Abstract
We investigated the interactive effect of the exercise pressor reflex (EPR) and the chemoreflex (CR) on the cardiovascular response to exercise. Eleven healthy participants (5 females) completed a total of six bouts of single-leg knee-extension exercise (60% peak work rate, 4 min each) either with or without lumbar intrathecal fentanyl to attenuate group III/IV afferent feedback from lower limbs to modify the EPR, while breathing either ambient air, normocapnic hypoxia (SaO2 ~79%, PaO2 ~43 mmHg, PaCO2 ~33 mmHg, pH ~7.39), or normoxic hypercapnia (SaO2 ~98%, PaO2 ~105 mmHg, PaCO2 ~50 mmHg, pH ~7.26) to modify the CR. During co-activation of the EPR and the hypoxia-induced CR (O2-CR), mean arterial pressure and heart rate were significantly greater, whereas leg blood flow and leg vascular conductance were significantly lower than the summation of the responses evoked by each reflex alone. During co-activation of the EPR and the hypercapnia-induced CR (CO2-CR), the haemodynamic responses were not different from the summated responses to each reflex response alone (P ≥ 0.1). Therefore, while the interaction resulting from the EPR:O2-CR co-activation is hyper-additive for blood pressure and heart rate, and hypo-additive for peripheral haemodynamics, the interaction resulting from the EPR:CO2-CR co-activation is simply additive for all cardiovascular parameters. Thus, EPR:CR co-activation results in significant interactions between cardiovascular reflexes, with the impact differing when the CR activation is achieved by hypoxia or hypercapnia. Since the EPR:CR co-activation with hypoxia potentiates the pressor response and restricts blood flow to contracting muscles, this interaction entails the most functional impact on an exercising human.
Keywords: autonomic control, blood flow, hypercapnia, hypoxia, sympathetic vasoconstriction
Introduction
The cardiovascular response to exercise is primarily determined by three autonomic neurocirculatory control mechanisms. These include a feed-forward mechanism, known as central command (Goodwin et al. 1972), and two feedback mechanisms, the baroreflex (Bristow et al. 1971a) and the exercise pressor reflex (EPR; McCloskey & Mitchell, 1972). Specific to the latter, triggered by exercise-induced excitation of mechano- and metabosensitive group III and IV muscle afferents (Kaufman et al. 1983), animal (Kim et al. 2005; Sala-Mercado et al. 2006) and human (Amann et al. 2011; Sidhu et al. 2015) studies have demonstrated that the EPR raises sympathetic outflow, mean arterial pressure (MAP), and heart rate (HR), and facilitates blood flow to a working skeletal muscle. More recent work has now suggested the chemoreflex (CR) as an additional feedback influence on the sympathetic and cardiovascular response to exercise (Stickland et al. 2007). The peripheral CR is predominantly activated by stimulation of O2-sensitive arterial chemoreceptors located in the carotid body, while the central CR is predominantly triggered by activation of CO2-sensitive medullary chemoreceptors. The CR also facilitates sympathetic outflow, MAP, and vascular resistance during exercise, but restricts active skeletal muscle blood flow (Stickland et al. 2011). However, it remains largely unknown whether the haemodynamic changes during exercise which activates both EPR and CR, are the result of a simple addition of the sympathetic and cardiovascular effects of each reflex (i.e. an additive effect), or the consequence of significant hyper- or hypo-additive interaction.
While studies examining interactions between the EPR and the baroreflex (Kaur et al. 2016; Hureau et al. 2018) and between the CR and the baroreflex (Katayama et al. 2014, 2016) have revealed interactive influences on the circulatory response to exercise, investigations on the haemodynamic consequences of the EPR:CR interaction are scarce and rather indirect. McCoy and colleagues (1987) found the EPR-evoked increases in blood pressure to inhibit the discharge of aortic chemoreceptors in anaesthetised cats. However, the cardiovascular consequences of the interaction between the two reflexes was not addressed. Earlier work in humans has altered arterial blood gases (to stimulate chemoreceptors) during post-exercise circulatory occlusion (PECO, to stimulate metabosensitive muscle afferents) or during passive limb movement (to stimulate mechanosensitive muscle afferents), and then focused on the interaction between the CR and the metaboreflex, or the mechanoreflex, component of the EPR at rest (Seals et al. 1991; Hanada et al. 2003; Gujic et al. 2007; Lykidis et al. 2010; Bruce & White, 2012; Edgell & Stickland, 2014; Delliaux et al. 2015; Silva et al. 2018). Unfortunately, taken together the outcome of these studies was somewhat equivocal and this may, at least in part, be because the use of PECO as a surrogate for the EPR activation has clear limitations. For instance, the background autonomic activity at rest differs from that during exercise (O’Leary & Seamans, 1993; O’Leary, 2006) and the PECO paradigm not only neglects the interplay between mechano- and metabosensitive muscle afferents (Bell & White, 2005; Cui et al. 2008), but mainly engages metabo-nociceptors, a subset of metabosensitive afferent fibres not active during conventional exercise (Light et al. 2008; Jankowski et al. 2013; Pollak et al. 2014). Indeed, Seals and colleagues (1991) found that the increase in the sympathetic tone was potentiated during exercise under hypoxic conditions, whereas this effect could not be reproduced when the circulatory occlusion was applied to resting muscles after exercise. Hence, the exact nature of the EPR:CR interaction and the resultant impact on the haemodynamic response to exercise are uncertain.
It was therefore the purpose of this study to investigate the impact of the EPR:CR interaction on the haemodynamic response to exercise. Since it was technically not feasible to quantify muscle blood flow during electrically evoked quadricep contraction in our preliminary studies and the known importance of central command in manifesting the EPR effect on cardiovascular responses (O’Leary, 1993; Lam et al. 2019), we employed voluntary knee extensions as the modality of exercise. Lower limb muscle afferents were pharmacologically blocked to manipulate the EPR during leg exercise (Amann et al. 2009) while the CR was manipulated via hypoxia (O2-CR) and hypercapnia (CO2-CR) (Xie et al. 2001; Steinback et al. 2009). We hypothesized that the EPR:CR interaction would potentiate sympathetically-mediated responses to exercise and restrain vascular conductance and blood flow to the exercising skeletal muscle.
