Abstract
Most head and neck cancers are squamous cell carcinomas that develop in the upper aerodigestive epithelium after exposure to carcinogens such as tobacco and alcohol. Human papillomavirus has also been strongly implicated as a causative agent in a subset of these cancers. The complex anatomy and vital physiological role of the tumour-involved structures dictate that the goals of treatment are not only to improve survival outcomes but also to preserve organ function. Major improvements have been accomplished in surgical techniques and radiotherapy delivery. Moreover, systemic therapy including chemotherapy and molecularly targeted agents—namely, the epidermal growth factor receptor inhibitors—has been successfully integrated into potentially curative treatment of locally advanced squamous-cell carcinoma of the head and neck. In deciding which treatment strategy would be suitable for an individual patient, important considerations include expected functional outcomes, ability to tolerate treatment, and comorbid illnesses. The collaboration of many specialties is the key for optimum assessment and decision making. We review the epidemiology, molecular pathogenesis, diagnosis and staging, and the latest multimodal management of squamous cell carcinoma of the head and neck.
Introduction
Head and neck cancer is a broad term that encompasses epithelial malignancies that arise in the paranasal sinuses, nasal cavity, oral cavity, pharynx, and larynx. Almost all of these epithelial malignancies are squamous cell carcinoma of the head and neck (SCCHN), for which the most important risk factors are tobacco and alcohol consumption.1 However, increasing evidence has documented human papillomavirus (HPV) as a cause of specific subsets of SCCHN.2 About two-thirds of patients with SCCHN present with advanced stage disease, commonly involving regional lymph nodes. Distant metastasis at initial presentation is uncommon, arising in about 10% of patients.3
Treatment decisions in SCCHN are often complicated, involving many specialists, including head and neck surgeons, medical oncologists, radiation oncologists, radiologists, plastic surgeons, and dentists. Primary tumour site, stage and resectability, and patient factors —including swallowing and airway considerations, desire for organ preservation, and comorbid illnesses— are used to guide appropriate management. Surgery and radiotherapy have long been the major treatment approaches. Improved surgical and radiation treatment approaches and incorporation of systemic agents into curative therapy have improved clinical outcomes. A new class of agents, the epidermal growth factor receptor (EGFR) inhibitors, has shown clinical benefit in this disease.
SCCHN survivors face lifetime risks of dying from cardiac and respiratory illnesses, and second primary tumours,4 which are commonly related to smoking. Second primary tumours develop at rates of 3–5% every year and can affect the entire aerodigestive tract.5 Presently, no established biomarker or evidenced-based imaging for patient surveillance exists, and no chemopreventive agent is of proven benefit. Despite promising early data, several well designed randomised clinical trials that assessed the effect of retinoids in chemoprevention yielded negative results.5 Continued smoking and alcohol use is harmful and should be avoided.6 Further elucidation of molecular events in SCCHN development are expected to accelerate the development of novel, potentially efficacious anticancer agents and identification of biomarkers, which could optimise treatment.
This Seminar provides an update on epidemiology, pathogenesis, diagnosis and staging, and latest treatment for SCCHN. Our primary focus is the four common sites of head and neck—ie, oral cavity, oropharynx, hypopharynx, and larynx. We exclude nasopharyngeal cancer, which is examined as a separate clinicopathological entity and reviewed separately.7
Epidemiology and risk factors
Head and neck cancer is the sixth most common type of cancer, representing about 6% of all cases and accounting for an estimated 650 000 new cancer cases and 350 000 cancer deaths worldwide every year.8 High-risk regions for oral cavity cancer include Melanesia (a subregion of Oceania, northeast of Australia) and southcentral Asia (including in women), western and southern Europe, and southern Africa, and for laryngeal cancer southern and eastern Europe, South America, and western Asia.8 In the USA alone, an estimated 45 660 new cases and 11 210 deaths caused by head and neck cancer occurred in 20079 The median age for diagnosis is in a patient’s early 60s, with a male predominance, especially in laryngeal cancer.3,8 A slight decrease in the overall incidence of head and neck cancer has been detected in the past two decades;3 however, an increase in cancer in the base of tongue and tonsillar cancer has been noted,10 which could be more pronounced in young adults in the USA and European countries.11,12 The 5-year survival for all stages combined on the basis of Surveillance Epidemiology and End Results (SEER) data is about 60%; survival is worse for specific primary sites such as the hypopharynx.3
Tobacco and alcohol consumption are implicated in 75% of all SCCHN and have a multiplicative combined effect.13–15 In people who have never smoked, substantial alcohol consumption (ie, three or more drinks per day) has been associated with an increased risk of SCCHN.16 Selected genetic polymorphisms in enzymes that metabolise tobacco and alcohol have been linked with an increased risk for SCCHN.16,17 Smokeless tobacco and chewing of betel quid—a preparation of various ingredients, including tobacco and the seeds of the betel palm (betel or areca nut), wrapped in a betel leaf—are well recognised risk factors for cancer of the oral cavity.18,19 Consumption of fruits and vegetables has been associated with a reduced risk of SCCHN.20 Additionally, the role of specific anthropometric variables21 and occupational factors have also been assessed.22,23 Although SCCHN arises sporadically, familial inheritance has been noted.24 Furthermore, the risk for SCCHN increases in individuals with cancer susceptibility syndromes, such as hereditary non-polyposis colorectal cancer, Li-Fraumeni syndrome, Fanconi’s anaemia, and ataxia telangiectasia.25,26
HPV, mainly HPV type 16 and to a lesser extent type 18, is a newly identified causal factor for SCCHN.2 Oncogenic HPV subtypes can be detected with in-situ hybridisation and p16 immunohistochemistry. About 25% of SCCHN contain HPV genomic DNA.27 The association between HPV and SCCHN is strongest for cancers of the tonsil, intermediate for the rest of the oropharynx, and weakest for the oral cavity and larynx.28 With in-situ hybridisation, HPV16 genomic DNA can be detected in up to 72% of oropharyngeal cancers.2 HPV-related SCCHN occurs more frequently in individuals who are not smokers, drinkers, or immunosuppressed, and are often poorly differentiated and of basaloid histology. The role of HPV16 in SCCHN might be independent of other carcinogens.2 Some sexual practices, because of their higher risk for transmission of HPV infection, were also noted to be risk factors for oropharyngeal cancer.2,29 High-risk oncogenic HPV types (eg, HPV16, HPV18) mediate their carcinogenic effect through E6 and E7 viral oncoproteins, which inactivate the tumour-suppressor proteins, P53 and PRb, respectively.30 Substantial differences in gene expression profiles and chromosomal defects have been documented between HPV-positive and HPV-negative tumours.31
The association between HPV and SCCHN has potentially important implications for prevention, treatment, and prognosis. HPV positivity is a favourable prognostic factor in SCCHN.32 HPV-positive tumours have better responsiveness to radiation, chemotherapy, or both, and might be more susceptible to immune surveillance of tumour-specific antigens than are HPV-negative tumours. Therefore, HPV status is an important predictive biomarker that should be taken into consideration in the design of prospective clinical studies in SCCHN. For example, it is possible that patients with locally advanced, HPV-positive SCCHN could do well with standard therapy, but might not benefit from treatment intensification. Finally, vaccination against HPV has shown efficacy in the prevention of cervical cancer and is of potential interest for prevention of SCCHN.
Pathogenesis
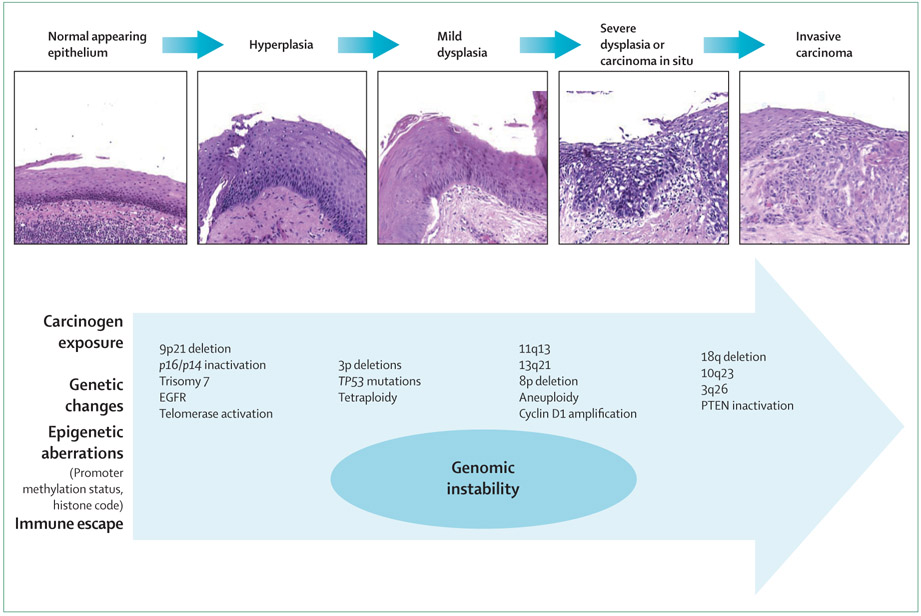
A plethora of genetic events leading to the inactivation of tumour-suppressor genes or activation of proto-oncogenes, or both, govern the development of SCCHN. Molecular techniques can identify genetic and epigenetic alterations in premalignant and invasive lesions, and allow the delineation of a hypothetical progression model for SCCHN carcinogenesis (figure 1).33–35 Stromal and immune or inflammatory cells contribute to carcinogenesis and treatment resistance. Telomerase, which is involved in telomere maintenance and immortalisation (thus protecting the acquired genetic changes), has been found to be reactivated in 90% of SCCHN and in premalignant lesions.36 The loss of 9p21 is a very common genetic aberration, because it is seen in 70–80% of SCCHN.37 Inactivation of p16, which is caused by homozygous deletion, point mutations, or promoter hypermethylation, and loss of 3p, could be early events in SCCHN carcinogenesis.34,38 Loss of heterozygocity of 17p and TP53 point mutations are seen in over 50% of cases of SCCHN.39 The prognostic significance of TP53 mutations is rather controversial; however, disruptive TP53 mutations in the DNA of the tumour were shown to be associated with reduced survival after surgical treatment of SCCHN.40 Amplification of 11q13 and overexpression of cyclin D1 are also detected in SCCHN, and could correlate with more aggressive tumour behaviour.41,42
Figure 1: Presentation of phenotypical progression and accumulated molecular alterations in head and neck carcinogenesis.
