BACKGROUND AND EPIDEMIOLOGY
Hypertension is the most common medical disorder occurring during pregnancy, complicating 5% to 10% of all pregnancies.1 It is also the leading cause of maternal mortality in industrialized countries, and its prevalence is increasing.2 From 1998 to 2006, the prevalence of hypertension during delivery hospitalizations increased from 67.2 to 81.4 per 1000 deliveries.3 This increase may in part be caused by the increasing prevalence of cardiometabolic disease in women of childbearing age.1 Maternal age more than 40 years, prepregnancy obesity, excess weight gain during pregnancy, and gestational diabetes are all associated with increased risks of maternal hypertension.4 (Box 1).
Box 1. Hypertensive disorders of pregnancy.
Chronic hypertension
Gestational hypertension
Preeclampsia/eclampsia
Chronic hypertension with superimposed preeclampsia/eclampsia
An often-overlooked epidemiologic aspect of hypertensive disorders of pregnancy (HDPs) is that their prevalence and associated mortalities vary by race and ethnicity (Fig. 1A).4,5 A study of the 2014 to 2015 US birth cohort data noted significant racial disparities in the prevalence of hypertension, with the highest burden in non-Hispanic black women (9.8%) followed by American Indian/Alaska Native (AIAN) (8.9%), and Filipino (7.74%) women compared with 7.2% in white women. Rates of eclampsia follow similar trends (Fig. 1B).4 From 2011 to 2015, in the United States, the overall pregnancy-related mortality was 17.2 per 100,000 live births. Non-Hispanic black women and AIAN women had the highest mortalities at 42.8 and 32.5 per 100,000 live births respectively.5 Furthermore, non-Hispanic black women and Hispanic white women with HDPs have a 2-fold greater risk of stroke during delivery admission. Among women with chronic hypertension, the highest risk of stroke was seen among Asian and Pacific Islander women.6 These variations in hypertension-associated maternal morbidity and mortality likely reflect underlying systemic disparities in access to care and differences in social determinants of health, rather than an underlying physiologic difference between women.
Fig. 1.
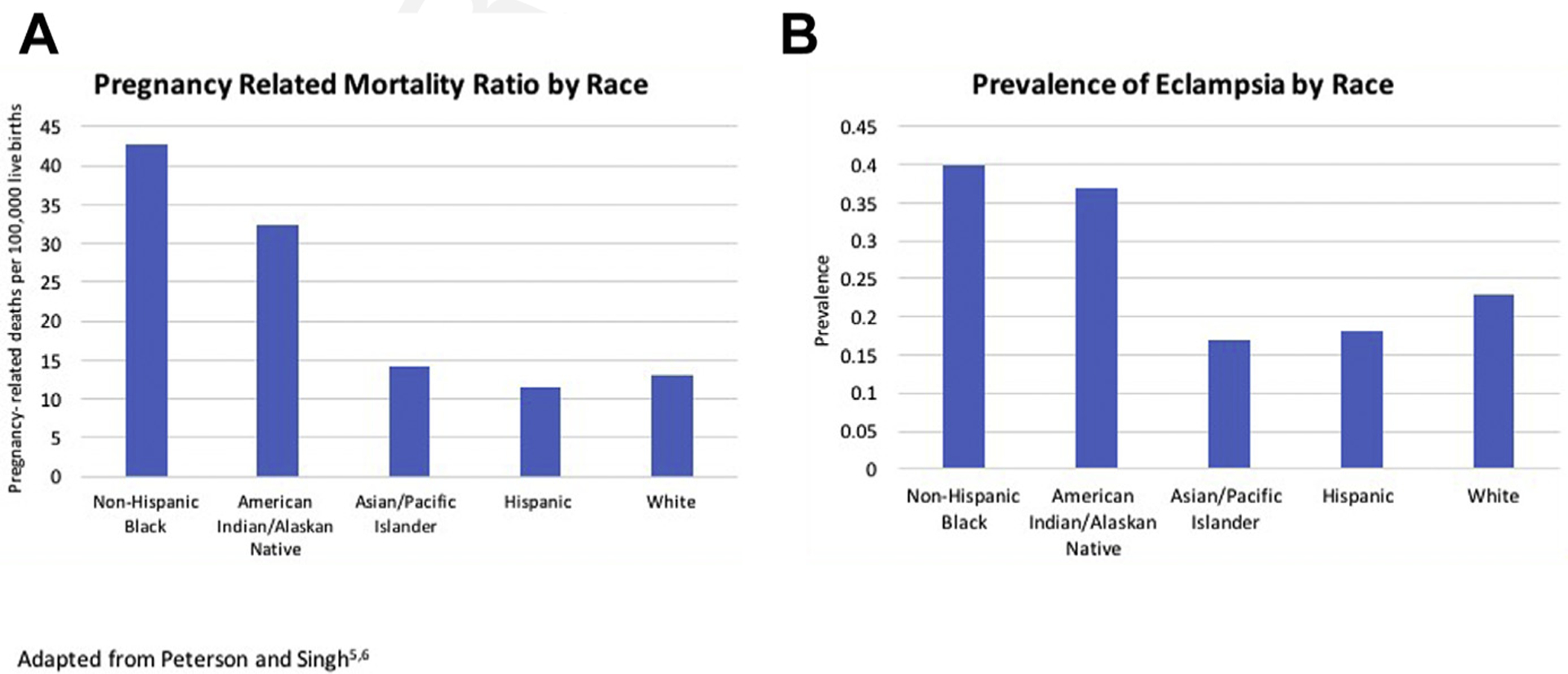
Racial disparities in morbidity and mortality associated with hypertensive disorders. (Adapted from Singh GK, Siahpush M, Liu L, Allender M. Racial/Ethnic, Nativity, and Sociodemographic Disparities in Maternal Hypertension in the United States, 2014–2015. Int J Hypertens. 2018;2018:7897189 and Petersen EE DN, Goodman D, et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morb Mortal Wkly Rep 2019. 2019;68:423–429.)
NORMAL PHYSIOLOGIC RESPONSE TO PREGNANCY
Pregnancy is a dynamic process during which there is a marked increase in metabolic demand and hemodynamic adaptations that vary by trimester and regress toward normal during the postpartum period (Fig. 2).7 The major maternal hemodynamic adaptations during pregnancy include increased cardiac output and plasma volume along with a concurrent reduction in systemic vascular resistance. In light of these rapid and dynamic changes, pregnancy is often considered a physiologic stress test, because insufficient adaptations result in maternal and fetal morbidity and mortality.
Fig. 2.
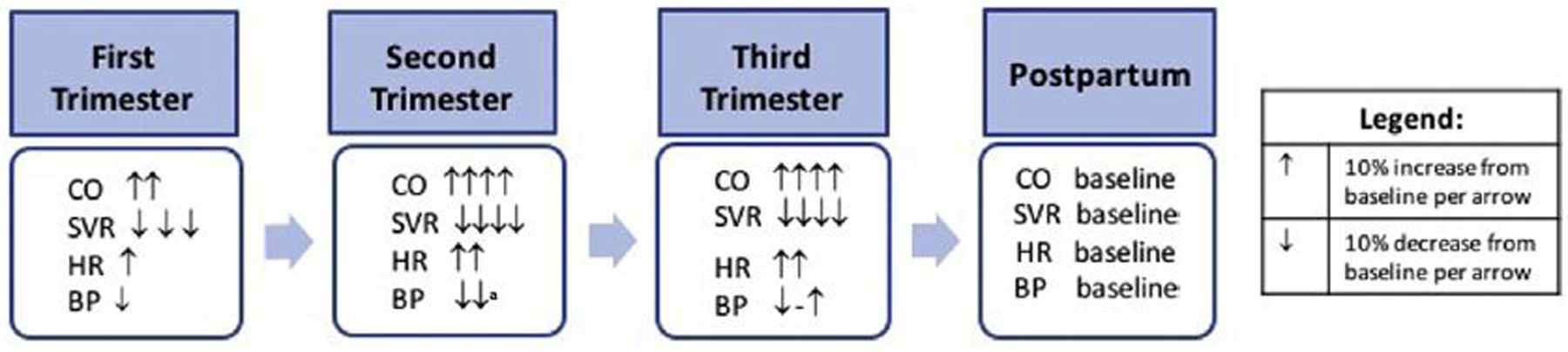
Hemodynamic changes associated with pregnancy. a Blood pressure nadir reached at 18.6 weeks. BP, blood pressure; CO, cardiac output; HR, heart rate; SVR, systemic vascular resistance.