Methods
Ethical approval
All experimental procedures conformed to the Declaration of Helsinki, except for registration in a database, and were approved by the Institutional Review Boards of the University of Utah and the Veterans Affairs Medical Centre – Salt Lake City (IRB #62889). Written informed consent was obtained from each participant. Eleven recreationally active, young, apparently healthy volunteers participated in the study (5 females and 6 males; age: 26 ± 3 years; height: 176 ± 12 cm; body mass: 75 ± 15 kg). The subjects were non-smokers, non-medicated, and asymptomatic for cardiovascular or respiratory disease. All subjects refrained from exercise for 24 h and from caffeine and alcohol for 12 h before each study visit. Female subjects either had normal menstruation (n = 1) or were asked to pause oral contraceptives such that they were studied during the follicular phase of the menstrual cycle. Circulatory sex hormones were assessed during each experimental session from a blood draw using standard clinical assays.
Experimental protocol
Subjects performed an incremental single-leg knee-extension test (10 + 10 W·min−1) to task failure to determine their peak work rate (Wpeak, 55 ± 15 W) and were then familiarized with all experimental procedures during a practice session. Thereafter, on separate days, subjects completed a control session (Ctrl) and an identical session during which feedback from μ-opioid receptor sensitive muscle afferents was blocked via lumbar intrathecal fentanyl (Fent). All visits were separated by ≥48 h and conducted at the same time of day. Upon arrival at the laboratory, subjects underwent radial artery catheterization and subsequently performed three bouts of rhythmic, single-leg knee-extension exercise with the right leg at 60% of their Wpeak (33 ± 9 W at 60 rpm, 4 min each). The three bouts of exercise in each of the two experimental sessions (Ctrl and Fent) were conducted while breathing room air, i.e. normoxic conditions (NormCtrl and NormFent), or under normocapnic hypoxemic (HypoCtrl and HypoFent) and normoxic hypercapnic (HyperCtrl and HyperFent) conditions (Fig. 1). The exercise bouts were separated by a 6 min break on room air. All experimental sessions and conditions were randomized and counterbalanced.
Figure 1. Schematic illustration of the individual and the concurrent activation of the exercise pressor reflex (EPR) and the chemoreflex (CR) during exercise.
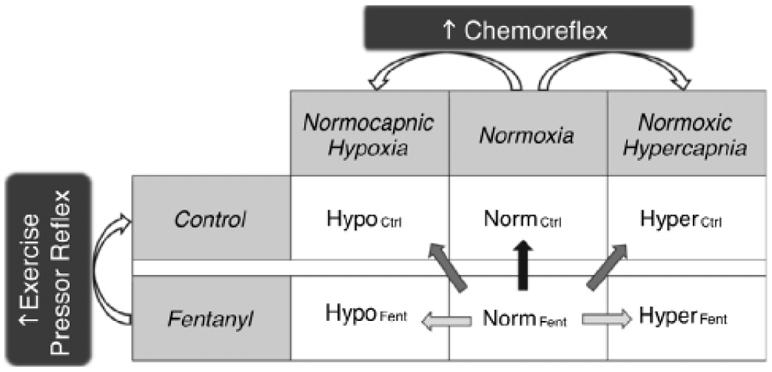
Each of the two experimental sessions (Control and Fentanyl) included 3 conditions (Normoxia, Normocapnic Hypoxia, and Normoxic Hypercapnia). This design resulted in 6 bouts during which single-leg knee-extension exercise was performed at a constant workload. The straight arrows indicate the comparisons utilized to estimate the cardiovascular effects of activation of the EPR ( ), activation of the CR (
), activation of the CR ( ), and co-activation of the EPR and CR (
), and co-activation of the EPR and CR ( ).
).
Procedures and measurements
Intrathecal fentanyl administration.
Subjects were seated in a flexed position and 0.5 ml of fentanyl solution (0.05 mg·ml−1·fentanyl) was delivered intrathecally at the vertebral interspace between L3 and L4 (Amann et al. 2009). To assess whether fentanyl migrated beyond the cervical level, the cardiorespiratory response to arm cranking exercise (15 and 30 W, 3 min each; Monark-Crescent AB, Varberg, Sweden) was assessed before the fentanyl injection and at the end of testing for all subjects (Amann et al. 2010). The arm cranking test was performed for both Ctrl and Fent to ensure a consistent protocol across all sessions.
Normocapnic hypoxia and normoxic hypercapnia.
Ventilatory responses and pulmonary gas exchange were monitored breath-by-breath using an open circuit calorimetry system (Innocor, Innovision, Glamsbjerg, Denmark). Oxyhaemoglobin saturation (SpO2) was estimated using pulse oximetry (OxiMax N-600x, Nellcor, Minneapolis, MN, USA) using a forehead sensor. To induce normocapnic hypoxia, subjects inspired from a Douglas bag containing 8% O2, 3%–4% CO2 and balance N2 to achieve a decrease in SpO2 to ~80%. SpO2 and the partial pressure of end-tidal CO2 (PETCO2) were then held constant by adding pure O2 or CO2 to the inspirate. In the normoxic hypercapnia conditions, an increase in PETCO2 to ~50 mmHg was induced by inspiring a gas mixture of 6%–7% CO2, 21% O2 and balance N2; PETCO2 was maintained by titrating pure CO2 as needed. During each exercise bout, radial arterial blood samples were collected anaerobically and assessed in a co-oximeter and blood gas analyser (GEM 4000, Instrumentation Laboratory, Bedford, MA, USA).
Cardiovascular responses.