Histological evolution shown in haematoxylin and eosin specimens (×200) parallels genetic and epigenetic events. Modified from references 33 and 34. EGFR=epidermal growth factor receptor. PTEN=phosphatase and tensin homologue.
Leukoplakia and erythroplakia are clinically detected lesions that can histologically represent hyperplasia, dysplasia, and even in-situ or invasive carcinoma.43 A subsequent invasive cancer might develop at the site of a known dysplastic lesion or at other mucosal sites; the reported risk depends on histology and length of follow-up, and varies considerably between studies (10–40%).44 Although standard treatment options for head and neck epithelial dysplasia range from watchful waiting to laser surgery and aggressive resection, none of these approaches are of clear clinical benefit in preventing malignant transformation.45 Chemoprevention (topical or systemic) has been used to prevent the evolution of premalignant lesions into invasive SCCHN, with variable results.46
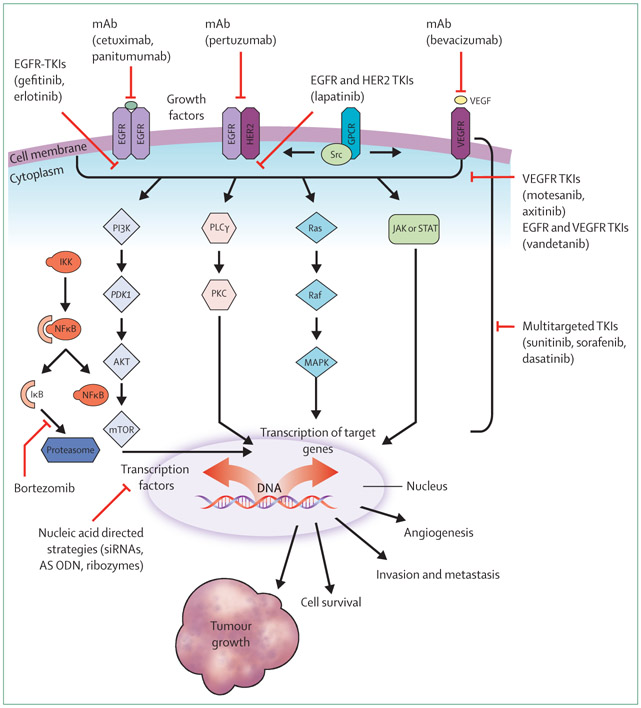
EGFR is a member of the ErbB growth factor receptor tyrosine kinase family, which is central to SCCHN biology.47 Ligand binding (eg, epidermal growth factor) results in EGFR homodimerisation or heterodimerisation with other members of the ErbB family. Thus, a molecular cascade is triggered, activating the receptor-linked tyrosine kinase and many downstream pathways, which regulate cell proliferation, apoptosis, metastatic potential, and angiogenesis (figure 2). EGFR activation can be also achieved through cross-talk with other receptors—eg, G-protein-coupled, platelet-derived growth factor, insulin-like growth factor, and hormone receptors.47 EGFR protein expression is seen in 90% or more of cases of SCCHN.48 Many retrospective analyses have shown poor outcome for patients with SCCHN that overexpress EGFR.49 Targeting of this receptor has been successfully exploited for therapeutic purposes.47
Figure 2: Molecular signalling pathways and novel targeted agents for the treatment of SCCHN.
There is considerable cross-talk between various signalling pathways. Various strategies have been developed to inhibit these pathways, such as mAbs; single-selective, dual-selective, or multi-selective tyrosine kinase inhibitors; and nucleic acid-directed gene silencing molecules (eg, AS ODN and RNA interference—siRNA and ribozymes). Representative agents that are used in human beings are listed in the figure. AS ODN=antisense oligodeoxynucleotide. EGFR=epidermal growth factor receptor. GPCR=G-protein-coupled receptor. HER2= human epidermal receptor 2. IKK=inhibitor κB. JAK=Janus kinase. mAb=monoclonal antbodies. MAPK=mitogen-activated protein kinase. mTOR=mammalian target of rapamycin. NF=nuclear factor. PDKI=pyruvate dehydrogenase kinase, isozyme 1. PI3K=phosphatidylinositol-3-kinase. PLCγ=phospholipase-Cγ. PKC=protein kinase. SCCHN=squamous cell carcinoma of the head and neck. STAT=signal transducers and activators of transcription. TKI=tyrosine kinase inhibitor. VEGFR=vascular endothelial growth factor receptor.
Angiogenesis is fundamental to cancer growth and metastasis, and is regulated by many endogenous proangiogenic and antiangiogenic factors—the most important being the vascular endothelial growth factor (VEGF) and its receptors.50 VEGF can be upregulated and has prognostic significance in SCCHN.51 Antiangiogenesis therapeutic strategies have been extensively studied in other solid tumours50 and are under assessment in the treatment of SCCHN (figure 2).
Malignant cells, including SCCHN, escape from immune-mediated destruction not only by evading immune recognition but also by directly inhibiting or exploiting antitumour immune defences. Patients with SCCHN showed reduced peripheral blood concentrations of CD3+, CD4+, and CD8+ T cells, which might persist even several years after curative surgery.52 Many mechanisms for immune evasion have been proposed, including escape from immune recognition and elimination caused by tumour factors,53,54 impaired T-lymphocyte activity, activity of immunosuppressive cells, and cytokines mediating local and systemic effects.
Diagnosis and staging
Early recognition of symptoms and signs of SCCHN is important for prompt diagnosis (panel 1 and figure 3). No proven screening methods, except population screening with oral visual inspection in high-risk regions for oral cavity cancer, are known to exist.55 Referral criteria have been developed to expedite specialist assessment for biopsy of suspected malignant lesions, which is always needed to confirm the diagnosis.56 Histological variants of SCCHN are rare and include verrucous, basaloid, spindle cell, and adenosquamous carcinomas. Poorly differentiated or undifferentiated carcinomas of the head and neck are managed in a manner similar to SCCHN.
Panel 1: Diagnostic considerations in squamous cell carcinoma of the head and neck.
Symptoms
Hoarseness, sore throat, tongue pain, mouth ulcer, poorly fitting dentures, otalagia, dysphagia and odynophagia, cough, mouth bleeding, stridor
Physical examination findings
Mass or ulceration in oral cavity or oropharynx, neck mass, vocal cord paralysis, swallowing dysfunction
Staging and assessment
Office nasopharyngeal examination, imaging studies (chest radiograph, CT, MRI, PET), direct laryngoscopy and oesophagoscopy, bimanual examination under anaesthesia, barium swallow, creation of tumour map, biopsy of tumour and adjacent sites, chemistry and complete blood count
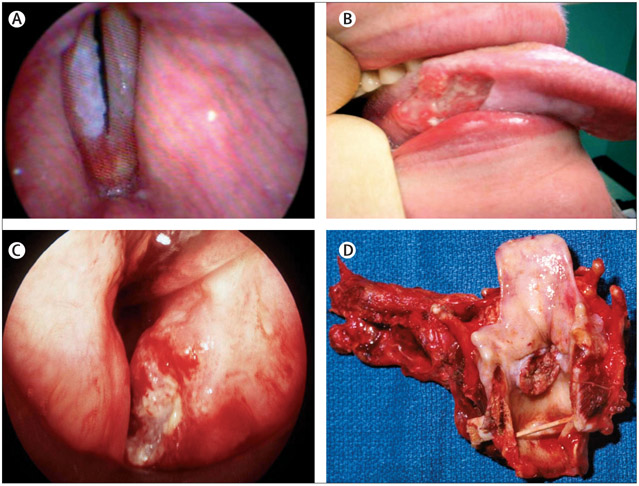
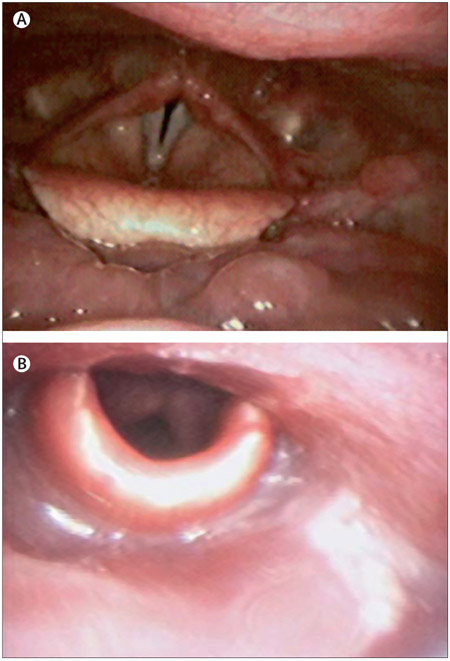
Figure 3: Various stages of squamous cell carcinoma of the head and neck.
(A) Premalignancy evidenced by leukoplakia on the right true vocal fold; (B) early oral tongue cancer in a young, non-smoking woman; (C) stage T2 laryngeal carcinoma, potentially treatable with radiation or surgery; and (D) pathology specimen post-total laryngectomy, which is a standard procedure for large volume, destructive laryngeal cancers.
Accurate staging is the most important factor that guides therapeutic decision making. Staging methods include examination by a head and neck surgeon and radiological assessment that usually entails imaging of the neck with CT or MRI, or both. The most common site of distant metastasis is the lung, followed by the mediastinal lymph nodes, liver, and bones. Chest imaging is routinely done at initial assessment, and might detect a second primary lung cancer or, especially in locally advanced SCCHN, the presence of lung metastasis. The sixth edition of the American Joint Committee on Cancer (AJCC) staging classification is widely used;57 staging criteria vary according to the primary site. Although the AJCC system is helpful in defining the anatomical extent of cancer, it does not include pathological or biological parameters of tumour behaviour.
PET scanning with [18F]fluoro-2-deoxyglucose is increasingly used, especially for the detection of nodal or distant metastasis, or both.58 Combined PET and CT scans are more accurate than either method alone59 for identifying malignant lesions in the head and neck.59 PET-CT scanning is also potentially valuable for assessment of response (figure 4) and detection of persistent or recurrent SCCHN.
