pass, with approximately 30–40% of patients reporting symptoms of hypoglycaemia in one study.22 Biochemically, patients show hypoglycaemia with exaggerated insulin and glucagon-like peptide-1 (GLP-1) responses following meal ingestion with early enhancements of hepatic insulin sensitivity and later improvements in peripheral insulin sensitivity.23 GLP-1 has a stimulatory and trophic effect on pancreatic β-cells, and plasma levels increase after a meal and can lead to hyperinsulinaemia and hypoglycaemia.24,25
Background to SST
Krulich et al.25 first observed that hypothalamic extracts of rats and sheep contain a growth-inhibiting factor. Further, Brazeau et al.26 isolated and characterized a peptide that inhibited the release of growth hormone from rat anterior pituitary cells. This peptide was named somatostatin (somatotroph release-inhibiting factor). It was hypothesized to be a neurohumoral substance that regulates growth hormone secretion from the pituitary gland through inhibition. A similar substance was extracted by Hellman and Lernmark from pigeon pancreatic islets that inhibited insulin release.27 These and subsequent observations identified it as a chemical substance that inhibits hormone secretion in the hypothalamus, pancreas and other secretory cells in the gastrointestinal tract (gut), salivary glands and other excretory systems. It has several important roles and functions as paracrine, autocrine or as true hormones.28 SST, variously known as SMS, SOM, SST, growth hormone-inhibiting hormone or somatotropin release-inhibiting factor (SRIF), is a cyclic tetradecapeptide. While there are six different SST genes in vertebrates, humans only have a single gene (SST).29,30
Immunoreactive SST is initially synthesized as a preprohormone peptide that consists of 116 amino acids which is cleaved into prosomatostatin, which has 92 amino acids. Prosomatostatin further undergoes C-terminal post-translational processing to generate somatostatin-14 (SST14) and somatostatin-28 (SST28).30 SST is a peptide hormone that binds with G protein-coupled SST receptors to regulate a variety of functions which include neurotransmission, cell proliferation and inhibition of insulin, glucagon and other hormones.31 Somatostatinergic neurons are also active inhibitors in the anterior and neural lobe of the pituitary glands, limbic system, brain stem and spinal cord that control the output of excitatory neurons.32
Role of SST in pancreatic islet cells
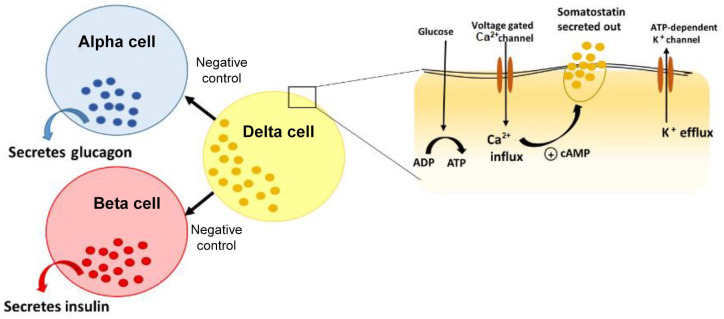
The endocrine pancreas consists of beta (β), alpha (α), delta (δ), pancreatic polypeptide and epsilon (ε) cells (Figure 1) present in the Islet of Langerhans.33 The predominant type of cell in the islet is the centrally located β-cell (50–60%), which is responsible for secreting insulin in response to rising blood glucose levels, whereas the SST δ-cells comprise about 5%.34 Glucagon and SST are secreted and released by the α-cells and δ-cells respectively. The close proximity of these cells within the islet facilitates paracrine regulation of hormone production and activity.35 Metabolites such as glucose, ketone bodies or amino acids act as stimuli to regulate the islet cell hormone production.36 Although there is no direct evidence that suggests an important role for SST in glucose homeostasis, it has been proposed that disruptions to paracrine interactions (between the β-cells and δ-cells) could lead to alterations in glucose metabolism.34,37
Figure 1.
Pancreatic islet is shown here with alpha, beta, delta cells and secreted hormones. This figure illustrates the mechanism of release of somatostatin from delta cells through the activation of glucose transporters and voltage gated calcium channels.33,36
ADP: Adenosine Diphosphate; ATP: Adenosine Triphosphate.
Role of SST in regulation of insulin secretion and β-cell physiology
SST is involved in the regulation of both insulin and glucagon secretion in response to changes in glucose levels by negative feedback mechanism.34 In response to high blood glucose levels, insulin is secreted and, once normoglycaemia is attained, the loss of physiological stimulus inhibits insulin secretion from β-cells. Also, there is inhibition from within the islet which is proposed to be mediated by SST.38 The exact molecular mechanism behind this regulation is unknown, but several mediators have been proposed.39 SST14 and SST28 are the biologically active forms of SST.40 SST14 is the more predominant inhibitor of α-cell-mediated glucagon secretion, while SST28 inhibits insulin secretion from β-cells.41 However, SST treatment at very low glucose levels does not attenuate insulin secretion. This suggests that the mechanism of action could be glucose metabolism dependent.42
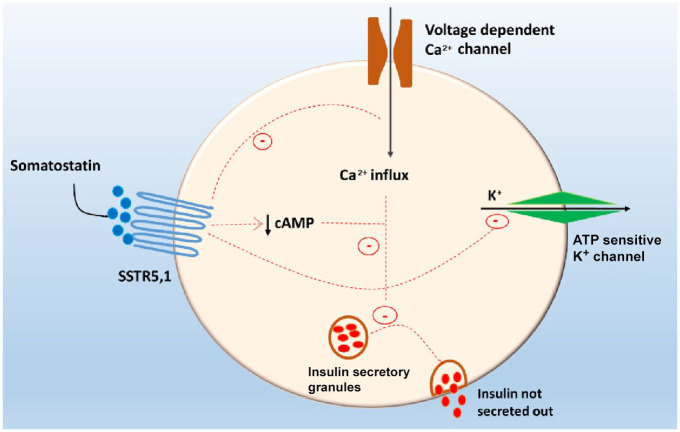
SST acts primarily via five transmembrane G-protein coupled SST receptor (SSTR) types (SSTR1–SSTR5) with SSTR1, SSTR2 and SSTR5 being highly expressed in β-cells.43 The five receptors share common signalling pathways such as the inhibition of adenylyl cyclase, activation of phosphotyrosine phosphatase and modulation of mitogen-activated protein kinase through G-protein-dependent mechanisms. Also, each receptor may have specific signalling mechanisms such as SSTR2–5 being coupled to inward rectifying K+ channels, SST binds to the SST receptor on the β-cell and inhibits the activation of voltage gated calcium channels and ATP sensitive K+ channels. This reduces cyclic adenosine monophosphate (cAMP) levels thereby inhibiting the release of insulin.44,45 Activation of SSTR by G-protein coupling inhibits adenylyl cyclase activity that leads to reduced cyclic AMP and diminished insulin secretion,46 as seen in Figure 2.
Figure 2.
Mechanism of action of somatostatin (SST) on insulin secretion by negative feedback. SST binds to the SST receptor on the β-cell and inhibits the activation of voltage gated calcium channels and ATP sensitive K+ channels. This reduces cyclic adenosine monophosphate (cAMP) levels thereby inhibiting the release of insulin.44–46
It has been postulated that the inhibitory effect of SST on insulin secretion may be through alterations in voltage-gated calcium channels, particularly inhibition of L type channel.47 β-cells at stimulatory glucose concentrations, exposed to SST, show a significant reduction in intracellular calcium levels that results in diminished insulin secretion, suggesting a Gβγ complex-mediated signalling.39 In MIN6 cell lines, SST can inhibit glucose-induced electrical activity by the combined activation of ATP sensitive potassium (KATP) channel and G-protein coupled inward rectifying potassium channel, suggesting that there may be some involvement of the KATP channels in response to SST.48 Further, gamma-aminobutyric acid (GABA), which is an inhibitory neurotransmitter, is released by the β-cells, which mediates negative feedback for the δ-cells.49 GABA is involved in both activities: in autocrine positive feedback, which helps to produce a quick response to hypoglycaemia, as well as in paracrine negative feedback control of the β-cells, thus maintaining an optimal islet cell output after attaining normoglycaemia.49 Another peptide hormone, Urocortin3 (Ucn3) is expressed by β-cells and α-cells in humans, which stimulates secretion of both insulin and glucagon.50 Ucn3 is stored in insulin granules and in the event of hyperglycaemia is released along with insulin. This provides a negative feedback loop via corticotropin-releasing hormone receptor 2a receptors to SST, which in turn inhibits the release of insulin.51 More recent data in mice and in human islets show that insulin-stimulated SST secretion plays a key role in regulating serum glucagon secretion.52
Interaction of SST with other pancreatic hormones
Endogenous SST secreted from δ-cells has paracrine effects on the regulation of insulin and glucagon secretion but the physiological significance of this regulation is still unclear. While SSTR5 seems to be a negative regulator of insulin secretion, SSTR2 inhibits glucagon secretion.53 SST receptor knockout mice have been generated in order to understand the physiological roles of SSTR receptors. Global SSTR knockout mice were generated in order to investigate the role of δ-cell-secreted SST in the regulation of insulin and glucagon secretion in vitro and in vivo.38 This study showed that δ-cell SST exerts a tonic inhibitory influence on insulin and glucagon secretion. Viable, fertile SSTR5 knockout mice that are otherwise healthy, exhibit no apparent phenotypic abnormalities.54 Pancreatic islets from these mice show increased insulin content as compared with wild-type islets. These mice exhibited decreased blood glucose and plasma insulin levels and increased leptin and glucagon concentrations compared with wild-type mice. SST analogue 28 was not able to inhibit insulin secretion in knockout islets as effectively as from wild-type islets. In contrast, islets from SSTR2 knockout mice show no difference in basal glucagon and insulin secretion while potassium/arginine-stimulated glucagon secretion was approximately 2-fold higher when compared with wild-type islets.42 Neither SST nor any SSTR-selective agonist inhibited basal glucagon or insulin release. SST14 potently inhibited stimulated glucagon secretion in islets from wild mice and much less effectively in islets from SSTR2 knockout mice. Both of these studies in mice suggest that SSTR5 is important for regulating insulin secretion and SSTR2 for glucagon secretion.
Ghrelin positive ε-cells are located in the periphery of the pancreatic islet. Although ghrelin mainly controls appetite, it can also regulate the secretion of insulin and SST. The effects of ghrelin on insulin secretion and glucose physiology are complex and not completely understood. Broglio et al.55 first showed that an intravenous injection of ghrelin leads to a decrease in insulin secretion and an increase in the blood glucose level. In another study an infusion of ghrelin led to an increase in the serum level of SST. This increase in the SST level could explain the reduction in the insulin level.56 Thus, ghrelin has complex effects on insulin and SST secretion and blood glucose levels.
Background to SST analogues
SST has anti-proliferative, anti-secretory and anti-angiogenic properties, which makes it useful in the treatment of certain diseases of the gastrointestinal system, pancreas, peripheral neurons and the retina, among others.57 However, the half-life of SST is only 1–3 min due to quick degradation by peptidases in plasma and tissues. This requires a direct continuous intravenous injection to produce the therapeutic effect. These limitations prompted the discovery of octapeptide analogues octreotide and lanreotide.58 Their mode of delivery is either subcutaneous injections or infusions or by intravenous route.