MAP was measured continuously using a pressure transducer (Transpac IV, ICU Medical, San Clemente, CA, USA) connected to the radial artery catheter (20 gauge, Arrow International, Reading, PA, USA). HR was monitored using a 3-lead electrocardiogram (CardioCard, Nasiff Associates, Central Square, NY, USA). Beat-to-beat MAP and HR were recorded at 1 kHz in a computer equipped with commercially available software (Spike 2, Cambridge Electronic Design, Cambridge, UK). To determine blood flow to the active leg (LBF), blood velocity and vessel diameter were measured in the common femoral artery, distal to the inguinal ligament and proximal to the bifurcation of the deep and superficial femoral arteries, using Doppler ultrasound (LOGIQ 7, General Electric Medical Systems, Milwaukee, WI, USA). LBF was calculated as blood velocity × π × (vessel diameter ÷ 2)2 × 60. All blood velocity measurements were performed with the probe positioned to maintain an insonation angle of 60° or less. Leg vascular conductance (LVC) was calculated as LBF ÷ MAP.
Data processing
For all cardiorespiratory responses, averages were calculated from the last minute of each exercise bout. Based on the assumption that normoxic exercise performed with fentanyl blockade is characterized by minimal EPR and CR activity, the cardiovascular response during NormFent was considered as the baseline. The individual and interactive effects of the reflexes on the cardiovascular response to exercise were determined as follows (Fig. 1): (1) the individual effects of the EPR, the O2-CR, and the CO2-CR were estimated by the differences between NormCtrl and NormFent (ΔNormCtrl-NormFent), between HypoFent and NormFent (ΔHypoFent-NormFent), and between HyperFent and NormFent (ΔHyperFent-NormFent), respectively; (2) the interactive effects of the EPR and O2-CR and of the EPR and CO2-CR during the reflex co-activation were calculated as the differences between HypoCtrl and NormFent (ΔHypoCtrl-NormFent), and between HyperCtrl and NormFent (ΔHyperCtrl-NormFent), respectively; (3) to investigate the mode of interaction between the reflexes, the observed cardiovascular changes during the co-activation of the EPR and the CR (O2-CR or CO2-CR) were compared with the arithmetic sum of the changes induced by each reflex alone (ΔHypoCtrl-NormFent vs. ΔNormCtrl-NormFent + ΔHypoFent-NormFent, or, ΔHyperCtrl-NormFent vs. ΔNormCtrl-NormFent + ΔHyperFent-NormFent). The interaction mode was defined as follows: when the observed responses during the co-activation of the reflexes were larger than the sum of the responses evoked by each reflex alone, the interaction was considered as hyper-additive; when the observed responses during the co-activation of the reflexes equalled the summated responses to the stimulation of each reflex alone, the interaction was considered as additive; when the observed responses during the co-activation of the reflexes were smaller than the sum of the responses evoked by each reflex alone, the interaction was considered as hypo-additive.
Statistical analysis
Data were analysed using statistical analysis software (SPSS 22, IBM, Armonk, NY, USA). Descriptive statistics were used for subject characteristics. The Mauchly’s test was used to determine sphericity and the Geiser–Greenhouse correction was applied when the data failed the sphericity test. The Student’s paired t test was used to examine the effect of fentanyl blockade on cardiorespiratory responses during the arm cranking exercise and baseline haemodynamics. Based upon the purpose of this study, to activate the CR with hypoxia or hypercapnia and to investigate its interaction with the EPR, data obtained from the hypoxic and hypercapnic conditions were separately compared with those from the normoxic conditions. Two-way ANOVA (session × condition) with repeated measures was used to test alterations in the arterial blood chemistry and cardiorespiratory responses during exercise as a consequence of changes in inspirate. If the ANOVA detected a significant main effect or interaction, the Tukey’s post hoc analysis was performed to identify the differences. To directly test our hypotheses, a priori planned comparisons were made to determine the individual/interactive effects and interaction mode of the reflexes on the changes in the cardiovascular response to exercise, using the Holm–Bonferroni method to correct for familywise error of multiple comparisons. All data are presented as mean ± SD. Statistical significance was set at P < 0.05.
Results
Sex hormones
In the female subjects (n = 5), arterial levels of sex hormones were not different between the Ctrl and Fent days (oestrogen: 133 ± 73 vs. 107 ± 53 pg·mL−1; progesterone: 0.1 ± 0.1 vs. 0.8 ± 1.2 ng·mL−1; follicle stimulating hormone: 4.7 ± 2.5 vs. 3.0 ± 3.0 mIU·mL−1; luteinizing hormone: 5.3 ± 5.9 vs. 5.2 ± 6.6 mIU·mL−1; all P ≥ 0.3), confirming that these subjects were studied during the early follicular phase of the menstrual cycle.
Cardiorespiratory responses to arm exercise
The cardiorespiratory response to arm cranking (15/30 W) was not affected by lumbar intrathecal fentanyl (HR: ~111/~121 beats·min−1; breathing frequency, fB: ~23/~25 breaths·min−1; tidal volume, VT: ~1.1/~1.3 L; ventilatory equivalent for CO2: ~38/~36; all P ≥ 0.1), supportive of an absence of a significant cephalad migration and direct binding of fentanyl to cerebral μ-opioid receptors (Lalley, 2003). Resting MAP (~87 mmHg) was not different between Ctrl and Fent in all subjects (P = 0.2).
Arterial blood gases, humoral chemoreceptor stimuli, and ventilatory responses to exercise
Hypoxia and hypercapnia altered arterial blood gases during exercise in both Ctrl and Fent (Table 1). In hypoxia, arterial haemoglobin saturation (SaO2) and partial pressure of O2 (PaO2) were significantly decreased while arterial partial pressure of CO2 (PaCO2), pH, K+ and lactate levels were not different from normoxia. Pulmonary ventilation , fB and VT were significantly increased by ~107%, ~24% and ~65%, respectively, during hypoxic exercise compared with normoxic exercise. During hypercapnic exercise, arterial K+ and lactate concentrations were not different to those during normoxic exercise, while SaO2, PaO2 and PaCO2 were higher and arterial pH was lower with hypercapnia (all P < 0.05). , fB and VT were also significantly increased by ~146%, ~21% and ~98%, respectively, during hypercapnic exercise compared with normoxic exercise.
Table 1.
