Abstract
Despite considerable progress has been achieved in the treatment of acute myeloid leukemia over the past decades, relapse remains a major problem. Novel therapeutic options aimed at attaining minimal residual disease-negative complete remission are expected to reduce the incidence of relapse and prolong survival. Natural killer cell-based immunotherapy is put forward as an option to tackle the unmet clinical needs. There have been an increasing number of therapeutic dimensions ranging from adoptive NK cell transfer, chimeric antigen receptor-modified NK cells, antibodies, cytokines to immunomodulatory drugs. In this review, we will summarize different forms of NK cell-based immunotherapy for AML based on preclinical investigations and clinical trials.
Keywords: Acute myeloid leukemia, Natural killer cells, Immunotherapy, Adoptive NK cell transfer, Chimeric antigen receptor-modified NK cells, Antibodies, Cytokines
Background
Acute myeloid leukemia (AML) is a clinically and genetically heterogeneous disease with unsatisfactory outcomes. Over the last few years, considerable progress has been achieved in the treatment of AML with the development and implementation of new drugs [1, 2]. However, allogeneic hematopoietic cell transplantation (HCT) has been recognized as the only way to cure AML so far and relapse remains a major problem. Novel therapeutic options aimed at attaining minimal residual disease (MRD)-negative complete remission (CR) are expected to reduce the incidence of relapse and prolong survival. Thus, immunotherapy becomes an option to tackle unmet clinical needs in AML [3, 4].
Immunotherapy has been recognized as an incredibly promising therapeutic strategy for numerous cancers [5]. The adoption of this treatment modality is based on mechanisms of immune surveillance/response and cancer escape [6]. Under physiological conditions, immune cells and substances in the immune system play pivotal roles in detecting and destroying pathogen-infected or neoplastically transformed cells. But they become less potent in cancer elimination when malignant cells display the loss of antigenicity and/or immunogenicity and are surrounded by an immunosuppressive microenvironment [6]. Thus, immunotherapy with strategies of reboosting patients’ own immune system or initiating new immune response to fight cancers has been demonstrated with the capacity of producing sustainable clinical benefits against both solid and hematological malignancies [7–9].
Natural killer (NK) cell-based immunotherapy represents one of the novel immunotherapeutic strategies recently, unleashing immune suppression of NK cells to attack various cancers [10–12]. With the progressive elucidation of NK cell immunobiology and the development of manipulative techniques, the field of NK cell-based immunotherapy in hematological malignancies has been expanding and accelerating over the past years, including adoptive NK cell transfer [13–16], chimeric antigen receptor (CAR)-modified NK cells [17–22], antibodies [23–25], cytokines [26, 27] and drug treatment [28–31]. Despite remarkable progress has been made, the application in AML is still at the initial stage. Firstly, clinical trials with results showing the efficacy and safety of these therapeutic approaches are limited, most of which are currently still in progress. Secondly, preclinical studies of NK cell-based immunotherapy are constantly emerging, in the aspect of new methodologies to utilize NK cells and strategies to enhance the response [32, 33].
Herein, in this review, we provide an overview of NK cell biology, the pathology of NK cells in AML and the recent advances in NK cell-based immunotherapy for AML based on preclinical investigations and clinical trials.
Biology of NK cells
NK cells belong to innate lymphoid cells that contribute to immune system’s first-line defense against infections and malignant diseases [34]. They can be categorized into two subsets on the basis of surface expression levels of CD56 and CD16, as measured by the intensity of immunofluorescence. The canonical CD56dimCD16+ NK cell subset comprises around 90% of the total population in peripheral blood and exerts strong cytolytic activity through releasing cytotoxic granules containing perforin and granzymes. The rest 10% of NK cell population, known as CD56brightCD16−, is a potent producer of immunoregulatory cytokines including interferon (IFN)-γ, tumor necrosis factor (TNF)-α/β and interleukin (IL)-10 [35].
The cytotoxic function of NK cells is finely regulated by a complex array of surface inhibitory receptors [e.g., inhibitory killer immunoglobulin-like receptors (KIRs), leukocyte immunoglobulin-like receptors (LIRs) and CD94/natural killer group 2A (NKG2A)] and activating receptors [e.g., activating KIRs, CD94/NKG2C, NKG2D and natural cytotoxicity receptors (NCRs)] that deliver suppressive and stimulatory signals, respectively (Fig. 1) [36, 37]. In line with the diversity of major histocompatibility complex (MHC) molecules in populations, KIRs are genetically determined and display a high level of polymorphism. There are two main groups of KIR haplotypes, termed as “A” and “B”, as classified by the distinct gene content. KIR A haplotypes mainly contain inhibitory KIR genes and only one activating KIR gene KIR2DS4, whereas KIR B haplotypes carry, besides inhibitory KIR genes, various numbers and combinations of activating KIR genes [38, 39]. The considerable differences of both allelic polymorphism and KIR gene content account for the high variability of KIR gene family among different individuals.
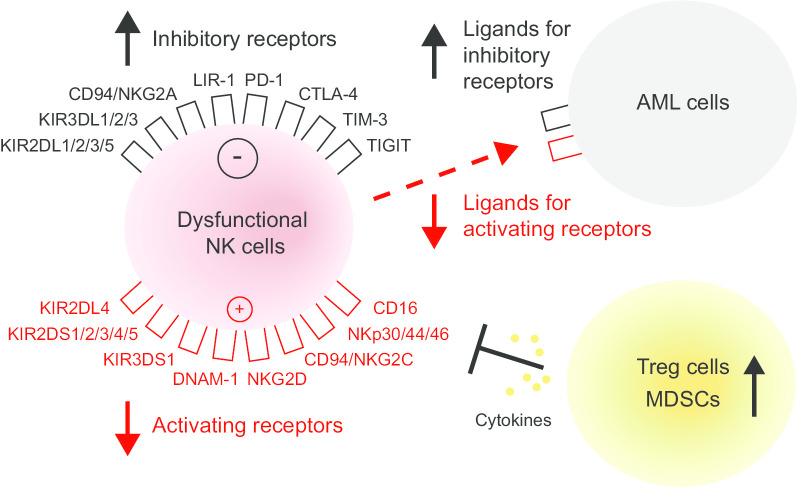
Fig. 1.
Mechanisms of immune escape from NK cell-mediated recognition in AML. Dysfunctional NK cells exhibit an imbalanced receptor expression with the overexpression of inhibitory receptors and the underexpression of activating receptors. AML cells display a defective expression of cognate ligands for NK cell activating and inhibitory receptors. The tumor microenvironment consisting of Treg cells and MDSCs can interfere with the function of NK cells through the secreting of cytokines. MDSC myeloid-derived suppressor cell, NK natural killer cell; Treg regulatory T cell
NK cell-mediated cytotoxicity is based on the notion of “missing self-recognition” and “induced self-recognition” [40]. During NK cell development, inhibitory KIR receptors encounter with MHC class I (MHC-I) ligands on their own hematopoietic cells, leading to the acquisition of functional competence and self-tolerance [41, 42]. Both the reduction/absence of MHC-I molecules and the upregulation/de novo expression of ligands for activating receptors on tumor cells can elicit NK cell immune response against “non-self,” through releasing cytotoxic granules, secreting cytokines and inducing death receptor-dependent apoptosis [36, 43]. Apart from the direct receptor-based recognition between NK cells and tumor cells that potentiates the anti-tumor function of NK cells, they can kill tumor cells by antibody-dependent cell-mediated cytotoxicity (ADCC) as well, which is mediated by the IgG Fc receptor CD16 [44].
In addition, the activation of NK cells can be induced by other immune cells such as macrophages and dendritic cells (DCs) as well, either through direct cell-to-cell contacts or the release of cytokines such as IL-12, IL-15, IL-18 and IFN-ɑ/β, promoting NK cell cytotoxicity and IFN-γ production [45, 46].
Dysfunction of NK cell-mediated anti-leukemia responses in patients with AML
In AML, leukemia cells can escape from NK cell-mediated recognition as a consequence of NK cell abnormalities, immunosuppressive properties of AML cells or interactions between NK cells and other immune cells in favor of immune escape (Fig. 1) [47].
Since the function of NK cells is tightly regulated by their sophisticated repertoire of inhibitory and activating receptors, imbalanced receptor expressions can lead to NK cell dysfunction. Studies evaluating the expression of these molecular receptors on NK cells showed the underexpression of activating receptors such as NKG2D, NCRs and DNAX accessory molecule-1 (DNAM-1) as well as overexpression of inhibitory receptors such as KIR2DL2/L3 and NKG2A in AML patients as compared with healthy controls [48–52]. Direct contact between AML cells and NK cells, high expression of CD200 on AML cells, soluble NKG2D ligands (NKG2DLs) in the sera and suppressive tumor microenvironment are factors that lead to defective receptor expression changes [49, 53, 54].
In addition to NK cell abnormalities, leukemia cells themselves displaying a defective expression of ligands for NK cell activating/inhibitory receptors give rise to the attenuation of NK cell-mediated anti-leukemia responses as well. For instance, the low expression of NKG2DLs [MHC class I chain-related proteins (MIC) and UL16-binding proteins (ULBP)], NCR ligands and DNAM-1 ligands (CD112 and CD155) on AML cells can render them resistant to NK cell killing [55, 56]. The deficient NKG2DL expression on AML cells may be caused by aberrant epigenetic mechanisms or the release of soluble forms from the cell surface by metalloproteinases [57, 58]. Whereas, upregulation of inhibitory immune checkpoint molecules programmed cell death ligand-1 (PD-L1) and PD-L2 is observed in AML blasts [59].
The tumor microenvironment, which possesses immunosuppressive cells, such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs) and tolerogenic DCs as well as immunosuppressive factors such as transforming growth factor (TGF)-β, IL-10 and indoleamine 2,3 dioxygenase (IDO), is another major limitation to the effectiveness of NK cells in AML [60, 61].
It is worth noting that expressions of NK receptors and their cognate ligands on leukemic cells as well as the signals deriving from tumor microenvironment are deemed to impact clinical outcomes and relapse in AML patients [47]. These NK cell function-related adverse prognostic parameters including hypomaturation NK cell profile (CD56bright and KIR−/CD57−), increased NKG2A and decreased NCR on NK cells, increased CD200 and decreased ULBP1 on AML cells [49, 51, 53, 62–66]. Moreover, persistence of dysfunctional NK cells was found even in patients who achieve first CR after intensive chemotherapy [67]. Thus, the presence of dysfunctional NK cells in AML and their prognostic relevance provide the rationale for the use of NK cell-based immunotherapy to restore impaired NK cell cytotoxicity against AML.
NK cell-based immunotherapy in AML
Adoptive NK cell transfer
The strategy of adoptive NK cell transfer was put forward based on beneficial effects of NK cell alloreactivity in the setting of allogeneic HCT (allo-HCT). NK cell alloreactivity is triggered by the mismatch between KIRs on donor NK cells and human leukocyte antigen (HLA) class I molecules on recipient cells, the effectiveness of which in leukemia was initially described by Perugia group [68, 69]. Alloreactions mediated by donor NK cells can kill leukemia through graft-versus-leukemia (GvL) effect, promote engraftment through ablation of recipient T cells and protect against graft-versus-host disease (GvHD) through depleting recipient antigen-presenting cells and producing IL-10 [70, 71]. Transplantation from NK alloreactive donors is considered as a strong independent factor predicting survival in allo-HCT recipients, especially from donors with more KIR B gene-content motifs [72–75]. Besides, rapid NK cell recovery post-HCT is associated with improved outcomes, while impaired NK function may be the cause of relapse [76–79]. Taken together, given the basic notions of NK cell alloreactivity and the prognostic effects of functional NK cell counts, adoptive transfer of NK cells for the management of AML has been explored in clinical applications (Fig. 2a).
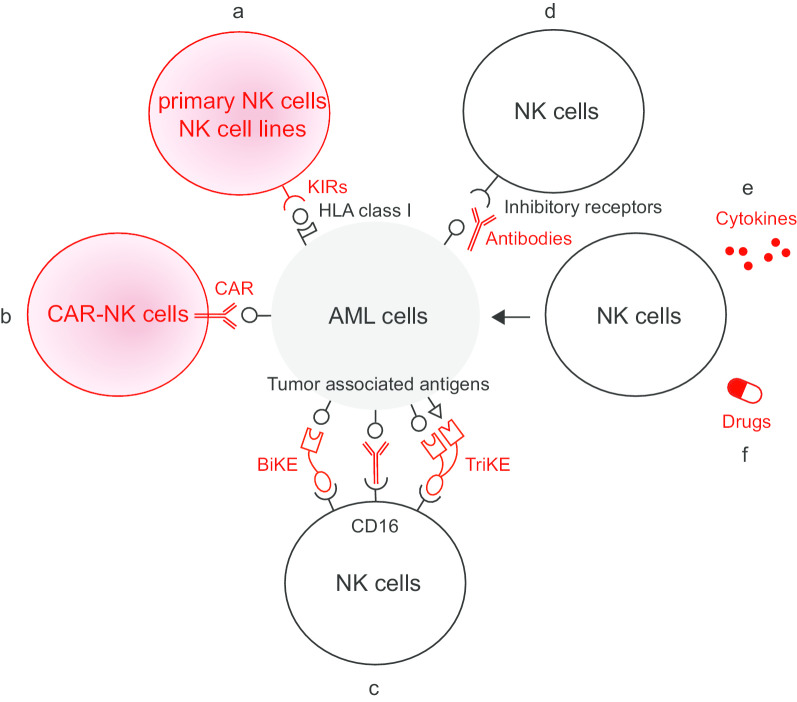
Fig. 2.
Strategies of NK cell-based immunotherapy in activating the reconstitution of NK cells against AML. a Adoptive NK cell transfer. b CAR-NK cell therapy. c Antibodies targeting tumor associated antigens, BiKE and TriKE. d Antibodies targeting NK cell inhibitory receptors. e Cytokines. f Drugs with immunomodulatory function. BiKE bi-specific killer cell engager, CAR chimeric antigen receptor, HLA human leukocyte antigen, KIR killer immunoglobulin-like receptor, TriKE tri-specific killer cell engager
Despite HCT has yielded a high rate of curability for AML, it is associated with transplant-related morbidity and mortality. Besides, not every patient is a candidate for HCT and relapse after HCT remains the most frequent cause of treatment failure. Therefore, adoptive NK cell transfer seems to be an ideal option as adjuvant and alternative treatment, and it has already been performed in the context of HCT as well as in the non-HCT setting.
Adoptive NK cell transfer in the context of HCT
Donor-derived NK cells are most commonly obtained from donor leukapheresis products using a magnetic cell sorting (MACS) system by CD3 depletion with or without CD56 enrichment [80–84]. They can also be generated by ex vivo differentiation from donor CD34 + hematopoietic progenitor cells [85]. NK cell transfer after HLA-haploidentical HCT is well tolerated and consolidates engraftment [80, 86]. Remarkably, a phase I study investigating the clinical effect of IL-15 plus IL-21 stimulated CD3-depleted NK cells given 2 and 3 weeks after HCT demonstrated that leukemia progression reduced compared with historical patients who have undergone HCT after the same conditioning regimen without NK cell infusion (hazard ratio 0.527, p = 0.042) [81]. Another phase I study showed that multiple doses of NK cells (days—2, 7 and 28 post-HCT) expanded ex vivo with K562-mbIL21-41BBL feeder cells, which were genetically modified K562 leukemia cell line expressing membrane-bound IL-21 and the 41BB ligand, could be effective in controlling leukemia relapse as well [82]. However, another study showed that compared with NK cell transfer at weeks 2 and 3 post-HCT, additional early transfer (days 6 and 9 post-HCT) was associated with significant cytokine release syndrome (CRS)-related toxicity and was not associated with less leukemia progression in patients with relapsed/refractory (R/R) AML [83]. Notably, high expression of NKp30 on donor NK cells was an independent predictor of high CR and low leukemia progression [83].