Octreotide was the first US Food and Drug Administration (FDA) approved synthetic SST analogue (SMS 201-995), marketed as Sandostatin® and available in a conventional and a long acting formulation (Sandostatin LAR®). The Sandostatin LAR® formulation contains octreotide distributed within polymer microspheres and it is available for intramuscular injection.
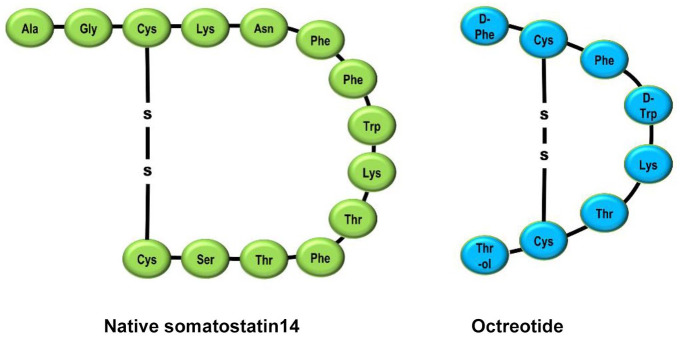
Octreotide is an octapeptide in which the four amino acid sequence essential for the biological activity of the native hormone is retained. However, incorporation of N-phenylalanine, L-terminal amino alcohol, D-tryptophan and a cysteine bridge makes the molecule very resistant to metabolic degradation.59 The half-life of octreotide is 90–120 min via subcutaneous administration and has a pharmacodynamic action lasting 8–12 h. Octreotide binds primarily to SSTR2 and SSTR5 with a low affinity to SSTR3. The selective binding to SSTR2 results in increased therapeutic benefits and fewer adverse effects. Octreotide has 40 times greater potency than SST in inhibiting growth hormone (GH) secretion. It has also demonstrated the ability to inhibit insulin and glucagon secretion in the pancreas.60 Figure 3 shows the structure of SST and SST analogue octreotide.
Figure 3.
Structure of somatostatin (SST) and SST analogue octreotide.30,40
The development of long-acting formulations of SST analogues which require only weekly or monthly injections can improve patient compliance. In particular, lanreotide (LAN) Autogel®, which is a viscous aqueous formulation supplied in ready-to-use prefilled syringes, can be administered every 28–56 days. Lanreotide is a cyclic octapeptide that is widely used for adults with neuroendocrine tumours. Pasireotide is a novel short synthetic SST analogue which exhibits high affinity binding to four of the five human SRIF receptor subtypes and has potent, long lasting inhibitory effects on GH and Insulin like Growth Factor (IGF-I) release.61
The oldest and most common use of these SST analogues is in the treatment of acromegaly and hormone dependent pituitary tumours17 where they control the hormonal imbalance as well as produce a tumour stabilizing effect.62 These are drugs of choice in gastrointestinal tumours such as VIP-oma, gastrinoma, glucagonoma, insulinoma and both typical and atypical carcinoid syndrome for symptomatic relief. Other, newer, uses for these drugs include endocrine tumours such as well differentiated thyroid tumours, pheochromocytoma and adrenocortical tumours as well as non-endocrine tumours such as small cell lung cancer and melanoma. They are very effective in conditions such as portal hypertension and oesophageal variceal bleeding, peptic ulcer and short bowel syndrome. Ophthalmologic indications include Graves’ ophthalmopathy and diabetic retinopathy among others.63
Octreotide has been shown to be beneficial in insulin dependent diabetes mellitus by increasing insulin sensitivity and in non-insulin dependent diabetes mellitus it reduces insulin resistance.64 It is also proposed to be useful in the prevention of diabetic complications such as diabetic retinopathy.65 However, the use of octreotide in diabetes mellitus and diabetic retinopathy is not registered and is experimental. Another potential use of octreotide is in imaging, namely somatostatin receptor scintigraphy (octreoscan), which helps to visualize autoimmunological processes in conditions such as lymphoma, medullary thyroid cancer and breast cancer.66
The role of SST analogues in treatment of HH
Use of SST analogues in children with HH
The congenital forms of HH typically present with severe and persistent hypoglycaemia. The management of HH typically involves having a stepwise approach to assessing the response to different forms of medical therapy.2 In the acute situation blood glucose levels are stabilized with concentrated intravenous glucose infusions and/or feeds. A bolus or a subcutaneous infusion of glucagon may also be used in the acute scenario. Once the biochemical diagnosis of HH is established the first line therapy is with diazoxide. Genetic testing for the important causes of HH is now a key component in the management of patients with HH. Currently, diazoxide is the only drug approved by the FDA for the treatment of children with HH. Patients with mutations in ABCC8/KCNJ11 encoding the SUR1/KIR6.2 components of the pancreatic β-cell typically do not respond to treatment with diazoxide (most of the other genetic forms of HH will respond to therapy with diazoxide). In these cases, when diazoxide fails, second-line treatment options for children are limited. In 1977 Hirsch et al.67 first described the infusion of synthetic cyclic SST in an infant with severe HH. This improved the blood glucose levels and reduced circulating serum insulin levels. The authors then treated the patient with single subcutaneous injections of 50 µg of protamine zinc SST, which also improved pre-prandial blood glucose levels. In another early study, in 1981, Roti et al.68 demonstrated an improvement in the blood glucose level in a patient with HH in response to an intravenous infusion of SST but no improvement in the blood glucose level in response to protamine zinc-SST, a long acting SRIF preparation. In the same year Aynsley-Green et al.69 reported the effect of SST infusion in two infants presenting at 9 weeks and 5 days of age with severe HH. In both infants normoglycaemia was restored with suppression of insulin secretion. The plasma concentrations of glucagon, cortisol, growth hormone, motilin, pancreatic polypeptide, gastric inhibitory of polypeptide, neurotensin, gastrin and vasoactive intestinal peptide decreased markedly during the SST infusion. In 1985 Bougnères et al.70 used native cyclic SST in an infusion pump to treat a child with HH and achieved normal blood glucose levels. Also, in 1985 Wendal et al.71 treated 11 infants with SST and showed that it was very reliable in the treatment of neonatal hypoglycaemia due to hyperinsulinism for a limited period of time until subtotal pancreatectomy was performed. These initial studies established the physiological basis for using SST in infants with HH. Then in 1986 Bruining et al.72 reported the use of long-acting SST analogue SMS 201-995 (octreotide) in a newborn with severe HH. The patient presented with severe hypoglycaemia and seizures. The intravenous infusion of this analogue (gradually increasing the dose from 2 to 50 µg/24 h) dramatically reduced circulating insulin levels and stabilized blood glucose levels.
SST analogues (such as octreotide) are now frequently used as second line therapy for children who are not responsive to diazoxide and there are now a large number of studies supporting the use of SST analogues for childhood HH.73 Many studies document the use of SST analogues for treating childhood HH, dating back to 1977.14,27,67–175 However some patients with HH do not respond to SST analogue treatment.
Interestingly, in some parts of the world where diazoxide is not available then octreotide has been used even as a first line therapy.176 SST analogues have also been used in the long term where in some patients with severe HH the need for pancreatectomy can be avoided.14 The dose range is typically between 5 and 25 µg/kg per day (maximum dose can go to 40 µg/kg per day) administered either intravenously, subcutaneously by continuous infusion, or as 6–8 hourly subcutaneous injections for short- and long-term treatment of hyperinsulinaemia.2
The development of long-acting formulations of SST analogues which require only weekly or monthly injections can improve patient compliance. In particular, lanreotide (LAN) Autogel®, which is a viscous aqueous formulation supplied in ready-to-use prefilled syringes, can be administered every 28–56 days.177 Since its introduction into clinical practice, several studies have evaluated the clinical utility of LAN Autogel® in the medical treatment of children with HH. The first study described two patients with HH who were initially managed with diazoxide and octreotide but then at the ages of 4 and 4.5 years were started on lanreotide acetate (Somatuline Autogel), administered by deep subcutaneous injection of 30 mg once a month.98 This allowed the patients to come off the diazoxide and the daily octreotide injections and normoglycaemia was maintained. In the study by Le Quan Sang et al.102 10 patients underwent replacement of their three daily subcutaneous octreotide (Sandostatin, Novartis) injections by a single and monthly intramuscular injection of long-acting release (LAR) octreotide (Sandostatin LP, Novartis). The study concluded that LAR octreotide was efficient, well tolerated and contributed to a clear simplification of the medical care. In another multicentre study,106 involving six different centres in Europe, data were obtained retrospectively from 27 patients with HH who received long-acting SST analogues. These included information on glycaemic stability, auxology and adverse effect profile in clinical follow-up assessments. The study concluded that long-acting SST analogues are effective in glycaemic control of patients with HH. Several other studies have also now documented the beneficial effects of long-acting octreotide formulations in children with different forms (focal and diffuse) of HH.110
The pharmacodynamics and pharmacokinetics of octreotide have largely been studied in adults with no studies in children. In blood, octreotide is mainly distributed in the plasma with about 65% bound to lipoproteins. Octreotide administered by subcutaneous injection is rapidly absorbed, with peak concentrations of 5.2 ng/mL occurring around 25 min after a 100-µg dose in adults. The apparent half-life of octreotide is approximately 1.7 h, which is significantly greater than the 1–3-min half-life of SST (in adults).178 The effects of octreotide are variable, but can last for up to 12 h, and approximately 32% of a dose is excreted in the urine as unchanged drug.179 Dosage adjustments may be required in infants with impaired renal function which leads to decreased clearance and increased half-life.178 Table 2 compares the pharmacology of short and long acting octreotide formulations.
Table 2.
Comparison of octreotide, lanreotide and Sandostatin LAR.
| Parameter | Octreotide | Lanreotide | Sandostatin LAR |
|---|---|---|---|
| Dose range | 5–25 µg/kg per day; maximum daily dose: 35 µg/kg per day | 30–90 mg per month | Cumulative 31-day subcutanous dose, which will equal the monthly intramuscular dose |
| Half-life | 90–120 min | 23–30 days | Steady state achieved after three injections |
| Time to peak action | 0.4 h | 7–12 h | 1 h |
| Mode of administration | Subcutaneous daily | Deep subcutaneous or intramuscular injection once a month | Intramuscular injection once a month |
LAR, long-acting release.