The first trimester, from conception to 13 weeks and 6 days of gestation, is associated with an overall decrease in blood pressure of approximately 10%. Significant vasodilation of the peripheral vasculature begins at approximately 5 weeks of gestation, partially caused by increases in estrogen and progesterone levels. In addition, serum concentration of relaxin increases and peaks at the end of the first trimester. Relaxin is a peptide hormone that has an endothelium-dependent vasodilatory effect that results in enhanced nitric oxide production.8 These changes result in a significant decrease in systemic vascular resistance and blood pressure, and a 50% increase in renal flow and glomerular filtration rates by the end of the first trimester. To maintain adequate blood pressure in this setting, additional maternal hemodynamic adaptations take place. There is an increased sympathetic and as well as maternal baroreceptor sensitivity. In addition, the reninangiotensin-aldosterone system is activated, counteracting the salt and water loss secondary to renal vasodilatation and leading to an increase in heart rate and cardiac output.9
During the second trimester, which is defined as 14 to 27 weeks and 6 days of gestation, there is a plateau in the reduction in systemic vascular resistance, as relaxin decreases to an intermediate value once the uteroplacental circulation is formed, resulting in a sink of low vascular resistance. In addition, arterial pressures reach a nadir during the second trimester, whereas cardiac output continues to increase to 45% above baseline by 24 weeks. Excessive sympathetic activity after 20 weeks of gestation is thought to be associated with gestational hypertension or preeclampsia.
The third trimester lasts from 28 weeks and 0 days of gestation through delivery. There is a peak in cardiac output in the early third trimester and blood pressure begins to increase back to baseline levels. In addition, the ratio between plasma volume and red cell mass peaks at 30 to 34 weeks, resulting in a physiologic anemia. The resultant decrease in blood viscosity further decreases resistance to flow and in turn allows improved placental perfusion to support the growing fetus. In addition, plasma volume increases to 50% greater than nonpregnant values near term, allowing a reserve against blood loss during delivery. Heart rate peaks in the late third trimester, with a 20% to 25% increase relative to baseline.7 During active labor, systolic and diastolic blood pressures can increase an additional 15% to 25% and 10% to 15% respectively. Cardiac output is increased by 15% in early labor and 25% during the active phase.
MEASURING BLOOD PRESSURE IN PREGNANCY
As previously described, each trimester of pregnancy involves marked hemodynamic changes, so the accurate measurement of blood pressure in pregnant women is essential for the diagnosis and treatment of hypertension. Although considered the gold standard for blood pressure measurement, the mercury sphygmomanometer is rarely available in the modern clinical setting where oscillometric devices are in widespread use. Note that all blood pressure monitor validation protocols recommend devices be specifically validated for accuracy in pregnant women in light of the previously mentioned alterations in the vasculature that occur and that may result in inaccuracies.10 Best practices for blood pressure measurement include taking blood pressure using an appropriately sized cuff, in a patient with an empty bladder, preferably at least 30 minutes after ingestion of caffeine or nicotine use, and after 5 minutes of quiet rest. The patient should be comfortably seated with uncrossed feet resting on the floor, in a chair with appropriate back and arm support, with the arm comfortably resting at the level of the heart (Fig. 3).11,12
Fig. 3.
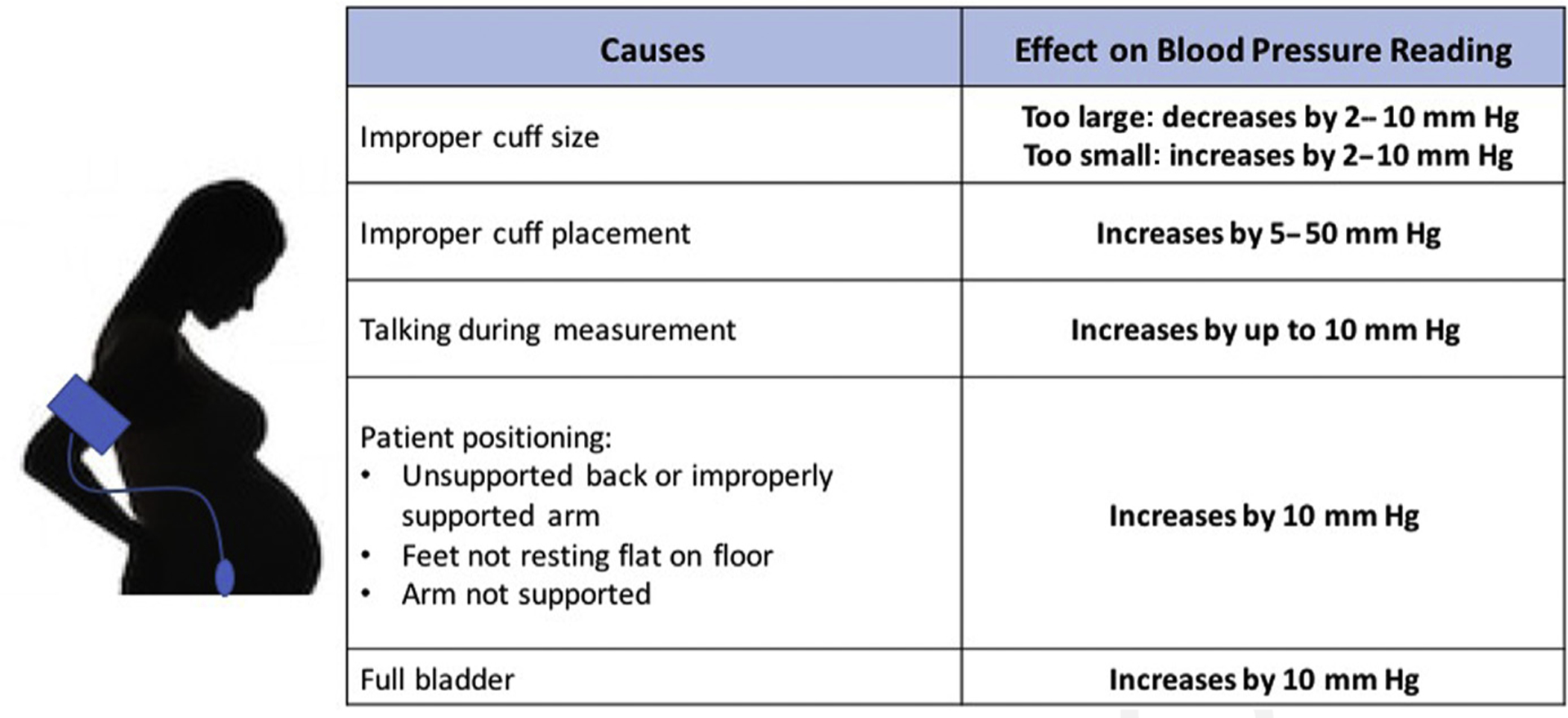
Identifying inaccuracies resulting from improper blood pressure measurement.
