Abstract
Introduction:
Geriatric hip fractures are a major, costly public health issue, expected to increase in incidence and expense with the aging population. As healthcare transitions towards value-based care, understanding cost drivers of hip fracture treatment will be necessary to perform adequate risk adjustment. Historically, cost has been variable and difficult to determine. This study was purposed to identify variables that can predict the overall cost of care for geriatric intertrochanteric (IT) hip fractures and provide a better cost prediction to ensure the success of future bundled payment models.
Methods:
A retrospective review of operatively-managed geriatric hip fractures was performed at single urban level I academic trauma center between 2013 and 2017. Patient variables were collected via the electronic medical record (EMR) including CCI, ACCI, ASA, overall length of stay (LOS), AO/OTA fracture classification and demographics. Direct and indirect costs were calculated by activity-based costing by the hospital’s accounting software. Multivariable linear regression models evaluated which parameters predicted total inpatient cost of care.
Results:
The mean cost of care was $19,822, ranging from $9,128 to $64,211. Critical care comprised 16.9% of total costs, followed by implant costs (13.6%), and nursing costs (12.6%). Regression analysis identified both ASA (p < 0.01) and ACCI (p = 0.01) as statistically significant associative parameters, but only LOS (r 2 = 0.77) as a strong correlative measure for inpatient care cost.
Conclusion:
This study found no correlation between ACCI or ASA and the total inpatient cost of care in isolated intertrochanteric geriatric hip fractures, suggesting that the inpatient episode-of-care costs cannot be accurately predicted by the patient demographics/comorbidities alone. Future bundled care payment models would have to be adjusted to account for variables beyond just patient characteristics.
Level of Evidence:
Diagnostic Level IV.
Keywords: hip fracture, geriatric, intertrochanteric fracture, implant costs, inpatient cost of care
Introduction
Geriatric hip fractures are a major and costly public health issue. Hip fractures comprise only 14% of all fractures yet account for 72% of costs attributed to musculoskeletal fracture care, estimated at $19 billion in 2005.1 Additionally, the US Census Bureau projects that persons aged 65 and older will reach 24% of the total US population in 2060, totaling 98 million.2 Given the high incidence of hip fractures within this age demographic, the costs attributable to geriatric hip fractures are projected to increase dramatically with the aging population, causing significant fiscal concerns for health care institutions.1,3
There has been a move in health care expenditures away from the traditional fee-for-service (FFS) model towards bundled payments in order to improve the value of care.4 The bundled payment model provides a reimbursement based on a target price for a predetermined episode of care. This shifts financial risk onto hospitals and incentivizes providers to minimize variations in care and cost.5 Effective utilization of bundled payments has the potential to significantly decrease cost burdens for health care practices. However, if target prices are not adequately adjusted for risk, reimbursements will fall short of incurred expenses. Therefore, a strong and accurate understanding of healthcare costs are crucial for the success of bundled payments.
The first step toward bundling geriatric hip fractures is to understand the current cost drivers of treatment.1 Unfortunately, costs have been variable and unpredictable; when episodes of care exceed predictions, they become unsustainable to participating healthcare institutions.6,7 To create sustainable bundled models, it is imperative to identify measurable parameters that are correlative of incurred costs.4 The goal of this study was to determine potential variables that could predict the overall inpatient cost-of-care in geriatric intertrochanteric hip fractures. We hypothesized that a correlation exists between patient comorbidity indexes, Age-Adjusted Charlson Comorbidity Index (ACCI) and the American Society of Anesthesiology (ASA) classification to our primary outcome, the overall inpatient cost-of-care for geriatric intertrochanteric hip fracture patients.
Methods
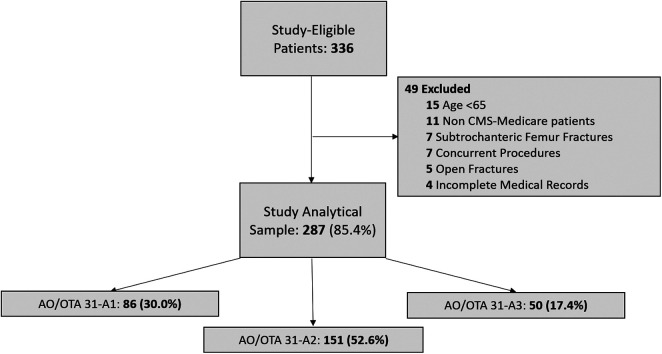
Following IRB approval in 2018, a retrospective review was performed of 287 isolated geriatric hip fractures managed operatively, 2013-2017, at a single level I metropolitan academic trauma center (Figure 1). Patients included were: ≥65 years, presenting with an isolated closed intertrochanteric femur fracture, were treated with open reduction internal fixation (ORIF) or intramedullary fixation, and were insured via the Centers for Medicare & Medicaid Services. Current procedural terminology (CPT) codes 27244 and 27245 were used to identify patients. Patients with incomplete medical records were excluded.