Use of SST analogues in the management of PPHH after Roux-en-Y gastric by-pass
A number of different treatment options have been proposed for the management of PPHH after Roux-en-Y gastric by-pass.180 These include having frequent high-fibre meals and reduction or elimination of simple carbohydrates in the diet, and using medications such as acarbose, diazoxide, calcium channel blockers and octreotide.181 Long-acting octreotide formulations (such as Sandostatin-LAR) have been used in the management of PPHH due to Roux-en-Y-gastric-bypass with equal efficacy as octreotide in suppressing hypoglycaemic symptoms.182 In one large study comparing the efficacy of subcutaneous octreotide with that of the long-acting LAR formulation on 30 patients, it was found that the administration of LAR octreotide improved the oral glucose tolerance results, symptoms and quality of life in patients with postoperative dumping.183 Several other studies have described the use of short- and long-acting SST formulations for the management of PPHH due to Roux-en-Y-gastric-bypass.24,184,185
Use of octreotide in HH due to sulphonylureas
HH due to sulphonylureas (either due to treatment or to intentional overdose) can also be managed with octreotide. Sulphonylurea poisoning can lead to severe hypoglycaemia especially in children and in the elderly, which may be refractory to intravenous glucose infusion. In these cases, octreotide can be used to treat hypoglycaemia.186 Even in children with accidental poisoning due to sulphonylureas, octreotide has been safely used to restore normoglycaemia.187 In 1993 Boyle et al.188 tested the hypothesis that octreotide could reverse sulphonylurea induced HH in eight normal subjects administered glipizide on three occasions. They found that octreotide reduced and in four of eight subjects entirely eliminated the need for exogenous glucose after a large overdose of glipizide and concluded that octreotide was safe and effective and should be considered as a logical therapeutic alternative for this metabolic emergency. Patients have also been described with sulphonylurea induced HH who are refractory to treatment with boluses of dextrose but respond to octreotide.189 Since the first reports, there have been numerous studies190–217 describing treatment of sulphonylurea overdoses with octreotide. In virtually all the studies that reported the use of octreotide, there were no serious adverse effects.
Use of SST analogues in patients with insulinoma
In 1975 Curnow et al. and Christensen et al.218,219 both reported the use of SST in patients with insulinoma. In both cases the infusion of SST improved the blood glucose profile of the patients. Following these two studies, several other studies were then also published which documented the efficacy of SST as well as a potential aid in diagnosis in patients with both benign and malignant insulinoma.220–225
For adults with insulinoma, the first report on the use of a SST analogue was in 1979, when a new long-acting octapeptide analogue of SST, Des AA1, 2, 4, 5, 12, 13 D Try8 SST, was tested in eight patients with different pancreatic endocrine tumours.226 The analogue was given subcutaneously and suppressed the tumour-derived hormones in patients with insulinomas, glucagonomas and gastrinomas for up to 24 h. The prolonged action appeared to be the result of slow release from the injection site and there were no side effects reported. Long-acting SST analogue (SMS 201-995) was used in the early 1980s in patients with, again, both benign and malignant insulinomas.227,228 Since these early studies numerous studies have documented the beneficial effects of SST analogues in insulinoma.
The use of SST analogues in the scintigraphic imaging of insulinomas and in the medical management of these tumours seems to be restricted to a subgroup of patients. SSTR2 and 5 are the predominant subtype receptors expressed in about 70% of malignant insulinomas whereas SSTR1 is present in about 50% and SSTR3 and 4 in approximately 15–20%.229 Interestingly some insulinomas do not express any SSTR. In insulinomas where there are no SST receptors, octreotide will aggravate hypoglycaemia due to suppression of glucagon and growth hormone.230 So, a test dose of octreotide is suggested before commencing treatment. Pasireotide, another SST analogue which can target multiple receptors, was reported to achieve better glycaemic control than octreotide and lanreotide in a patient with malignant insulinoma.231
Octreotide during pregnancy
The use of octreotide during pregnancy seems to be associated with poor outcome, as reported in two studies,232,233 but in another study114 octreotide treatment was found to be effective in controlling endogenous hyperinsulinism during pregnancy and did not affect physiological changes during pregnancy such as insulin-resistance or placental GH level with no abnormalities in the foetus.
In the report by Skajaa et al.,232 a woman with a genetic form of HH was given octreotide during the first four pregnancies, resulting in two cases of early termination of pregnancy on parental request and two cases of inappropriate foetal growth and unviable outcome. The following two pregnancies, treated with diet only, had a successful outcome. In the second report, by Geilswijk et al.,233 treatment of the mother with octreotide (who was also affected by a genetic form of HH) led to foetal growth restriction. These two observations suggest that octreotide should be used with caution during pregnancy in mothers who have genetic forms of HH.
Octreotide use in small for gestational age infants with HH
A case series of neonates born small for gestational age with HH were successfully treated with octreotide infusion and the blood glucose was corrected with no complications.176
Octreotide use in Beckwith–Wiedemann syndrome and HH
There have been two cases of newborns with Beckwith–Wiedemann syndrome and HH who were managed with SST analogues, again with no complications.118,122
Side effects of SST analogues in patients with HH
The local subcutaneous injection of octreotide can cause pain, a sensation of stinging and burning as well as redness and swelling. Common gastrointestinal adverse effects of octreotide include delayed gastric emptying, reducing gallbladder contraction, nausea, abdominal cramps, diarrhoea, malabsorption of fat, and flatulence.234 STT analogues inhibit the secretion of GH, prolactin, thyrotropin, cholecystokinin, gastric inhibitory peptide, gastrin, motilin, neurotensin, secretin, glucagon, insulin and pancreatic polypeptide. They also inhibit the exocrine secretion of amylase of salivary glands; hydrochloric acid, pepsinogen, and intrinsic factor of gastrointestinal mucosa; and enzymes and bicarbonate of pancreas and bile in the liver.235 The suppression of GH (and IGF-I) and thyroid hormones, in theory, can lead to stunting of growth; however, in clinical practice, the suppressive effects of octreotide seem to be overridden with no major long-term problems.3 In one study assessing the long-term impact of octreotide in children with HH, where the mean ± standard deviation duration of follow-up on octreotide therapy was 52.4 (±33.8) months (range 6 months to 9.5 years), it was found that transient elevation of liver enzymes and asymptomatic gallbladder pathology were the most prevalent long-term side effects of octreotide therapy.126 There was no correlation between the dose or the duration of octreotide therapy and the development of liver dysfunction and gallbladder pathology and no long-term effects on growth. Octreotide can decrease gallbladder contractility and bile secretion, leading to steatorrhoea, cholestasis, hepatic dysfunction and cholelithiasis.236,237 Blood flow to the splanchnic circulation is decreased by octreotide; hence, it must be used cautiously in babies at risk of necrotizing enterocolitis (see below). Table 3 lists all the side effects reported due to the use of SST analogues in children with HH.
Table 3.
Reported side effects of somatostatin analogues.
| Side effects | References |
|---|---|
| Hepatitis, cholestasis and gallstones | Demirbilek et al.,126 Levy-Khademi et al.,236 Avatapalle et al.,162 Ben-Ari et al.,77 Malik et al.,181 Koren et al.,78 Radetti et al.,124 Glaser et al.139 |
| Necrotizing enterocolitis | Laje et al.,238 Hawkes et al.,134 Abdel Khalek and Kandil138 Alsaedi et al.,150 McMahon et al.,108 Reck-Burneo et al.239 |
| Tachyphylaxis | Thornton et al.,3 Hawdon et al.130 |
| Inhibition of other hormones | Aynsley-Green et al.69 |
| Gastrointestinal dysmotility | Glaser et al.139 |
| Paradoxical hyperglycaemia and bradycardia | Batra et al.173 |
| Prolonged QT interval | Celik et al.83 |
| Deceleration of growth | Yorifuji et al.113 |
| Seizure after stopping octreotide | Bas et al.169 |
Necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is a disease mainly of premature infants and associated with a high mortality rate. It typically presents with abdominal distension, bilious vomiting and bloody stools, which can lead to sepsis and shock in severe cases. NEC is characterized by coagulation necrosis of the intestine, bacterial overgrowth and inflammation.240 The first report in the literature of octreotide-related NEC was in 2010 by Laje et al.238 In this study, out of 197 neonates who developed NEC, four had no risk factor for NEC, except that they were being treated with octreotide. The authors reported that NEC occurred in 2% of neonates who were treated with octreotide for HH. See Table 3, which lists the studies reporting NEC in patients with HH due to SST analogue therapy. All reported cases of NEC associated with octreotide use in infants with HH occurred in neonates younger than 1 month of age and within 15 days of commencing octreotide therapy, at doses of 15–27 µg/kg per day.241 However, a case of late-onset NEC in a patient with Beckwith–Wiedemann syndrome and HH, who was treated with a relatively low dose of octreotide, has also been reported.134
The mechanism of octreotide induced NEC in patients with HH is not completely clear. But it is known that octreotide reduces superior mesenteric artery blood flow as well as portal venous blood flow and blunts the normal postprandial rise. The reduction in blood flow seems to be dose dependent.242
Tachyphylaxis
The major limiting factor for the use of octreotide in children with HH is the associated tachyphylaxis. Nearly all patients show resistance to the continued use of octreotide, even to increasing doses.3,130 Some patients develop tachyphylaxis soon after treatment whereas in others it may take weeks or months to develop. The underlying mechanisms leading to tachyphylaxis are not known but might involve SSTR down-regulation, or G protein uncoupling and/or receptor internalization.243
Future directions
Although most children with HH show a good initial response to treatment with octreotide the response becomes less effective over time due to the tolerance effect. Therefore, more potent SST analogues are required with less tolerance. Pasireotide is a relatively new SST analogue with increased affinity for SSTR5 and with a longer half-life.244 Its affinity for SSTR5 is thought to be 40 times that of the other analogues. It has been used primarily for the treatment of Cushing’s disease due to increased adrenocorticotrophic hormone secretion. However, in adults with PPHH due to gastric by-pass, it has been shown to be effective in comparison with octreotide.184 Pasireotide has not been trialled in children with congenital forms of HH but it might offer potential treatment options for some forms of HH in the childhood period. Thus, one of the future directions could be to undertake a clinical trial of pasireotide use in children with HH and assess its effectiveness.
Peptide receptor radionuclide therapy (PRRT) is a relatively new mode of treatment for patients (mostly adults) with inoperable, usually metastasized, neuroendocrine tumours. SST analogues are radiolabelled and can bind with high affinity to the cancer cell expressing the SST receptor. PRRT has been reported to be effective in adults with malignant insulinomas, with one study reporting successful control of blood glucose in five patients with malignant insulinomas.245 PRRT treatment has not been reported in children with HH, thus this could be another potential treatment modality which needs further exploration.
Conclusion
SST has a key physiological role in the inhibition of insulin and glucagon secretion from the pancreatic β-cells and δ-cells respectively, hence the use of its analogues in the treatment of different forms of HH. The precise molecular mechanisms involved in the inhibition of insulin and glucagon secretion are not known. Due to SST’s short half-life SST analogues have been developed which are used to treat various conditions, including different forms of HH in children and adults. Both octreotide and the long-acting formulations are effective in the management of patients with HH. In the neonatal period, octreotide must be used with caution as there is a risk of developing NEC.
Footnotes
Author contribution(s): Basma Haris: Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Writing-original draft; Writing-review & editing.
Saraswathi Sundararajan: Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Writing-original draft.
Khalid Hussain: Conceptualization; Data curation; Methodology; Project administration; Resources; Supervision; Writing-original draft; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Khalid Hussain  https://orcid.org/0000-0002-5480-7112
https://orcid.org/0000-0002-5480-7112
Contributor Information
Basma Haris, Department of Paediatric Medicine, Division of Endocrinology, Sidra Medicine, Doha, Qatar.
Saras Saraswathi, Department of Paediatric Medicine, Division of Endocrinology, Sidra Medicine, Doha, Qatar.
Khalid Hussain, Professor of Paediatrics, Weill Cornell Medicine-Qatar, Division Chief – Endocrinology, Department of Paediatric Medicine, Division of Endocrinology, Sidra Medicine, OPC, C6-340 |PO Box 26999, Al Luqta Street, Education City North Campus, Doha, Qatar.
