Abstract
An outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus capable of causing coronavirus disease 2019 (COVID-19), was declared as a global public health emergency on January 30, 2020, by the World Health Organization. In this devastating situation, precautionary measures, early diagnosis, and repurposed drugs appear to be timely and decisive factors by which to handle this problem until the discovery of an effective, dedicated vaccine or medicine is made. Currently, some researchers and clinicians have claimed evidence exists in favor of the use of some antimalarial drugs (chloroquine, hydroxychloroquine) antiviral drugs (remdesivir, favipiravir, lopinavir, ritonavir, umifenovir) vitamins, traditional Chinese medicines, and herbal medicines against SARS-CoV-2 infection. Based on the available literature, this review article sought to highlight the current understanding of the origin, transmission, diagnosis, precautionary measures, infection and drug action mechanisms, therapeutic role, and toxicities of targeted drugs for the prevention and cure of COVID-19. This review may be useful for developing further strategies as a blueprint and understanding the mentioned drugs’ mechanisms to elucidate the possible target of action by which to successfully freeze the replication of the SARS-CoV-2 virus.
Keywords: SARS-CoV-2, COVID-19, Diagnosis, Precautionary measures, Chloroquine, Hydroxychloroquine, Remdesivir, Favipiravir, Lopinavir, Ritonavir, Umifenovir, Drug toxicities
Introduction
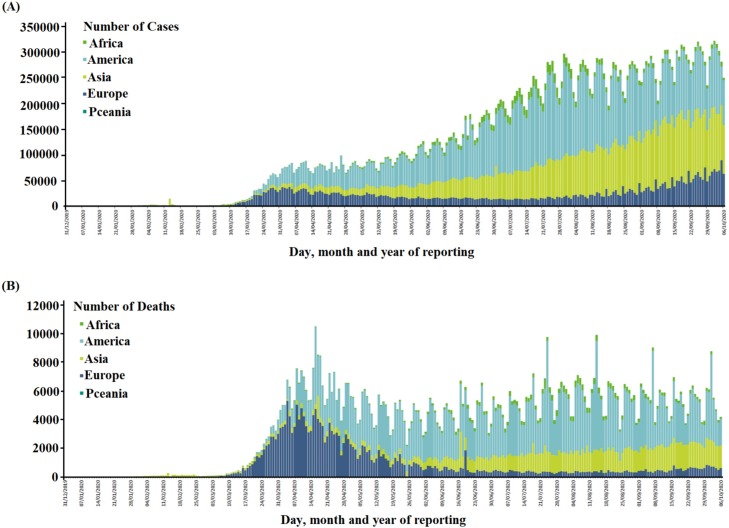
The man-to-man transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global threat and was declared as the coronavirus disease 2019 (COVID-19) pandemic by the World Health Organization (WHO) in March 2020. Reporting indicates SARS-CoV-2 was first observed in cases of unexplained pneumonia in Wuhan, China in November 2019 but has since infected millions of people all over the world [1,2]. The rapid proliferation of this virus has impacted more than 200 countries and territories, with 35,523,518 confirmed cases around the globe and 1,042,398 deaths as shown in Fig. 1 [1,3,4]. It has also had significant impacts on the global economy, more than US$ 1.0 trillion, due to the long quarantine periods required to control the spread of the SARS-CoV-2 together with the hospitalization and high complexity of clinical care required by a proportion of patients and the resultant deaths in some cases [1,5]. SARS-CoV-2, a single-strand RNA virus measuring 80–120 nm in diameter, is the seventh known member of the coronavirus family, which also contains severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome–related coronavirus (MERS-CoV) viruses in spite of substantial diff ;erences in their epidemiology, pathology, and proteins [6,7]. COVID-19 patients commonly present with respiratory symptoms, including fever, cough, and dyspnea (shortness of breath), and the severity of infection may become lethal due to the onset of pneumonia, severe acute respiratory syndrome, and kidney failure [4,6]. However, to date, no proper medicine or treatment of COVID-19 has been reported [8,9].The ongoing and significant increase in the number of confirmed cases requires the urgent identification of appropriate treatments for the prevention and control of COVID-19 spread [10]. Instead, current treatments for COVID-19 patients mainly address their symptoms and a related decrease in the viral load is not apparent. Therefore, efficient approaches for drug development and the use of several existing drugs, including antimalarial and antiviral agents, vitamins, herbal medicines, and antipyretics [10,11] are being explored promptly. Among the drugs presently undergoing testing in vitro and in vivo, chloroquine (CQ), hydroxychloroquine (HCQ), remdesivir, ribavirin, favipiravir, lopinavir/ritonavir, umifenovir, traditional Chinese medicines (TCM), and micronutrients, have shown favorable clinical results in inhibiting the viral replication of SARS-CoV-2 [11,12]. The possible importance of these medications triggers the demand to understand their mechanisms of action, therapeutic aspects, and toxicity profiles. Keeping these points in consideration, this review article summarizes and describes the mechanisms of action, therapeutic characteristics, and toxicological effects of the drugs mentioned above in view of their possible role in the prevention and control of the COVID-19 pandemic.