DIAGNOSING AND CLASSIFYING HYPERTENSION IN PREGNANCY
In contrast with nonpregnant adults, the diagnosis of hypertension in pregnancy is based primarily on office blood pressure measurements, and concordant diagnostic thresholds between office and ambulatory or home blood pressure measurements have not been defined.13 American College of Obstetricians and Gynecologists (ACOG) defines hypertension in pregnant women as clinic maternal systolic blood pressure greater than or equal to 140 mm Hg and/or diastolic blood pressure greater than or equal to 90 mm Hg on 2 or more occasions at least 4 hours apart. ACOG further categorizes severe-range hypertension as sustained systolic blood pressure greater than or equal to 160 mm Hg and/or diastolic blood pressure greater than or equal to 110 mm Hg; in this setting, verification should be performed in as few as 15 minutes to avoid delays in treatment11 (Table 1).
Table 1.
Classification of hypertensive disorders of pregnancy
| Chronic hypertension |
|
| OR | |
| |
| Or | |
| |
| Chronic hypertension with superimposed preeclampsia |
|
| Or | |
| |
| Or | |
| |
| And | |
| |
| Gestational hypertension |
|
| Preeclampsia |
|
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Classification of Hypertensive Disorders During Pregnancy
Another distinction in the classification of pregnant women with hypertension compared with nonpregnant adults is that the category of HDP depends on how far along in pregnancy the woman is when first diagnosed. Twenty weeks’ gestation is the cut point used, reflecting the return to an approximate baseline blood pressure after the first-trimester decline (Fig. 4).11
Fig. 4.
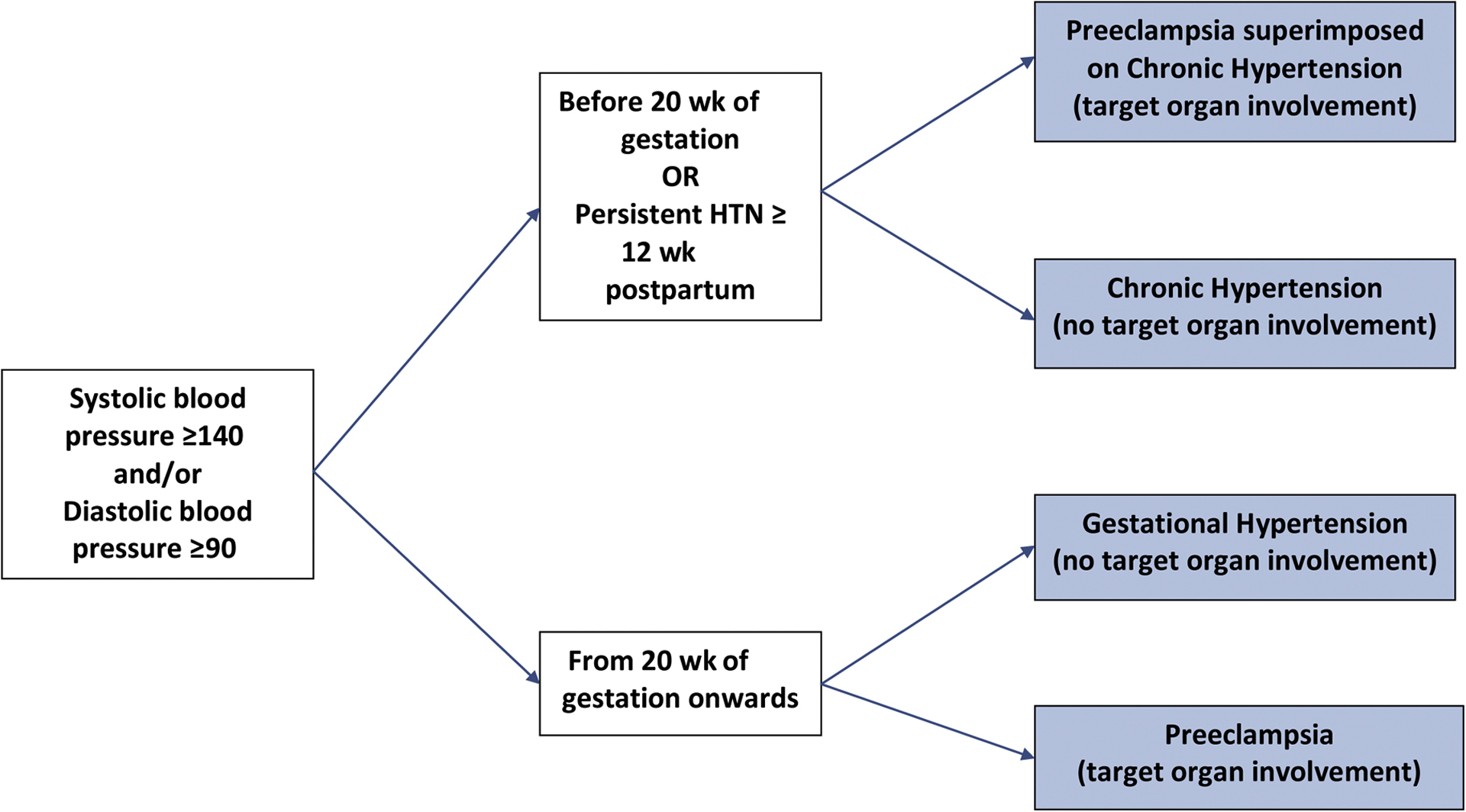
Classification of hypertensive disorders in pregnancy. HTN, hypertension.
Chronic Hypertension
Chronic hypertension is defined as systolic blood pressure greater than or equal to 140 mm Hg and/or diastolic blood pressure greater than or equal to 90 mm Hg before pregnancy or before 20 weeks of gestation, the use of antihypertensives before pregnancy, or the persistence of hypertension more than 12 weeks after delivery.11 Around 3% to 5% of pregnancies are estimated to be afflicted with chronic hypertension.
Gestational Hypertension
Gestational hypertension is defined as systolic blood pressure greater than or equal to 140 mm Hg and/or diastolic blood pressure greater than or equal to 90 mm Hg after 20 weeks of gestation in a woman who was at baseline normotensive.12 If a woman diagnosed with gestational hypertension has persistent postpartum increases in blood pressure, she should be reclassified as having chronic hypertension.
Preeclampsia with and Without Severe Features
Preeclampsia is an HDP that is typically associated with new-onset hypertension with proteinuria, which occurs most often after 20 weeks of gestation.12 Proteinuria is defined by ACOG as (1) 300 mg or more per 24-hour urine collection; (2) protein to creatinine ratio greater than or equal to 0.3 mg/dL; or (3) a dipstick reading of 2+ if quantitative methods are not available. However, preeclampsia can also manifest in the absence of proteinuria, and additional diagnostic criteria include (1) thrombocytopenia, defined as a platelet count less than 100,000 × 109/L; (2) impaired hepatic function, defined as transaminase level greater than 2 times the upper limit of normal; (3) severe right upper right quadrant or epigastric pain that is not associated with other diagnoses; (4) renal insufficiency, defined as serum creatinine greater than 1.1 mg/dL or a doubling of serum creatinine level in the absence of other renal disease; (5) pulmonary edema; (6) new-onset headache unresponsive to acetaminophen and not associated with other diagnosis or visual symptoms.
Preeclampsia with severe features is defined as systolic blood pressure of 160 mm Hg or more and diastolic blood pressure of 110 mm Hg or more on 2 occasions at least 4 hours apart. The clinical presentation of hemolysis, increased liver enzyme levels, and low platelet count (HELLP syndrome) is a form of preeclampsia with severe features that generally occurs in the third trimester and has been associated with increased maternal morbidity and mortality. The diagnostic criteria for HELLP are (1) lactate dehydrogenase increased to 60 IU/L or more; (2) aspartate aminotransferase and alanine aminotransferase levels increased more than twice the upper limit of normal; (3) platelet count less than 100,000 × 109/L.12 However, right upper quadrant pain and generalized fatigue are the main pre-senting symptoms in 90% of cases.
