Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent disorder characterized by symptoms of inattention, hyperactivity, and/or impulsivity, as well as executive dysfunction. Recent work underlines the importance in understanding the role of emotion reactivity and regulatory deficits in the context of the disorder. One study (i.e., Musser et al. 2011) utilized a positive and negative emotion induction and suppression task, as well as indexes of autonomic nervous system reactivity, to examine emotional functioning in youth with ADHD. This study revealed inflexible parasympathetic-based regulation across emotion conditions among youth with ADHD compared to typically developing youth. The present study sought to replicate and extend these findings to a clinically recruited, diverse sample, while also examining sympathetic functioning. Two hundred fifty-nine participants (160 youth with ADHD), aged 5 to 13, completed the task utilized in Musser et al. 2011, while indexes of parasympathetic (i.e., respiratory sinus arrhythmia [RSA]) and sympathetic (i.e., pre-ejection period [PEP] and electrodermal activity [EDA]) reactivity were obtained. ADHD was associated with significantly elevated parasympathetic (i.e., augmented RSA) and sympathetic (as indexed by EDA) reactivity. Overall, results replicate and extend Musser et al. 2011, revealing sympathetic-linked disruptions in emotion reactivity and parasympathetic-linked disruptions in emotion regulation among youth with ADHD. Future studies of behavioral therapies for ADHD should consider the efficacy of adding an emotion regulation skills training component.
Keywords: Attention-deficit/hyperactivity disorder (ADHD), Autonomic reactivity, Electrodermal activity (EDA), Emotion regulation, Pre-ejection period (PEP), Respiratory sinus arrhythmia (RSA)
Attention-deficit/hyperactivity disorder (ADHD) is characterized by inattention, hyperactivity, and/or impulsivity, as well as impairment across contexts (American Psychiatric Association 2013). ADHD is one of the most prevalent psychiatric disorders of childhood, affecting seven to 11 % of youth, ages four to seventeen years, in the United States (Visser et al. 2014; Vitola et al. 2017). Although ADHD is often conceptualized as a disorder of deficits in attention or executive functioning, recent work has established the need to integrate emotional functioning into the conceptualization of this disorder (Graziano and Garcia 2016; Musser et al. 2011; Shaw et al. 2014), as well as clinical care (Barkley and Fischer 2010; Wehmeier et al. 2010). Several reviews (e.g., Martel 2009; Shaw et al. 2014) and a recent meta-analysis (e.g., Graziano and Garcia 2016) support this assertion. For example, a recent meta-analysis of 77 studies revealed youth with ADHD display both greater emotion dysregulation (weighted ES d = .80) and excessive negative emotion reactivity (weighted ES d = .95), with effect sizes similar to, if not larger than, those reported in the literature on executive dysfunction (d = .46–.69; Graziano and Garcia 2016; Willcutt et al. 2005). These findings emphasize the importance of considering emotion reactivity and regulation in order to further understand ADHD.
Emotion reactivity, in the context of this study, is the intensity of the “bottom-up” emotional response to a stimulus (Rothbart and Derryberry 1981). Relatedly, emotion regulation is defined as the “top-down” manipulation of an emotional response, which can occur behaviorally, via effortful cognitive control, and/or physiologically (Gross 1998). Behaviors that commonly characterize emotion dysregulation among youth with ADHD include emotional impulsiveness and difficulty managing the intensity of emotional states (Barkley 2010; Bunford et al. 2015; Graziano and Garcia 2016). Emotion reactivity and regulation are associated with changes in autonomic nervous system functioning, which can be indexed via psychophysiological measures (Bunford et al. 2015).
Autonomic Indexes of Emotional Functioning
Psychophysiological measurements via electrocardiogram, impedance cardiography, and electrodermagraphy can be used to derive indexes of autonomic nervous system functioning. Respiratory sinus arrhythmia (RSA), derived from electrocardiogram data, is a reliable and valid index of parasympathetic nervous system activity. RSA is specifically related to parasympathetic control of heartrate through efferent vagus nerve activity, as empirically demonstrated by pharmacological blockade studies (Beauchaine 2001; Berntson et al. 1993; Hayano et al. 1991). Prior work demonstrates that, in specific contexts, greater RSA reactivity is associated with emotion dysregulation (Berntson et al. 1997; Calkins 2007; Eisenberg et al. 1995; Porges et al. 1996).
Relatedly, impedance cardiography can be utilized to derive cardiac pre-ejection period (PEP), an index of sympathetic nervous system activity (Sherwood et al. 1991). PEP is the interval between contraction of the left ventricle and the onset of ejection of blood into the aorta and is a commonly utilized index of beta-adrenergic influence over the heart (Beauchaine 2001; Berntson et al. 1997). PEP has been associated with a variety of sympathetically mediated functions, including emotional reactivity, mental effort, reward sensitivity, and approach behaviors (Beauchaine 2001; Crowell et al. 2006; Kelsey et al. 2007). Shortening of PEP in response to emotionally evocative contexts generally indexes greater sympathetic influence over heartrate associated with emotional reactivity (Brenner and Beauchaine 2011; Brenner et al. 2005).
Similarly, electrodermal activity (EDA; Fowles 1986) has been utilized to index sympathetic activity. Increased activity in cholinergic fibers, which directly affect the activity of the eccrine sweat glands, is associated with greater sympathetic activity (Beauchaine 2001; Cacioppo et al. 2007; Fowles 1986; Shields et al. 1987; Uno, 1977). Previous literature has indicated EDA as being highly correlated with sympathetic activity (Wallin 1981), as well as emotional arousal/reactivity (Bradley et al. 1990); particularly for negative, avoidance-based emotions (e.g., anxiety and/or stress; Salminen et al. 2013). Thus, EDA and PEP serve as indexes of sympathetic-based emotion reactivity, while RSA is an index of parasympathetically-based emotion regulation.
Emotion Dysregulation in Youth with ADHD via Autonomic Indexes
Over the past decade, several studies have expanded the understanding of emotion functioning among youth with ADHD via autonomic indexes. Prior studies have utilized psychophysiological measures to index autonomic functioning during emotion regulation tasks (e.g., social rejection and frustration tasks) or in response to emotionally evocative stimuli (e.g., International Affective Picture System [IAPS]; Beauchaine et al. 2013; Conzelmann et al. 2014; Lang et al. 1999; Leaberry et al. 2018; McQuade and Breaux 2017; Taskiran et al. 2018). However, these studies often examine a singular process (i.e., only emotional reactivity or only emotion regulation) or use methodology that does not adequately distinguish the two processes. Thus, these studies do not allow for an investigation of both emotion reactivity and regulatory mechanisms underlying emotion-related functioning in youth with ADHD. To our knowledge, only one prior study has specifically probed both emotion reactivity and regulation of both negative and positive emotion, with the use of an emotion induction and suppression design, in youth with and without ADHD (i.e., Musser et al. 2011).
Examining Emotion Regulation by the Elicitation of Induction and Suppression of Emotion
Suppression of an emotion, here, is defined as the purposeful act of inhibiting one’s emotional expression during an emotionally arousing activity, whereas induction involves prompting for the active experience and expression of said emotion (Gross 1998; Gross and Levenson 1993, 1997). Suppression and induction of emotions within an artificial setting (e.g., research lab) has been successfully completed through instructing participants to either conceal or exhibit the emotion during the presentation of an emotionally arousing video clip (e.g., Beauchaine et al. 2001; Ehring et al. 2010; Gross 1998; Musser et al. 2011).
Of particular interest with regard to the present study, Musser et al. 2011 evaluated emotion reactivity and regulation within the context of induction and suppression conditions, while utilizing positive and negative valence film clips. Electrocardiogram and impedance cardiography data were obtained during each condition (i.e., negative suppression, positive suppression, negative induction, and positive induction of emotions) within a well-characterized group of youth with ADHD and typically developing youth. Results indicated that among youth with ADHD, RSA was augmented (i.e., increased) from baseline levels of functioning across task conditions. Thus, less flexible emotion regulation was observed in response to task demands among youth with ADHD compared to typically developing youth. However, no group differences in PEP reactivity were observed, implying that sympathetic functioning among youth with ADHD in an emotional context is intact.
