Abstract
Outer ear infections (OE) affect millions of people annually with significant associated healthcare costs. Incorrect administration or non-compliance with the treatment regimen can lead to infection persistence, recurrence, antibiotic resistance, and in severe cases aggravation to malignant otitis externa. Such issues are particularly pertinent for military personnel, patients in nursing homes, the geriatric population, for patients with head or hand tremors and for those with limited or no access to proper healthcare. With the intent of using traditional material science principles to deconvolute material design while increasing relevance and efficacy, we developed a single application, cold-chain independent thixotropic drug delivery system. This can be easily applied into the ear as a liquid, then gels to deliver effective concentrations of antibiotics against bacterial strains commonly associated with OE. The system maintains thixotropic properties over several stress/no stress cycles, shows negligible swelling and temperature dependence, and does not impact the minimum inhibitory concentration or bactericidal effects of relevant antibiotics. Moreover, the thixogels are biocompatible and are well tolerated in the ear. This drug delivery system can readily translate into a user-friendly product, could improve compliance via a single application by the diagnosing health care provider, is expected to effectively treat OE and minimize the development of antibiotic resistance, infection recurrence or exacerbation.
One Sentence Summary:
We introduce a single use drug delivery system to safely and effectively treat otitis externa.
INTRODUCTION
In the United States, according to a comprehensive 5-year report published by the Centers of Disease Control and Prevention, annually, approximately 2.4 million health care visits are diagnosed with outer ear infections (otitis externa or OE)1. Both the pediatric and adult population are affected by OE, with a higher prevalence recorded in adults (approximately 53% of cases)1. OE affects the external auditory canal, which connects the outside of the ear to the tympanic membrane (eardrum). The most common cause accounting for approximately 98% of all ear infections is bacterial infection typically attributable to Pseudomonas aeruginosa or Staphylococcus aureus2,3. Treatment of OE involves patient or caregiver administered topical antibiotic drops for 7–14 days with multiple daily applications3,4. Compliance with the treatment regimen, however, poses significant challenges for the target population, especially for military personnel, patients in nursing homes, the geriatric population, and for patients with head or hand tremors. Incorrect application or non-compliance with the administration schedule of antibiotics often translates to ineffective drug doses at the infection site and leads to infection persistence, recurrence and development of antibiotic resistant bacterial strains5,6. In elderly, diabetic or immunocompromised patients, OE can spread to the surrounding tissue as necrotizing or malignant otitis externa (MOE), causing bone erosion, cranial nerve deficits, abscesses or even death7,8. MOE had been primarily associated with P. aeruginosa infections and ciprofloxacin-resistant strains have been found in as many as 33% of isolates9,10. Alarmingly, several recent studies have reported MOE rates to be increasing worldwide and it is of most concern in developing countries with limited or inadequate access to medical care11–14. Patients diagnosed with MOE currently undergo more than six weeks of bacteria-culture specific oral or parenteral systemic antibiotic treatment15. Consequently, the need for a user-friendly, safe and effective therapeutic approach, for both developed and developing countries, is real.
Recently a thermosensitive and thermoreversible poloxamer-based ciprofloxacin containing material was approved by the Food and Drug Administration for the treatment of otitis externa16. This product is a single-dose, health care professional-administered formulation that eliminates treatment compliance issues. However, the thermosensitive aspect of this therapeutic implies multiple preparation steps that open the possibility of user error and the product is cold chain dependent16,17. This product, and traditional ear drops, mostly address the OE-specific clinical needs for the developed world and patients with easy access to medical care. However, under conditions where a patient cannot consistently and at prescribed intervals self-administer ear drops, has inadequate access to medical care, or where there is no access to electricity and/or refrigeration, such products are inadequate. To address these limitations, we developed a thixotropic drug delivery system that would maintain the convenience of single, point-of-care application by the health professional during the initial office visit, but would be cold-chain independent and would not require any preparation steps.
Two thixotropic formulations were evaluated in this study. They both satisfy the requirements of an easy-to-use, single application, cold chain independent product, are compatible with antibiotics currently used for the treatment of OE, effectively targets OE-specific bacterial infections and are well tolerated.
RESULTS
Thixogels
Formulations.
Two delivery systems were formulated by blending activated tetraethyl orthosilicate with large molecular weight polymers (Table S1). One of the design requirements for these systems was their ability to be deployed as liquids, then to rapidly gel in place to ensure site-specific delivery of the therapeutic cargo. This particular property would enable this system to be deployed to the infection site from a prepacked cannulated syringe or application tube, without any necessary preparation steps, via shear stress. Two specific formulations H-Tx and S-Tx were assessed for thixotropy. H-Tx contains 10 % w/v hyaluronan, a polymeric glycosaminoglycan, in a 2:1 v/v ratio to the activated tetraethyl orthosilicate (aTEOS), while S-Tx is based on 0.5% w/v silk fibroin, a polymeric protein, in a 1:1 v/v ratio to the activated TEOS. The H-Tx hydrogel is optically transparent and can be easily deployed though a syringe equipped 26G ½ needle (Fig. 1). S-Tx has a slightly opaque appearance but deploys just as easily.
Figure 1. Empirical evaluation of thixogels.
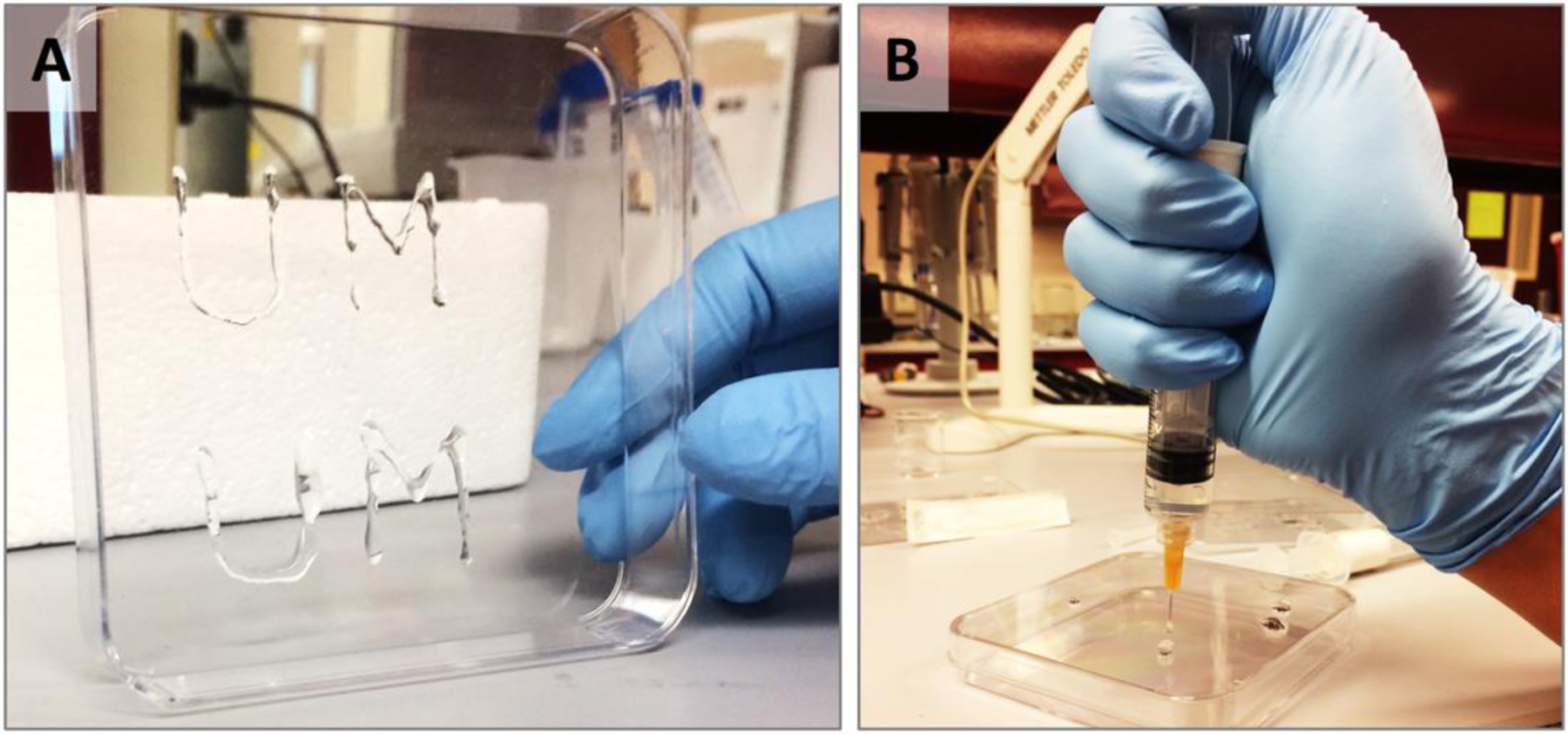
A – the University of Montana initials (UM) drawn by deploying our H-Tx thixogel though a 26G 1/2 needle (right). B – the thixogel liquefies when pushed through the needle/syringe nozzle and rapidly gels once deployed.
Rheological evaluations of thixogels
Thixotropy evaluation.