Fig. 1.
(A) Total number of confirmed cases of SARS-CoV-2 infection from December 31, 2019 to October 6, 2020 around the world, (B) Total number of deaths by COVID-19 around the world. (The numbers are retrieved from ECDC) [3].
Origin and transmission of SARS-CoV-2
In response to the emerging COVID-19 pandemic, the WHO Director-General announced a Public Health Emergency of International Concern on January 30, 2020. According to the South Morning China Post, in Hubei province, China, a 55-year-old individual was the first person worldwide to contract COVID-19 in a case that dates back to November 17, 2019, more than a month before doctors began broadly reporting cases of a pneumonia of unknown origin in Wuhan, China, also in Hubei province, at the end of December 2019 [[13], [14], [15], [16]]. Since the first clinical reports of the novel coronavirus emerged in Wuhan, Hubei province, China, there has been considerable discussion on the origin of the causative virus, SARS-CoV-2. Earlier, an assumption was made that the virus escalated from the wet market into the city. However, it's now clear that the pandemic had no connection to the wet market, which was reported in January 2020 in The Lancet [8]. The worldwide escalation of this epidemic remains in a gray area; as of October 6, 2020, 35,523,518 cases of SARS-CoV-2 infection in more than 200 countries with 1,042,398 deaths have been confirmed [3]. Andersen et al. studied the comparative analysis of the SARS-CoV-2 genome and reported its origin while also discussing scenarios by which the virus could have appeared; notably, their analyses clearly show that SARS-CoV-2 is not a laboratory construct or a purposefully manipulated virus [16]. Instead, given it was initially predicted that SARS-CoV-2 originated from the wet market of Huwan, China, it was suggested that some natural source or an animal host had existed before zoonotic transfer. The phylogenetic analysis of SARS-CoV-2 genome suggested that the virus is closely identical to bat-derived SARS (bat CoV, RaTG13, 96%) which indicates that bats serve as reservoir hosts for its progenitor [6,[17], [18], [19]]. The role of the intermediate host is also notable in the transmission of viruses, as, in earlier reported cases of SARS-CoV and MERS-CoV, the intermediate hosts were civet cats and camels, respectively. In this case, the pangolin is suspected to be the intermediate host of the SARS-CoV-2 virus [20]. Others also suggested the pangolin may be an intermediate host because of the genome similarities (85.5%–92.4%) between SARS-CoV-2 and pangolin CoV [21]. Hence, it can be easily understood that natural selection in humans following zoonotic transfer of SARS-CoV-2 spread the infection into human beings. Once the progenitor of SARS-CoV-2 jumped into humans and acquired the genomic features through adaptation during undetected human-to-human transmission, the pandemic began taking off on a large scale. Human-to-human transmission through binding between cellular receptors (i.e., angiotensin-converting enzyme 2; ACE2) and receptor-binding domains of the virus’ spikes could be a possible method for SARS-CoV-2 infection [17,22,23]. However, direct contact, respiratory droplets, and aerosols released by an infected person through coughing or sneezing facilitated the spread of SARS-CoV-2 in the community. The direct or indirect exposure of the eyes, mouth, and nose mucous membranes may also play a role in SARS-CoV-2 infection as the virus also remains in the air for a limited period of time and functions as an airborne pathogen [[24], [25], [26]]. Recently, the WHO announced that asymptomatic patients are not infectious [4]. In some cases, the digestive tract may have been the potential route of SARS-CoV-2 transmission rather than the respiratory tract, but further studies are required to confirm this possibility [27]. Breastfeeding mothers should also be studied regarding virus transmission because pregnant women have an increased chance of experiencing respiratory infections and extreme pneumonia [19,22]. Precautionary measures such as quarantine, isolation, social distancing, and sanitization have been adopted to limit the escalation of the pandemic.
