Abstract
Inhibition of the epithelial sodium channel (ENaC) represents an important, mutation-agnostic therapeutic approach to restore airway surface liquid in patients with cystic fibrosis (CF). A phase II trial of the ENaC inhibitor BI 1265162, inhaled via the Respimat® Soft Mist™ inhaler, in patients aged ≥12 years with CF is being conducted to assess the efficacy and safety of BI 1265162, on top of standard CF treatment (www.clinicaltrials.gov identifier NCT04059094).
BALANCE-CF™ 1 is a multinational, randomised, double-blind, placebo-controlled, parallel-group, dose-ranging trial consisting of 2 weeks’ screening, 4 weeks’ randomised treatment and 1 week follow-up. 98 patients, including ≥21 adolescents, will be randomised. First, 28 patients will be allocated to the highest dose of BI 1265162 (200 µg twice daily) or placebo in a 1:1 ratio. The remaining 70 patients will be allocated to one of five treatment arms (200 µg, 100 µg, 50 µg, 20 µg or placebo twice daily), with a final distribution ratio of 2:1:1:1:2. Recruitment and randomisation will begin with adult patients. An independent data monitoring committee will review safety data to advise on inclusion of adolescents and study continuation. A futility analysis will be conducted after 28 patients to prevent exposure of further patients in case of insufficient evidence of clinical efficacy. The design ensures that potential for effect is assessed ahead of wider enrolment, allowing investigation of a dose–response effect with minimal patient numbers.
The results will increase understanding of efficacy, safety and optimal dosing of the inhaled ENaC inhibitor BI 1265162 in adults and adolescents with CF.
Short abstract
BALANCE-CF™-1 is an innovative phase II trial in patients aged ≥12 years with CF to assess the efficacy and safety of the inhaled ENaC inhibitor BI 1265162 vs placebo. The results will increase understanding and support further investigation in phase III trials. https://bit.ly/2B8WOPe
Introduction
Cystic fibrosis (CF) is a life-threatening, autosomal recessive genetic disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which encodes an ion channel protein [1, 2]. The CFTR protein mediates the transport of chloride and other anions across the apical membrane of epithelial cells. Defective or absent CFTR protein in the airway epithelial cells leads to reduced chloride and bicarbonate excretion and thus reduced mucus hydration in the airways [2–4].
Loss of function of CFTR is further affected by the epithelial sodium channel (ENaC) protein, which is influenced by CFTR [5]. The nature of the interaction between CFTR and ENaC modulation is not fully understood, but it is known that in CF, ENaC activity is hyperactivated when CFTR function is reduced or absent [2, 5, 6]. Upregulation of ENaC activity leads to increased absorption of sodium ions and water from the luminal surface into the epithelial cell, causing reduced airway surface liquid (ASL) volume and dehydrated mucus. This results in poor mucociliary clearance (MCC) [2, 6] and promotes bacterial colonisation, infection, lung inflammation and airway obstruction [7].
CFTR modulator therapies have become available that target specific dysfunctional CFTR proteins. Combinations of modulators extend the number of eligible mutations. Despite the approval of CFTR modulator therapies, there is still a need for symptomatic treatment to reduce the risk of CF-related exacerbations, improve individual quality of life, and prolong patient survival [8]. This applies both for patients eligible to take a CFTR modulator and for patients who do not take this class of medication. ENaC inhibition, a mutation-agnostic approach, has the potential to restore ASL hydration and enhance MCC [9]. Treatments that target ENaC in the presence of CFTR modulators could have a synergistic electrophysiological effect, helping to normalise ASL volume and MCC [2]. ENaC inhibition (aimed at reducing cation absorption) could provide an enhanced electrical drive increasing CFTR anion secretion [2]. Thus, ENaC inhibition could potentially enhance the effect of CFTR modulation, thereby further improving CFTR function [2, 10].