As indicated, thixotropy or the ability of our materials to liquefy under shear stress then gel once the stress is removed, is a key value driver of our product. The stress-dependent behavior of the two hydrogels was evaluated rheologically (Fig. 2A and 2B). Both hydrogels transitioned between gel (blue curve)-sol (green curve) states as a function of stress over the three cycles tested. The storage moduli values (G’) of the H-Tx formulation, reflective of the hydrogel’s stiffness were in the 0.99 ± 0.32 kPa range. The G’ values for S-Tx were in the 2.40 ± 0.68 kPa range indicating that this formulation produces stiffer materials than H-Tx. Based on the determined G’ values, both materials fall under the category of soft materials18,19. This is a desired feature for the thixotropic hydrogels (thixogels) and is expected to translate to minimal hearing disruption when they are placed in the ear. A slight increase in G’ values was observed in both materials throughout the testing procedure. The effect is more pronounced for the S-Tx formulations and represents an experimental artifact due to shear compression induced water loss. Considering that silk fibroin is hydrophobic and hyaluronan is hydrophilic, the observed differences in water loss are justified. From a product perspective, our data clearly indicate that both formulations would deploy as liquids and would gel within seconds once placed to the infection location.
Figure 2. Rheological evaluation of thixogels.
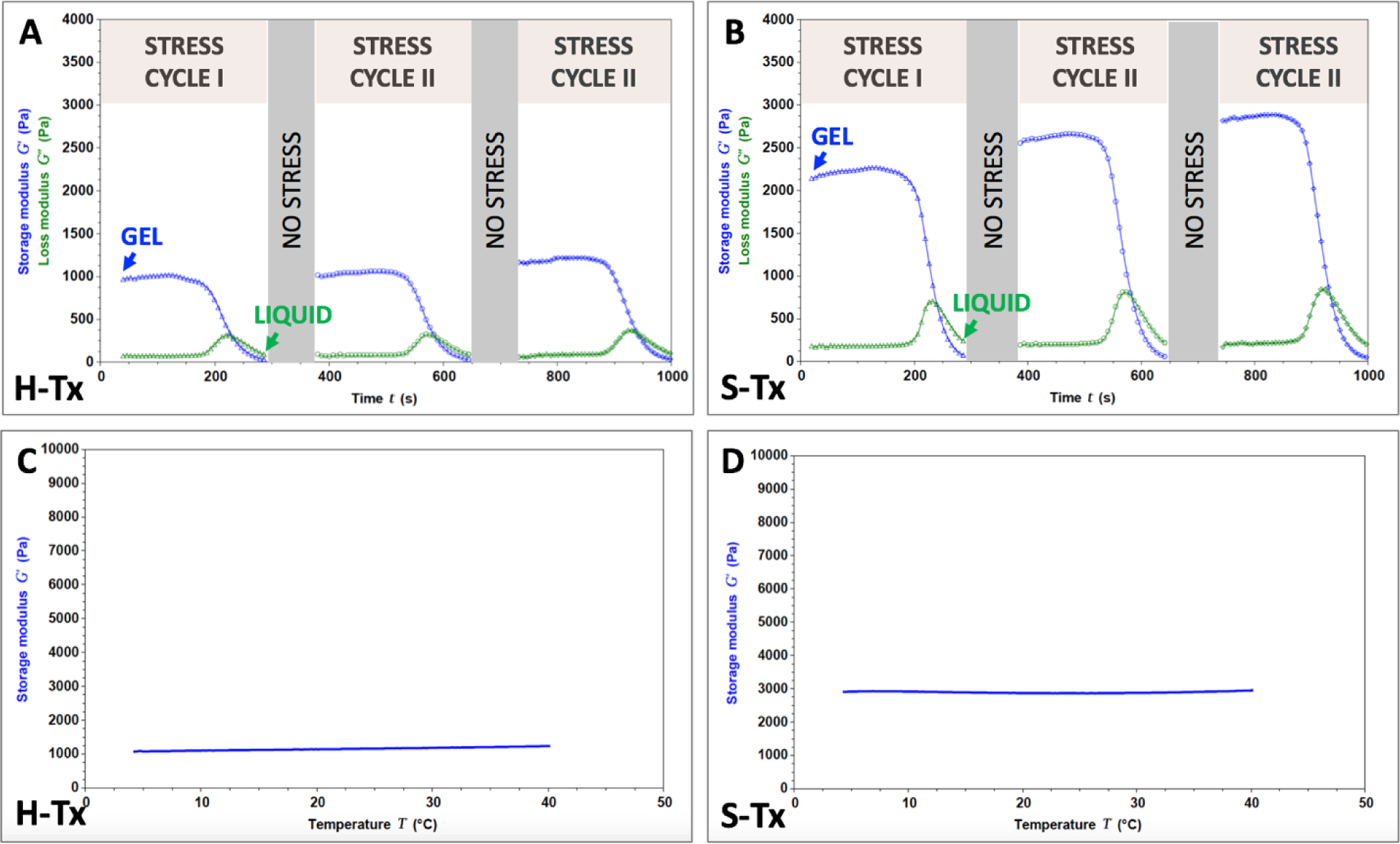
A – thixotropy evaluation of H-Tx. B – thixotropy evaluation of S-Tx. Blue lines indicate storage moduli (G’) and green lines indicate loss moduli (G”). C – temperature dependence of H-Tx. D – temperature dependence of S-Tx. The lines indicate storage moduli (G’).
Swelling evaluation.
Swelling is a major adverse event typically associated with hydrogels and for our intended application could impact the patient’s comfort and hearing while in place. We therefore rheologically tested the thixogels by measuring the gap change between rheometer fixtures in response to swelling20. During the 30 minutes testing time, at 37°C immersed in phosphate buffered saline (physiological conditions but with exacerbated fluid present), the volumes of the thixogels increased by 0.23 ± 0.19% for H-Tx and by 1.72 ± 0.43% for S-Tx (Table S2). These results indicate that both thixogels’ swelling is negligible and that a product based on either of these formulations would not cause swelling related adverse effects.
Temperature dependence.
The thixotropic drug delivery systems were designed to be cold chain independent, with storage and handling at ambient temperature. However, given that certain antibiotics require cold storage and that product and shipment conditions might deviate from room temperature, we sought to understand the temperature dependence of these materials in the range of conditions mimicking refrigeration (4°C/39.2°F), physiological conditions (37°C/98.6°F), and slightly higher temperatures that might occur during typical shipping/transportation (40°C/104°F). Our data indicate that both formulations tested maintain their viscoelastic behavior (stay as gels) in the tested temperature range (Fig. 2C and 2D), only showing modest changes in the storage moduli compared to the room temperature values (up to 7% decrease in G’ at low temperatures and 8.5% increase at high temperatures for H-Tx; up to 4.7% increase in G’ for S-Tx, see Table 1). Overall, these results indicate that the thixogels are stable in gel form in the application-specific temperature range.
Table 1.
Effect of temperature changes on thixogels’ storage moduli.
| Thixogel | HA | SF | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | 4 | 22 | 40 | 4 | 22 | 40 |
| Storage modulus G’ (Pa) | 1072.73 | 1155.24 | 1238.94 | 2899.69 | 2868.36 | 3052.42 |
| 675.51 | 712.41 | 759.88 | 2905.38 | 2837.56 | 2955.79 | |
| 836.76 | 911.52 | 1015.2 | 2586.76 | 2653.24 | 2743.20 | |
| Average | 861.67 | 926.39 | 1004.67 | 2797.28 | 2786.39 | 2917.14 |
| STDEV | 199.78 | 221.79 | 239.70 | 182.33 | 116.33 | 158.19 |
| Change in G’ (%) | −6.99 | 0.00 | +8.45 | +0.39 | 0.00 | +4.69 |
Antimicrobial release from thixogels
Thixogel effects on antibiotic minimum inhibitory concentrations.
Clinically used antibiotics such as ciprofloxacin and imipenem are potent and effective compounds. The minimum inhibitory concentration (MIC) defines the lowest antibiotic concentration that inhibits 99.9% of bacterial growth. As we plan to use such antibiotics in a thixogel delivery system it is important to show that the thixogel has no adverse effects upon the potency of the antibiotic. For this experiment we chose to measure the MIC concentrations of vancomycin, ciprofloxacin, imipenem and gentamycin (Table 2) when the antibiotic was delivered in a thixogel.
Table 2. Effect of the thixogel formulation upon the Minimum Inhibitory Concentrations (MIC) of antibiotics against the main two bacterial pathogens associated with otitis externa.