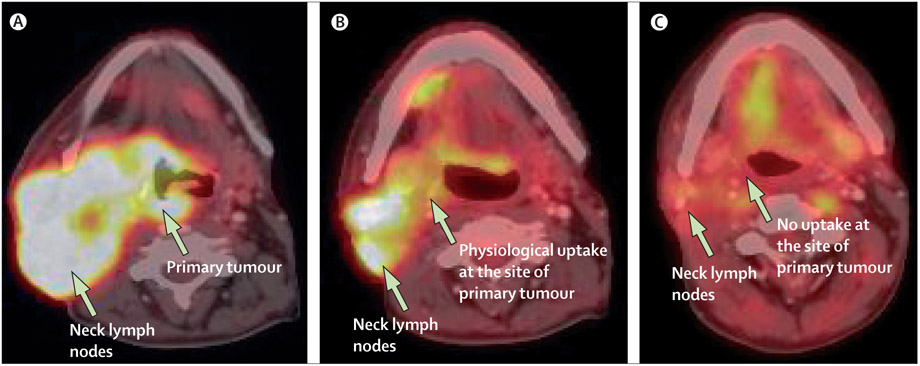
Figure 4: Combined PET-CT scan used for staging and response assessment in locally advanced squamous cell carcinoma of the head and neck.
Axial fused images showing (A) locally advanced oropharyngeal squamous cell carcinoma with massive lymphadenopathy, staged as T4N3; (B) induction treatment with cisplatin, docetaxel, and cetuximab (on a research protocol) resulted in complete response in the primary and partial response in regional lymph nodes; and (C) further improvement after completion of radiotherapy with concurrent cisplatin and cetuximab. Physiological uptake is seen in the floor of the mouth. Subsequent neck dissection and biopsy of primary tumour showed a complete pathological response.
Patients might also present with neck lymphadenopathy without an apparent primary tumour. Endoscopic assessment and biopsy of Waldeyer’s ring (nasopharynx, tonsil, and base of tongue), including tonsillectomy, along with PET, could identify the occult primary tumour.60 However, in up to 5% of cases of SCCHN the primary site remains unknown. Management of this entity is controversial, but prognosis seems favourable.61
Innovations in treatment
Surgery
Surgery is a standard treatment for SCCHN but is frequently limited by the anatomical extent of tumour and desire to achieve organ preservation. Advances in microsurgical free tissue transfer for reconstruction of surgical defects have made major reconstructive procedures commonplace at many centres, helping in the resection of locally advanced tumours. By use of modern surgical techniques, substantially improved functional outcomes are often possible for patients who need extensive surgical resections, even in the setting of salvage surgery after failure of organ-preserving treatment. No uniform unresectability criteria exist, but most surgeons accept invasion of the carotid artery, base of the skull, or prevertebral musculature as unresectable.
Surgical management provides pathological staging of primary tumour and regional nodes, often upstaging clinically uninvolved neck (ie, clinical stage N0) by histological identification of micrometastasis,62 which will guide adjuvant treatment decisions. For small, transorally accessible cancers of the oral cavity, pharynx, and larynx, surgical excision can be achieved with functional preservation of much of the involved organ and good oncological results.63,64 Microsurgical treatment, which uses endoscopic laser or robotic techniques and high resolution magnified optics, has become technically feasible and could be cost saving compared with open surgical procedures or radiotherapy for early stage laryngeal cancer with acceptable voice outcomes.65
When surgery is the primary treatment, neck dissection is carried out as part of surgical management. However, after treatment with primary chemoradiotherapy, neck dissection is usually recommended when residual disease is suspected, whereas its role remains controversial in the setting of complete response.66,67 The adaptation of less morbid selective neck dissection procedures is yielding important staging information for guiding therapy and assessing prognosis.68 Selective neck dissection is also a reasonable therapeutic procedure for clinically uninvolved necks, which can harbour micrometastasis in up to a third of cases,62 and clinically N1 disease in the absence of adverse histological features.69,70 Sentinel lymph node biopsy has gained attention for its ability to reliably detect nodal metastasis in oral and oropharyngeal SCCHN, and was validated by a large multi-institutional trial done by the American College of Surgeons’ Oncology Group. In the future, risk of metastasis could be ascertained through molecular gene signatures in the sentinel lymph node70 or in the primary tumour.71,72
Radiotherapy
Radiotherapy is an integral part of primary or adjuvant treatment of SCCHN. Radiotherapy alone results in high tumour control and cure rates for early stage glottic, base of tongue, and tonsillar cancer. Advances in imaging and radiation delivery have dramatically changed management approaches. Planning CT scans are now frequently combined with diagnostic CT, MRI, or PET datasets to improve tumour delineation in three dimensions. Intensity-modulated radiation therapy represents an advanced form of radiation in three dimensions. A computer-controlled treatment machine is used to produce many radiotherapy beams in which the intensity is optimised to deliver a high dose of radiation to specified volumes, while reducing the dose and, theoretically, the toxic effect on adjacent non-target tissues. Intensity-modulated radiation therapy uses inverse planning to protect healthy tissue from chronic damage by limiting the dose delivered to areas such as the salivary tissue. Another advantage of this technique is that it allows for delivery of a synchronous integrated boost in which treatment field modifications, such as off-cord laterals and posterior electron strips, are no longer needed. Although initial clinical results of intensity-modulated radiation therapy in the treatment of SCCHN are very promising,73 several issues have to be addressed before its use becomes widespread, either as a single treatment method or in combination with chemotherapy.74,75 Additional advances in radiotherapy include tomotherapy (integration of CT or PET-CT technology into a linear accelerator) heavy particle radiation, proton therapy, neutron beam radiation, brachytherapy, and stereotactic radiosurgery; however, in most instances these methods have not been validated in prospective clinical trials.76,77
Radiation therapy for treatment of SCCHN is typically given in daily fractions of 2·0 Gy, 5 days a week, up to a total dose of 70 Gy over 7 weeks. Higher dose per fraction schemas have been attempted for early-stage laryngeal SCCHN, with excellent results (2·25 Gy per fraction) and no increase in late toxic effects.78 Postoperatively, radiation doses of 60–66 Gy are usually prescribed; increasing the total dose to at least 63 Gy improves locoregional control when extracapsular extension is present.79
Long-term interruptions to radiotherapy or delays in starting postoperative radiotherapy are potentially harmful, presumably because of repopulation of cancer cells.80–82 To address tumour cell kinetics and exploit differences in damage repair between healthy and tumour cells, altered fractionation radiotherapy regimens were introduced. Two major fractionation variants that make it possible for multiple fractions per day to be delivered have been tested: hyperfractionation and accelerated fractionation.83
Hyperfractionation was designed to improve effectiveness by delivering two to three fractions every day with a reduced dose per fraction (usually 1· 10–1· 25 Gy), while maintaining or improving the chronic toxic effect profile of standard fractionation, despite an increased total dose. Accelerated fractionation was designed to increase radiation dose intensity by delivering fractions of 1·6–1·8 Gy more than once daily with a planned dose of 10 Gy per week in a reduced time period compared with hyperfractionation, but maintaining the same or slightly reduced dose of conventional radiation treatment.84 Phase III trials showed that altered fractionation improves locoregional control with increased infield toxic effects but with marginal effects on survival, compared with conventional radiotherapy.85 A meta-analysis of 15 randomised trials with more than 5000 participants, mostly with oropharyngeal and laryngeal SCCHN, showed that altered fractionation radiotherapy yielded an absolute 5-year survival benefit of 3·4% (HR 0·92, 95% CI 0·86–0·97; p=0.004).86 Additionally, the combination of altered fractionation schedules and chemotherapy has been tested in clinical trials with promising results (table 1). The results of a completed phase III trial (Radiation Therapy Oncology Group [RTOG] 01-29) that compared conventional fractionation with accelerated boost fractionation in patients receiving concurrent cisplatin are pending.
Table 1:
Selected randomised phase III studies with concurrent chemoradiotherapy in locally advanced SCCHN
| Number of patients |
Treatment schedule | Control arm | Primary endpoint |
Treatment outcome | |
|---|---|---|---|---|---|
| Chemoradiotherapy vs conventional radiotherapy alone as postoperative treatment | |||||
| Oral cavity, oropharynx, hypopharynx, larynx100 | 334 | Radiotherapy+C (100 mg/m2) d 1,22,43 | Radiotherapy | Progression-free survival | 47% vs 36% at 5 years, p=0·04 Overall survival 53% vs 40% at 5 years, p=0·04 |
| Oral cavity, oropharynx, hypopharynx, larynx101,102 |
416 | Radiotherapy+C (100 mg/m2) d 1,22,43 | Radiotherapy | Locoregional control | HR 0·61, 95% CI 0·41–0·91; p=0·01 Updated HR 0·72, 95% CI 0·48–1·06; p=0·08 |
| Oral cavity, oropharynx, hypopharynx, larynx103 | 444 | Radiotherapy+C (20 mg/m2 ci)+F (600 mg/m2 ci) d 1–5, 29–33 | Radiotherapy | Locoregional control | 88·3% vs 61·9% at 5 years, p=0·0006 Overall survival 58·1% vs 48·6%, p=0·11 |
| Chemoradiotherapy vs conventional radiotherapy alone as primary treatment | |||||
| Oropharynx104,105 | 222 (unresectable) | Radiotherapy+Cb (70 mg/m2, d 1–4)+F (600 mg/m2 ci, d 1–4) for 3 cycles | Radiotherapy | Overall survival | 51% vs 31% at 3 years, p=0·02 22·4% vs 15·8% at 5 years, p=0·05 |
| Oral cavity, oropharynx, hypopharynx, larynx106 | 295 (unresectable) | Radiotherapy+C (100 mg/m2) d 1,22,43 (A) or Radiotherapy split course+C (75 mg/m2 d 1)+F (1000 mg/m2 ci, d 1–4) for 3 cycles (B) | Radiotherapy (C) | Overall survival | (A) 23% vs (C) 37% (p=0·014) vs (B) 27% (p=NS) at 3 years |
| Larynx107,108 | 510 (resectable) | Radiotherapy+C (100 mg/m2) d 1,22,43 (A) or radiotherapy alone (B) | C (100 mg/m2 d 1)+F (1000 mg/m2/d ci d 1–5) for 3 cycles followed by radiotherapy alone in responders (C) | Laryngeal preservation | (A) 84% vs (B) 66% (p=0·00017) vs (C) 70% (p=0·0029), at 5 years |
| Chemoradiotherapy vs altered fractionation radiotherapy alone as primary treatment | |||||
| Oral cavity, oropharynx, hypopharynx, larynx109 | 270 (unresectable) | Hfx radiotherapy+C (60 mg/m2, d 1)+F (350 mg/m2 bolus, d 2)+Fo (50 mg/m2 bolus, d 2) or F (350 mg/m2 ci, d 2–5)+Fo (100 mg/m2 ci, d 2–5) for 3 cycles | Hfx radiotherapy | Locoregional control | 35% vs 17% at 3 years, p<0·004 Overall survival 49% vs 24%, p<0·0003 |
| Oral cavity, oropharynx, hypopharynx, larynx110 | 116 (62 unresectable) | Hfx radiotherapy (70 Gy)+C (12 mg/m2, d 1–5)+F (600 mg/m2 ci, d 1–5) weeks 1,6 | Hfx radiotherapy (75 Gy) | Locoregional control | 70% vs 44% at 3 years, p<0·01 Overall survival 55% vs 34%, p=0·07 |
| Oral cavity, oropharynx, hypopharynx, larynx111 | 130 | Hfx radiotherapy+C (6 mg/m2) per day | Hfx radiotherapy | Overall survival | 46% vs 25% at 5 years, p=0·0075 |
| Oropharynx112 | 192 (unresectable) | Radiotherapy (66–70 Gy)+Cb (75 mg/m2, d 1–4)+F (1000 mg/m2 ci, d 1–4) for 3 cycles | Conventional radiotherapy or Hfx Acc radiotherapy (64–67·2 Gy) | Overall survival | 51% vs 40% vs 37% at 2 years, p=0·129 |
| Oral cavity, oropharynx, hypopharynx, larynx113 | 224 (78 unresectable) | Hfx radiotherapy+C (20 mg/m2, d 1–5) for 2 cycles | Hfx radiotherapy | Time to treatment failure | 27% vs 24% at 5 years, p>0·05 Overall survival 46% vs 32% at 5 years, p=0·15 |
| Oral cavity, oropharynx, hypopharynx114 | 384 (unresectable) | Hfx Acc radiotherapy (70·6 Gy)+F (600 mg/m2 ci, d 1–5)+M (10 mg/m2 bolus) d 1,36 | Hfx Acc radiotherapy (77·6 Gy) | Locoregional control | 49·9% vs 37·4% at 5 years, p=0·001 Overall survival 28·6% vs 23·7% at 5 years, p=0·023 |
| Oropharynx, hypopharynx115,116 | 240 (unresectable) | Hfx Acc radiotherapy+Cb (70 mg/m2 d 1–4)+F (600 mg/m2 ci, d 1–4) for 2 cycles | Hfx Acc radiotherapy | Locoregional control | 22·7% vs 12·6% at 5 years, p=0·01 Overall survival 26·1% vs 13% at 5 years, p=0·008 |
Acc=accelerated. C=cisplatin. Cb=carboplatin. ci=continuous infusion. d=day of treatment cycle. F=fluorouracil. Fo=folinate. Hfx=hyperfractionated. HR=hazard ratio. M=mitomycin. NS=not significant. SCCHN=squamous cell carcinoma of the head and neck. Unresectable (inoperable) SCCHN is not defined uniformly; it is associated with worse outcomes vs resectable (operable) SCCHN.