Chronic Hypertension with Superimposed Preeclampsia
Chronic hypertension occurs in 1% to 5% of pregnant women, and 20% to 50% of these women go on to develop superimposed preeclampsia.14 The risk of superimposed preeclampsia in women with chronic hypertension is increased in women who are black, obese, smoke, have a diastolic blood pressure greater than 100 mm Hg, have had chronic hypertension for more than 4 years, and have a history of preeclampsia during a prior pregnancy.15 The incidence of superimposed preeclampsia is even higher in women with end-organ failure or secondary hypertension and approaches 75%.16 However, in women with chronic hypertension and baseline proteinuria, superimposed preeclampsia can be difficult to distinguish from worsening chronic hypertension, and a high index of suspicion is required. The presence of new-onset thrombocytopenia or a sudden increase of liver enzyme levels is often the first sign of superimposed preeclampsia in this group.11
TREATMENT OF HYPERTENSIVE DISORDERS DURING PREGNANCY
The treatment of HDPs is mostly based on expert opinion and observational studies because there are few randomized controlled trials in this population, which was traditionally considered by institutional review boards to be a vulnerable population. Balancing the risks and benefits of the treatment of increased blood pressure in pregnant women on both the mother and fetus is an important consideration. In turn, the exact blood pressure at which pharmacologic treatment is initiated in pregnant women is the subject of ongoing research. Overall, based on the current evidence, ACOG recognizes that pregnant women with severe hypertension (blood pressure ≥160/110 mm Hg) should be treated with antihypertensives to prevent maternal vascular complications such as stroke and placental abruption.
Chronic Hypertension
There remains a controversy about treatment of pregnant women with chronic hypertension. This article presents the best evidence underscoring the major recommendations for antihypertensive use among pregnant women with nonsevere and severe chronic hypertension. First, a 2014 Cochrane Review of 49 trials noted that treatment of pregnant women with mild to moderate hypertension did not reduce the incidence of complications such as developing preeclampsia, preterm birth, or maternal and fetal mortality. However, treatment did reduce their risk of developing severe hypertension.17 Second, the 2015 CHIPS trial, which randomized 987 pregnant women to less tight control (target diastolic blood pressure of 100 mm Hg) versus tight control (target diastolic blood pressure <85 mm Hg) found that tight control of hypertension again reduced progression to severe hypertension.18 There was no benefit or increased risk of harm to the fetus from tight diastolic blood pressure control, though a secondary analysis found severe hypertension was associated with an increased risk of adverse perinatal outcomes (birth weight <10th percentile, preeclampsia, preterm delivery, increased liver enzyme levels, low platelet count, and prolonged hospital stay) in both arms of the trial, and has been used to promote tighter diastolic blood pressure control in this population.19 The third pivotal trial is currently ongoing. The Chronic Hypertension and Pregnancy (CHAP) project (clinicaltrials.gov NCT02299414) is a multicenter, pragmatic randomized trial with the primary aim to evaluate the benefits and harms of pharmacologic treatment of mild chronic hypertension in pregnancy treated to a goal blood pressure less than 140/90 mm Hg compared with current ACOG recommendation (no initiation of antihypertensive medication unless blood pressure ≥160/110 mm Hg). It is anticipated that the results of this trial will be available in 2021.
Because the current data show no direct benefit to the fetus with tight control of hypertension, according to ACOG, the initiation of antihypertensive medications is only recommended for persistent chronic hypertension when systolic pressure is greater than or equal to 160 mm Hg or diastolic is greater than or equal to 110 mm Hg. For pregnant women without a prior diagnosis of chronic hypertension with stage 1 hypertension (systolic blood pressure of 130–139 mm Hg or diastolic blood pressure of 80–89 mm Hg) before 20 weeks of gestation, antihypertensive initiation is not recommended.
The effects of continuing antihypertensive therapy for women with chronic hypertension diagnosed previous to pregnancy and with less than severe hypertension remain unclear. The current evidence base is conflicting and, at the current time, this decision is left to the treating physician’s discretion. One prospective observational study noted that the discontinuation of antihypertensive treatment during pregnancy did not affect the incidence of preeclampsia or fetal complications such as growth restriction and perinatal death, but it was associated with a higher incidence of severe hypertension, placental abruption, and preterm delivery.20 Another study found no increase in preeclampsia, placental abruption, and perinatal death with discontinuation of antihypertensive therapy during pregnancy.21 High-quality, large, randomized controlled trials examining risks and benefits of antihypertensive treatment of both mothers and babies in nonsevere hypertension are sorely needed. The CHAP trial is working to respond to that demand.
Gestational Hypertension and Preeclampsia
The management of pregnant women with gestational hypertension, with no evidence of severe hypertension or progression to preeclampsia, can safely be performed as outpatients. Other than weekly in-office blood pressure monitoring and urine protein excretion, as well as twice-weekly home blood pressure measurements, nonpharmacologic interventions are deemed by ACOG to be appropriate for management. Nonpharmacologic interventions include monitoring activity and diet. However, up to 50% of women with gestational hypertension may develop preeclampsia. Women with gestational hypertension with severe-range blood pressures should start antihypertensive therapy and be managed as women with severe preeclampsia.12
PRIMARY AND SECONDARY PREVENTION OF PREECLAMPSIA
Numerous studies have been performed examining potential preventive therapies for preeclampsia. including supplementation with aspirin, calcium, vitamin C, vitamin E, fish oil, garlic, vitamin D, and folic acid. Low-dose aspirin is the 1 agent that has consistently been shown to provide a significant reduction in the risk of preeclampsia.22 When administered before 16 weeks of gestation, low-dose aspirin (60–150 mg daily) has a modest impact on the risk of preeclampsia, severe preeclampsia, and fetal growth restriction.23 Therefore, both ACOG and the United States Preventive Services Task Force (USPSTF) recommend the use of low-dose aspirin for women at increased risk of preeclampsia. ACOG recommends women at high risk, or with more than 1 moderate-risk factor, start aspirin 81 mg/d between 12 and 28 weeks of gestation, preferably before 16 weeks, to be continued through delivery.24 The USPSTF recommends the use of low-dose aspirin (60–150 mg daily) between 12 and 28 weeks of gestation for women with 1 or more high-risk factors and consideration for use in women with more than 1 moderate-risk factor for preeclampsia.25 High-risk factors for the development of preeclampsia according to both the USPSTF and ACOG include history of preeclampsia, multifetal gestation, chronic hypertension, type 1 or 2 diabetes, renal disease, and autoimmune disease. ACOG and the USPSTF both consider the following moderate-risk factors: first pregnancy, body mass index greater than 30 kg/m2, family history of preeclampsia, and age greater than or equal to 35 years, whereas the USPSTF additionally recognizes low socioeconomic status, black race, history of low birthweight, and history of adverse pregnancy outcome to be moderate-risk factors. Current evidence does not support the universal use of low-dose aspirin for preeclampsia prevention.24
CHOICE OF ANTIHYPERTENSIVE MEDICATIONS IN PREGNANCY
There are no large randomized trials on which to base recommendations for the use of one antihypertensive medication rather than any other. All antihypertensive medications cross the placenta, but there is scant evidence on the impact of most antihypertensive medication classes on pregnancy outcomes and fetal risk. The exception to this is the known teratogenicity of angiotensin receptor blockers, angiotensin-converting enzyme (ACE) inhibitors, and direct renin inhibitors, which are always contraindicated in pregnancy. A large 2017 systemic review and meta-analysis of newborn outcomes after exposure to antihypertensive medications found there were inadequate data to recommend any specific therapy because of methodologic weakness of the available evidence.26 A 2018 international cohort study with pooled data from 6 countries, the InPreSS consortium, found no relative or absolute risks for major congenital malformations, including cardiac malformations, with first-trimester β-blocker exposure.27
ACOG recommends the use of β-blockers and calcium channel blockers as first-line agents for the treatment of HDPs. Labetalol, a mixed alpha-adrenergic and beta-adrenergic blocker, is the most common beta-blocker used in pregnancy (Table 2).28 In addition, pindolol and long-acting metoprolol are less studied in pregnancy but are considered acceptable alternatives, especially for women with concurrent heart failure who are chronically treated with metoprolol.29 Atenolol should be avoided in pregnancy because of its association with a heightened risk of fetal growth restriction and low birth weight.30
Table 2.