Figure 1.
Strobe distributional flow chart of the study patient population.
Variables were collected via review of each patient’s electronic medical record (EMR), including: CCI,8 ASA grade,9 details of the patient’s episode of care and surgical admission, overall patient length of stay (LOS), the AO/Orthopaedic Trauma Association (AO/OTA)10 fracture classification grade, and 1-year readmission and reoperation. Of note, ACCI is the CCI with modifying addition relative to the patients’ age.8 Patient all-cause mortality and their date of death were determined from a query of the National Death Index (USA National Center for Health Statistics).
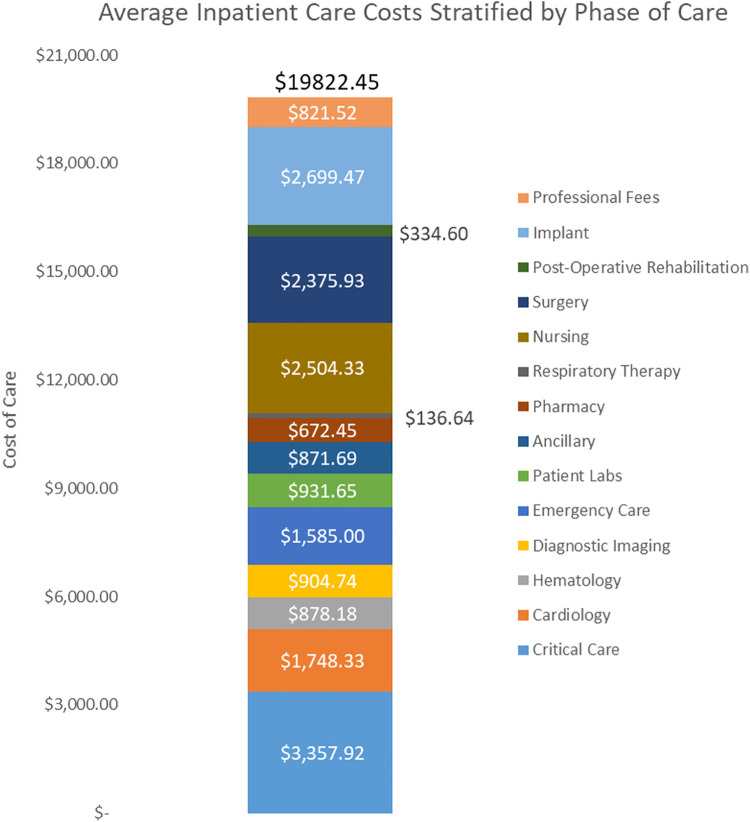
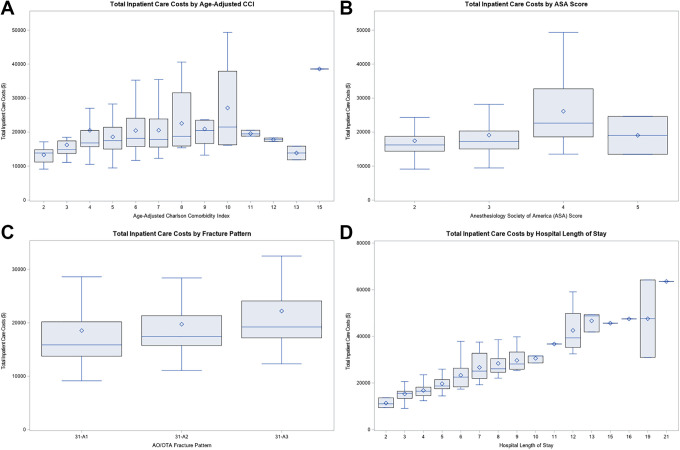
The total inpatient cost of care incorporates both direct and indirect costs (Figure 2). Direct costs included costs incurred for: surgical implants, inpatient post-op rehabilitation, surgical costs, nursing, respiratory therapy, pharmacy, patient labs, emergency care, diagnostic imaging, hematology, cardiology, and critical care costs. Indirect costs were related to general hospital operations and maintenance, such as: administration, information technologies support, human resources, etc. Patient-specific costs were calculated via activity-based costing, a product of the relative value of the cost of treatment to the amount of time that is spent delivering patient care.11,12 Of note, activity-based costing is an alternative to time-driven activity-based costing, because the relative time measures are obtained via timestamps in the hospital’s electronic medical record (EMR) and not reflective of the actual time of the procedure itself. This cost derivation was performed by the hospital’s accounting software, McKesson (San Francisco, CA). It should be noted that these costs are not representative of the hospital charges, which utilizes a different derivation formula in the hospital accounting software and pertains its own unique column. The costs for this study only evaluate the costs associated to the inpatient care episode linked to the primary surgical encounter. This would not include costs associated to reoperations, readmissions, or post-discharge care. The primary study outcome of interest was the total inpatient cost and its relationship with ACCI (Figure 3A). Secondary study outcomes included the relationship of the total inpatient cost with ASA (Figure 3B), AO/OTA fracture classifications (Figure 3C), and patient LOS (Figure 3D).
