Abstract
Background
This retrospective study aimed to describe the effects of convalescent plasma therapy in 24 patients diagnosed with coronavirus disease 2019 (COVID-19) pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during February and March 2020 in Wuhan, China.
Materia/Methods
The confirmation of SARS-CoV-2 infection was made by the reverse transcription-polymerase chain reaction test. We retrospectively analyzed the clinical data and laboratory test reports of patients with severe COVID-19 pneumonia who received a convalescent plasma transfusion.
Results
A total of 24 patients with COVID-19 pneumonia who were transfused with ABO-compatible convalescent plasma were enrolled in the study. Convalescent plasma transfusion showed an effective clinical outcome in 14 of 24 patients (an effective rate of 58.3%). No patients had an adverse reaction to the transfusion. Compared with before convalescent plasma transfusion, the lymphocyte count after convalescent plasma transfusion increased to a normal level (median: 0.80×109/L vs. 1.12×109/L, P=0.004). Other laboratory indicators such as white blood cells, high-sensitivity C-reactive protein, procalcitonin, alanine aminotransferase, and aspartate transaminase showed a decreasing trend after transfusion.
Conclusions
This retrospective observational clinical study showed that convalescent plasma therapy could have beneficial effects on patient outcomes. Recently, regulatory authorization has been given for the use of convalescent plasma therapy, and clinical guidelines have been developed for the collection and use of convalescent plasma and hyperimmune immunoglobulin in patients with COVID-19.
MeSH Keywords: COVID-19, Plasma, SARS Virus
Background
Wuhan, China, in December 2019, was suddenly exposed to coronavirus disease 2019 (COVID-19), which caught all people immersed in the Spring Festival atmosphere unprepared and quickly became the focus of world attention [1]. The Chinese government immediately took measures to control the epidemic and conducted an etiological investigation. During the period of epidemic prevention and control, our hospital adopted the “1+3” model to take over 3 designated hospitals, becoming one of the largest COVID-19 treatment hospitals in China. COVID-19 was identified as the third zoonotic coronavirus disease after severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS), and its cause was SARS coronavirus 2 (SARS-CoV-2) [2]. The onset of COVID-19 in patients was acute and the population was generally susceptible. Patients with latent disease and asymptomatic carriers of the virus were thought to be infectious [3]. To date, more than 1 million people have died from COVID-19 worldwide, placing a heavy burden on countries and bringing untold suffering to affected families. For this novel beta-coronavirus, the World Health Organization (WHO) approved the reverse transcription-polymerase chain reaction (RT-PCR) assay as its diagnostic test and recommended adopting quality control procedures to set up a multicenter collaborative network for reducing false negative and false positive test results [4]. Approved vaccines or specific antiviral drugs are still not available; therefore, it is urgent to find an alternative COVID-19 treatment strategy.
Convalescent plasma therapy by transfusion is a classical passive immunity therapy, which was employed in MERS and 2009 influenza A (H1N1) [5]. A systematic review of SARS-CoV infection revealed shorter hospital stays and lower overall mortality in patients receiving convalescent plasma transfusion compared with patients not receiving the convalescent plasma transfusion [6]. A recent study also suggested that neutralizing antibodies, obtained from COVID-19 convalescent plasma might provide an alternative treatment for patients with severe COVID-19 pneumonia [7]. Because convalescent plasma is donated voluntarily by patients who have fully recovered from COVID-19, the available amount of plasma is quite limited. In addition, most patients with COVID-19 have mild symptoms, which can resolve by using routine symptomatic supportive therapy. Therefore, to reserve the convalescent plasma resources to the maximum extent, only patients with severe disease receive convalescent plasma treatment. Currently, there are 47 ongoing studies assessing the efficacy of COVID-19 convalescent plasma and 1 study assessing hyperimmunoglobulin, 22 of which are randomized controlled trials [8], suggesting that convalescent plasma may be a promising treatment for COVID-19. However, the evidence on the efficacy of convalescent plasma in the cure of COVID-19 remains limited. Therefore, this retrospective study describes the effects of using convalescent plasma therapy in 24 patients diagnosed with COVID-19 pneumonia due to SARS-CoV-2 infection during February and March 2020 at 2 centers in Wuhan, China.