A number of ENaC inhibitors have been tested both pre-clinically and clinically in patients with CF. These pre-clinical and clinical studies showed positive initial results, followed by nonsignificant results in later clinical studies. Reasons for discontinuation of development included adverse events related to systemic hyperkalaemia, unfavourable pharmacokinetics and pharmacodynamics, weak affinity for the channel and short duration of action [2].
BI 1265162 is a novel ENaC inhibitor, inhaled via the Respimat® Soft Mist™ inhaler (Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany). This device was chosen due to its high efficiency in drug delivery and high lung deposition, as well as ease of use for patients. It has already been successfully deployed in therapeutics for COPD and asthma [11], and has been used previously in studies with tiotropium in patients with CF [12].
Pre-clinical analysis demonstrated that BI 1265162 effectively inhibits sodium ion absorption, leading to a reduction in water resorption in human and murine cell lines, and liquid absorption in a rat lung model, and accelerates MCC in a sheep model without any relevant changes in plasma electrolyte concentrations [13]. BI 1265162 has a 30–70-fold higher potency compared with the prototypical ENaC inhibitor, amiloride, in pre-clinical studies [13]. In normal and CF human airway epithelia models, BI 1265162 decreased water transport and increased MCC, alone and in combination with CFTR modulators (ivacaftor/lumacaftor (IVA/LUM)), with BI 1265162 increasing the effects of IVA/LUM in CF epithelium to reach the effect size seen in healthy epithelium with IVA/LUM alone, supporting a possible synergistic effect of BI 1265162 with CFTR modulators [13].
Phase I studies with healthy volunteers who received doses of up to 1200 µg per day showed that BI 1265162 was safe and well tolerated in a single-dose trial (www.clinicaltrials.gov identifier NCT03349723) and multiple-dose trial (www.clinicaltrials.gov identifier NCT03576144) over a 1-week period [14]. Renal excretion was a very minor elimination route for the unchanged parent compound [15].
Based on promising pre-clinical and phase I results, BI 1265162 is now in phase II development. BALANCE-CF™ 1 is a 4-week, dose-ranging, double-blind, parallel-group, phase II trial in patients aged ≥12 years with CF. The trial is being conducted to assess the efficacy and safety of four dose levels of BI 1265162 versus placebo, on top of standard CF treatment (www.clinicaltrials.gov identifier NCT04059094). Here, we present and discuss the trial methodology.
Research methods
Objectives
The primary objective of BALANCE-CF™ 1 is to assess the efficacy and safety of four doses of twice-daily BI 1265162 (20 µg, 50 µg, 100 µg and 200 µg), inhaled via the Respimat® Soft Mist™ inhaler, compared with placebo on top of standard CF therapies, including CFTR modulators, in patients aged ≥12 years with CF. Pharmacokinetics will also be assessed.
Study design
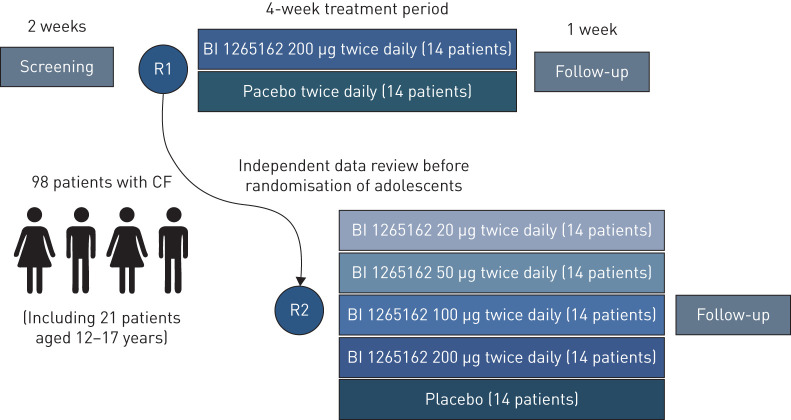
BALANCE-CF™ 1 is a multicentre, multinational, randomised, double-blind, placebo-controlled, parallel-group, dose-ranging trial, consisting of a 2-week screening period, a 4-week randomised treatment period and a 1-week follow-up period (figure 1). A total of 98 patients, including ≥21 adolescents, will be randomised. First, 28 patients will be allocated to the highest dose of BI 1265162 (200 µg twice daily) or placebo in a 1:1 ratio. Once the first 28 patients are randomised, the remaining 70 patients will be allocated to one of five treatment arms (200 µg twice daily, 100 µg twice daily, 50 µg twice daily, 20 µg twice daily or placebo twice daily) in an equal ratio.