Figure 2.
Component breakdown of the average inpatient care costs in the setting of operated isolated intertrochanteric hip fracture (N = 287).
Figure 3.
Graphical visualization of the total incurred inpatient cost-of-care as a function of: (A) age-adjusted Charlson Comorbidity Index (ACC), (B) the American Society of Anesthesiologists (ASA) score, and the (C) AO Foundation/Orthopaedic Trauma Association (AO/OTA) fracture classification grade, and (D) the overall patient length-of-stay (LOS).
Statistical analyses consisted of descriptive statistics to characterize the study population (Table 1). Quantitative measures were reported in the format of mean ± standard deviation, followed by the 95% confidence interval in brackets. The Inpatient care cost was then stratified and compared across both AO/OTA fracture classification and the selected fixation implant (Table 2). Multivariable linear regression (MLR) models were employed to evaluate which parameters functioned as effective predictors for the derived total inpatient cost of care, both isolated and in-conjunction (Table 3).13 Regression analysis involved the evaluation of both the statistical significance, p-value, of the studied parameters and their correlation, r-squared value, with the primary outcome, inpatient cost of care. Least absolute shrinkage selection operator (LASSO) regression analysis was utilized to identify statistically relevant parameters and identify which parameters could be utilized in MLR (Table 3). All statistical analysis was performed in SAS 9.4 (SAS Institute; Cary, NC). Study data was consolidated and retained in Microsoft Excel on a secure hospital server (Microsoft Corporation; Redmond, WA). Statistical significance was set at an alpha (α) value of 0.05, with all p-values below 0.05 being deemed statistically significant.
Table 1.
Study Population Characteristics, Between 2013-2018 (N = 287).
| N (% of N) | Mean ± SD [95% CI] | |
|---|---|---|
| Gender | Male: 83 (28.9%) Female: 204 (71.1%) | |
| Age | 83.1 ± 8.5 [82.1, 84.1] | |
| Body Mass Index (BMI) | 24.5 ± 5.5 [23.9, 25.1] | |
| AO/OTA Fracture Classification | 31-A1: 86 (30.0%)
31-A2: 151 (52.6%) 31-A3: 50 (17.4%) |
|
| Anesthesiologist Society of America (ASA) Score | 2: 43 (15.0%)
3: 204 (71.1%) 4: 38 (13.2%) 5: 2 (0.7%) |
|
| Charlson Comorbidity Index (CCI) | 5.7 ± 2.1 [5.5, 6.0] | |
| Construct Design | Dynamic Hip Screw: 50 (17.4%)
Short Intramedullary Nail: 111 (38.7%) Long Intramedullary Nail: 126 (43.9%) |
|
| Hospital Length of Stay | 4.9 ± 2.7 [4.6, 5.2] | |
| Total Inpatient Cost(s) | $19,822 ± $8,078 [$18,888, $20,755] | |
| Total Implant Cost(s) | $2,699 ± $879 [$2,598, $2,701] | |
| 1-Year Readmissiona | 16 (5.6%) | |
| 1-Year Reoperationa | 12 (4.2%) | |
| Mortality | 90-Day Mortality: 39 (13.6%) 1-Year Mortality: 71 (24.7%) | |
A summary of the study populations’ characteristics.
a1-Year readmissions and reoperations only include admissions/procedures that are related to the index hip fracture procedure and only those captured within the original hospital system.
