INTRODUCTION
Aortic aneurysm is defined as segmental dilation of ≥50% of the full thickness of the vessel involving any segment from the aortic root to the abdominal aorta. Aneurysms involving the descending thoracic and abdominal aorta are commonly associated with risk factors for atherosclerosis such as smoking, hypercholesterolemia, and hypertension. Conversely, thoracic aortic aneurysms (TAA) proximal to the ligamentum arteriosum often occur in patients with connective tissue disorders including Marfans, Ehlers-Danlos, and Loeyz-Dietz syndromes as well as patients with congenital bicuspid aortic valves [1]. In addition, familial patterns of thoracic aortic aneurysms have been described [2]. The prevalence of TAA is less than 0.5 % of the population, though the incidence is increasing likely due to population aging, earlier detection through imaging, and different cut-off values for defining aneurysmal dilatation [3,4]. Concomitant abdominal aortic aneurysm (AAA) occurs in more than a quarter of patients with TAA [5]. Aneurysms of the abdominal aorta, often defined as dilation ≥ 3 cm, are more common than TAA occurring in approximately 5% of screened males > 65 years. In addition to male gender, smoking and advanced age are the major risk factors for AAA. There may also be a genetic predisposition as a family history of AAA in first-degree family members is associated with a two-fold risk [6]. According to the Centers for Disease Control, AAA is the 15th leading cause of death in adults > 60y in the US and, similar to TAA, the incidence of AAA is also increasing [7].
The vast majority of patients with aortic aneurysms remain asymptomatic until they develop a complication such as aortic dissection or rupture which are the primary causes of morbidity and mortality. The risk of a complication increases as the aneurysm expands. For TAA, the 5-year risk of rupture as a function of aneurysm size is 0% for aneurysms less than 4 cm in diameter, 16% for those 4 to 5.9 cm, and 31% for aneurysms 6 cm or more [8]. Like TAA, there is an exponential increase in AAA rupture risk with increasing aneurysm diameter with the 12-month risk being ≤1 % for AAA diameter ≤ 5.5 cm, 9 % for diameter 5.5 to 5.9 cm, 10% for diameter 6.0 to 6.9 cm and 33 % for diameter ≥7.0 cm [6]. Other risk factors for AAA rupture include rapid expansion, active tobacco use, uncontrolled hypertension, elevated peak wall stress, prior cardiac or renal transplant, and female gender.
PATHOBIOLOGY
Aortic aneurysms distal to the ligamentum arteriosum are strongly associated with atherosclerosis, whereas those proximal are largely non-atherosclerotic. Embryological differences in the origin of smooth muscle cells in the ascending and descending aorta may account for this variation. TAA arises from progressive structural alterations, including cystic medial degeneration, resulting in weakening of the vessel wall. The breakdown of extracellular matrix proteins including elastin and collagen by enzymes such as matrix metalloproteinases (MMPs) is a primary factor in the pathobiology of aneurysm formation and expansion, both in TAA and AAA [9]. Especially in the case of AAA, these enzymes are derived largely from inflammatory cells infiltrating the vessel wall. Animal models demonstrating a correlation between increase in metalloproteinase activity and aneurysm rupture and the prevention of this with inhibitors of MMPs have been instrumental in our understanding of this process [10]. Biomechanical forces acting in conjunction with these structural and inflammatory processes lead to aneurysm expansion, rupture, and dissection. A more thorough discussion of aortic aneurysm pathogenesis can be found in recent reviews [11,12].
CURRENT APPROACH TO RISK ASSESSMENT
Given the risk of complications in patients with aortic aneurysms as a function of aneurysm size, current decision-making for prophylactic surgery or endovascular repair is based largely on aneurysm diameter and/or rate of expansion. Guidelines generally recommend elective surgery when the diameter is >5.5 cm for TAA and >5.0-5.5 cm for AAA depending on location, gender, and suitability for endovascular repair [6,13]. Diameter cutoffs for elective surgery are generally lower in patients with connective tissue disorders. Rapid aneurysm expansion of > 5-10 mm over a 6-12-month surveillance period is another accepted indication for repair in otherwise asymptomatic patients. Urgent surgical intervention is almost always indicated when symptoms occur.