FIGURE 1.
Trial design. CF: cystic fibrosis; R: randomisation.
Approximately 50 investigational sites in nine countries in Europe and North America are involved. Recruitment and randomisation will begin with adult patients only. Enrolment of adolescent patients will be based on periodic reviews of adult patient safety data after every seven patients have completed the trial treatment period, performed by an independent data monitoring committee (DMC; in collaboration with the Cystic Fibrosis Foundation). The DMC will advise on continuation of the study and the initiation of enrolment of adolescent patients. Once the DMC has permitted enrolment of adolescent patients, the periodic safety reviews will occur every 3 months.
An interim futility analysis will be conducted on the primary efficacy end-point once the first 28 patients have completed the 4-week treatment period to prevent exposure of further patients in case of insufficient evidence of clinical efficacy. For the primary end-point, insufficient evidence of clinical efficacy is defined, for example, as an improvement in trough forced expiratory volume in 1 s (FEV1) of <1.5% predicted. The effect of single patients; point estimates, confidence intervals and variance; stability of the treatment effect; baseline comparability; and other potentially important factors may be considered.
If the independent DMC has permitted inclusion of adolescents by this time, the interim futility analysis may also include adolescent data. Recruitment will continue during preparation and conduct of the interim analysis.
Due to its mutation-agnostic treatment approach, which allows for combination with all other standard-of-care CF drugs including CFTR modulators, BI 1265162 will be assessed as add-on therapy to standard of care, as the same effect size is assumed and is to be assessed in pre-defined subgroup analyses at the final trial evaluation. Therefore, patients will remain on their current treatment (including CFTR modulator therapy) as long as their therapy is stable (periodic or continuous) and has been established for ≥4 weeks before randomisation. For patients who are receiving a stable regimen of inhaled cycling antibiotics, visit 2 (first treatment day) should occur on day 1 of an “on-cycle” (±1 or ±2 days), regardless of the antibiotic. For patients cycling different inhaled antibiotics, visit 2 should occur on day 1 of a new antibiotic cycle. To reduce short-term impact on lung function, a standardised washout of bronchodilators prior to spirometry is required. In addition, lung clearance index (LCI) will be assessed using a standardised washout recording system. Standardisation of physiotherapy is required at visit days, i.e. to be performed in the same fashion as at screening. Detailed subgroup analysis will be planned for the final analysis.
The protocol for BALANCE-CF™ 1 was reviewed by the Cystic Fibrosis Foundation Therapeutics Development Network and the European Cystic Fibrosis Society – Clinical Trials Network and approved by competent authorities and institutional review board/independent ethics committee of the respective investigational sites.
End-points
The primary end-point of BALANCE-CF™ 1 is change from baseline in percentage predicted trough (within 30 min prior to dosing) FEV1 after 4 weeks of treatment. The primary end-point will be assessed in the first 28 patients in the interim futility analysis, and evaluated further in all 98 patients at study completion. FEV1 was selected as the primary end-point as it correlates with clinical status and mortality in CF and it is expected that improved mucus clearance associated with BI 1265162 would improve FEV1. In addition, the study includes a wide range of patients (FEV1 40–90%), a population for which end-points such as LCI might be more difficult to assess at the lower range of the FEV1 spectrum.
Secondary efficacy, safety and pharmacokinetics end-points are described in table 1.
TABLE 1.