In addition, NK cells are also safe and feasible to be infused prior to HCT. A phase I study infusing escalating doses of donor-derived NK cells as a component of the preparative regimen for allo-HCT (day—8 pre-HCT) demonstrated that relapse-free survival was highly associated with the number of NK cells delivered [87]. Besides, NK cell transfer can also be applied as a bridge to HCT in R/R AML, which is useful in the reduction in disease burden to make patients eligible to proceed to HCT [84].
Adoptive NK cell transfer in the non-HCT setting
Since the limitations of HCT make it not applicable to all patients, it is conceivable to propel the development of adoptive NK cell transfer outside the transplantation setting.
Miller et al. was the first to conduct NK cell transfer in adult AML patients without prior HCT, reporting that haploidentical NK cell transfer with the intense high-dose cyclophosphamide and fludarabine immune suppression regimen, CD3 depletion and IL-2 administration both ex vivo and in vivo was a safe treatment with successful NK cell proliferation and activation in R/R AML (CR 5/19) [88]. Over the years, modifications to this approach have led to remarkable progress, ranging from donor selection according to KIR-ligand mismatch to improve outcomes, NK cell purification using CD3 depletion followed by CD56 enrichment to avoid side effects caused by residual cells, to milder conditioning regimens and lower dose of IL-2 in vivo to make it a well-tolerated regimen. Adoptive NK cell transfer is a feasible strategy for AML not only to induce remission, but also to maintain CR [89–93]. The combination of consolidation therapies of NK cell transfer and chemotherapy contributed to the further remission with decreased MRD and the reduction in long-term recurrence in AML patients at CR [94]. Though a phase II trial reported that NK cell transfer as a consolidation therapy for pediatric AML in first CR did not decrease relapse and increase overall survival (OS), the result of another just concluded phase II trial (NCT02763475) with a higher number of NK cell administration is worth the wait [95, 96].
Since the higher number of donor alloreactive NK cells correlates with better outcomes, ex vivo generation and in vivo expansion of an adequate number of donor NK cells with robust anti-leukemia potential are highly warranted [92]. In terms of ex vivo manipulating methods, Miller et al. demonstrated the superiority of CD3 and CD19 depletion method compared with CD3 depletion alone and CD3 depletion followed by CD56 enrichment methods, with no cause of negative effects by co-infused monocytes [97]. NK cell expansion and functional activity can be significantly enhanced by co-culturing donor's peripheral blood mononuclear cells (PBMC) with cytokines (mainly IL-2 and IL-15) or feeder cells bearing membrane-bound cytokines (such as K562-mbIL15-41BBL or K562-mbIL21-41BBL) [94, 98–100]. The feeder-free approach of using plasma membrane particles derived from K562-mbIL15-41BBL feeder cells resulted in great expansion of NK cells as well and avoided tumor-derived feeder cells being injected into patients [101]. Two phase I studies demonstrated NK cells primed with the lysate of CTV-1 leukemia cell line could prolong CR in high-risk AML patients [102, 103]. Despite IL-2 has the effect of stimulating NK cells, it stimulates host Treg cells in the meanwhile, which can inhibit NK cell proliferation and expansion in vivo. IL-15 was proposed as an alternative to IL-2 without such drawback [104, 105]. The first-in-human trial of using in vivo recombinant human IL-15 to potentiate haploidentical NK cell transfer in R/R AML showed better rates of NK cell expansion and remission compared with previous trials with IL-2, but CRS was observed when IL-15 was administered subcutaneously [106]. Furthermore, Miller et al. proposed a method of incorporating Treg depletion with IL-2 diphtheria toxin (IL2DT) into adoptive transfer platform. IL2DT was delivered to patients 1 or 2 days before NK cell transfer and it improved CR rate (53% versus 21%; P = 0.02) and disease-free survival (33% versus 5%; P < 0.01) for R/R AML patients [97]. It was showed that the use of IL2DT or low-dose irradiation as part of conditioning resulted in increased NK cell homing and persistence in the bone marrow, which correlated with better leukemia control [107].
Apart from quantity demands for NK cells, alternative sources for NK cells can facilitate their clinical applications as well. A phase I clinical trial evaluated the feasibility and safety of transferring activated human NK-92 cell lines to patients with R/R AML. NK-92 cells possess advantages of easy cultivation and expansion and can be repeatedly infused in the context of lymphodepletion [108]. Its derivative cell line NK-92MI without the presence of surface sialic acid-binding immunoglobulin-like lectins (siglec)-7 exhibited high and sustainable cytotoxicity against NK-92MI-resistant leukemia cells [109]. Besides, a study established the proof-of-concept of the feasibility of NK cells generated from CD34 + hematopoietic stem and progenitor cells (HSPC) isolated from cryopreserved umbilical cord blood (UCB) in a preclinical AML xenograft model [110]. The first-in-human study exploiting UCB-derived HSPC-NK cells in the treatment of elderly AML patients in morphologic CR found NK cell expansion and further maturation in vivo as well as a reduction in MRD without the induction of NK cell-related toxicity [111]. Another study evaluating placental-derived HSPC-NK cells (PNK-007) in R/R AML demonstrated an encouraging safety profile, but larger scale studies are needed to assess clinical outcomes [112]. A clinical trial investigating the feasibility of CYNK-001, the cryopreserved successor product to PNK-007, has recently been initiated (NCT04310592). Moreover, FT516, a NK cell product derived from a clonal master engineered induced pluripotent stem cell (iPSC) line, as a monotherapy for R/R AML is in clinical investigation (NCT04023071). These “off-the-shelf” products have significant benefits over primary NK cells from adult donors in the aspect of low costs, high therapeutic dosages, immediately application, choosing appropriate KIR B haplotype alloreactive donors and doing genetic modifications.
Further clinical trials are underway to evaluate the safety and efficacy of adoptive NK cell transfer, with the exploration of optimal NK cell dosages and resources, the optimal time points in relation to HCT and potential combination therapies. A list of currently ongoing clinical trials of NK cell transfer is provided in Table 1.
Table 1.
Overview of ongoing clinical trials of adoptive NK cell transfer in AML
| Identifier | Phase | Indication | In vivo cytokine | Transplantation | Outcome measure |
|---|---|---|---|---|---|
| PBMC-derived NK cell infusion | |||||
| NCT04221971 | I | R/R AML | None | No | AE, response, NK cell metabolism, migration and reconstruction, cell count recovery, relapse |
| NCT04220684 | I | R/R AML or MDS | None | No | MTD, AE, response, survival, cell count recovery, number of patients able to proceed to HCT |
| NCT04209712 | I | AML with MRD | IL-2 | No | MRD, AE |
| NCT02890758 | I | AML, MDS, et al | ALT-803 | No | AE, response, survival, in vivo NK level |
| I/II | R/R AML | None | No | MTD, response, NK cell expansion | |
| NCT03300492 | I/II | AML or MDS | None | Days + 10, + 15 and + 20 post-HCT | AE, survival, response, NK cell dose |
| NCT01823198 | I/II | High-risk AML or MDS | IL-2 | Day -8 pre-HCT | Optimal NK cell dose, survival, AE |
| NCT01904136 | I/II | AML, MDS or CML | None | Days 7 and 28–90 post-HCT | MTD, AE, survival, time to engraftment |
| NCT04395092 | II | High-risk AML or MDS | None | Days—2, + 7 and + 28 post-HCT | Relapse, AE, survival |
| NCT04166929 | II | AML or MDS | None | Day + 7 post-HCT | Relapse |
| NCT03050216 | II | R/R AML | ALT-803 | No | Response, NK cell expansion, AE |
| NCT03955848 | NA | AML in remission | IL-2 | No | Survival |
| Placental-derived HSPC-NK cell (CYNK-001) infusion | |||||
| NCT04310592 | I | AML | None | No | MTD, AE, MRD, survival |
| UCB-derived HSPC-NK cell infusion | |||||
| NCT01619761 | I | AML, MDS, et al | None | Day-2 pre-CBT | AE, survival |
| NCT04347616 | I/II | R/R AML | IL-2 | No | AE, MRD, NK cell lifespan, expansion and functional activity, plasma cytokine concentration, number of patients able to proceed to HCT |
| NCT02727803 | II | AML, MDS, et al | None | Days 30–180 post-CBT | Survival, AE |
| iPSC-derived NK cell (FT516) infusion | |||||
| NCT04023071 | I | R/R AML or B-cell lymphoma | IL-2 | No | AE, response, pharmacokinetic data |
| Cytokine-induced memory-like NK cell infusion | |||||
| NCT03068819 | I | Relapsed AML after HCT | None | No | AE, response, survival |
| NCT04024761 | I | Relapsed AML, MDS or MPN after HCT | IL-2 | No | AE, response, survival |
| NCT01898793 | I/II | R/R AML or MDS | IL-2 | No | MTD, response, AE, survival |
| NCT04354025 | II | R/R AML | IL-2 | No | Response, number of patients able to proceed to HCT, survival, MRD, AE |
| NCT02782546 | II | R/R AML | ALT-803 | Day + 7 post-HCT | Survival, response |
| CMV-induced memory-like NK cell (FATE-NK100) infusion | |||||
| NCT03081780 | I | R/R AML | IL-2 | No | MTD, response, NK cell expansion, AE, MRD, survival |
AE adverse event, AML acute myeloid leukemia, CBT cord blood transplantation, CML chronic myeloid leukemia, CMV cytomegalovirus, HCT hematopoietic cell transplantation, HSPC hematopoietic stem and progenitor cell, IL interleukin, iPSC induced pluripotent stem cell, MDS myelodysplastic syndrome, MPN myeloproliferative neoplasm, MRD minimal residual disease, MTD maximum tolerated dose, NA not applicable, NK natural killer cell, PBMC peripheral blood mononuclear cell, R/R relapsed/refractory, UCB umbilical cord blood
CAR-NK cell therapy
In adoptive NK cell transfer, the ability of NK cells to mount an immune response against AML cells is largely dependent on the interactions between NK cell activating/inhibitory receptors with their cognate ligands on target cells. In order to augment the specificity and cytotoxicity, genetically modified NK cells such as CAR-modified-NK cells are designed (Fig. 2b). Since the success of CAR-T therapy in the treatment of B-lineage acute lymphoblastic leukemia and B-cell lymphoma has not yet been translated into the treatment of AML and its wide applications are limited by adverse effects such as CRS [113, 114], NK cells with short lifespan are being considered as promising alternatives to modified T cells with favorable toxicity profiles and low manufacturing costs [115]. Nowadays, the actions of CAR-NK cells are being extensively studied in a variety of tumor models, but the applications in AML are relatively limited and mainly at the preclinical stage.
The optimal choice of leukemia specific markers that can be targeted by CAR-NK cells is a major obstacle, since AML shares some phenotypic markers with normal hematopoietic stem cells (HSCs). Myeloid differentiation antigen CD33 is detected on blasts of > 85% of AML patients and also on leukemia stem cells (LSCs) [116]. A preclinical investigation ascertained the targeting effect of NK cell line YT with gene transfer of a CD33-specific immunoglobulin-based humanized chimeric T cell receptor (cIgTCR) to CD33 + AML cell lines [117]. The first-in-man reported phase I trial of CAR-NK cells demonstrated the safety of irradiated CD33-CD28-4-1BB-CD3ζ CAR-NK-92 cells infusion in 3 patients with R/R AML, but it did not demonstrate obvious clinical efficacy [118]. Larger-scale clinical trials are warranted to determine the effects (NCT02944162). CD4 is another antigen present on AML blasts without ubiquitous expression on HSPCs and non-hematopoietic cells. Salman et al. established the role of CD4-CD28-4-1BB-CD3ζ CAR-NK-92 cells in robustly eliminating CD4 + AML cells ex vivo and in mouse xenografts [119]. CD7 is detected in approximately 30% of AML cases and also presents as an attractive target [120, 121]. CD7-CD28-4-1BB-CD3ζ CAR-NK-92MI cells have significantly improved killing efficiency against CD7 + AML cells as compared with NK-92MI cells without genetic modifications, which provides a basis for clinical investigation (NCT02742727) [122].
As for the sources of CAR-NK cells, a preclinical study showed that CD123-CAR-NK-92 cell lines represented better CAR effector cells than primary human donor CD123-CAR-NK cells in terms of cytotoxic activities [123].
The lessons learned from CAR-T and CAR-NK cells in the treatment of other cancers are worthy to be exploited in CAR-NK cell therapy in AML in the future, including optimizing targets and structures of CAR-NK cells as well as investigating the ideal patient populations for this type of immunotherapy.
Antibodies
In the normal physiologic setting, the interaction of receptors-ligands and the process of ADCC are involved in the NK cell activation. Taking advantage of this functionality, monoclonal antibodies become another method of boosting patients’ NK cells against AML. On the one hand, antibodies targeting tumor-associated antigens endow NK cells with the power of activation via ADCC effects. On the other hand, antibodies targeting NK cell inhibitory receptors have the potential to weaken inhibitory signals and let activating signals dominate the process. Great progress has been made in the field of antibody therapies, and the overview of ongoing clinical trials concerning novel antibodies for AML is presented in Table 2.
Table 2.