References
- 1. Hussain K, Aynsley-Green A. Management of hyperinsulinism in infancy and childhood. Ann Med 2000; 32: 544–551. [DOI] [PubMed] [Google Scholar]
- 2. Aynsley-Green A, Hussain K, Hall J, et al. Practical management of hyperinsulinism in infancy. Arch Dis Child Fetal Neonatal Ed 2000; 82: F98–F107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thornton PS, Alter CA, Katz LE, et al. Short- and long-term use of octreotide in the treatment of congenital hyperinsulinism. J Pediatr 1993; 123: 637–643. [DOI] [PubMed] [Google Scholar]
- 4. Kane C, Shepherd RM, Squires PE, et al. Loss of functional KATP channels in pancreatic beta-cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nat Med 1996; 2: 1344–1347. [DOI] [PubMed] [Google Scholar]
- 5. Stanley CA. Hypoglycemia in the neonate. Pediatr Endocrinol Rev 2006; 4(Suppl. 1): 76–81. [PubMed] [Google Scholar]
- 6. Fafoula O, Alkhayyat H, Hussain K. Prolonged hyperinsulinaemic hypoglycaemia in newborns with intrauterine growth retardation. Arch Dis Child Fetal Neonatal Ed 2006; 91: F467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galcheva S, Al-Khawaga S, Hussain K. Diagnosis and management of hyperinsulinaemic hypoglycaemia. Best Pract Res Clin Endocrinol Metab 2018; 32: 551–573. [DOI] [PubMed] [Google Scholar]
- 8. Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet 1996; 5: 1809–1812. [DOI] [PubMed] [Google Scholar]
- 9. Thomas PM, Cote GJ, Wohllk N, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 1995; 268: 426–429. [DOI] [PubMed] [Google Scholar]
- 10. Verkarre V, Fournet JC, de Lonlay P, et al. Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest 1998; 102: 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sempoux C, Capito C, Bellanne-Chantelot C, et al. Morphological mosaicism of the pancreatic islets: a novel anatomopathological form of persistent hyperinsulinemic hypoglycemia of infancy. J Clin Endocrinol Metab 2011; 96: 3785–3793. [DOI] [PubMed] [Google Scholar]
- 12. de Lonlay-Debeney P, Poggi-Travert F, Fournet JC, et al. Clinical features of 52 neonates with hyperinsulinism. N Engl J Med 1999; 340: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 13. Fékété CN, de Lonlay P, Jaubert F, et al. The surgical management of congenital hyperinsulinemic hypoglycemia in infancy. J Pediatr Surg 2004; 39: 267–269. [DOI] [PubMed] [Google Scholar]
- 14. Glaser B, Hirsch HJ, Landau H. Persistent hyperinsulinemic hypoglycemia of infancy: long-term octreotide treatment without pancreatectomy. J Pediatr 1993; 123: 644–650. [DOI] [PubMed] [Google Scholar]
- 15. Han B, Mohamed Z, Estebanez MS, et al. Atypical forms of congenital hyperinsulinism in infancy are associated with mosaic patterns of immature islet cells. J Clin Endocrinol Metab 2017; 102: 3261–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Service FJ, McMahon MM, O’Brien PC, et al. Functioning insulinoma-incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 1991; 66: 711–719. [DOI] [PubMed] [Google Scholar]
- 17. Oberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer 2016; 23: R551–R566. [DOI] [PubMed] [Google Scholar]
- 18. Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol 2005; 19: 753–781. [DOI] [PubMed] [Google Scholar]
- 19. Padidela R, Fiest M, Arya V, et al. Insulinoma in childhood: clinical, radiological, molecular and histological aspects of nine patients. Eur J Endocrinol 2014; 170: 741–747. [DOI] [PubMed] [Google Scholar]
- 20. Kar P, Price P, Sawers S, et al. Insulinomas may present with normoglycemia after prolonged fasting but glucose-stimulated hypoglycemia. J Clin Endocrinol Metab 2006; 91: 4733–4736. [DOI] [PubMed] [Google Scholar]
- 21. Service GJ, Thompson GB, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005; 353: 249–254. [DOI] [PubMed] [Google Scholar]
- 22. Øhrstrøm CC, Worm D, Hansen DL. Postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: an update. Surg Obes Relat Dis 2017; 13: 345–351. [DOI] [PubMed] [Google Scholar]
- 23. Bojsen-Møller KN. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Dan Med J 2015; 62: B5057. [PubMed] [Google Scholar]
- 24. Spanakis E, Gragnoli C. Successful medical management of status post-Roux-en-Y-gastric-bypass hyperinsulinemic hypoglycemia. Obes Surg 2009; 19: 1333–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krulich L, Dhariwal AP, McCann SM. Stimulatory and inhibitory effects of purified hypothalamic extracts on growth hormone release from rat pituitary in vitro. Endocrinology 1968; 83: 783–790. [DOI] [PubMed] [Google Scholar]
- 26. Brazeau P, Vale W, Burgus R, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973; 179: 77–79. [DOI] [PubMed] [Google Scholar]
- 27. Hellman B, Lernmark A. Inhibition of the in vitro secretion of insulin by an extract of pancreatic alpha-1 cells. Endocrinology 1969; 84: 1484–1488. [DOI] [PubMed] [Google Scholar]
- 28. Reichlin S. Somatostatin. N Engl J Med 1983; 309: 1495–1501. [DOI] [PubMed] [Google Scholar]
- 29. Shen LP, Rutter WJ. Sequence of the human somatostatin I gene. Science 1984; 224: 168–171. [DOI] [PubMed] [Google Scholar]
- 30. Patel YC, Galanopoulou A. Processing and intracellular targeting of prosomatostatin-derived peptides: the role of mammalian endoproteases. Ciba Found Symp 1995; 190: 26–40; discussion 40–50. [DOI] [PubMed] [Google Scholar]
- 31. O’Toole TJ, Sharma S. Physiology, somatostatin. Treasure Island, FL: StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 32. Alpert LC, Brawer JR, Patel YC, et al. Somatostatinergic neurons in anterior hypothalamus: immunohistochemical localization. Endocrinology 1976; 98: 255–258. [DOI] [PubMed] [Google Scholar]
- 33. Folli F, La Rosa S, Finzi G, et al. Pancreatic islet of Langerhans’ cytoarchitecture and ultrastructure in normal glucose tolerance and in type 2 diabetes mellitus. Diabetes Obes Metab 2018; 20(Suppl. 2): 137–144. [DOI] [PubMed] [Google Scholar]
- 34. Rorsman P, Huising MO. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat Rev Endocrinol 2018; 14: 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taborsky GJ, Jr, Smith PH, Porte D., Jr. Interaction of somatostatin with the A and B cells of the endocrine pancreas. Metabolism 1978; 27: 1299–1302. [DOI] [PubMed] [Google Scholar]
- 36. Braun M. The somatostatin receptor in human pancreatic β-cells. Vitam Horm 2014; 95: 165–193. [DOI] [PubMed] [Google Scholar]
- 37. Li N, Yang Z, Li Q, et al. Ablation of somatostatin cells leads to impaired pancreatic islet function and neonatal death in rodents. Cell Death Dis 2018; 9: 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hauge-Evans AC, King AJ, Carmignac D, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 2009; 58: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwetz TA, Ustione A, Piston DW. Neuropeptide Y and somatostatin inhibit insulin secretion through different mechanisms. Am J Physiol Endocrinol Metab 2013; 304: E211–E221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cuevas-Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. J Mol Endocrinol 2014; 52: R223–R240. [DOI] [PubMed] [Google Scholar]
- 41. Ballian N, Brunicardi FC, Wang XP. Somatostatin and its receptors in the development of the endocrine pancreas. Pancreas 2006; 33: 1–12. [DOI] [PubMed] [Google Scholar]
- 42. Strowski MZ, Parmar RM, Blake AD, et al. Somatostatin inhibits insulin and glucagon secretion via two receptor subtypes: an in Vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 2000; 141: 111–117. [DOI] [PubMed] [Google Scholar]
- 43. Strowski MZ, Blake AD. Function and expression of somatostatin receptors of the endocrine pancreas. Mol Cell Endocrinol 2008; 286: 169–179. [DOI] [PubMed] [Google Scholar]
- 44. Kumar U, Sasi R, Suresh S, et al. Subtype-selective expression of the five somatostatin receptors (hSSTR1-5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes 1999; 48: 77–85. [DOI] [PubMed] [Google Scholar]
- 45. Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999; 20: 157–198. [DOI] [PubMed] [Google Scholar]
- 46. Patel YC, Greenwood MT, Warszynska A, et al. All five cloned human somatostatin receptors (hSSTR1-5) are functionally coupled to adenylyl cyclase. Biochem Biophys Res Commun 1994; 198: 605–612. [DOI] [PubMed] [Google Scholar]
- 47. Arrojo EDR, Jacob S, García-Prieto CF, et al. Structural basis for delta cell paracrine regulation in pancreatic islets. Nat Commun 2019; 10: 3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith PA, Sellers LA, Humphrey PP. Somatostatin activates two types of inwardly rectifying K+ channels in MIN-6 cells. J Physiol 2001; 532: 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol 2013; 24: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li C, Chen P, Vaughan J, et al. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology 2003; 144: 3216–3224. [DOI] [PubMed] [Google Scholar]
- 51. van der Meulen T, Donaldson CJ, Cáceres E, et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med 2015; 21: 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vergari E, Knudsen JG, Ramracheya R, et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat Commun 2019; 10: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rohrer SP, Birzin ET, Mosley RT, et al. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science 1998; 282: 737–740. [DOI] [PubMed] [Google Scholar]
- 54. Strowski MZ, Kohler M, Chen HY, et al. Somatostatin receptor subtype 5 regulates insulin secretion and glucose homeostasis. Mol Endocrinol 2003; 17: 93–106. [DOI] [PubMed] [Google Scholar]
- 55. Broglio F, Arvat E, Benso A, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 2001; 86: 5083–5086. [DOI] [PubMed] [Google Scholar]
- 56. Arosio M, Ronchi CL, Gebbia C, et al. Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. J Clin Endocrinol Metab 2003; 88: 701–704. [DOI] [PubMed] [Google Scholar]