Actual doses of ciprofloxacin (3000 μg/mL) and imipenem (10,000 μg/mL) used in ear and eye drop applications are over three orders of magnitude greater than the MIC concentrations. The MIC concentration inhibits 99.9% of bacterial growth.
| Minimum Inhibitory Concentrations (MIC) (μg/mL) | ||||||
|---|---|---|---|---|---|---|
| S. aureus 13709 | P. aeruginosa 27853 | |||||
| No Gel | S-Tx | H-Tx | No Gel | S-Tx | H-Tx | |
| Vancomycin | 2 | 4 | 16 | > 4 | > 32 | > 32 |
| Ciprofloxacin | 0.25 | 0.125 | 0.125 | 0.25 | 0.25 | 0.125 |
| Imipenem | 0.032 | 0.125 | < 0.032 | 2 | > 4 | 4 |
| Gentamycin | 1 | 8 | 32 | 2 | 32 | 32 |
Gentamycin was found to be undesirable as both gels tested (S-Tx and H-Tx) have a deleterious effect upon potency. Vancomycin was also found to be unsuitable as it did not offer coverage of both S. aureus (Gram positive) and P. aeruginosa (Gram negative) organisms. Imipenem and ciprofloxacin, however, were selected for further development as they showed potency against both pathogenic bacteria and neither of the gels had a significant impact upon potency. It is important to note that these tests are determining the MIC concentration – customary dosing of ciprofloxacin17,21 (3,000 μg/mL) and imipenem22 (10,000 μg/mL) in eye and ear drops are 12,000 and 5,000 times higher, respectively, than these MIC concentrations. Overall, the MIC evaluation data indicates that thixogels do not negatively impact the potency of relevant antibiotics.
Thixogel-loaded antibiotics are effective at sub-pharmaceutical concentrations.
To understand how the thixogel might affect the rate at which a loaded antibiotic affects S. aureus and P. aeruginosa growth we measured the kill kinetics of ciprofloxacin, with or without H-Tx and S-TX, respectively. Ciprofloxacin is the most commonly employed antibiotic in topical ear drops. Our data indicate that growth rate in media only, and in media above HA and SF gels was identical, and as expected, reached cell densities of 109 CFU/mL within twelve hours. The thixogels alone do not inhibit bacterial growth. However, H-Tx and S-Tx, respectively, containing ciprofloxacin concentrations 100 times lower than the 0.3% found in commercial otic solutions (30 μg/mL or ~0.003% w/v), did completely inhibit bacterial growth in around four hours for both bacterial strains tested (Fig. 3A and 3B). The thixogels loaded drug inhibited bacterial growth for both stains more than 3log10 (99.999 % inhibition) which corresponds to bactericidal drug effects. This key result completely validates our overall premise that antibiotics delivered in thixogels can be an effective and straightforward therapy for otitis externa.
Figure 3. Antibiotic release profiles from thixogels.
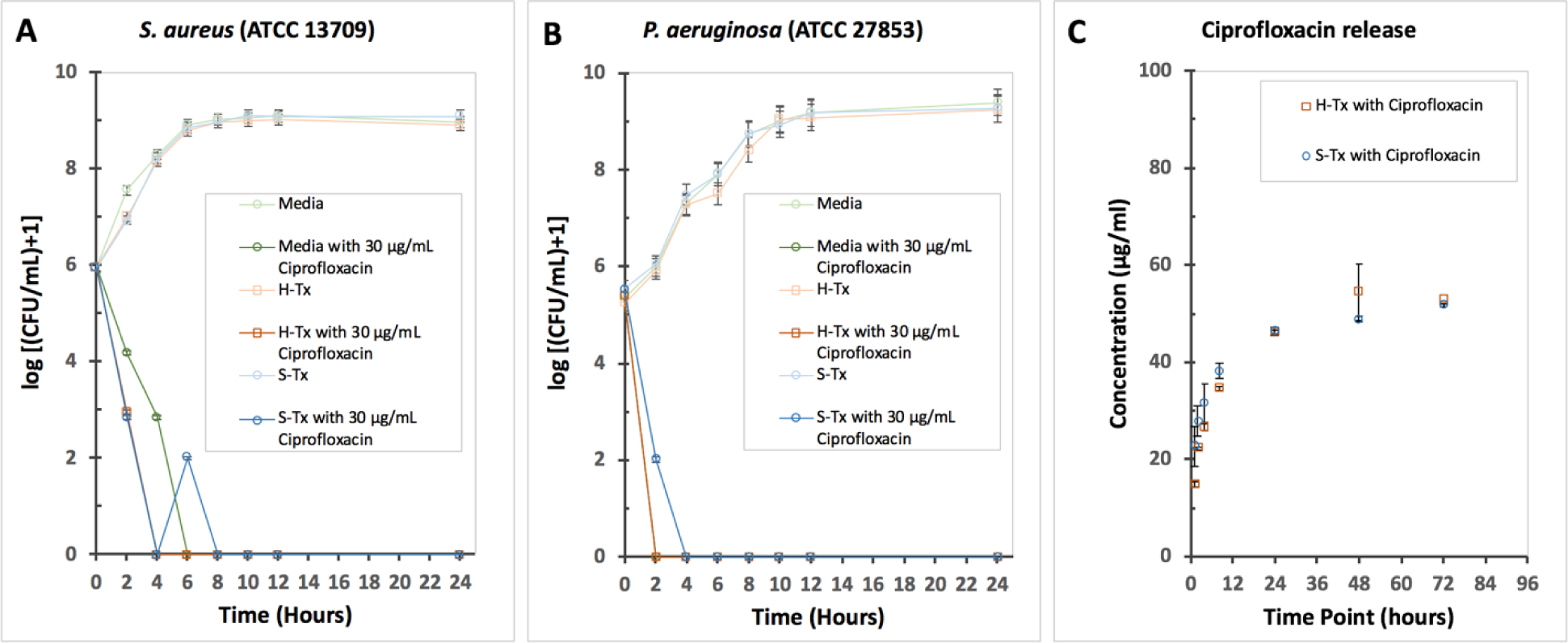
A – effects of H-Tx and B – S-Tx on the kill kinetics of ciprofloxacin against S. aureus 13709 and P. aeruginosa 27853. The MIC for ciprofloxacin is 0.25 μg/mL (from Table 2); the 30 μg/mL is a dose that is ~100 times lower than the intended clinical dose of ciprofloxacin (3,000 μg/mL or 0.3% w/v). Growth was measured by sampling media placed above the thixogel and then quantifying colony forming units (CFU’s) by serial dilution. C – Ciprofloxacin release profiles from H-Tx and S-Tx. Both thixogels released the loaded drug at comparable rates via diffusion (n = 3). The equilibrium concentration between gels and supernatant saline was reached within 24 hours.
Thixogel-loaded antibiotics are released via diffusion.
Another important aspect of our drug delivery system we sought to interrogate was the mechanism of drug release. For this, thixogels loaded with Ciprofloxacin were covered with saline and were assessed for drug release over a period of 72 hours (Fig. 3C). Our data indicate that most of the drug is released within 24 hours via diffusion. Data fitting best matched the Makoid-Banakar mathematical release model for both thixogels (Fig. S1) indicating diffusion as the primary mechanism of drug release23,24. This rapid drug release profile is promising as this would rapidly ensure the delivery of a therapeutically effective dose and the maintenance of this concentration at the infection site.
Thixogels biocompatibility evaluations.
Cytocompatibility.
The H-Tx and S-Tx systems were evaluated for cytocompatibility with primary human adult dermal fibroblasts. Per ISO 10993–5, a material that reduces cell viability to <70% of the negative control (NC) has cytotoxic potential25. When tested with the aforementioned primary cells, both our thixogels met the acceptance criteria for cytocompatibility, with the H-Tx formulation seemingly better tolerated than the S-Tx (Fig. 4A).
Figure 4. Biocompatibility evaluation of thixogels.