Replication and Extension of the Study of Emotion Induction and Suppression in ADHD
While the Musser et al. 2011 study has many notable strengths, including the well-characterized sample, use of a task that allowed for induction and suppression of both negative and positive emotions, as well as indexing of both autonomic branches, it also included limitations. These limitations include a relatively small sample size (i.e., 32 youth with ADHD and 34 typically developing youth) with insufficient power to fully examine ADHD heterogeneity with respect to Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5; American Psychiatric Association 2013) presentation and/or which symptom domain(s) contributed to the results. Additionally, the sample was highly limited in ethnic and racial diversity and was recruited from the community, resulting in limitations in generalizability. Finally, the index of sympathetic functioning utilized (e.g., PEP activity) in Musser et al. 2011 may be more appropriately interpreted as an index of approach-based reward responding (Brenner et al. 2005) than a broad index of sympathetic control. Thus, specific measures associated with emotional functioning in negative or avoidance-based emotional domains, such as EDA, are needed. Prior work suggests reduced EDA levels in youth with ADHD during rest and emotionally evocative tasks, when compared to typically developing peers (Barry et al. 2012; Conzelmann et al. 2014; Losoya 1995; Satterfield and Dawson 1971). However, such prior work has not simultaneously examined indexes of sympathetic (e.g., EDA) and parasympathetic (e.g., RSA) functioning in youth with and without ADHD.
The Present Study
The current study seeks to replicate Musser et al. 2011, while extending the study’s methods to include EDA measurements in a larger, more ethnically and racially diverse sample that is clinically recruited. Given the use of a clinical sample, which are generally believed to be characterized by greater symptom severity/impairment (Surman et al. 2010), results and effect sizes are expected to be similar to or greater than those observed in Musser et al. 2011. That is, with respect to RSA reactivity, youth with ADHD are expected to be elevated from baseline during task conditions and less flexible across task conditions in comparison to typically developing youth. As Musser et al. 2011 did not identify significant group differences in PEP reactivity, no hypotheses regarding PEP reactivity are predicted in the current study. However, the results of analyses examining PEP are reported, as Musser et al. 2011 may have been under powered to detect effects. With respect to EDA, youth with ADHD are expected to experience reduced EDA reactivity across task conditions compared to typically developing youth. Further, prior work has demonstrated significant differences in emotion reactivity and regulation according to ADHD presentation and symptom domain (i.e., inattention, hyperactivity/impulsivity) at the behavioral level of analysis (see Martel 2009; Maedgen and Carlson 2000), such that youth with predominantly inattentive presentation (ADHD-I) have been shown to have poor emotion regulation, while youth with the predominantly hyperactivity/impulsive presentation (ADHD-HI) have been shown to demonstrate atypical emotion reactivity. Thus, the current study additionally investigates whether such differences were also present at the psychophysiological level of analysis, and explored these associations in follow-up analyses examining 1) ADHD DSM-5 presentation and 2) ADHD-I and ADHD-HI symptom domains.
Method
Participants
Two hundred fifty-nine youth, ages five to thirteen years (M = 8.93, SD = 1.84), participated in the current study. One hundred sixty met DSM-5 (American Psychiatric Association 2013) criteria for ADHD, while 99 were typically developing comparison youth. The majority of the sample identified as Hispanic/Latinx (i.e., 88.66% Hispanic/Latino), consistent with the geographic location of the study. Further, 80.62% identified as racially Caucasian, 9.30% as African American, 1.16% as Asian, and 8.92% as another race or multiple races, which is also consistent with the demographics of the geographic location of the study. Thus, overall, the sample represents a demographic which has traditionally been underrepresented in research on mental health. The age range of youth (i.e., five to thirteen years spanning middle childhood), was specifically selected as it is the most common period in which youth are diagnosed with ADHD (Polanczyk et al. 2007) and corresponds with the developmental period selected in the original study (i.e., Musser et al. 2011).
Recruitment and identification
All participants were approved through the institutional review board at Florida International University, with youth ascenting and parents consenting to the study. However, in contrast to Musser et al. 2011, which utilized a community sample, the present study utilized a clinical sample. Specifically, youth with ADHD were recruited from a double-masked, crossover study examining tolerance to stimulant medication via an annual Summer Treatment Program (STP). Recruitment for the STP was completed through multiple platforms, including the university’s clinical treatment center, school personnel (e.g., teachers), physicians, and advertisements (e.g., billboard, newspaper, postal service, and radio). Inclusion criteria for STP included a current DSM-5 diagnosis of ADHD and an estimated Full-Scale Intelligence Quotient (IQ) score > 80. Exclusion criteria for STP included demonstrated intolerance to methylphenidate or OROS methylphenidate at the highest therapeutic dose (e.g., hypertension, Tourette’s disorder, arrhythmias, and mania/psychosis). Meeting full diagnostic criteria for autism was also exclusionary. Additional exclusion criteria specific to the present study, based upon parent report, included the use of psychotropic medication for any disorders other than ADHD in the previous six months, as well as the presence of any cardiovascular, developmental, neurological disorders, major depressive disorder, and/or mania or psychosis.
In contrast to the original study (i.e., Musser et al. 2011), in which youth with ADHD and typically developing youth were both collected through the community, only typically developing youth were recruited through the community in the current study. As such, typically developing comparison youth were recruited through advertisements (e.g., newspapers, electronic mails, and flyers in the university’s treatment center) and community events (e.g., family-oriented expos and local school events). Exclusion criteria for typically developing youth included the use of any psychoactive medication (including stimulants), estimated Full-Scale IQ score < 80, the presence of any cardiovascular, developmental disorder, neurological disorder, major depressive disorder, and/or mania or psychosis. Finally, typically developing youth were excluded if they presented with more than three symptoms of ADHD.
To examine eligibility, as well as obtain clinical, demographic, and diagnostic information, parents of participants completed a demographic survey and the Diagnostic Interview Schedule for Children Version Four (DISC-IV; Shaffer et al. 2000). Parents and teachers of youth with ADHD completed the Disruptive Behavior Disorders Rating Scale (Pelham et al. 1992) and Pittsburgh Modified Conner’s Rating Scale (Pelham et al. 2005a). Likewise, parents of typically developing youth completed similar measures; however teacher ratings of typically developing youth were not available. Both youth with ADHD and typically developing youth completed the Wechsler Abbreviated Scale of Intelligence Second Edition (Wechsler 2011) to obtain an estimated Full-Scale IQ.
Final ADHD and other diagnoses
The diagnostic process for identifying youth with ADHD was completed using best-practice recommendations (Pelham et al. 2005b). Specifically, parent and teacher rating scales were utilized to identify ADHD symptoms according to DSM-5 (i.e., Disruptive Behavior Disorder Scale and Pittsburgh Modified Conner’s Rating Scale 2005a); Pelham et al. 1992). Parent and teacher ratings of impairment were utilized to identify cross-situational impairment (i.e., Impairment Rating Scale; Fabiano et al. 2006). A parent clinical interview was utilized to obtain corroborating information, as well as to obtain information regarding comorbid diagnoses and symptoms (e.g., DISC-IV; Shaffer et al. 2000). Two Ph.D. level clinicians reviewed all information to determine final diagnoses of ADHD and comorbid disruptive disorders (e.g., anxiety, conduct disorder [CD], oppositional defiant disorder [ODD]). In the event that consensus was not obtained (i.e., less than 1% of cases), a third clinician was consulted, and the majority opinion was utilized. Diagnoses other than ADHD, CD, and ODD (e.g., anxiety disorders) were determined by parental endorsement on the DISC-IV.