Antihypertensive medications for pregnant women with nonsevere hypertension
| Agent | Dose | Side Effects | |
|---|---|---|---|
| First line | Labetalol (PO) | 100–200 mg BID (increase Q 2–3 d to max dosage 2400 mg/d) | Hypotension, increased liver enzyme Levels, persistent fetal bradycardia, and neonatal hypoglycemia |
| Avoid in asthma because of risk of bronchospasm | |||
| Extended-release nifedipine (PO) | 30–60 mg Q day (increase Q 7–14 d to max dosage 120 mg/d) | Severe headache, peripheral edema | |
| Methyldopa (PO) | 250 mg BID or TID (increase Q 2 d to max dose 3000 mg/d) | Sedative, peripheral edema, anxiety, nightmares, dry mouth, hypotension, | |
| Contraindicated in depression | |||
| Second or Third line | Hydrochlorothiazide | 12.5 mg Q day (increase Q 7–14 d to max dosage 50 mg/d) | Volume depletion, fetal growth restriction, oligohydramnios |
| Hydralazine | 10 mg QID (increase Q 2–5 d to max dosage 200 mg/d) | Tachycardia, headache, flushing, fetal distress, hypotension, and inhibition of labor especially when combined with magnesium sulfate | |
| Should never be used in isolation because of reflex tachycardia |
Abbreviations: BID, twice a day; PO, by mouth; max, maximum; Q, every; QID, 4 times a day; TID, 3 times a day.
Extended-release nifedipine, a dihydropyridine calcium channel blocker, is also recommended by ACOG to treat hypertension in pregnant women.11 Extended-release nifedipine reduces blood pressure within an hour. A 2019 randomized control trial noted that nifedipine reduced blood pressure more rapidly and was easier to administer, although it was equal in efficacy and safety to labetalol.31
Alpha-methyldopa has been widely used in pregnant women around the globe because it has a long safety record and is available generically. A follow-up study on children of women treated with methyldopa during pregnancy noted its long-term safety.32 However, it is less favored in the United States, where other options are readily available because of decreased efficacy compared with β-blockers and its association with adverse effects such as depression.17
Second-line agents for the treatment of HDPs include thiazide diuretics and hydralazine. The use of thiazide diuretics can be associated with significant volume depletion within the first 2 weeks, and close monitoring of volume status is recommended. Although this effect has not been noted within randomized control trials, the concern for potential fetal growth restriction or oligohydramnios is the reason thiazide diuretics are a second-line agent.33 Care should be taken when using hydralazine in pregnancy because approximately half of women have associated side effects, including hypotension, headaches, flushing, tremors, and fluid retention. It is also associated with reflex tachycardia and therefore should never be used in isolation without a nodal blocking agent. A 2003 meta-analysis found hydralazine was more effective in reducing blood pressure than labetalol, but was also associated with more adverse maternal and fetal outcomes because of the unpredictable nature of resultant extreme hypotension in some patients.34
Other agents, such as clonidine, can be considered for recalcitrant hypertension but are considered third line. As previously mentioned, the use of ACE inhibitors, angiotensin II receptor blockers, direct renin inhibitors, and mineralocorticoid receptor antagonists are absolutely contraindicated in pregnancy because of their significant associations with fetal renal abnormalities and failure, growth restriction, congenital malformations, and death.
Nonpharmacologic Interventions to Reduce Blood Pressure in Pregnant Women
Moderate exercise can be continued in pregnancy, and ACOG recommends that 30 minutes of moderate exercise several days of the week can offer benefits such as decreased risk of developing gestational diabetes, operative vaginal delivery, risk of cesarean birth, postpartum recovery time, and risk of postpartum depression.35
Weight loss and extremely low-sodium diets (<100 mEq/d) are not recommended for the management of HDPs. Overall, evidence is limited, but few studies of diet and lifestyle modification to reduce blood pressure have shown any effects on pregnancy outcomes. A 2020 observational study on the relationship between the DASH (Dietary Approaches to Stop Hypertension) diet and maternal blood pressure in pregnancy found that the DASH dietary pattern was associated with lower maternal blood pressure in women without HDPs.36 However, in the 2013 executive summary, ACOG reports that there is no adequate evidence that salt restriction reduces preeclampsia risk.37
TREATMENT OF SEVERE-RANGE HYPERTENSION AND HYPERTENSIVE EMERGENCIES IN PREGNANCY
Severe-range hypertension is defined as blood pressure values exceeding 160/110 mm Hg. Hypertensive emergency of pregnancy is classified as (1) acute increase in blood pressures greater than or equal to 160/110 mm Hg, (2) development of symptoms consistent with severe preeclampsia, and (3) symptoms of end-organ damage. Severe hypertension is noted to cause central nervous system injury, and two-thirds of maternal deaths during 2003 to 2005 were caused by cerebral hemorrhage or infarction.38 Therefore, any pregnant women with acute-onset severe hypertension that is persistent (15 minutes or more) should be initiated on antihypertensive treatment within 30 to 60 minutes to acutely reduce blood pressure. First-line agents include intravenous (IV) labetalol and hydralazine for management of acute-onset, severe hypertension in pregnant women and women in the postpartum period. IV hydralazine has been used for the acute treatment of severe hypertension for more than 65 years and is recommended by ACOG (Table 3).39 In addition, immediate-release oral nifedipine may also be used. Current ACOG guidelines recommend the use of immediate-release oral nifedipine as a first-line alternative to labetalol in the absence of IV access for the treatment of acute, severe hypertension. IV nitroglycerin can also be used in the treatment of severe pregnancy-induced hypertension complicated by pulmonary edema.40
Table 3.
Antihypertensive medications for pregnant women with severe hypertension
| Agent | Dose | Side Effects |
|---|---|---|
| Labetalol (IV) | 5–20 mg (increase every 10–15 min to max ag 220 mg/d) | Hypotension, increased liver enzyme levels, persistent fetal bradycardia, and neonatal hypoglycemia |
| Avoid in asthma because of risk of bronchospasm and congestive heart failure | ||
| Hydralazine (IV) | 5–10 mg (increase every 20 min to max dosage 30 mg/d) | Severe headache, peripheral edema, flushing, reflex bradycardia, hypotension. |
| Avoid use in isolation because of reflex tachycardia | ||
| Immediate-release nifedipine (PO) | 5–10 mg (increase every 30 min to max dosage 50 mg/d) | First-line alternative to labetalol only in the absence of IV access for treatment of acute, severe hypertension |
Abbreviation: IV, intravenous.
If blood pressure remains increased or initial blood pressure is greater than or equal to 180/120 mm Hg, then accelerated maternal and obstetric management should occur. In addition, if the blood pressure exceeds 240/150 mm Hg or there is any evidence of acute end-organ damage, accelerated management by a maternal fetal medicine or a critical care specialist is highly recommended.39
IV magnesium sulfate is not recommended as an antihypertensive agent, although it has blood pressure–reducing effects.41 Magnesium remains the most common seizure prophylaxis in pregnant women with acute-onset severe hypertension, because it has been shown to be more effective than phenytoin, diazepam, and nimodipine in reducing the risk of eclampsia and is the drug of choice to prevent eclampsia in intrapartum and postpartum women.42 The current preferred dosage is a loading dose of 4 to 6 g intravenously followed by a maintenance dosage of 1 to 2 g/h. The use of nifedipine with magnesium sulfate should be avoided because of the risk of synergistic hypotension.
