‘Any fool can know. The point is to understand’.
Albert Einstein.
Coronavirus disease 2019 (COVID-19) has sneakily entered our lives early on in 2020 and the adverse health effects associated with it continue. We now understand more about the virus and about how to manage patients. Large-scale studies have been initiated across the globe and one provided an ally in our fight against COVID-19 in an old fashioned drug, dexamethasone reducing mortality [1]. Others showed reduction in hospital stay [2] or simply reinforced the safety of continuing medications during COVID-19 [3]. We have also realized how catastrophic the virus can be. We learned that severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), which causes COVID-19, enters the cells using Angiotensin-converting enzyme 2 (ACE2) receptors. These receptors are found abundantly not only in the pulmonary but also other organ systems such as the cardiovascular and gastrointestinal (GI) systems. Consequently, extra-pulmonary effects of COVID-19 are not at all surprising. With an increasing number of cases and a forthcoming second wave infection, clinicians must be aware of these multisystem manifestations. In this article, we aim to discuss the short and long-term impact of COVID-19 on the respiratory and some of the other major organ systems in the human body.
RESPIRATORY SYSTEM
The principal manifestation of COVID-19 is in the lungs, where respiratory failure is a leading cause of death. SARS-CoV-2 enters human cells by binding to the ACE2 receptors, that are highly expressed in the alveolar type 2 cells (AT2), explaining the vulnerability of the respiratory system [4].
Post mortem studies elucidate the observed pathology upon macroscopic and microscopic examinations [5]. Gross lung findings include patchy to diffuse consolidation, severe interstitial congestion, and thromboembolism while histology demonstrates hyaline membranes, extensive capillary congestion, and microthrombi in alveolar capillaries, consistent with exudative diffuse alveolar damage (DAD) (Fig. 1). Whilst aforementioned findings resemble those found in acute respiratory distress syndrome (ARDS), COVID-19-associated ARDS displays a peculiar phenotype owing to dissociation between severity of hypoxaemia and relative maintenance of lung mechanics [6].
Figure 1.
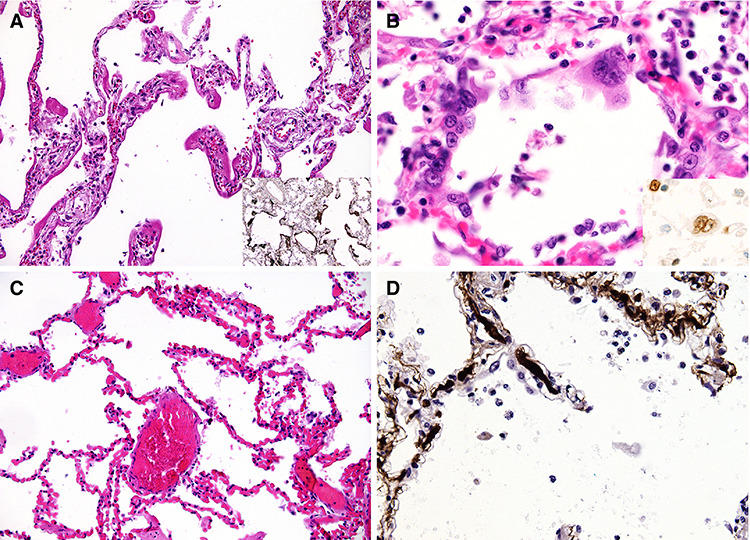
Post mortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction; microscopic lung findings: (A) Exudative DAD showing discrete hyaline membranes and prominent capillary congestion; (B) syncytial cells of pneumocyte II origin; (C) Extensive capillary congestion without DAD; (D) Microthrombi in alveolar capillaries; image reproduced from Menter et al. [5] under the terms of the creative commons attribution license
Despite COVID-19-associated ARDS meeting the criteria, it has been proposed that patients developing it could be categorized into type 1 ‘non-ARDS’ and type 2 ‘ARDS’ depending on lung compliance [6]. Distinction between them can be made based on computed tomography (CT) scans (Fig. 2) or, albeit substandard, response to positive end-expiratory pressure (PEEP). Type 1 patients exhibit severe hypoxaemia with reasonable lung compliance while type 2 patients exhibit severe hypoxaemia with poor lung compliance. This has important clinical implications when deciding management in the intensive care unit (ICU).