Medication washout
Youth diagnosed with ADHD were required to partake in a washout period of seven half-lives of their prescribed stimulant medication dosage (i.e., approximately forty-eight hours) prior to completing study tasks.
Task and Psychophysiology Recording Procedures
Procedures for this study were identical to those utilized in Musser et al. 2011 and are described here in brief.
Baseline task conditions
Psychophysiology data was recorded during a two-minute resting baseline prior to the task and during two neutral baselines, while participants viewed a set of 10 neutral pictures from the IAPS (Lang et al. 1999). During the neutral periods, which occurred before the task and between valence conditions to account for and reduce carry-over effects, participants rated each neutral picture using the Self-Assessment Manikin (SAM) valence and arousal scales (Bradley and Lang 1994). Neutral baselines were utilized to calculate change scores to account for psychophysiological responses associated with attending and orienting.
Emotion induction and suppression task
Participants were informed they would be watching clips from the movie Homeward Bound, in which two dogs and a cat are separated and reunited with their human family. Each clip from Homeward Bound has been shown in prior work to induce either positive or negative emotions, with the first two clips being associated with negative emotions (e.g., separation from family) and the last two clips associated with positive emotions (e.g., reunion with family; Musser et al. 2011).
Using procedures identical to Musser et al. 2011, induction and suppression demands were incorporated via instructions to the participants. Specifically, during the induction conditions, participants were instructed to express the emotion they believed was experienced by the main character (e.g., if the child believed the main character was happy, the child would express that emotion). In the suppression conditions, participants were instructed not to think about the emotion of the main character and to “keep it a secret” by retaining a neutral face (i.e., suppressing the emotion). In order to further validate the valence and arousal level associated with each clip, participants completed SAM valence and arousal scales after each clip. In keeping with Musser et al. 2011, all participants completed the task in the same order: resting baseline, neutral period, negative induction, negative suppression, neutral period, positive induction, and positive suppression.
Psychophysiology recording and processing overview
Identical psychophysiological indexes were obtained to those in Musser et al. 2011, with the addition of EDA. To obtain psychophysiological indexes continuously across task conditions, disposable silver/silver-chloride electrodes were placed in an electrocardiogram and impedance cardiography configuration (for added details see Musser et al. 2011). Additionally, EDA electrodes with 0 % chloride were placed on the palm of the non-dominant hand at roughly the thenar and hypothenar muscles. In processing all psychophysiological data, in the event that 10 s or more of a 60 s epoch were determined to contain artifact or missing data, then the entire epoch was excluded and subsequently imputed (below). This occurred in fewer than 5 % of cases across all psychophysiological variables.
RSA.
RSA was derived in 60 s epochs using the detrended R-R time series, which was derived from electrocardiogram, and then submitted to a Fourier transformation. The high frequency respiratory band (ms2) was set over the respiratory frequency band of 0.24 to 1.04 Hz and estimated via ICG. Respiratory rates were derived from the impedance cardiography signal (Z0) to verify that signals remained within the analytic bandwidth. R-R waves were inspected for artifacts by visual inspection and MindWare Heart Rate Variability V.3.1. Among typically developing youth 3.50% of cases were edited for artifacts, while among youth with ADHD, on average 2.80% of cases were edited for artifacts. Thus, groups did not differ with respect to presence of artifacts (χ2 = 0.10, p = 0.75). Inter-rater reliability (k > 0.90) was established by two raters examining 20% of the data from each condition.
PEP.
PEP was derived at 60 s epochs from electrocardiogram and impedance cardiography with MindWare Impedance Cardiography V.3.1. PEP was indexed as milliseconds from the onset of the Q-wave to the B-point of the dZ/dt wave. Artifacts were examined and removed through the MindWare software and through visual inspection. Among typically developing youth 4.50% of cases were edited for artifacts. Similarly, among youth with ADHD, 3.90% of cases were edited for artifacts. Thus, groups did not differ with respect to presence of artifacts (χ2 = 0.06, p = 0.81). Inter-rater reliability (k > 0.85) was established by two raters examining 20% of the data obtained from each condition.
EDA.
EDA was recorded at a rate of 1000 samples per second and derived at 60 s epochs. Artifacts were examined and removed by MindWare EDA V.3.1.1 and through visual inspection. Criteria for a skin conductance response (SCR) included at least 0.05 microsiemens of a difference from peak and to trough, and an SCR duration of no more than 10 s with at least 0.25 s between each SCR. The value used during analysis was mean skin conductance. Among typically developing youth 4.30% of cases were edited for artifacts. Among youth with ADHD, on average 4.30% of cases were edited for artifacts. Thus, groups did not differ with respect to presence of artifacts (χ2 < 0.01, p = 0.99). Inter-rater reliability (k > 0.90) was established by two raters examining 20% of the data obtained from each condition.
Analytic Plan
Primary analyses were completed in a manner identical to Musser et al. 2011 to compare replicability of results. As such, a repeated measures ANCOVA (RM-ANCOVA) was conducted with covariates identical to those in Musser et al. 2011, including youth’s biological sex and total number of comorbid diagnoses of ODD, CD, and/or anxiety. Missing data was handled through multiple imputation. Here, missing data ranged from 1.5% to 12.8% of cases missing RSA, PEP, and/or EDA baseline and/or reactivity scores for one or more task conditions. More specifically, typically developing youth were missing approximately 1.01% to 12.20% cases, while youth with ADHD were missing approximately 1.20% to 11.20% cases. No demographic information or SAM valence and arousal ratings were determined to be missing. Overall, data was determined to be missing at random, and groups did not differ with respect to the amount or type of data missing.
Specificity of effect via ADHD DSM-5 presentation and symptom domains.
Post-hoc analyses were conducted in order to examine the effects of each DSM-5 ADHD presentation (via RM-ANCOVA) and each continuous ADHD symptom domain (i.e., inattention and hyperactivity/impulsivity via Linear Mixed Effects Multi Level Models [MLM]).
Power Analysis
G*Power revealed with a sample size of 259, a post-hoc power analysis for RM-ANOVA with two levels would have adequate power (b = 0.99, p < 0.05) to detect moderate effects (Cohen’s d > 0.30). With the addition of youth’s biological sex and total number of comorbid diagnoses (i.e., anxiety, CD, and ODD) as covariates, power was reduced only slightly.
Results
Preliminary Analyses
Descriptive and diagnostic statistics
As presented in Table 1, age, ethnicity, and IQ did not differ between groups; inclusion of these variables did not affect results, and thus, these variables were excluded from further analyses. However, groups differed significantly according to youth’s biological sex, χ2(1) = 14.41, p < 0.001, Cramer’s V > 0.23, with ADHD group being more likely to be male, as in prior literature (Anderson et al. 1987; Gaub and Carlson 1997; Gershon 2002; see Table 1). Thus, youth’s biological sex was covaried in all results. Although family income differed significantly between ADHD and typically developing youth, F(1,187) > 5.54, p = 0.02, ηp2 > 0.02, family income did not affect results; and was not included as a covariate.
Table 1.