Chemotherapy and novel agents
The role of chemotherapy in SCCHN treatment has evolved from palliative care to a central component of curative programmes for locally advanced SCCHN.87 Various classes of agents such as platinum compounds, antimetabolites, and taxanes have shown single-agent activity against SCCHN.88 The platinum compound cisplatin is regarded as a standard agent in combination with radiation or with other agents. Carboplatin is well tolerated but less active than cisplatin as a component of combination regimens,89,90 although the radiosensitising properties of the two platinum agents could be comparable.91 Taxane-based combinations are very active and have been tested in the induction chemotherapy of locally advanced SCCHN.92 Moreover, EGFR inhibition has emerged as a novel treatment strategy for SCCHN, and cetuximab is the first molecularly targeted agent that has been introduced into standard practice.47 Other ways of targeting EGFR and other dysregulated molecular pathways in SCCHN, using monoclonal antibodies, single-selective or multi-selective tyrosine kinase inhibitors, and nucleic acid-directed approaches, are also being explored (figure 2). The combination of EGFR inhibitors with other molecularly targeted agents (eg, angiogenesis inhibitors) has surfaced as a novel strategy, whereas the combination of these novel agents with chemotherapy and radiotherapy is under investigagion.
Treatment of early stage disease
About a third of all patients with SCCHN present with stage I or II disease. These patients are treated with surgery or radiation therapy with the intent of curing the disease, which is achieved in up to 90% of patients with stage I disease and about 70% of those with stage II disease. Treatment approaches differ according to the primary tumour site. In early stages of SCCHN in the oral cavity, surgery or radiotherapy could be used. Although efficacy is comparable between the two methods, surgery is usually preferred so that the late toxic effects of radiation can be avoided and so the most accurate staging can be obtained. Elective functional neck dissection of ipsilateral cervical lymph nodes in the clinically uninvolved neck remains a standard procedure in patients with a high risk for occult neck lymphadenopathy.93 Radiotherapy, as well as open or endoscopic surgery that spare the larynx, are acceptable options for treatment of early-stage laryngeal SCCHN; the treatment choice depends on tumour location, the treating centre’s expertise, and patient preference.94–96 For early-stage oropharyngeal and hypopharyngeal carcinomas, radiotherapy generally represents the first option since it results in cure rates comparable with surgery and is usually associated with lower morbidity.97,98
Treatment of locally advanced disease
Surgery, radiotherapy, and chemotherapy are the main means for curative management of locally advanced SCCHN (ie, stage III or IV). A major advancement in the treatment of this stage of disease has been the introduction of concurrent administration of chemotherapy and radiotherapy (chemoradiotherapy). Adequate preclinical and clinical rationales exist to support the use of chemoradiotherapy.99 Several phase III clinical trials have shown that chemoradiotherapy yields better results than radiotherapy alone or the sequential administration of chemotherapy and radiotherapy (table 1).100–116 The survival advantage that was noted with chemoradiotherapy over radiotherapy alone could, in most cases, be attributed to improved locoregional control.100–116 Benefit from chemoradiotherapy has also been documented in the postoperative setting. After encouraging preliminary data,117,118 phase III trials compared postoperative radiotherapy with or without concurrent chemotherapy with cisplatin100–102 or cisplatin and fluorouracil103 in patients with high-risk resected SCCHN (table 1). The definition of high-risk pathological features was not uniform in these studies and included the involvement of many lymph nodes, extracapsular extension, and involved margins. These studies show that the addition of cisplatin-based chemotherapy to radiotherapy can improve locoregional control, disease-free survival, or survival. A combined analysis of two of these studies suggested that the addition of cisplatin to postoperative radiotherapy was most beneficial when extracapsular spread or positive margins were present.119
A meta-analysis of 63 trials with nearly 11 000 patients with SCCHN showed that the addition of chemotherapy to locoregional treatment resulted in an absolute survival benefit of 4% at 5 years (HR 0·90, 95% CI 0·85-0–94; p<0·0001).120 This benefit was confined to chemoradiotherapy (HR 0·81, 95% CI 0·76–0·88; p<0·0001) that resulted in an absolute survival improvement of 8% at 5 years,120 which was also supported by an updated meta-analysis that included 24 additional studies.121 Treatment benefit was maintained in stage III or IV disease, each major primary site, definitive or postoperative radiotherapy, and when altered fractionation radiotherapy was used in the control arm.121 However, chemoradiotherapy has an insignificant effect on distant recurrence rate and results in increased acute complications including mucositis, dermatitis, and myelosuppression. Nevertheless, being cured is of paramount importance to patients, overshadowing potential toxic effects.122
In patients with unresectable, locally advanced SCCHN chemoradiotherapy is standard,106 unless the addition of chemotherapy is not indicated because of poor performance status or comorbid illnesses. In patients with resectable, locally advanced SCCHN, surgery usually followed by postoperative chemoradiotherapy or chemoradiotherapy alone could be the primary treatment choice. Guidelines that provide useful practical approaches have been developed.66 Although an important treatment goal is organ preservation, physicians recognise that such attempts might leave an anatomically intact but dysfunctional organ. No conclusive data exist regarding quality of life in surgical versus non-surgical approaches. In general, oral cavity primaries are usually treated with surgery, since the cosmetic and functional result is regarded as satisfactory and patients might be spared aggresive chemoradiotherapy. However, locally advanced oropharyngeal primaries are usually treated with chemoradiotherapy with good efficacy and functional results.123 Patients with locally advanced hypopharyngeal and laryngeal primaries should be considered for organ preservation.
In the 1990s, induction chemotherapy followed by radiotherapy in responders offered comparable survival rates to laryngectomy.124,125 However, only a few patients with T4 tumours were enrolled in these two randomised trials. In bulky T4 tumours, especially with baseline organ dysfunction, many experts advocate upfront surgery because of high failure rates with non-surgical approaches and questionable benefit from preservation of a poorly functional larynx. A randomised trial (RTOG 91-11) assessed the optimum non-surgical treatment of patients with locally advanced laryngeal cancer, using organ preservation as the primary endpoint (table 1).107,108 Although no survival difference was evident between the three arms in the study, which could be partially attributed to the effect of salvage laryngectomy,126 the laryngeal preservation rate was highest in patients given chemoradiotherapy with radiation and concurrent cisplatin.
Although various chemoradiotherapy regimens are being used, cisplatin-containing regimens are generally viewed as standard and have been studied extensively. Moreover, even though chemoradiotherapy with two-drug combinations has produced promising data,127 it has not yet proved better than chemoradiotherapy with one chemotherapy agent alone in randomised comparisons.120,121 Cetuximab, an IgG1 chimeric monoclonal antibody directed against EGFR, is the first molecularly targeted agent to yield positive survival data in SCCHN. The combination of radiation and cetuximab was compared with radiation alone in patients with locally advanced SCCHN and showed improved locoregional control (47% vs 34% at 3 years; HR 0·68, 95% CI 0·52–0·89; p=0·005), progression-free survival (42% vs 31% at 3 years; HR 0·70, 95% CI 0· 54–0· 90; p=0·006), and overall survival (55% vs 45% at 3 years; HR 0·74, 95% CI 0·74–0·97; p=0·03) but not distant control (17% vs 16% at 2 years).128 Although results with cetuximab were comparable with those achieved with platinum-based regimens, no randomised trial has yet compared radiation plus cetuximab versus radiation plus cisplatin. Radiation plus cetuximab is an alternative to chemoradiotherapy that should be strongly considered in patients who cannot tolerate chemotherapy, since this regimen is not associated with increased myelosuppression or mucositis, although side-effects due to inhibition of EGFR are seen (eg, rash and hypomagnesaemia). A focus of continuing investigation is the incorporation of EGFR inhibitors into various chemoradiotherapy regimens. A phase II study assessed the combination of cetuximab with cisplatin and accelerated boost radiotherapy with promising preliminary efficacy, but there were also concerns about possibly enhanced toxic effects.129 An ongoing phase III trial by the RTOG is comparing this regimen with radiation and cisplatin alone.