Imaging modalities commonly employed to measure and monitor aneurysm size include abdominal ultrasound, CT, MRI, and echocardiography. Due to inherent differences in the approach and spatial resolution of these techniques, there may be disagreement in measurements made across modalities. In addition, measurements are typically performed by different providers, introducing inter-observer variability as well as inaccuracies when measurements are obtained from oblique planes. There is also a lack of general consensus regarding the best approach to measure aortic diameter including which level of the aorta and whether to include or exclude the vessel wall. Other modality-specific issues include incomplete visualization of the ascending aorta with echocardiography, differences with and without contrast enhancement, and motion artifact in CT. These issues can lead to additional variability in measurements up to several millimeters which could have a substantial impact on clinical decision-making. Whether or not to index aortic diameter measurements to body surface area is another area of uncertainty, which is especially relevant in very small or large individuals [4].
Finally, although aneurysm diameter is an important determinant of risk for rupture or dissection, these complications do occur in individuals with aortic aneurysms below the diameter criteria for prophylactic repair [14]. Conversely, not all patients with aneurysms exceeding size criteria for surgery will have a complication. Novel approaches to better identify patients at increased risk for aneurysm expansion and rupture or dissection are needed to address these gaps.
MOLECULAR IMAGING
Molecular imaging involves the use of targeted approaches to identify, measure, and monitor cellular and molecular processes underlying health and disease with the goal of addressing diagnostic gaps and furthering precision medicine. Successful implementation of molecular imaging in cardiovascular disease relies heavily upon an imaging tracer’s ability to target a biologic process with high specificity in a reproducible and quantifiable manner. The biological processes involved in aortic aneurysm formation, expansion, and rupture which are potential targets for imaging include inflammation and extracellular matrix remodeling (figure 1). The ability to non-invasively identify and characterize these processes may offer additional risk stratification and identify appropriate candidates for interventions to reduce morbidity and mortality, including surgical or endovascular repair (figure 2), and help develop targeted medical therapies that favorably impact the molecular processes contributing to aneurysm expansion and rupture. Nuclear (PET and SPECT) and magnetic resonance (MR) imaging approaches hold much promise with several tracers targeting key processes in aneurysm pathogenesis currently being evaluated in pre-clinical and early clinical studies (table 1) [15]. While high sensitivity is an advantage for nuclear imaging techniques, their limited spatial resolution is a potential barrier for vascular imaging. Co-registration of nuclear molecular imaging data with high resolution anatomical imaging, such as CT or MRI, addresses this limitation and allows for precise localization of the biological process being evaluated.
Figure 1:
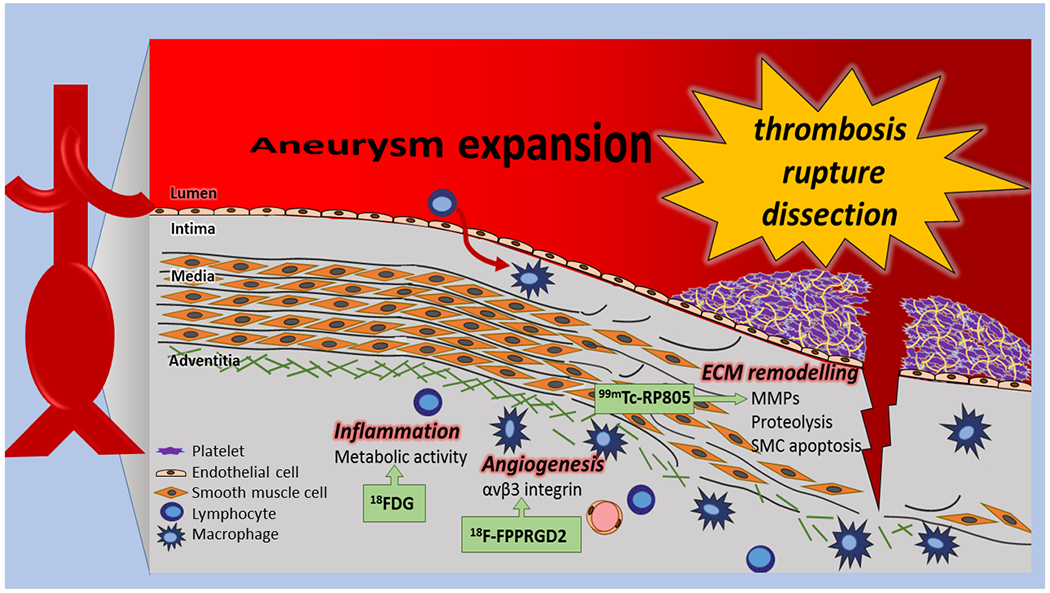
Schematic representation of major biological processes involved in abdominal aortic aneurysm formation, expansion, and rupture. Some targets for molecular imaging are highlighted and representative tracers are shown.