Table 2.
A 2-Way Comparative Matrix of Hospital Inpatient Costs Between OTA Fracture Patterns and the Construct Design.
| Inpatient cost(s) | OTA | p-value | ||||
|---|---|---|---|---|---|---|
| 31-A1 | 31-A2 | 31-A3 | ||||
| Implant Choice | DHS | $17279.40 ± $10515.66 [$13488.10, $21070.70] | $16736.18 ± $4750.60 [$14446.47, $19205.90] | N/A | 0.80 | |
| Short IMN | $18509.66 ± $6971.30 [$16337.25, $20682.07] | $19801.88 ± $8536.21 [$17615.65, $21988.11] | $21180.52 ± $8766.87 [$13072.52, $29288.53] | 0.60 | ||
| Long IMN | $21852.59 ± $8918.57 [$16463.16, $27242.03] | $20579.75 ± $6737.69 [$18961.17, $22198.32] | $22381.20 ± $8651.46 [$19750.92, $25011.49] | 0.47 | ||
| p-value | 0.28 | 0.13 | 0.75 | <0.012 | ||
| <0.011 | ||||||
Two-way comparative analysis matrix of implant costs versus construct design and AO/OTA fracture pattern.
1Resulting p-value from a 1-way analysis (ANOVA) F-test of implant choice: SHS: $17077.03 ± $8760.93; Short CMN: $19396.22 ± $7951.15; Long CMN: $21340.15 ± $7668.59.
2Resulting p-value from a 1-way analysis (ANOVA) F-test of AO/OTA fracture classifications: 31-A1: $18556.67 ± $8739.46; 31-A2: $19771.17 ± $7391.28; 31-A3: $22216.40 ± $8588.73.
IMN—Intramedullary Nail
DHS—Dynamic Hip Screw
AO/OTA—AO Foundation/Orthopaedic Trauma Association
Table 3.
Multivariable Linear Regression (MLR) Models to Evaluate Inpatient Costs Relative to Study Parameters (N = 287).
| Model | Variable(s) | β-estimate(s) | p-value | R 2 |
|---|---|---|---|---|
| Demographics | Age | $26.16 | 0.64 | 0.04 |
| Gender1 | $2532.45 | 0.01 | ||
| BMI | $28.03 | 0.31 | ||
| Injury-Independent Characteristics | LOS | $2619.10 | <0.01 | 0.77 |
| Injury Characteristics | ASA | $3825.30 | <0.01 | 0.09 |
| AO/OTA | ||||
| 31-A1 | (ref) | |||
| 31-A2 | $1048.13 | 0.31 | ||
| 31-A3 | $3646.80 | 0.01 | ||
| Comorbidity Parameters | CCI | $567.79 | 0.02 | 0.02 |
| ACCI | $572.62 | 0.01 | 0.04 | |
| Demographics + Injury-Independent + Injury Characteristics + ACCI | Age | $21.60 | 0.44 | 0.69 |
| Gender1 | -$469.52 | 0.37 | ||
| BMI | $44.71 | 0.34 | ||
| ACCI | $544.65 | 0.15 | ||
| LOS | $2573.63 | <0.01 | ||
| ASA | $718.73 | 0.11 | ||
| AO/OTA | ||||
| 31-A1 | (ref) | |||
| 31-A2 | $1330.45 | 0.01 | ||
| 31-A3 | $2172.59 | <0.01 |
A summary of multiple linear regression results for evaluating correlation between patient demographics and inpatient costs.
BMI = Body Mass Index; LOS = Length-of-Stay; ASA = Anesthesiologist Society of America Score; AO/OTA = AO Foundation/Orthopaedic Trauma Association; CCI = Charlson Comorbidity Index; ACCI = Age-Adjusted Charlson Comorbidity Index
1Female was set as the reference group for the parameter of gender.
Results
A total of 287 (85.4%) isolated closed geriatric intertrochanteric hip fractures were identified (Figure 1). The overall mean age was 83.1 ± 8.5 years [82.1, 84.1] and 205 (71.1%) were female. The average BMI was 24.5 ± 5.5 [23.9, 25.1]. The average CCI was 2.1 ± 2.0 [1.9, 2.3] and 5.7 ± 2.1 [5.5, 6.0] when adjusting for age, ACCI. The most common fracture pattern was 31-A2 (52.6%), followed by 31-A1 (30.0%), then 31-A3 (17.4%). On average, patients underwent surgery for their hip fractures within 24 hours of admission.