Material and Methods
Study population
This study was conducted during February and March 2020, at 2 participating hospitals (Zhongnan Hospital of Wuhan University and Wuhan Leishenshan Hospital). All patients were confirmed as having severe COVID-19 pneumonia according to the latest interim guidance of the WHO. If patients satisfied any of the following criteria [9], they were recruited to receive convalescent plasma transfusion: (1) respiratory distress with a respiration rate ≥30 breaths per min; (2) oxygen saturation at rest of less than 93%; (3) partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤300 mmHg (1 mmHg=0.133 kPa); and (4) septic shock, respiratory failure requiring mechanical ventilation, or failure of organs other than the lung. A diagnosis of severe COVID-19 was made when patients met these inclusion criteria. Patients were excluded if they met any of the following criteria: (1) refusal to sign informed consent for convalescent plasma treatment; (2) pregnancy or lactation; or (3) immunoglobulin allergy or IgA deficiency. These exclusion criteria were based on the patients’ refusal or physical condition preventing convalescent plasma transfusion.
Confirmation of COVID-19 pneumonia
All patients included in the study were diagnosed by RT-PCR assay. We collected throat swab samples from patients and extracted SARS-CoV-2 RNA using an RNA viral kit (Tiangen, Beijing, China). The RT-PCR test was conducted using the SARS-CoV-2 nucleic acid detection kit (Shengxiang, Shanghai, China). In the RT-PCR test, 2 target genes, including open reading frame 1AB and nucleocapsid protein, were simultaneously amplified, and the PCR parameter was set to 45°C for 10 min and 95°C for 3 min, followed by 45 cycles of 95°C for 15 s and 58°C for 30 s. Then, the ABI 7500 real-time PCR System analyzed the amplification products. A cycle threshold value of less than 40 was regarded as a positive result and a cycle threshold value of 40 or more was regarded as a negative result.
Acquisition and storage of COVID-19 convalescent plasma
Convalescent plasma was donated by volunteers who had fully recovered from COVID-19 pneumonia. Volunteers suitable for donating convalescent plasma satisfied the following conditions: (1) the time from hospital discharge was more than 14 days and there was no fever within and no history of exposure or close contact with infected persons within those 14 days; (2) age was between 18 and 55 years; (3) no blood-borne disease; (4) the throat swab for SARS-CoV-2 was negative for 2 consecutive times by RT-PCR test (sampling interval 1 day). Because the recruitment of donors and the preparation and invocation of convalescent plasma usually take longer than 1 day, the convalescent plasma collected from donors cannot be immediately transfused to patients. After convalescent plasma collection, the Haemonetics MCS+LN90 00-220E blood cell separator (Haemonetics, Boston, MA, USA) was used to perform apheresis. ABO-compatible plasma samples of 200 mL or 400 mL were collected from each donor, then each sample was divided and stored as 100 mL or 200 mL aliquots at 4°C. Next, the convalescent plasma was placed in a medical plasma virus inactivation cabinet and handled with methylene blue and light treatment for 30 min.
Transfusion and Administration of COVID-19 Convalescent Plasma
The transfusion dose of convalescent plasma depended on the body weight of the recipient and was approximately 4 mL/kg to 5 mL/kg. This transfusion dose was inspired by the different proportions of tangible components and plasma in the blood, of which the plasma accounted for about 4% to 5% of the recipient patient’s body weight. Additionally, the ABO blood type of convalescent plasma was compatible with the patients’ ABO blood type. Convalescent plasma should be administered slowly at a rate of no more than 20 drops per min in the first 15 min. During this period, adverse blood transfusion reactions were closely monitored, and if no adverse reactions occurred, clinicians could appropriately adjust the transfusion speed. After convalescent plasma transfusion, patients were measured by observing the outcomes of clinical symptoms such as body temperature and cough and by dynamically monitoring the blood and biochemical indicators every 2 or 3 days. The improvement of clinical symptoms was defined as follows: (1) body temperature returned to normal; (2) dyspnea was relieved; (3) oxygen saturation gradually returned to normal; and (4) lung lesions were absorbed in different degrees.