Secondary efficacy, safety and pharmacokinetics end-points
| Efficacy | Safety | Pharmacokinetics |
| Change from baseline in LCI for patients with FEV1 >60% pred values at screening after 4 weeks of treatment | Percentage of patients with treatment-emergent AEs up to day 36 | Concentration of the analyte in plasma at time t following dose N (Ct.N) |
| Change from baseline in CFQ-R after 4 weeks of treatment | Pre-dose concentration measured for dose N (Cpre.N) | |
| Change from baseline in CASA-Q after 4 weeks of treatment | Area under the concentration–time curve of the analyte in plasma until t hours after dose N (AUC0–t,N) |
LCI: lung clearance index; FEV1: forced expiratory volume in 1 s; AE: adverse event; CFQ-R: CF Questionnaire – Revised; CASA-Q: Cough and Sputum Assessment Questionnaire.
Recruitment and sample size calculation
Patients will be screened from ∼50 trial sites to ensure randomisation of ≥98 patients including ≥21 adolescents. Screening of patients is competitive, with screening at trial sites stopping once a sufficient number of patients have been screened. It is expected that two to three patients will be randomised at each trial site.
In order to ensure 12 evaluable patients per arm with primary end-point data for interim analysis, ≥14 patients per arm will be randomised (total 28). In order to ensure an additional 12 patients per group are evaluable for final analysis, ≥14 patients will be randomised per treatment group (total 70). Overall, 98 patients will be randomised in the trial. This guarantees an overall power of ≥80% for all true candidate monotone shapes; and overall Type I error is also controlled <5%. Pre-specified candidate models and assumptions for treatment effect size and standard deviation will be required for performing the Multiple Comparison Procedures – Modelling (MCP-Mod) analysis. This calculation has been performed using R software, version 3.2.2.
Inclusion and exclusion criteria
Males and females with CF aged ≥12 years at screening will be included in BALANCE-CF™ 1. Further inclusion and exclusion criteria are detailed in table 2.
TABLE 2.
Inclusion and exclusion criteria for study participants
| Inclusion criteria | Exclusion criteria |
| Male or female, aged ≥12 years at screening | Acute upper or lower respiratory tract infection ≤4 weeks prior to randomisation |
| Diagnosis of CF (positive sweat chloride ≥60 mEq·L−1, or genotype with two identifiable mutations and ≥1 clinical phenotypic feature of CF) | Pulmonary exacerbation requiring the use of antibiotics or oral corticosteroids ≤4 weeks prior to randomisation |
| FEV1 (according to Global Lung Initiative) ≥40 and ≤90% pred at screening and pre-dose at visit 2 |
CF: cystic fibrosis; FEV1: forced expiratory volume in 1 s.
Randomisation of patients
Prior to patient participation in BALANCE-CF™ 1, written informed consent will be obtained from each patient (or the patient's legally accepted representative) according to International Conference on Harmonisation Good Clinical Practice (ICH-GCP) guidelines and to the regulatory and legal requirements of the participating country.
28 adult patients (or adolescent patients, if permitted by the DMC) will be randomised to receive either the highest dose (200 µg twice daily) or placebo. An interim futility analysis will be conducted by the study sponsor, independent of the trial team, to assess whether the drug has the potential to show efficacy in order to prevent further enrolment in the case of insufficient clinical potential. Patients, investigators and all those involved in trial conduct or analysis will remain blinded with regard to the randomised treatment assignments until after database unlock.
Recruitment will continue during conduct of the interim analysis. After randomisation of the first 28 patients, the remaining 70 patients will be randomised to all study arms in an equal allocation for this second portion of the study, leading to a final distribution ratio of 2:1:1:1:2 to receive twice-daily placebo, 20 µg, 50 µg, 100 µg or 200 µg BI 1265162 via the Respimat® Soft Mist™ inhaler (figure 1). This unbalanced design follows the MCP-Mod approach [16] and ensures that a dose–response signal is established using multiple comparison procedures before the dose–response curve and target doses of interest are subsequently estimated using modelling techniques. The doses were selected in order to cover the expected therapeutic range (50–200 µg) and a subtherapeutic dose (20 µg), which is required for modelling of the dose–response shape.