Arterial blood chemistry and cardiovascular responses during the final minute of exercise
| Normoxia |
Normocapnic hypoxia |
Normoxic hypercapnia |
||||
|---|---|---|---|---|---|---|
| Ctrl | Fent | Ctrl | Fent | Ctrl | Fent | |
| SaO2 (%) | 97 ± 0 | 96 ± 2 | 79 ± 2 | 79 ± 1 | 98 ± 1 | 97 ± 2* |
| PaO2 (mmHg) | 84 ± 4 | 81 ± 3* | 43 ± 3 | 44 ± 5 | 105 ± 4 | 104 ± 7 |
| PaCO2 (mmHg) | 32 ± 3 | 33 ± 2* | 33 ± 3 | 33 ± 2 | 50 ± 2 | 50 ± 1 |
| Arterial pH | 7.39 ± 0.03 | 7.37 ± 0.04 | 7.39 ± 0.03 | 7.37 ± 0.04 | 7.26 ± 0.03 | 7.26 ± 0.03 |
| Arterial K+ (mmol·L−1) | 3.9 ± 0.3 | 4.0 ± 0.3 | 3.9 ± 0.2 | 3.9 ± 0.2 | 4.0 ± 0.4 | 3.8 ± 0.4 |
| Arterial lactate (mmol·L−1) | 3.0 ± 1.2 | 3.3 ± 1.1 | 3.0 ± 1.0 | 3.3 ± 0.8 | 2.4 ± 0.6 | 2.8 ± 0.9 |
| MAP (mmHg) | 108 ± 7 | 99 ± 11* | 113 ± 8 | 98 ± 11* | 115 ± 7 | 105 ± 9*’ |
| SYS (mmHg) | 152 ± 14 | 139 ± 18* | 162 ± 17 | 137 ± 15* | 164 ± 16 | 149 ± 17*’ |
| DIA (mmHg) | 86 ± 5 | 80 ± 9* | 87 ± 6 | 78 ± 9* | 90 ± 4 | 82 ± 6* |
| HR (beats·min−1) | 109 ± 20 | 107 ± 21 | 130 ± 20 | 121 ± 24*’ | 123 ± 18 | 114 ± 15* |
| LBF (L·min−1) | 3.0 ± 0.7 | 2.6 ± 0.7* | 3.0 ± 0.6 | 2.8 ± 0.9 | 3.1 ± 0.7 | 2.8 ± 0.9* |
| LVC (mL·min−1·mmHg−1) | 27.6 ± 7.0 | 25.9 ± 7.2 | 26.5 ± 5.3 | 28.3 ± 7.0 | 27.0 ± 6.2 | 27.1 ± 9.1 |
Exercise was performed at 60% of peak work rate (33 ± 9 W; = 11). Ctrl: exercise performed with intact leg muscle afferent feedback; Fent: exercise performed with attenuated leg muscle afferent feedback; SaO2: arterial haemoglobin saturation; PaO2: arterial partial pressure of O2; PaCO2: arterial partial pressure of CO2; MAP: mean arterial pressure; SYS: systolic blood pressure; DIA: diastolic blood pressure; HR: heart rate; LBF: leg blood flow; LVC: leg vascular conductance
< 0.05 . Ctrl; < 0.05 .normoxia. Data were analysed using two-way repeated-measure ANOVA with Tukey’s test and are presented as mean ± SD.
Individual and interactive cardiovascular effects of the EPR, the O2-CR and the CO2-CR
The cardiovascular responses to exercise in normoxia, hypoxia and hypercapnia are documented in Table 1. Fig. 2 illustrates the separate and combined cardiovascular effects triggered by the activation of the EPR and the O2-CR during exercise. Individual activation of the EPR (i.e. ΔNormCtrl-NormFent) significantly increased MAP, systolic blood pressure (SYS), diastolic blood pressure (DIA), LBF and LVC during exercise. Individual activation of the O2-CR (i.e. ΔHypoFent-NormFent) significantly increased HR, LBF and LVC during exercise. When the EPR and the O2-CR were activated simultaneously (i.e. ΔHypoCtrl-NormFent), MAP, SYS, DIA, HR and LBF significantly increased during exercise. As for the mode of interaction between the EPR and the O2-CR (i.e. ΔHypoCtrl-NormFent vs. ΔNormCtrl-NormFent + ΔHypoFent-NormFent), the MAP, SYS, DIA and HR responses during exercise were significantly greater during the co-activation of the two reflexes compared with the sum of the responses induced by each reflex alone, reflecting a hyper-additive interaction. In contrast, the LBF and LVC responses were significantly lower during the co-activation of the EPR and the O2-CR compared with the summated responses to the stimulation of each reflex alone, reflecting a hypo-additive interaction.
Figure 2. Cardiovascular consequences of the individual and the concurrent activation of the exercise pressor reflex (EPR) and the hypoxia-induced chemoreflex (O2-CR).
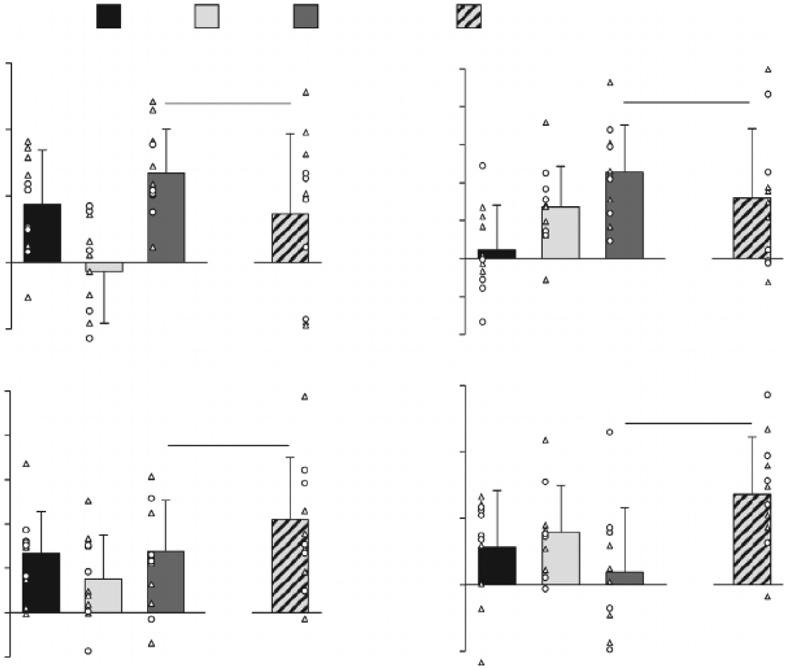
EPR & O2-CR: observed changes during co-activation of the EPR and O2-CR; EPR + O2-CR: sum of the changes elicited by each reflex alone. MAP: mean arterial pressure; HR: heart rate; LBF: leg blood flow; LVC: leg vascular conductance. Individual subject data: ○ females, Δ males; n = 11. * Significantly different from zero, P 0.05; †significantly different between EPR & O2-CR and EPR + O2-CR, P 0.05. Data were analysed using a priori planned comparisons with the Holm–Bonferroni correction and are presented as mean ± SD.