For women with preeclampsia who have severely increased blood pressure that is unresponsive to treatment, the definitive treatment is always delivery. Delivery is always beneficial for the mother but may be harmful to the fetus depending on its gestational age. Decisions weighing the risks of being born premature with the potential benefit of removing the fetus from the preeclamptic environment where it is at risk for intrauterine growth restriction and stillbirth must be made in collaboration by maternal fetal medicine specialists and neonatologists. For women with a preterm fetus who have gestational hypertension or preeclampsia without severe features, continued observation is often appropriate. However, the HYPITAT trial found that women with gestational hypertension and preeclampsia without severe features after 36 weeks who had induction of labor had a significant reduction of adverse maternal outcomes, including HELLP syndrome, eclampsia, pulmonary edema, and placental abruption. In addition, induction of labor versus expectant management resulted in no significant differences in neonatal complications.43
THE FOURTH TRIMESTER
The postpartum period, defined as the 12 weeks after delivery, is now commonly referred to as the fourth trimester. Marked fluid shifts occur during the early postpartum period and they are associated with fluctuations in blood pressure.7 There is an initial decrease in the first 48 hours and subsequent increase during postpartum days 3 to 6 as fluids mobilize. Contributing factors include IV fluid administration and loss of pregnancy-associated vasodilation. The use of nonsteroidal antiinflammatory drugs and ergot derivatives, which are used to treat postpartum hemorrhage, can also contribute to blood pressure variability.44 Blood volume returns to nonpregnant values by 8 weeks postpartum because of diuresis. Pregnancy-related hypertension should resolve within 12 weeks; for increases in blood pressure beyond this period, clinicians should consider a secondary cause of hypertension because it is found in around 10% of patients.45
The weeks following birth are a critical period for women and her infants. There are scarce data regarding the evaluation and management of blood pressure in the fourth trimester, although ACOG recommends that women with severe hypertension should have a blood pressure evaluation within 72 hours after delivery, and no more than 7 to 10 days postpartum for women with HDPs.46 The incidence of new-onset postpartum hypertension is uncertain, with an estimate ranging from 0.3% to 28%. In 1 study of 203 women with planned postpartum inpatient stays of 1 week or longer, 12% of previously normotensive women became hypertensive and more than 50% of women with a hypertensive disorder of pregnancy had a systolic/diastolic blood pressure greater than or equal to 150/100 mm Hg.47 Since those studies were performed, the length of a hospital admission for delivery has shortened and most women are now discharged within 24 hours of delivery. In addition, many do not return for a blood pressure check until the standard 6-week postpartum visit, leading to missed blood pressure measurements and adjustment of treatment.
Hypertension is a leading cause of postpartum readmission in the United States.48 More than one-half of pregnancy-related maternal deaths occur in the fourth trimester.49 Postpartum maternal complications also include de novo postpartum preeclampsia; around one-third of eclampsia occurs postpartum. Women with postpartum preeclampsia most often present with new-onset headaches or visual changes in addition to increases in blood pressure. Early identification and management is pivotal, because these women are at an increased risk of stroke, seizures, pulmonary edema, renal failure, congestive heart failure, and death.50 Half of all intracerebral hemorrhage caused by preeclampsia occurs in the postpartum period.51
As previously discussed, significant racial disparities exist in postpartum complication and readmission rates. Black women with cardiovascular risk factors are more likely to be readmitted postpartum, to have severe morbidity, and to have life-threatening complications, including pulmonary edema and acute heart failure, compared with white women.52 Many of these complications are related to HDPs, and targeted interventions such as the use of self-measured blood pressure and telehealth may play an important role in prevention of postpartum hypertension and associated complications, such as postpartum stroke.53 Future studies are needed to examine the impact and acceptability of these interventions.
In addition to the health burden imposed by preexisting or new-onset hypertension, new mothers are often faced with other medical concerns, such as pain, depression, and anxiety. Despite the fourth trimester being a pivotal time of transition in the health of postpartum women and newborn infants, it is also frequently a time of health care transition and loss of coverage for many US women, which likely contributes to high rates of maternal mortality as well as disparities in care. Medicaid is the largest single payer of maternity care, covering 43% of all births in the United States in 2017. However, postpartum pregnancy-related coverage for mothers only lasts 60 days into the fourth trimester. Thirty-six states and Washington DC have expanded Medicaid under the Affordable Care Act (ACA), allowing pregnant women to remain covered beyond this period, but in the 14 non–Medicaid expansion states, many new mothers become uninsured because they no longer meet Medicaid income eligibility requirements (Fig. 5).54,55 These women fall out of care and are at an even higher risk for adverse outcomes; uninsured women are more likely to have advanced-stage diseases such as HDPs and higher associated mortality.56,57 At baseline, pregnant women with Medicaid have an increased risk for complications in pregnancy and poor fetal outcomes compared with women with private insurance, thus the most vulnerable women are exposed to even greater adversity when they lose coverage.57 Although a 2019 observational study of births from 2011 to 2016 found state Medicaid expansion did not result in significant changes in rates of low birth weight or preterm births, it did result in the reduction of health disparities experienced by black infants.58 Further work is needed to examine the effects of Medicaid expansion on maternal health in the fourth trimester.
Fig. 5.
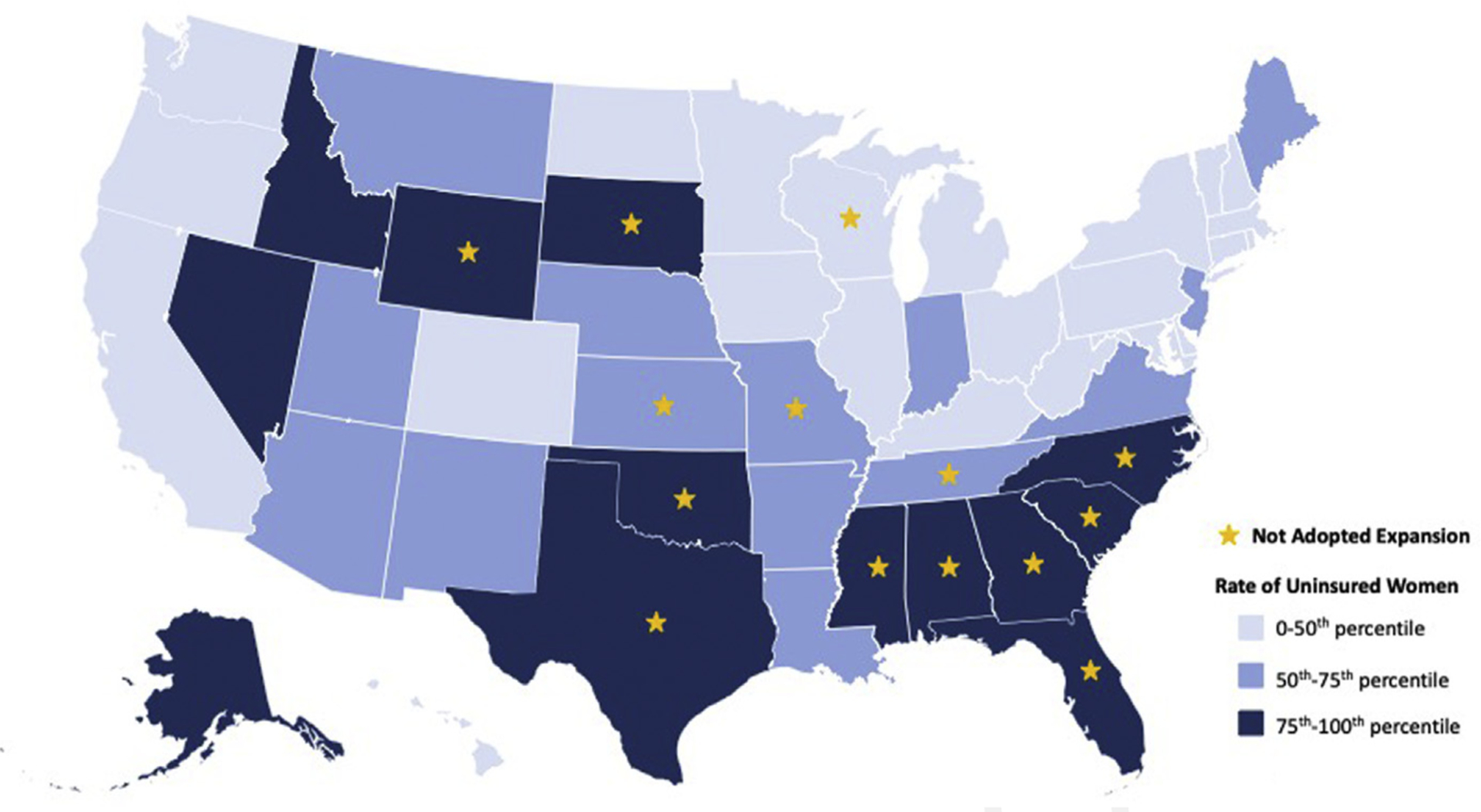
Map of the United States showing the relationship between rate of uninsured women by state in 2019 and the Medicaid expansion status of the state as of March 2020. (Data from Status of State Medicaid Expansion Decisions: Interactive Map. Kaiser Family Foundation. Published 2020. Accessed 2020 and America’s Health Rankings analysis of U.S. Census Bureau. United Health Foundation. Published 2019. Accessed2020.)