Role of induction chemotherapy
Induction (neoadjuvant) chemotherapy has the potential to reduce the incidence of distant metastases, which are increasingly recognised as sites of disease recurrence, as a result of improved locoregional control with chemoradiotherapy.130 SCCHN is a highly responsive malignancy at initial presentation; cisplatin-based induction chemotherapy has produced response rates of 80–90%, with complete response rates of 20–40% in locally advanced SCCHN.92 Despite high antitumour activity, many phase III trials that compared induction chemotherapy followed by locoregional treatment (surgery or radiotherapy, or both) with locoregional treatment alone failed to show survival benefit,92 except for two studies in which benefit was shown, although one in a subset analysis only (table 2).131–133 Many of these trials reported a reduction in the risk of distant metastases in patients given induction chemotherapy, but the effect on locoregional control was insignificant. In a meta-analysis, induction chemotherapy resulted in a non-significant survival improvement of 2% at 5 years (HR 0·95, 95% CI 0·88–1–01; p=0·10).120 However, in the 15 trials with a platinum agent plus fluorouracil, a marginal survival benefit was evident (HR 0·88, 95% CI 0·79–0·97; p=0·05).120 The introduction of more active, taxane-containing chemotherapy combinations has strengthened the rationale for using induction chemotherapy. In phase III clinical trials, a taxane (docetaxel or paclitaxel) plus cisplatin and fluorouracil resulted in improved survival or organ preservation compared with cisplatin and fluorouracil alone (table 2).134–137 However, induction chemotherapy is not yet regarded a standard treatment for locally advanced SCCHN. Several randomised trials comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone are underway.
Table 2:
Selected phase III randomised trials of cisplatin-based induction chemotherapy in locally advanced SCCHN
| Number of patients |
Treatment schedule | Control arm | Primary endpoint |
Treatment outcome | |
|---|---|---|---|---|---|
| Induction chemotherapy followed by locoregional treatment vs locoregional treatment alone | |||||
| Larynx124 | 332 (resectable) | C (100 mg/m2, d 1)+F (1000 mg/m2 ci, d 1–5) every 3 weeks for 3 cycles followed by radiotherapy in responders | Surgery (followed by radiotherapy) | Survival or laryngeal preservation | 64% of survivors achieved laryngeal preservation. No difference in overall survival |
| Hypopharynx125 | 194 (resectable) | C (100 mg/m2, d 1)+F (1000 mg/m2 ci, d 1–5) every 3 weeks for 3 cycles followed by radiotherapy in responders | Surgery (followed by radiotherapy) | Efficacy equivalence | 35% laryngeal preservation at 5 years. Overall survival 57% vs 43% at 3 years (favouring induction) |
| Oropharynx133 | 318 | C (100 mg/m2, d 1)+F (1000 mg/m2 ci, d 1–5) every 3 weeks for 3 cycles followed by surgery or radiotherapy, or both | Surgery or radiotherapy, or both | Overall survival | Median survival, 5·1 years vs 3·3 years, p=0·03 |
| Oral cavity, oropharynx, hypopharynx, paranasal sinus131,132 | 237 (171 unresectable) | C (100 mg/m2, d 1)+F (1000 mg/m2 ci, d 1–5) every 3 weeks followed by surgery or radiotherapy, or both | Surgery or radiotherapy, or both | Overall survival | In unresectable, at 5 years, 21% vs 8%; 16% vs 6%, at 10 years, p=0·04 No statistically significant differences in resectable |
| Taxane plus cisplatin or fluorouracil vs cisplatin or fluorouracil alone | |||||
| Oral cavity, oropharynx, hypopharynx, larynx135 | 382 (248 unresectable) | P (175 mg/m2 d 1)+C (100 mg/m2, d 2)+F (500 mg/m2 ci, d 2–6) every 3 weeks for 3 cycles | P (100 mg/m2 d 1)+F (1000 mg/m2 ci, d 1–5) every 3 weeks for 3 cycles | Complete response rate | 33% vs 14%, p<0·001 Overall survival 66·5% vs 53·6% at 2 years, p=0·06 |
| Oral cavity, oropharynx, hypopharynx, larynx137 | 358 (unresectable) | D (75 mg/m2 d 1)+C (75 mg/m2, d 1)+F (750 mg/m2 ci, d 1–5) every 3 weeks for 4 cycles followed by radiotherapy | C (100 mg/m2 d 1)+F (1000 mg/m2, ci, d 1–5) every 3 weeks for 4 cycles followed by radiotherapy | Progression-free survival | Median progression-free survival, 11 months vs 8·2 months, p=0·007 Overall survival 37% vs 26% at 3 years, p=0·02 |
| Oral cavity, oropharynx, hypopharynx, larynx136 | 501 (176 unresectable) | D (75 mg/m2, d 1)+C (100 mg/m2, d 1)+F (1000 mg/m2 ci, d 1–4) every 3 weeks for 3 cycles followed by radiotherapy+Cb (AUC=1·5) every week | C (100 mg/m2, d 1)+F (1000 mg/m2, ci, d 1–5) every 3 weeks for 3 cyles followed by radiotherapy+Cb (AUC=1·5) every week | Overall survival | Median overall survival, 71 months vs 30 months, p=0·006,62% vs 48% at 3 years |
| Hypopharynx, larynx134 | 220 (resectable) | D (75 mg/m2 d 1)+C (75 mg/m2 d 1)+F (750 mg/m2 ci, d 1–5) every 3 weeks for 3 cycles followed by radiotherapy for responders | C (100 mg/m2 d 1)+F (1000 mg/m2 ci, d 1–5) every 3 weeks for 3 cycles followed by radiotherapy for responders | Laryngeal preservation rate | 73% vs 63% at 3 years, p=0·036 |
C=cisplatin. Cb=carboplatin. ci=continuous infusion. d=day of treatment cycle. D=docetaxel. F=fluorouracil. P=paclitaxel. HR=hazard ratio. SCCHN=squamous cell carcinoma of the head and neck. Unresectable (inoperable) SCCHN is not defined uniformly; it is associated with worse outcomes versus resectable (operable) SCCHN.
Acute and late complications
Patients with SCCHN develop acute and late complications as a result of their disease and its treatment. Common acute toxic effects associated with radiation are mucositis (which is severe in 50% or more of patients receiving chemoradiotherapy), increased secretions, dysphagia, occasionally with aspiration, loss of taste, hoarseness caused by laryngeal oedema, and dermatitis.138,139 Supportive care during chemoradiotherapy is often demanding and includes oral and skin care, narcotic analgesics, intravenous hydration, and enteral nutrition, as necessary. Swallowing function and quality of life usually improve in the first year after treatment, but swallowing dysfunction might be permanent.140,141 Other possible late sequelae of treatment include osteoradionecrosis, dental caries, subcutaneous fibrosis, trismus, thyroid dysfunction, sensorineural hearing loss, pharyngeal or oesophageal stenosis (figure 5), and myelitis. Radiation-induced xerostomia is universal in long-term survivors, which is moderate or severe in about 60% of patients.142 Several strategies seek to prevent xerostomia, including surgical transfer of salivary glands, radioprotectants (eg, amifostine), and radiation techniques that spare the salivary glands.143 Supportive care measures include oral hygiene with fluoride agents and antimicrobials to prevent dental caries and oral infections, saliva substitutes, and cholinergic stimulants (eg, pilocarpine). Treatment and disease-induced anatomical and functional defects might lead to many social and psychological problems;141,144 thus, patients with SCCHN should be cautiously monitored and encouraged to participate in long-term supportive care programmes.
Figure 5: Late effects of radiation might result in poor organ function and quality of life.
Laryngoscopic view of (A) normal pharynx; and (B) pharynx with severe fibrosis several years after curative chemoradiotherapy.
At present, there is no radioprotectant with proven efficacy in decreasing the severity of mucositis during chemoradiotherapy for SCCHN. Granulocyte-macrophage colony stimulating factor or granulocyte colony stimulating factor has been investigated for amelioration of radiation mucositis without convincing results; moreover, there are concerns about poor disease control when such factors are added to radiation.115,145 Erythropoietin could also have an adverse effect on survival presumably because of the presence of erythropoietin receptors in cancer cells.146 Therefore, the use of cytokines during curative radiation or chemoradiotherapy should be generally avoided. Many novel agents with potential cytoprotective effect, such as palifermin (recombinant human keratinocyte growth factor), are under assessment in clinical studies.
Recurrent or metastatic disease
At least 50% of patients with locally advanced SCCHN develop locoregional or distant relapses, which are usually detected within the first 2 years of treatment. Salvage surgery is a likely curative option for the few patients with potentially resectable locoregional recurrence.126 Investigators have also studied re-irradiation alone or in combination with chemotherapy for patients with locoregionally recurrent SCCHN. A randomised study that assessed re-irradiation combined with chemotherapy compared with observation after salvage surgery reported an improvement of progression-free survival (HR 1·6, 95% CI 1· 1–2 ·4; p=0·01) with acceptable toxic effects.147 However, chemotherapy remains a standard-of-care treatment option for patients with recurrent or metastatic SCCHN. The main objective in these patients is not only to provide symptom palliation but also to extend survival. Methotrexate, bleomycin, carboplatin, and fluorouracil are active as single agents in recurrent or metastatic SCCHN.88 Well designed randomised trials to compare chemotherapy versus supportive care have not been done. In a small study, cisplatin monotherapy resulted in a 10-week prolongation in median survival, compared with no treatment.148 Two-drug combinations improved response rates but not overall survival in randomised trials.88 Cisplatin plus fluorouracil has been widely accepted as a reference regimen in patients with recurrent or metastatic SCCHN; however, single-agent cisplatin or methotrexate are acceptable alternatives. Among the new drugs, taxanes have substantial activity as monotherapy.88 Phase II and III studies assessed the combination of taxanes with platinum compounds and showed promising results, although they were not better than cisplatin plus fluorouracil.149 At present, there is no standard second-line chemotherapy regimen for the treatment of recurrent or metastatic SCCHN.