Overview of ongoing clinical trials of antibodies for AML
| Antibody | Target | Regimen | Indication | Phase | Identifier |
|---|---|---|---|---|---|
| Antibodies targeting tumor-associated antigens | |||||
| BI 836858 | CD33 | BI 836858 + decitabine | AML | II | NCT02632721 |
| GO | CD33 | GO + CPX-351 | Relapsed AML | I | NCT03904251 |
| GO + venetoclax | R/R CD33 + AML | I | NCT04070768 | ||
| GO + pracinostat | R/R CD33 + AML | I | NCT03848754 | ||
| GO + allo-HCT | Average-risk CD33 + AML or MDS or JMML | I | NCT01020539 | ||
| GO, midostaurin, cytarabine and daunorubicin | Newly diagnosed FLT3 mutated AML | I | NCT03900949 | ||
| GO + talazoparib | R/R CD33 + AML | I/II | NCT04207190 | ||
| GO, midostaurin, cytarabine and daunorubicin | Newly diagnosed AML | I/II | NCT04385290 | ||
| GO, PF-04518600, venetoclax, avelumab, glasdegib and azacitidine | R/R AML | I/II | NCT03390296 | ||
| GO, G-CSF, cladribine, cytarabine and mitoxantrone | Newly diagnosed AML | I/II | NCT03531918 | ||
| GO | CD33 + AML | II | NCT03737955 | ||
| GO + allo-HCT | Average-risk CD33 + AML or MDS | II | NCT02117297 | ||
| GO + azacitidine | Newly diagnosed elderly AML | II | NCT00658814 | ||
| GO + bortezomib | R/R AML | II | NCT04173585 | ||
| GO + CPX-351 | R/R CD33 + AML or high-risk MDS | II | NCT03672539 | ||
| GO + DLI | R/R AML | II | NCT03374332 | ||
| GO, mitoxantrone and etoposide | Refractory CD33 + AML | II | NCT03839446 | ||
| GO, cyclophosphamide, busulfan and allo-HCT | High-risk CD33 + AML or MDS | II | NCT02221310 | ||
| GO, fludarabine, cytarabine, filgrastim-sndz and idarubicin | Newly diagnosed AML or high-risk MDS | II | NCT00801489 | ||
| GO, daunorubicin, cytarabine and glasdegib | Newly diagnosed AML | II | NCT04168502 | ||
| GO + standard chemotherapy | Pediatric AML | II | NCT04326439 | ||
| GO + cytarabine | Newly diagnosed AML | II/III | NCT02473146 | ||
| GO + daunorubicin + cytarabine | Elderly AML | II/III | NCT02272478 | ||
| GO | Newly diagnosed AML | III | NCT04093505 | ||
| GO + standard chemotherapy | Newly diagnosed NPM1 mutated AML | III | NCT00893399 | ||
| GO + standard chemotherapy + HCT | AML | III | NCT00049517 | ||
| GO, CPX-351, gilteritinib and standard chemotherapy | Newly diagnosed AML | III | NCT04293562 | ||
| GO, liposomal daunorubicin, mitoxantrone, fludarabine, cytarabine, busulfan and cyclophosphamide | Pediatric AML | III | NCT02724163 | ||
| GO | R/R CD33 + AML | IV | NCT03727750 | ||
| Lintuzumab Ac-225 | CD33 | Lintuzumab Ac-225, cladribine, cytarabine, mitoxantrone and G-CSF | R/R CD33 + AML | I | NCT03441048 |
| Lintuzumab-Ac225 + venetoclax + spironolactone | R/R CD33 + AML | I/II | NCT03867682 | ||
| Lintuzumab-Ac225 + venetoclax + azacitidine | R/R CD33 + AML | I/II | NCT03932318 | ||
| Daratumumab | CD38 | Daratumumab | R/R AML or high-risk MDS | II | NCT03067571 |
| Daratumumab + DLI | Relapsed AML after HCT | I/II | NCT03537599 | ||
| Isatuximab | CD38 | Isatuximab + standard chemotherapy | Pediatric R/R ALL or AML | II | NCT03860844 |
| Magrolimab | CD47 | Magrolimab + atezolizumab | R/R AML | I | NCT03922477 |
| Magrolimab + azacitidine | AML or MDS | I | NCT03248479 | ||
| Magrolimab + azacitidine + venetoclax | AML | I/II | NCT04435691 | ||
| Cusatuzumab | CD70 | Cusatuzumab, azacitidine and venetoclax | AML | I | NCT04150887 |
| Cusatuzumab + azacitidine | Newly diagnosed AML or high-risk MDS | I | NCT04241549 | ||
| Cusatuzumab + azacitidine | Newly diagnosed AML or high-risk MDS | I/II | NCT03030612 | ||
| Cusatuzumab + azacitidine | Newly diagnosed AML | II | NCT04023526 | ||
| SEA-CD70 | CD70 | SEA-CD70 | AML or MDS | I | NCT04227847 |
| IMGN632 | CD123 | IMGN632 | R/R CD123 + AML or other hematologic malignancies | I/II | NCT03386513 |
| IMGN632, venetoclax and azacitidine | CD123 + AML | I/II | NCT04086264 | ||
| ASP1235 | FLT3 | ASP1235 | AML | I | NCT02864290 |
| FLYSYN | FLT3 | FLYSYN | AML | I/II | NCT02789254 |
| Atezolizumab | PD-L1 | Atezolizumab + magrolimab | R/R AML | I | NCT03922477 |
| Atezolizumab + gilteritinib | R/R FLT3 mutated AML | I/II | NCT03730012 | ||
| Atezolizumab + guadecitabine | R/R AML, CML or MDS | I/II | NCT02935361 | ||
| Avelumab | PD-L1 | Avelumab, GO, PF-04518600, venetoclax, glasdegib and azacitidine | R/R AML | I/II | NCT03390296 |
| Durvalumab | PD-L1 | Durvalumab + azacitidine | Newly diagnosed MDS or elderly AML | II | NCT02775903 |
| Antibodies targeting NK cell inhibitory receptors | |||||
| Pembrolizumab | PD-1 | Pembrolizumab | Relapsed AML or MDS after HCT | I | |
| Pembrolizumab + decitabine | AML or MDS | I | NCT03969446 | ||
| Pembrolizumab + AMG 330 | R/R AML | I | NCT04478695 | ||
| Pembrolizumab | Non-favorable risk AML | II | NCT02771197 | ||
| Pembrolizumab | Elderly AML in remission | II | NCT02708641 | ||
| Pembrolizumab + cytarabine | R/R AML | II | NCT02768792 | ||
| Pembrolizumab + azacitidine | NPM1 mutated AML | II | NCT03769532 | ||
| Pembrolizumab + azacitidine | R/R AML or newly diagnosed elderly AML | II | NCT02845297 | ||
| Pembrolizumab, azacitidine and venetoclax | Elderly newly diagnosed AML | II | NCT04284787 | ||
| Pembrolizumab, cytarabine, idarubicin, daunorubicin and HCT | Newly diagnosed AML | II | NCT04214249 | ||
| Nivolumab | PD-1 | Nivolumab | High-risk AML or MDS after HCT | I | NCT04361058 |
| Nivolumab | Relapsed AML after HCT | I | NCT01822509 | ||
| Nivolumab + ipilimumab | AML or MDS | I | NCT02846376 | ||
| Nivolumab + ipilimumab | High-risk R/R AML or MDS | I | NCT03600155 | ||
| Nivolumab, CDX-1401, poly ICLC and decitabine | AML or MDS | I | NCT03358719 | ||
| Nivolumab + azacytidine | Pediatric R/R AML | I/II | NCT03825367 | ||
| Nivolumab | AML in remission at high-risk for relapse | II | NCT02532231 | ||
| Nivolumab | AML in remission | II | NCT02275533 | ||
| Nivolumab, azacitidine and ipilimumab | AML | II | NCT02397720 | ||
| Nivolumab, azacitidine, midostaurin, decitabine and cytarabine | Elderly newly diagnosed AML or high-risk MDS | II/III | NCT03092674 | ||
| Tislelizumab | PD-1 | Tislelizumab, DNA hypomethylating agent and chemotherapy | AML | II | NCT04541277 |
| Spartalizumab | PD-1 | Spartalizumab, MBG453 and decitabine | AML or high-risk MDS | I | NCT03066648 |
| Ipilimumab | CTLA-4 | Ipilimumab | Relapsed AML after HCT | I | NCT01822509 |
| Ipilimumab + nivolumab | High-risk R/R AML or MDS | I | NCT03600155 | ||
| Ipilimumab + nivolumab | AML or MDS | I | NCT02846376 | ||
| Ipilimumab + decitabine | R/R AML or MDS | I | NCT02890329 | ||
| Ipilimumab + DLI | Relapsed AML, MDS or MPN after HCT | I | NCT03912064 | ||
| Ipilimumab, nivolumab and azacitidine | AML | II | NCT02397720 | ||
| MBG453 | TIM-3 | MBG453, HDM201 and venetoclax | AML or high-risk MDS | I | NCT03940352 |
| MBG453, spartalizumab and decitabine | AML or high-risk MDS | I | NCT03066648 | ||
| MBG453, azacitidine and venetoclax | Newly diagnosed AML | II | NCT04150029 | ||
| BiKE or TriKE | |||||
| GTB-3550 | CD16/IL-15/CD33 | GTB-3550 | CD33 + R/R AML or high-risk MDS | I/II | NCT03214666 |
ALL acute lymphoblastic leukemia, allo-HCT allogeneic hematopoietic cell transplantation, AML acute myeloid leukemia, BiKE bi-specific killer cell engager, CML chronic myeloid leukemia, CTLA-4 cytotoxic T lymphocyte-associated antigen-4, DLI donor lymphocyte infusion, FLT3 FMS-like tyrosine kinase 3, G-CSF granulocyte colony-stimulating factor, GO gemtuzumab ozogamicin, HCT hematopoietic cell transplantation, JMML juvenile myelomonocytic leukemia, MDS myelodysplastic syndrome, MPN myeloproliferative neoplasm, NPM1 nucleophosmin 1, PD-1 programmed cell death-1, PD-L1 programmed cell death ligand-1, R/R relapsed/refractory, TIM-3 T-cell immunoglobulin domain and mucin domain-3, Treg regulatory T cell, TriKE tri-specific killer cell engager
Antibodies targeting tumor-associated antigens
Antibodies targeting tumor-associated antigens are attractive means of immunotherapy for cancers, the mechanisms of which are in great part the induction of ADCC via NK cells (Fig. 2c). The outcomes of unconjugated antibodies were generally poor when used alone [124–126]. The effects could be enhanced by engineering antibodies’ Fc parts to increase affinity to CD16 or integrating with other therapies [127–129]. Preclinical studies investigating the efficacy of novel Fc-optimized antibodies targeting various potential antigens such as CD133, CD33, CD157 and IL-1 receptor accessory protein (IL1RAP) as well as new regimens of antibodies combined with NK cell transfer exhibited promising results and these strategies can be valuable to be conducted in future clinical trials [130–136]. Antibody-drug conjugates (ADCs) and antibody-radio conjugates are promising strategies to enhance the antibody potency as well, and they yield superior clinical impacts on AML patients [137–141]. Gemtuzumab ozogamicin (GO), the combination of anti-CD33 antibody with anti-neoplastic agent calicheamicin, is currently the only ADC approved by the Food and Drug Administration (FDA) for the treatment of newly diagnosed and R/R CD33 + AML [142–144]. Latest preclinical findings of more novel ADCs targeting CD33, CD37, FLT3, C-type lectin-like molecule 1 (CLL-1; also known as C-type lectin domain family 12, member A, CLEC12A) and leukocyte immunoglobulin-like receptor subfamily B4 (LILRB4) highlight their clinical potential for the treatment of AML [145–151].
In addition, ligands of NK cell inhibitory or activating receptors on AML cells can also be the targets of antibodies. It was reported that NK-resistant feature of mixed lineage leukemia (MLL)-rearranged leukemia could be overcome by anti-CD19 antibody and anti-CD33 antibody-induced ADCC, and the effects could be further amplified with pan-MHC-I antibodies, suggesting the utilization of a triple immunotherapy approach, including KIR-mismatched NK cell transfer, antibodies against tumor-associated antigens and inhibitory KIR blockade, for the treatment of MLL-rearranged leukemia [152]. The expression level of inhibitory immune checkpoint molecule PD-L1 on AML blasts is an important negative prognostic factor [153]. Hypomethylating agents, while enhancing anti-tumor immune response, can concurrently dampen immune response by upregulating PD-1 and PD-L1 expression, providing the rationale of combination therapies of PD-L1 inhibitors and hypomethylating agents [154, 155]. Other antibodies targeting TNF family members on AML cells, such as glucocorticoid-induced TNFR-related protein ligand (GITRL) and receptor activator for NF-κB ligand (RANKL), were manifested against primary AML cells in preclinical studies through the prevention of inhibitory signals into NK cells as well as the induction of ADCC [156–158]. Despite the inevitable reduction in activating signals upon antibodies binding to ligands of activating receptors, NKG2D-Fc and NKp80-Fc fusion proteins were shown to be able to compensate for it by inducing ADCC to potentiate NK cell killing of AML cells [159, 160].
Antibodies targeting NK cell inhibitory receptors
Inhibitory receptors in NK cells serve as the sources of cancer immune escape, making them ideal targets for immunotherapy (Fig. 2d). Over the past decades, the number of inhibitory receptors identified in NK cells has been increasing. Apart from MHC-I-specific inhibitory receptors KIRs, LIRs and CD94/NKG2A, other immune checkpoints on NK cells have been shown to cause dysfunction such as programmed cell death-1 (PD-1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), T-cell immunoglobulin domain and mucin domain-3 (TIM-3), T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), siglec-7/9 and CD200R [161].
Just as the benefit of KIR-ligand mismatch between donors and recipients in improving the outcome of HCT, pharmacologic KIR blockade by anti-KIR antibodies can prevent the KIR-HLA-C interaction and augment NK cell function as well. IPH2101 and IPH2102 (lirilumab) are antibodies targeting KIR2D and both were reported to be safe in the treatment of elderly patients with AML in first CR, though the leukemia-free survival with lirilumab did not compare favorably to placebo in a phase II study [162–164]. The combination of lirilumab with azacitidine also did not display significant improvement in R/R AML in terms of response rate (overall response rate, ORR 14%) or survival (median OS 4.2 months), and the relevant clinical trial (NCT02399917) was terminated early due to unsatisfactory results [165]. LIR-1 or NKG2A blockade resulted in increased NK cell cytotoxicity against AML, suggesting that the cocktail consisting of anti-KIR, anti-LIR-1 and anti-NKG2A antibodies may be a necessary option for better efficacy [166, 167]. Anti-PD-1 antibody (nivolumab and pembrolizumab) and anti-CTLA4 antibody (ipilimumab) are FDA-approved immune checkpoint inhibitors mainly for the treatment of various solid tumors, while their applications in the field of AML are still at the exploratory stage. Nivolumab in combination with idarubicin and cytarabine produced an encouraging response rate (ORR 80%) and OS (median OS 18.5 months) in patients with newly diagnosed AML [168]. The combination therapy of nivolumab and azacitidine was feasible in patients with R/R AML, and the addition of ipilimumab further upregulated the clinical efficacy (ORR 33% vs 44%; median OS 6.4 vs 10.5 months) [169, 170]. And nivolumab maintenance was safe and feasible in high-risk AML patients in CR (1-year CR duration 71%; 1-year OS 86%) [171]. The outcomes of pembrolizumab administered in combined with decitabine or following high-dose cytarabine in R/R AML (ORR 10% and 46%; median OS 7 and 8.9 months, respectively) suggested that immune checkpoint inhibitors after intensive cytotoxic chemotherapy may be a better option [172, 173]. A phase I/Ib study demonstrated the safety and efficacy of ipilimumab monotherapy in AML patients with post-HCT relapse (ORR 32%; 1-year OS 49%) [174]. As for anti-TIM-3 antibody MBG453, the combination therapy with decitabine was safe and well-tolerated and exhibited encouraging preliminary response rates for AML in a phase Ib study (ORR 29% for both newly diagnosed and R/R AML) [175]. However, caution should be paid to checkpoint inhibitors, since exposure can lead to a significantly increased risk of GvHD [168, 174, 176, 177]. Furthermore, the prognostic effect of TIGIT in the bone marrow post-HCT as well as the involvement of CD137-CD137L and CD200-CD200R interactions in immune evasion raise the possibility of attacking other inhibitory receptors with antibodies as potent immunotherapeutic strategies in the near future [53, 178–180].
BiKE and TriKE
Bi-specific killer cell engager (BiKE) and tri-specific killer cell engager (TriKE) are the recombinant agents of bivalent and trivalent single-chain variable fragments (scFv), serving as immunologic synapses between NK cells and tumor cells. They retain the specificity of original antibodies and, at the same time, minimize the size of antibodies to increase distribution. CD16-directed BiKE and TriKE trigger NK cell activation through CD16 signaling and against tumor cells with target antigens in a highly efficient manner (Fig. 2c) [181].