Detection of the titer of SARS-CoV-2 IgG antibody
The enzyme-linked immunoassay (ELISA) method was used to test the SARS-CoV-2 IgG antibody titer according to the following instructions: (1) 100 uL purified SARS-CoV-2 RBD protein was coated on ELISA plates with 100 ng/well; (2) the plates were washed 3 times to remove unbound RBD protein, 200 μL of 0.05% dried skimmed milk powder was added to each well, and the plates were incubated at 37°C for 90 min; (3) the plates were washed, each well was blocked with 100 μL of 10-fold serial diluted human serum, and the plates were incubated at 37°C for 60 min; (4) the plates were washed, each well was blocked with 100 μL of anti-Human IgG-HRP conjugated monoclonal antibody at a 1: 5000 dilution in phosphate-buffered saline, and the plates were incubated at 37°C for 30 min; (5) the plates were washed 5 times, 100 μL of TMB solution was added, then the plates were incubated at 37°C for 15 min; (6) the reaction was terminated by adding 50 μL of 2M H2SO4; (7) finally, the optical density (OD) value was measured at 450 nm and 630 nm using an Infinite 200 PRO microplate reader. The OD cutoff was determined as follows, OD=0.093+mean OD (blank control). If the OD value of the sample was greater than or equal to the cutoff OD value, the SARS-CoV-2 IgG antibody was positive.
Data collection
Clinical information of patients before and after convalescent plasma transfusion was extracted from electronic medical records and included baseline demographic data (age and sex), comorbidities, laboratory test reports, dose of convalescent plasma transfusion, number of hospitalization days, and some symptomatic treatments such as auxiliary mechanical ventilation, nasal oxygen inhalation, and medication scheme. The main criteria for the evaluation of a curative effect were the improvement of clinical symptoms, blood and biochemical indicators, and radiological images after receiving convalescent plasma treatment. The clinical outcomes were discharge from hospital and death. The efficacy of the convalescent plasma treatment was estimated by the attending physician.
Statistical analysis
SPSS 22.0 software was used to analyze the data and a P value less than 0.05 was considered statistically significant. Categorical variables were depicted as frequencies and percentages and continuous variables were depicted as the mean (SD) or, if non-normally distributed, the median and interquartile range (IQR).
Results
Patient characteristics
A total of 24 patients (13 men and 11 women) with severe COVID-19 pneumonia who received convalescent plasma transfusion were included in this study. The average age was 67.0 years and the median time from hospital admission to convalescent plasma transfusion and convalescent plasma transfusion to hospital discharge was 18 days (IQR, 11.3 days to 28.8 days) and 6 days (IQR, 3.3 days to 12.0 days), respectively. Twenty-two patients had chronic diseases on admission, including hypertension, diabetes, coronary heart disease, and hyperlipidemia (Table 1).
Table 1.
The clinical characteristics of the 24 patients with COVID-19 pneumonia who were treated with COVID-19 convalescent plasma during February and March 2020 at 2 centers in Wuhan, China.
| Patient no. | Sex | Age | Clinical classification | Comorbidiy | Days from admission to CCP transfusion | Days from CCP transfusion to discharge | Total transfusion volume, mL |
|---|---|---|---|---|---|---|---|
| 1 | F | 53 | Critical | Hypertension | 13 | 3 | 700 |
| 2 | F | 80 | Critical | None | 19 | 12 | 400 |
| 3 | F | 56 | Critical | Others | 19 | 8 | 800 |
| 4 | M | 31 | Critical | Others | 14 | 18 | 200 |
| 5 | M | 62 | Severe | Hypertension | 29 | 5 | 400 |
| 6 | F | 62 | Critical | Diabetes, CHD | 4 | 3 | 200 |
| 7 | M | 62 | Severe | Hyperlipidemia, fatty liver | 8 | 6 | 800 |
| 8 | M | 62 | Critical | Hypertension | 11 | 3 | 600 |
| 9 | M | 71 | Critical | Hypertension, CHD | 42 | 14 | 200 |
| 10 | F | 77 | Critical | Hypertension, CHD | 15 | 31 | 300 |
| 11 | M | 83 | Critical | Diabetes | 44 | 10 | 400 |
| 12 | M | 81 | Critical | Hypertension, CHD | 43 | 5 | 200 |
| 13 | M | 86 | Severe | Hypertension, CHD | 36 | 8 | 200 |
| 14 | M | 57 | Critical | Hypertension | 28 | 12 | 1200 |
| 15 | M | 64 | Severe | Diabetes, lymphoma | 24 | 15 | 200 |
| 16 | M | 47 | Critical | Diabetes, others | 17 | 2 | 400 |
| 17 | M | 68 | Severe | Hypertension, diabetes | 16 | 6 | 800 |
| 18 | F | 62 | Severe | Hypertension | 36 | 2 | 425 |
| 19 | F | 58 | Severe | None | 28 | 5 | 600 |
| 20 | F | 79 | Severe | Hypertension | 11 | 4 | 600 |
| 21 | F | 81 | Critical | Chronic heart failure, others | 12 | 6 | 200 |
| 22 | F | 81 | Severe | Ventricular contraction, lymphoma | 9 | 13 | 200 |
| 23 | F | 81 | Severe | Hypertension, MI, RA, uremia | 6 | 4 | 800 |
| 24 | M | 63 | Critical | Hypertension, diabetes, CRF | 19 | 2 | 400 |
F – Female; M – Male; CHD – coronary heart disease; MI -– myocardial infarction; RA – rheumatoid arthritis; CRF –chronic renal failure; CCP – COVID-19 convalescent plasma.