The mean cost of care was $19,822 ± $8.078 [$18,888, $20,755] and ranged from $9,128 to $64,211 (Table 1). Critical care was the largest cost component averaging $3,357.92 (16.9%), followed by implant costs, $2,699 (13.6%), and then nursing costs, $2,504 (12.6%). Inpatient costs were greatest for patients treated with a long intramedullary nail, $21,372 ± $7,647 [$20,030, $22,715], followed by patients treated with a short intramedullary nail, $19,314 ± $7,906 [$17,834, $20,794], and a DHS, $17,077 ± $8,761 [$14,613, $19,541] (p < 0.01). When stratified by AO/OTA fracture classifications, 31-A3 fractures averaged the greatest cost, $22,216 ± $8,588 [$19,801, $24,632], followed by 31-A2, $19,771 ± $7,391 [$18,564, $20,922], and 31-A1, $18,557 ± $8,739 [$16,694, $20,419] (Table 2). There was no identifiable linear correlation between the AO/OTA fracture classification and the resulting inpatient cost of care (r 2 = 0.03).
Variables of ACCI (p < 0.01) (Figure 3A), ASA (p < 0.01) (Figure 3B), time between admit and surgery (p < 0.01), LOS (p < 0.01), and AO/OTA classification (p < 0.01) (Figure 3C) had a positive linear association with the total cost of care when evaluated singularly in linear regression models. However, ACCI (r2 = 0.04), ASA (r2 = 0.07), age (r2 = 0.01), time between admit and surgery (r2 = 0.12), and AO/OTA fracture classification (r2 = 0.02) had a poor correlation with overall cost of care. LOS had a relatively strong correlation (r2 = 0.77) with overall inpatient cost of care (Figure 3D).
When evaluated in a combined linear regression model, LOS (p < 0.01) and AO/OTA fracture classification (31-A2: p = 0.01 & 31-A3: p < 0.01) were the only statistically significant parameters. The combined linear regression model demonstrated an overall strong correlation to the designated outcome of inpatient cost (r 2 = 0.69). The combined linear model identifies an increase of $545 per each point increase in ACCI (p = 0.15) and $719 per each point increase in ASA (p = 0.11), but neither were statistically significant parameters in the combined model (Table 3).
Discussion
Patients with geriatric hip fractures have complex and costly hospital courses. Identifying cost drivers will be necessary as we move towards bundled payments so that reimbursement can be adjusted for risk. The purpose of this study was to determine potential variables that predict overall cost of care in geriatric hip fractures. We had hypothesized that a measure of patient comorbidity would be correlated to the overall cost of inpatient care for geriatric hip fracture patients, and retrospectively reviewed cases of isolated geriatric hip fractures from 2013-2017 that were managed operatively at an urban level 1 academic trauma center. Overall inpatient cost was broken into component costs to analyze where greatest cost burden in hip fracture patients were attributed. It was found that the largest percent of cost was from critical care expenses, which was followed closely by implant costs and nursing.
Current bundled payment models function under the assumption that there is a positive correlation with patient overall health status and financial burden. However, this study found no correlation between ACCI or ASA with overall cost of care. LOS was the only patient variable with correlation to overall cost, but LOS is difficult to predict prior to hospitalization. While it would seem that patients with increased comorbidities would incur increased expenses compared to healthier patients, this relationship was not demonstrated.
CCI and (age-adjusted) ACCI are metrics commonly used to assess patient health status that we assumed would predict cost in geriatric hip fractures. However, our study did not find a correlation between CCI (r2 = 0.02) or ACCI (r2 = 0.04) and overall cost and there was wide variability in cost of inpatient stays. On the contrary, in 2015, Johnson et al found that there was a correlation between CCI and both LOS and overall cost in hip fracture patients. Cost was calculated based on number of hospital days and set average cost per day.14 However, with medically complex patients it is difficult to assume that spending by inpatient day is equal since costs vary widely among days and patients.