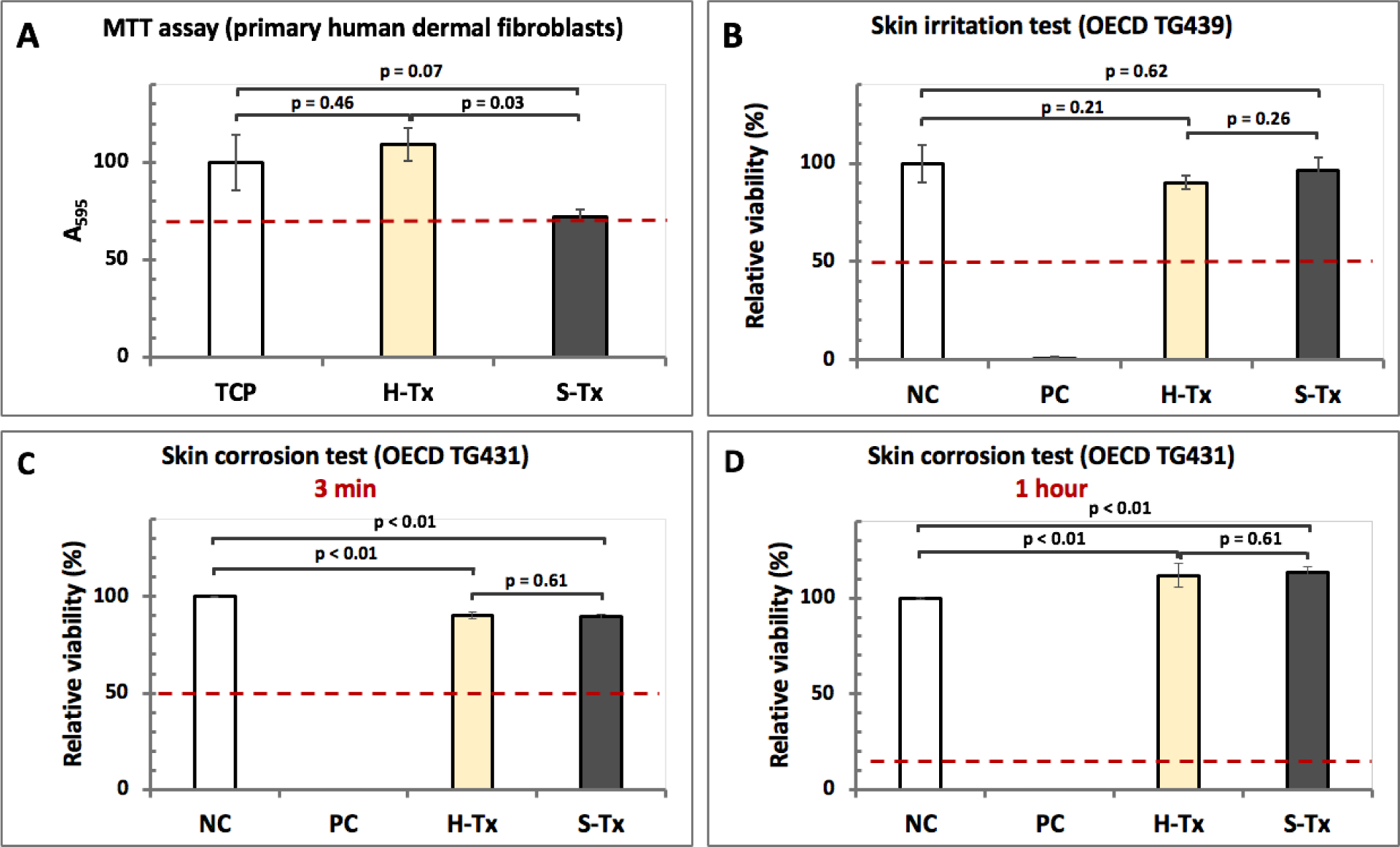
A – cytocompatibility of thixogel formulations as determined by a colorimetric methyltetrazolium assay (MTT) with primary human dermal fibroblasts (n = 3). Cells with no treatment on tissue culture plate (TCP) were used as control. The dotted red line indicates the cut-off for cytocompatibility per ISO 10993–5. ANOVA analysis: p = 0.013 for alpha = 0.05, F = 9.61, F crit = 5.14, DF = 3. B – standardized skin irritation test with EpiDerm™ indicating that the thixogels are non-irritant per UN GHS (n = 3). NC – negative control. An irritant is defined as a test substance that reduces tissue viability ≤ 50% of the mean viability (dotted red line) of the negative controls (NC); NC – Dulbecco’s Phosphate Buffered Saline; PC – 5% Sodium Dodecyl Sulfate solution. ANOVA analysis: p = 4.41E-06 for alpha = 0.05, F = 19.07, F crit = 3.09, DF = 3. C – skin corrosion testing of thixogels with EpiDerm™. After 3 minutes of exposure to HA or SF formulations, the viability of tissues was above the 50% acceptance threshold (red dotted line). ANOVA analysis: p = 2.25E-31 for alpha = 0.05, F = 8814.54, F crit = 3.09, DF = 3. D – skin irritation test after the 1-hour exposure, tissues treated with both formulations fully recovered and had better viability that the negative control (NC) H2O treated tissues. As positive control (PC) an 8N KOH solution was used (acceptance threshold after 1 hour is ≥ 15%, indicated by the red dotted line). ANOVA analysis: p = 8.21E-23 for alpha = 0.05, F = 1221.19, F crit = 3.09, DF = 3.
Organotypic testing.
To further confirm the biocompatibility of the thixogels we sought to evaluate them in standardized biocompatibility assays. The outer ear canal and ear drum are lined with epithelial tissue similar to skin26. We therefore chose EpiDerm™, a validated and regulatory body-accepted in vitro skin model, representative of in vivo outcomes of chemical, pharmaceutical and skin care testing, to assess the biocompatibility of the two thixogels27. EpiDerm™ consists of normal human-derived epidermal keratinocytes cultured to form organized basal, spinous and granular layers, and a multilayered stratum corneum containing intracellular lamellar lipid layers arranged in patterns analogous to those found in the human epidermis. Two separate material biocompatibility aspects were conducted: skin irritability and skin corrosion.
For skin irritability testing we used a standardized protocol (OECD TG 439) in which pre-conditioned EpiDerm™ human skin samples (surface area of 0.63 cm2) are treated with 30 μL test substance for 60 min, followed by removal of test substance and tissue washing28. Subsequently, the tissue viability is assessed with a methyltetrazolium-based colorimetric assay after a 24-hour post-incubation period. Skin irritation is defined by the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (UN GHS) as the production of reversible damage to the skin following the application of a test substance. A test substance is predicted to have skin irritation potential if the mean relative tissue viability of three individual tissues exposed to it is reduced below 50% of the mean viability of the negative controls (NC). Our results (Fig. 4B) indicate that both H-Tx and S-Tx are non-irritant, with tissue samples treated with both H-Tx and S-Tx having mean viability values statistically equivalent to the mean viability of NC (TTEST: H-Tx versus NC, p = 0.21; S-Tx versus NC, p = 0.62; H-Tx versus S-Tx, p = 0.26; p ≤ 0.05 indicates statistically significant differences).
Similarly, for skin corrosion assessments H-Tx and S-Tx thixogels were tested according to OECD TG 43129. Skin corrosion is defined by the UN GHS as the production of irreversible tissue damage in the skin following the application of a test material. The test involves treating the EpiDerm™ tissue samples with 50 μL of test material for 3 minutes and 1 hour, respectively. A material is classified as definitely corrosive if the relative tissue viability after 3 minutes treatment with a test material is decreased below 50%. However, materials classified non-corrosive after the 3-minute treatment (viability ≥ 50%) will be re-classified as corrosive if the relative tissue viability after the 1-hour treatment with the test material is decreased below 15%. For the 3-minute exposure, H-Tx and S-Tx treated tissues showed a slight decrease in mean sample viability compared to the NC (TTEST: H-Tx and S-Tx, respectively, versus NC, p < 0.01), but there was no statistical difference between the mean viability of samples treated with H-Tx or S-Tx (TTEST: H-Tx versus S-Tx p = 0.61; p ≤ 0.05 indicates statistically significant differences) (Fig. 4C). After 1-hour exposure, tissues treated with both H-Tx and S-Tx fully recovered (TTEST: H-Tx and S-Tx, respectively, versus NC, p < 0.01), and there was no statistical difference between the mean viability of samples treated with H-Tx or S-Tx (TTEST: H-Tx versus S-Tx, p = 0.61; p ≤ 0.05 indicates statistically significant differences) (Fig. 4D).
Overall, our results indicate that both after 3-minute and 1-hour treatments our thixogels are classified as non-corrosive. The aforementioned tests are validated and accepted by regulatory bodies to be stand-alone in vitro replacements for animal skin irritation testing. Our skin irritation and skin corrosion results indicate that both the H-Tx and S-Tx thixogels are biocompatible and therefore expected to be well tolerated when applied into the outer ear canal.
In vivo evaluation of antibiotic loaded thixogels
Physiological effects of thixogel application into ears.
Our group has previously reported that based on in vitro evaluations, thixogels dehydrate and revert to a small amount of dry matter within 10 days, and are expected to be eliminated via normal cerumen (ear wax)20. Otological evaluation of mouse ears before, right after and 10 days post thixogel applications confirmed out previous data on the anticipated in situ persistence of thixogels (Fig. 5A–C). No detectable amounts of material were observed at the 10-day data point (Fig. 5C). Histological examination of the ear canal and tympanic membrane indicated minimal to no inflammation or tissue reaction in all treated specimens (Fig. 5D–F). For all histology images, the tissue under the most external layer of darkly stained cells consists mainly of fibrous connective tissue. Fibroblasts are darkly stained cells with an oblong, fuselage appearance. Overall these results indicate that the thixogels are well tolerated in the ear and are expected to be completely resolved within 10-days post application.
Figure 5. Physiological effects of thixogels of thixogel application into ears.