Figure 3 illustrates the separate and combined cardiovascular effects triggered by the activation of the EPR and the CO2-CR during exercise. Individual activation of the CO2-CR (i.e. ΔHyperFent-NormFent) significantly increased MAP and SYS during exercise. When the EPR and the CO2-CR were activated concurrently (i.e. ΔHyperCtrl-NormFent), MAP, SYS, DIA, HR and LBF significantly increased during exercise. The mode of interaction between the EPR and the CO2-CR (i.e. ΔHyperCtrl-NormFent vs. ΔNormCtrl-NormFent + ΔHyperFent-NormFent) was additivefor all variables, as the observed and the summated changes were not different (all P ≥ 0.1).
Figure 3. Cardiovascular consequences of the individual and the concurrent activation of the exercise pressor reflex (EPR) and the hypercapnia-induced chemoreflex (CO2-CR).
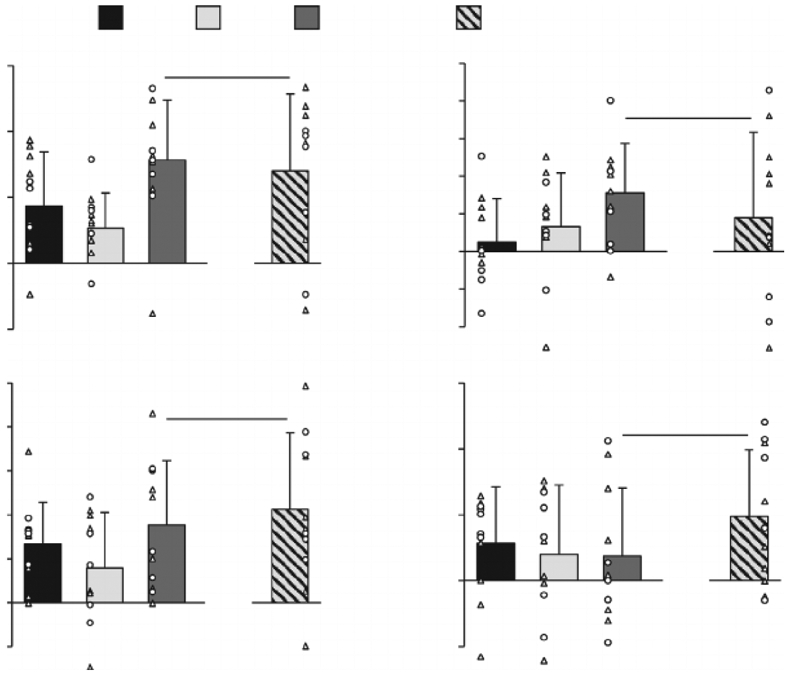
EPR & CO2-CR: observed changes during co-activation of the EPR and CO2-CR; EPR + CO2-CR: sum of the changes elicited by each reflex alone. MAP: mean arterial pressure; HR: heart rate; LBF: leg blood flow; LVC: leg vascular conductance. Individual subject data: ○ females, Δ males; n = 11. *Significantly different from zero, P 0.05. No differences between EPR & CO2-CR and EPR + CO2-CR. Data were analysed using a priori planned comparisons with the Holm–Bonferroni correction and are presented as mean ± SD.
Discussion
This investigation utilized spinal anaesthesia and arterial blood gases to manipulate the EPR and the CR, respectively, with the goal of determining the individual and interactive effects of these reflexes on the haemodynamic response to exercise in humans. The findings suggest that the EPR:O2-CR interaction potentiates the tachycardic and pressor responses to exercise, with both HR and MAP rising higher than the sum of the responses resulting from the activation of each reflex alone, while restricting exercise-induced hyperaemia and vasodilation in the working muscle. The mode of interaction resulting from the EPR:CO2-CR co-activation is somewhat different and characterized by a simple addition of the haemodynamic response evoked by each reflex in isolation. This distinction suggests that the type of CR activation can affect the mode of the EPR:CR interaction and thus the cardiovascular implication of exercise in humans. Therefore, EPR:CR co-activation results in significant interactions between cardiovascular reflexes, with the impact being different when the CR activation is achieved by either hypoxia or hypercapnia. In particular, peripheral haemodynamics are further restricted during the EPR:CR co-activation with hypoxia and this likely has the most functional impact on an exercising human.
Haemodynamic effects of the individual activation of the EPR and the CR
In keeping with our prior findings (Amann et al. 2011; Sidhu et al. 2015), the individual activation of the EPR during normoxic exercise (i.e. little activation of the CR) increased MAP and LBF (Fig. 2). Moreover, the individual, predominantly peripheral, CR activation during exercise, attained with arterial hypoxemia and fentanyl blockade (i.e. little activation of the EPR), enhanced LBF and LVC with no effect on MAP. This differs from a previous human study where intravascular dopamine, used to attenuate the peripheral CR during exercise, increased LBF and LVC and decreased MAP; the authors attributed these cardiovascular changes to a diminution in CR-mediated sympathetic vasoconstriction (Stickland et al. 2011). The disparity between the previous findings and the current observations during the hypoxic trial may be explained by the potent vasodilatory effect of low SaO2 (Ellsworth et al. 1995; Gonzalez-Alonso et al. 2001) which, presumably, outweighed the CR-mediated sympathetic vasoconstriction in the present study. In fact, results from our hypercapnic trial indirectly confirm the idea that the CR activation raises vasoconstrictor outflow. Specifically, despite an increase in MAP, the individual CO2-CR activation did not change LBF (Fig. 3), suggesting that the CR-mediated increase in sympathetic vasoconstrictor tone might have counterbalanced the formerly documented regional vasodilation induced by hypercapnia (Kontos et al. 1971).