A randomised, placebo-controlled, trial compared cisplatin with or without the EGFR inhibitor cetuximab as first-line treatment of 123 patients with recurrent or metastatic SCCHN.150 This trial showed significant improvement in response rates (p=0·03) with a trend towards improved progression-free survival (p=0·09) with the addition of cetuximab; however, it was underpowered. A phase III trial with a larger sample size (420 patients) than the previous trial investigated the combination of a platinum agent (cisplatin or carboplatin) and fluorouracil with or without cetuximab. Preliminary results showed survival benefit with the addition of cetuximab, with an improvement of median survival from 7 · 4 months to 10·1 months (HR 0·797, 95% CI 0·64–0·98; p=0·036).151 Finally, cetuximab showed activity either as a single agent152 or in combination with platinum-based regimens in patients with platinum-refractory SCCHN,47 however, it has not been assessed in comparison with other agents or with best supportive care in patients with previously treated recurrent or metastatic SCCHN.
EGFR-tyrosine kinase inhibitors, gefitinib or erlotinib, have shown modest activity when given alone, with response rates of 1–11%, disease control rates of 34–53%, and median overall survival of 5 ·5–8· 1 months in patients with recurrent or metastatic SCCHN.47 A multicentre phase III trial showed that gefitinib (at either 250 mg or 500 mg per day) is not better than methotrexate in terms of overall survival.153 A phase III, placebo-controlled study, which is in progress in the USA (Eastern Cooperative Oncology Group, E1302), is comparing docetaxel with or without gefitinib as first-line or second-line treatment in recurrent or metastatic SCCHN.47
Future perspectives
Clinical investigations in SCCHN now being actively pursued include the use of induction chemotherapy and the incorporation of EGFR, angiogenesis inhibitors, and other molecularly targeted agents (figure 2) in the treatment of locally advanced SCCHN. Treatment intensification is expected to not only improve the locoregional and distant control, but also improve the overall survival of potentially curable patients with SCCHN. Consequently, an important area of investigation is focused on ways of ameliorating treatment-induced toxic effects. New, more accurate, radiation delivery methods and radioprotectant agents are being tested. Additionally, the understanding of the molecular changes that underlie SCCHN development has created optimism that more effective strategies for chemoprevention could be developed. Although many clinicopathological variables and molecular markers (eg, EGFR and HPV) are of prognostic value (panel 2), wide heterogeneity in clinical outcomes is seen. Certainly, there is an unmet need for identification of biomarkers that will guide treatment decisions. Advances in basic research and application of genomic and proteomic profiling are expected to provide powerful methods for the individualisation of treatment approaches in patients with SCCHN.
Panel 2: Prognostic factors in squamous cell carcinoma of the head and neck.
Locally advanced
Patient characteristics: performance status, haemoglobin
Tumour characteristics: T and N stage, site of primary tumour, tumour differentiation, involved margins of resection, extracapsular spread, perineural invasion
Molecular markers: epidermal growth factor receptor, human papillomavirus
Recurrent or metastatic
Clinical characteristics: performance status, weight loss, time of recurrence, prior treatment (radiotherapy, chemotherapy)
Tumour characteristics: tumour differentiation, site of primary tumour
Search strategy and selection criteria.
We did an extensive search of the English-language publications on head and neck cancer through Medline (1966–2007), Cochrane Library (1990–2007), and EmBase (1998–2007). Search terms included “head and neck cancer”, “head and neck squamous cell carcinomas”, “oral cavity”, “pharynx”, “larynx”, “surgery”, “radiotherapy”, “chemotherapy”, “chemoradiotherapy”, “clinical trials”, “randomised clinical trials”, “meta-analysis”, “epidemiology”, “chemoprevention”, “molecular biology”, “EGFR”, “predictive factors”, “prognostic markers”, “supportive care”, and “novel agents”. Additionally, we manually reviewed relevant bibliographies for extra material. We prioritised the selection of randomised clinical trials. Further information was obtained in oral and abstract form from recent pertinent conferences, such as the American Society of Clinical Oncology (ASCO) and American Association for Cancer Research (AACR) annual meetings. Published National Comprehensive Cancer Network (NCCN) and ASCO guidelines were also reviewed. We largely selected recent publications and preferentially included original articles, but reviews and book chapters were also cited to provide readers with more details.
Acknowledgments
We thank Raja Seethala and Ricardo Carrau of the University of Pittsburgh, PA, USA for providing slides and commentary. This paper was supported in part by the National Cancer Institute, Head and Neck Cancer SPORE: P50CA097190.
Footnotes
Conflict of interest statement
AA has been a consultant to Amgen and Imclone. He has received research support from the National Cancer Institute, Genentech, Lilly Oncology, Bristol-Myers Squibb, Pfizer, Novartis, Millenium Pharmaceuticals, M’s Science Corporation, and AstraZeneca. He also received honoraria from Genentech and AstraZeneca. MVK has no conflict of insterest to declare. DR has been a consultant to BMS, Amgen, Imclone, Lilly Oncology, Sanofi-Aventis, Array Biopharm, and GenMab. He is on the speaker’s bureau for BMS, Imclone, and Medimmune, and has received grants from AstraZeneca and Millennium Pharmaceuticals. RLF has received research support from the National Cancer Institute, the National Institute of Dental and Craniofacial Disorders, Amgen, and Altor Biosciences. He has been a consultant to Bristol-Myers Squibb, Imclone, and Altor Biosciences.
Contributor Information
Athanassios Argiris, Division of Hematology-Oncology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Head and Neck Cancer Program, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA.
Michalis V Karamouzis, Division of Hematology-Oncology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; Head and Neck Cancer Program, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA.
David Raben, Department of Radiation Oncology, University of Colorado, Denver, CO, USA.
Robert L Ferris, Head and Neck Cancer Program, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA; Department of Otolaryngology, University of Pittsburgh, Pittsburgh, PA, USA; Department of Immunology, University of Pittsburgh, Pittsburgh, PA, USA; Cancer Immunology, Immunotherapy, and Immunoprevention Program, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA.
References
- 1.Argiris A, Eng C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat Res 2003; 114: 15–60. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356: 1944–56. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute, 2006. [Google Scholar]
- 4.Argiris A, Brockstein BE, Haraf DJ, et al. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res 2004; 10: 1956–62. [DOI] [PubMed] [Google Scholar]
- 5.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst 2006; 98: 441–50. [DOI] [PubMed] [Google Scholar]
- 6.Do KA, Johnson MM, Doherty DA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States). Cancer Causes Control 2003; 14: 131–38. [DOI] [PubMed] [Google Scholar]
- 7.Wei WI, Sham JST. Nasopharyngeal carcinoma. Lancet 2005; 365: 2041–54. [DOI] [PubMed] [Google Scholar]
- 8.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43–66. [DOI] [PubMed] [Google Scholar]
- 10.Saraiya M, Kawaoka K. Incidence of human papillomavirus (HPV)-related head and neck cancers in the U.S. from 1998–2003: Pre-HPV vaccine licensure. J Clin Oncol 2007; 25 (suppl 18): 6003. [Google Scholar]
- 11.Annertz K, Anderson H, Biorklund A, et al. Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. Int J Cancer 2002; 101: 95–99. [DOI] [PubMed] [Google Scholar]
- 12.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer 2005; 103: 1843–49. [DOI] [PubMed] [Google Scholar]
- 13.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst 2004; 96: 99–106. [DOI] [PubMed] [Google Scholar]
- 14.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988; 48: 3282–87 [PubMed] [Google Scholar]
- 15.Tuyns AJ, Esteve J, Raymond L, et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). Int J Cancer 1988; 41: 483–91. [DOI] [PubMed] [Google Scholar]
- 16.Hashibe M, Boffetta P, Zaridze D, et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev 2006; 15: 696–703. [DOI] [PubMed] [Google Scholar]
- 17.Sturgis EM, Wei Q. Genetic susceptibility—molecular epidemiology of head and neck cancer. Curr Opin Oncol 2002; 14: 310–17 [DOI] [PubMed] [Google Scholar]
- 18.Proia NK, Paszkiewicz GM, Nasca MA, Franke GE, Pauly JL. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer—a review. Cancer Epidemiol Biomarkers Prev 2006; 15: 1061–77 [DOI] [PubMed] [Google Scholar]
- 19.Warnakulasuriya S. Areca nut use following migration and its consequences. Addict Biol 2002; 7: 127–32. [DOI] [PubMed] [Google Scholar]
- 20.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr 2006; 83: 1126–34. [DOI] [PubMed] [Google Scholar]
- 21.Garavello W, Randi G, Bosetti C, et al. Body size and laryngeal cancer risk. Ann Oncol 2006; 17: 1459–63. [DOI] [PubMed] [Google Scholar]
- 22.Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck 2007; 29: 779–92. [DOI] [PubMed] [Google Scholar]
- 23.Shangina O, Brennan P, Szeszenia-Dabrowska N, et al. Occupational exposure and laryngeal and hypopharyngeal cancer risk in central and eastern Europe. Am J Epidemiol 2006; 164: 367–75. [DOI] [PubMed] [Google Scholar]
- 24.Suarez C, Rodrigo JP, Ferlito A, Cabanillas R, Shaha AR, Rinaldo A. Tumours of familial origin in the head and neck. Oral Oncol 2006; 42: 965–78. [DOI] [PubMed] [Google Scholar]
- 25.Foulkes WD, Brunet JS, Sieh W, Black MJ, Shenouda G, Narod SA. Familial risks of squamous cell carcinoma of the head and neck: retrospective case-control study. BMJ 1996; 313: 716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trizna Z, Schantz SP. Hereditary and environmental factors associated with risk and progression of head and neck cancer. Otolaryngol Clin North Am 1992; 25: 1089–103. [PubMed] [Google Scholar]
- 27.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14: 467–75. [DOI] [PubMed] [Google Scholar]