- 57. Rai U, Thrimawithana TR, Valery C, et al. Therapeutic uses of somatostatin and its analogues: current view and potential applications. Pharmacol Ther 2015; 152: 98–110. [DOI] [PubMed] [Google Scholar]
- 58. de Herder WW, Lamberts SWJ. Somatostatin and somatostatin analogues: diagnostic and therapeutic uses. Curr Opin Oncol 2002; 14: 53–57. [DOI] [PubMed] [Google Scholar]
- 59. Rosenthal LE, Yamashiro DJ, Rivier J, et al. Structure-activity relationships of somatostatin analogs in the rabbit ileum and the rat colon. J Clin Invest 1983; 71: 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anthony L, Freda PU. From somatostatin to octreotide LAR: evolution of a somatostatin analogue. Curr Med Res Opin 2009; 25: 2989–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bruns C, Lewis I, Briner U, et al. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 2002; 146: 707–716. [DOI] [PubMed] [Google Scholar]
- 62. Igarashi H, Hijioka M, Lee L, et al. Biotherapy of pancreatic neuroendocrine tumors using somatostatin analogs. J Hepatobiliary Pancreat Sci 2015; 22: 618–622. [DOI] [PubMed] [Google Scholar]
- 63. Pawlikowski M, Melen-Mucha G. Perspectives of new potential therapeutic applications of somatostatin analogs. Neuro Endocrinol Lett 2003; 24: 21–27. [PubMed] [Google Scholar]
- 64. Moller N, Bagger JP, Schmitz O, et al. Somatostatin enhances insulin-stimulated glucose uptake in the perfused human forearm. J Clin Endocrinol Metab 1995; 80: 1789–1793. [DOI] [PubMed] [Google Scholar]
- 65. Krassas GE, Tzotzas T, Papazisis K, et al. The efficacy of somatostatin analogues in the treatment of diabetic retinopathy and thyroid eye disease. Clin Ophthalmol 2007; 1: 209–215. [PMC free article] [PubMed] [Google Scholar]
- 66. Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993; 20: 716–731. [DOI] [PubMed] [Google Scholar]
- 67. Hirsch HJ, Loo S, Evans N, et al. Hypoglycemia of infancy and nesidioblastosis. Studies with somatostatin. N Engl J Med 1977; 296: 1323–1326. [DOI] [PubMed] [Google Scholar]
- 68. Roti E, Ghinelli C, Bandini P, et al. Effects of somatostatin in a case of severe hypoglycemia due to nesidioblastosis. J Endocrinol Invest 1981; 4: 209–212. [DOI] [PubMed] [Google Scholar]
- 69. Aynsley-Green A, Barnes ND, Adrian TE, et al. Effect of somatostatin infusion on intermediary metabolism and entero-insular hormone release in infants with hyperinsulinaemic hypoglycaemia. Acta Paediatr Scand 1981; 70: 889–895. [DOI] [PubMed] [Google Scholar]
- 70. Bougneres PF, Landier F, Garnier P, et al. Treatment of insulin excess by continuous subcutaneous infusion of somatostatin and glucagon in an infant. J Pediatr 1985; 106: 792–794. [DOI] [PubMed] [Google Scholar]
- 71. Wendel U, Kardorff C, Dorittke P, et al. [Somatostatin in the emergency treatment of persistent hypoglycemias caused by hyperinsulinism (nesidioblastosis of the pancreas)]. Monatsschr Kinderheilkd 1985; 133: 527–531. [PubMed] [Google Scholar]
- 72. Bruining GJ, Bosschaart AN, Aarsen RS, et al. Normalization of glucose homeostasis by a long-acting somatostatin analog SMS 201-995 in a newborn with nesidioblastosis. Acta Endocrinol Suppl (Copenh) 1986; 279: 334–339. [DOI] [PubMed] [Google Scholar]
- 73. Delemarre-van de, Waal HA, Veldkamp EJ, Schrander-Stumpel CT. Long-term treatment of an infant with nesidioblastosis using a somatostatin analogue. N Engl J Med 1987; 316: 222–223. [DOI] [PubMed] [Google Scholar]
- 74. Behrens R, Prenbe AK, Barmeier H. Unusual course of neonatal hyperinsulinaemic hypoglycaemia (nesidioblastosis). Arch Dis Child Fetal Neonatal Ed 1998; 78: F156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kondo T, Tomita S, Adachi H, et al. A case of hyperinsulinemia of undetermined origin, successfully treated with long-acting octreotide. Endocr J 2005; 52: 511–517. [DOI] [PubMed] [Google Scholar]
- 76. Sullivan MJ, Taylor BJ, Broadbent RS, et al. Somatostatin analogue SMS 201-995 in the short-term management of neonatal hyperinsulinism due to nesidioblastosis. Aust Paediatr J 1988; 24: 375–378. [DOI] [PubMed] [Google Scholar]
- 77. Ben-Ari J, Greenberg M, Nemet D, et al. Octreotide-induced hepatitis in a child with persistent hyperinsulinemia hypoglycemia of infancy. J Pediatr Endocrinol Metab 2013; 26: 179–182. [DOI] [PubMed] [Google Scholar]
- 78. Koren I, Riskin A, Barthlen W, et al. Hepatitis in an infant treated with octreotide for congenital hyperinsulinism. J Pediatr Endocrinol Metab 2013; 26: 183–185. [DOI] [PubMed] [Google Scholar]
- 79. Takahashi N, Nagamine M, Fukuda M, et al. Octreotide-treated diabetes accompanied by endogenous hyperinsulinemic hypoglycemia and protein-losing gastroenteropathy. Case Rep Med 2011; 2011: 381203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Blakely ML, Lobe TE, Cohen J, et al. Laparoscopic pancreatectomy for persistent hyperinsulinemic hypoglycemia of infancy. Surg Endosc 2001; 15: 897–898. [DOI] [PubMed] [Google Scholar]
- 81. Kuno T, Fujita I, Ohta M, et al. Normal growth after administration of octreotide: report on a case of persistent hyperinsulinemic hypoglycemia of infancy treated by continuous subcutaneous injection of octreotide. Endocr J 1999; 46(Suppl): S47–S49. [DOI] [PubMed] [Google Scholar]
- 82. Tauber MT, Harris AG, Rochiccioli P. Clinical use of the long acting somatostatin analogue octreotide in pediatrics. Eur J Pediatr 1994; 153: 304–310. [DOI] [PubMed] [Google Scholar]
- 83. Celik N, Cinaz P, Emeksiz HC, et al. Octreotide-induced long QT syndrome in a child with congenital hyperinsulinemia and a novel missense mutation (p.Met115Val) in the ABCC8 gene. Horm Res Paediatr 2013; 80: 299–303. [DOI] [PubMed] [Google Scholar]
- 84. Leibowitz G, Glaser B, Higazi AA, et al. Hyperinsulinemic hypoglycemia of infancy (nesidioblastosis) in clinical remission: high incidence of diabetes mellitus and persistent beta-cell dysfunction at long-term follow-up. J Clin Endocrinol Metab 1995; 80: 386–392. [DOI] [PubMed] [Google Scholar]
- 85. Unal S, Gonulal D, Ucakturk A, et al. A novel homozygous mutation in the KCNJ11 gene of a neonate with congenital hyperinsulinism and successful management with sirolimus. J Clin Res Pediatr Endocrinol 2016; 8: 478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chandran S, Peng FY, Rajadurai VS, et al. Paternally inherited ABCC8 mutation causing diffuse congenital hyperinsulinism. Endocrinol Diabetes Metab Case Rep 2013; 2013: 130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Levy-Shraga Y, Pinhas-Hamiel O, Kraus-Houminer E, et al. Cognitive and developmental outcome of conservatively treated children with congenital hyperinsulinism. J Pediatr Endocrinol Metab 2013; 26: 301–308. [DOI] [PubMed] [Google Scholar]
- 88. Vajravelu ME, Congdon M, Mitteer L, et al. Continuous intragastric dextrose: a therapeutic option for refractory hypoglycemia in congenital hyperinsulinism. Horm Res Paediatr 2019; 91: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Christesen HB, Brusgaard K, Beck Nielsen H, et al. Non-insulinoma persistent hyperinsulinaemic hypoglycaemia caused by an activating glucokinase mutation: hypoglycaemia unawareness and attacks. Clin Endocrinol (Oxf) 2008; 68: 747–755. [DOI] [PubMed] [Google Scholar]
- 90. Liberatore Junior Rdel R, Negri AA, Martinelli Junior CE, et al. [Hyperinsulinemic hypoglycemia of the infancy: analysis of clinical data from a Brazilian sample]. Arq Bras Endocrinol Metabol 2012; 56: 666–671. [DOI] [PubMed] [Google Scholar]
- 91. Vieira TC, Bergamin CS, Gurgel LC, et al. Hyperinsulinemic hypoglycemia evolving to gestational diabetes and diabetes mellitus in a family carrying the inactivating ABCC8 E1506K mutation. Pediatr Diabetes 2010; 11: 505–508. [DOI] [PubMed] [Google Scholar]
- 92. Corda H, Kummer S, Welters A, et al. Treatment with long-acting lanreotide autogel in early infancy in patients with severe neonatal hyperinsulinism. Orphanet J Rare Dis 2017; 12: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Loechner KJ, Akrouh A, Kurata HT, et al. Congenital hyperinsulinism and glucose hypersensitivity in homozygous and heterozygous carriers of Kir6.2 (KCNJ11) mutation V290M mutation: K(ATP) channel inactivation mechanism and clinical management. Diabetes 2011; 60: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wabitsch M, Lahr G, Van de, Bunt M, et al. Heterogeneity in disease severity in a family with a novel G68V GCK activating mutation causing persistent hyperinsulinaemic hypoglycaemia of infancy. Diabet Med 2007; 24: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 95. Costa RR, Maia FF, Araujo LR. [Endogenous persistent hypoglycemia of adult: case report]. Arq Bras Endocrinol Metabol 2007; 51: 125–130. [DOI] [PubMed] [Google Scholar]
- 96. Ludwig A, Ziegenhorn K, Empting S, et al. Glucose metabolism and neurological outcome in congenital hyperinsulinism. Semin Pediatr Surg 2011; 20: 45–49. [DOI] [PubMed] [Google Scholar]
- 97. Wang WY, Sun Y, Zhao WT, et al. Congenital hyperinsulinism in China: a review of Chinese literature over the past 15 years. J Clin Res Pediatr Endocrinol 2017; 9: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Modan-Moses D, Koren I, Mazor-Aronovitch K, et al. Treatment of congenital hyperinsulinism with lanreotide acetate (Somatuline Autogel). J Clin Endocrinol Metab 2011; 96: 2312–2317. [DOI] [PubMed] [Google Scholar]
- 99. Dacou-Voutetakis C, Psychou F, Maniati-Christidis M. Persistent hyperinsulinemic hypoglycemia of infancy: long-term results. J Pediatr Endocrinol Metab 1998; 11(Suppl. 1): 131–141. [DOI] [PubMed] [Google Scholar]
- 100. Marquard J, Palladino AA, Stanley CA, et al. Rare forms of congenital hyperinsulinism. Semin Pediatr Surg 2011; 20: 38–44. [DOI] [PubMed] [Google Scholar]
- 101. Welters A, Lerch C, Kummer S, et al. Long-term medical treatment in congenital hyperinsulinism: a descriptive analysis in a large cohort of patients from different clinical centers. Orphanet J Rare Dis 2015; 10: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Le Quan Sang K-H, Arnoux J-B, Mamoune A, et al. Successful treatment of congenital hyperinsulinism with long-acting release octreotide. Eur J Endocrinol 2012; 166: 333–339. [DOI] [PubMed] [Google Scholar]