Randomisation will be stratified by age (12–17 years and ≥18 years) to ensure that adolescent patients are equally randomised to each treatment group as per the final distribution ratio (at least six adolescents in both the placebo and 200 µg BI 1265162 twice daily group, and at least three adolescents in each of the 20 µg, 50 µg and 100 µg BI 1265162 twice daily groups).
It is anticipated that this study will complete enrolment by quarter 2/3 of 2020.
Outcome assessments
Efficacy will be assessed using measures of pulmonary function (FEV1 and forced vital capacity evaluated using Global Lung Initiative lung function reference equations [17]), LCI (by nitrogen multiple-breath washout test), and questionnaires on health-related quality of life (CF Questionnaire – Revised) and symptoms (Cough and Sputum Assessment Questionnaire).
Safety will be monitored throughout the trial by adverse event reporting, safety laboratory tests, vital signs measurements, 12-lead electrocardiogram and periodic safety data reviews by the DMC. Discontinuation criteria in case of confirmed increased levels of serum potassium are implemented.
Pharmacokinetics analysis will be performed in blood using a sparse population pharmacokinetics approach that jointly analyses the combined data from this study along with those from the phase I study in healthy volunteers.
Planned analyses and assessments
The analyses for proof of concept and dose-finding will be performed using multiple comparison and modelling techniques, whereby several possible dose–response models will be evaluated.
To assess the change from baseline in FEV1 % pred at 4 weeks of treatment, a restricted maximum likelihood-based approach using a mixed model with repeated measurements will be performed. The analysis will include the fixed, categorical effects of treatment at each visit, age (adolescents versus adults) and the fixed continuous effects of baseline at each visit. “Visit” will be treated as the repeated measure with an unstructured covariance structure used to model the within-patient measurements.
In addition, the linear mixed-effects model will be used to analyse the secondary end-points, with resulting confidence intervals and p-values considered as nominal or descriptive.
Patients should remain on their usual stable therapy throughout the study. However, if patients change other CF treatments during the trial period, these subgroups of patients will be investigated and descriptive analyses will be conducted. In addition, the impact of baseline characteristics, including lung function FEV1 categories and CF gene status, will be considered as possible subgroups.
Ethical approval
The trial was initiated after review and approval by the respective institutional review board/independent ethics committee and competent authority according to national and international regulations.
Funding of study
This study (BALANCE-CF™ 1) is funded by Boehringer Ingelheim.
Discussion
Following promising pre-clinical and phase I results, the results of the phase II clinical trial, BALANCE-CF™ 1, will increase understanding of safety, efficacy and optimal dosing of the new inhaled ENaC inhibitor BI 1265162 in adults and adolescents with CF.
Despite the approval of CFTR modulator therapies, there is still a need for a mutation-agnostic therapy that operates independently of CFTR function and mutation to benefit all patients with CF [2]. In murine models overexpressing ENaC with resultant CF-like disease characteristics, including mucus plugging and impaired MCC, ENaC inhibition with amiloride resulted in significant therapeutic benefits [18]. In addition, in patients with CF homozygous for F508del, rare loss-of-function mutations in genes encoding the ENaC may be associated with a long-term, nonprogressive phenotype, further supporting the potential of ENaC inhibition [19].
The ENaC inhibitor BI 1265162 has higher pre-clinical potency than previously studied ENaC inhibitors [13]. A number of previous ENaC inhibitors have failed in clinical development. The Hydration for Optimal Pulmonary Effectiveness (HOPE)-1 phase II trial of SPX-101, a peptide analogue that promotes ENaC channel internalisation [4], showed no effect of SPX-101 on FEV1 % pred in the full dataset, despite promising interim phase II data and despite phase I and pre-clinical experiments demonstrating positive results [4, 7, 20]. The HOPE-1 trial has now been discontinued [21]. Unlike HOPE-1, BALANCE-CF™ 1 assesses not only FEV1 % pred, but also change from baseline in LCI. Compared with FEV1, LCI reflects ventilation inhomogeneity and disease effects in the peripheral airways, effects which may occur earlier in the disease process and are not detected with spirometric measures such as FEV1 [22]. This phase II trial builds upon the robust pre-clinical evidence of BI 1265162, together with safety data derived from a single-rising dose and a multiple-rising dose phase I trial [14].