Table 2 lists other drug treatments and respiratory support treatments administered before convalescent plasma transfusion. Four patients were treated with extracorporeal membrane oxygenation, 12 with high-flow nasal cannula, 5 with intermittent positive pressure ventilation, and 3 with low-flow nasal cannula. Antibacterial or antifungal treatments were used when patients had complications of infection, and 13 patients were given corticosteroids as needed (Table 2). The demographic baseline characteristics of the donors were not available because the recruitment of donors and the collection and preparation and deployment of convalescent plasma were managed by the Wuhan Blood Center. To ensure the safety of blood donation and blood use, only the blood donation code was available when convalescent plasma products were distributed to the hospitals.
Table 2.
Other supportive treatments given to the 24 patients with COVID-19 pneumonia who were treated with COVID-19 convalescent plasma during February and March 2020 at 2 centers in Wuhan, China.
| Patient no. | Respiratory support | Antiviral therapy | Antibiotics or antifungal therapy | Corticosteroids therapy |
|---|---|---|---|---|
| 1 | IPPV | Arbidol 0.2 g q8h po. | Tigecycline i.v., polymyxin B i.v. | None |
| 2 | HFNC+NPPV | Lopinavir and Ritonavir Tablets (Take 3 tablets twice a day) | Cefoperazone Sodium and Sulbactam Sodium i.v., polymyxin B i.v. Voriconazole i.v. | Methylprednisolone i.v. |
| 3 | HFNC+NPPV | Arbidol 0.2 g q8h po. | Moxifloxacin i.v., Voriconazole i.v. | Methylprednisolone i.v. |
| 4 | IPPV+ECMO | Arbidol 0.2 g q8h po. | Imipenem i.v., Linezolid i.v. | None |
| 5 | HFNC | Arbidol 0.2 g q8h po. IFN-α 500 MIU qd inh. |
Moxifloxacin i.v., Cefoperazone Sodium and Sulbactam Sodium i.v. | Methylprednisolone i.v. |
| 6 | IPPV+ECMO | Arbidol 0.2 g q8h po. | Imipenem-Sitastatin Sodium i.v., Cefoperazone Sodium and Sulbactam Sodium i.v., Caspofungin i.v. | None |
| 7 | HFNC | Lopinavir and Ritonavir Tablets (Take 3 tablets twice a day) | Moxiflxacin i.v., Imipenem-Sitastatin Sodium i.v. | Methylprednisolone i.v. |
| 8 | HFNC | Arbidol 0.2 g q8h po. | Imipenem-Sitastatin Sodium i.v., Cefoperazone Sodium and Sulbactam Sodium i.v. | Methylprednisolone i.v. |
| 9 | IPPV | Arbidol 0.2 g q8h po. | Levofloxaxin i.v., Cefoperazone Sodium and Sulbactam Sodium i.v., Moxiflxacin i.v. | None |
| 10 | IPPV | Arbidol 0.2 g q8h po. | Moxiflxacin i.v., Imipenem i.v., Linezolid i.v. | None |
| 11 | IPPV | Arbidol 0.2 g q8h po. Ribavirin 0.5 g qd i.v. |
Moxiflxacin i.v., Cefoperazone Sodium and Sulbactam Sodium i.v. | Methylprednisolone i.v. |
| 12 | HFNC | Arbidol 0.2 g q8h po. | Moxiflxacin i.v., Cefoperazone Sodium and Sulbactam Sodium i.v. | None |
| 13 | LFNC | Ribavirin 0.5 g qd i.v. IFN-α 500 MIU qd inh. |
Moxiflxacin i.v., Levofloxaxin i.v. | Methylprednisolone i.v. |
| 14 | IPPV+ECMO | Ribavirin 0.5 g qd i.v. | Imipenem-Sitastatin Sodium i.v., Linezolid i.v. | Methylprednisolone i.v. |
| 15 | HFNC | Arbidol 0.2 g q8h po. IFN-α 500MIU qd inh. |
Moxiflxacin i.v., Cefoperazone Sodium and Sulbactam Sodium i.v. | Methylprednisolone i.v. |
| 16 | IPPV+ECMO | Arbidol 0.2 g q8h po. | Moxiflxacin i.v., Imipenem i.v., Tigecycline i.v. | Methylprednisolone i.v. |