Figure 2.
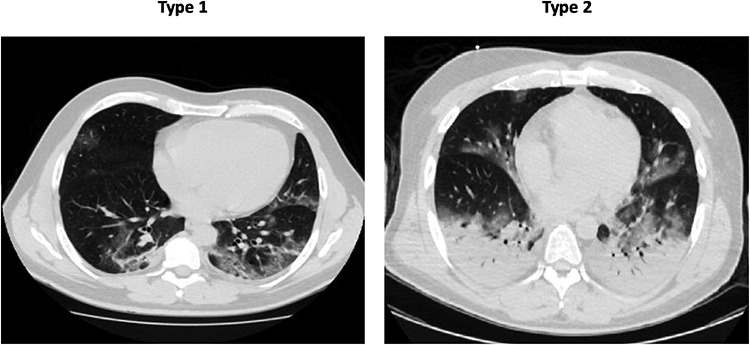
In these two patients, following variables were recorded: Type 1 lung weight (1192 g), gas volume (2774 ml), percentage of non-aerated tissue (8.4%), venous admixture (56%), P/F (68) and respiratory system compliance (80 ml/cmH2O); Type 2 lung weight (1441 g), gas volume (1640 ml), percentage of non-aerated tissue (39%), venous admixture (49%), P/F (61) and respiratory system compliance (43 ml/cmH2O). Image reproduced from Gattinoni et al. [6] under the terms of the creative commons attribution 4.0 international license
CARDIOVASCULAR SYSTEM
Systemic inflammation in response to the SARS-CoV2 infection, in addition to ongoing hypoxia secondary to severe pneumonia or ARDS, causes increased cardiometabolic demand and myocardial oxygen supply/demand mismatch [7] leading to myocardial damage [8]. Myocardial injury, in turn, has been shown to be independently associated with high mortality and various complications such as ARDS and electrolyte disturbances [9], increasing the risk of arrhythmias. There is also rising awareness of acute plaque rupture and consequently, acute coronary events in patients with underlying coronary artery disease [7].
Additionally, the surge in pro-inflammatory cytokines as part of the systemic inflammatory response to COVID-19 can result in myocarditis and other changes including myocardial fibrosis (Fig. 3), oedema and pericarditis [8, 10]. Interestingly, all these changes were seen in much younger non-hospitalized patients and were associated with adverse events and poor prognosis [8].
Figure 3.
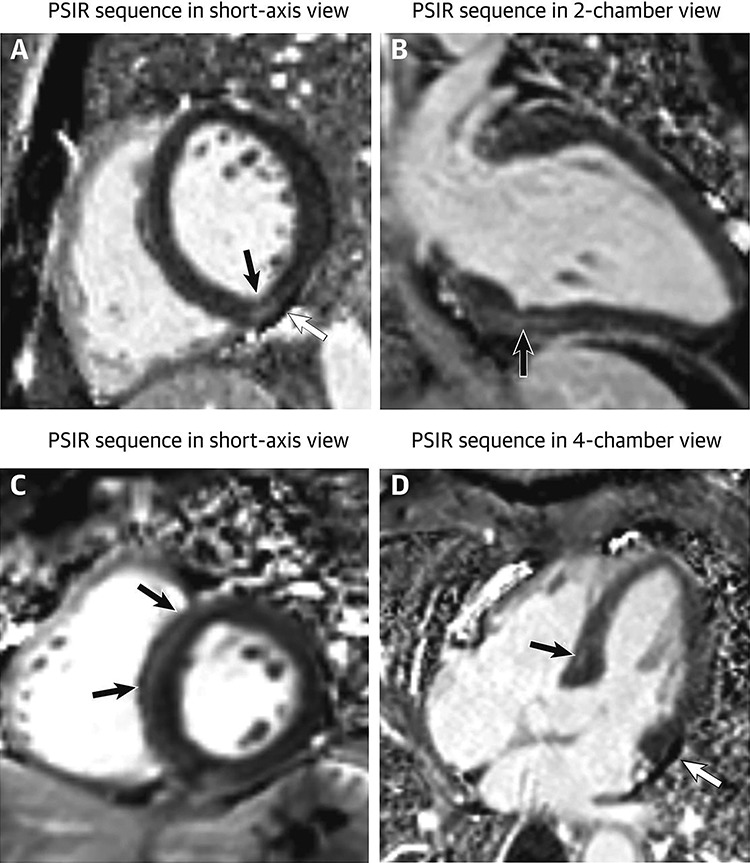
Focal myocardial fibrosis in patients recovered from COVID-19: a 29-year-old male patient (first row) underwent cardiac CMR 1 month after the onset of palpitations. A 60-year-old male patient (second row) underwent cardiac CMR 2 months after the onset of palpitations. PSIR sequences in short-axis view (A, C) showed focal LGE (black arrows) in inferior and septal segments of left ventricle, respectively. Results were confirmed on the PSIR sequences in 2-chamber view (C) and 4-chamber view (D). Images A and D demonstrated a small pericardial effusion (white arrow) in both patients.; LGE, late gadolinium enhancement; CMR, cardiac magnetic resonance; PSIR, phase-sensitive inversion recovery. Image reproduced from Huang et al. [8] under the terms of the creative commons attribution 4.0 international license.