ASA score is another way patient health status is measured and has been found in previous studies to be predictive of LOS and inpatient cost. Garcia et al used a calculated average cost per day of patients who had an uncomplicated post-operative course and multiplied this by the number of hospital days.3 They found that an increase in ASA of 1 increased LOS by approximately 2 days and accordingly increased cost estimates.3 Similarly, Kay et al’s 2014 chart review showed ASA as the strongest predictor of postop LOS and a significant predictor of inpatient costs for orthopaedic procedures assigned to CPT code 27536.15 Conversely, Nikkel et al examined the relationship between the number of comorbidities and estimated hospital costs in hip fracture patients and found only a weak correlation (r2 = 0.22). They also found that as the number of comorbidities increased, variability in estimated costs also increased.16 Our study finds low correlation between inpatient cost and ASA (r2 = 0.07) as well as high cost variability, especially in complex patients.
Bundled models have been successful in other areas of orthopedics and show tremendous opportunity for decreasing expenditure. A study by Healy and Iorio et al on Comprehensive Care for Joint Replacement (CJR) bundling over a 3-year period showed decreased 90-day readmissions and post-acute facility costs with no change in patient outcomes.17 Another study by Gray et al over a 1 year period also showed improved quality and value of care with implementation of CJR bundled model compared with the previous year.18 Surgical Hip and Femur Fracture Treatment (SHFFT) is a 90-day bundle modeled after CJR that nearly became mandatory in 2018. It is only a matter of time before alternative payment models for hip fracture care become mandatory. However, cost drivers must be further clarified for geriatric hip patients before SHFFT can be as successful as CJR.
Determination of bundling inclusion and risk stratification of hip fracture patients are essential to allow hospitals to reduce costs and manage risk associated with these payment models.6,7 In a younger healthier patient population, such as TKA patients, bundling is straightforward and has already been successful; while frail patient populations with multiple comorbidities inherently have increased variability over 90-days of care and thus contributing to the difficulty in determining which conditions and treatments relate to a given procedure and which do not.4,16 Risk-adjustment of patients is necessary so that hospitals and physicians are not losing money or avoiding complex patients for whom increased costs are associated.4,7 Another challenge associated with hip fractures is that cases come in emergently, and patients cannot be medically optimized before surgery.19 Healy and Iorio indicated that risk stratification and delayed surgery in high risk patients were invaluable ways costs were decreased in their CJR study, which is not something that can be done in hip fracture patients.7
This study attempted to elucidate patient variables associated with increased cost, but no correlation with overall cost of care was found outside LOS, which is difficult to predict prior to hospitalization. The highly variable and unpredictable costs seen in our study indicate the need for guidelines to standardize care in geriatric hip fractures for more predictable costing before bundled payment models can be successfully implemented.
This study had multiple strengths and weaknesses. Where previous studies included patients with heterogeneous hip fracture profiles, this study had a uniform patient population (of isolated geriatric intertrochanteric femur fractures treated operatively) on which to analyze predictors of cost, minimizing the risk of confounding results. This study also had several limitations. As a review at a single tertiary center, the patients studied may not be representative of the orthopaedic population as a whole and the results may not be generalizable. In addition, this study only evaluates costs related to the inpatient care episode for the original index procedure, not including costs incurred for the after-discharge care, surgically-related hospital readmissions, or reoperations. These costs would be relevant to the full patient episode-of-care, but was limited due to logistical challenges in accessing long-term hospital financial cost data. Therefore, at the risk of providing misleading, incomplete, or inaccurate data, this study was purposed to only evaluate the inpatient care costs for the primary surgical encounter. Lastly, as a retrospective review, this study can only identify potential associations and cannot infer causal relationships.
Conclusion
Our study found no correlation between CCI, ACCI, or ASA and the total inpatient cost of care in isolated intertrochanteric geriatric hip fractures. This suggests that the inpatient episode-of-care costs cannot be accurately described by patient comorbidities and demographics alone. However, our study does highlight significant variability in the inpatient care costs for these patients, potentially interfering with the identification of a discernable relationship between patient factors and the derived inpatient cost-of-care. For the successful implementation of bundled payments in geriatric hip fractures, further investigations and interventions dedicated to understanding and controlling the variability in care costs will be crucial.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Cunningham reports a grant from Integra during the conduct of the study. Outside the submitted work, wife is founder and CEO of CODE Technology.