Diagnosis of COVID-19
In the emergence of a virulent pandemic, the straightforward point-of-care (diagnosis), should be robust in terms of both handling and analysis. Until scientists and clinicians can contrive proper treatments for COVID-19 and they enter into daily practice, making an appropriate diagnosis is the only tool by which to help mitigate the current situation. Currently, the use of molecular-based polymerase chain reaction (PCR) tests and serological assays, which detect the presence of antibodies in a blood sample, have been recommended by the WHO and United States (US) Centers for Disease Control and Prevention (CDC) [4,28,29]. Additionally, demographical; clinical; laboratory assessments including lymphopenia, prolonged prothrombin time, elevated lactate dehydrogenase, elevated alanine aminotransferase, elevated aspartate aminotransferase, elevated d-dimer, elevated neutrophils, eosinopenia, elevated C-reactive protein, and elevated troponin (including high-sensitivity troponin) [8,[30], [31], [32], [33], [34], [35]]; and radiological assessments such as chest X-ray or computed tomography imaging of COVID-19 patients can establish a line of action for the clinician to take regarding patient management. Moreover, other secondary infections/problems could be ruled out or, if present, the cure of the secondary infection/problem might be helpful to increase the immunity and lessen the traumatized condition of the patient.
The WHO and CDC have issued an advisory on the clinical and epidemiological findings of COVID-19 patients [4,28,29]. The preliminary diagnosis of a suspected case may include fever, respiratory symptoms, low lymphocyte count, travel history, direct contact with an infected patient, musculoskeletal pain, and diarrhea [36]. A molecular-based real-time reverse-transcriptase PCR (RT-PCR) test is currently being implemented as a standard method for COVID-19 patient diagnosis. In this test, the extracted viral RNA from oro-/nasopharyngeal (OP/NP) swab samples of the patient are input into the RT-PCR system, which is followed by the synthesis of cDNA using the reverse-transcriptase enzyme. The synthesized complementary DNA template accelerates the amplification of target sequences of the viral genome that could be highly homologous to SARS-CoV-2 [8,37,38]. However, such RT-PCR test kits have many limitations, including timing and handling. During RT-PCR, the concentration and quality of viral RNA affect the speed of target-sequence amplification of the viral genome, leading it to be considered only as a semi-quantitative assay. Thus, the amplification rate can be used as a proxy for the sample viral load and false amplification can yield negative results, which could be a severe concern in the case of poor quality of the patient sample, a sample taken during an early stage of the disease, or poor experience among the analyzing personnel.