Moreover, new evidence demonstrates COVID-19 induced cardiac changes might not be limited to the short term (Fig. 4) [8]. A study by Puntmann et al. [10] involving 100 COVID-19 recovered patients showed lower left ventricular ejection fraction compared to healthy and risk factor-matched controls and significantly raised troponin T levels. After a follow-up of 71 days, 78% of patients had abnormal cardiac magnetic resonance findings. These patients might be in the very early stage of cardiac involvement and perhaps need longer follow-up [8] to identify any potential long-term complications, especially in those patients with persistent symptoms attributed to COVID-19.
Figure 4.
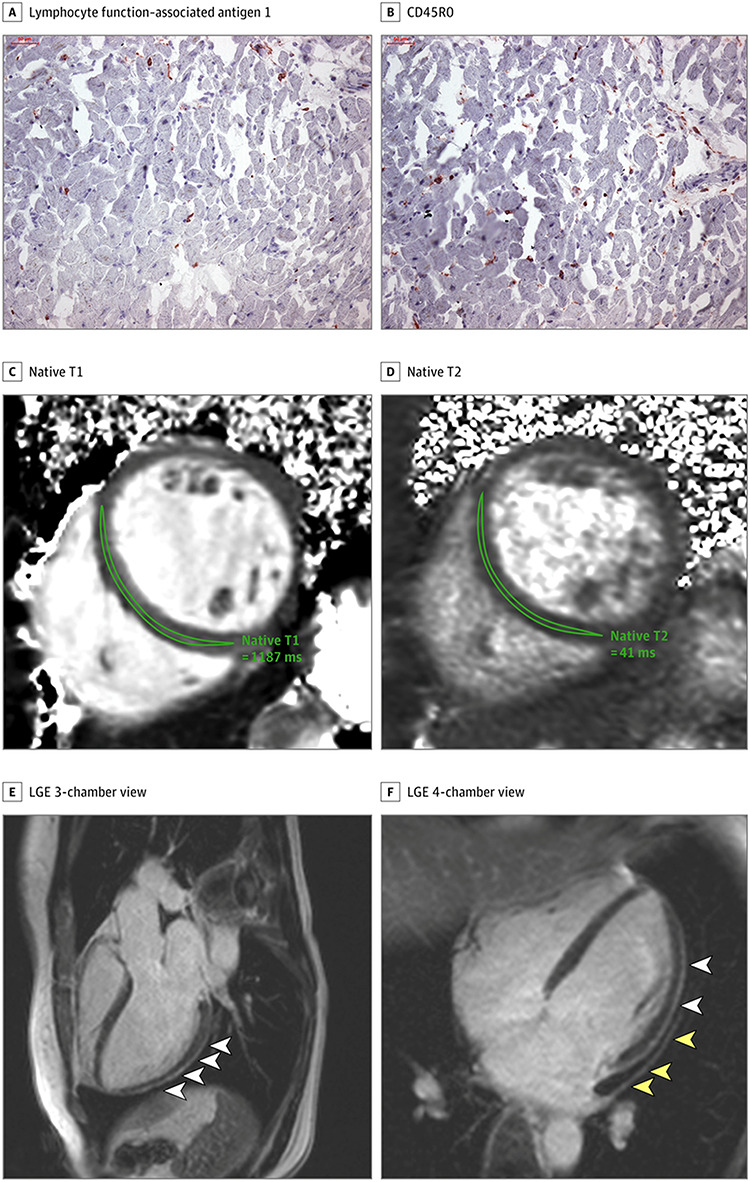
A and B, Histologic findings in an adult man with severe CMR imaging abnormalities 67 days after COVID-19 diagnosis. High-sensitivity troponin T level on the day of CMR imaging was 16.7 pg/ml. The patient recovered at home from COVID-19 illness with minimal symptoms, which included loss of smell and taste and only mildly increased temperature lasting 2 days. There were no known previous conditions or regular medication use. Histology revealed intracellular oedema as enlarged cardiomyocytes with no evidence of interstitial or replacement fibrosis. Panels A and B show immunohistochemical staining, which revealed acute lymphocytic infiltration (lymphocyte function–associated antigen 1 and activated lymphocyte T antigen CD45R0) as well as activated intercellular adhesion molecule 1. C–F, Representative CMR images of an adult woman with COVID-19–related perimyocarditis. Panels C and D show significantly raised native T1 and native T2 in myocardial mapping acquisitions. Panels E and F show pericardial effusion and enhancement (yellow arrowheads) and epicardial and intramyocardial enhancement (white arrowheads) in LGE acquisition. Image reproduced from Puntmann et al. [10] under the terms of the CC-BY License