Haemodynamic effect of the EPR:CR co-activation
This study compared the haemodynamic response during exercise performed with high EPR and CR activity (i.e. hypoxic/hypercapnic exercise with intact muscle afferent feedback) to the haemodynamic response during exercise performed with low activity of both reflexes (i.e. normoxic exercise with attenuated muscle afferent feedback). The differences in the cardiovascular response between the two conditions were, as expected, profound. Specifically, without affecting LVC, the EPR:CR co-activation during exercise increased MAP, HR and LBF (dark grey bars in Figs. 2 and 3). Based on the previous findings of an elevated muscle sympathetic nerve activity (MSNA) during handgrip (Seals et al. 1991; Hanada et al. 2003; Houssiere et al. 2005) and cycling (Katayama et al. 2011) exercise in hypoxia, it is conceivable that these haemodynamic changes may, at least in part, be mediated by an increase in sympathetic outflow. However, exposure to hypoxia, or hypercapnia, per se, not only activates the CR, but also facilitates the EPR by exaggerating intramuscular metabolic perturbation. It is therefore difficult to discern whether the cardiovascular and, likely, sympathoexcitatory consequences are a simple addition of the effect of the CR stimulation and an increased EPR, or due to the interactive effect of co-activating the two reflexes. To address this issue, as discussed below, we further examined the EPR and the CR interaction by comparing the cardiovascular response observed during the co-activation of these reflexes with the sum of the responses resulting from the activation of each reflex alone.
Mode of the EPR:CR interaction
The co-activation of the EPR and the O2-CR resulted in various modes of interaction with pronounced consequences for the cardiovascular response to exercise. Particularly, the EPR:O2-CR co-activation was characterized by a hyper-additive interaction in terms of MAP and HR (Fig. 2A and B). Since the individual activation of the O2-CR had no effect on MAP during exercise, this finding suggests that the O2-CR enhances the EPR-induced blood pressure response to exercise. In contrast, the EPR:O2-CR interaction had a hypo-additive effect on LVC amplifying leg vasoconstriction which, considering the hyper-additive effect of the EPR:O2-CR interaction on MAP, accounts for the restriction in blood flow to the working skeletal muscle (Fig. 2C).
Although the exact mechanisms have yet to be determined, the origin of the reflex interaction likely occurs at the brainstem where neural feedback of muscle afferents and chemoreceptors are both processed and integrated (Fisher et al. 2015). Given that the tachycardic response to moderate intensity exercise is, despite some cardiovagal contribution, mainly modulated by the sympathetic nervous system (White & Raven, 2014), the observed hyper-additive effect on HR suggests that the mode of EPR:O2-CR interaction resulted in a hyper-additive effect on centrally-mediated sympathoexcitation. Based on the assumption of a solid transduction of sympathetic nerve activity into the vascular and pressor responses (Seals, 1989), the hyper-additive effect on sympathetic outflow, therefore, also explains the exaggerated MAP response and the restricted LVC and LBF resulting from the EPR:O2-CR interaction.
Differing from the findings of the EPR:O2-CR trial, the mode of interaction evoked by simultaneously triggering the EPR and the CO2-CR was simply additive for all cardiovascular variables (Fig. 3). The distinct cardiovascular implications associated with the CR activation induced by hypoxia compared with hypercapnia are not necessarily surprising. Earlier investigations have already alluded to this difference by documenting that the baroreflex buffering of CR-mediated increases in MSNA is more effective in hypoxic than hypercapnic conditions (Somers et al. 1991) and that the impact of hypoxia on altering baroreflex sensitivity (Cooper et al. 2005; Steinback et al. 2009) and ventilatory responsiveness during exercise (Weil et al. 1972; Martin et al. 1978) differs from hypercapnia. Perhaps the most obvious mechanism responsible for the differences in the interaction mode between EPR:O2-CR and EPR:CO2-CR stems from the specificity of O2 and CO2 in terms of chemoreceptor stimulation. While normocapnic hypoxia predominately activates the peripheral chemoreceptors, normoxic hypercapnia stimulates both the central and, albeit to a lesser degree, peripheral chemoreceptors (Dahan et al. 2007). Importantly, excitatory inputs from peripheral and central chemoreceptors to pre-sympathetic neurons in the rostral ventrolateral medulla are conveyed through divergent neural circuits and modulated by different afferent influences (Guyenet, 2014; Toledo et al. 2017). In addition, strong interactions between the central and peripheral CRs influence the sympathetic (Guyenet, 2014) and the ventilatory (Smith et al. 2015) responses to CR activation. The circulatory outcomes observed during hypercapnic exercise may therefore be affected by the interactive effect of the central and peripheral CRs (Bristow et al. 1971b; Shoemaker et al. 2002). Hence, CR-related neural inputs to the medulla may have been quite different in hypoxia compared with hypercapnia, potentially altering the central integration of the feedback from the muscle afferents and chemoreceptors, and, ultimately, resulting in differential modes of interaction.