| 17 | HFNC | Arbidol 0.2 g q8h po. | Moxiflxacin i.v. | None |
| 18 | LFNC | Arbidol 0.2 g q8h po. Lopinavir and Ritonavir Tablets (Take 3 tablets twice a day) |
Piperacillin Sodium and Tazobactam Sodium i.v. | Methylprednisolone i.v. |
| 19 | LFNC | Arbidol 0.2 g q8h po. | Moxiflxacin i.v. | Methylprednisolone i.v. |
| 20 | HFNC | Ribavirin 0.5 g qd i.v. | Moxiflxacin i.v., Imipenem-Sitastatin Sodium i.v. | None |
| 21 | IPPV | Arbidol 0.2 g q8h po. | Tegacycline i.v., Imipenem i.v. | None |
| 22 | HFNC | Arbidol 0.2 g q8h po. Ribavirin 0.5 g qd i.v. IFN-α 500MIU qd inh. |
Moxiflxacin i.v., Imipenem-Sitastatin Sodium i.v. | Methylprednisolone i.v. |
| 23 | HFNC | Lopinavir and Ritonavir Tablets (Take 3 tablets twice a day) | Piperacillin Sodium and Tazobactam Sodium i.v. | None |
| 24 | HFNC | Arbidol 0.2 g q8h po. | Moxiflxacin i.v. | None |
IPPV – Intermittent positive pressure ventilation; HFNC – high-flow nasal cannula; NPPV – noninvasive positive pressure ventilation; ECMO – extracorporeal membrane oxygenation; LFNC – low-flow nasal cannula; q8h – every 8 h; po. – per os; MIU – million IU; qd – every day; inh. – inhalation; i.v. – injection.
Outcomes of patients receiving convalescent plasma transfusion
The titer of SARS-CoV-2 IgG antibody in all convalescent plasma samples involved in this study was more than 1: 160. 8 patients were given a single dose (200 mL) of convalescent plasma transfusion and the remaining 16 patients were given multiple doses of convalescent plasma transfusion. 4 patients died and the remaining 20 patients were discharged from the hospital, with a median length of hospital stay of 29.0 days (IQR, 16.5–39.8 days). After the comprehensive evaluation of the attending physician, it was found that adverse events such as fever, chills, rash, or allergy during or after convalescent plasma transfusion did not occur in any patients. Convalescent plasma transfusion showed an effective clinical outcome in 14 of 24 patients, an effective rate of 58.3%. Since the viral titer of SARS-CoV-2 can only be detected by special personnel in a biosafety level 3 laboratory, and the hospital laboratories did not have the conditions necessary to carry out SARS-CoV-2 titer testing, all patients’ SARS-CoV-2 viral titers involved in this study were unavailable.
Improvement of laboratory parameters before and after convalescent plasma transfusion
An important feature of COVID-19 in patients is lymphocytopenia. Thus, the lymphocyte count of patients with COVID-19 pneumonia can be used to estimate the outcomes of treatment. In this study, lymphocytes gradually increased to the normal level in patients with COVID-19 pneumonia receiving convalescent plasma transfusion (median: before, 0.80×109/L vs. after, 1.12×109/L). The difference of lymphocyte count before and after convalescent plasma transfusion was statistically significant (P=0.004). For laboratory indicators related to inflammation and liver dysfunction, we observed a downward trend after transfusion compared with those before transfusion (Table 3). In particular, the high-sensitivity C-reactive protein levels in patients with severe COVID-19 pneumonia declined rapidly after convalescent plasma transfusion (median: before, 64.14 mg/L vs. after, 26.08 mg/L, P=0.008).