Figure 2:
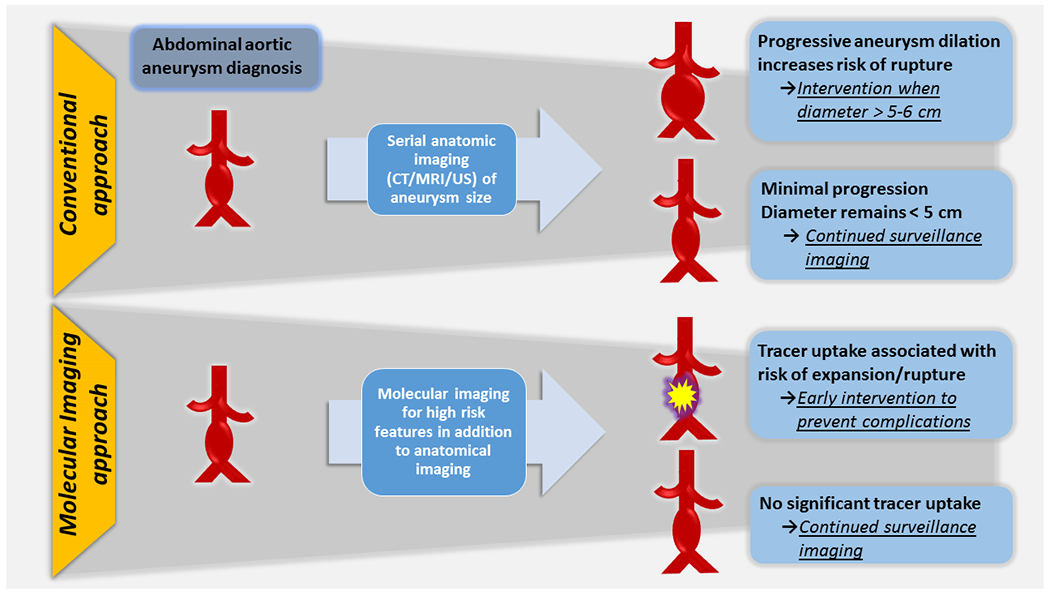
Proposed application of molecular imaging in AAA clinical decision-making.
Table 1:
Representative biological targets and tracers for molecular imaging of aortic aneurysms [15]
| Biologic Process | Target | Tracer | Modality |
|---|---|---|---|
| Inflammation | Metabolism | 18F-FDG | PET |
| Phagocytosis | 18F nanoparticles | PET | |
| (U)SPIO | MRI | ||
| Extracellular matrix remodeling | MMP activation | 99mTc-RP805 | SPECT |
| P947 | MRI | ||
| Matrix proteins | CNA-35 | MRI | |
| Angiogenesis | αvβ3 integrin | 99mTc-NC100692 | SPECT |
| (and inflammation) | 18F-FPPRGD2 | PET |
(FDG, Flurodeoxyglucose; MMP, matrix metalloproteinase; (U)SPIO, (ultrasmall) superparamagnetic iron oxide
Tracers currently being investigated clinically for molecular imaging of vascular inflammation in aortic aneurysms include 18F-fluorodeoxyglucose (FDG) and ultrasmall superparamagnetic particles of iron oxide (USPIO). FDG is a glucose analog PET tracer which is taken up and retained in highly metabolically active cells, including macrophages (figure 3). Histologic studies have correlated FDG uptake with vessel wall inflammation and small clinical studies in patients with aortic aneurysms have demonstrated an association between FDG uptake with increased event rates [16]. However, other studies have demonstrated opposite results underscoring the biological heterogeneity of the disease (including differences between TAA and AAA) and patient population, and the need to standardize image acquisition, analysis, and quantification [17,18]. Another limitation of using FDG for predicting outcomes in AAA is related to its relatively nonspecific uptake in inflammatory states. USPIO are taken up in macrophages creating signal voids from the iron particles on T2 sequences. In patients with AAA, USPIO signal patterns have identified those with increased aneurysm expansion rates [19]. Limitations of this approach include lack of quantification, difficulties in interpretation due to endogenous iron, and uptake by non-inflammatory cells. Of note, not FDA-approved 18F-labelled nanoparticles, which are taken up by macrophages have shown promise in micro-PET/CT imaging of angiotensin-II-induced murine AAA [20].