The suspected diagnosis of COVID-19 through RT-PCR requires analysis of an OP/NP swab, sputum, or bronchoalveolar lavage sample in the reference laboratory. The time between sample collection, transfer to the laboratory, and generation of the results take at least 24–72 h, which seems to be time-consuming. At the same time, this process should become much faster with a streamlined approach enacted physically near to the patient (on-site) in urgent clinical scenarios. As such, the true prevalence of SARS-CoV-2 infection is currently unknown and the sensitivity of PCR to detect infection is also unclear. Another tremendously used diagnosis method is serological testing [39]. In serological testing, an enzyme-linked immunosorbent assay (ELISA) is used detect the presence of immunoglobulins M and G (IgM and IgG) antibodies in whole blood, plasma, or a serum sample, which is a highly recommended approach relative to immunochromatography testing (Card test) due to its higher sensitivity and specificity, although the latter is convenient, inexpensive, and offers rapid turnover [40]. ELISA also boasts a quick turnover time; however, it may yield false-positive results due to the N (Nucleocapsid) proteins of SARS-CoV-2 [41,42]. Cross-reactivity is expected with SARS-CoV-1 infection since there is 90% homology with the genetic sequence of SARS-CoV-2. Hence, ELISA kits should be explicitly developed to assess SARS-CoV-2 S proteins (transmembrane glycoprotein spikes) [43]. This is why the application of the serological test to SARS-CoV-2 is based on the choice of antigen and why the choice of an assay is less well-defined for SARS-CoV-2. Moreover, both serological and RT-PCR tests are complementary to each other and offer sustainable results according to their sensitivities at different stages of the infection. Therefore, it is recommended to combine both the modalities of diagnosis, which could be conducive for the enhanced sensitivity in the early detection and diagnosis of COVID-19 patients [44,45]. Indeed, IgM-ELISA, in combination with RT-PCR, achieves a greater rate of detection than RT-PCR only at 98.6% vs. 51.9%, respectively [42,44,45]. Additionally, lung radiography is a correlative diagnosis that provides potential shreds of evidence in the case of asymptomatic patients. Imaging modalities such as high-resolution computed tomography could be quite useful in the diagnosis and observation of lung abnormalities (e.g., bilateral pulmonary parenchymal ground-glass and consolidative pulmonary opacities) in patients [8,31,[46], [47], [48], [49]]. The findings of abnormalities in computed tomography scans may be correlated with disease progression and prognosis. Based on the diagnosis, the patient’s condition could be monitored and a line of action established for the delivery of further or intensive treatment or follow-up could be planned accordingly [50].
Precautionary measures for COVID-19
Given the scarcity of proper medications of COVID-19, precautions and preventive measures are the key strategies by which to limit the escalation of the pandemic. Currently, the WHO and CDC have issued various advisories in support of preventing further spread of COVID-19 [29,51]. The essential precautionary measures such as social distancing, quarantine of infected individuals, and hygiene of the environment are now in common practice for the prevention of SARS-CoV-2 spread. The measures described above have been adopted by human beings involved with COVID-19 patients or who face greater chances of infection such as local pharmacists, laboratory professionals, and skilled and unskilled human support in the industries. Additionally, health care professionals have been recommended to use personal protective equipment kits for their protection. Meanwhile, environmental hygiene includes proper handwashing after touching surfaces in public places; covering the mouth and nose with the elbow or a tissue when you cough or sneeze; avoiding exposure to the public lavatory (e.g., wear a mask and gloves); carefully disposing of material containing nasal and oral secretions; and sanitizing standard tools, utensils, and frequently used surfaces regularly using disinfectants. Restraining from touching the face, eyes, nose, and mouth can curtail the chances of respiratory mucosal contamination [52,53]. A blueprint for preventing the spread of SARS-CoV-2 infection is presented as follows:
-
•
Avoid large events, mass gatherings, and close contact (less than six feet) with symptomatic individuals
-
•
Suspend travel to high-risk areas of serious illness
-
•
Use an alcohol-based hand sanitizer that contains at least 60% alcohol
-
•
Avoid sharing dishes, glasses, bedding, and other household items if you’re sick
-
•
Stay home from work, school, and public areas and avoid public transportation if you’re sick
The WHO and CDC have recommended wearing a face mask only when in contact with individuals with symptoms or who are under quarantine, because the inappropriate use and disposal of masks may increase the infection risk [4,54,55]. Moreover, health care workers are advised to use personal protective equipment and particulate respirators such as N95 or FFP2 masks for protection from infection during aerosol-generating procedures and when treating suspected or confirmed cases [4,56]. Globally, government officials have been implementing considerable policies such as lockdown, social distancing, and self-quarantining to minimize public interaction, which helps to break the chain of transmission and results in a pause of COVID-19 spread in the community [57]. Additionally, immunity boosters are commonly recommended by clinicians to ensure a better response against the causative agent. The adequate intake of nutrients having vitamin C and D, fruits and vegetables, whole grains, nuts, and unsaturated fatty acids might play a role as immune boosters. Six to eight glasses of water daily can keep adults properly hydrated, which strengthens the physiological system of human beings and reduces fatigue and inflammation. All foodstuffs can be purchased through an online shop and delivered to the home to maintain social distancing [58,59].