Wiernik et al. designed a novel full humanized BiKE that specifically binds to both CD16 and CD33 (CD16 × 33 BiKE). NK cell cytotoxicity and cytokine release were specifically triggered by CD16 × 33 BiKE when cultured with CD33 + AML cell lines and primary AML cells, and the effector functions of NK cells were further enhanced when combined with adisintegrin and metalloprotease-17 (ADAM17) inhibitor which prevents CD16 shedding [182]. Lately, the same research group designed a TriKE by incorporating a novel modified human IL-15 crosslinker into CD16 × 33 BiKE, which provided a signal for NK cell self-sustaining proliferation and activation [183]. A phase I/II clinical trial of CD16 × 33 × IL-15 TriKE (GTB-3550) for the treatment of CD33 + R/R AML is underway (NCT03214666). TriKEs of linking anti-CD16 scFv to either two scFv against the same antigen (such as CD16 × 33 × 33 TriKE) or two scFv against two different antigens (such as CD16 × 33 × 123 TriKE) displayed greater binding affinity and superior NK cell cytotoxic potency toward AML cells compared to BiKE [184, 185]. Since CD33 is abundantly expressed on healthy myeloid cells as well, NKG2DLs, which are leukemia cell-restricted expressed, become promising targets. CD16 × NKG2D BiKE displayed increased affinity to CD16 and induced superior leukemia cell killing compared to the engineered NKG2D-Fc fusion protein [186]. Besides, CD16 × CLL-1 × IL-15 TriKE displayed robust NK cell activity against AML in vitro and in vivo [187]. These molecules constitute attractive candidates for personalized immunotherapy for AML based on preclinical findings.
Cytokines
Cytokines, including IL-2, IL-12, IL-15, IL-18 and IL-21, play an important role in NK cell proliferation, activation and effector function (Fig. 2e). Ex vivo stimulation with 10 ng/mL IL-2 or 50 ng/mL IL-15 was reported to be optimal for NK cell expansion and enable NK cells of AML patients with recovered function through upregulating activating receptors such as NKp30, NKp46, NKG2C and NKG2D [188–190]. IL-2 monotherapy may not be clinically efficacious in AML patients [191–194]. But, IL-2 in conjunction with histamine dihydrochloride has been proposed as a maintenance therapy in AML, resulting in improved leukemia-free survival [195, 196]. The mechanism of this therapy may partially be the induction of a striking expansion of immunocompetent CD56bright NK cell subpopulations [197]. A phase I study identified IL-15 superagonist complex ALT-803 as a safe agent in the treatment of elderly AML patients who relapsed after HCT and the potential efficacy is expected to be reported (NCT01885897) [198]. And the feasibility of using ALT-803 as an relapse prophylaxis for AML patients after HCT is under assessment (NCT02989844). Furthermore, genetically engineered AML cells with DNA encoding IL-12 or IL-15 have been constructed to reduce toxicities associated with systemic administration of cytokines [199, 200]. A clinical trial (NCT02483312) is ongoing to test engineered AML cells expressing IL-12 in AML patients that cannot have HCT.
Cytokines have also been widely incorporated in the NK cell transfer as a process of ‘priming or arming’ in order to increase NK cell proliferation and expansion. However, the effect is short-lasting and the short-term NK cell persistence within patients might limit their clinical use. Remarkably, NK cells preactivated with a cocktail of cytokines (IL-12, IL-15 and IL-18) exhibited augmented anti-leukemia responses to restimulation for weeks to months regardless of inhibitory KIR-KIR ligand interactions [201–203]. Those cytokine-induced memory-like (CIML) NK cells with adaptive immune properties represent a promising approach to enhancing adoptive NK cell transfer. The first-in-human trial of adoptive transfer of CIML NK cells in elderly patients with R/R AML showed successful induction of remission (ORR 67%) without the cause of CRS, GvHD or neurotoxicity [204, 205]. Patient outcomes were negatively associated with the frequency of CD8α + donor NK cells and the expression of NKG2A on CIML NK cells within patients [205]. Encouraging preliminary data give us confidence on more ongoing early phase clinical trials of CIML NK cells for R/R AML (NCT04354025, NCT02782546, NCT01898793, NCT03068819) [206, 207].
Drugs with immunomodulatory function
Many anti-tumor drugs have been illustrated with immunomodulatory properties to enhance endogenous NK cell function against AML in recent years (Fig. 2f). Since AML cells resist to NK cell-mediated killing by changing the expression of their surface ligands for various NK cell receptors and these phenotypic defects correlate with clinical outcomes, drugs that are capable of restoring ligand expressions on AML cells can render them more susceptible to NK cell killing [64].
Firstly, hypomethylating agents azacitidine and decitabine can upregulate the expression of NKG2DL on AML cells by reversing epigenetically silenced genes, resulting in enhanced NK cell-mediated immunity through the immune recognition mediated by NKG2D-NKG2DL engagement [208]. They concurrently increase the expression of tissue inhibitor of metalloproteinases-3 (TIMP3), an ADAM17 inhibitor, thus reducing the shedding of soluble NKG2DLs from AML cells [209]. Histone deacetylase inhibitors (trichostatin A and valproic acid), differentiation-promoting drugs (vitamin D3, bryostatin 1 and all-trans-retinoic acid) and hydroxyurea all somehow show the potential of upregulating the expression of NKG2DLs on AML cells, while dinaciclib-treated AML is associated with the downregulation of inhibitory NK ligand HLA-E on AML cells, consequently inducing potent NK cell anti-tumor immunity [208, 210–213]. Then, immunomodulatory drugs lenalidomide and pomalidomide exert anti-leukemia effects both directly and via NK cell-mediated immunostimulatory activities along with downregulation of HLA-class I on AML blasts [214]. The combination therapies containing the aforementioned drugs for AML are widely used in clinical practice and also in clinical trials. Besides, natural compounds or their derivatives such as safrole, α-phellandrene, casticin and ouabain can also promote NK cell activity against AML cells [215–218]. In addition, novel agents with immunomodulatory function were proposed in fundamental researches, providing therapeutic implications in AML. For instance, vascular endothelial growth factor receptor (VEGFR)-3 antagonist restored NK cell cytotoxicity with an increased IFN-γ level [219, 220], and the therapeutic efficacy of adoptive NK cell transfer could be enhanced by a TGF-β receptor kinase inhibitor galunisertib [221]. With the clarification of mechanisms of anti-tumor drugs, combining pharmacological approaches with other NK cell-based immunotherapies may strengthen the efficacy and provide a clinical benefit for AML patients.
Conclusions and perspectives
Results from current preclinical studies and clinical trials highlight the significant contribution of numerous NK cell-based immunotherapies in activating the reconstitution of NK cells against AML. Adoptive NK cell transfer has expanded the option of cellular immunotherapy as a feasible strategy to induce and maintain remission. Strategies of manipulating adoptively transferred NK cells, such as CAR modification and cytokine induction, may further enhance the therapeutic efficacy. Other strategies, such as immune checkpoint inhibitors, BiKE/TriKE and immunomodulatory drugs, can reverse endogenous NK cell anergy, contributing to an increasing dimensions of utilizing NK cells to fight AML.
There are several advantages in NK cell-based immunotherapy. Firstly, NK cells detect tumor cells through native receptors in a non-MHC-restricted manner and also mediate ADCC, expanding their clinical applications. Secondly, as compared with T-cell therapy, NK-cell therapy has better safety profiles with rare instances of GvHD and CRS due to limited lifespan and distinct cytokines produced [71]. Thirdly, NK cells have the advantage of “off-the-shelf” manufacturing, making it easy to be prepared under good manufacturing practice standards and convenient to universally treat patients with increased speed of administration [222–225]. However, the field of NK cell-based immunotherapy still faces several challenges. In fact, short lifespan of NK cells narrows the therapeutic window, leading to a relatively short duration of response in most patients [88, 90, 95, 226]. Besides, tumors can escape from NK cell cytotoxicity via immunosuppressive tumor microenvironment or by shedding soluble ligands that activate NK receptors [54, 60]. Finally, transduction efficiency of CAR-NK cells is another aspect needed to be improved [227].
In the future, the efficacy of NK cell-based immunotherapy is waiting to be confirmed in large sample sizes and in great detail. The optimal dosage and schedule of adoptive NK cell transfer as well as the feasible sources and manipulation methods for NK cells have yet to be evaluated [228]. It seems logical to combine various NK cell-based immunotherapies to utilize the full potential of NK cells, such as stimulating both target-specific lysis and ADCC effects as well as simultaneously boosting endogenous NK cells and receiving exogenous NK cells [131, 135, 136, 229, 230]. Also, it is reasonable to integrate them with well-established AML treatments or novel agents which may provide synergistic effects and improve clinical response [94]. As for preclinical researches, a better knowledge of the mechanisms of NK cell dysfunction and NK cell-based immunotherapy in AML could broaden the application of NK cells and help the discovery of additional new therapeutic opportunities, including new targets and potential combination therapies. Strategies of wisely using cytokines, such as CMIL NK cells and the transduction of genes encoding cytokines into NK cells, seem to prolong the duration of NK cell persistence in some degree, but more efforts are warranted to figure out approaches to enhance tumor-immune surveillance long term [17, 183, 206, 231]. Taking advantage of multi-omics and information technology, investigation of both donor NK cell-intrinsic and host factors which may contribute to treatment response or resistance can provide an array of biomarkers in donor and patient selection. Overall, there is a bright future in NK cell-based immunotherapy for AML.
Acknowledgements
Not applicable.
Abbreviations
- ADAM17
A disintegrin and metalloprotease-17
- ADC
Antibody-drug conjugate
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- AE
Adverse event
- ALL
Acute lymphoblastic leukemia
- allo-HCT
Allogeneic hematopoietic cell transplantation
- AML
Acute myeloid leukemia
- BiKE
Bi-specific killer cell engager
- CAR
Chimeric antigen receptor
- CBT
Cord blood transplantation
- CD
Cluster of differentiation
- cIgTCR
Immunoglobulin-based chimeric T cell receptor
- CIML
Cytokine-induced memory-like
- CML
Chronic myeloid leukemia
- CMV
Cytomegalovirus
- CR
Complete remission
- CRS
Cytokine release syndrome
- CTLA-4
Cytotoxic T lymphocyte-associated antigen-4
- CLEC12A
C-type lectin domain family 12, member A
- DC
Dendritic cell
- DLI
Donor lymphocyte infusion
- CLL-1
C-type lectin-like molecule 1
- DNAM-1
DNAX accessory molecule-1
- FDA
Food and Drug Administration
- FLT3
FMS-like tyrosine kinase 3
- G-CSF
Granulocyte colony-stimulating factor
- GITR
Glucocorticoid-induced TNFR-related protein
- GO
Gemtuzumab ozogamicin
- GvHD
Graft-versus-host disease
- GvL
Graft-versus-leukemia
- HCT
Hematopoietic cell transplantation
- HLA
Human leukocyte antigen
- HSC
Hematopoietic stem cell
- HSPC
Hematopoietic stem and progenitor cell
- IDO
Indoleamine 2,3 dioxygenase
- IFN
Interferon
- IL
Interleukin
- IL1RAP
IL-1 receptor accessory protein
- IL2DT
IL-2 diphtheria toxin
- iPSC
Induced pluripotent stem cell
- JMML
Juvenile myelomonocytic leukemia
- KIR
Killer immunoglobulin-like receptor
- LILRB4
Leukocyte immunoglobulin-like receptor subfamily B4
- LIR
Leukocyte immunoglobulin-like receptor
- LSC
Leukemia stem cell
- MACS
Magnetic cell sorting
- MDS
Myelodysplastic syndrome
- MDSC
Myeloid-derived suppressor cell
- MHC
Major histocompatibility complex
- MIC
MHC class I chain-related protein
- MLL
Mixed lineage leukemia
- MPN
Myeloproliferative neoplasm
- MRD
Minimal residual disease
- MTD
Maximum tolerated dose
- NA
Not applicable
- NCR
Natural cytotoxicity receptor
- NK
Natural killer cell
- NKG2A
Natural killer group 2A
- NKG2C
Natural killer group 2C
- NKG2D
Natural killer group 2D
- NKG2DL
NKG2D ligand
- NPM1
Nucleophosmin 1
- ORR
Overall response rate
- PBMC
Peripheral blood mononuclear cell
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand-1
- PD-L2
Programmed cell death ligand-2
- RANKL
Receptor activator for NF-κB ligand
- R/R
Relapsed/refractory
- scFv
Single chain variable fragment
- Siglec
Sialic acid-binding immunoglobulin-like lectin
- TAM
Tumor-associated macrophage
- TGF
Transforming growth factor
- TIGIT
T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain
- TIM-3
T-cell immunoglobulin domain and mucin domain-3
- TIMP3
Tissue inhibitor of metalloproteinases-3
- TNF
Tumor necrosis factor
- TNFR
Tumor necrosis factor receptor
- Treg
Regulatory T cell
- TriKE
Tri-specific killer cell engager
- UCB
Umbilical cord blood
- ULBP
UL16-binding protein
- VEGFR
Vascular endothelial growth factor receptor
Authors’ contributions
JX wrote the manuscript and prepared the tables and figures. TN critically reviewed and edited the manuscript. Both authors read and approved the final manuscript.
Funding
No funding was provided for this review.
Availability of data and materials
The material supporting the information of this review has been included within the article.