GI-including hepatic system
GI manifestations of COVID-19, have been well-documented [11, 12] but the underlying mechanisms remain to be further elucidated. ACE2 receptors, found in enterocytes and oesophageal epithelial cells, play an important role in regulating intestinal inflammation [12] which may, at least in part, explain typical GI symptoms such as diarrhoea, nausea and vomiting, which can occur with or without respiratory symptoms [12]. Unsurprisingly, there has been evidence of the virus in faecal samples even after clearance of the virus in the respiratory tract [12], posing questions and challenges for the future.
In addition, a radiology study [13] of 412 patients found bowel wall abnormalities in 31% of CT scans and pneumatosis (Fig. 5) or portal venous gas in 20% of CT scans in ICU patients. Furthermore, 54% of abdominal ultrasounds, performed mainly for abnormal LFTs, were suggestive of cholestasis [13], a precursor to liver injury. SARS-CoV2-mediated liver injury can be direct through binding of the virus to ACE2 receptors found in cholangiocytes and hepatocytes, or indirect through release of cytokines as a part of the immune response [11]. This aggravates liver cell damage and bile duct cell dysfunction [11]. In addition, the liver as the principal site of drug metabolism of antiviral drugs such as lopinavir/ritonavir that are widely used for COVID-19 may endure further iatrogenic damage during treatment [11].
Figure 5.
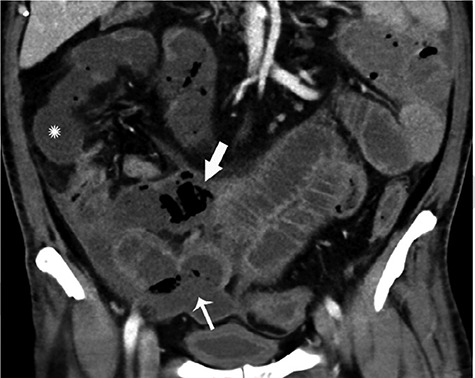
Coronal CT of the abdomen and pelvis with IV contrast in a 47-year-old man with abdominal tenderness demonstrates typical findings of mesenteric ischemia and infarction, including pneumatosis intestinalis (arrow) and non-enhancing bowel (asterisk); Frank discontinuity of a thickened loop of small bowel in the pelvis (thin arrow) is in keeping with perforation. Permissions granted to reproduce image from Bhayana et al. [13].