Finally, although the present level of hypoxia (SaO2 ~79%) and hypercapnia (PaCO2) ~50 mmHg) are both effective chemoreceptor stimuli, the magnitude of CR activation could have differed between the trials and a potential dose-response divergence might have resulted in unequal sympathetic changes. A previous human study, however, has documented that normocapnic hypoxia (~80% SpO2) and nomoxic hypercapnia (PETCO2 ~45 mmHg) elicit similar increases in sympathetic outflow at rest (Xie et al. 2001). Since it is unknown whether this similarity remains during exercise, we only compared the normoxic condition with the hypoxic condition and with the hypercapnic condition and did not make direct comparisons between the hypoxic and the hypercapnic responses. Furthermore, because the mode of interaction was determined by comparing the observed response with the summated response within each condition, the potential discrepancy in the CR-induced sympathetic activation was taken into account and should therefore not have affected the determination of the interaction mode.
Conclusion
The EPR:CR co-activation results in significant interactions between the cardiovascular reflexes, with the impact differing when the CR activation is achieved by hypoxia or hypercapnia. Since the EPR:CR co-activation with hypoxia potentiates the exercise pressor response and restricts blood flow to the contracting limb muscles, this interaction entails the most functional impact on an exercising human.
Supplementary Material
Key points.
Although the exercise pressor reflex (EPR) and the chemoreflex (CR) are recognized for their sympathoexcitatory effect, the cardiovascular implication of their interaction remains elusive.
We quantified the individual and interactive cardiovascular consequences of these reflexes during exercise and revealed various modes of interaction.
The EPR and hypoxia-induced CR interaction is hyper-additive for blood pressure and heart rate (responses during co-activation of the two reflexes are greater than the summation of the responses evoked by each reflex) and hypo-additive for peripheral haemodynamics (responses during co-activation of the reflexes are smaller than the summated responses).
The EPR and hypercapnia-induced CR interaction results in a simple addition of the individual responses to each reflex (i.e. additive interaction).
Collectively, EPR:CR co-activation results in significant cardiovascular interactions with restriction in peripheral haemodynamics, resulting from the EPR:CR interaction in hypoxia, likely having the most crucial impact on the functional capacity of an exercising human.
Acknowledgements
We highly appreciate our subjects’ enthusiasm to participate in this demanding study. We thank Prof Jerry Dempsey for his advice and feedback on the paper.
Funding
This study was supported by the National Heart, Lung, and Blood Institute (HL-116579 and HL-139451) and the U.S. Department of Veterans Affairs (E6910-R, E1697-R, E1433-P, E9275-L and E1572-P).
Biography

Hsuan-Yu Wan received his VMD at National Chung Hsing University (Taiwan) and then completed his PhD in Exercise Physiology at Indiana University Bloomington (USA) in 2016. He is currently a postdoctoral fellow at the University of Utah Vascular Research Laboratory under the mentorship of Professor Markus Amann. Hsuan-Yu is interested in the reflex regulation of cardiovascular and ventilatory responses to exercise under environmental stress and in health and disease. His long-term research goal is to better understand the mechanisms for the neural integration of autonomic and cardiorespiratory control and associated development of muscle fatigue in exercising humans.
Footnotes
Competing interests
The authors declare no conflict of interest.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Statistical Summary Document
References
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2009). Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR & Richardson RS (2011). On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MP & White MJ (2005). Cardiovascular responses to external compression of human calf muscle vary during graded metaboreflex stimulation. Exp Physiol 90, 383–391. [DOI] [PubMed] [Google Scholar]
- Bristow JD, Brown EB, Cunningham DJ, Howson MG, Petersen ES, Pickering TG & Sleight P (1971a). Effect of Bicycling on Baroreflex Regulation of Pulse Interval. Circulation Research 28, 582-+. [Google Scholar]
- Bristow JD, Brown EB Jr., Cunningham DJ, Goode RC, Howson MG & Sleight P (1971b). The effects of hypercapnia, hypoxia and ventilation on the baroreflex regulation of the pulse interval. J Physiol 216, 281–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RM & White MJ (2012). Muscle afferent activation causes ventilatory and cardiovascular responses during concurrent hypercapnia in humans. Exp Physiol 97, 208–218. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Pearson SB, Bowker CM, Elliott MW & Hainsworth R (2005). Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia - a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol 568, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Mascarenhas V, Moradkhan R, Blaha C & Sinoway LI (2008). Effects of muscle metabolites on responses of muscle sympathetic nerve activity to mechanoreceptor(s) stimulation in healthy humans. Am J Physiol Regul Integr Comp Physiol 294, R458–466. [DOI] [PubMed] [Google Scholar]
- Dahan A, Nieuwenhuijs D & Teppema L (2007). Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med 4, e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delliaux S, Ichinose M, Watanabe K, Fujii N & Nishiyasu T (2015). Cardiovascular responses to forearm muscle metaboreflex activation during hypercapnia in humans. Am J Physiol Regul Integr Comp Physiol 309, R43–50. [DOI] [PubMed] [Google Scholar]
- Edgell H & Stickland MK (2014). Activation of the carotid chemoreflex secondary to muscle metaboreflex stimulation in men. Am J Physiol Regul Integr Comp Physiol 306, R693–700. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG & Dietrich HH (1995). The erythrocyte as a regulator of vascular tone. Am J Physiol-Heart C 269, H2155–H2161. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN & Fadel PJ (2015). Autonomic adjustments to exercise in humans. Compr Physiol 5, 475–512. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Richardson RS & Saltin B (2001). Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530, 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI & Mitchell JH (1972). Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujic M, Laude D, Houssiere A, Beloka S, Argacha JF, Adamopoulos D, Xhaet O, Elghozi JL & van de Borne P (2007). Differential effects of metaboreceptor and chemoreceptor activation on sympathetic and cardiac baroreflex control following exercise in hypoxia in human. J Physiol 585, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG (2014). Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4, 1511–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada A, Sander M & Gonzalez-Alonso J (2003). Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol 551, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssiere A, Najem B, Ciarka A, Velez-Roa S, Naeije R & van de Borne P (2005). Chemoreflex and metaboreflex control during static hypoxic exercise. Am J Physiol Heart Circ Physiol 288, H1724–1729. [DOI] [PubMed] [Google Scholar]