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J Hematol Oncol. 2019;12(1):100. doi: 10.1186/s13045-019-0774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum WG, Mims AS. Treating acute myeloid leukemia in the modern era: a primer. Cancer. 2020;126:4668–4677. doi: 10.1002/cncr.32904. [DOI] [PubMed] [Google Scholar]
- 3.Ball B, Stein EM. Which are the most promising targets for minimal residual disease-directed therapy in acute myeloid leukemia prior to allogeneic stem cell transplant? Haematologica. 2019;104(8):1521–1531. doi: 10.3324/haematol.2018.208587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenegger FS, Krupka C, Haubner S, Köhnke T, Subklewe M. Recent developments in immunotherapy of acute myeloid leukemia. J Hematol Oncol. 2017;10(1):142. doi: 10.1186/s13045-017-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14(1):1–18. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21(4):687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathyanarayanan V, Neelapu SS. Cancer immunotherapy: strategies for personalization and combinatorial approaches. Mol Oncol. 2015;9(10):2043–2053. doi: 10.1016/j.molonc.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasekar M, Rizvi S, Liu X, Vrana KE, Zheng H. Novel immunotherapies for hematological malignancies. Curr Mol Pharmacol. 2016;9(3):264–271. doi: 10.2174/1874467208666150716121253. [DOI] [PubMed] [Google Scholar]
- 9.Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: past, present, and future. J Hematol Oncol. 2017;10(1):94. doi: 10.1186/s13045-017-0453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. 2017;31:37–54. doi: 10.1016/j.smim.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valipour B, Abedelahi A, Naderali E, Velaei K, Movassaghpour A, Talebi M, Montazersaheb S, Karimipour M, Darabi M, Chavoshi H. Cord blood stem cell derived CD16+ NK cells eradicated acute lymphoblastic leukemia cells using with anti-CD47 antibody. Life Sci. 2020;242:117223. doi: 10.1016/j.lfs.2019.117223. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka J, Tanaka N, Wang Y-H, Mitsuhashi K, Ryuzaki M, Iizuka Y, Watanabe A, Ishiyama M, Shinohara A, Kazama H. Phase I study of cellular therapy using ex vivo expanded natural killer cells from autologous peripheral blood mononuclear cells combined with rituximab-containing chemotherapy for relapsed CD20-positive malignant lymphoma patients. Haematologica. 2020;105(4):e190. doi: 10.3324/haematol.2019.226696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachanova V, Sarhan D, DeFor TE, Cooley S, Panoskaltsis-Mortari A, Blazar BR, Curtsinger JM, Burns L, Weisdorf DJ, Miller JS. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother. 2018;67(3):483–494. doi: 10.1007/s00262-017-2100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H, Blum RH, Bjordahl R, Gaidarova S, Rogers P, Lee TT, Abujarour R, Bonello GB, Wu J, Tsai P-F. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135(6):399–410. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oelsner S, Waldmann A, Billmeier A, Röder J, Lindner A, Ullrich E, Marschalek R, Dotti G, Jung G, Große-Hovest L. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive B-ALL and inhibit in vivo leukemia growth. Int J Cancer. 2019;145(7):1935–1945. doi: 10.1002/ijc.32269. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Liu Q, Zhong M, Wang Z, Chen Z, Zhang Y, Xing H, Tian Z, Tang K, Liao X. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol. 2019;12(1):49. doi: 10.1186/s13045-019-0732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You F, Wang Y, Jiang L, Zhu X, Chen D, Yuan L, An G, Meng H, Yang L. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am J Cancer Res. 2019;9(1):64–78. [PMC free article] [PubMed] [Google Scholar]
- 21.Daher M, Basar R, Gokdemir E, Baran N, Uprety N, Nunez Cortes AK, Mendt M, Kerbauy LN, Banerjee PP, Hernandez Sanabria M. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood. 2020 doi: 10.1182/blood.2020007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gang M, Marin ND, Wong P, Neal CC, Marsala L, Foster M, Schappe T, Meng W, Tran J, Schaettler M. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood. 2020;136:2308–2318. doi: 10.1182/blood.2020006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmied BJ, Lutz MS, Riegg F, Zekri L, Heitmann JS, Bühring H-J, Jung G, Salih HR. Induction of NK cell reactivity against B-cell acute lymphoblastic leukemia by an Fc-optimized FLT3 antibody. Cancers. 2019;11(12):1966. doi: 10.3390/cancers11121966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyas M, Schneider A-C, Shatnyeva O, Reiners KS, Tawadros S, Kloess S, Köhl U, Hallek M, Hansen HP, Pogge Von trandmann E. Mono-and dual-targeting triplebodies activate natural killer cells and have anti-tumor activity in vitro and in vivo against chronic lymphocytic leukemia. Oncoimmunology. 2016;5(9):e1211220. doi: 10.1080/2162402X.2016.1211220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst D, Williams BA, Wang X-H, Yoon N, Kim K-P, Chiu J, Luo ZJ, Hermans KG, Krueger J, Keating A. Humanized anti-CD123 antibody facilitates NK cell antibody-dependent cell-mediated cytotoxicity (ADCC) of Hodgkin lymphoma targets via ARF6/PLD-1. Blood Cancer J. 2019;9(2):1–11. doi: 10.1038/s41408-018-0168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta U, Hira SK, Singh R, Paladhi A, Srivastava P, Manna PP. Essential role of TNF-α in gamma c cytokine aided crosstalk between dendritic cells and natural killer cells in experimental murine lymphoma. Int Immunopharmacol. 2020;78:106031. doi: 10.1016/j.intimp.2019.106031. [DOI] [PubMed] [Google Scholar]
- 27.Hemati M, Nejad ZR, Shokri M-R, Ghahremanfard F, Mohammadkhani MM, Kokhaei P. IL-27 impact on NK cells activity: implication for a robust anti-tumor response in chronic lymphocytic leukemia. Int Immunopharmacol. 2020;82:106350. doi: 10.1016/j.intimp.2020.106350. [DOI] [PubMed] [Google Scholar]
- 28.Villa-Álvarez M, Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, Payer AR, Gonzalez-Garcia E, Villa-Álvarez MC, López-Soto A, Gonzalez S. Ig-like transcript 2 (ILT2) blockade and lenalidomide restore NK cell function in chronic lymphocytic leukemia. Front Immunol. 2018;9:2917. doi: 10.3389/fimmu.2018.02917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang M-C, Cheng H-I, Hsu K, Hsu Y-N, Kao C-W, Chang Y-F, Lim K-H, Chen CG. NKG2A down-regulation by dasatinib enhances natural killer cytotoxicity and accelerates effective treatment responses in patients with chronic myeloid leukemia. Front Immunol. 2019;9:3152. doi: 10.3389/fimmu.2018.03152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizoguchi I, Yoshimoto T, Katagiri S, Mizuguchi J, Tauchi T, Kimura Y, Inokuchi K, Ohyashiki JH, Ohyashiki K. Sustained upregulation of effector natural killer cells in chronic myeloid leukemia after discontinuation of imatinib. Cancer Sci. 2013;104(9):1146–1153. doi: 10.1111/cas.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cucè M, Cantafio MEG, Siciliano MA, Riillo C, Caracciolo D, Scionti F, Staropoli N, Zuccalà V, Maltese L, Di Vito A. Trabectedin triggers direct and NK-mediated cytotoxicity in multiple myeloma. J Hematol Oncol. 2019;12(1):32. doi: 10.1186/s13045-019-0714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha C, Cunningham LC. An overview of the potential strategies for NK cell-based immunotherapy for acute myeloid leukemia. Pediatr Blood Cancer. 2016;63(12):2078–2085. doi: 10.1002/pbc.26171. [DOI] [PubMed] [Google Scholar]
- 33.Baragano Raneros A, López-Larrea C, Suárez-Álvarez B. Acute myeloid leukemia and NK cells: two warriors confront each other. Oncoimmunology. 2019;8(2):e1539617. doi: 10.1080/2162402X.2018.1539617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 35.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 36.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 37.Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013;132(3):536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Handgretinger R, Lang P, André MC. Exploitation of natural killer cells for the treatment of acute leukemia. Blood. 2016;127(26):3341–3349. doi: 10.1182/blood-2015-12-629055. [DOI] [PubMed] [Google Scholar]
- 39.Pende D, Falco M, Vitale M, Cantoni C, Vitale C, Munari E, Bertaina A, Moretta F, Del Zotto G, Pietra G. Killer Ig-like receptors (KIRs): their role in NK cell modulation and developments leading to their clinical exploitation. Front Immunol. 2019;10:1179. doi: 10.3389/fimmu.2019.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ljunggren H-G, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song Y-J, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 42.Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42(4):501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Gómez Román VR, Murray JC, Weiner LM. Chapter 1-Antibody-dependent cellular cytotoxicity (ADCC). Antibody Fc 2014:1–27.
- 45.Thomas R, Yang X. NK-DC crosstalk in immunity to microbial infection. J Immunol Res. 2016;2016:6374379. doi: 10.1155/2016/6374379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Z, Zhang C, Zhang J, Tian Z. Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa-1. PLoS ONE. 2012;7(5):e36928. doi: 10.1371/journal.pone.0036928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lion E, Willemen Y, Berneman Z, Van Tendeloo V, Smits E. Natural killer cell immune escape in acute myeloid leukemia. Leukemia. 2012;26(9):2019–2026. doi: 10.1038/leu.2012.87. [DOI] [PubMed] [Google Scholar]
- 48.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci M-J, Reviron D, Gastaut J-A, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell–triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99(10):3661–3667. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 49.Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood. 2007;109(1):323–330. doi: 10.1182/blood-2005-08-027979. [DOI] [PubMed] [Google Scholar]
- 50.Sandoval-Borrego D, Moreno-Lafont MC, Vazquez-Sanchez EA, Gutierrez-Hoya A, López-Santiago R, Montiel-Cervantes LA, Ramírez-Saldaña M, Vela-Ojeda J. Overexpression of CD158 and NKG2A inhibitory receptors and underexpression of NKG2D and NKp46 activating receptors on NK cells in acute myeloid leukemia. Arch Med Res. 2016;47(1):55–64. doi: 10.1016/j.arcmed.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, Pavlu J, Brisley G, de Lavallade H, Sarvaria A. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014;99(5):836–847. doi: 10.3324/haematol.2013.087536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, Tarazona R. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol. 2012;90(1):109–115. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 53.Coles S, Wang ECY, Man S, Hills RK, Burnett AK, Tonks A, Darley RL. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25(5):792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hilpert J, Grosse-Hovest L, Grünebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol. 2012;189(3):1360–1371. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 55.Nowbakht P, Ionescu MCS, Rohner A, Kalberer CP, Rossy E, Mori L, Cosman D, De Libero G, Wodnar-Filipowicz A. Ligands for natural killer cell–activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105(9):3615–3622. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 56.Kearney CJ, Ramsbottom KM, Voskoboinik I, Darcy PK, Oliaro J. Loss of DNAM-1 ligand expression by acute myeloid leukemia cells renders them resistant to NK cell killing. Oncoimmunology. 2016;5(8):e1196308. doi: 10.1080/2162402X.2016.1196308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baragaño Raneros A, Martín-Palanco V, Fernandez AF, Rodriguez RM, Fraga MF, Lopez-Larrea C, Suarez-Alvarez B. Methylation of NKG2D ligands contributes to immune system evasion in acute myeloid leukemia. Genes Immun. 2015;16(1):71–82. doi: 10.1038/gene.2014.58. [DOI] [PubMed] [Google Scholar]
- 58.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102(4):1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 59.Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng Q-R, Fang Z, Nguyen M, Pierce S, Wei Y. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280–1288. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol. 2014;44(6):1582–1592. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- 61.Curran EK, Godfrey J, Kline J. Mechanisms of immune tolerance in leukemia and lymphoma. Trends Immunol. 2017;38(7):513–525. doi: 10.1016/j.it.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tajima F, Kawatani T, Endo A, Kawasaki H. Natural killer cell activity and cytokine production as prognostic factors in adult acute leukemia. Leukemia. 1996;10(3):478. [PubMed] [Google Scholar]
- 63.Chretien A-S, Fauriat C, Orlanducci F, Galseran C, Rey J, Bouvier Borg G, Gautherot E, Granjeaud S, Hamel-Broza J-F, Demerle C. Natural killer defective maturation is associated with adverse clinical outcome in patients with acute myeloid leukemia. Front Immunol. 2017;8:573. doi: 10.3389/fimmu.2017.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mastaglio S, Wong E, Perera T, Ripley J, Blombery P, Smyth MJ, Koldej R, Ritchie D. Natural killer receptor ligand expression on acute myeloid leukemia impacts survival and relapse after chemotherapy. Blood Adv. 2018;2(4):335–346. doi: 10.1182/bloodadvances.2017015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martner A, Rydström A, Riise RE, Aurelius J, Anderson H, Brune M, Foà R, Hellstrand K, Thorén FB, Re: Mission Study G Role of natural killer cell subsets and natural cytotoxicity receptors for the outcome of immunotherapy in acute myeloid leukemia. Oncoimmunology. 2016;5(1):e1041701. doi: 10.1080/2162402X.2015.1041701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martner A, Rydström A, Riise RE, Aurelius J, Brune M, Foà R, Hellstrand K, Thorén FB. NK cell expression of natural cytotoxicity receptors may determine relapse risk in older AML patients undergoing immunotherapy for remission maintenance. Oncotarget. 2015;6(40):42569–42574. doi: 10.18632/oncotarget.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dauguet N, Récher C, Demur C, Fournié JJ, Poupot M, Poupot R. Pre-eminence and persistence of immature natural killer cells in acute myeloid leukemia patients in first complete remission. Am J Hematol. 2011;86(2):209–213. doi: 10.1002/ajh.21906. [DOI] [PubMed] [Google Scholar]
- 68.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–339. doi: 10.1182/blood.V94.1.333.413a31_333_339. [DOI] [PubMed] [Google Scholar]
- 69.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 70.Ruggeri L, Mancusi A, Perruccio K, Burchielli E, Martelli MF, Velardi A. Natural killer cell alloreactivity for leukemia therapy. J Immunother. 2005;28(3):175–182. doi: 10.1097/01.cji.0000161395.88959.1f. [DOI] [PubMed] [Google Scholar]