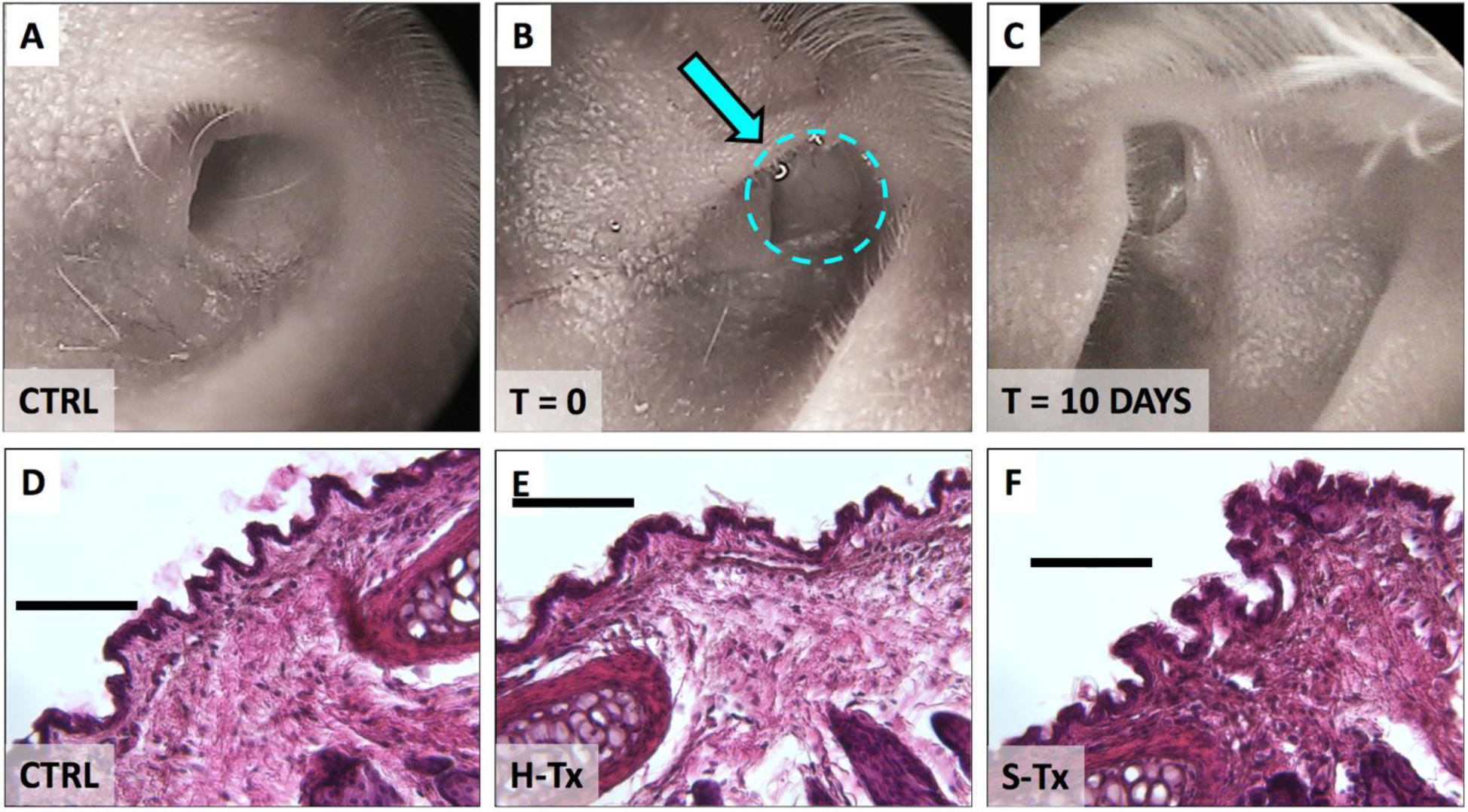
Top panels illustrate the otoscopic evaluation of the mouse ear canal: A – prior to thixogel application; B – immediately after thixogel application (thixogel highlighted by dotted area and indicated by the arrow); and C – 10 days post deployment. No detectable thixogel was present 10 days post-deployment. The bottom row illustrates the histological evaluation of tissue treated with D – saline (control); E – H-Tx and F – S-Tx. Scale bar is 100 μm.
Hearing effects.
To analyze the consequences of thixogel deployment into the ear canal on hearing in mice, we applied H-Tx (7 ears), S-Tx (7 ears) or saline control (6 ears), respectively, and we measured the minimum electrophysiological inputs required to evoke a threshold response in our experimental animals using auditory brainstem response (ABR), and distortion product otoacoustic emission (DPOAE). ABR testing measures the physiological response of the entire auditory pathway. In contrast, DPOAE measures sound produced by the structures of the inner ear. In both cases, increased thresholds required to elicit measurable responses indicate hearing impairment. Immediately after thixogel application, both ABR and DPOAE measurements showed a slight, but significant, threshold elevation (p < 0.005) (Fig. 6B–C and 6E–F); however, this trend was similar to that observed for the saline control application (mimicking typical ear drops) (Fig. 6A and 6D). A 2-way ANOVA analysis of the t = 0 ABR data for saline, H-Tx and S-Tx yielded a p = 0.09 for treatment group comparisons (DF = 2), with a post-hoc Dunnett’s multiple comparisons test indicating that ABR thresholds for H-Tx and S-Tx treatments were significantly higher at 32 Hz compared to saline (p = 0.03 for H-Tx and p = 0.02 for S-Tx, respectively). For DPOAE, no statistical differences were noted between the saline control, H-Tx and S-Tx (p = 0.54, DF = 2). Ten days after thixogels application, statistical analyses of both ABR and DPOAE data indicate that hearing returned to identical levels as the saline control for all tested frequencies (p = 0.95, DF = 2 for ABR; p = 0.42, DF = 2, for DPOAE) (Fig. S2). Overall, our findings indicate that both ABR and DPOAE thresholds reverted back to normal levels 10-days post thixogel deployment, and that the application of thixogels into the ear canal only transiently impacts hearing with effects similar to traditional ear drop applications.
Figure 6. Effects of thixogel topical application on hearing.
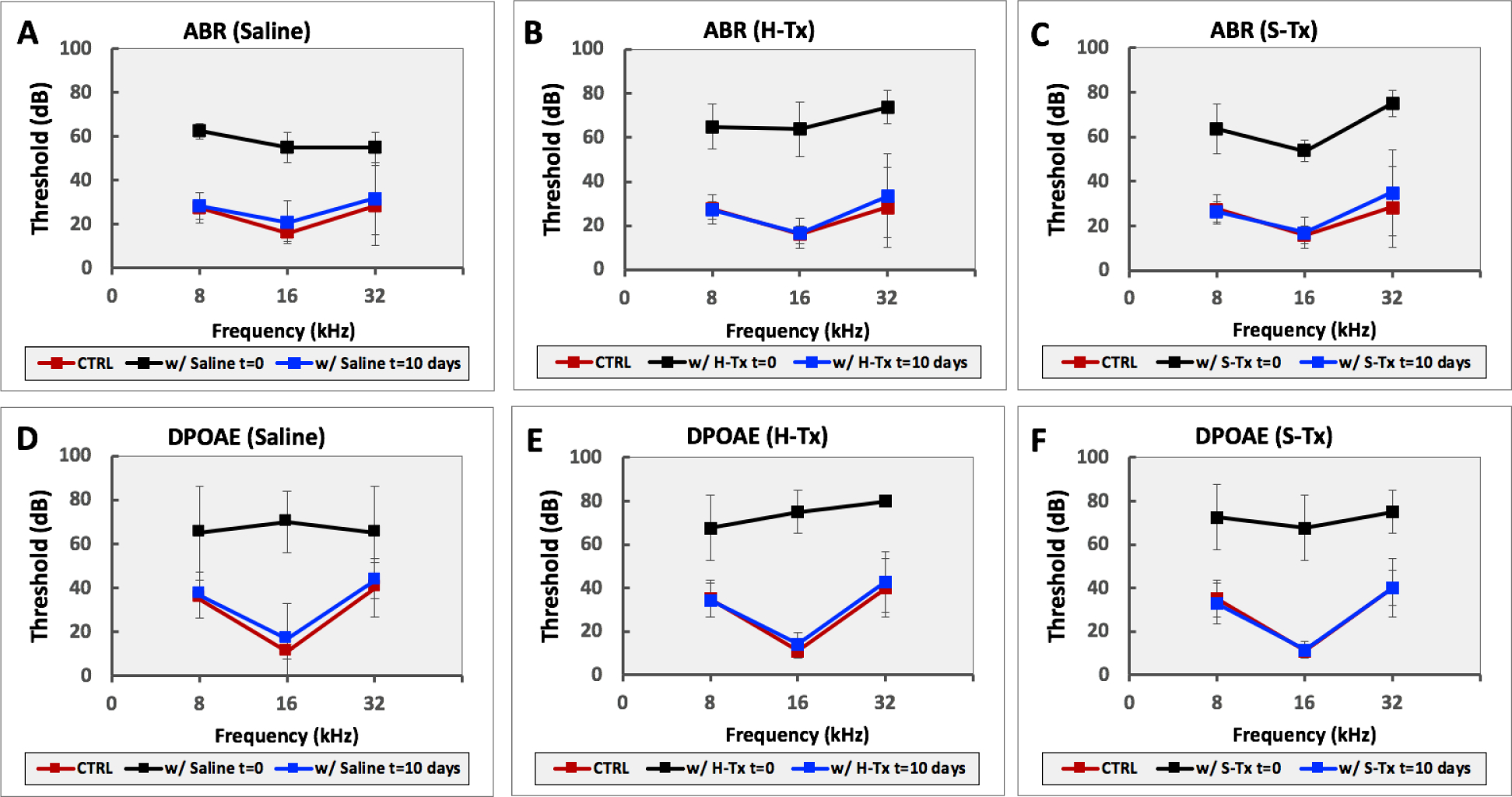
ABR measurements of hearing upon application of equivalent volumes of A – saline (control); B – HA thixogel; and C – SF thixogel in the outer ear canal. DPOAE measurements of hearing upon application of equivalent volumes of D – saline (control); E – HA thixogel; and F – SF thixogel in the outer ear canal. CTRL = measurements recorded prior to administration of saline, H-Tx or S-Tx, respectively.