- 103. Daneman D, Ehrlich RM. The enigma of persistent hyperinsulinemic hypoglycemia of infancy. J Pediatr 1993; 123: 573–575. [DOI] [PubMed] [Google Scholar]
- 104. Martini S, Aceti A, Lima M, et al. Octreotide in a critically Ill extremely preterm infant with perforated necrotizing enterocolitis. Pediatrics 2016; 138: e20160467. [DOI] [PubMed] [Google Scholar]
- 105. Welters A, Meissner T, Grulich-Henn J, et al. Characterization of diabetes following pancreatic surgery in patients with congenital hyperinsulinism. Orphanet J Rare Dis 2018; 13: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van der Steen I, van Albada ME, Mohnike K, et al. A multicenter experience with long-acting somatostatin analogues in patients with congenital hyperinsulinism. Horm Res Paediatr 2018; 89: 82–89. [DOI] [PubMed] [Google Scholar]
- 107. Dastamani A, Guemes M, Pitfield C, et al. The use of a long-acting somatostatin analogue (Lanreotide) in three children with focal forms of congenital hyperinsulinaemic hypoglycaemia. Horm Res Paediatr 2019; 91: 56–61. [DOI] [PubMed] [Google Scholar]
- 108. McMahon AW, Wharton GT, Thornton P, et al. Octreotide use and safety in infants with hyperinsulinism. Pharmacoepidemiol Drug Saf 2017; 26: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yadav D, Dhingra B, Kumar S, et al. Persistent hyperinsulinemic hypoglycemia of infancy. J Pediatr Endocrinol Metab 2012; 25: 591–593. [DOI] [PubMed] [Google Scholar]
- 110. Kuhnen P, Marquard J, Ernert A, et al. Long-term lanreotide treatment in six patients with congenital hyperinsulinism. Horm Res Paediatr 2012; 78: 106–112. [DOI] [PubMed] [Google Scholar]
- 111. DeClue TJ, Malone JI, Bercu BB. Linear growth during long-term treatment with somatostatin analog (SMS 201-995) for persistent hyperinsulinemic hypoglycemia of infancy. J Pediatr 1990; 116: 747–750. [DOI] [PubMed] [Google Scholar]
- 112. Mosdell KW, Visconti JA. Emerging indications for octreotide therapy, Part 1. Am J Hosp Pharm 1994; 51: 1184–1192. [PubMed] [Google Scholar]
- 113. Yorifuji T, Kawakita R, Hosokawa Y, et al. Efficacy and safety of long-term, continuous subcutaneous octreotide infusion for patients with different subtypes of KATP-channel hyperinsulinism. Clin Endocrinol (Oxf) 2013; 78: 891–897. [DOI] [PubMed] [Google Scholar]
- 114. Boulanger C, Vezzosi D, Bennet A, et al. Normal pregnancy in a woman with nesidioblastosis treated with somatostatin analog octreotide. J Endocrinol Invest 2004; 27: 465–470. [DOI] [PubMed] [Google Scholar]
- 115. Demirbilek H, Arya VB, Ozbek MN, et al. Clinical characteristics and phenotype-genotype analysis in Turkish patients with congenital hyperinsulinism; predominance of recessive KATP channel mutations. Eur J Endocrinol 2014; 170: 885–892. [DOI] [PubMed] [Google Scholar]
- 116. Murakami M, Mushiake S, Kashiwagi H, et al. A case of persistent hyperinsulinemic hypoglycemia of infancy successfully managed with subcutaneous octreotide injection and nocturnal intravenous glucose supply. Clin Pediatr Endocrinol 2007; 16: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Alberts AS, Falkson G. Rapid reversal of life-threatening hypoglycaemia with a somatostatin analogue (octreotide). A case report. S Afr Med J 1988; 74: 75–76. [PubMed] [Google Scholar]
- 118. Gerver WJ, Menheere PP, Schaap C, et al. The effects of a somatostatin analogue on the metabolism of an infant with Beckwith–Wiedemann syndrome and hyperinsulinaemic hypoglycaemia. Eur J Pediatr 1991; 150: 634–637. [DOI] [PubMed] [Google Scholar]
- 119. Du Y, Ju R, Xi Y, et al. A newborn with congenital hyperinsulinism. Fetal Pediatr Pathol 2019; 38: 1–6. [DOI] [PubMed] [Google Scholar]
- 120. Park SE, Flanagan SE, Hussain K, et al. Characterization of ABCC8 and KCNJ11 gene mutations and phenotypes in Korean patients with congenital hyperinsulinism. Eur J Endocrinol 2011; 164: 919–926. [DOI] [PubMed] [Google Scholar]
- 121. El Tonbary K, Robinson P, Banerjee I, et al. Congenital hyperinsulinism: management and outcome, a single tertiary centre experience. Eur J Pediatr 2020; 179: 947–952. [DOI] [PubMed] [Google Scholar]
- 122. Zarate YA, Shur N, Robin A, et al. Persistent congenital hyperinsulinism in two patients with Beckwith-Wiedemann syndrome due to mosaic uniparental disomy 11p. J Pediatr Endocrinol Metab 2014; 27: 951–955. [DOI] [PubMed] [Google Scholar]
- 123. Durmaz E, Flanagan SE, Parlak M, et al. A combination of nifedipine and octreotide treatment in an hyperinsulinemic hypoglycemic infant. J Clin Res Pediatr Endocrinol 2014; 6: 119–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Radetti G, Gentili L, Paganini C, et al. Cholelithiasis in a newborn following treatment with the somatostatin analogue octreotide. Eur J Pediatr 2000; 159: 550. [DOI] [PubMed] [Google Scholar]
- 125. Hindmarsh P, Brook CG. Short-term management of nesidioblastosis using the somatostatin analogue SMS 201-995. N Engl J Med 1987; 316: 221–222. [DOI] [PubMed] [Google Scholar]
- 126. Demirbilek H, Shah P, Arya VB, et al. Long-term follow-up of children with congenital hyperinsulinism on octreotide therapy. J Clin Endocrinol Metab 2014; 99: 3660–3667. [DOI] [PubMed] [Google Scholar]
- 127. Ferraz DP, Almeida MA, Mello BF. [Octreotide therapy for persistent hyperinsulinemic hypoglycemia of infancy]. Arq Bras Endocrinol Metabol 2005; 49: 460–467. [DOI] [PubMed] [Google Scholar]
- 128. Ramadan DG, Badawi MH, Zaki M, et al. Persistent hyperinsulinaemic hypoglycaemia of infancy (nesidioblastosis): a report from Kuwait. Ann Trop Paediatr 1999; 19: 55–59. [DOI] [PubMed] [Google Scholar]
- 129. Jackson JA, Hahn HB, Jr, Oltorf CE, et al. Long-term treatment of refractory neonatal hypoglycemia with long-acting somatostatin analog. J Pediatr 1987; 111: 548–551. [DOI] [PubMed] [Google Scholar]
- 130. Hawdon JM, Platt MPW, Lamb WH, et al. Tolerance to somatostatin analogue in a preterm infant with islet cell dysregulation syndrome. Arch Dis Child 1991; 66: 341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Flanagan SE, Kapoor RR, Banerjee I, et al. Dominantly acting ABCC8 mutations in patients with medically unresponsive hyperinsulinaemic hypoglycaemia. Clin Genet 2011; 79: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ramirez-Gonzalez LR, Sotelo-Alvarez JA, Rojas-Rubio P, et al. [Nesidioblastosis in the adult: a case report]. Cir Cir 2015; 83: 324–328. [DOI] [PubMed] [Google Scholar]
- 133. Jackson JA, Hahn HB, Jr, Oltorf CE. Long-acting somatostatin analog in refractory neonatal hypoglycemia: follow-up information. J Pediatr 1988; 113: 1118. [DOI] [PubMed] [Google Scholar]
- 134. Hawkes CP, Adzick NS, Palladino AA, et al. Late presentation of fulminant necrotizing enterocolitis in a child with hyperinsulinism on octreotide therapy. Horm Res Paediatr 2016; 86: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Giri D, Price V, Yung Z, et al. Fluoxetine-induced hypoglycaemia in a patient with congenital hyperinsulinism on lanreotide therapy. J Clin Res Pediatr Endocrinol 2016; 8: 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Salomon-Estebanez M, Flanagan SE, Ellard S, et al. Conservatively treated Congenital Hyperinsulinism (CHI) due to K-ATP channel gene mutations: reducing severity over time. Orphanet J Rare Dis 2016; 11: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kaiser N, Corcos AP, Tur-Sinai A, et al. Regulation of insulin release in persistent hyperinsulinaemic hypoglycaemia of infancy studied in long-term culture of pancreatic tissue. Diabetologia 1990; 33: 482–488. [DOI] [PubMed] [Google Scholar]
- 138. Abdel Khalek M, Kandil E. Is octreotide safe for the management of persistent hyperinsulinemic hypoglycemia of infancy? Eur J Pediatr Surg 2011; 21: 188–189. [DOI] [PubMed] [Google Scholar]
- 139. Glaser B, Landau H, Smilovici A, et al. Persistent hyperinsulinaemic hypoglycaemia of infancy: long-term treatment with the somatostatin analogue Sandostatin. Clin Endocrinol (Oxf) 1989; 31: 71–80. [DOI] [PubMed] [Google Scholar]
- 140. Semiz S, Bircan I, Akcurin S, et al. Persistent hyperinsulinaemic hypoglycaemia of infancy: case report. East Afr Med J 2002; 79: 554–556. [DOI] [PubMed] [Google Scholar]
- 141. Kirk JM, Di Silvio L, Hindmarsh PC, et al. Somatostatin analogue in short term management of hyperinsulinism. Arch Dis Child 1988; 63: 1493–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Al-Agha AE, Ahmad IA. Characterization of the ABCC8 gene mutation and phenotype in patients with congenital hyperinsulinism in western Saudi Arabia. Saudi Med J 2013; 34: 1002–1006. [PubMed] [Google Scholar]
- 143. Glaser B, Landaw H. Long-term treatment with the somatostatin analogue SMS 201-995: alternative to pancreatectomy in persistent hyperinsulinaemic hypoglycaemia of infancy. Digestion 1990; 45(Suppl. 1): 27–35. [DOI] [PubMed] [Google Scholar]
- 144. Senniappan S, Alexandrescu S, Tatevian N, et al. Sirolimus therapy in infants with severe hyperinsulinemic hypoglycemia. N Engl J Med 2014; 370: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 145. Krentz AJ, Pace J, Somerville W, et al. Effects of octreotide on circulating islet B cell products in endogenous hyperinsulinism. Postgrad Med J 1993; 69: 735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Al-Nassar S, Sakati N, Al-Ashwal A, et al. Persistent hyperinsulinaemic hypoglycaemia of infancy in 43 children: long-term clinical and surgical follow-up. Asian J Surg 2006; 29: 207–211. [DOI] [PubMed] [Google Scholar]
- 147. Gong C, Huang S, Su C, et al. Congenital hyperinsulinism in Chinese patients: 5-yr treatment outcome of 95 clinical cases with genetic analysis of 55 cases. Pediatr Diabetes 2016; 17: 227–234. [DOI] [PubMed] [Google Scholar]