Descriptive and diagnostic statistics for Attention-Deficit/Hyperactivity Disorder (ADHD) and typically developing (TD) groups
| Variable | TD (n = 99) | ADHD (n = 160) | F / χ2 | ηp2< / Cramer’s V < |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 9.08 (1.93) | 8.78 (1.74) | 1.63 | 0.01 |
| Gender (% male) | 56.57 | 78.75 | 14.41*** | 0.24 |
| Ethnicity (% Hispanic or Latino) | 86.81 | 89.74 | 0.49 | 0.05 |
| WASI-II FSIQ, mean (SD) | 100.21 (12.69) | 97.01 (12.82) | 3.59 | 0.02 |
| Household Income, mean (SD) | 88,248.60 (50,231.79) | 63,978.36 (63,978.36) | 5.54* | 0.03 |
| Previously received Medication for behavior, emotional or psychiatric problems (% received) | – | 71.25 | – | – |
| Parent Disruptive Behavior Disorder Rating Scale | ||||
| Inattention Symptoms | 0.36 (0.42) | 2.07 (0.64) | 517.45*** | 0.68 |
| Hyperactivity/Impulsivity Symptoms | 0.29 (0.31) | 1.78 (1.00) | 399.71*** | 0.62 |
| Total ADHD Symptoms | 0.33 (0.31) | 1.93 (0.67) | 618.60*** | 0.72 |
| CD Symptoms | 0.02 (0.04) | 0.20 (0.20) | 71.89*** | 0.23 |
| ODD Symptoms | 0.21 (0.30) | 1.25 (0.68) | 191.03*** | 0.44 |
| Teacher Disruptive Behavior Disorder Rating Scale | ||||
| Inattention Symptoms | – | 2.14 (0.72) | – | – |
| Hyperactivity/Impulsivity Symptoms | – | 1.77 (0.85) | – | – |
| Total ADHD Symptoms | – | 1.95 (0.66) | – | – |
| CD Symptoms | – | 1.91 (0.78) | – | – |
| ODD Symptoms | – | 1.20 (0.89) | – | – |
| Comorbid Disorders (% Diagnosis) | ||||
| Anxiety | 7.53 | 14.47 | 2.69 | 0.11 |
| CD | 0 | 8.75 | 9.16** | 0.19 |
| ODD | 2.02 | 64.38 | 98.65*** | 0.62 |
indicates p < 0.001;
indicates p < 0.01;
indicates p < 0.05;
% = percentage; SD = Standard Deviation; WASI-II = Wechsler Abbreviated Scale of Intelligence-Second Edition; FSIQ = Full-Scale Intelligence Quotient (estimated); ODD = Oppositional Defiant Disorder; CD = Conduct Disorder; Missing data handled via listwise deletion
Clinical characteristics are also included in Table 1. Scores on the Parent Disruptive Behavior Disorder Rating Scale differed significantly between parents of ADHD and typically developing youth, as expected, F(1,248) > 618.60, p < 0.001, ηp2 > 0.71. The total number of comorbid diagnoses (i.e., anxiety, CD, and ODD) also differed significantly between the groups and was treated as a covariate, χ2(3) > 90.17, p < 0.001, Cramer’s V > 0.10.
Effectiveness of emotion induction by self-report
A 2 ×2×2 RM-ANOVA (valence[negative/positive] × regulation [induction/suppression] × group[control/ADHD]) was used to assess SAM valence and arousal scores across the four task conditions (i.e., negative induction, negative suppression, positive induction, and positive suppression).
With respect to valence (i.e., ranging from 1 [unpleasant] to 5 [pleasant]), similar to Musser et al. 2011, there was a significant main effect of valence conditions (i.e., negative versus positive), F(1,245) = 44.02, p < 0.001, ηp2 = 0.15, and a significant main effect of regulation conditions (i.e., induction versus suppression), F(1,245) = 5.87, p = 0.02, ηp2 = 0.02. Across the full sample, youth rated the positive conditions as more pleasant (M = 4.54, S.E. = 0.05) compared to the negative conditions (M = 2.47, S.E. = 0.07). Additionally, youth rated the induction condition as more pleasant (M = 3.77, S.E. = 0.06) compared to the suppression conditions (M = 3.24, S.E. = 0.06). Similar to Musser et al. 2011, none of the interactions by ADHD group status were significant, all F(1,245) < 0.02, p > 0.80, ηp2 < 0.001. Further, the main effect of group was not significant, F(1,245) = 0.07, p = 0.79, ηp2 < 0.001. Thus, youth with ADHD and typically developing youth did not differ significantly in their SAM valence ratings across task conditions.
With respect to SAM arousal ratings, neither the main effects of task valence conditions, F(1,245) = 0.16, p = 0.69, ηp2 = 0.001, nor regulation conditions were significant, F(1,245) = 0.09, p = 0.77, ηp2 < 0.001, respectively. Additionally, the interaction of valence by regulation by group was not significant, F(1,245) = 0.21, p > 0.64, ηp2 < 0.001. Thus, overall SAM ratings of arousal on the task conditions did not vary according to group.
Effects of Emotion Induction and Suppression on PEP, RSA, and EDA
Baseline effects
As in Musser et al. 2011, groups did not differ significantly in their SAM ratings of the IAPS neutral pictures utilized during the neutral pictures, all F(1,252) < 2.65, p > 0.10, ƞp2 < 0.012 (see Table 2). However, in contrast to Musser et al. 2011, significant group differences in RSA were present during both resting baseline and each of the neutral baselines, all F(1,255) > 18.52, p < 0.001, ηp2 > 0.06, with youth with ADHD exhibiting lower levels of RSA across baselines than typically developing youth (see Table 3). Additionally, significant group differences in EDA were observed during resting baseline and the first neutral baseline, F(1, 255) > 4.58, p < 0.05, ηp2 > 0.01, but not the second neutral baseline, F(1, 255) < 1.94, p > 0.16, ηp2 < 0.008, with youth with ADHD exhibiting lower EDA activity across baseline conditions compared to typically developing youth (see Table 3). No significant group differences in PEP were observed during baseline conditions, all F(1, 255) < 3.23, p > 0.07, ηp2 < 0.02 (see Table 3).
Table 2.
Self-assessment manikin (SAM) scores across task conditions for Attention-Deficit/Hyperactivity Disorder (ADHD) and typically developing (TD) groups
| Variable (mean, SD) | TD (n = 99) | ADHD (n = 160) | F(1,258) | ηp2< |
|---|---|---|---|---|
| SAM Valence/Pleasure | ||||
| Picture Baseline 1 | 3.34, 0.73 | 3.40, 0.84 | 0.92 | 0.01 |
| Negative Induction | 2.95, 1.28 | 2.82, 1.51 | 0.03 | 0.01 |
| Negative Suppression | 2.12, 1.18 | 2.06, 1.26 | 0.01 | 0.01 |
| Picture Baseline 2 | 3.26, 0.61 | 3.35, 0.86 | 2.64 | 0.02 |
| Positive Induction | 4.69, 0.72 | 4.67, 0.83 | 0.18 | 0.01 |
| Positive Suppression | 4.40, 0.95 | 4.40, 1.09 | 0.04 | 0.01 |
| SAM Intensity/Arousal | ||||
| Picture Baseline 1 | 2.64, 0.85 | 2.56, 0.95 | 0.01 | 0.01 |
| Negative Induction | 3.31, 1.28 | 3.33, 1.54 | 1.83 | 0.01 |
| Negative Suppression | 2.64, 1.34 | 2.70, 1.43 | 0.80 | 0.01 |
| Picture Baseline 2 | 2.80, 0.79 | 2.68, 1.00 | 0.77 | 0.01 |
| Positive Induction | 3.50, 1.41 | 3.21, 1.72 | 0.49 | 0.01 |
| Positive Suppression | 3.71, 1.30 | 3.44, 1.63 | 0.46 | 0.01 |
indicates p < 0.001;
indicates p < 0.01;
indicates p < 0.05;
SD = Standard Deviation; Covariates include youth’s biological sex and comorbidity (i.e., anxiety, CD, and ODD); Missing data handled via listwise deletion
Table 3.