If all states were to implement Medicaid expansion, the percentage of uninsured women aged 19 to 64 years could decrease from 20% to 8%.59 ACOG fully supports the expansion of Medicaid as proposed in the ACA to help reduce health disparities and improve outcomes.57 Many steps were taken in 2019 to ensure that mothers were able to have Medicaid coverage through 12 months postpartum, including the passage of the bipartisan legislation H.R. 4996, the Helping MOMs Act. Expanded fourth-trimester coverage is a pivotal part of care for pregnant women and new mothers, and further legislative steps need to be taken to ensure equitable outcomes for all women in the United States.
HYPERTENSIVE DISORDERS DURING PREGNANCY AND FUTURE CARDIOVASCULAR RISK
HDPs are associated with an increased risk of complications for both mother and baby during pregnancy and beyond.60 Whether the HDP itself leads to increased risk or it is merely a marker of underlying increased risk remains an area of intense debate and ongoing research. Despite this debate, it is irrefutable that a history of HDP is associated with an increased risk of future maternal myocardial infarction, heart failure, chronic hypertension, and stroke.61 In addition, the severity of preeclampsia has been shown to be positively correlated with severity of cardiovascular disease and earlier age of onset of disease.62 These findings have led many professional societies, including the American Heart Association, American College of Cardiology, American Stroke Association, and American College of Obstetrics and Gynecology, to include preeclampsia as a clinical risk factor that should be screened for as part of a comprehensive cardiovascular risk assessment.12,63 The increased time between diagnosis of HDP and management is associated with an increased risk of maternal cardiovascular risk.64 Therefore, early identification and treatment of women at risk for and with HDPs is important to prevent not only immediate but also future mortality and morbidity. As such, the authors recommend the inclusion of a reproductive history as part of the comprehensive assessment of all women, to identify risk-modifying factors such as HDP, and appropriately risk stratify women for primary cardiovascular disease prevention.
SUMMARY
HDPs are a significant cause of maternal and fetal morbidity and mortality, and their prevalence is increasing. Moreover, the prevalence as well as the morbidity and mortality associated with HDP vary by race/ethnicity. Improvements in the prevention, diagnosis, and management of HDPs are needed to reduce maternal morbidity and mortality and reduce disparities in care for pregnant and postpartum women.
KEY POINTS.
Hypertensive disorders of pregnancy (HDPs) include chronic hypertension, gestational hypertension, preeclampsia, and chronic hypertension with superimposed preeclampsia.
American College of Obstetricians and Gynecologists (ACOG) defines hypertension in pregnant women as clinic systolic and diastolic blood pressure greater than or equal to 140 and/or 90 mm Hg. Severe-range hypertension, a medical emergency, is defined as blood pressure greater than or equal to 160/110 mm Hg.
Labetalol and extended-release nifedipine are first-line agents for the outpatient management of hypertension in pregnancy. First-line agents for treatment of severe-range hypertension include intravenous labetalol and hydralazine, and oral immediate-release nifedipine.
Both ACOG and the United States Preventive Services Task Force (USPSTF) recommend the use of low-dose aspirin between 12 and 28 weeks of gestation for women at increased risk of preeclampsia.
The fourth trimester is a vulnerable time period when postpartum women are at risk for adverse out comes, and in many states lose access to health care. Expanded fourth-trimester Medicaid coverage has the potential to reduce disparities in care and improve maternal outcomes.
DISCLOSURE
N.A. Bello reports grant support from the National Institutes of Health, National Heart, Lung, and Blood Institute (K23 HL136853), and the Katz Foundation. A.M. Khedagi has nothing to disclose.
REFERENCES
- 1.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25(4):391–403. [DOI] [PubMed] [Google Scholar]
- 2.Data on Selected pregnancy complications in the United States. Center for Disease Control and Prevention: Center for Disease Control and Prevention; 2019. [Google Scholar]
- 3.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009;113(6): 1299–306. [DOI] [PubMed] [Google Scholar]
- 4.Singh GK, Siahpush M, Liu L, et al. Racial/ethnic, nativity, and sociodemographic disparities in maternal hypertension in the United States, 2014–2015. Int J Hypertens 2018;2018:7897189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep 2019;68: 423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller EC, Espinoza MDZ, Huang Y, et al. Maternal race/ethnicity, hypertension, and risk for stroke during delivery admission. J Am Heart Assoc 2020; 9(3):e014775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustafa R, Ahmed S, Gupta A, et al. A comprehensive review of hypertension in pregnancy. J Pregnancy 2012;2012:105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher C, MacLean M, Morecroft I, et al. Is the pregnancy hormone relaxin also a vasodilator peptide secreted by the heart? Circulation 2002;106(3): 292–5. [DOI] [PubMed] [Google Scholar]
- 9.Lumbers ER, Pringle KG. Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol 2014;306(2):R91–101. [DOI] [PubMed] [Google Scholar]
- 10.Bello NA, Woolley JJ, Cleary KL, et al. Accuracy of blood pressure measurement devices in pregnancy: a systematic review of validation studies. Hypertension 2018;71(2):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ACOG practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol 2019;133(1): e26–50. [DOI] [PubMed] [Google Scholar]
- 12.ACOG practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol 2019; 133(1):e1–25. [DOI] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, Detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American heart association Task Force on clinical practice guidelines. Hypertension 2018;71(6): 1269–324. [DOI] [PubMed] [Google Scholar]
- 14.Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation 2014;129(11):1254–61. [DOI] [PubMed] [Google Scholar]
- 15.Lecarpentier E, Tsatsaris V, Goffinet F, et al. Risk factors of superimposed preeclampsia in women with essential chronic hypertension treated before pregnancy. PLoS One 2013;8(5):e62140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chappell LC, Enye S, Seed P, et al. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension 2008;51(4):1002–9. [DOI] [PubMed] [Google Scholar]
- 17.Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2014; (2): Cd002252. [DOI] [PubMed] [Google Scholar]
- 18.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med 2015;372(5):407–17. [DOI] [PubMed] [Google Scholar]
- 19.Magee LA, von Dadelszen P, Singer J, et al. The CHIPS randomized controlled trial (control of hypertension in pregnancy study): is severe hypertension just an elevated blood pressure? Hypertension 2016;68(5):1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezk M, Ellakwa H, Gamal A, et al. Maternal and fetal morbidity following discontinuation of antihypertensive drugs in mild to moderate chronic hypertension: a 4-year observational study. Pregnancy Hypertens 2016;6(4):291–4. [DOI] [PubMed] [Google Scholar]