Pre-clinical animal model data demonstrate that BI 1265162 reduces liquid absorption in rat lungs and increases MCC in sheep, without any systemic effects [13]. In addition, inhibition of ENaC was maintained for 5 days, as indicated by attenuation of water transport in normal and CF airway epithelia in vitro [13]. In clinical phase I trials in which healthy volunteers were given inhaled total daily doses of up to 1200 µg, BI 1265162 was well tolerated, and renal excretion was a very minor elimination route for the unchanged parent compound. Further evaluation of the absorption, distribution, metabolism and excretion of BI 1265162 is ongoing. These data demonstrate a good safety profile for the ENaC inhibitor BI 1265162, supporting the present study design where the first patients are allocated to the highest dose of BI 1265162 (200 µg twice daily).
The study design of BALANCE-CF™ 1 has several advantages. The unbalanced design, which follows the MCP-Mod approach [16], allows assessment of efficacy and dose ranging with minimal patient numbers. By first including patients in the highest dose versus placebo with an interim futility analysis, patient exposure is minimised should there be little probability that efficacy can ultimately be detected. This also facilitates minimal exposure to potentially ineffective lesser doses. Moreover, adolescents are enrolled into the study based on periodic DMC review of safety data (every seven participants who have been enrolled and exposed to 4 weeks of treatment). By including adolescents in this study, a bridge of the pharmacokinetics and efficacy to adults can be made to allow a rational phase III study design that includes adolescents and adults.
Another strength of the study is that BI 1265162 is taken on top of standard of care, including CFTR modulator therapy (provided the medication regimen for CF has been stable for ≥4 weeks prior to randomisation). BI 1265162 is believed to work on top of other CF medication, independently of mutation status, as demonstrated in in vitro studies where BI 1265162 decreased water transport and increased MCC, with BI 1265162 increasing the effects of the CFTR modulators IVA/LUM on CF epithelium to reach the effect size seen in healthy human epithelium with IVA/LUM alone [13]. CFTR modulators may only restore ∼20–50% of CFTR function [10]; therefore, BI 1265162 could potentially further improve CFTR function. The study design on top of standard care may result in further heterogeneity of patients. However, the potential effects of variability based on underlying therapy being introduced should be minimised by the requirements that standard of care treatments remain stable for ≥4 weeks prior to and during the trial, and that changes relative to baseline will be assessed. In addition, effect size is to be assessed in predefined subgroup analyses at the final trial evaluation.
Conclusion
BALANCE-CF™ 1 includes an innovative trial design, where patients are first randomised to receive the highest dose of BI 1265162 or placebo before an interim futility analysis is performed, which prevents exposure in case of insufficient ability to detect clinical efficacy. An unbalanced MCP-Mod design ensures that a dose–response signal can be investigated while requiring minimal patient numbers. Adolescents will only be enrolled once safety data from adults are reviewed and adolescents’ inclusion recommended by an independent DMC. BALANCE-CF™ 1 includes both FEV1 and LCI, which assess different aspects of airway physiology, as efficacy end-points.
The results of BALANCE-CF™ 1 will increase understanding of safety, efficacy and optimal dosing of the new inhaled ENaC inhibitor BI 1265162 in adults and adolescents with CF. If efficacy and safety are demonstrated, the results will support further investigation of BI 1265162 in phase III trials.
Acknowledgements
Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by Boehringer Ingelheim and provided by Rosie Robson at MediTech Media (Manchester, UK), under the authors’ conceptual direction and based on feedback from the authors.