Respiratory sinus arrhythmia (RSA; ms2), pre-ejection period (PEP; ms), and electrodermal activity (EDA; μS) in task epochs for Attention-Deficit/Hyperactivity Disorder (ADHD) and typically developing (TD) groups
| Variable (mean, SD) | TD (n = 99) | ADHD (n = 160) | F(1, 255) | ηp2< |
|---|---|---|---|---|
| Baseline Physiology Data | ||||
| Rest Baseline | ||||
| RSA | 6.85, 1.10 | 6.27, 1.24 | 18.53*** | 0.07 |
| PEP | 98.36, 13.91 | 96.40, 11.57 | 2.62 | 0.01 |
| EDA | 5.09, 3.39 | 3.89, 3.03 | 8.04** | 0.03 |
| Picture Baseline 1 | ||||
| RSA | 6.73, 0.95 | 6.18, 1.28 | 18.90*** | 0.08 |
| PEP | 98.60, 14.92 | 97.77, 12.19 | 0.44 | 0.01 |
| EDA | 6.37, 3.51 | 5.62, 3.31 | 4.59* | 0.02 |
| Picture Baseline 2 | ||||
| RSA | 6.71, 0.98 | 6.07, 1.25 | 19.28*** | 0.07 |
| PEP | 98.84, 14.23 | 97.07, 11.62 | 3.22 | 0.02 |
| EDA | 7.51, 3.07 | 6.94, 3.15 | 1.93 | 0.01 |
| Task Physiological Data | ||||
| Negative Induction | ||||
| RSA | 6.81, 0.99 | 6.41, 1.16 | 12.09** | 0.05 |
| PEP | 99.05, 13.55 | 97.10, 13.26 | 4.06* | 0.02 |
| EDA | 6.76, 3.29 | 6.55, 3.10 | 1.33 | 0.01 |
| Negative Suppression | ||||
| RSA | 6.92, 1.00 | 6.53, 1.21 | 9.57** | 0.04 |
| PEP | 99.05, 13.49 | 96.92, 13.62 | 3.56 | 0.02 |
| EDA | 7.09, 2.99 | 6.88, 3.04 | 0.44 | 0.01 |
| Positive Induction | ||||
| RSA | 6.69, 1.02 | 6.41, 1.21 | 8.12** | 0.06 |
| PEP | 99.27, 13.98 | 98.40, 12.60 | 3.48 | 0.01 |
| EDA | 7.41, 2.99 | 7.14, 2.97 | 0.63 | 0.01 |
| Positive Suppression | ||||
| RSA | 6.85, 0.96 | 6.54, 1.19 | 6.55** | 0.03 |
| PEP | 99.44, 13.21 | 97.58, 12.99 | 3.36 | 0.01 |
| EDA | 7.48, 2.96 | 7.32, 2.94 | 0.52 | 0.01 |
| Physiology Change Scores | ||||
| Negative Induction | ||||
| RSA | 0.10, 0.61 | 0.24, 0.64 | 3.87* | 0.02 |
| PEP | 0.35, 8.03 | −0.66, 8.75 | 4.45* | 0.02 |
| EDA | 0.40, 1.59 | 0.93, 1.88 | 3.92* | 0.02 |
| Negative Suppression | ||||
| RSA | 0.19, 0.65 | 0.36, 0.72 | 5.07* | 0.02 |
| PEP | 0.58, 6.64 | −0.77, 9.15 | 4.30* | 0.02 |
| EDA | 0.75, 1.78 | 1.26, 2.26 | 5.84* | 0.03 |
| Positive Induction | ||||
| RSA | 0.04, 0.72 | 0.43, 0.79 | 7.00** | 0.03 |
| PEP | −0.31, 5.80 | 1.20, 10.53 | 0.18 | 0.01 |
| EDA | −0.07, 1.42 | 0.24, 1.65 | 2.04 | 0.01 |
| Positive Suppression | ||||
| RSA | 0.14, 0.64 | 0.47, 0.73 | 10.22** | 0.05 |
| PEP | 0.62, 7.21 | 0.34, 11.72 | 0.19 | 0.01 |
| EDA | −0.01, 1.50 | 0.42, 1.72 | 2.36 | 0.01 |
indicates p < 0.001;
indicates p < 0.01;
indicates p < 0.05;
SD = Standard Deviation; Covariates include youth’s biological sex and comorbidity (i.e., anxiety, CD, and ODD); Missing data handled via multiple imputation
Overall effects on RSA
RSA raw and reactivity (i.e., change scores from neutral period) scores for each task epoch are listed according to group in Table 3. A 2×2×2 RM-ANOVA (valence[negative/positive] × regulation[induction/suppression] × group[control/ADHD]) examined the effects of task condition on RSA reactivity according to ADHD group status. In contrast to Musser et al. 2011, none of the interactions were significant, all F(1,255) < 0.94, p > 0.34, ηp2 < .004. However, there was a significant main effect of group on RSA reactivity, F(1,255) = 13.02, p < 0.001, ηp2 = 0.049; such that, across task conditions, youth with ADHD exhibited greater RSA augmentation (i.e., increase from neutral baseline to task; M = 0.38, S.E. = 0.06) compared to typically developing youth (M = 0.10, S.E. = 0.08; see Fig. 1).
Fig. 1.
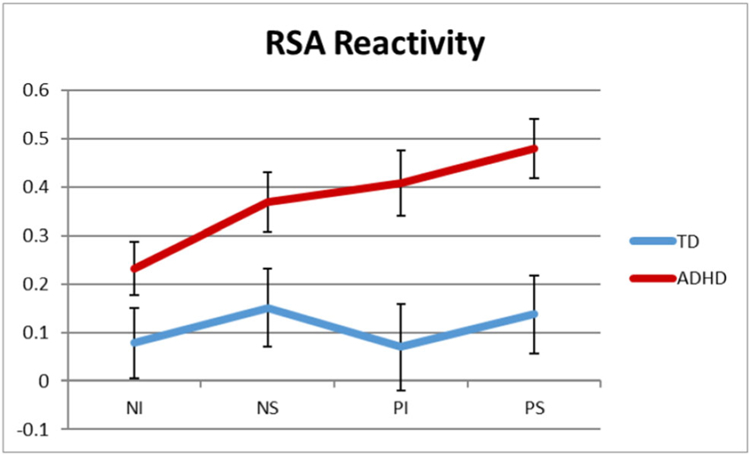
Mean change scores in respiratory sinus arrhythmia (RSA) from neutral period to each of the task epochs for youth with ADHD and typically developing (TD) youth. Neutral period 1 to negative induction (NI), neutral period 1 to negative suppression (NS), neutral period 2 to positive induction (PI), and neutral period 2 to positive suppression (PS). Error bars represent standard error
Overall effects on PEP
PEP raw and reactivity (i.e., change scores from neutral period) scores for each task epoch are listed according to group in Table 3. A 2×2×2 RM-ANOVA examined the main and interaction effects of task valence and regulation condition (by ADHD diagnostic group) on PEP reactivity. Consistent with Musser et al. 2011, neither the main effect of diagnostic status, all F(1,255) < 2.11, p = 0.17, ηp2 = 0.008, nor the interactions by diagnostic status, all F(1,255) < 0.26, p > 0.66, ηp2 = 0.001, were significant (see Fig. 2).
Fig. 2.
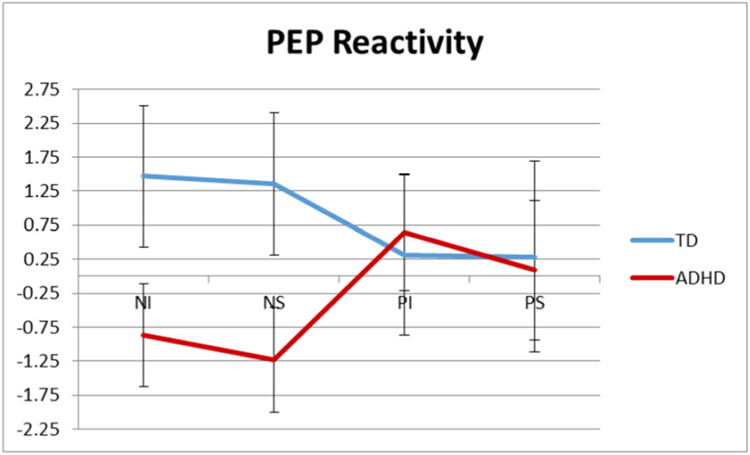
Mean change scores in pre-ejection period (PEP) from neutral period to each of the task epochs for youth with ADHD and typically developing (TD) youth. Neutral period 1 to negative induction (NI), neutral period 1 to negative suppression (NS), neutral period 2 to positive induction (PI), and neutral period 2 to positive suppression (PS). Error bars represent standard error
Overall effects on EDA
To extend Musser et al. 2011, the effects of emotion induction and suppression on EDA were examined. Raw and reactivity (i.e., change scores from neutral period) EDA scores for each task epoch are listed according to group in Table 3. A 2×2×2 RM-ANOVA examined the effects of task condition on EDA reactivity according to ADHD group status. Neither the interactions by diagnostic group were significant, all F(1,255) < 0.78, p > 0.28, ηp2 < 0.005. However, the main effect of group was significant, F(1,255) > 5.78, p = 0.018, ηp2 > 0.02; such that, overall (i.e., across all four task conditions) youth with ADHD exhibited significantly greater EDA augmentation (i.e., increase from neutral baseline to task epoch; M = 0.74, S.E. = 0.15) compared to typically developing youth (M = 0.22, S.E. = 0.20; see Fig. 3).