Dermatological
Skin manifestations were first reported in an Italian cohort where ~20% of hospitalized patients with COVID-19 and no exposure to new medications showed signs of cutaneous involvement [14]. Rashes appeared either before (9%) or after (11%) hospitalization and were described as erythematous, widespread urticarial or resembling chickenpox. Since then skin manifestations have been classified, most comprehensively, in a cross-sectional study of 375 patients with COVID-19 and cutaneous lesions [15]. The five groups identified were (i) pseudo-chilblain lesions, i.e. acral areas of erythema–oedema with some vesicles or pustules (19%, Fig. 6a and b), (ii) other vesicular eruptions (9%, Fig. 6c), (iii) urticarial lesions (19%, Fig. 6d), (iv) other maculopapules (47%, Fig. 7a–c) and (v) livedo or necrosis (6%, Fig. 7d) [15]. Eruptions occurred before (6%), at the same time (57%), or after (37%) the onset of other symptoms and the type of lesion was associated with age and disease severity, both increasing from Group 1 to Group 5. In this study, patients received a variety of drug treatments and not all skin manifestations may therefore be directly caused by SARS-CoV-2. For example, hydroxychloroquine may cause skin reactions including the rare Stevens-Johnson Syndrome presenting with a pruritic erythematous maculopapular eruption that rapidly spreads from distal extremities (Fig. 8) to the whole body with orolabial and genital mucosal involvement [16]. An awareness of clinicians to possible skin involvement may help in the diagnosis and management of patients with COVID-19.
Figure 6.
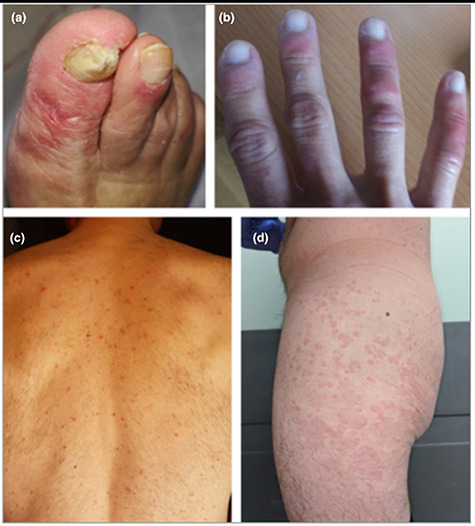
All of the patients shown had confirmed COVID-19; (A, B) acral areas of erythema–oedema with vesicles or pustules (pseudo-chilblain); (C) monomorphic (i.e. at same stages) disseminated vesicles; (D) urticarial lesions; permissions granted to reproduce image from Casas [15].
Figure 7.
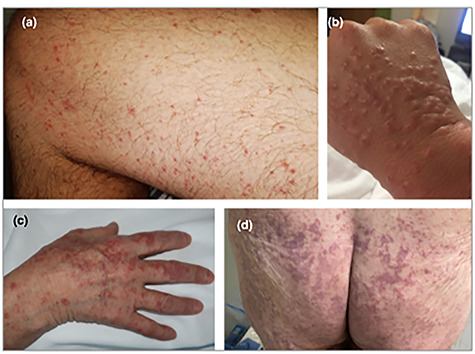
All of the patients shown had confirmed COVID-19; (A) maculopapular eruption. Some of the lesions are perifollicular; (B) acral infiltrated papules (pseudovesicular); (C) acral papules (erythema multiforme like); (D) livedoid areas; permissions granted to reproduce image from Casas [15].
Figure 8.

Pruritic erythematous maculopapular rash on the distal extremities due to HCQ consumption; image reproduced from Davoodi et al. [16] under the terms of the creative commons CC-BY-NC license.
Neurological
Neurological manifestations of COVID-19 include anosmia and ageusia, which are extremely common and are now recognized as key symptoms. A systematic review [17] of 41 studies found the neurological manifestations of COVID-19 could be divided into three main categories: (i) neurological diseases comorbid with COVID-19, i.e. symptoms prior to the infection and neurological diseases that also act as a risk factor for developing the virus, (ii) non-specific neurological manifestations caused by systemic responses and partly the neuro-invasive behaviour of the virus and (iii) specific neurological symptoms and diseases including encephalitis, myelitis and seizures.