- 71.Chan YLT, Zuo J, Inman C, Croft W, Begum J, Croudace J, Kinsella F, Maggs L, Nagra S, Nunnick J. NK cells produce high levels of IL-10 early after allogeneic stem cell transplantation and suppress development of acute GVHD. Eur J Immunol. 2018;48(2):316–329. doi: 10.1002/eji.201747134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, Marsh SG, Geraghty D, Spellman S, Haagenson MD, Ladner M, Trachtenberg E, Parham P, Miller JS. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stringaris K, Adams S, Uribe M, Eniafe R, Wu CO, Savani BN, Barrett AJ. Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010;16(9):1257–1264. doi: 10.1016/j.bbmt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, Guethlein LA, Trachtenberg EA, Haagenson M, Horowitz MM. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, Zeilah J, Kurlander R, Srinivasan R, Childs R, Hensel N, Barrett AJ. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21(10):2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 77.Pittari G, Fregni G, Roguet L, Garcia A, Vataire A, Wittnebel S, Amsellem S, Chouaib S, Bourhis J, Caignard A. Early evaluation of natural killer activity in post-transplant acute myeloid leukemia patients. Bone Marrow Transplant. 2010;45(5):862–871. doi: 10.1038/bmt.2009.265. [DOI] [PubMed] [Google Scholar]
- 78.Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, Brunstein CG, Blazar BR, Wagner J, Diamond DJ. CD56 dim CD57+ NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2016;30(2):456–463. doi: 10.1038/leu.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michelis FV, Messner HA, Loach D, Uhm J, Gupta V, Lipton JH, Seftel MD, Kuruvilla J, Kim DD. Early lymphocyte recovery at 28 d post-transplant is predictive of reduced risk of relapse in patients with acute myeloid leukemia transplanted with peripheral blood stem cell grafts. Eur J Haematol. 2014;93(4):273–280. doi: 10.1111/ejh.12338. [DOI] [PubMed] [Google Scholar]
- 80.Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kühne T, Favre G, Gratwohl A. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18(11):1835–1838. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 81.Choi I, Yoon SR, Park S-Y, Kim H, Jung S-J, Jang YJ, Kang M, Yeom YI, Lee J-L, Kim D-Y. Donor-derived natural killer cells infused after human leukocyte antigen–haploidentical hematopoietic cell transplantation: a dose-escalation study. Biol Blood Marrow Transplant. 2014;20(5):696–704. doi: 10.1016/j.bbmt.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 82.Ciurea SO, Schafer JR, Bassett R, Denman CJ, Cao K, Willis D, Rondon G, Chen J, Soebbing D, Kaur I, Gulbis A, Ahmed S, Rezvani K, Shpall EJ, Lee DA, Champlin RE. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130(16):1857–1868. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi I, Yoon SR, Park S-Y, Kim H, Jung S-J, Kang Y-L, Lee J-H, Lee J-H, Kim D-Y, Lee J-L. Donor-derived natural killer cell infusion after human leukocyte antigen-haploidentical hematopoietic cell transplantation in patients with refractory acute leukemia. Biol Blood Marrow Transplant. 2016;22(11):2065–2076. doi: 10.1016/j.bbmt.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Rubnitz JE, Inaba H, Kang G, Gan K, Hartford C, Triplett BM, Dallas M, Shook D, Gruber T, Pui CH, Leung W. Natural killer cell therapy in children with relapsed leukemia. Pediatr Blood Cancer. 2015;62(8):1468–1472. doi: 10.1002/pbc.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoon SR, Lee Y, Yang S, Ahn K, Lee J-H, Lee J-H, Kim D, Kang Y, Jeon M, Seol M. Generation of donor natural killer cells from CD34+ progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant. 2010;45(6):1038–1046. doi: 10.1038/bmt.2009.304. [DOI] [PubMed] [Google Scholar]
- 86.Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, Paulussen M, Gratwohl A, Klingebiel T, Bader P. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant. 2013;48(3):433–438. doi: 10.1038/bmt.2012.162. [DOI] [PubMed] [Google Scholar]
- 87.Lee DA, Denman CJ, Rondon G, Woodworth G, Chen J, Fisher T, Kaur I, Fernandez-Vina M, Cao K, Ciurea S. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol Blood Marrow Transplant. 2016;22(7):1290–1298. doi: 10.1016/j.bbmt.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 89.Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118(12):3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 90.Björklund AT, Carlsten M, Sohlberg E, Liu LL, Clancy T, Karimi M, Cooley S, Miller JS, Klimkowska M, Schaffer M. Complete remission with reduction of high-risk clones following haploidentical NK-cell therapy against MDS and AML. Clin Cancer Res. 2018;24(8):1834–1844. doi: 10.1158/1078-0432.CCR-17-3196. [DOI] [PubMed] [Google Scholar]
- 91.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui C-H, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Curti A, Ruggeri L, Parisi S, Bontadini A, Dan E, Motta MR, Rizzi S, Trabanelli S, Ocadlikova D, Lecciso M. Larger size of donor alloreactive NK cell repertoire correlates with better response to NK cell immunotherapy in elderly acute myeloid leukemia patients. Clin Cancer Res. 2016;22(8):1914–1921. doi: 10.1158/1078-0432.CCR-15-1604. [DOI] [PubMed] [Google Scholar]
- 93.Shaffer BC, Le Luduec J-B, Forlenza C, Jakubowski AA, Perales M-A, Young JW, Hsu KC. Phase II study of haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(4):705–709. doi: 10.1016/j.bbmt.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang CJ, Huang XJ, Gong LZ, Jia JS, Liu XH, Wang Y, Yan CH, Chang YJ, Zhao XS, Shi HX, Lai YY, Jiang H. Observation on the efficacy of consolidation chemotherapy combined with allogeneic natural killer cell infusion in the treatment of low and moderate risk acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2019;40(10):812–817. doi: 10.3760/cma.j.issn.0253-2727.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen R, Wu H, Pounds S, Inaba H, Ribeiro RC, Cullins D, Rooney B, Bell T, Lacayo NJ, Heym K, Degar B, Schiff D, Janssen WE, Triplett B, Pui CH, Leung W, Rubnitz JE. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J Immunother Cancer. 2019;7(1):81. doi: 10.1186/s40425-019-0564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Builes MM, Cuenca MV, Soler JLF, Astigarraga I, Martínez AP, Valero JMV, Tong HY, Quiroga JV, Casanova LF, López AE. Study protocol for a phase II, multicentre, prospective, non-randomised clinical trial to assess the safety and efficacy of infusing allogeneic activated and expanded natural killer cells as consolidation therapy for paediatric acute myeloblastic leukaemia. BMJ Open. 2020;10(1):e029642. doi: 10.1136/bmjopen-2019-029642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vela M, Corral D, Carrasco P, Fernández L, Valentín J, González B, Escudero A, Balas A, de Paz R, Torres J, Leivas A, Martinez-Lopez J, Pérez-Martínez A. Haploidentical IL-15/41BBL activated and expanded natural killer cell infusion therapy after salvage chemotherapy in children with relapsed and refractory leukemia. Cancer Lett. 2018;422:107–117. doi: 10.1016/j.canlet.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 99.Zhao XY, Jiang Q, Jiang H, Hu LJ, Zhao T, Yu XX, Huang XJ. Expanded clinical-grade membrane-bound IL-21/4-1BBL NK cell products exhibit activity against acute myeloid leukemia in vivo. Eur J Immunol. 2020;50:1374–1385. doi: 10.1002/eji.201948375. [DOI] [PubMed] [Google Scholar]
- 100.Wu Y, Li Y, Fu B, Jin L, Zheng X, Zhang A, Sun R, Tian Z, Wei H. Programmed differentiated natural killer cells kill leukemia cells by engaging SLAM family receptors. Oncotarget. 2017;8(34):57024–57038. doi: 10.18632/oncotarget.18659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oyer JL, Igarashi RY, Kulikowski AR, Colosimo DA, Solh MM, Zakari A, Khaled YA, Altomare DA, Copik AJ. Generation of highly cytotoxic natural killer cells for treatment of acute myelogenous leukemia using a feeder-free, particle-based approach. Biol Blood Marrow Transplant. 2015;21(4):632–639. doi: 10.1016/j.bbmt.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 102.Fehniger TA, Miller JS, Stuart RK, Cooley S, Salhotra A, Curtsinger J, Westervelt P, DiPersio JF, Hillman TM, Silver N. A phase 1 trial of CNDO-109-activated natural killer cells in patients with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant. 2018;24(8):1581–1589. doi: 10.1016/j.bbmt.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kottaridis PD, North J, Tsirogianni M, Marden C, Samuel ER, Jide-Banwo S, Grace S, Lowdell MW. Two-stage priming of allogeneic natural killer cells for the treatment of patients with acute myeloid leukemia: a phase I trial. PLoS ONE. 2015;10(6):e0123416. doi: 10.1371/journal.pone.0123416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tao Q, Chen T, Tao L, Wang H, Pan Y, Xiong S, Zhai Z. IL-15 improves the cytotoxicity of cytokine-induced killer cells against leukemia cells by upregulating CD3+ CD56+ cells and downregulating regulatory T cells as well as IL-35. J Immunother. 2013;36(9):462–467. doi: 10.1097/CJI.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 105.Rettinger E, Meyer V, Kreyenberg H, Volk A, Kuçi S, Willasch A, Koscielniak E, Fulda S, Wels W, Boenig H. Cytotoxic capacity of IL-15-stimulated cytokine-induced killer cells against human acute myeloid leukemia and rhabdomyosarcoma in humanized preclinical mouse models. Front Oncol. 2012;2:32. doi: 10.3389/fonc.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cooley S, He F, Bachanova V, Vercellotti GM, DeFor TE, Curtsinger JM, Robertson P, Grzywacz B, Conlon KC, Waldmann TA. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019;3(13):1970–1980. doi: 10.1182/bloodadvances.2018028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grzywacz B, Moench L, McKenna D, Jr, Tessier KM, Bachanova V, Cooley S, Miller JS, Courville EL. Natural killer cell homing and persistence in the bone marrow after adoptive immunotherapy correlates with better leukemia control. J Immunother (Hagerstown, Md: 1997) 2019;42(2):65. doi: 10.1097/CJI.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boyiadzis M, Agha M, Redner RL, Sehgal A, Im A, Hou JZ, Farah R, Dorritie KA, Raptis A, Lim SH, Wang H, Lapteva N, Mei Z, Butterfield LH, Rooney CM, Whiteside TL. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy. 2017;19(10):1225–1232. doi: 10.1016/j.jcyt.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 109.Huang C-H, Liao Y-J, Fan T-H, Chiou T-J, Lin Y-H, Twu Y-C. A developed NK-92MI cell line with siglec-7neg phenotype exhibits high and sustainable cytotoxicity against leukemia cells. Int J Mol Sci. 2018;19(4):1073. doi: 10.3390/ijms19041073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cany J, van der Waart AB, Tordoir M, Franssen GM, Hangalapura BN, de Vries J, Boerman O, Schaap N, van der Voort R, Spanholtz J. Natural killer cells generated from cord blood hematopoietic progenitor cells efficiently target bone marrow-residing human leukemia cells in NOD/SCID/IL2Rg null mice. PLoS ONE. 2013;8(6):e64384. doi: 10.1371/journal.pone.0064384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dolstra H, Roeven MW, Spanholtz J, Hangalapura BN, Tordoir M, Maas F, Leenders M, Bohme F, Kok N, Trilsbeek C. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin Cancer Res. 2017;23(15):4107–4118. doi: 10.1158/1078-0432.CCR-16-2981. [DOI] [PubMed] [Google Scholar]
- 112.Cooley S, Hari P, McCloskey J, Byrne M, Wang E, Hussein M, Giarritta E, Zhang X, Hariri R, Miller JS. Abstract CT079: a phase I study of PNK-007, allogeneic, off the shelf NK cell in relapsed/refractory AML (NCT02781467). In: AACR; 2019.
- 113.Cummins KD, Gill S. Chimeric antigen receptor T-cell therapy for acute myeloid leukemia: how close to reality? Haematologica. 2019;104(7):1302–1308. doi: 10.3324/haematol.2018.208751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yáñez L, Sánchez-Escamilla M, Perales M-A. CAR T cell toxicity: current management and future directions. HemaSphere. 2019;3(2):e186. doi: 10.1097/HS9.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth MT, Fritsch G, Schernthaner GH, Wacheck V, Selzer E, Sperr WR. Expression of the target receptor CD33 in CD34+/CD38−/CD123+ AML stem cells. Eur J Clin Investig. 2007;37(1):73–82. doi: 10.1111/j.1365-2362.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 117.Schirrmann T, Pecher G. Specific targeting of CD33(+) leukemia cells by a natural killer cell line modified with a chimeric receptor. Leuk Res. 2005;29(3):301–306. doi: 10.1016/j.leukres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, Yin J, You F, Zhu M, Shen W, Chen G, Zhu X, Wu D, Yu J. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083–1089. [PMC free article] [PubMed] [Google Scholar]
- 119.Salman H, Pinz KG, Wada M, Shuai X, Yan LE, Petrov JC, Ma Y. Preclinical targeting of human acute myeloid leukemia using CD4-specific chimeric antigen receptor (CAR) T cells and NK cells. J Cancer. 2019;10(18):4408–4419. doi: 10.7150/jca.28952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang H, Salma F, Yi Q, Patterson B, Brien B, Minden MD. Prognostic relevance of immunophenotyping in 379 patients with acute myeloid leukemia. Leuk Res. 2004;28(1):43–48. doi: 10.1016/S0145-2126(03)00180-2. [DOI] [PubMed] [Google Scholar]
- 121.Zhu MY, Zhu Y, Chen RR, Zhu LX, Zhu JJ, Li XY, Zhou D, Yang XD, Zheng YL, Xie MX. CD7 expression and its prognostic significance in acute myeloid leukemia patients with wild-type or mutant CEBPA. Nature. 2020;41(2):100–105. doi: 10.3760/cma.j.issn.0253-2727.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu X-Y, Liu X, Wang X-B, Wang A-Y, Wang M, Liu N-N, You F-T, Pan G-F, Yang L. Killing effect of A CD7 chimeric antigen receptor-modified NK-92MI cell line on CD7-positive hematological malignant cells. Zhongguo shi yan xue ye xue za zhi. 2020;28(4):1367–1375. doi: 10.19746/j.cnki.issn.1009-2137.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 123.Kloess S, Oberschmidt O, Dahlke J, Vu X-K, Neudoerfl C, Kloos A, Gardlowski T, Matthies N, Heuser M, Meyer J. Preclinical assessment of suitable natural killer cell sources for chimeric antigen receptor natural killer-based “off-the-shelf” acute myeloid leukemia immunotherapies. Hum Gene Ther. 2019;30(4):381–401. doi: 10.1089/hum.2018.247. [DOI] [PubMed] [Google Scholar]
- 124.He SZ, Busfield S, Ritchie DS, Hertzberg MS, Durrant S, Lewis ID, Marlton P, McLachlan AJ, Kerridge I, Bradstock KF, Kennedy G, Boyd AW, Yeadon TM, Lopez AF, Ramshaw HS, Iland H, Bamford S, Barnden M, DeWitte M, Basser R, Roberts AW. A phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leuk Lymphoma. 2015;56(5):1406–1415. doi: 10.3109/10428194.2014.956316. [DOI] [PubMed] [Google Scholar]
- 125.Sekeres MA, Lancet JE, Wood BL, Grove LE, Sandalic L, Sievers EL, Jurcic JG. Randomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia. Haematologica. 2013;98(1):119–128. doi: 10.3324/haematol.2012.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O'Connor J, Wedel N, Roboz GJ, Miller C, Chopra R, Jurcic JC, Brown R, Ehmann WC, Schulman P, Frankel SR, De Angelo D, Scheinberg D. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23(18):4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 127.Sallman DA, Asch AS, Al Malki MM, Lee DJ, Donnellan WB, Marcucci G, Kambhampati S, Daver NG, Garcia-Manero G, Komrokji RS. The first-in-class anti-CD47 antibody magrolimab (5F9) in combination with azacitidine is effective in MDS and AML patients: ongoing phase 1b results. Blood. 2019;134(Supplement_1):569. doi: 10.1182/blood-2019-126271. [DOI] [Google Scholar]