Fig. 3.
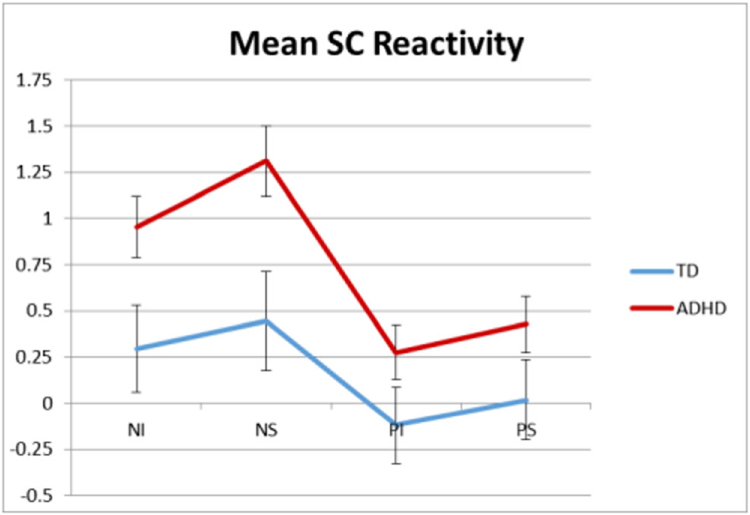
Mean change scores in electrodermal activity (EDA) from neutral period to each of the task epochs for youth with ADHD and typically developing (TD) youth. Neutral period 1 to negative induction (NI), neutral period 1 to negative suppression (NS), neutral period 2 to positive induction (PI), and neutral period 2 to positive suppression (PS). Error bars represent standard error
ADHD presentation and symptom domain effects
A post-hoc analysis was conducted in order to examine the effects of each DSM-5 ADHD presentation (i.e., ADHD-I or ADHD combined presentation [ADHD-C]; ADHD-HI was excluded due to low sample size [n = 15]). Here, 4×2×2 RM-ANOVA examined the effects of task condition on RSA, PEP, and EDA reactivity according to DSM-5 ADHD presentation (and TD status; available in Table S1).
None of the interactions were significant for RSA, PEP, and EDA reactivity, all F(1,253) < 1.14, p> 0.37, ηp2 < 0.02. While group main effects were not significant for PEP reactivity, F(3,253) < 1.82, p > 0.17, ηp2 < 0.03, nor for EDA reactivity, F(3,253) < 2.44, p > 0.11, ηp2 < 0.03, group main effects were significant for RSA reactivity, F(3,253) = 6.14, p < 0.02, ηp2 > .06. However, when the RSA reactivity main effect was probed, significant differences were not observed between youth with ADHD-I and ADHD-C, F(1,255) = 3.49, p= 0.10, ηp2 = 0.03. Similar results were observed when RM-ANOVA was repeated with covariates of youth’s biological sex and any comorbid diagnosis (i.e., anxiety, CD, and ODD), F(3,253) = 4.37, p= 0.08, ηp2 = 0.03. Thus, the effect appears to not be specific to a particular ADHD presentation type.
A series of Linear Mixed Effects MLM were used to predict physiological measures (i.e., RSA, PEP, and EDA in separate models) from task conditions, inattention symptoms, hyperactive/impulsive symptoms, and the interactions of task conditions and symptom type. For RSA, the overall model (i.e., including inattention, hyperactivity/impulsivity, task conditions, and the interaction of task conditions with each symptom domain) was determined to fit the data for RSA, Log-Likelihood = −1000.53, χ2(11) = 30.38, p = 0.0001. Here, none of the symptom or symptom by condition terms were found to be significantly associated with RSA, all B< 0.04, p > 0.09. However, given the marginal effect of the inattention domain, B= 0.04, p= 0.09 and the high correlation between inattention and hyperactivity/impulsivity domains, r= 0.82, p < 0.001, a follow-up model was fit removing the hyperactivity/impulsivity terms. This model was determined to have a similar fit, Log-Likelihood = −1001.62, χ2(7) = 28.13, p = 0.0001. Here, the inattention main effect was found to be significant, B = 0.03, p < 0.001, f2 = 0.02. Neither the full model examining PEP nor the full model examining EDA fit the data, all Log Likelihood<−1800.00, χ2(5) < 5, p> 0.50. Thus, the RSA effect observed in the main analysis appears to be primarily driven by inattention symptoms.
Discussion
This study sought to replicate and extend Musser et al. 2011 by examining indexes of parasympathetic (i.e., RSA) and sympathetic (i.e., PEP and EDA) functioning during an emotion induction and suppression task among youth with and without ADHD. Results were predicted to be parallel to those of Musser et al. 2011. Specifically, youth with ADHD were expected to experience augmented levels of RSA reactivity across task conditions in comparison to typically developing youth, indicating that youth with ADHD experience emotion dysregulation compared to typically developing youth.
Although not exact, the present study’s pattern of RSA results observed were similar to those of Musser et al. 2011, with a significant between groups difference across task conditions varying within the small effect size range (Cohen 1988). Specifically, while in Musser et al. 2011 youth with ADHD showed a pattern marked by slight augmentation from neutral periods across each task condition, in the present study, youth with ADHD showed a pattern marked by significant augmentation from neutral periods across task conditions, varying within the medium effect sizes range (Cohen 1988). Thus, these differences across task conditions, as documented in two independent studies, suggest youth with ADHD experience difficulties in regulating emotional response(s) during emotionally evocative situations. The differences observed in the level of RSA augmentation among the ADHD sample in this study and the Musser et al. 2011 study may be due to differences in the nature of the samples. Specifically, the current study included a clinical sample of youth with ADHD, while Musser et al. 2011 included a community sample. Further, the present sample was more racially diverse and likely to identify ethnically as Hispanic/Latinx than the Musser et al. 2011 sample. These results hold in both a clinical sample and across racial and ethnic groups, which boosts confidence in the results. Further, levels of RSA reactivity of typically developing youth in both studies were similar, further instilling confidence in the results.
With respect to PEP reactivity, similar to Musser et al. 2011, no significant differences in PEP reactivity were observed when comparing youth with ADHD and typically developing youth, which was determined to be in the small effect range (Cohen 1988). Prior literature has suggested that PEP may be specifically linked to approach-based emotion reactivity, which the task described herein is unlikely to engage, and the fact that PEP reactivity from neutral period was modest across both groups.