- 28.Hobbs CG, Sterne JA, Bailey M, Heyderman RS, Birchall MA, Thomas SJ. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol 2006; 31: 259–66. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst 1998; 90: 1626–36. [DOI] [PubMed] [Google Scholar]
- 30.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res 2002; 89: 213–28. [DOI] [PubMed] [Google Scholar]
- 31.Slebos RJ, Yi Y, Ely K, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res 2006; 12: 701–09. [DOI] [PubMed] [Google Scholar]
- 32.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 2006; 24: 5630–36. [DOI] [PubMed] [Google Scholar]
- 33.Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res 1996; 56: 2488–92. [PubMed] [Google Scholar]
- 34.Perez-Ordonez B, Beauchemin M, Jordan RC. Molecular biology of squamous cell carcinoma of the head and neck. J Clin Pathol 2006; 59: 445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ha PK, Califano JA. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. Lancet Oncol 2006; 7: 77–82. [DOI] [PubMed] [Google Scholar]
- 36.McCaul JA, Gordon KE, Clark LJ, Parkinson EK. Telomerase inhibition and the future management of head-and-neck cancer. Lancet Oncol 2002; 3: 280–88. [DOI] [PubMed] [Google Scholar]
- 37.Mao L, Lee JS, Fan YH, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med 1996; 2: 682–85. [DOI] [PubMed] [Google Scholar]
- 38.Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res 2001; 264: 42–55. [DOI] [PubMed] [Google Scholar]
- 39.Balz V, Scheckenbach K, Gotte K, Bockmuhl U, Petersen I, Bier H. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2-11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res 2003; 63: 1188–91. [PubMed] [Google Scholar]
- 40.Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med 2007; 357: 2552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pignataro L, Pruneri G, Carboni N, et al. Clinical relevance of cyclin D1 protein overexpression in laryngeal squamous cell carcinoma. J Clin Oncol 1998; 16: 3069–77. [DOI] [PubMed] [Google Scholar]
- 42.Capaccio P, Pruneri G, Carboni N, et al. Cyclin D1 expression is predictive of occult metastases in head and neck cancer patients with clinically negative cervical lymph nodes. Head Neck 2000; 22: 234–40. [DOI] [PubMed] [Google Scholar]
- 43.Brennan M, Migliorati CA, Lockhart PB, et al. Management of oral epithelial dysplasia: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: S19 e1–12. [DOI] [PubMed] [Google Scholar]
- 44.Lee JJ, Hong WK, Hittelman WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res 2000; 6: 1702–10. [PubMed] [Google Scholar]
- 45.Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev 2006; 4: CD001829. [DOI] [PubMed] [Google Scholar]
- 46.Lippman SM, Lee JJ, Martin JW, et al. Fenretinide activity in retinoid-resistant oral leukoplakia. Clin Cancer Res 2006; 12: 3109–14. [DOI] [PubMed] [Google Scholar]
- 47.Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA 2007; 298: 70–82. [DOI] [PubMed] [Google Scholar]
- 48.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 1993; 53: 3579–84. [PubMed] [Google Scholar]
- 49.Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 1998; 90: 824–32. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara N. VEGF as a therapeutic target in cancer. Oncology 2005; 69 (Suppl 3): S11–16. [DOI] [PubMed] [Google Scholar]
- 51.Smith BD, Smith GL, Carter D, Sasaki CT, Haffty BG. Prognostic significance of vascular endothelial growth factor protein levels in oral and oropharyngeal squamous cell carcinoma. J Clin Oncol 2000; 18: 2046–52. [DOI] [PubMed] [Google Scholar]
- 52.Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res 2004; 10: 3755–62. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Albaitero A, Nayak JV, Ogino T, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol 2006; 176: 3402–09. [DOI] [PubMed] [Google Scholar]
- 54.Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical signifi cance. Immunol Res 2005; 33: 113–33. [DOI] [PubMed] [Google Scholar]
- 55.Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet 2005; 365: 1927–33. [DOI] [PubMed] [Google Scholar]
- 56.Witcher TP, Williams MD, Howlett DC. “One-stop” clinics in the investigation and diagnosis of head and neck lumps. Br J Oral Maxillofac Surg 2007; 45: 19–22. [DOI] [PubMed] [Google Scholar]
- 57.Patel SG, Shah JP. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin 2005; 55: 242–58. [DOI] [PubMed] [Google Scholar]
- 58.Ng SH, Yen TC, Chang JT, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol 2006; 24: 4371–76. [DOI] [PubMed] [Google Scholar]
- 59.Branstetter BF, Blodgett TM, Zimmer LA, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology 2005; 235: 580–86. [DOI] [PubMed] [Google Scholar]
- 60.Schmalbach CE, Miller FR. Occult primary head and neck carcinoma. Curr Oncol Rep 2007; 9: 139–46. [DOI] [PubMed] [Google Scholar]
- 61.Argiris A, Smith SM, Stenson K, et al. Concurrent chemoradiotherapy for N2 or N3 squamous cell carcinoma of the head and neck from an occult primary. Ann Oncol 2003; 14: 1306–11. [DOI] [PubMed] [Google Scholar]
- 62.Greenberg JS, El Naggar AK, Mo V, Roberts D, Myers JN. Disparity in pathologic and clinical lymph node staging in oral tongue carcinoma. Implication for therapeutic decision making. Cancer 2003; 98: 508–15. [DOI] [PubMed] [Google Scholar]
- 63.Ambrosch P. The role of laser microsurgery in the treatment of laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg 2007; 15: 82–88. [DOI] [PubMed] [Google Scholar]
- 64.Lefebvre JL. Laryngeal preservation in head and neck cancer: multidisciplinary approach. Lancet Oncol 2006; 7: 747–55. [DOI] [PubMed] [Google Scholar]
- 65.Myers EN, Wagner RL, Johnson JT. Microlaryngoscopic surgery for T1 glottic lesions: a cost-effective option. Ann Otol Rhinol Laryngol 1994; 103: 28–30. [DOI] [PubMed] [Google Scholar]
- 66.Pfister DG, Laurie SA, Weinstein GS, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol 2006; 24: 3693–704. [DOI] [PubMed] [Google Scholar]
- 67.Argiris A, Stenson KM, Brockstein BE, et al. Neck dissection in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck 2004; 26: 447–55. [DOI] [PubMed] [Google Scholar]
- 68.Ferlito A, Rinaldo A, Silver CE, et al. Elective and therapeutic selective neck dissection. Oral Oncol 2006; 42: 14–25. [DOI] [PubMed] [Google Scholar]
- 69.Simental AA Jr, Duvvuri U, Johnson JT, Myers EN. Selective neck dissection in patients with upper aerodigestive tract cancer with clinically positive nodal disease. Ann Otol Rhinol Laryngol 2006; 115: 846–49. [DOI] [PubMed] [Google Scholar]
- 70.Ferris RL, Xi L, Raja S, et al. Molecular staging of cervical lymph nodes in squamous cell carcinoma of the head and neck. Cancer Res 2005; 65: 2147–56. [DOI] [PubMed] [Google Scholar]
- 71.Chung CH, Parker JS, Karaca G, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 2004; 5: 489–500. [DOI] [PubMed] [Google Scholar]
- 72.Roepman P, Kemmeren P, Wessels LF, Slootweg PJ, Holstege FC. Multiple robust signatures for detecting lymph node metastasis in head and neck cancer. Cancer Res 2006; 66: 2361–66. [DOI] [PubMed] [Google Scholar]
- 73.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys 2004; 59: 28–42. [DOI] [PubMed] [Google Scholar]
- 74.Konski A, Watkins-Bruner D, Feigenberg S, et al. Using decision analysis to determine the cost-effectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int J Radiat Oncol Biol Phys 2006; 66: 408–15. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki M, Nishimura Y, Nakamatsu K, et al. Analysis of interfractional set-up errors and intrafractional organ motions during IMRT for head and neck tumors to define an appropriate planning target volume (PTV) and planning organs at risk volume (PRV)-margins. Radiother Oncol 2006; 78: 283–90. [DOI] [PubMed] [Google Scholar]
- 76.Ding M, Newman F, Raben D. New radiation therapy techniques for the treatment of head and neck cancer. Otolaryngol Clin North Am 2005; 38: 371–95. [DOI] [PubMed] [Google Scholar]
- 77.Voynov G, Heron DE, Burton S, et al. Frameless stereotactic radiosurgery for recurrent head and neck carcinoma. Technol Cancer Res Treat 2006; 5: 529–35. [DOI] [PubMed] [Google Scholar]
- 78.Le QT, Fu KK, Kroll S, et al. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys 1997; 39: 115–26. [DOI] [PubMed] [Google Scholar]
- 79.Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys 1993; 26: 3–11. [DOI] [PubMed] [Google Scholar]
- 80.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys 2007; 68: 654–61. [DOI] [PubMed] [Google Scholar]
- 81.Bentzen SM. Repopulation in radiation oncology: perspectives of clinical research. Int J Radiat Biol 2003; 79: 581–85. [DOI] [PubMed] [Google Scholar]
- 82.Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys 2003; 56: 399–412. [DOI] [PubMed] [Google Scholar]
- 83.Bernier J. Alteration of radiotherapy fractionation and concurrent chemotherapy: a new frontier in head and neck oncology? Nat Clin Pract Oncol 2005; 2: 305–14. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen LN, Ang KK. Radiotherapy for cancer of the head and neck: altered fractionation regimens. Lancet Oncol 2002; 3: 693–701. [DOI] [PubMed] [Google Scholar]
- 85.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000; 48: 7–16. [DOI] [PubMed] [Google Scholar]
- 86.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006; 368: 843–54. [DOI] [PubMed] [Google Scholar]
- 87.Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol 2004; 22: 1743–52. [DOI] [PubMed] [Google Scholar]
- 88.Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2006; 24: 2644–52. [DOI] [PubMed] [Google Scholar]
- 89.De Andres L, Brunet J, Lopez-Pousa A, et al. Randomized trial of neoadjuvant cisplatin and fluorouracil versus carboplatin and fluorouracil in patients with stage IV-M0 head and neck cancer. J Clin Oncol 1995; 13: 1493–500. [DOI] [PubMed] [Google Scholar]
- 90.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol 1992; 10: 1245–51. [DOI] [PubMed] [Google Scholar]
- 91.Jeremic B, Shibamoto Y, Stanisavljevic B, Milojevic L, Milicic B, Nikolic N. Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomized trial. Radiother Oncol 1997; 43: 29–37 [DOI] [PubMed] [Google Scholar]
- 92.Argiris A. Induction chemotherapy for head and neck cancer: will history repeat itself? J Natl Compr Canc Netw 2005; 3: 393–403. [DOI] [PubMed] [Google Scholar]
- 93.Duvvuri U, Simental AA Jr, D’Angelo G, et al. Elective neck dissection and survival in patients with squamous cell carcinoma of the oral cavity and oropharynx. Laryngoscope 2004; 114: 2228–34. [DOI] [PubMed] [Google Scholar]
- 94.Jones AS, Fish B, Fenton JE, Husband DJ. The treatment of early laryngeal cancers (T1-T2 N0): surgery or irradiation? Head Neck 2004; 26: 127–35. [DOI] [PubMed] [Google Scholar]