DISCUSSION
OE is currently treated with topically administered ear drops, but noncompliance with the prescribed treatment regimen can lead to severe complications. A single administration product designed to release the therapeutic drug in situ would significantly improve the treatment, especially for military or elderly patients with hand or head tremors. Furthermore, the incidence of OA and MOE is higher in tropical and developing countries3,13, and these market segments where cold chain preservation or access to adequate medical care are limited, would be well served by such a product. Herein, we described a drug delivery system that is patient and health care provider friendly, would require a single application, is cold chain independent and effectively delivers antibacterials against OE-specific strains.
Two formulations were derived from a range of combinations tested - H-Tx containing hyaluronan, a glycosaminoglycan ubiquitous to mammalian systems, and S-Tx, containing silk fibroin, a biocompatible and biodegradable insect polymeric protein. These two systems performed well in empirical evaluations of usability, and their appearance is elegant as soft, optically transparent hydrogels. Rheological evaluations highlighted their ability to transition through several gel/sol cycles without drastic changes in their storage or loss moduli. However, from a product perspective, the materials would be required to undergo one single transition during deployment into the ear, as they would be pre-packaged prior to initial gelation into a single application, ready to use format. As indicated by our temperature dependence results, both thixogels maintain their properties over the range of temperatures commonly associated with product handling. Temperatures below freezing were not tested, given that the product is intended to be cold chain independent and accidental freezing during shipment and transportation would be unlikely. Some hydrogel-type products have been reported to undergo significant increase in their volume post-deployment due to water uptake, leading to adverse events associate with product use30–32. We therefore assessed the swelling of the two thixogels and found them to be negligible even under exacerbated ‘worst case’ scenario conditions, with samples immersed in saline (the ear canal would contain limited amounts of fluid even in severe infection cases).
With the desired material properties of the H-Tx and S-Tx thixogels confirmed we next sought to evaluate their effect on antibiotic efficacy. Specifically, we sought to understand if the thixogels impact the efficiency or MICs of antibiotics, and if the release rates of the drug from the thixogels is adequate for killing bacterial strains commonly associated with OE. Our results indicated that the effectiveness of certain antibiotics can be affected when incorporated into thixogels. For vancomycin and gentamycin, the MIC values were increased while for ciprofloxacin they were comparable to the no-gel values. This result is particularly promising in the context of our target application as ciprofloxacin, a fluoroquinolone, is the commonly used active pharmaceutical ingredient in topical ear drops17,21. Moreover, from the perspective of a cold chain independent product, ciprofloxacin can also be stored at room temperature and does not require refrigeration21,33,34. The assessment of the release rates of antibiotics from thixogels indicated that when ciprofloxacin was incorporated into either X-Tx and S-Tx, even at concentrations 100-times lower than those in commercial products, it was bactericidal for both S. aureus and P. aeruginosa.
As next step the cytocompatibility and biocompatibility of the thixogels was assessed. For this, in in vitro tests we employed standardized testing methods, specifically ISO 10993–5, OECD 431 and 43925,28,29. All of these tests indicated that both thixogels met the acceptance criteria for cytocompatibility, non-irritability and non-corrosiveness. H-Tx and S-Tx’s biocompatibility was further assessed in mice, with results indicating that they were both well tolerated when deployed into the ear canal. The in vivo evaluation also confirmed our in vitro testing-based prediction regarding the expected 10-day in vitro residence time for the thixogels20.
Finally, we sought to understand the potential effects that the deployment of thixogels into the ear canal could have on hearing. Two different tests were used – one that comprehensively assesses the functionality of the cochlea and the brain pathways for hearing (ABR), and a second one (DPOAE) that specifically interrogates the performance of the inner ear. Both tests indicated that immediately after thixogel application, the hearing levels were affected, but to a similar extent to the application of saline, representative of normal ear drops. Importantly, in both tests, the hearing levels of the mice fully reverted to pre-treatment values, indicating that the application of thixogels only transiently impacts hearings and to the same extent as traditional ear drops.
Collectively, our data highlights the value drivers of these thixotropic material as single application delivery systems for OE-specific antibiotics. Specifically, we confirmed: a – their ability to be easily deployed as liquid but to gel in place immediately after application; b – their cold-chain independence; c – ability to release effective, bactericidal antibiotic concentrations; d – biocompatibility; and e – minimal impact on hearing. While our current study was focused on assessing the suitability of this thixotropic drug delivery system for otitis externa treatment, this material is expected to be readily transferable to other pathologies benefitting from in situ deployment of drugs, especially in developing countries.
MATERIALS AND METHODS
Materials.
Tetraethyl orthosilicate (TEOS) was purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI). Hyaluronic acid was from Lifecore Biomedical, Chaska, MN. Silk fibroin was extracted from commercial, medical device grade Bombyx mori silk yarn (Bratac, Brazil)35 according to a published protocols36–38. Acetic acid (HOAc) was from EMD Millipore (Billerica, MA), ammonium hydroxide (NH4OH) was from Fisher Chemical (Fair Lawn, NJ), phosphate-buffered saline (PBS) was from ATCC (Manassas, VA). Gentamycin Sulfate 600 IU/mg was from Alfa Aesar (Ward Hill, MA). Vancomycin HCl, Ciprofloxacin, and Imipenem were from Sigma-Aldrich (Milwaukee, WI).
Thixogels.
TEOS was activated via hydrolysis with 0.15 M HOAc for 1.5 hour at a 1:9 v/v ratio. Activated TEOS (aTEOS) was then combined with aqueous hyaluronan (10% w/v) or silk solution (2% w/v), respectively (Table S1). The mixtures were vortexed and the pH was adjusted to ~ 2 with 3.0 N HOAc. After 3 hours, the pH was raised to 8.5 with 1.5 N NH4OH. Gels formed when mixtures were left unstirred overnight at room temperature. For antibiotic incorporation, drugs were added in at desired concentrations after thixogel pH adjustment, prior to gelation.
Minimum inhibitory concentrations (MIC) determination.
MICs were assessed for S. aureus (ATCC 13709) and P. aeruginosa (ATCC 27853) using a broth microdilution approach based on CLSI standards and the use of the colorimetric reporter resazurin. Stock solutions of the antibiotics Vancomycin, Ciprofloxacin, Imipenem, and Gentamycin were loaded into 75 μL of thixogel to achieve final test concentrations ranging from 0.0039 – 4.0 μg/mL. For the susceptibility testing, an overnight culture of bacteria was grown in chemically defined Iso-sensitest media (Oxoid). The overnight culture was diluted to 1 × 106 CFU/mL in media and added to 96-well test plates (200 μL per well) containing the antibiotic loaded thixogel. Test plates were then incubated at 37°C for either a period determined by the strain specific doubling time or 24 hours, followed by the addition of 20 μL of a 0.03% w/v aqueous solution of resazurin. After incubation, the test wells were scored for dye reduction. The MIC value was taken as the well with no visible reduction of resazurin to resorufin, marked by the color change from blue to pink.
Kill curves.
Time-kill analyses were performed by culturing S. aureus (ATCC 13709) and P. aeruginosa (ATCC 27853) in Iso-sensitest media overlaid on thixogel loaded with Ciprofloxacin at either final test concentrations of 0.3 or 30 μg/mL. Overnight cultures of each strain were diluted to 1 × 106 CFU/mL in Iso-sensitest media and 2 mL of this dilution was carefully overlaid over 1 mL of Ciprofloxacin loaded thixogel in sterile test tubes. The test tubes were incubated at 37°C and shaken at 50 rpm for 24 hours. Samples of 20 μL were taken every 2 hours (0, 2, 4, 6, 8, 10, 12, and 24), serially diluted in PBS, and plated on Iso-sensitest agar. After incubation overnight at 37°C, bacterial colonies were enumerated and the CFU/mL concentration was calculated for each timepoint. A growth curve was visualized by plotting the log [(CFU/mL) + 1] vs Time.
Drug release studies.
In vitro release studies were performed in triplicate by adding 500 μL PBS to the top of each thixogel followed by incubation at 37°C with gentle agitation (50 rpm) in an incubator shaker (New Burnswick Scientific Co., Classic C24, Model M1247–0002). At each time point (1, 2, 4, 8, 24, 48, and 72 hours), 10 μL of PBS layer on top of gel were removed and mixed with 490 μL diluent (90% 0.02M phosphate buffer, 10% ACN) followed by aliquoting 100 uL into high performance liquid chromatography (HPLC) vial for analysis. An equal volume of PBS (10 μL) was added back to each sample followed by returning samples to incubator until the next time point. The stock standard of ciprofloxacin was prepared at 1.8 mg/mL in 0.1M hydrochloric acid. Intermediate standards (0, 5, 12.5, 25, 37.5, 50, 125, and 250 μg/mL) were prepared from the stock standard at 50x working standard concentrations in PBS. Working standards (0, 0.1, 0.25, 0.5, 0.75, 1, 2.5, and 5 μg/mL) were prepared by mixing 10 μL of each intermediate standard with 490 μL diluent and aliquoting 100 μL into HPLC vial for analysis. Analysis was performed on an Agilent HPLC system equipped with an autosampler (Agilent 1260 Infinity, Model G1329B), a quaternary pump (Agilent 1260 Infinity, Model G1311B), and a diode array detector (Agilent 1260 Infinity, Model G4212B). Data was analyzed with Agilent OpenLAB CDS software (Version C.01.02[14]). A Phenomenex Gemini (3.0mm × 150 mm, 100 Å, 5 μm, Part No. 00F-4435-Y0) column with an attached Phenomenex SecurityGuard™ (C18, 4 × 2.0 mm, Part No. AJ0–4286) guard column was used for analysis. Chromatographic separation was achieved by gradient elution (Table S3) with mobile phase A (0.02M phosphate buffer in water) and mobile phase B (acetonitrile) at a flow rate of 1.0 mL/min. Column temperature was ambient, injection volume was 50 μL, and the total run time was 5.0 minutes. UV absorbance was collected at a wavelength of 277 nm.