As an extension to Musser et al. 2011, EDA reactivity was included to index sympathetic arousal to negative emotions. Based on previous literature (e.g., Barry et al. 2012; Conzelmann et al. 2014; Losoya 1995), it was predicted that youth with ADHD would experience under arousal (i.e., lower EDA reactivity) during the emotionally evocative task compared to typically developing youth. However, in contrast to our hypothesis, EDA reactivity was significantly elevated among youth with ADHD compared to typically developing youth, varying within the small to medium effect size range (Cohen 1988). This suggests that youth with ADHD in this sample were characterized by elevated sympathetic activity in response to task conditions compared to typically developing youth. Of note, the present study statistically controlled for comorbid disruptive behavior disorders, which have been associated in prior work with reduced sympathetic reactivity (Lazzaro et al. 1999; O’connell et al. 2004; Odle and Ouellette 2016); thus, our results (similar to prior literature) suggest that when controlling for such comorbidity, youth with ADHD experience elevated sympathetic-based emotional reactivity (Mangeot et al. 2001).
As a further extension to Musser et al. 2011, the current study examined DSM-5 ADHD presentations and symptom domain specificity. No significant differences according to ADHD presentation were observed among any of the psychophysiological indexes of emotion reactivity or regulation, varying within the small effect size range (Cohen 1988). However, the RSA effect appears to be driven primarily by inattention symptoms, suggesting that inattention may be more closely related to emotion dysregulation than hyperactivity/impulsivity. This is also in line with prior work by Martel (2009), as well as Maedgen and Carlson (2000), at the behavioral level of analyses which suggests that inattention may be more strongly associated with emotion dysregulation, while hyperactivity/impulsivity may be more strongly associated with disruptions in emotion reactivity.
Similar to Musser et al. 2011, results in this study were not due to differences in youth’s biological sex, use of medication, nor presence of comorbid diagnoses. Additionally, as in Musser et al. 2011, this study did not observe group differences in SAM valence or arousal scales, varying within the small effect size range (Cohen 1988). Thus, youth with ADHD and typically developing youth did not differ with respect to understanding the nature of the task.
Due to identical methodology implemented in the current study and Musser et al. 2011, similar limitations are applicable. By utilizing a task with conditions based on positive and negative valence, rather than specific emotional states (e.g., happiness, sadness, and anger), interpretations of these results are limited in relation to specific emotions. Additionally, analysis of ADHD subtypes was insufficiently powered to detect effects of the ADHD-HI presentation. Furthermore, order effects due to consistency between task conditions per participant were not meaningful to the interpretations of the results in Musser et al. 2011, thus similar assumptions can be made in the current study as identical measures were used. On a similar note, altering the order of task conditions would have confounded the story line of the movie. Additionally, ethnic and racial diversity was also limited in this sample. However, it is important to note that while Musser et al. 2011 included a sample which predominantly identified as Non-Hispanic/Latinx and White, the current study includes a sample that identified as majority Hispanic/Latinx. Thus, while additional work in more ethnically and racially representative samples is needed, of note, this study provided a much needed extension to a demographic which has traditionally been underrepresented in research on mental health (i.e., Hispanic/Latinx youth). Importantly, this work demonstrates that results replicate in such a sample. Additionally, future studies should consider utilizing paired sample recruitment through matched clinical or community samples in order to yield more precise effect size estimates. A final noted limitation of the current study is that a single author involved in the prior study was involved in and integral to the completion of the current study. As such, we recognize and encourage other novel investigators to engage in a study designed to examine the replicability of these findings.
In conclusion, this study adds to the growing literature on emotion dysregulation among youth with ADHD. Findings generally support those observed in the original Musser et al. 2011 study, in that overall dysregulation across positive and negative valence conditions was present within youth with ADHD in comparison to typically developing youth. These findings may be applicable in clinical settings, given that they have been shown to replicate in a clinical sample. As such, assessment for emotional functioning during assessment procedures may be helpful in further understanding presenting problems and in treatment planning. Further, the development of future evidence-based treatment programs for youth with ADHD should target the emotion reactivity and regulation difficulties common among these youth.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Institute of Mental Health (PI: Musser, 1R03MH110812-01) and (PI: Pelham: 1R01MH099030).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10802-019-00593-8) contains supplementary material, which is available to authorized users.
Conflict of Interest The study was approved by the Florida International University Institutional Review Board under IRB Protocol #14–0088. Written informed consent was obtained by all parents, while written ascent was obtained by all youth who participated in the current study.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Anderson JC, Williams SM, McGee R, & Silva PA (1987). DSM-III disorders in preadolescent children: Prevalence in a large sample from the general population. Archives of General Psychiatry, 44(1), 69–76. 10.1001/archpsyc.1987.01800130081010. [DOI] [PubMed] [Google Scholar]
- Barkley RA (2010). Deficient emotional self-regulation: A core component of attention-deficit/hyperactivity disorder. Journal of ADHD & Related Disorders, 1(2), 5–37. [Google Scholar]
- Barkley RA, & Fischer M (2010). The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Academy of Child & Adolescent Psychiatry, 49(5), 503–513. 10.1097/00004583-201005000-00011. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, MacDonald B, & Dupuy FE (2012). Caffeine effects on resting-state electrodermal levels in AD/HD suggest an anomalous arousal mechanism. Biological Psychology, 89(3), 606–608. 10.1016/j.biopsycho.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2001). Vagal tone, development, and gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13(2), 183–214. 10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, & Snarr J (2001). Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology, 110(4), 610 10.1037/0021-843X.110.4.610. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J, Reid MJ, & Webster-Stratton C (2013). Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. Journal of Consulting and Clinical Psychology, 81(3), 481–493. 10.1037/a0032302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993). Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183–196. 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1994). Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry, 25(1), 49–59. 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, & Lang PJ (1990). Startle reflex modification: Emotion or attention? Psychophysiology, 27(5), 513–522. 10.1111/j.1469-8986.1990.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Brenner SL, & Beauchaine TP (2011). Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: A pilot study. Psychophysiology, 48(11), 1588–1596. 10.1111/j.1469-8986.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, & Sylvers PD (2005). A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology, 42(1), 108–115. 10.1111/j.1469-8986.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Bunford N, Evans SW, & Wymbs F (2015). ADHD and emotion dysregulation among children and adolescents. Clinical Child and Family Psychology Review, 18(3), 185–217. 10.1007/s10567-015-0187-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, & Berntson G (Eds.). (2007). Handbook of psychophysiology. Cambridge University Press. [Google Scholar]
- Calkins SD (2007). The emergence of self-regulation: Biological and behavioral control mechanisms supporting toddler competencies In Brownell CA, & Kopp CB (Eds.), Socioemotional development in the toddler years: Transitions and transformations; socioemotional development in the toddler years: Transitions and transformations (pp. 261–284, Chapter xi, 497 Pages) Guilford Press, New York. [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: Erlbaum. [Google Scholar]
- Conzelmann A, Gerdes ABM, Mucha RF, Weyers P, Lesch K, Bähne CG, et al. (2014). Autonomic hypoactivity in boys with attention-deficit/hyperactivity disorder and the influence of methylphenidate. The World Journal of Biological Psychiatry, 15(1), 56–65. 10.3109/15622975.2013.829584. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, & Chipman-Chacon J (2006). Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology, 115(1), 174–178. 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Ehring T, Tuschen-Caffier B, Schnülle J, Fischer S, & Gross JJ (2010). Emotion regulation and vulnerability to depression: spontaneous versus instructed use of emotion suppression and reappraisal. Emotion, 10(4), 563 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, & Karbon M (1995). The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development, 66(5), 1360–1384. 10.2307/1131652. [DOI] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE Jr., Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, et al. (2006). A practical measure of impairment: Psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology, 35, 369–385. 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Fowles DC (1986). The eccrine system and electrodermal activity. Psychophysiology: Systems, Processes, and Applications, 1, 51–96. [Google Scholar]
- Gaub M, & Carlson CL (1997). Gender differences in ADHD: a meta-analysis and critical review. Journal of the American Academy of Child & Adolescent Psychiatry, 36(8), 1036–1045. 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Gershon J (2002). A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders, 5(3), 143–154. 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Graziano PA, & Garcia A (2016). Attention-deficit hyperactivity disorder and children’s emotion dysregulation: A meta-analysis. Clinical Psychology Review, 46, 106–123. 10.1016/j.cpr.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Gross JJ (1998). Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74(1), 224–237. 10.1037/0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1993). Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology, 64(6), 970–986. 10.1037/0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1997). Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology, 106(1), 95–103. 10.1037/0021-843X.106.1.95. [DOI] [PubMed] [Google Scholar]
- Hayano J, Sakakibara Y, Yamada A, Yamada M, Mukai S, Fujinami T, et al. (1991). Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. American Journal of Cardiology, 67(2), 199–204. 10.1016/0002-9149(91)90445-Q. [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Ornduff SR, & Alpert BS (2007). Reliability of cardiovascular reactivity to stress: Internal consistency. Psychophysiology, 44(2), 216–225. 10.1111/j.1469-8986.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley MM, & Cuthbert BN (1999). International affective picture system (IAPS): Instruction manual and affective ratings University of Florida; Gainesville. FL: 2005. Technical Report A-6. [Google Scholar]
- Lazzaro I, Gordon E, Li W, Lim CL, Plahn M, Whitmont S, et al. (1999). Simultaneous EEG and EDA measures in adolescent attention deficit hyperactivity disorder. International Journal of Psychophysiology, 34(2), 123–134. 10.1016/S0167-8760(99)00068-9. [DOI] [PubMed] [Google Scholar]
- Leaberry KD, Rosen PJ, Fogleman ND, Walerius DM, & Slaughter KE (2018). Physiological emotion regulation in children with adhd with and without comorbid internalizing disorders: A preliminary study. Journal of Psychopathology and Behavioral Assessment. 10.1007/s10862-018-9644-z. [DOI]
- Losoya SH (1995). Patterns of emotional responding in children with and without attention-deficit hyperactivity disorder.