One national registry of 125 COVID-19 patients investigating neurological or psychiatric disease over a three-week period found 31% to have altered mental status including 16 (13%) with encephalopathy (Fig. 9A–D) [18] and 23 (18%) with a neuropsychiatric diagnosis. Of the 23 patients, ten had psychosis; six neuro-cognitive dementia-like syndrome and four demonstrated an affective disorder [18]. Cerebrovascular events were prevalent at 62% (77/125); 46% with ischaemic strokes (Fig. 9E and F) and 7% with intracerebral haemorrhages [18]. Many patients had known risk factors and were over 60 years old. Predominately we are seeing large vessel disease and markers of a prothrombotic state, suggesting a causal relationship [18]. However, the extremely high number of cases of SARS-CoV-2 and the large percentage of those with stroke having other risk factors makes proving causality a challenge. Small asymptomatic infarcts have been identified on magnetic resonance imaging, and blood D-dimer concentrations were raised in many COVID-19 patients [18]. The best current explanation for the cerebrovascular events is the hyper-coagulable state SARS-CoV-2 triggers owing to the activation of inflammatory and thrombotic pathways and consequently destabilization of carotid plaque.
Figure 9.
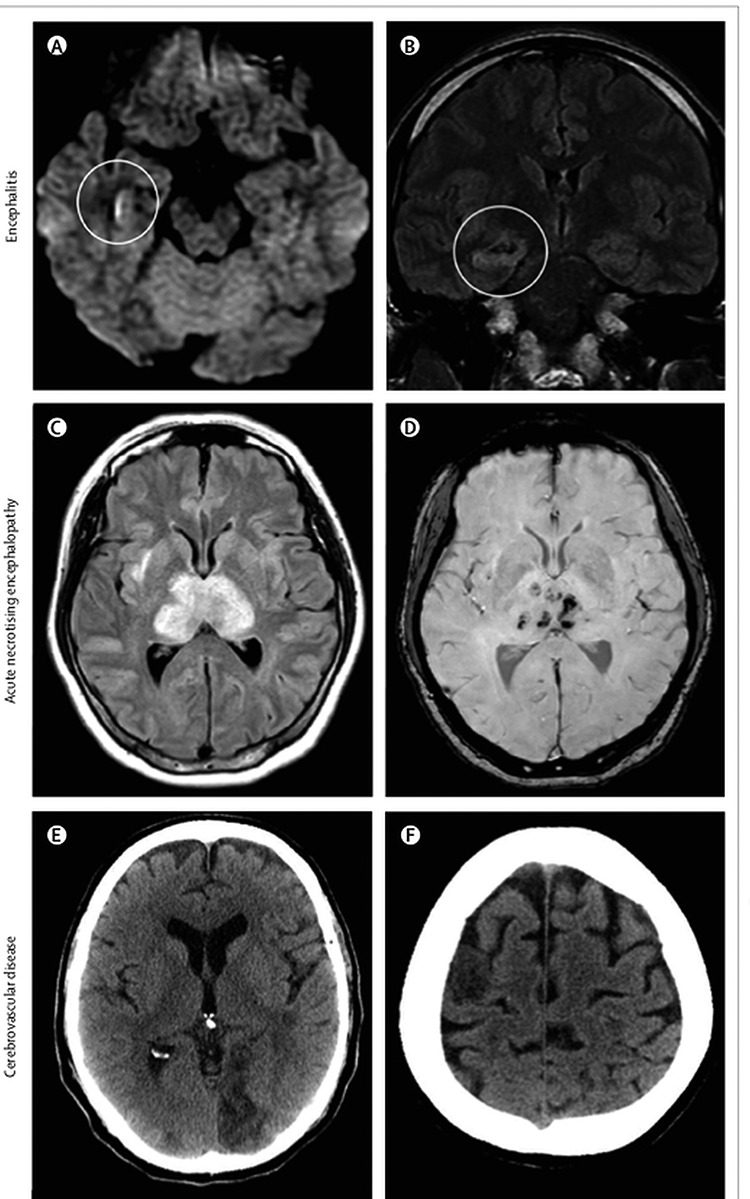
Brain imaging in patients with neurological disease associated with COVID-19; (A) hyperintensity along the wall of inferior horn of right lateral ventricle on diffusion-weighted imaging, indicating ventriculitis; (B) hyperintense signal changes in the right mesial temporal lobe and hippocampus with slight hippocampal atrophy, consistent with encephalitis; (C) hyperintensity within the bilateral medial temporal lobes and thalami; (D) evidence of haemorrhage, indicated by hypointense signal on susceptibility-weighted images, consistent with acute necrotising encephalopathy; (E) CT showing ischaemic lesions involving the left occipital lobe; (F) right frontal precentral gyrus of the brain in a man aged 64 years who deteriorated neurologically after admission to hospital with COVID-19 and was diagnosed with acute stroke. Permissions granted to reproduce image from Ellul et al. [18]
CONCLUSION
This article provides a succinct description of how COVID-19 affects a wide range of organs. SARS-CoV2 exerts direct damage of target tissues through binding at the ACE2 receptor and indirect effects mediated by systemic inflammation and a prothrombotic state. Although the short-term consequences of COVID-19 are becoming clearer over time, the long-term effects of this novel virus are not transparent yet. Highlighting the pulmonary and extra-pulmonary manifestations of COVID-19 in an open access platform like the one provided by Oxford Medical Case Reports will help clinicians to better understand and manage the multi-organ manifestations of this disease during an imminent second wave of infection.