Table 3.
The laboratory findings before and after convalescent plasma transfusion in 14 patients with COVID-19 pneumonia who had an effective response to this treatment.
| Laboratory indicators | Normal range | Before CP transfusion | After CP transfusion | P values |
|---|---|---|---|---|
| WBC, 109/L | 3.5–9.5 | 9.28 (6.91–10.96) | 8.84 (7.16–11.37) | 0.982 |
| Lymphocyte, 109/L | 1.1–3.2 | 0.80 (0.58–0.91) | 1.12 (0.87–2.11) | 0.004* |
| CRP, mg/L | 0–4 | 64.14 (32.85–96.10) | 26.08 (8.34–42.09) | 0.008* |
| PCT, ng/ml | 0–0.05 | 0.55 (0.19–1.71) | 0.31 (0.09–0.48) | 0.273 |
| ALT, IU/L | 7–45 | 30.50 (22.50–64.50) | 24.50 (12.00–39.25) | 0.175 |
| AST, IU/L | 13–35 | 37.50 (25.00–55.25) | 28.00 (22.50–40.00) | 0.375 |
| Urea, mmol/L | 3.1–8.8 | 7.90 (5.73–11.93) | 7.45 (3.85–15.53) | 0.581 |
WBC – white blood cell; CRP – high-sensitivity C-reactive protein; PCT – procalcitonin; ALT – alanine aminotransferase; AST – aspartate transaminase; urea – urea nitrogen.
Indicated statistical significance (P<0.05).
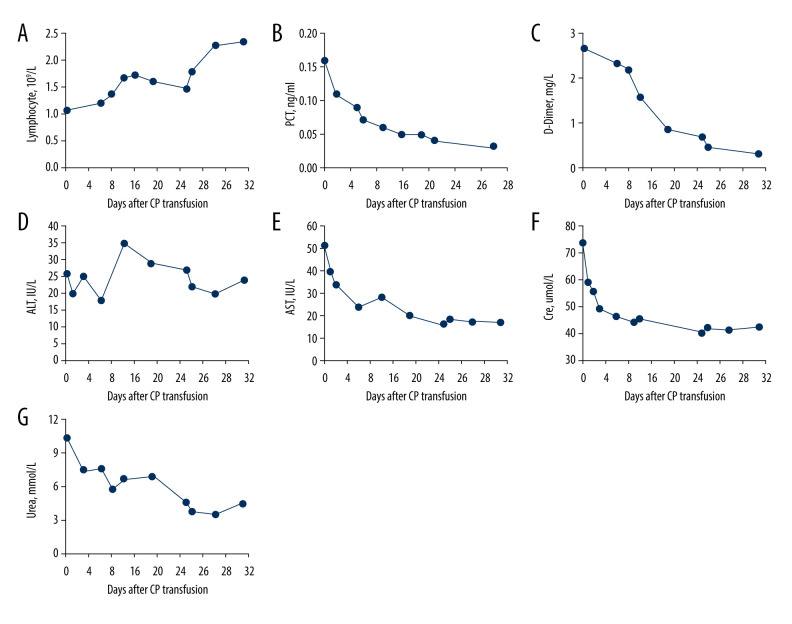
It is worth noting that 1 of the 14 patients with COVID-19 pneumonia who was effectively treated with convalescent plasma transfusion was the most representative of treatment response. Although this was a 77-year-old woman in poor health with a 20-year history of hypertension and coronary heart disease and severe COVID-19 pneumonia who had developed type I respiratory failure on admission, she achieved remarkable outcomes after convalescent plasma transfusion. After admission, this patient was given intermittent positive pressure ventilation for respiratory support, arbidol for antiviral treatment, and moxifloxacin, imipenem, and linezolid for antiinfection, but all of these treatments failed to improve her condition. After consultation, clinicians unanimously decided to perform a convalescent plasma transfusion for this patient. To everyone’s delight, her body temperature decreased from 39°C to normal and her clinical symptoms and laboratory indicators were significantly improved after 2 or 3 days of convalescent plasma transfusion (Figure 1). In addition, the results presented in Table 3 can be reproduced in this patient. We observed that after convalescent plasma transfusion, the lymphocyte count of this patient gradually increased, and the levels of procalcitonin, D-dimer, aspartate aminotransferase, and creatinine decreased, suggesting that the immune, liver, and kidney function of the patient had gradually improved. Finally, this patient was cured by convalescent plasma treatment after the failure of antiviral and antiinfective therapy and was discharged from the hospital.