Figure 3: 18F-FDG PET imaging in AAA.
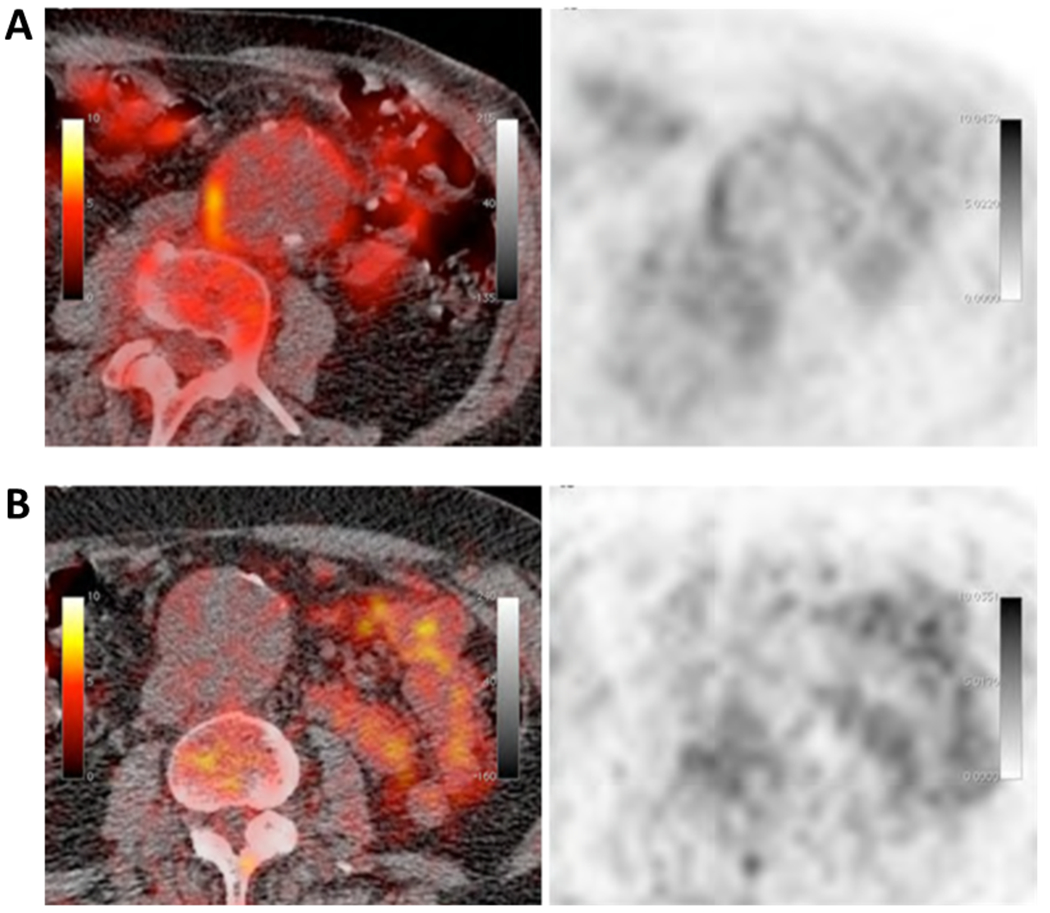
Axial fused PET/CT (left) and PET (right) images of an AAA showing high (A) or minimal (B) 18F-FDG uptake in the wall of the aorta. Reproduced from Kotze et al17 with permission from Springer.