Rheological characterization.
All hydrogels were characterized within the materials pseudo-linear viscoelastic range with a 1.00 mm gap, at 20°C unless otherwise specified. Oscillatory strain sweeps for thixotropy investigation were conducted with a 8 mm parallel plate geometry within a strain range of 1–250% and an angular frequency of 10 rad/sec. The wait time between the cycles was 30 sec. For temperature dependent hydrogel behavior evaluation, samples were loaded onto the Peltier plate and a 20 mm parallel plate geometry (to provide a larger temperature exchange surface) was used for characterization. The hydrogel samples were equilibrated to 4°C then subjected to a temperature ramp of 5°C/min up to 40°C, at a stain rate of 0.4% and an angular frequency of 10 rad/sec. Swelling tests were performed with the 20 mm flat plate geometry and an active axial force control of 0.1 N. Hydrogels were loaded onto the Peltier plate and equilibrated at 37°C for 6 minutes. PBS pre-warmed to 37°C was then added and the gap distance between the plate and geometry was monitored for 30 minutes post addition. Initial gap heights varied from 1000 to 2000 μm depending on sample loading variations. All volumes were calculated assuming a perfect cylinder with a radius of 10 mm and a height measured by the rheometer.
Cytocompatibility.
Primary adult human dermal fibroblasts (PCS-201–012, ATCC, Manassas, VA) were used to assess the cytocompatibility of the thixogels according to ISO −10993–5:2009 (Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity, Annex C). Cells were seeded in a 96-well plate at a density of 105 cells/well in 100 μL in serum-free fibroblast conditioned media (ATCC, Manassas, VA) and incubated for 24 hours. Subsequently, the media was removed and replaced with 50 μL of thixogel (different formulations) and 50 μL of fresh media followed by incubation for 24 h at 37°C/5% CO2. Cellular viability was quantified with an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid) (Promega, Madison, WI) and detected via absorbance at 595 nm with a FilterMax F5 microplate reader (Molecular Devices, Sunnyvale, CA).
Organotypic testing.
Organotypic tests kits (EPI-SIT and EPI-SCT) were purchased from MatTek Corporation, Ashland, MA. Testing was conducted per standardized protocols (OECD 431 and OECD 439)28,29.
In vivo studies.
All animal studies were approved by the University of Utah Institutional Animal Care and Use Committee (protocol number 17–06012), performed in compliance with relevant institutional policies, local, state, and federal laws, and conducted following National Research Council Guide for the Care and Use of Laboratory Animals, Eighth Edition. Four-week-old BALB/c mice were used for experiments as indicated. Animals were housed and bred under pathogen-free conditions at the Central Animal Facility at the University of Utah.
For this study a total of 10 mice (8 females and 2 males, based on availability) were used. Mice were anesthetized, then their outer ear canal was filled with thixogels or saline control, respectively. Otoscopic imaging was performed with a Medcam Pro camera (with an 8mm optical lens) with a 70-degree Karl Storz 7230CA 4.0mm 18cm rigid endoscope, captured on a Sony DPP-SV88 at 403 DPI.
Hearing assessments.
For auditory brainstem response (ABR) and distortion product otoacoustic emission (DPOAE) testing, mice (10 total, 8 male and 2 female) were anesthetized with a combination of ketamine and xylazine at 100 and 10 mg/kg body weight, respectively. Their outer ear canals were then filled with 5 μl H-Tx (7 ears), S-Tx (7 ears) or saline control (6 ears), respectively. ABRs/DPOAEs were performed in a double-walled sound chamber (IAC Acoustics, North Aurora, IL, USA). The body temperature was maintained at ~37°C via a heating pad. For ABR testing, an electrostatic speaker (EC-1, Tucker-Davis Technology, Alachua, FL, USA) fitted with a 1.5 cm long polyethylene tube was placed abutting the ear canal. Needle electrodes were placed subcutaneously at the mastoid of the tested side and vertex, with a remote ground electrode placed in the rump area. ABR thresholds were measured bilaterally in all mice. ABR signals were amplified with a TDT RA4 pre-amplifier (Tucker-Davis Technology), filtered from 100 to 3000 Hz, averaged and digitized with a TDT RA16BA processor controlled by BioSigRP software (Tucker-Davis Technology). Acoustic stimuli were digitally generated and processed by a RX6 real-time processor and passed through a PA5 attenuator prior to delivery to the speaker amplifier at a rate 24±32 times/sec. Responses to 1,000 sweeps were averaged for a series of responses to tone pips ranging from 8 to 32 kHz (5 ms with 0.5 ms cos2 rise and fall) using 5- or 10-dB intensity steps, over a 15±90 dB of sound pressure level (dB SPL) range. ABR traces were visually inspected after plotting the amplitude of each peak against stimulus intensity. The DPOAEs were measured using an ER-10B+ (Etymotic Research, Elk Grove, IL, USA) microphone coupled with two EC1 speakers. Stimuli of two primary tones f1 and f2 (f2/f1 = 1.2) were presented with f2 = f1±10 dB. Primary tones were stepped from 30 to 80 dB SPL (for f1) in 10 dB increments and swept from 8 to 32 kHz in octave steps. Stimuli were generated and attenuated digitally (200 kHz sampling). The ear canal sound pressure was pre-amplified and digitized. A fast Fourier transformation was computed, and the sound pressures at f1, f2, and 2f1- f2 were extracted after spectral averaging from 50 serial waveform traces (each corresponding to 84 ms of digitized ear canal sound pressure waveform). The mice hearing reaches mature thresholds by 3 weeks of age39. To avoid potential confounding results due to immaturity of the auditory system, we chose to test our animals at 4 weeks of age.
Histology.
Temporal bones/ear canals/tympanic membranes were harvested ten days after thixogel application. The temporal bones were subsequently decalcified for 48 hours as previously published39,40. Paraffin-embedded samples were then cryosectioned in a plane perpendicular to the tympanic membrane at a thickness of 10 μm, then stained with hematoxylin and eosin. Samples were analyzed by a pathologist blinded to the identity of the treatment groups.
Statistical analyses.
Two-tail type 3 Student’s TTESTs were employed for 2-group comparisons with α = 0.05. One-way ANOVA was employed for multiple comparisons with single variables with confidence limits of 95% considered significant. Two-way ANOVA was used for multiple comparisons with two variables with confidence limits of 95% considered significant and post-hoc Dunnett’s multiple comparisons tests. For all figures the error bars represent standard deviation values.
Supplementary Material
Table S1. Formulation of the two thixogels evaluated.
Table S2. Swelling parameters for the two thixogels.
Table S3. Mobile phase used for gradient elution in the drug release studies.
Fig. S1. Ciprofloxacin release data fitting with the Makoid-Banakar model.
Fig. S2. Two-way ANOVA analyses for treatment groups.
ACKNOWLEDGEMENTS
We thank Dr. Theodore Pysher, MD, pathologist from University of Utah, for his assistance with the interpretation of the histology data, and Dr. Travis Hughes, PhD from the University of Montana for his support with the 2-way ANOVA data analyses.
Funding: This work was financially supported by a National Institutes of Health Phase I Small Business Technology Transfer the Center grant to M.S. and Promiliad BioPharma, Inc. (R41DC017641 - ‘Single application thixotropic antibiotic delivery systems for otitis externa’).
Footnotes
Competing interests: N.P. serves as CEO for Promiliad BioPharma. N.P., J.A. and J. H. received compensation from Promiliad BioPharma for their work on this project. The other authors declare that they have no competing interests. An international patent application (PCT/US20/39551) was filed on 06/26/2020 pertaining to the results presented in the paper.
Data and materials availability: All data for this study have been included in this article. All materials used in this study are commercially available.