Figure 1.
The dynamic changes in the laboratory results in the days following treatment with convalescent plasma transfusion (x-axis) in a 77-year-old female patient with severe COVID-19 pneumonia. (A) Changes in the lymphocyte count during 32 days following convalescent plasma transfusion; (B) Changes in the procalcitonin (PCT) levels during the 28 days following convalescent plasma transfusion; (C) Changes in the D-dimer levels during the 32 days following convalescent plasma transfusion; (D) Changes in the serum aminotransferase (ALT) levels during the 32 days following convalescent plasma transfusion; (E) Changes in the serum aspartate transaminase (AST) levels during the 32 days following convalescent plasma transfusion; (F) Changes in the creatinine levels during the 32 days following convalescent plasma transfusion; (G) Changes in the urea levels during the 32 days following convalescent plasma transfusion.
Discussion
COVID-19 was first reported in Wuhan, China. To increase the cure rate and reduce the mortality rate as much as possible, the National Health Commission of China was the first to issue the use of convalescent plasma to treat patients with severe COVID-19 pneumonia. Results from one of the earliest studies from Wuhan revealed that COVID-19 convalescent plasma therapy may have a significant effect on patients with severe COVID-19 pneumonia [10]. To further explore the feasibility of convalescent plasma therapy in an emergency situation lacking vaccines and specific drugs, we carried out this study. In mid to late February, our hospital was approved for the use of convalescent plasma to treat patients with severe COVID-19 pneumonia, according to the clinical treatment plan of COVID-19 convalescent plasma (trial second edition). During this period, a total of 24 patients with severe COVID-19 pneumonia were treated with convalescent plasma, and all patients showed good tolerance without any adverse events. Most patients receiving convalescent plasma treatment experienced significant improvements in clinical symptoms such as fever and cough and their SARS-CoV-2 nucleic acid test results gradually changed from positive to negative or from strong positive to weak positive.
Convalescent plasma was acquired from donor patients who had recovered from COVID-19 pneumonia and established humoral immunity to the virus. The plasma contains large amounts of neutralizing antibodies that neutralize SARS-CoV-2 and remove pathogens from circulating blood and lung tissue [11]. The earliest study of 436 convalescent plasma donors from England showed that most donors with confirmed SARS-CoV-2 infection could produce detectable neutralizing antibodies [12]. It is becoming increasingly clear that convalescent plasma is a safe and promising treatment for COVID-19. Results from the latest large-scale study showed the incidence of severe adverse events in 5000 patients with severe COVID-19 pneumonia who received convalescent plasma transfusion was less than 1% [13]. The study by Joyner et al. of COVID-19 convalescent plasma therapy in 2000 patients also suggested that the treatment was safe and does not increased the risk of complications [14]. In addition, COVID-19 convalescent plasma can bring benefits to immunomodulatory by improving macrophage activation and systemic inflammation or “cytokine storms” [15]. A new randomized clinical trial of COVID-19 convalescent plasma therapy reported that the transfusion in patients with severe COVID-19 pneumonia improved clinical symptoms [16]. Also of note, Young et al. revealed that after convalescent plasma transfusion, the inflammatory indicators in patients with COVID-19 pneumonia decreased significantly, while the oxyhemoglobin saturation gradually increased to a normal level [17]. All of these findings support the results of our current study.