- Hureau TJ, Weavil JC, Thurston TS, Broxterman RM, Nelson AD, Bledsoe AD, Jessop JE, Richardson RS, Wray DW & Amann M (2018). Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. J Physiol 596, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE & Koerber HR (2013). Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol 109, 2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Ishida K, Iwamoto E, Iemitsu M, Koike T & Saito M (2011). Hypoxia augments muscle sympathetic neural response to leg cycling. Am J Physiol Regul Integr Comp Physiol 301, R456–464. [DOI] [PubMed] [Google Scholar]
- Katayama K, Ishida K, Saito M, Koike T, Hirasawa A & Ogoh S (2014). Enhanced muscle pump during mild dynamic leg exercise inhibits sympathetic vasomotor outflow. Physiol Rep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K, Ishida K, Saito M, Koike T & Ogoh S (2016). Hypoxia attenuates cardiopulmonary reflex control of sympathetic nerve activity during mild dynamic leg exercise. Exp Physiol 101, 377–386. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH & Mitchell JH (1983). Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol 55, 105–112. [DOI] [PubMed] [Google Scholar]
- Kaur J, Alvarez A, Hanna HW, Krishnan AC, Senador D, Machado TM, Altamimi YH, Lovelace AT, Dombrowski MD, Spranger MD & O’Leary DS (2016). Interaction between the muscle metaboreflex and the arterial baroreflex in control of arterial pressure and skeletal muscle blood flow. Am J Physiol Heart Circ Physiol 311, H1268–H1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ & O’Leary DS (2005). Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288, H1374–1380. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Thames MD, Lombana A, Watlington CO & Jessee F Jr (1971). Vasodilator effects of local hypercapnic acidosis in dog skeletal muscle. Am J Physiol 220, 1569–1572. [DOI] [PubMed] [Google Scholar]
- Lalley PM (2003). Mu-opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol 285, R1287–1304. [DOI] [PubMed] [Google Scholar]
- Lam E, Greenhough E, Nazari P, White MJ & Bruce RM (2019). Muscle metaboreflex activation increases ventilation and heart rate during dynamic exercise in humans. Exp Physiol 104, 1472–1481. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z & Lee J (2008). Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100, 1184–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykidis CK, Kumar P, Vianna LC, White MJ & Balanos GM (2010). A respiratory response to the activation of the muscle metaboreflex during concurrent hypercapnia in man. Exp Physiol 95, 194–201. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Weil JV, Sparks KE, McCullough RE & Grover RF (1978). Exercise ventilation correlates positively with ventilatory chemoresponsiveness. J Appl Physiol Respir Environ Exerc Physiol 45, 557–564. [DOI] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy KW, Rotto DM & Kaufman MP (1987). Inhibition of aortic chemoreceptor discharge by pressor response to muscular contraction. J Appl Physiol (1985) 62, 2258–2263. [DOI] [PubMed] [Google Scholar]
- O’Leary DS (1993). Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol (1985) 74, 1748–1754. [DOI] [PubMed] [Google Scholar]
- O’Leary DS (2006). Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol 91, 73–77. [DOI] [PubMed] [Google Scholar]
- O’Leary DS & Seamans DP (1993). Effect of exercise on autonomic mechanisms of baroreflex control of heart rate. J Appl Physiol (1985) 75, 2251–2257. [DOI] [PubMed] [Google Scholar]
- Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M & Light AR (2014). Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol 99, 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW & O’Leary DS (2006). Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290, H751–757. [DOI] [PubMed] [Google Scholar]
- Seals DR (1989). Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol (1985) 66, 2472–2478. [DOI] [PubMed] [Google Scholar]
- Seals DR, Johnson DG & Fregosi RF (1991). Hypoxia potentiates exercise-induced sympathetic neural activation in humans. J Appl Physiol (1985) 71, 1032–1040. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Vovk A & Cunningham DA (2002). Peripheral chemoreceptor contributions to sympathetic and cardiovascular responses during hypercapnia. Can J Physiol Pharmacol 80, 1136–1144. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Venturelli M, Rossman MJ, Gmelch BS, Bledsoe AD, Richardson RS & Amann M (2015). Aging alters muscle reflex control of autonomic cardiovascular responses to rhythmic contractions in humans. Am J Physiol Heart Circ Physiol 309, H1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva TM, Aranda LC, Paula-Ribeiro M, Oliveira DM, Medeiros WM, Vianna LC, Nery LE & Silva BM (2018). Hyperadditive ventilatory response arising from interaction between the carotid chemoreflex and the muscle mechanoreflex in healthy humans. J Appl Physiol (1985) 125, 215–225. [DOI] [PubMed] [Google Scholar]
- Smith CA, Blain GM, Henderson KS & Dempsey JA (2015). Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2: role of carotid body CO2. J Physiol 593, 4225–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL & Abboud FM (1991). Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest 87, 1953–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback CD, Salzer D, Medeiros PJ, Kowalchuk J & Shoemaker JK (2009). Hypercapnic vs. hypoxic control of cardiovascular, cardiovagal, and sympathetic function. Am J Physiol Regul Integr Comp Physiol 296, R402–410. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Fuhr DP, Haykowsky MJ, Jones KE, Paterson DI, Ezekowitz JA & McMurtry MS (2011). Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J Physiol 589, 6219–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Miller JD, Smith CA & Dempsey JA (2007). Carotid chemoreceptor modulation of regional blood flow distribution during exercise in health and chronic heart failure. Circ Res 100, 1371–1378. [DOI] [PubMed] [Google Scholar]
- Toledo C, Andrade DC, Lucero C, Schultz HD, Marcus N, Retamal M, Madrid C & Del Rio R (2017). Contribution of peripheral and central chemoreceptors to sympatho-excitation in heart failure. J Physiol 595, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil JV, Byrne-Quinn E, Sodal IE, Kline JS, McCullough RE & Filley GF (1972). Augmentation of chemosensitivity during mild exercise in normal man. J Appl Physiol 33, 813–819. [DOI] [PubMed] [Google Scholar]
- White DW & Raven PB (2014). Autonomic neural control of heart rate during dynamic exercise: revisited. J Physiol 592, 2491–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS & Morgan BJ (2001). Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol (1985) 91, 1555–1562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