Footnotes
This study is registered at www.clinicaltrials.gov with identifier number NCT04059094. To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfil their role and obligations as authors under the ICMJE criteria. Furthermore, clinical study documents (e.g. study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the BI Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/. Prior to providing access, documents will be examined, and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants. Clinical Study Reports and Related Clinical Documents can also be requested via the link https://trials.boehringeringelheim.com/. All requests will be governed by a Document Sharing Agreement. Bona fide, qualified scientific and medical researchers may request access to de-identified, analysable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request. Researchers should use the https://trials.boehringer-ingelheim.com/ link to request access to study data.
Support statement: This study was supported by Boehringer Ingelheim. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: C.H. Goss reports funding for conducting exacerbation studies in cystic fibrosis (CF) from the Cystic Fibrosis Foundation, funding to study the role of microbiome in the treatment of exacerbation in CF from the European Commission, funding to conduct a study of home monitoring in pulmonary exacerbation from the NIH (NHLBI), and funding to support clinical research in CF and clinical trials in other disease entities from the NIH (NIDDK and NCRR), during the conduct of the study; personal fees for serving as a Chair of a Grant Review Committee from Gilead Sciences, personal fees for serving as a DSMB Chair for a trial supported by Novartis and the European Commission from Novartis, funding to study a novel antimicrobial in CF from the NIH and the FDA, serving a US lead in a phase 2 trial of novel therapy for CF, receiving travel expenses and an honorarium for meeting participation, and a future site contract for trial participation from Boehringer Ingelheim, and honoraria and travel expenses for talk at Nottingham LEAD conference from Vertex Pharmaceuticals, outside the submitted work. None of the work presented in this opinion piece was influenced by the funding sources noted above. The funding sources that support other ongoing research played no role in writing this manuscript or in the decision to submit for publication.
Conflict of interest: R. Jain reports grants and personal fees from Vertex Pharmaceuticals, personal fees from Gilead Sciences, and grants from the CF Foundation, outside the submitted work.
Conflict of interest: W. Seibold is an employee of Boehringer Ingelheim.
Conflict of interest: A-C. Picard is an employee of Boehringer Ingelheim International GmbH.
Conflict of interest: M-C. Hsu is an employee of Boehringer Ingelheim (China) Investment Co. Ltd.
Conflict of interest: A. Gupta is an employee of Boehringer Ingelheim International GmbH.
Conflict of interest: I. Fajac reports grants and personal fees for study conduct and expert advice from Boehringer during the conduct of the study, and from Proteostasis Therapeutics and Vertex Pharmaceuticals outside the submitted work.
References
- 1.Farrell PM, White TB, Ren CL, et al. Diagnosis of cystic fibrosis: consensus guidelines from the Cystic Fibrosis Foundation. J Pediatr 2017; 181: S4–S15. doi: 10.1016/j.jpeds.2016.09.064 [DOI] [PubMed] [Google Scholar]
- 2.Shei R-J, Peabody JE, Kaza N, et al. The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis. Curr Opin Pharmacol 2018; 43: 152–165. doi: 10.1016/j.coph.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mall MA, Galietta LJV. Targeting ion channels in cystic fibrosis. J Cystic Fibros 2015; 14: 561–570. doi: 10.1016/j.jcf.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Couroux P, Farias P, Rizvi L, et al. First clinical trials of novel ENaC targeting therapy, SPX-101, in healthy volunteers and adults with cystic fibrosis. Pulm Pharmacol Ther 2019; 58: 101819. doi: 10.1016/j.pupt.2019.101819 [DOI] [PubMed] [Google Scholar]