Based on these results, the US FDA announced on March 24, 2020, that COVID-19 convalescent plasma therapy was approved as an emergency investigational new drug [18] and was granted emergency use authorization [19]. Moreover, the FDA released 3 pathways for the use of COVID-19 convalescent plasma: (1) clinical trials, (2) expanded access program (EAP), and (3) the single patient emergency investigational new drug pathway [20]. To date, more than 34 000 patients with COVID-19 pneumonia have received convalescent plasma transfusion under the federally supported expanded access program [21]. Taken together, this evidence indicates that convalescent plasma is a promising treatment option for COVID-19. The national health commission of China strongly called on the patients who had recovered from COVID-19 pneumonia to actively donate their precious plasma to save more lives, with informed consent and ethics conditions considered. Now, as winter approaches, the Chinese government is making an orderly deployment of prevention efforts to prevent any possibility of a rebound of the epidemic. No serious adverse reactions related to convalescent plasma transfusion were found in the present study. This result could be supported by the evidence that SARS-CoV-2 was not regarded as an associated infection transmitted through blood transfusion [22]; it has been noted that only 1% of patients with COVID-19 pneumonia have detectable SARS-CoV-2 RNA in their blood.
This study has several limitations. First and foremost, this was an observational study that relied on the availability of patient data in the hospital records, the study population was small, and the study demonstrated the experiences of a single center in Wuhan. Importantly, the study included patients who were diagnosed with COVID-19 at the beginning of the pandemic when evidence-based guidelines for the diagnosis of SARS-CoV-2 infection and patient management, including the use of convalescent plasma, were awaiting development. Second, all patients with COVID-19 pneumonia included in the study received antiviral therapy in addition to convalescent plasma transfusion. Therefore, whether the improvement in patient condition was related to treatment other than convalescent plasma transfusion cannot be fully determined. Third, the severity of COVID-19 pneumonia in convalescent plasma donors is unknown, so determining if there is a difference in the therapeutic effect of convalescent plasma donated by patients who had recovered from COVID-19 pneumonia with different severity levels requires more in-depth research.
Conclusions
This retrospective clinical study from 2 centers in Wuhan, China, in early 2020 showed that convalescent plasma therapy could have beneficial effects on patient outcomes. More recently, regulatory authorization has been given for the use of convalescent plasma therapy, and clinical guidelines have been developed for the collection and use of convalescent plasma and hyperimmune immunoglobulin in patients with COVID-19.
Acknowledgments
We would like to thank Zhongnan Hospital of Wuhan University and Wuhan Leishenshan Hospital for their assistance in completing this study and all COVID-19 patients who participated in this study for their active cooperation.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilocca B, Soggiu A, Sanguinetti M, et al. Comparative computational analysis of SARS-CoV-2 nucleocapsid protein epitopes in taxonomically related coronaviruses. Microbes Infect. 2020;22(4):188–94. doi: 10.1016/j.micinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Use of laboratory methods for SARS diagnosis. 2020. https://www.who.int/csr/sars/labmethods/en/
- 5.Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–56. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A rapid review. Cochrane Database Syst Rev. 2020;5(5):CD13600. doi: 10.1002/14651858.CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng H, Wang D, Nie J, et al. The efficacy assessment of convalescent plasma therapy for COVID-19 patients: A multi-center case series. Signal Transduct Target Ther. 2020;5:219. doi: 10.1038/s41392-020-00329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117:9490–96. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marano G, Vaglio S, Pupella S, et al. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–57. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvala H, Mehew J, Robb ML, et al. Convalescent plasma treatment for SARS-CoV-2 infection: Analysis of the first 436 donors in England, 22 April to 12 May 2020. Euro Surveill. 2020;25(28):2001260. doi: 10.2807/1560-7917.ES.2020.25.28.2001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130(9):4791–97. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95:1888–97. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas M, Rodríguez Y, Monsalve DM, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev. 2020;19:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA. 2020;324(5):1–11. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal P, Ibrahim M, Niu A, et al. Safety and efficacy of COVID-19 convalescent plasma in severe pulmonary disease: A report of 17 patients. Transfus Med. 2020 doi: 10.1111/tme.12729. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Tanne JH. COVID-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. doi: 10.1136/bmj.m1256. [DOI] [PubMed] [Google Scholar]
- 19.Factsheet F. US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) recommendations for the use of convalescent plasma for patients hospitalized with COVID-19. 2020. https://www.fda.gov/media/141479/download.
- 20.US Food and Drug Administration. COVID-19 convalescent plasma-emergency INDs. 2020. http://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds.
- 21.World Health Organization. An EU programme of COVID-19 convalescent plasma collection and transfusion. Guidance on collection, testing, processing, storage, distribution and monitored use. 2020. https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf.
- 22.Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–65. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]