ORCID iD: Brian P. Cunningham, MD  https://orcid.org/0000-0002-6653-2451
https://orcid.org/0000-0002-6653-2451
References
- 1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475. doi:10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- 2. Colby SL, Ortman JM. Population Estimates and Projections Current Population Reports. US Department of Commerce; 2015. [Google Scholar]
- 3. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620–623. doi:10.1097/BOT.0b013e3182695416 [DOI] [PubMed] [Google Scholar]
- 4. Bozic KJ, Ward L, Vail TP, Maze M. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction knee. Clin Orthop Relat Res. 2014;472(1):188–193. doi:10.1007/s11999-013-3034-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacofsky DJ. Episodic payments (bundling). Bone Jt J. 2017;99B(10):1280–1285. doi:10.1302/0301-620X.99B10.BJJ-2017-0355.R1 [DOI] [PubMed] [Google Scholar]
- 6. Seth Greenwald A, Bassano A, Wiggins S, Froimson MI. Alternative reimbursement models: bundled payment and beyond: AOA critical issues. J Bone Jt Surg-Am Vol. 2016;98(11):e45 doi:10.2106/JBJS.15.01174 [DOI] [PubMed] [Google Scholar]
- 7. Elbuluk A, Iorio R, Egol KA, Bosco JA. The surgical hip and femur fracture treatment model. JBJS Rev. 2017;5(10):e2 doi:10.2106/jbjs.rvw.17.00036 [DOI] [PubMed] [Google Scholar]
- 8. Yang CC, Chen PC, Hsu CW, Chang SL, Lee CC. Validity of the age-adjusted Charlson comorbidity index on clinical outcomes for patients with nasopharyngeal cancer post radiation treatment: a 5-year nationwide cohort study. PLoS One. 2015;10(1):1–11. doi:10.1371/journal.pone.0117323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitz-Henry J. The ASA classification and peri-operative risk. Ann R Coll Surg Engl. 2011;93(3):185 doi:10.1308/147870811X565070A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marsh JL, Slongo TF, Agel J, et al. Fracture and Dislocation Classification Compendium—2007: Orthopaedic Trauma Association Classification, Database and Outcomes Committee. J Orthop Trauma. 2007;21(10):S1–S133. doi:10.1097/00005131-200711101-00001 [DOI] [PubMed] [Google Scholar]
- 11. McCreary DL, White M, Vang S, Plowman B, Cunningham BP. Time-driven activity-based costing in fracture care: is this a more accurate way to prepare for alternative payment models?. J Orthop Trauma. 2018;32(7):344–348. doi:10.1097/BOT.0000000000001185 [DOI] [PubMed] [Google Scholar]
- 12. Parikh HR, O’Hara N, Levy JF, Cunningham BP. Value denominator: the fundamentals of costing for orthopaedic surgeons. J Orthop Trauma. 2019;33(S):56–61. [DOI] [PubMed] [Google Scholar]
- 13. Nathans LL, Oswald FL, Nimon K. Interpreting multiple linear regression: a guidebook of variable importance. Pract Assess Res Eval. 2012;17:9 doi:10.7275/5fex-b874 [Google Scholar]
- 14. Johnson DJ, Greenberg SE, Sathiyakumar V, et al. Relationship between the Charlson comorbidity index and cost of treating hip fractures: implications for bundled payment. J Orthop Traumatol. 2015;16(3):209–213. doi:10.1007/s10195-015-0337-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kay HF, Sathiyakumar V, Yoneda ZT, et al. The effects of American Society of Anesthesiologists physical status on length of stay and inpatient cost in the surgical treatment of isolated orthopaedic fractures. J Orthop Trauma. 2014;28(7):e153–e159. doi:10.1097/01.bot.0000437568.84322.cd [DOI] [PubMed] [Google Scholar]
- 16. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Jt Surg-Am Vol. 2012;94(1):9–17. doi:10.2106/JBJS.J.01077 [DOI] [PubMed] [Google Scholar]
- 17. Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty. Clin Orthop Relat Res. 2007;457:57–63. doi:10.1097/BLO.0b013e31803372e0 [DOI] [PubMed] [Google Scholar]
- 18. Gray CF, Prieto HA, Duncan AT, Parvataneni HK. Arthroplasty care redesign related to the Comprehensive Care for Joint Replacement model: results at a tertiary academic medical center. Arthroplast Today. 2018;4(2):221–226. doi:10.1016/j.artd.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernstein J. Not the last word: learned helplessness and Medicare’s bungled bundled payment program. Clin Orthop Relat Res. 2016;474(9):1919–1923. doi:10.1007/s11999-016-4935-8 [DOI] [PMC free article] [PubMed] [Google Scholar]