- 95.Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1-T2 glottic carcinomas. Cancer 2004; 100: 1786–92. [DOI] [PubMed] [Google Scholar]
- 96.Dey P, Arnold D, Wight R, MacKenzie K, Kelly C, Wilson J. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst Rev 2002; 2: CD002027. [DOI] [PubMed] [Google Scholar]
- 97.Mendenhall WM, Morris CG, Amdur RJ, et al. Definitive radiotherapy for tonsillar squamous cell carcinoma. Am J Clin Oncol 2006; 29: 290–97. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura K, Shioyama Y, Kawashima M, et al. Multi-institutional analysis of early squamous cell carcinoma of the hypopharynx treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2006; 65: 1045–50. [DOI] [PubMed] [Google Scholar]
- 99.Vokes EE, Weichselbaum RR. Concomitant chemoradiotherapy: rationale and clinical experience in patients with solid tumors. J Clin Oncol 1990; 8: 911–34. [DOI] [PubMed] [Google Scholar]
- 100.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004; 350: 1945–52. [DOI] [PubMed] [Google Scholar]
- 101.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004; 350: 1937–44. [DOI] [PubMed] [Google Scholar]
- 102.Cooper JS, Pajak TF, Forastiere AA, et al. Long-term survival results of a phase III intergroup trial (RTOG 95-01) of surgery followed by radiotherapy vs. radiochemotherapy for resectable high risk squamous cell carcinoma of the head and neck. Annual Meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO); Philadelphia, USA; Nov 5–6, 2006. Abstract 25. [Google Scholar]
- 103.Fietkau R, Lautenschlager C, Sauer R, et al. Postoperative concurrent radiochemotherapy versus radiotherapy in high-risk SCCA of the head and neck: Results of the German phase III trial ARO 96–3. J Clin Oncol 2006; 24 (suppl 18): 5507. [Google Scholar]
- 104.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 1999; 91: 2081–86. [DOI] [PubMed] [Google Scholar]
- 105.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004; 22: 69–76. [DOI] [PubMed] [Google Scholar]
- 106.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92–98. [DOI] [PubMed] [Google Scholar]
- 107.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349: 2091–98. [DOI] [PubMed] [Google Scholar]
- 108.Forastiere AA, Maor M, Weber RS, et al. Long-term results of Intergroup RTOG 91-11: a phase III trial to preserve the larynx— induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. J Clin Oncol 2006; 24 (suppl 18): 5517 [Google Scholar]
- 109.Wendt TG, Grabenbauer GG, Rodel CM, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol 1998; 16: 1318–24. [DOI] [PubMed] [Google Scholar]
- 110.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 1998; 338: 1798–804. [DOI] [PubMed] [Google Scholar]
- 111.Jeremic B, Shibamoto Y, Milicic B, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol 2000; 18: 1458–64. [DOI] [PubMed] [Google Scholar]
- 112.Olmi P, Crispino S, Fallai C, et al. Locoregionally advanced carcinoma of the oropharynx: conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy—a multicenter randomized trial. Int J Radiat Oncol Biol Phys 2003; 55: 78–92. [DOI] [PubMed] [Google Scholar]
- 113.Huguenin P, Beer KT, Allal A, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol 2004; 22: 4665–73. [DOI] [PubMed] [Google Scholar]
- 114.Budach V, Stuschke M, Budach W, et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: final results of the radiotherapy cooperative clinical trials group of the German Cancer Society 95-06 Prospective Randomized Trial. J Clin Oncol 2005; 23: 1125–35. [DOI] [PubMed] [Google Scholar]
- 115.Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy: results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001; 50: 1161–71. [DOI] [PubMed] [Google Scholar]
- 116.Semrau R, Mueller RP, Stuetzer H, et al. Efficacy of intensified hyperfractionated and accelerated radiotherapy and concurrent chemotherapy with carboplatin and 5-fluorouracil: updated results of a randomized multicentric trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006; 64: 1308–16. [DOI] [PubMed] [Google Scholar]
- 117.Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys 1996; 36: 999–1004. [DOI] [PubMed] [Google Scholar]
- 118.Laramore GE, Scott CB, al-Sarraf M, et al. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck: report on Intergroup Study 0034. Int J Radiat Oncol Biol Phys 1992; 23: 705–13. [DOI] [PubMed] [Google Scholar]
- 119.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005; 27: 843–50. [DOI] [PubMed] [Google Scholar]
- 120.Pignon JP, Bourhis J, Domenge C, Designé L, on behalf of the MACH-NC Collaborative Group. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet 2000; 355: 949–55. [PubMed] [Google Scholar]
- 121.Bourhis J, Amand C, Pignon JP. Update of MACH-NC (Meta-Analysis of Chemotherapy in Head & Neck Cancer) database focused on concomitant chemoradiotherapy. J Clin Oncol 2004; 22: 5505. [Google Scholar]
- 122.List MA, Stracks J, Colangelo L, et al. How do head and neck cancer patients prioritize treatment outcomes before initiating treatment? J Clin Oncol 2000; 18: 877–84. [DOI] [PubMed] [Google Scholar]
- 123.Allal AS, Nicoucar K, Mach N, Dulguerov P. Quality of life in patients with oropharynx carcinomas: assessment after accelerated radiotherapy with or without chemotherapy versus radical surgery and postoperative radiotherapy. Head Neck 2003; 25: 833–39. [DOI] [PubMed] [Google Scholar]
- 124.The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med 1991; 324: 1685–90. [DOI] [PubMed] [Google Scholar]
- 125.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst 1996; 88: 890–99. [DOI] [PubMed] [Google Scholar]
- 126.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg 2003; 129: 44–49. [DOI] [PubMed] [Google Scholar]
- 127.Garden AS, Harris J, Vokes EE, et al. Preliminary results of Radiation Therapy Oncology Group 97-03: a randomized phase II trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol 2004; 22: 2856–64. [DOI] [PubMed] [Google Scholar]
- 128.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567–78. [DOI] [PubMed] [Google Scholar]
- 129.Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol 2006; 24: 1072–78. [DOI] [PubMed] [Google Scholar]
- 130.Brockstein B, Haraf DJ, Rademaker AW, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol 2004; 15: 1179–86. [DOI] [PubMed] [Google Scholar]
- 131.Paccagnella A, Orlando A, Marchiori C, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst 1994; 86: 265–72. [DOI] [PubMed] [Google Scholar]
- 132.Zorat PL, Paccagnella A, Cavaniglia G, et al. Randomized phase III trial of neoadjuvant chemotherapy in head and neck cancer: 10-year follow-up. J Natl Cancer Inst 2004; 96: 1714–17. [DOI] [PubMed] [Google Scholar]
- 133.Domenge C, Hill C, Lefebvre JL, et al. Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d’Etude des Tumeurs de la Tete et du Cou (GETTEC). Br J Cancer 2000; 83: 1594–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Calais G, Pointreau Y, Alfonsi M, et al. Randomized phase III trial comparing induction chemotherapy using cisplatin (P) fluorouracil (F) with or without docetaxel (T) for organ preservation in hypopharynx and larynx cancer. Preliminary results of GORTEC 2000-01. J Clin Oncol 2006; 24 (suppl 18): 5506. [Google Scholar]
- 135.Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 2005; 23: 8636–45. [DOI] [PubMed] [Google Scholar]
- 136.Posner MR, Hershock DM, Blajman C, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007; 357: 1705–15. [DOI] [PubMed] [Google Scholar]
- 137.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007; 357: 1695–704. [DOI] [PubMed] [Google Scholar]
- 138.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003; 66: 253–62. [DOI] [PubMed] [Google Scholar]
- 139.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol 2006; 24: 2636–43. [DOI] [PubMed] [Google Scholar]
- 140.List MA, Siston A, Haraf D, et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol 1999; 17: 1020–28. [DOI] [PubMed] [Google Scholar]
- 141.Abendstein H, Nordgren M, Boysen M, et al. Quality of life and head and neck cancer: a 5 year prospective study. Laryngoscope 2005; 115: 2183–92. [DOI] [PubMed] [Google Scholar]
- 142.Dirix P, Nuyts S, Van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer 2006; 107: 2525–34. [DOI] [PubMed] [Google Scholar]
- 143.Garden AS, Lewin JS, Chambers MS. How to reduce radiation-related toxicity in patients with cancer of the head and neck. Curr Oncol Rep 2006; 8: 140–45. [DOI] [PubMed] [Google Scholar]
- 144.Kendal W. Suicide and cancer: a gender-comparative study. Ann Oncol 2007; 18: 381–87 [DOI] [PubMed] [Google Scholar]
- 145.Ryu JK, Swann S, Leveque F, et al. The impact of concurrent granulocyte macrophage-colony stimulating factor on radiation-induced mucositis in head and neck cancer patients: a double-blind placebo-controlled prospective phase III study by Radiation Therapy Oncology Group 9901. Int J Radiat Oncol Biol Phys 2007; 67: 643–50. [DOI] [PubMed] [Google Scholar]
- 146.Henke M, Mattern D, Pepe M, et al. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol 2006; 24: 4708–13. [DOI] [PubMed] [Google Scholar]
- 147.Janot F, De Raucourt D, Castaing M, et al. Re-irradiation combined with chemotherapy after salvage surgery in head and neck carcinoma: a randomized trial from the GETTEC and GORTEC groups. J Clin Oncol 2006; 24 (suppl 18): 5508. [Google Scholar]
- 148.Morton RP, Rugman F, Dorman EB, et al. Cisplatinum and bleomycin for advanced or recurrent squamous cell carcinoma of the head and neck: a randomised factorial phase III controlled trial. Cancer Chemother Pharmacol 1985; 15: 283–89. [DOI] [PubMed] [Google Scholar]
- 149.Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2005; 23: 3562–67. [DOI] [PubMed] [Google Scholar]
- 150.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 2005; 23: 8646–54. [DOI] [PubMed] [Google Scholar]
- 151.Vermorken J, Mesia R, Vega V, et al. Cetuximab extends survival of patients with recurrent or metastatic SCCHN when added to first line platinum based therapy: results of a randomized phase III (Extreme) study. J Clin Oncol 2007; 25 (suppl 18): 6091. [Google Scholar]
- 152.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 2007; 25: 2171–77. [DOI] [PubMed] [Google Scholar]
- 153.Stewart S, Cohen E, Licitra L, et al. A phase III randomized parallel-group study of gefitinib (Iressa) versus methotrexate (IMEX) in patients with recurrent squamous cell carcinoma of the head and neck. Annual Meeting of the American Association of Cancer Research (AACR); Los Angeles, USA; April 14–18, 2007 (Abstr 3522). [Google Scholar]