REFERENCES
- (1).Centers for Disease Control and Prevention. Estimated Burden of Acute Otitis Externa --- United States, 2003–2007; 2011. [Google Scholar]
- (2).Hui CP; Canadian Paediatric Society; Infectious Diseases and Immunization Committee. Acute Otitis Externa. Paediatrics & Child Health 2013, 18 (2), 96–98. 10.1093/pch/18.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Rosenfeld RM; Schwartz SR; Cannon CR; Roland PS; Simon GR; Kumar KA; Huang WW; Haskell HW; Robertson PJ Clinical Practice Guideline: Acute Otitis Externa. Otolaryngology–Head and Neck Surgery 2014, 150 (1_suppl), S1–S24. 10.1177/0194599813517083. [DOI] [PubMed] [Google Scholar]
- (4).Lee H; Kim J; Nguyen V Ear Infections. Primary Care: Clinics in Office Practice 2013, 40 (3), 671–686. 10.1016/j.pop.2013.05.005. [DOI] [PubMed] [Google Scholar]
- (5).Agius AM; Reid AP; Hamilton C Patient Compliance with Short-Term Topical Aural Antibiotic Therapy. Clin Otolaryngol Allied Sci 1994, 19 (2), 138–141. 10.1111/j.1365-2273.1994.tb01198.x. [DOI] [PubMed] [Google Scholar]
- (6).England RJA; Homer JJ; Jasser P; Wilde AD Accuracy of Patient Self-Medication with Topical Eardrops. The Journal of Laryngology & Otology 2000, 114 (1), 24–25. 10.1258/0022215001903834. [DOI] [PubMed] [Google Scholar]
- (7).Kumar SP Malignant Otitis Externa-A Review. Journal of Infectious Diseases and Therapy 2014, 03 (01). 10.4172/2332-0877.1000204. [DOI] [Google Scholar]
- (8).Medina-Blasini Y; Sharman T Otitis Externa In StatPearls; StatPearls Publishing: Treasure Island (FL), 2020. [PubMed] [Google Scholar]
- (9).Berenholz L; Katzenell U; Harell M Evolving Resistant Pseudomonas to Ciprofloxacin in Malignant Otitis Externa: The Laryngoscope 2002, 112 (9), 1619–1622. 10.1097/00005537-200209000-00017. [DOI] [PubMed] [Google Scholar]
- (10).Hobson CE; Moy JD; Byers KE; Raz Y; Hirsch BE; McCall AA Malignant Otitis Externa: Evolving Pathogens and Implications for Diagnosis and Treatment. Otolaryngology-Head and Neck Surgery 2014, 151 (1), 112–116. 10.1177/0194599814528301. [DOI] [PubMed] [Google Scholar]
- (11).Bhasker D; Hartley A; Agada F Is Malignant Otitis Externa on the Increase? A Retrospective Review of Cases. Ear Nose Throat J 2017, 96 (2), E1–E5. [DOI] [PubMed] [Google Scholar]
- (12).Chawdhary G; Liow N; Democratis J; Whiteside O Necrotising (Malignant) Otitis Externa in the UK: A Growing Problem. Review of Five Cases and Analysis of National Hospital Episode Statistics Trends. The Journal of Laryngology & Otology 2015, 129 (06), 600–603. 10.1017/S002221511500105X. [DOI] [PubMed] [Google Scholar]
- (13).Kumar SP; Ravikumar A; Somu L; Ismail NM Malignant Otitis Externa: An Emerging Scourge. Journal of Clinical Gerontology and Geriatrics 2013, 4 (4), 128–131. 10.1016/j.jcgg.2013.02.003. [DOI] [Google Scholar]
- (14).Loh TL; Renger L; Latis S; Patel H Malignant Otitis Externa in Australian Aboriginal Patients: A 9-Year Retrospective Analysis from the Northern Territory. Australian Journal of Rural Health 2019, 27 (1), 78–82. 10.1111/ajr.12468. [DOI] [PubMed] [Google Scholar]
- (15).Carlton DA; Perez EE; Smouha EE Malignant External Otitis: The Shifting Treatment Paradigm. American Journal of Otolaryngology 2018, 39 (1), 41–45. 10.1016/j.amjoto.2017.05.010. [DOI] [PubMed] [Google Scholar]
- (16).Ciprofloxacin Otic Suspension (Otiprio) for Acute Otitis Externa. JAMA 2018, 320 (13), 1375 10.1001/jama.2018.12787. [DOI] [PubMed] [Google Scholar]
- (17).Otonomy, Inc. Otiprio Directions for Use. March 2018. [Google Scholar]
- (18).Handorf AM; Zhou Y; Halanski MA; Li W-J Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11 (1), 1–15. 10.1080/15476278.2015.1019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Jiao T; Clifton RJ; Converse GL; Hopkins RA Measurements of the Effects of Decellularization on Viscoelastic Properties of Tissues in Ovine, Baboon, and Human Heart Valves. Tissue Engineering Part A 2012, 18 (3–4), 423–431. 10.1089/ten.tea.2010.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Serban B; Stipe K; Alverson J; Johnston E; Priestley N; Serban M A Controlled Antibiotic Release System for the Development of Single-Application Otitis Externa Therapeutics. Gels 2017, 3 (4), 19 10.3390/gels3020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Novartis. Ciprodex - Indications and Usage; 2019. [Google Scholar]
- (22).Merck. Primaxin - Prescribing Information; 2010. [Google Scholar]
- (23).Costa P; Sousa Lobo JM Evaluation of Mathematical Models Describing Drug Release from Estradiol Transdermal Systems. Drug Development and Industrial Pharmacy 2003, 29 (1), 89–97. 10.1081/DDC-120016687. [DOI] [PubMed] [Google Scholar]
- (24).Makoid Michael; Dufoure A; Banakar U. Makoid Michael & Dufoure A & Banakar U (1993). Modelling of Dissolution Behaviour of Controlled Release Systems. STP Pharma. 3. 49 – 58. STP Pharma 1993, 3, 49–58. [Google Scholar]
- (25).Biological Evaluation of Medical Devices: Tests for in Vitro Cytotoxicity; BSI British Standards. 10.3403/30160958. [DOI]
- (26).Yang R; Wei T; Goldberg H; Wang W; Cullion K; Kohane DS Getting Drugs Across Biological Barriers. Advanced Materials 2017, 29 (37), 1606596 10.1002/adma.201606596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Kandárová H; Liebsch M; Gerner I; Schmidt E; Genschow E; Traue D; Spielmann H The EpiDerm Test Protocol for the Upcoming ECVAM Validation Study on in Vitro Skin Irritation Tests--an Assessment of the Performance of the Optimised Test. Altern Lab Anim 2005, 33 (4), 351–367. 10.1177/026119290503300408. [DOI] [PubMed] [Google Scholar]
- (28).OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. OECD Publishing; July 28, 2015. 10.1787/9789264242845-en. [DOI] [Google Scholar]
- (29).OECD. Test No. 431: In Vitro Skin Corrosion: Human Skin Model Test. OECD Publishing; November 23, 2004. 10.1787/9789264071148-en. [DOI] [Google Scholar]
- (30).Baxter Healthcare Co. CoSeal Surgical Sealant - Instructions for Use. 2014. [Google Scholar]
- (31).Integra LifeSciences Corporation. DuraSeal - Instructions for Use. 2014. [Google Scholar]
- (32).Serban MA; Panilaitis B; Kaplan DL Silk Fibroin and Polyethylene Glycol-Based Biocompatible Tissue Adhesives. J Biomed Mater Res A 2011, 98 (4), 567–575. 10.1002/jbm.a.33149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Bayer HealthCare Pharmaceuticals Inc. CIPRO® (Ciprofloxacin Hydrochloride) Tablet, for Oral Use CIPRO® (Ciprofloxacin Hydrochloride), for Oral Suspension - Medication Guide. 2016. [Google Scholar]
- (34).Donnelly RF Stability of Ciprofloxacin in Polyvinylchloride Minibags. The Canadian Journal of Hospital Pharmacy 2011, 64 (4). 10.4212/cjhp.v64i4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Jewell M; Daunch W; Bengtson B; Mortarino E The Development of SERI ® Surgical Scaffold, an Engineered Biological Scaffold: The Development of SERI ® Surgical Scaffold. Annals of the New York Academy of Sciences 2015, 1358 (1), 44–55. 10.1111/nyas.12886. [DOI] [PubMed] [Google Scholar]
- (36).Rockwood DN; Preda RC; Yücel T; Wang X; Lovett ML; Kaplan DL Materials Fabrication from Bombyx Mori Silk Fibroin. Nature Protocols 2011, 6 (10), 1612–1631. 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Johnston ER; Miyagi Y; Chuah J-A; Numata K; Serban MA Interplay between Silk Fibroin’s Structure and Its Adhesive Properties. ACS Biomaterials Science & Engineering 2018, 4 (8), 2815–2824. 10.1021/acsbiomaterials.8b00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Love CJ; Serban BA; Katashima T; Numata K; Serban MA Mechanistic Insights into Silk Fibroin’s Adhesive Properties via Chemical Functionalization of Serine Side Chains. ACS Biomater Sci Eng 2019, 5 (11), 5960–5967. 10.1021/acsbiomaterials.9b01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Almishaal AA; Mathur PD; Hillas E; Chen L; Zhang A; Yang J; Wang Y; Yokoyama WM; Firpo MA; Park AH Natural Killer Cells Attenuate Cytomegalovirus-Induced Hearing Loss in Mice. PLOS Pathogens 2017, 13 (8), e1006599 10.1371/journal.ppat.1006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Pecha PP; Almishaal AA; Mathur PD; Hillas E; Johnson T; Price MS; Haller T; Yang J; Rajasekaran NS; Firpo MA; Park AH Role of Free Radical Formation in Murine Cytomegalovirus–Induced Hearing Loss. Otolaryngology–Head and Neck Surgery 2020, 162 (5), 709–717. 10.1177/0194599820901485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Formulation of the two thixogels evaluated.
Table S2. Swelling parameters for the two thixogels.
Table S3. Mobile phase used for gradient elution in the drug release studies.
Fig. S1. Ciprofloxacin release data fitting with the Makoid-Banakar model.
Fig. S2. Two-way ANOVA analyses for treatment groups.