- 21.Nakhai-Pour HR, Rey E, Berard A. Discontinuation of antihypertensive drug use during the first trimester of pregnancy and the risk of preeclampsia and eclampsia among women with chronic hypertension. Am J Obstet Gynecol 2009;201(2): 180.e1–8. [DOI] [PubMed] [Google Scholar]
- 22.Meher S, Duley L, Hunter K, et al. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta-analysis. Am J Obstet Gynecol 2017;216(2): 121–8.e2. [DOI] [PubMed] [Google Scholar]
- 23.Roberge S, Nicolaides K, Demers S, et al. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 2017;216(2): 110–20.e6. [DOI] [PubMed] [Google Scholar]
- 24.ACOG committee opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol 2018;132(1): e44–52. [DOI] [PubMed] [Google Scholar]
- 25.LeFevre M Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:819–26. [DOI] [PubMed] [Google Scholar]
- 26.Boesen EI. Consequences of in-utero exposure to antihypertensive medication: the search for definitive answers continues. J Hypertens 2017;35(11): 2161–4. [DOI] [PubMed] [Google Scholar]
- 27.Bateman BT, Heide-Jorgensen U, Einarsdottir K, et al. Beta-blocker use in pregnancy and the risk for congenital malformations: an international cohort study. Ann Intern Med 2018;169(10):665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster LM, Myers JE, Nelson-Piercy C, et al. Labetalol versus nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: a randomized controlled trial. Hypertension 2017;70(5): 915–22. [DOI] [PubMed] [Google Scholar]
- 29.Ferrer RL, Sibai BM, Mulrow CD, et al. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol 2000;96(5, Part 2):849–60. [DOI] [PubMed] [Google Scholar]
- 30.Easterling TR, Carr DB, Brateng D, et al. Treatment of hypertension in pregnancy: effect of atenolol on maternal disease, preterm delivery, and fetal growth. Obstet Gynecol 2001;98(3):427–33. [DOI] [PubMed] [Google Scholar]
- 31.Zulfeen M, Tatapudi R, Sowjanya R. IV labetalol and oral nifedipine in acute control of severe hypertension in pregnancy–A randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2019;236:46–52. [DOI] [PubMed] [Google Scholar]
- 32.Cockburn J, Moar VA, Ounsted M, et al. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet 1982;1(8273):647–9. [DOI] [PubMed] [Google Scholar]
- 33.Collins R, Yusuf S, Peto R. Overview of randomised trials of diuretics in pregnancy. Br Med J (Clin Res Ed 1985;290(6461):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magee LA, Cham C, Waterman EJ, et al. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ 2003;327(7421):955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Physical activity and exercise during pregnancy and the postpartum period: ACOG committee opinion, number 804. Obstet Gynecol 2020;135(4):e178–88. [DOI] [PubMed] [Google Scholar]
- 36.Courtney AU, O’Brien EC, Crowley RK, et al. DASH (Dietary Approaches to Stop Hypertension) dietary pattern and maternal blood pressure in pregnancy. J Hum Nutr Diet 2020;33(5):686–97. [DOI] [PubMed] [Google Scholar]
- 37.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet Gynecol 2013;122(5):1122–31. [DOI] [PubMed] [Google Scholar]
- 38.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. the eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118(Suppl 1): 1–203. [DOI] [PubMed] [Google Scholar]
- 39.ACOG committee opinion No. 767: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol 2019;133(2):e174–80. [DOI] [PubMed] [Google Scholar]
- 40.Cotton DB, Jones MM, Longmire S, et al. Role of intravenous nitroglycerin in the treatment of severe pregnancy-induced hypertension complicated by pulmonary edema. Am J Obstet Gynecol 1986; 154(1):91–3. [DOI] [PubMed] [Google Scholar]
- 41.Crowther CA, Brown J, McKinlay CJ, et al. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev 2014; (8):Cd001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke 2009;40(4):1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet 2009;374(9694): 979–88. [DOI] [PubMed] [Google Scholar]
- 44.Bramham K, Nelson-Piercy C, Brown MJ, et al. Postpartum management of hypertension. BMJ 2013; 346:f894. [DOI] [PubMed] [Google Scholar]
- 45.Powles K, Gandhi S. Postpartum hypertension. CMAJ 2017;189(27):E913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ACOG committee opinion No. 736: Optimizing postpartum care. Obstet Gynecol 2018;131(5):e140–50. [DOI] [PubMed] [Google Scholar]
- 47.Walters BN, Walters T. Hypertension in the puerperium. Lancet 1987;2(8554):330. [DOI] [PubMed] [Google Scholar]
- 48.Clapp MA, Little SE, Zheng J, et al. A multi-state analysis of postpartum readmissions in the United States. Am J Obstet Gynecol 2016;215(1):113.e1–10. [DOI] [PubMed] [Google Scholar]
- 49.Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol 2017;130(2):366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol 2012;206(6):470–5. [DOI] [PubMed] [Google Scholar]
- 51.Bateman BT, Schumacher HC, Bushnell CD, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology 2006;67(3): 424–9. [DOI] [PubMed] [Google Scholar]
- 52.Aziz A, Gyamfi-Bannerman C, Siddiq Z, et al. Maternal outcomes by race during postpartum readmissions. Am J Obstet Gynecol 2019;220(5): 484.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bello NA, Miller E, Cleary K, et al. Out of office blood pressure measurement in pregnancy and the postpartum period. Curr Hypertens Rep 2018; 20(12):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Status of state Medicaid expansion decisions: Interactive Map. Kaiser Family Foundation; 2020. Accessed, 2020.
- 55.America’s health Rankings analysis of U.S. Census Bureau. United Health Foundation; 2019. Accessed, 2020.
- 56.Sicker Hadley J. and poorer–the consequences of being uninsured: a review of the research on the relationship between health insurance, medical care use, health, work, and income. Med Care Res Rev 2003;60(2 Suppl):3S–75S [discussion: 76S–112S]. [DOI] [PubMed] [Google Scholar]
- 57.ACOG Committee opinion no. 552: benefits to women of Medicaid expansion through the Affordable Care Act. Obstet Gynecol 2013;121(1):223–5. [DOI] [PubMed] [Google Scholar]
- 58.Brown CC, Moore JE, Felix HC, et al. Association of state medicaid expansion status with low birth weight and preterm birth. JAMA 2019;321(16): 1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyon SM, Douglas IS, Cooke CR. Medicaid expansion under the Affordable Care Act. Implications for insurance-related disparities in pulmonary, critical care, and sleep. Ann Am Thorac Soc 2014; 11(4):661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thoulass JC, Robertson L, Denadai L, et al. Hypertensive disorders of pregnancy and adult offspring cardiometabolic outcomes: a systematic review of the literature and meta-analysis. J Epidemiol Community Health 2016;70(4):414–22. [DOI] [PubMed] [Google Scholar]
- 61.Vahedi FA, Gholizadeh L, Heydari M. Hypertensive disorders of pregnancy and risk of future cardiovascular disease in women. Nurs Womens Health 2020;24(2):91–100. [DOI] [PubMed] [Google Scholar]
- 62.Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Ther Adv Cardiovasc Dis 2008;2(4): 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the american heart association. Circulation 2011; 123(11):1243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenbloom JI, Lewkowitz AK, Lindley KJ, et al. Expectant management of hypertensive disorders of pregnancy and future cardiovascular morbidity. Obstet Gynecol 2020;135(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