- 148. Senniappan S, Hussain K. An evaluation of growth hormone and IGF-1 responses in neonates with hyperinsulinaemic hypoglycaemia. Int J Endocrinol 2013; 2013: 638257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lehnert H, Beyer J, Weber P, et al. Treatment of severe reactive hypoglycemia with a somatostatin analogue (SMS 201-995). Arch Intern Med 1990; 150: 2401–2402. [PubMed] [Google Scholar]
- 150. Alsaedi AA, Bakkar AA, Kamal NM, et al. Late presentation of necrotizing enterocolitis associated with rotavirus infection in a term infant with hyperinsulinism on octreotide therapy: a case report. Medicine (Baltimore) 2017; 96: e7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Güemes M, Shah P, Silvera S, et al. Assessment of nifedipine therapy in hyperinsulinemic hypoglycemia due to mutations in the ABCC8 Gene. J Clin Endocrinol Metab 2017; 102: 822–830. [DOI] [PubMed] [Google Scholar]
- 152. Shah P, Arya VB, Flanagan SE, et al. Sirolimus therapy in a patient with severe hyperinsulinaemic hypoglycaemia due to a compound heterozygous ABCC8 gene mutation. J Pediatr Endocrinol Metab 2015; 28: 695–699. [DOI] [PubMed] [Google Scholar]
- 153. Amendt P, Kohnert KD, Kunz J. The hyperinsulinaemic hypoglycaemias in infancy: a study of six cases. Eur J Pediatr 2020; 148: 107–112. [DOI] [PubMed] [Google Scholar]
- 154. Al-Shanafey S, Habib Z, AlNassar S. Laparoscopic pancreatectomy for persistent hyperinsulinemic hypoglycemia of infancy. J Pediatr Surg 2009; 44: 134–138; discussion 138. [DOI] [PubMed] [Google Scholar]
- 155. Guo D, Liu H, Ruzi A, et al. Modeling congenital hyperinsulinism with ABCC8-deficient human embryonic stem cells generated by CRISPR/Cas9. Sci Rep 2017; 7: 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Shah P, Nicholson J, Cosgrove KE, et al. The use of a long-acting somatostatin analogue (Lanreotide) in three children with focal forms of congenital hyperinsulinaemic hypoglycaemia. Diabet Med 2019; 91: 56–61. [DOI] [PubMed] [Google Scholar]
- 157. Pincemaille O, Chouraqui JP, Andrini P, et al. [Somatostatin in a case of neonatal hyperinsulinism caused by focal adenomatosis of the head of pancreas]. Arch Fr Pediatr 1987; 44: 803–805. [PubMed] [Google Scholar]
- 158. Apak RA, Yurdakok M, Oran O, et al. Preoperative use of octreotide in a newborn with persistent hyperinsulinemic hypoglycemia of infancy. J Pediatr Endocrinol Metab 1998; 11(Suppl. 1): 143–145. [DOI] [PubMed] [Google Scholar]
- 159. Hosokawa Y, Kawakita R, Yokoya S, et al. Efficacy and safety of octreotide for the treatment of congenital hyperinsulinism: a prospective, open-label clinical trial and an observational study in Japan using a nationwide registry. Endocr J 2017; 64: 867–880. [DOI] [PubMed] [Google Scholar]
- 160. Shah P, Nicholson J, Cosgrove KE, et al. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. Diabet Med 2012; 166: 951–955. [DOI] [PubMed] [Google Scholar]
- 161. Shah P, Rahman SA, McElroy S, et al. Use of long-acting somatostatin analogue (Lanreotide) in an adolescent with diazoxide-responsive congenital hyperinsulinism and its psychological impact. Horm Res Paediatr 2015; 84: 355–360. [DOI] [PubMed] [Google Scholar]
- 162. Avatapalle B, Padidela R, Randell T, et al. Drug-induced hepatitis following use of octreotide for long-term treatment of congenital hyperinsulinism. BMJ Case Rep 2012; 2012: bcr2012006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hu SW, Liu M, Sang YM. [Treatment of congenital hyperinsulinism with octreotide: a case report]. Zhongguo Dang Dai Er Ke Za Zhi 2013; 15: 392–393. [PubMed] [Google Scholar]
- 164. Cao B, Wu Di, Su C, et al. Efficacy and safety of octreotide treatment for diazoxide-unresponsive congenital hyperinsulinism in China. Pediatr Investig 2020; 4: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Vezzosi D, Bennet A, Courbon F, et al. Short- and long-term somatostatin analogue treatment in patients with hypoglycaemia related to endogenous hyperinsulinism. Clin Endocrinol (Oxf) 2008; 68: 904–911. [DOI] [PubMed] [Google Scholar]
- 166. Barrons RW. Octreotide in hyperinsulinism. Ann Pharmacother 1997; 31: 239–241. [DOI] [PubMed] [Google Scholar]
- 167. Jindal R, Ahmad A, Siddiqui MA, et al. Novel mutation c.597_598dup in exon 5 of ABCC8 gene causing congenital hyperinsulinism. Diabetes Metab Syndr 2014; 8: 45–47. [DOI] [PubMed] [Google Scholar]
- 168. Sherif EM, Abdelmaksoud AA, Elbarbary NS, et al. An Egyptian case of congenital hyperinsulinism of infancy due to a novel mutation in KCNJ11 encoding Kir6.2 and response to octreotide. Acta Diabetol 2013; 50: 801–805. [DOI] [PubMed] [Google Scholar]
- 169. Bas VN, Ozkan M, Zenciroglu A, et al. Seizure due to somatostatin analog discontinuation in a case diagnosed as congenital hyperinsulinism novel mutation. J Pediatr Endocrinol Metab 2012; 25: 553–555. [PubMed] [Google Scholar]
- 170. John CM, Agarwal P, Govindarajulu S, et al. Congenital hyperinsulinism: diagnostic and management challenges in a developing country - case report. Ann Pediatr Endocrinol Metab 2017; 22: 272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Shirland L. When it is more than transient neonatal hypoglycemia: hyperinsulinemia–a case study challenge. Neonatal Netw 2001; 20: 5–11. [DOI] [PubMed] [Google Scholar]
- 172. Rasmussen AG, Melikian M, Globa E, et al. The difficult management of persistent, non-focal congenital hyperinsulinism: a retrospective review from a single, tertiary center. Pediatr Diabetes 2020; 21: 441–455. [DOI] [PubMed] [Google Scholar]
- 173. Batra YK, Rajeev S, Samra T, et al. Octreotide-induced severe paradoxical hyperglycemia and bradycardia during subtotal pancreatectomy for congenital hyperinsulinism in an infant. Paediatr Anaesth 2007; 17: 1117–1119. [DOI] [PubMed] [Google Scholar]
- 174. Kane C, Lindley KJ, Johnson PR, et al. Therapy for persistent hyperinsulinemic hypoglycemia of infancy. Understanding the responsiveness of beta cells to diazoxide and somatostatin. J Clin Invest 1997; 100: 1888–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Sotelo-Cruz N, Cordero-Olivares A, Ramirez-Rodriguez C, et al. [Persistent hyperinsulinemic hypoglycemia. Two case reports]. Cir Cir 2004; 72: 409–414. [PubMed] [Google Scholar]
- 176. Pan S, Zhang M, Li Y. Experience of octreotide therapy for hyperinsulinemic hypoglycemia in neonates born small for gestational age: a case series. Horm Res Paediatr 2015; 84: 383–387. [DOI] [PubMed] [Google Scholar]
- 177. Paragliola RM, Prete A, Papi G, et al. Clinical utility of lanreotide Autogel® in gastroenteropancreatic neuroendocrine tumors. Drug Des Devel Ther 2016; 10: 3459–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sheppard M, Shapiro B, Pimstone B, et al. Metabolic clearance and plasma half-disappearance time of exogenous somatostatin in man. J Clin Endocrinol Metab 1979; 48: 50–53. [DOI] [PubMed] [Google Scholar]
- 179. Marbach P, Briner U, Lemaire M, et al. From somatostatin to sandostatin: pharmacodynamics and pharmacokinetics. Digestion 1993; 54(Suppl. 1): 9–13. [DOI] [PubMed] [Google Scholar]
- 180. Mathavan VK, Arregui M, Davis C, et al. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc 2010; 24: 2547–2555. [DOI] [PubMed] [Google Scholar]
- 181. Malik S, Mitchell JE, Steffen K, et al. Recognition and management of hyperinsulinemic hypoglycemia after bariatric surgery. Obes Res Clin Pract 2016; 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Penning C, Vecht J, Masclee AA. Efficacy of depot long-acting release octreotide therapy in severe dumping syndrome. Aliment Pharmacol Ther 2005; 22: 963–969. [DOI] [PubMed] [Google Scholar]
- 183. Arts J, Caenepeel P, Bisschops R, et al. Efficacy of the long-acting repeatable formulation of the somatostatin analogue octreotide in postoperative dumping. Clin Gastroenterol Hepatol 2009; 7: 432–437. [DOI] [PubMed] [Google Scholar]
- 184. de Heide LJ, Laskewitz AJ, Apers JA. Treatment of severe postRYGB hyperinsulinemic hypoglycemia with pasireotide: a comparison with octreotide on insulin, glucagon, and GLP-1. Surg Obes Relat Dis 2014; 10: e31–e33. [DOI] [PubMed] [Google Scholar]
- 185. Vilarrasa N, Goday A, Rubio MA, et al. Hyperinsulinemic hypoglycemia after bariatric surgery: diagnosis and management experience from a Spanish multicenter registry. Obes Facts 2016; 9: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Glatstein M, Scolnik D, Bentur Y. Octreotide for the treatment of sulfonylurea poisoning. Clin Toxicol (Phila) 2012; 50: 795–804. [DOI] [PubMed] [Google Scholar]
- 187. Rath S, Bar-Zeev N, Anderson K, et al. Octreotide in children with hypoglycaemia due to sulfonylurea ingestion. J Paediatr Child Health 2008; 44: 383–384. [DOI] [PubMed] [Google Scholar]
- 188. Boyle PJ, Justice K, Krentz AJ, et al. Octreotide reverses hyperinsulinemia and prevents hypoglycemia induced by sulfonylurea overdoses. J Clin Endocrinol Metab 1993; 76: 752–756. [DOI] [PubMed] [Google Scholar]
- 189. Carr R, Zed PJ. Octreotide for sulfonylurea-induced hypoglycemia following overdose. Ann Pharmacother 2002; 36: 1727–1732. [DOI] [PubMed] [Google Scholar]
- 190. Krentz AJ, Boyle PJ, Justice KM, et al. Successful treatment of severe refractory sulfonylurea-induced hypoglycemia with octreotide. Diabetes Care 1993; 16: 184–186. [DOI] [PubMed] [Google Scholar]
- 191. Graudins A, Linden CH, Ferm RP. Diagnosis and treatment of sulfonylurea-induced hyperinsulinemic hypoglycemia. Am J Emerg Med 1997; 15: 95–96. [DOI] [PubMed] [Google Scholar]
- 192. O Hung, Eng J, Ho J, et al. Octreotide as an antidote for refractory sulfonylurea hypoglycemia. J Toxicol Clin Toxicol 1997; 35: 540–541. [Google Scholar]
- 193. Braatvedt GD. Octreotide for the treatment of sulphonylurea induced hypoglycaemia in type 2 diabetes. N Z Med J 1997; 110: 189–190. [PubMed] [Google Scholar]
- 194. McLaughlin SA, Crandall CS, McKinney PE. Octreotide: an antidote for sulfonylurea-induced hypoglycemia. Ann Emerg Med 2000; 36: 133–138. [DOI] [PubMed] [Google Scholar]