- 128.Riether C, Pabst T, Höpner S, Bacher U, Hinterbrandner M, Banz Y, Müller R, Manz MG, Gharib WH, Francisco D. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat Med. 2020;26:1–9. doi: 10.1038/s41591-020-0910-8. [DOI] [PubMed] [Google Scholar]
- 129.Kayser S, Heitmann JS, Dörfel D, Thol F, Heuser M, Märklin M, Müller-Tidow C, Steiner M, Grosse-Hovest L, Jung G, Schlenk RF, Salih HR. Interim results of a first in man study with the Fc-optimized FLT3 antibody Flysyn for treatment of acute myeloid leukemia with minimal residual disease. Blood. 2019;134(Supplement_1):3928. doi: 10.1182/blood-2019-125327. [DOI] [Google Scholar]
- 130.Koerner SP, André MC, Leibold JS, Kousis PC, Kübler A, Pal M, Haen SP, Bühring HJ, Grosse-Hovest L, Jung G, Salih HR. An Fc-optimized CD133 antibody for induction of NK cell reactivity against myeloid leukemia. Leukemia. 2017;31(2):459–469. doi: 10.1038/leu.2016.194. [DOI] [PubMed] [Google Scholar]
- 131.Vasu S, He S, Cheney C, Gopalakrishnan B, Mani R, Lozanski G, Mo X, Groh V, Whitman SP, Konopitzky R, Kössl C, Bucci D, Lucas DM, Yu J, Caligiuri MA, Blum W, Adam PJ, Borges E, Rueter B, Heider KH, Marcucci G, Muthusamy N. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood. 2016;127(23):2879–2889. doi: 10.1182/blood-2015-11-680546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ågerstam H, Karlsson C, Hansen N, Sandén C, Askmyr M, von Palffy S, Högberg C, Rissler M, Wunderlich M, Juliusson G. Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci. 2015;112(34):10786–10791. doi: 10.1073/pnas.1422749112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Venditti A, Buccisano F, Maurillo L, Del Principe MI, Coppola A, Palomba P, Arriga R, Bellarosa D, Bressan A, Wilson K. MEN1112/OBT357, an anti Bst1/CD157 humanized antibody inducing acute myelogenous leukemia (AML) blast depletion in an autologous ex vivo assay: a potential new targeted therapy for AML. Blood. 2015;126(23):788. doi: 10.1182/blood.V126.23.788.788. [DOI] [Google Scholar]
- 134.Krupka C, Lichtenegger FS, Köhnke T, Bögeholz J, Bücklein V, Roiss M, Altmann T, Do TU, Dusek R, Wilson K. Targeting CD157 in AML using a novel, Fc-engineered antibody construct. Oncotarget. 2017;8(22):35707. doi: 10.18632/oncotarget.16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams BA, Wang XH, Leyton JV, Maghera S, Deif B, Reilly RM, Minden MD, Keating A. CD16(+)NK-92 and anti-CD123 monoclonal antibody prolongs survival in primary human acute myeloid leukemia xenografted mice. Haematologica. 2018;103(10):1720–1729. doi: 10.3324/haematol.2017.187385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mani R, Rajgolikar G, Nunes J, Zapolnik K, Wasmuth R, Mo X, Byrd JC, Lee DA, Muthusamy N, Vasu S. Fc-engineered anti-CD33 monoclonal antibody potentiates cytotoxicity of membrane-bound interleukin-21 expanded natural killer cells in acute myeloid leukemia. Cytotherapy. 2020;22(7):369–376. doi: 10.1016/j.jcyt.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Narayan R, Blonquist TM, Emadi A, Hasserjian RP, Burke M, Lescinskas C, Neuberg DS, Brunner AM, Hobbs G, Hock H, McAfee SL, Chen YB, Attar E, Graubert TA, Bertoli C, Moran JA, Bergeron MK, Foster JE, Ramos AY, Som TT, Vartanian MK, Story JL, McGregor K, Macrae M, Behnan T, Wey MC, Rae J, Preffer FI, Lesho P, Duong VH, et al. A phase 1 study of the antibody-drug conjugate brentuximab vedotin with re-induction chemotherapy in patients with CD30-expressing relapsed/refractory acute myeloid leukemia. Cancer. 2020;126(6):1264–1273. doi: 10.1002/cncr.32657. [DOI] [PubMed] [Google Scholar]
- 138.Goldberg AD, Atallah E, Rizzieri D, Walter RB, Chung K-Y, Spira A, Stock W, Tallman MS, Cruz HG, Boni J. Camidanlumab tesirine, an antibody-drug conjugate, in relapsed/refractory CD25-positive acute myeloid leukemia or acute lymphoblastic leukemia: a phase I study. Leuk Res. 2020;95:106385. doi: 10.1016/j.leukres.2020.106385. [DOI] [PubMed] [Google Scholar]
- 139.Daver NG, Erba HP, Papadantonakis N, DeAngelo DJ, Wang ES, Konopleva MY, Sloss CM, Culm-Merdek K, Zweidler-McKay PA, Kantarjian HM. A phase I, first-in-human study evaluating the safety and preliminary antileukemia activity of IMGN632, a novel CD123-targeting antibody-drug conjugate, in patients with relapsed/refractory acute myeloid leukemia and other CD123-positive hematologic malignancies. Blood. 2018;132(Supplement_1):27. doi: 10.1182/blood-2018-99-112955. [DOI] [Google Scholar]
- 140.Agura E, Gyurkocza B, Nath R, Litzow MR, Tomlinson BK, Abhyankar S, Seropian S, Stiff PJ, Choe HK, Kebriaei P. Targeted conditioning of Iomab-B (131I-anti-CD45) prior to allogeneic hematopoietic cell transplantation versus conventional care in relapsed or refractory acute myeloid leukemia (AML): preliminary feasibility and safety results from the prospective, randomized phase 3 Sierra trial. Blood. 2018;132(Supplement_1):1017. doi: 10.1182/blood-2018-99-111914. [DOI] [Google Scholar]
- 141.Jurcic JG, Ravandi F, Pagel JM, Park JH, Smith BD, Douer D, Levy MY, Estey E, Kantarjian HM, Earle D, Cicic D, Scheinberg DA. Phase I trial of α-particle therapy with actinium-225 (225Ac)-lintuzumab (anti-CD33) and low-dose cytarabine (LDAC) in older patients with untreated acute myeloid leukemia (AML) J Clin Oncol. 2015;33(15_suppl):7050. doi: 10.1200/jco.2015.33.15_suppl.7050. [DOI] [Google Scholar]
- 142.Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN, Legrand O, Thomas X, Turlure P, Reman O, de Revel T, Gastaud L, de Gunzburg N, Contentin N, Henry E, Marolleau JP, Aljijakli A, Rousselot P, Fenaux P, Preudhomme C, Chevret S, Dombret H. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 143.Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M, Annino L, Venditti A, Voso MT, Mazzone C. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 144.Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N, Bouabdallah R, Pautas C, Turlure P, Reman O, Gardin C, Varet B, de Botton S, Pousset F, Farhat H, Chevret S, Dombret H, Castaigne S. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66–71. doi: 10.1038/sj.leu.2404434. [DOI] [PubMed] [Google Scholar]
- 145.Pereira DS, Guevara CI, Jin L, Mbong N, Verlinsky A, Hsu SJ, Aviña H, Karki S, Abad JD, Yang P. AGS67E, an anti-CD37 monomethyl auristatin E antibody–drug conjugate as a potential therapeutic for B/T-cell malignancies and AML: a new role for CD37 in AML. Mol Cancer Ther. 2015;14(7):1650–1660. doi: 10.1158/1535-7163.MCT-15-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zheng B, Yu S-F, Del Rosario G, Leong SR, Lee GY, Vij R, Chiu C, Liang W-C, Wu Y, Chalouni C. An anti-CLL-1 antibody-drug conjugate for the treatment of acute myeloid leukemia. Clin Cancer Res. 2019;25(4):1358–1368. doi: 10.1158/1078-0432.CCR-18-0333. [DOI] [PubMed] [Google Scholar]
- 147.Jiang Y-P, Liu BY, Zheng Q, Panuganti S, Chen R, Zhu J, Mishra M, Huang J, Dao-Pick T, Roy S. CLT030, a leukemic stem cell-targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood Adv. 2018;2(14):1738–1749. doi: 10.1182/bloodadvances.2018020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Anami Y, Deng M, Gui X, Yamaguchi A, Yamazaki CM, Zhang N, Zhang C, An Z, Tsuchikama K. LILRB4-targeting antibody-drug conjugates for the treatment of acute myeloid leukemia. Mol Cancer Ther. 2020;19:2330–2339. doi: 10.1158/1535-7163.MCT-20-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li F, Sutherland MK, Yu C, Walter RB, Westendorf L, Valliere-Douglass J, Pan L, Cronkite A, Sussman D, Klussman K. Characterization of SGN-CD123A, a potent CD123-directed antibody-drug conjugate for acute myeloid leukemia. Mol Cancer Ther. 2018;17(2):554–564. doi: 10.1158/1535-7163.MCT-17-0742. [DOI] [PubMed] [Google Scholar]
- 150.Snyder JT, Malinao M-C, Dugal-Tessier J, Atkinson JE, Anand BS, Okada A, Mendelsohn BA. Metabolism of an oxime-linked antibody drug conjugate, AGS62P1, and characterization of its identified metabolite. Mol Pharm. 2018;15(6):2384–2390. doi: 10.1021/acs.molpharmaceut.8b00225. [DOI] [PubMed] [Google Scholar]
- 151.Kovtun Y, Noordhuis P, Whiteman KR, Watkins K, Jones GE, Harvey L, Lai KC, Portwood S, Adams S, Sloss CM. IMGN779, a novel CD33-targeting antibody-drug conjugate with DNA-alkylating activity, exhibits potent antitumor activity in models of AML. Mol Cancer Ther. 2018;17(6):1271–1279. doi: 10.1158/1535-7163.MCT-17-1077. [DOI] [PubMed] [Google Scholar]
- 152.Chan WK, Kung Sutherland M, Li Y, Zalevsky J, Schell S, Leung W. Antibody-dependent cell-mediated cytotoxicity overcomes NK cell resistance in MLL-rearranged leukemia expressing inhibitory KIR ligands but not activating ligands. Clin Cancer Res. 2012;18(22):6296–6305. doi: 10.1158/1078-0432.CCR-12-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brodská B, Otevřelová P, Šálek C, Fuchs O, Gašová Z, Kuželová K. High PD-L1 expression predicts for worse outcome of leukemia patients with concomitant NPM1 and FLT3 mutations. Int J Mol Sci. 2019;20(11):2823. doi: 10.3390/ijms20112823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang H, Bueso-Ramos CE, Parmar S, Wei Y, Fang Z, Nguyen M, Fernandez M, Pierce SA, Geng Q, Kantarjian HM. Induction of PD-1 and PD-1 ligand expression by hypomethylating agents (HMA) in myelodysplastic syndromes and acute myelogenous leukemia suggest a role for T cell function in clinical resistance to Hmas. Blood. 2012;120(21):3810. doi: 10.1182/blood.V120.21.3810.3810. [DOI] [Google Scholar]
- 155.Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, Kantarjian H. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia. 2018;32(5):1094–1105. doi: 10.1038/s41375-018-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schmiedel BJ, Werner A, Steinbacher J, Nuebling T, Buechele C, Grosse-Hovest L, Salih HR. Generation and preclinical characterization of a Fc-optimized GITR-Ig fusion protein for induction of NK cell reactivity against leukemia. Mol Ther. 2013;21(4):877–886. doi: 10.1038/mt.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schmiedel BJ, Nuebling T, Steinbacher J, Malinovska A, Wende CM, Azuma M, Schneider P, Grosse-Hovest L, Salih HR. Receptor activator for NF-κB ligand in acute myeloid leukemia: expression, function, and modulation of NK cell immunosurveillance. J Immunol. 2013;190(2):821–831. doi: 10.4049/jimmunol.1201792. [DOI] [PubMed] [Google Scholar]
- 158.Baessler T, Krusch M, Schmiedel BJ, Kloss M, Baltz KM, Wacker A, Schmetzer HM, Salih HR. Glucocorticoid-induced tumor necrosis factor receptor-related protein ligand subverts immunosurveillance of acute myeloid leukemia in humans. Cancer Res. 2009;69(3):1037–1045. doi: 10.1158/0008-5472.CAN-08-2650. [DOI] [PubMed] [Google Scholar]
- 159.Steinbacher J, Baltz-Ghahremanpour K, Schmiedel BJ, Steinle A, Jung G, Kübler A, André MC, Grosse-Hovest L, Salih HR. An Fc-optimized NKG2D-immunoglobulin G fusion protein for induction of natural killer cell reactivity against leukemia. Int J Cancer. 2015;136(5):1073–1084. doi: 10.1002/ijc.29083. [DOI] [PubMed] [Google Scholar]
- 160.Deng G, Zheng X, Zhou J, Wei H, Tian Z, Sun R. Generation and preclinical characterization of an NKp80-Fc fusion protein for redirected cytolysis of natural killer (NK) cells against leukemia. J Biol Chem. 2015;290(37):22474–22484. doi: 10.1074/jbc.M115.678912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Khan M, Arooj S, Wang H. NK cell-based immune checkpoint inhibition. Front Immunol. 2020;11:167. doi: 10.3389/fimmu.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, Dombret H, Olive D. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 163.Vey N, Bourhis J-H, Recher C, Etienne A, Charbonnier A, Rey J, André P, Calmels F, Zerbib R, Buffet R. Repeated dosing of anti-KIR (IPH2101) as maintenance therapy in elderly patients with acute myeloid leukemia. Blood. 2013;122(21):2696. doi: 10.1182/blood.V122.21.2696.2696. [DOI] [Google Scholar]
- 164.Vey N, Dumas P-Y, Recher C, Gastaud L, Lioure B, Bulabois C-E, Pautas C, Marolleau J-P, Leprêtre S, Raffoux E, Thomas X, Hicheri Y, Bonmati C, Quesnel B, Rousselot P, Castaigne S, Jourdan E, Malfuson JV, Guillerm G, Bouhris JH, Ojeda M, Hunault M, Ifrah N, Gardin C, Delannoy A, Beautier L, Paturel C, Andre P, Zerbib R, Preudhomme C, et al. Randomized phase 2 trial of lirilumab (anti-KIR monoclonal antibody, mAb) as maintenance treatment in elderly patients (pts) with acute myeloid leukemia (AML): results of the Effikir trial. Blood. 2017;130(Supplement_1):889. [Google Scholar]
- 165.Daver NG, Garcia-Manero G, Cortes JE, Basu S, Ravandi F, Kadia TM, Borthakur G, Jabbour E, Dinardo CD, Pemmaraju N. Phase IB/II study of lirilumab with azacytidine (AZA) in relapsed AML. Blood. 2017;130(Supplement_1):2634. [Google Scholar]
- 166.Godal R, Bachanova V, Gleason M, McCullar V, Yun GH, Cooley S, Verneris MR, McGlave PB, Miller JS. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant. 2010;16(5):612–621. doi: 10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ruggeri L, Urbani E, André P, Mancusi A, Tosti A, Topini F, Bléry M, Animobono L, Romagné F, Wagtmann N, Velardi A. Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica. 2016;101(5):626–633. doi: 10.3324/haematol.2015.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ravandi F, Assi R, Daver N, Benton CB, Kadia T, Thompson PA, Borthakur G, Alvarado Y, Jabbour EJ, Konopleva M. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study. Lancet Haematol. 2019;6(9):e480–e488. doi: 10.1016/S2352-3026(19)30114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, Konopleva M, Ravandi-Kashani F, Jabbour E, Kadia T. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov. 2019;9(3):370–383. doi: 10.1158/2159-8290.CD-18-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Daver NG, Garcia-Manero G, Konopleva MY, Alfayez M, Pemmaraju N, Kadia TM, DiNardo CD, Cortes JE, Ravandi F, Abbas H. Azacitidine (AZA) with nivolumab (Nivo), and AZA with Nivo+ ipilimumab (Ipi) in relapsed/refractory acute myeloid leukemia: a non-randomized, prospective, phase 2 study. Blood. 2019;134(Supplement_1):830. doi: 10.1182/blood-2019-131494. [DOI] [Google Scholar]
- 171.Kadia TM, Cortes JE, Ghorab A, Ravandi F, Jabbour E, Daver NG, Alvarado Y, Ohanian M, Konopleva M, Kantarjian HM. Nivolumab (Nivo) maintenance (maint) in high-risk (HR) acute myeloid leukemia (AML) patients. J Clin Oncol. 2018;36:7014. doi: 10.1200/JCO.2018.36.15_suppl.7014. [DOI] [Google Scholar]
- 172.Lindblad KE, Thompson J, Gui G, Valdez J, Worthy T, Tekleab H, Hughes T, Goswami M, Oetjen K, Kim D-Y, Dillon L, DeStefano C, Lai CE, Hourigan CS. Pembrolizumab and decitabine for refractory or relapsed acute myeloid leukemia. Blood. 2018;132(Supplement_1):1437. doi: 10.1182/blood-2018-99-115097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zeidner JF, Vincent BG, Esparza S, Ivanova A, Moore DT, Foster MC, Coombs CC, Jamieson K, Van Deventer HW, Blanchard L. Final clinical results of a phase II study of high dose cytarabine followed by pembrolizumab in relapsed/refractory AML. Blood. 2019;134(Supplement_1):831. doi: 10.1182/blood-2019-126065. [DOI] [Google Scholar]