A number of other targets and tracers have been evaluated for aneurysm imaging in preclinical studies. Examples include the integrin αvβ3, which is highly expressed in proliferating endothelial and smooth muscle cells, and to a lower extent, in macrophages, and can be imaged using labelled arginine-glycine-aspartate peptides [21]. NC100692, a 99mTc SPECT tracer, and 18F-FPPRGD2, a PET tracer, have both been shown to detect vascular inflammation and angiogenesis in murine aneurysm models, but their predictive value for aneurysm expansion or rupture is not addressed [22,23].
As discussed above, extracellular matrix remodeling of the arterial wall facilitates aneurysm expansion and rupture. This process is mediated, at least in part, by MMPs, which have been targeted in preclinical investigations, both with nuclear and MR approaches. RP782, an 111In-labeled pan MMP-tracer can detect MMP activation in murine carotid aneurysm using micro SPECT/CT imaging [24]. Aortic signal on micro SPECT/CT images of a related 99mTc-labeled tracer, RP805 has been found to be predictive of AAA expansion and rupture in another murine model (figure 4). The value of this approach in predicting aneurysm outcome in the clinical setting remains to be determined [25].
Figure 4: Predictive value of RP805 imaging.
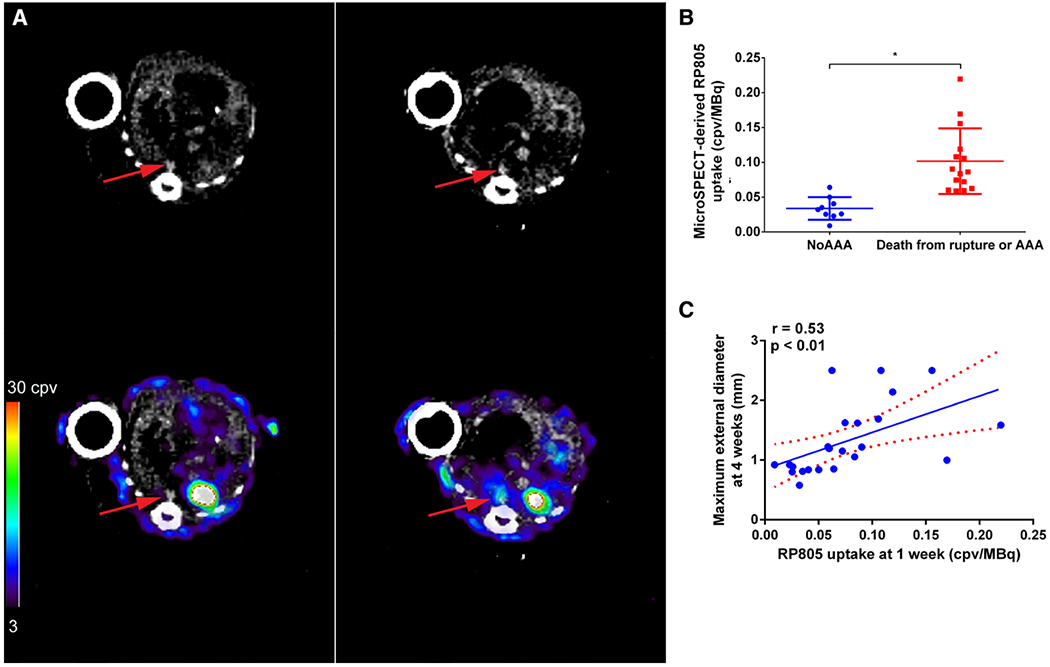
A, Examples of RP805 micro-SPECT/CT images at 1 week in an angiotensin II–infused mouse that did not (left) and a mouse that did (right) develop aneurysm at 4 weeks. Top row, transverse CT angiographic images and bottom row, transverse fused micro-SPECT and CT angiographic images. Arrows indicate suprarenal abdominal aorta. B, Quantification of micro–SPECT/CT-derived suprarenal aorta RP805 uptake in images obtained at 1 week in mice that developed abdominal aortic aneurysm (AAA) or died of AAA rupture by 4 weeks after starting angiotensin II infusion and those that did not develop AAA, *P=0.001. C, Correlation between RP805 in suprarenal abdominal aorta at 1 week and aortic diameter at 4 weeks after angiotensin II infusion. Dotted lines represent 95% confidence interval for the linear regression line. AU indicates arbitrary unit; cpv, counts per voxel; and MMP, matrix metalloproteinase. Reprinted from Golestani et al25 with permission of the publisher. Copyright @ 2015, Springer.