Contributor Information
Ranu Baral, Norwich Medical School, University of East Anglia, Norwich, UK; Norfolk and Norwich University Hospital, Norwich, UK.
Omar Ali, Norwich Medical School, University of East Anglia, Norwich, UK.
Iona Brett, Leicester Medical School, Leicester, UK.
Johannes Reinhold, Norwich Medical School, University of East Anglia, Norwich, UK; Norfolk and Norwich University Hospital, Norwich, UK.
Vassilios S Vassiliou, Norwich Medical School, University of East Anglia, Norwich, UK; Norfolk and Norwich University Hospital, Norwich, UK.
REFERENCES
- 1. Massachusetts Medical Society Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med 2020. [Google Scholar]
- 2. Rochwerg B, Agarwal A, Zeng L, Leo YS, Appiah JA, Agoritsas T et al. Remdesivir for severe covid-19: a clinical practice guideline. BMJ NLM (Medline) 2020;m2924:370. [DOI] [PubMed] [Google Scholar]
- 3. Baral R, White M, Vassiliou VS. Effect of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19: a systematic review and meta-analysis of 28,872 patients. Curr Atheroscler Rep Springer Science and Business Media LLC 2020;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med Higher Education Press 2020;14:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology Blackwell Publishing Ltd 2020;77:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care BioMed Central Ltd. 2020;154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J NLM (Medline) 2020;41:1798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C et al. Cardiac involvement in recovered COVID-19 patients identified by magnetic resonance imaging In: JACC Cardiovasc Imaging. Elsevier BV, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F et al. Association of Cardiac Injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol American Medical Association 2020;5:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhan K, Liao S, Li J, Bai Y, Lv L, Yu K et al. Risk factors in patients with COVID-19 developing severe liver injury during hospitalisation. Gut BMJ 2020; gutjnl-2020-321913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng SC, Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut BMJ Publishing Group 2020;973–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology NLM (Medline) 2020;201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatology Venereol Blackwell Publishing Ltd 2020;e212–3. [DOI] [PubMed] [Google Scholar]
- 15. Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol Blackwell Publishing Ltd 2020;183:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davoodi L, Jafarpour H, Kazeminejad A, Soleymani E, Akbari Z, Razavi A. Hydroxychloroquine-induced Stevens-Johnson syndrome in COVID-19: a rare case report. Oxf Med Case Reports Oxford University Press 2020;2020:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang L, Shen Y, Li M, Chuang H, Ye Y, Zhao H et al. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Neurol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A et al. Neurological associations of COVID-19. Lancet Neurol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delcuve GP, Lakowski TM, Su R-C, Beacon TH, Davie JR. SARS-CoV-2 multifaceted interaction with human host. Part I: what we have learnt and done so far, and the still unknown realities. IUBMB Life. IUBMB Life 2020. [DOI] [PubMed] [Google Scholar]
- 20. Tabary M, Khanmohammadi S, Araghi F, Dadkhahfar S, Tavangar SM. Pathologic features of COVID-19: a concise review. Pathol Res Pract Elsevier GmbH 2020;153097. [DOI] [PMC free article] [PubMed] [Google Scholar]