- Maedgen JW, & Carlson CL (2000). Social functioning and emotional regulation in the attention deficit hyperactivity disorder subtypes. Journal of Clinical Child Psychology, 29(1), 30–42. 10.1207/S15374424jccp2901_4. [DOI] [PubMed] [Google Scholar]
- Mangeot SD, Miller LJ, McIntosh DN, McGrath-Clarke J, Simon J, Hagerman RJ, & Goldson E (2001). Sensory modulation dysfunction in children with attention-deficit-hyperactivity disorder. Developmental Medicine & Child Neurology, 43(6), 399–406. 10.1017/S0012162201000743. [DOI] [PubMed] [Google Scholar]
- Martel MM (2009). Research review: A new perspective on attention-deficit hyperactivity disorder: Emotion dysregulation and trait models. Journal of Child Psychology and Psychiatry, 50(9), 1042–1051. 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- McQuade JD, & Breaux RP (2017). Are elevations in ADHD symptoms associated with physiological reactivity and emotion dysregulation in children? Journal of Abnormal Child Psychology. 10.1007/s10802-016-0227-8. [DOI] [PubMed]
- Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, & Nigg JT (2011). Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD). Journal of Abnormal Child Psychology, 39(6), 841–852. 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’connell RG, Bellgrove MA, Dockree PM, & Robertson IH (2004). Reduced electrodermal response to errors predicts poor sustained attention performance in attention deficit hyperactivity disorder. Neuroreport, 15(16), 2535–2538. [DOI] [PubMed] [Google Scholar]
- Odle M, & Ouellette JA (2016). Anticipatory Electrodermal Response as a Differentiating Somatic Marker Between Children with ADHD and Controls. Applied Psychophysiology and Biofeedback, 41(4), 375–380. [DOI] [PubMed] [Google Scholar]
- Pelham WE Jr., Gnagy EM, Greenslade KE, & Milich R (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 31(2), 210–218. 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Burrows-MacLean L, Gnagy EM, Fabiano GA, Coles EK, Tresco KE, et al. (2005a). Transdermal methylphenidate, behavioral and combined treatment for children with ADHD. Experimental and Clinical Psychopharmacology, 13, 111–126. 10.1037/1064-1297.13.2.111. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, & Massetti GM (2005b). Evidence-based assessment of attention-deficit/hyperactivity disorder in children and adolescents. Journal of Clinical Child and Adolescent Psychology, 34, 449–476. 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, & Rohde LA (2007). The worldwide prevalence of ADHD: A systematic review and metaregression analysis. The American Journal of Psychiatry, 164(6), 942–948. 10.1176/appi.ajp.164.6.942. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt J, Portales AL, & Greenspan SI (1996). Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology, 29(8), 697–712. . [DOI] [PubMed] [Google Scholar]
- Rothbart MK, & Derryberry D (1981). Development of individual differences in temperament. Advances in Developmental Psychology.
- Salminen M, Ravaja N, Kallinen K, & Saari T (2013). Mediated cues of group emotion during knowledge-work tasks: Effects on subjective and physiological responses. Interacting with Computers, 25(1), 60–73. 10.1093/iwc/iws006. [DOI] [Google Scholar]
- Satterfield JH, & Dawson ME (1971). Electrodermal correlates of hyperactivity in children. Psychophysiology, 8(2), 191–197. 10.1111/j.1469-8986.1971.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, & Leibenluft E (2014). Emotion dysregulation in attention deficit hyperactivity disorder. The American Journal of Psychiatry, 171(3), 276–293. 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Turner JR, Light KC, & Blumenthal JA (1991). Temporal stability of the hemodynamics of cardiovascular reactivity. International Journal of Psychophysiology, 10(1), 95–98. 10.1016/0167-8760(90)90050-N. [DOI] [PubMed] [Google Scholar]
- Shields SA, MacDowell KA, Fairchild SB, & Campbell ML (1987). Is mediation of sweating cholinergic, adrenergic, or both? A comment on the literature. Psychophysiology, 24(3), 312–319. 10.1111/j.1469-8986.1987.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Surman CBH, Monuteaux MC, Petty CR, Faraone SV, Spencer TJ, Chu NF, & Biederman J (2010). Representativeness of participants in a clinical trial for attention-deficit/hyperactivity disorder? comparison with adults from a large observational study. The Journal of Clinical Psychiatry, 71(12), 1612–1616. 10.4088/JCP.09m05344pur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskiran C, Karaismailoglu S, Cak Esen HT, Tuzun Z, Erdem A, Balkanci ZD, et al. (2018). Clinical features and subjective/physiological responses to emotional stimuli in the presence of emotion dysregulation in attention-deficit hyperactivity disorder. Journal of Clinical and Experimental Neuropsychology, 40(4), 389–404. 10.1080/13803395.2017.1353952. [DOI] [PubMed] [Google Scholar]
- Uno H (1977). Sympathetic innervation of the sweat glands and piloarrector muscles of macaques and human beings. The Journal of Investigative Dermatology, 69, 112–120. 10.1111/1523-1747.ep12497915. [DOI] [PubMed] [Google Scholar]
- Visser S, Danielson M, Bitsko R, Holbrook JR, Kogan MD, Ghandour RM, et al. (2014). Trends in the parent-report of health care provider-diagnosis and medication treatment for ADHD disorder: United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry, 53(1), 34–46. 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitola ES, Bau CHD, Salum GA, Horta BL, Quevedo L, Barros FC, et al. (2017). Exploring DSM-5 ADHD criteria beyond young adulthood: Phenomenology, psychometric properties and prevalence in a large three-decade birth cohort. Psychological Medicine, 47 (4), 744–754. 10.1017/S003329171600285. [DOI] [PubMed] [Google Scholar]
- Wallin BG (1981). Sympathetic nerve activity underlying electrodermal and cardiovascular reactions in man. Psychophysiology, 18(4), 470–476. 10.1111/j.1469-8986.1981.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler abbreviated scale of intelligence-second edition (WASI II). San Antonio: Psychological Corporation. [Google Scholar]
- Wehmeier PM, Schacht A, & Barkley RA (2010). Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. Journal of Adolescent Health, 46(3), 209–217. 10.1016/j.jadohealth.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention-Deficit/Hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57(11), 1336–1346. 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