CONCLUSIONS and FUTURE PERSPECTIVES
Despite considerable progress in recent years, important gaps remain in pathobiology, diagnosis and management of aortic aneurysms, which contribute to their place as a major cause of morbidity and mortality in contemporary vascular medicine (Table 2). Current approaches to diagnose and risk stratify patients are based primarily on anatomic imaging of aneurysm size which may be considered suboptimal for identifying patients at the highest and lowest risk for complications. In addition, aneurysms of the thoracic and abdominal aorta are biologically distinct entities which demand diagnostic and management approaches tailored to this understanding. Assessment of vessel wall biology at the patient-level using emerging molecular imaging techniques offers a promising approach in this regard. In addition, molecular imaging can advance our understanding of aneurysm pathobiology and lead to the development of novel approaches to prevent and manage aneurysms. Despite the promising results from early clinical and preclinical studies, there are limitations with current molecular imaging approaches and there is a need for larger-scale studies in humans. In spite of these challenges, the field of molecular cardiovascular imaging remains poised to contribute substantially to our ability to accurately risk stratify and appropriately manage patients with aortic aneurysms.
Table 2.
| Examples of existing gaps in Aortic Aneurysm pathobiology, diagnosis and management |
|---|
| Triggers and mediators * |
| Role of genetics and epigenetics |
| Role of biomechanics in aneurysm expansion and rupture |
| Standard methodology for measuring aneurysm size |
| Improved risk stratification of aneurysm expansion and rupture * |
| Targeted pharmacotherapy * |
| Appropriate selection of patients for surgical or endovascular intervention * |
| Prediction and management of endoleaks following endovascular repair * |
potential role of molecular imaging
Acknowledgments
Funding Sources
MMS research is supported by grants from NIH (R01-HL112992, R01-HL114703), Connecticut Department of Public Health (2016-0087) and Department of Veterans Affairs (I0-BX001750).
Footnotes
DISCLOSURES: No conflicts of interest to disclose.
REFERENCES
- 1.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. The New England journal of medicine. 2014; 05(370):1920–1929. [DOI] [PubMed] [Google Scholar]
- 2.Coady MA, Davies RR, Roberts M, Goldstein LJ, Rogalski MJ, Rizzo JA, Hammond GL, Kopf GS, Elefteriades JA. Familial patterns of thoracic aortic aneurysms. Arch Surg 1999; 4:361–7. [DOI] [PubMed] [Google Scholar]
- 3.Olsson CC. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 12(114)2611–2618. [DOI] [PubMed] [Google Scholar]
- 4.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm. Clinically pertinent controversies and uncertainties. J Am Coll Cariol 2010; 55:841–57. [DOI] [PubMed] [Google Scholar]
- 5.Pressler VV. Aneurysm of the thoracic aorta. Review of 260 cases. The Journal of thoracic and cardiovascular surgery 1985; 01(89):50–54. [PubMed] [Google Scholar]
- 6.Kent KC. Clinical practice: Abdominal aortic aneurysms. N Eng J Med 2014; 371:2101–2108. [DOI] [PubMed] [Google Scholar]
- 7.Acosta S, Ogren M, Bengtsson H, Bergqvist D, Lindblad B, Zdanowski Z. Increasing incidence of ruptured abdominal aorta aneurysm: a population-based study. J Vasc Surg 2006; 44:237–43. [DOI] [PubMed] [Google Scholar]
- 8.Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 12;280:1926–1929. [DOI] [PubMed] [Google Scholar]
- 9.Huusko T, Salonurmi T, Taskinen P, Liinamaa J, Juvonen T, Pääkkö P, Savolainen M, Kakko S. Elevated messenger RNA expression and plasma protein levels of osteopontin and matrix metalloproteinase types 2 and 9 in patients with ascending aortic aneurysms. The Journal of thoracic and cardiovascular surgery 2013; 04;145:1117–1123. [DOI] [PubMed] [Google Scholar]