- 174.Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, Lukez AP, Avigan D, Chen Y-B, McSweeney P. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375:143–153. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Borate U, Esteve J, Porkka K, Knapper S, Vey N, Scholl S, Garcia-Manero G, Wermke M, Janssen J, Traer E, Chua CC, Narayan R, Tovar N, Kontro M, Ottmann O, Sun H, Longmire T, Szpakowski S, Liao S, Patel A, Rinne ML, Brunner A, Wei AH. Phase Ib study of the anti-TIM-3 antibody MBG453 in combination with decitabine in patients with high-risk myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) Blood. 2019;134(Supplement_1):570. doi: 10.1182/blood-2019-128178. [DOI] [Google Scholar]
- 176.Gros F, Cazaubiel T, Forcade E, Lechevalier N, Leguay T, Servant V, Tabrizi R, Clement L, Dumas P, Bidet A. Severe acute GvHD following administration of ipilimumab for early relapse of AML after haploidentical stem cell transplantation. Bone Marrow Transplant. 2017;52(7):1047–1048. doi: 10.1038/bmt.2017.78. [DOI] [PubMed] [Google Scholar]
- 177.Ijaz A, Khan AY, Malik SU, Faridi W, Fraz MA, Usman M, Tariq MJ, Durer S, Durer C, Russ A. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol Blood Marrow Transplant. 2019;25(1):94–99. doi: 10.1016/j.bbmt.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Baessler T, Charton JE, Schmiedel BJ, Grünebach F, Krusch M, Wacker A, Rammensee HG, Salih HR. CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood. 2010;115(15):3058–3069. doi: 10.1182/blood-2009-06-227934. [DOI] [PubMed] [Google Scholar]
- 179.Hattori N, Kawaguchi Y, Sasaki Y, Shimada S, Murai S, Abe M, Baba Y, Watanuki M, Fujiwara S, Arai N. Monitoring TIGIT/DNAM-1 and PVR/PVRL2 immune checkpoint expression levels in allogeneic stem cell transplantation for acute myeloid leukemia. Biol Blood Marrow Transplant. 2019;25(5):861–867. doi: 10.1016/j.bbmt.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 180.Atfy M, Ebian HF, Elshorbagy SM, Atteia HH. CD200 suppresses the natural killer cells and decreased its activity in acute myeloid leukemia patients. J Leukemia. 2015;3:1–5. doi: 10.4172/2329-6917.1000190. [DOI] [Google Scholar]
- 181.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, Panoskaltsis-Mortari A, Weiner LM, Vallera DA, Miller JS. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11(12):2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, Vallera DA, Miller JS. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19(14):3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, Zhang B, Lenvik AJ, Panoskaltsis-Mortari A, Verneris MR. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res. 2016;22(14):3440–3450. doi: 10.1158/1078-0432.CCR-15-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, Schwemmlein M, Stein C, Mentz K, Mackensen A, Fey GH. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33(6):599–608. doi: 10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
- 185.Kügler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, Schubert I, Singer H, Oduncu F, Stockmeyer B, Mackensen A, Fey GH. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol. 2010;150(5):574–586. doi: 10.1111/j.1365-2141.2010.08300.x. [DOI] [PubMed] [Google Scholar]
- 186.Märklin M, Hagelstein I, Koerner SP, Rothfelder K, Pfluegler MS, Schumacher A, Grosse-Hovest L, Jung G, Salih HR. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins for induction of NK and T cell reactivity against acute myeloid leukemia. J Immunother Cancer. 2019;7(1):143. doi: 10.1186/s40425-019-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Arvindam US, van Hauten PMM, Schirm D, Schaap N, Hobo W, Blazar BR, Vallera DA, Dolstra H, Felices M, Miller JS. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia. 2020 doi: 10.1038/s41375-020-01065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Decot V, Voillard L, Latger-Cannard V, Aissi-Rothé L, Perrier P, Stoltz JF, Bensoussan D. Natural-killer cell amplification for adoptive leukemia relapse immunotherapy: comparison of three cytokines, IL-2, IL-15, or IL-7 and impact on NKG2D, KIR2DL1, and KIR2DL2 expression. Exp Hematol. 2010;38(5):351–362. doi: 10.1016/j.exphem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 189.Szczepanski MJ, Szajnik M, Welsh A, Foon KA, Whiteside TL, Boyiadzis M. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol Immunother. 2010;59(1):73. doi: 10.1007/s00262-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sanchez-Correa B, Bergua JM, Pera A, Campos C, Arcos MJ, Bañas H, Duran E, Solana R, Tarazona R. In vitro culture with interleukin-15 leads to expression of activating receptors and recovery of natural killer cell function in acute myeloid leukemia patients. Front Immunol. 2017;8:931. doi: 10.3389/fimmu.2017.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Baer MR, George SL, Caligiuri MA, Sanford BL, Bothun SM, Mrózek K, Kolitz JE, Powell BL, Moore JO, Stone RM. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: cancer and Leukemia Group B Study 9720. J Clin Oncol. 2008;26(30):4934. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lange BJ, Smith FO, Feusner J, Barnard DR, Dinndorf P, Feig S, Heerema NA, Arndt C, Arceci RJ, Seibel N. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children's oncology group. Blood. 2008;111(3):1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Petit A, Ducassou S, Leblanc T, Pasquet M, Rousseau A, Ragu C, Cachanado M, Nelken B, Bertrand Y, Michel G. Maintenance therapy with interleukin-2 for childhood AML: results of ELAM02 phase III randomized trial. HemaSphere. 2018;2(6):e159. doi: 10.1097/HS9.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, Bourhis J-H, Reman O, Turlure P, Contentin N. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28(5):808–814. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 195.Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann W-K, Hogge DE, Nilsson B, Or R, Romero AI. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108(1):88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 196.Nilsson MS, Hallner A, Brune M, Nilsson S, Thorén FB, Martner A, Hellstrand K. Immunotherapy with HDC/IL-2 may be clinically efficacious in acute myeloid leukemia of normal karyotype. Hum Vaccines Immunother. 2020;16(1):109–111. doi: 10.1080/21645515.2019.1636598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cuapio A, Post M, Cerny-Reiterer S, Gleixner KV, Stefanzl G, Basilio J, Herndlhofer S, Sperr WR, Brons NH, Casanova E. Maintenance therapy with histamine plus IL-2 induces a striking expansion of two CD56bright NK cell subpopulations in patients with acute myeloid leukemia and supports their activation. Oncotarget. 2016;7(29):46466. doi: 10.18632/oncotarget.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, Weisdorf DJ, Blazar BR, Ustun C, DeFor TE. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131(23):2515–2527. doi: 10.1182/blood-2017-12-823757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang J, Liu Y, Au BC, Barber DL, Arruda A, Schambach A, Rothe M, Minden MD, Paige CJ, Medin JA. Preclinical validation: LV/IL-12 transduction of patient leukemia cells for immunotherapy of AML. Mol Therapy Methods Clin Dev. 2016;3:16074. doi: 10.1038/mtm.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shi Y, Dincheva-Vogel L, Ayemoba CE, Fung JP, Bergamaschi C, Pavlakis GN, Farzaneh F, Gaensler KML. IL-15/IL-15Rα/CD80-expressing AML cell vaccines eradicate minimal residual disease in leukemic mice. Blood Adv. 2018;2(22):3177–3192. doi: 10.1182/bloodadvances.2018019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev. 2010;235(1):297–305. doi: 10.1111/j.0105-2896.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rosario M, Romee R, Schneider SE, Leong JW, Sullivan RP, Fehniger TA. Human cytokine-induced memory-like (CIML) NK cells are active against myeloid leukemia in vitro and in vivo. Blood. 2014;124(21):1117. doi: 10.1182/blood.V124.21.1117.1117. [DOI] [Google Scholar]
- 204.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CC, Yu L, Oh ST, Lee YS, Mulder A, Claas F, Cooper MA, Fehniger TA. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Berrien-Elliott MM, Cashen AF, Cubitt CC, Neal CC, Wong P, Wagner JA, Foster M, Schappe T, Desai S, McClain E. Multidimensional analyses of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Foltz JA, Berrien-Elliott MM, Neal C, Foster M, McClain E, Schappe T, Desai S, Becker-Hapak M, Cashen AF, Fehniger TA. Cytokine-induced memory-like (ML) NK cells persist for> 2 months following adoptive transfer into leukemia patients with a MHC-compatible hematopoietic cell transplant (HCT) Blood. 2019;134(Supplement_1):1954. doi: 10.1182/blood-2019-126004. [DOI] [Google Scholar]
- 207.Bednarski JJ, Zimmerman C, Cashen AF, Desai S, Foster M, Schappe T, McClain E, Becker-Hapak M, Berrien-Elliott MM, Fehniger TA. Adoptively transferred donor-derived cytokine induced memory-like NK cells persist and induce remission in pediatric patient with relapsed acute myeloid leukemia after hematopoietic cell transplantation. Blood. 2019;134(Supplement_1):3307. doi: 10.1182/blood-2019-126982. [DOI] [Google Scholar]
- 208.Rohner A, Langenkamp U, Siegler U, Kalberer CP, Wodnar-Filipowicz A. Differentiation-promoting drugs up-regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis. Leuk Res. 2007;31(10):1393–1402. doi: 10.1016/j.leukres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 209.Raneros AB, Puras AM, Rodriguez RM, Colado E, Bernal T, Anguita E, Mogorron AV, Gil AC, Vidal-Castiñeira JR, Márquez-Kisinousky L. Increasing TIMP3 expression by hypomethylating agents diminishes soluble MICA, MICB and ULBP2 shedding in acute myeloid leukemia, facilitating NK cell-mediated immune recognition. Oncotarget. 2017;8(19):31959. doi: 10.18632/oncotarget.16657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lu X, Ohata K, Kondo Y, Luis Espinoza J, Qi Z, Nakao S. Hydroxyurea upregulates NKG2D ligand expression in myeloid leukemia cells synergistically with valproic acid and potentially enhances susceptibility of leukemic cells to natural killer cell-mediated cytolysis. Cancer Sci. 2010;101(3):609–615. doi: 10.1111/j.1349-7006.2009.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yun HD, Schirm DK, Felices M, Miller JS, Eckfeldt CE. Dinaciclib enhances natural killer cell cytotoxicity against acute myelogenous leukemia. Blood Adv. 2019;3(16):2448. doi: 10.1182/bloodadvances.2019000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, Hofsteenge J, Gratwohl A, Tichelli A, Paluszewska M. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111(3):1428–1436. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
- 213.Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia. 2009;23(4):641–648. doi: 10.1038/leu.2008.354. [DOI] [PubMed] [Google Scholar]
- 214.Le Roy A, Prebet T, Castellano R, Goubard A, Riccardi F, Fauriat C, Granjeaud S, Benyamine A, Castanier C, Orlanducci F. Immunomodulatory drugs exert anti-leukemia effects in acute myeloid leukemia by direct and immunostimulatory activities. Front Immunol. 2018;9:977. doi: 10.3389/fimmu.2018.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yu FS, Yang JS, Yu CS, Chiang JH, Lu CC, Chung HK, Yu CC, Wu CC, Ho HC, Chung JG. Safrole suppresses murine myelomonocytic leukemia WEHI-3 cells in vivo, and stimulates macrophage phagocytosis and natural killer cell cytotoxicity in leukemic mice. Environ Toxicol. 2013;28(11):601–608. doi: 10.1002/tox.20756. [DOI] [PubMed] [Google Scholar]
- 216.Lin J-J, Lu K-W, Ma Y-S, Tang N-Y, Wu P-P, Wu C-C, Lu H-F, Lin J-G, Chung J-G. Alpha-phellandrene, a natural active monoterpene, influences a murine WEHI-3 leukemia model in vivo by enhancing macrophague phagocytosis and natural killer cell activity. Vivo. 2014;28(4):583–588. [PubMed] [Google Scholar]
- 217.Lai K-C, Lu H-F, Chen K-B, Hsueh S-C, Chung J-G, Huang W-W, Chen C-C, Shang H-S. Casticin promotes immune responses, enhances macrophage and NK cell activities, and increases survival rates of leukemia BALB/c mice. Am J Chin Med. 2019;47(01):223–236. doi: 10.1142/S0192415X19500113. [DOI] [PubMed] [Google Scholar]
- 218.Shih YL, Shang HS, Chen YL, Hsueh SC, Chou HM, Lu HF, Lee MZ, Hou HT, Chuang YY, Lee MH. Ouabain promotes immune responses in WEHI-3 cells to generate leukemia mice through enhancing phagocytosis and natural killer cell activities in vivo. Environ Toxicol. 2019;34(5):659–665. doi: 10.1002/tox.22732. [DOI] [PubMed] [Google Scholar]
- 219.Lee JY, Park S, Kim DC, Yoon J-H, Shin SH, Min W-S, Kim H-J. A VEGFR-3 antagonist increases IFN-γ expression on low functioning NK cells in acute myeloid leukemia. J Clin Immunol. 2013;33(4):826–837. doi: 10.1007/s10875-013-9877-2. [DOI] [PubMed] [Google Scholar]
- 220.Lee JY, Park S, Min W-S, Kim H-J. Restoration of natural killer cell cytotoxicity by VEGFR-3 inhibition in myelogenous leukemia. Cancer Lett. 2014;354(2):281–289. doi: 10.1016/j.canlet.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 221.Otegbeye F, Ojo E, Moreton S, Mackowski N, Lee DA, de Lima M, Wald DN. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS ONE. 2018;13(1):e0191358. doi: 10.1371/journal.pone.0191358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Morgan MA, Büning H, Sauer M, Schambach A. Use of cell and genome modification technologies to generate improved “off-the-shelf” CAR T and CAR NK Cells. Front Immunol. 1965;2020:11. doi: 10.3389/fimmu.2020.01965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, Tonn T. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65(4):485–492. doi: 10.1007/s00262-015-1761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, De Witte T, Schaap N, Dolstra H. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS ONE. 2011;6(6):e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shankar K, Capitini CM, Saha K. Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem cell Res Therapy. 2020;11(1):1–14. doi: 10.1186/s13287-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, Griffin GE, Taylor GP, Tough DF, Beverley PC, Macallan DC. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hu Y, Tian Z-G, Zhang C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol Sin. 2018;39(2):167–176. doi: 10.1038/aps.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pahl JHW, Koch J, Götz J-J, Arnold A, Reusch U, Gantke T, Rajkovic E, Treder M, Cerwenka A. CD16A activation of NK cells promotes NK cell proliferation and memory-like cytotoxicity against cancer cells. Cancer Immunol Res. 2018;6(5):517–527. doi: 10.1158/2326-6066.CIR-17-0550. [DOI] [PubMed] [Google Scholar]
- 230.Cany J, Roeven MWH, Hoogstad-van Evert JS, Hobo W, Maas F, Franco Fernandez R, Blijlevens NMA, van der Velden WJ, Huls G, Jansen JH. Decitabine enhances targeting of AML cells by CD34+ progenitor-derived NK cells in NOD/SCID/IL2Rgnull mice. Blood. 2018;131(2):202–214. doi: 10.1182/blood-2017-06-790204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jiang W, Zhang C, Tian Z, Zhang J. hIL-15 gene-modified human natural killer cells (NKL-IL15) augments the anti-human hepatocellular carcinoma effect in vivo. Immunobiology. 2014;219(7):547–553. doi: 10.1016/j.imbio.2014.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The material supporting the information of this review has been included within the article.