- 195. Schier JG, Hirsch ON and Chu J. Octreotide as antidote for sulfonylurea-induced hypoglycemia. Ann Emerg Med 2001; 37: 417–418. [DOI] [PubMed] [Google Scholar]
- 196. Green RS, Palatnick W. Effectiveness of octreotide in a case of refractory sulfonylurea-induced hypoglycemia. J Emerg Med 2003; 25: 283–287. [DOI] [PubMed] [Google Scholar]
- 197. Nzerue CM, Thomas J, Volcy J, et al. Use of octreotide to treat prolonged sulfonylurea-induced hypoglycemia in a patient with chronic renal failure. Int J Artif Organs 2003; 26: 86–89. [DOI] [PubMed] [Google Scholar]
- 198. Crawford BA, Perera C. Octreotide treatment for sulfonylurea-induced hypoglycaemia. Med J Aust 2004; 180: 540–541; author reply 541. [DOI] [PubMed] [Google Scholar]
- 199. Lheureux PE, Zahir S, Penaloza A, et al. Bench-to-bedside review: antidotal treatment of sulfonylurea-induced hypoglycaemia with octreotide. Crit Care 2005; 9: 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Soderstrom J, Murray L, Daly FF, et al. Toxicology case of the month: oral hypoglycaemic overdose. Emerg Med J 2006; 23: 565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Fleseriu M, Skugor M, Chinnappa P, et al. Successful treatment of sulfonylurea-induced prolonged hypoglycemia with use of octreotide. Endocr Pract 2006; 12: 635–640. [DOI] [PubMed] [Google Scholar]
- 202. Gul M, Cander B, Girisgin S, et al. The effectiveness of various doses of octreotide for sulfonylurea-induced hypoglycemia after overdose. Adv Ther 2006; 23: 878–884. [DOI] [PubMed] [Google Scholar]
- 203. Francis M, Langhan T, Prosser J, et al. Comparison of octreotide and standard therapy versus standard therapy alone for the treatment of sulfonylurea-induced hypoglycemia. Ann Emerg Med 2008; 51: 795–796; author reply 796–797. [DOI] [PubMed] [Google Scholar]
- 204. Fasano CJ, O’Malley G, Dominici P, et al. Comparison of octreotide and standard therapy versus standard therapy alone for the treatment of sulfonylurea-induced hypoglycemia. Ann Emerg Med 2008; 51: 400–406. [DOI] [PubMed] [Google Scholar]
- 205. Clerc D, Hugli O, Trueb L, et al. [Role of octreotide in the treatment of hypoglycemia induced by sulfonylureas]. Rev Med Suisse 2008; 4: 1764–1767. [PubMed] [Google Scholar]
- 206. Hanchard B, Boulouffe C, Vanpee D. Sulfonylurea-induced hypoglycaemia: use of octreotide. Acta Clin Belg 2009; 64: 56–58. [DOI] [PubMed] [Google Scholar]
- 207. Osterhoudt KC. Octreotide for paediatric sulfonylurea poisonings. J Paediatr Child Health 2009; 45: 473. [DOI] [PubMed] [Google Scholar]
- 208. Osterhoudt KC, Calello DP. Pediatric glipizide ingestion, onset of hypoglycemia, and octreotide. J Pediatr Endocrinol Metab 2009; 22: 1185. [DOI] [PubMed] [Google Scholar]
- 209. Fasano CJ, Rowden AK. Successful treatment of repaglinide-induced hypoglycemia with octreotide. Am J Emerg Med 2009; 27: 756.e3–756e4. [DOI] [PubMed] [Google Scholar]
- 210. Glatstein M, Garcia-Bournissen F, Scolnik D, et al. Sulfonylurea intoxication at a tertiary care paediatric hospital. Can J Clin Pharmacol 2010; 17: e51–e56. [PubMed] [Google Scholar]
- 211. Dougherty PP, Klein-Schwartz W. Octreotide’s role in the management of sulfonylurea-induced hypoglycemia. J Med Toxicol 2010; 6: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Maiza JC, Vezzosi D, Grunenwald S, et al. Treatment with somatostatin analogs and chemoembolization of liver metastases for severe hypoglycemia in malignant insulinomas. J Endocrinol Invest 2011; 34: e253–e258. [DOI] [PubMed] [Google Scholar]
- 213. Barkin JA, Block HM, Mendez PE. Octreotide: a novel therapy for refractory sulfonylurea-induced hypoglycemia. Pancreas 2013; 42: 722–723. [DOI] [PubMed] [Google Scholar]
- 214. Llamado R, Czaja A, Stence N, et al. Continuous octreotide infusion for sulfonylurea-induced hypoglycemia in a toddler. J Emerg Med 2013; 45: e209–e213. [DOI] [PubMed] [Google Scholar]
- 215. Dougherty PP, Lee SC, Lung D, et al. Evaluation of the use and safety of octreotide as antidotal therapy for sulfonylurea overdose in children. Pediatr Emerg Care 2013; 29: 292–295. [DOI] [PubMed] [Google Scholar]
- 216. Klein-Schwartz W, Stassinos GL, Isbister GK. Treatment of sulfonylurea and insulin overdose. Br J Clin Pharmacol 2016; 81: 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kumar S, Choudhary A, Ali M, et al. Sulfonylurea poisoning in a healthy toddler. Indian J Pediatr 2017; 84: 147–149. [DOI] [PubMed] [Google Scholar]
- 218. Curnow RT, Carey RM, Taylor A, et al. Somatostatin inhibition of insulin and gastrin hypersecretion in pancreatic islet-cell carcinoma. N Engl J Med 1975; 292: 1385–1386. [DOI] [PubMed] [Google Scholar]
- 219. Christensen SE, Hansen AP, Lundbaek K, et al. Letter: somatostatin and insulinoma. Lancet 1975; 1: 1426. [DOI] [PubMed] [Google Scholar]
- 220. Lorenzi M, Gerich JE, Karam JH, et al. Failure of somatostatin to inhibit tolbutamide-induced insulin secretion in patients with insulinomas: a possible diagnostic tool. J Clin Endocrinol Metab 1975; 40: 1121–1124. [DOI] [PubMed] [Google Scholar]
- 221. Van Kersen F. Inhibition by somatostatin of tolbutamide-induced insulin secretion in a patient with an insulinoma. Neth J Med 1976; 19: 5–7. [PubMed] [Google Scholar]
- 222. Scuro Lo LA, Cascio V, Adami S, et al. Somatostatin inhibition of insulin secretion in insulin-producing tumors. Metabolism 1976; 25: 603–609. [DOI] [PubMed] [Google Scholar]
- 223. Fajans SS, Floyd JC., Jr. Diagnosis and medical management of insulinomas. Annu Rev Med 1979; 30: 313–329. [DOI] [PubMed] [Google Scholar]
- 224. Khandekar JD. Islet cell tumors of the pancreas: clinico-biochemical correlations. Ann Clin Lab Sci 1979; 9: 212–218. [PubMed] [Google Scholar]
- 225. Fallucca F, Mirabella C, Tamburrano G, et al. Effects of somatostatin on insulin and glucagon in patients with insulinoma. J Endocrinol Invest 1979; 2: 257–260. [DOI] [PubMed] [Google Scholar]
- 226. Long RG, Barnes AJ, Adrian TE, et al. Suppression of pancreatic endocrine tumour secretion by long-acting somatostatin analogue. Lancet 1979; 2: 764–767. [DOI] [PubMed] [Google Scholar]
- 227. Osei K, O’Dorisio TM. Malignant insulinoma: effects of a somatostatin analog (compound 201-995) on serum glucose, growth, and gastro-entero-pancreatic hormones. Ann Intern Med 1985; 103: 223–225. [DOI] [PubMed] [Google Scholar]
- 228. Anderson JV, Bloom SR. Neuroendocrine tumours of the gut: long-term therapy with the somatostatin analogue SMS 201-995. Scand J Gastroenterol Suppl 1986; 119: 115–128. [DOI] [PubMed] [Google Scholar]
- 229. Bertherat J, Tenenbaum F, Perlemoine K, et al. Somatostatin receptors 2 and 5 are the major somatostatin receptors in insulinomas: an in Vivo and in Vitro study. J Clin Endocrinol Metab 2003; 88: 5353–5360. [DOI] [PubMed] [Google Scholar]
- 230. Perry RR, Vinik AI. Clinical review 72: diagnosis and management of functioning islet cell tumors. J Clin Endocrinol Metab 1995; 80: 2273–2278. [DOI] [PubMed] [Google Scholar]
- 231. Tirosh A, Stemmer SM, Solomonov E, et al. Pasireotide for malignant insulinoma. Hormones (Athens) 2016; 15: 271–276. [DOI] [PubMed] [Google Scholar]
- 232. Skajaa GO, Mathiesen ER, Iyore E, et al. Poor pregnancy outcome after octreotide treatment during pregnancy for familial hyperinsulinemic hypoglycemia: a case report. BMC Res Notes 2014; 7: 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Geilswijk M, Andersen LL, Frost M, et al. Octreotide therapy and restricted fetal growth: pregnancy in familial hyperinsulinemic hypoglycemia. Endocrinol Diabetes Metab Case Rep 2017; 2017: 16-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Heikenen JB, Pohl JF, Werlin SL, et al. Octreotide in pediatric patients. J Pediatr Gastroenterol Nutr 2002; 35: 600–609. [DOI] [PubMed] [Google Scholar]
- 235. Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C. Somatostatin analogs in clinical practice: a review. Int J Mol Sci 2020; 21: 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Levy-Khademi F, Irina S, Avnon-Ziv C, et al. Octreotide-associated cholestasis and hepatitis in an infant with congenital hyperinsulinism. J Pediatr Endocrinol Metab 2015; 28: 449–451. [DOI] [PubMed] [Google Scholar]
- 237. Camilleri M. Effects of somatostatin analogues on human gastrointestinal motility. Digestion 1996; 57(Suppl. 1): 90–92. [DOI] [PubMed] [Google Scholar]
- 238. Laje P, Halaby L, Adzick NS, et al. Necrotizing enterocolitis in neonates receiving octreotide for the management of congenital hyperinsulinism. Pediatr Diabetes 2010; 11: 142–147. [DOI] [PubMed] [Google Scholar]
- 239. Reck-Burneo CA, Parekh A, Velcek FT. Is octreotide a risk factor in necrotizing enterocolitis? J Pediatr Surg 2008; 43: 1209–1210. [DOI] [PubMed] [Google Scholar]
- 240. Ballance WA, Dahms BB, Shenker N, et al. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr 1990; 117: S6–S13. [DOI] [PubMed] [Google Scholar]
- 241. Wilson DC, Carson DJ, Quinn RJ. Long-term use of somatostatin analogue SMS 201-995 in the treatment of hypoglycaemia due to nesidioblastosis. Acta Paediatr Scand 1988; 77: 467–470. [DOI] [PubMed] [Google Scholar]
- 242. Schiedermaier P, Brensing KA, Goke B, et al. Effects of different octreotide dosages on splanchnic hemodynamics and glucagon in healthy volunteers. Digestion 1999; 60: 132–140. [DOI] [PubMed] [Google Scholar]
- 243. Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 2003; 24: 28–47. [DOI] [PubMed] [Google Scholar]
- 244. Sawicka-Gutaj N, Owecki M, Ruchala M. Pasireotide – mechanism of action and clinical applications. Curr Drug Metab 2018; 19: 876–882. [DOI] [PubMed] [Google Scholar]
- 245. van Schaik E, van Vliet EI, Feelders RA, et al. Improved control of severe hypoglycemia in patients with malignant insulinomas by peptide receptor radionuclide therapy. J Clin Endocrinol Metab 2011; 96: 3381–3389. [DOI] [PubMed] [Google Scholar]