- 10.Allaire E, Forough R, Clowes M, Starcher B, Clowes AW. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model.. The Journal of clinical investigation 1998; 10(102):1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Hamamsy I, Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol 2009; 6:771–86. [DOI] [PubMed] [Google Scholar]
- 12.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011; 8:92–102 [DOI] [PubMed] [Google Scholar]
- 13.2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cariol 2010; 55(14): e27–129. [DOI] [PubMed] [Google Scholar]
- 14.Pape LA, Tsai TT, Isselbacher EM, Oh JK, et al. Aortic diemeter > 5.5 cm is not a good predictor of type A aortic dissection- observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116:1120–7. [DOI] [PubMed] [Google Scholar]
- 15.Golestani R, Sadeghi MM. Emergence of molecular imaging of aortic aneurysm: Implications for risk stratification and management. J Nucl Cardiol. 2014; 21:251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nchimi A, Cheramy-Bien JP, Gasser TC, Namur G, Gomez P, Seidel L, Albert A, Defraigne JO, Labropoulos N, Sakalihasan N. Multifactorial relationship between 18F-fluoro-deoxy-glucose positron emission tomography signaling and biomechanical properties in unruptured aortic aneurysms. Circ Cardiovasc Imaging 2014; 7:82–91. [DOI] [PubMed] [Google Scholar]
- 17.Kotze CW, Groves AM, Menezes LJ, Harvey R, Endozo R, Kayani IA, et al. What is the relationship between (1)F-FDG aortic aneurysm uptake on PET/CT and future growth rate? Eur J Nucl Med Mol Imaging 2011; 38:1493–9. [DOI] [PubMed] [Google Scholar]
- 18.Jalalzadeh H, Indrakusuma R, Planken RN, Legemate DA, Koelemay MJW, Balm R. Inflammation as a predictor of abdominal aneurysm growth and rupture: A systematic review of imaging biomarkers. Eur J Vasc Endovasc Surg 2016; 52: 333–342. [DOI] [PubMed] [Google Scholar]
- 19.Richards JM, Semple SI, MacGillivray TJ, Gray C, Langrish JP, Williams M, Dweck M, Wallace W, McKillop G, Chalmers RT, Garden OJ, Newby DE. Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of iron oxide: a pilot study. Circ Cardiovasc Imaging 2011; 4:274–281. [DOI] [PubMed] [Google Scholar]
- 20.Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS,Gerszten RE, et al. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol 2011; 31:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toczek J, Meadows JL, Sadeghi MM. Novel molecular imaging approaches to abdominal aortic aneurysm risk stratification. Circ Cardiovasc Imaging 2016; 9(1):e003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razavian M, Marfatia R, Mongue-Din H, Tavakoli S, Sinusas AJ, Zhang J, Nie L, Sadeghi MM. Integrin-targeted imaging of inflammation in vascular remodeling. Arterioscler Thromb Vasc Biol. 2011; 31:2820–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitagawa T, Kosuge H, Chang E, James ML, Yamamoto T, Shen B, et al. Integrin-targeted molecular imaging of experimental abdominal aortic aneurysms by 18F-FPPRGD2 positron emission tomography. Circ Cardiovasc Imaging 2013; 6:950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razavian M, Zhang J, Nie L, Tavakoli S, Razavian N, Dobrucki LW, Sinusas AJ, Edwards DS, Azure M, Sadeghi MM. Molecular imaging of matrix metalloproteinase activation to predict murine aneurysm expansion in vivo. J Nucl Med. 2010;51:1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golestani R, Razavian M, Nie L, Zhang J, Jung JJ, Ye Y, de Roo M, Hilgerink K, Liu C, Robinson SP, Sadeghi MM. Imaging vessel wall biology to predict outcome in abdominal aortic aneurysm. Circ Cardiovasc Imaging. 2015; 8:e002471. [DOI] [PMC free article] [PubMed] [Google Scholar]