- 5.Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Mol Biosyst 2009; 5: 123–127. doi: 10.1039/B810471A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clunes MT, Boucher RC. Cystic fibrosis: the mechanisms of pathogenesis of an inherited lung disorder. Drug Discov Today Dis Mech 2007; 4: 63–72. doi: 10.1016/j.ddmec.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott DW, Walker MP, Sesma J, et al. SPX-101 is a novel epithelial sodium channel–targeted therapeutic for cystic fibrosis that restores mucus transport. Am J Respir Crit Care Med 2017; 196: 734–744. doi: 10.1164/rccm.201612-2445OC [DOI] [PubMed] [Google Scholar]
- 8.Edmondson C, Davies JC. Current and future treatment options for cystic fibrosis lung disease: latest evidence and clinical implications. Ther Adv Chronic Dis 2016; 7: 170–183. doi: 10.1177/2040622316641352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler R, Hunt T, Smith NJ. ENaC inhibitors for the treatment of cystic fibrosis. Pharm Pat Anal 2015; 4: 17–27. doi: 10.4155/ppa.14.51 [DOI] [PubMed] [Google Scholar]
- 10.Guimbellot J, Sharma J, Rowe SM. Toward inclusive therapy with CFTR modulators: progress and challenges. Pediatr Pulmonol 2017; 52: S4–S14. doi: 10.1002/ppul.23773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan CK, Say GQ, Geake JB. Long-term safety of tiotropium delivered by Respimat® SoftMist™ Inhaler: patient selection and special considerations. Ther Clin Risk Manag 2016; 12: 1433–1444. doi: 10.2147/TCRM.S109011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratjen F, Koker P, Geller DE, et al. Tiotropium Respimat in cystic fibrosis: phase 3 and pooled phase 2/3 randomized trials. J Cyst Fibros 2015; 14: 608–614. doi: 10.1016/j.jcf.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Nickolaus P, Jung B, Sabater J, et al. Preclinical evaluation of the ENaC inhibitor BI 1265162 for treatment of cystic fibrosis. ERJ Open Res 2020; [Epub ahead of print]: 10.1183/23120541.00429-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand T, Endriss V, Risse F, et al. Single and multiple doses of the inhaled ENaC inhibitor BI 1265162 are well tolerated in healthy males. Pediatr Pulmonol 2019; 54: S359. [Google Scholar]
- 15.Mackie AE, Endriss V, Rascher J, et al. Pharmacokinetics of BI 1265162, an inhaled ENaC inhibitor going into phase II. Pediatr Pulmonol 2019; 54: 24. [Google Scholar]
- 16.Bornkamp B. The MCP-Mod methodology – a statistical methodology for dose-response. 2014. www.page-meeting.org/pdf_assets/601-MCP-Mod_PAGE.pdf Date last accessed: December 18, 2019.
- 17.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Duerr J, Johannesson B, et al. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros 2011; 10: Suppl. 2, S172–S182. doi: 10.1016/S1569-1993(11)60021-0 [DOI] [PubMed] [Google Scholar]
- 19.Agrawal PB, Wang R, Li HL, et al. The epithelial sodium channel is a modifier of the long-term nonprogressive phenotype associated with F508del CFTR mutations. Am J Respir Cell Mol Biol 2017; 57: 711–720. doi: 10.1165/rcmb.2017-0166OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spyryx Biosciences Spyryx SPX-101 Phase 2 HOPE-1 Trial Shows Improvement in Lung Function in Patients with Cystic Fibrosis via Novel Modulation of ENaC. 2018. www.prnewswire.com/news-releases/spyryx-spx-101-phase-2-hope-1-trial-shows-improvement-in-lung-function-in-patients-with-cystic-fibrosis-via-novel-modulation-of-enac-300660000.html Date last accessed: December 18, 2019.
- 21.Cystic Fibrosis Foundation Drug Development Pipeline: SPX-101. 2019. www.cff.org/Trials/Pipeline/details/10128/SPX-101 Date last accessed: December 18, 2019.
- 22.Kent L, Reix P, Innes JA, et al. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cystic Fibros 2014; 13: 123–138. doi: 10.1016/j.jcf.2013.09.005 [DOI] [PubMed] [Google Scholar]