Mechanism of SARS-CoV-2 infection
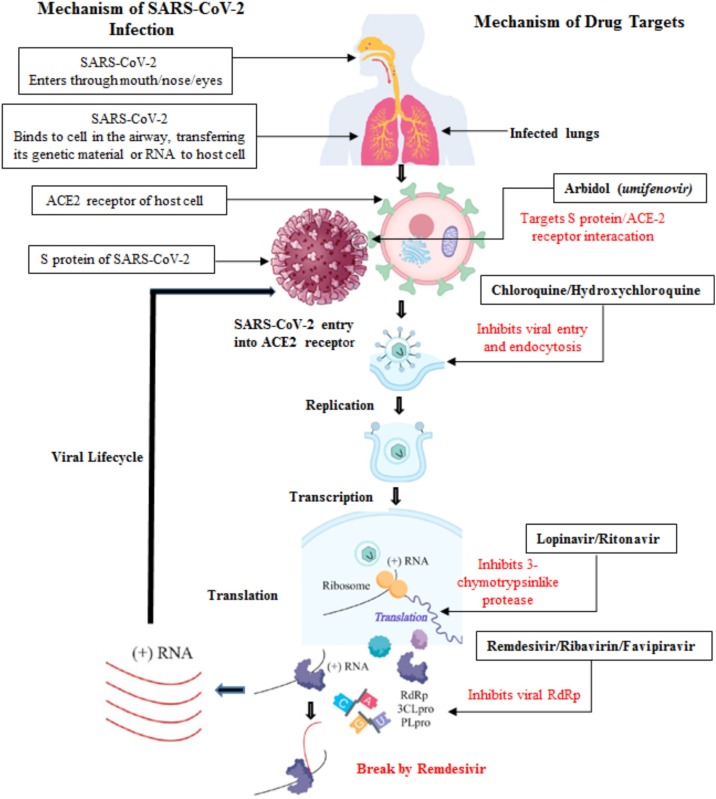
For the treatment of any kind of disease, either the route of disease occurrence, mechanism of infection, or the action mechanism of the targeted drug has to be known in order to follow the line of action for treatment. Although the SARS-CoV-2 infection mechanism has already been explored, target drugs or vaccines remain under development due to the rapid mutation in the species [[60], [61], [62]]. SARS-CoV-2 is a positive-stranded RNA-enveloped virus with crown-shaped glycoproteins (S-protein) on their surface (as seen under the electron microscope); hence, it is referred to as a coronavirus (the Latin term for crown is corona). Initially, the receptor-binding domain of S-protein recognizes and binds to the host’s ACE2 receptor. The transmembrane serine protease 2 on the surface of the host’s cell receptors facilitates cross-species transmission, resulting in exposure of virus RNA and the translation of its RNA replicase to an RNA replicase–transcriptase complex, which supports the synthesis of encoded viral polyproteins inside the host’s cells [[60], [61], [62]]. The viral RNA-dependent RNA polymerase synthesizes new RNA, which is encapsulated by structural proteins for the survival and infection of other host cells. Furthermore, this virulence migrates to other normal cells via exocytosis; consequently, the infection spreads inside other cells as illustrated in Fig. 2 [[63], [64], [65]]. The spread of the infection across cells reaches the bronchial alveoli and extrapulmonary organs, causing pneumonia and affecting the function of the kidneys and other organs. Notably, ACE2 as the target receptor is not expressed in the respiratory system only but also in the kidneys and gastrointestinal tract, rendering these ideal target organs for SARS-CoV-2 invasion [60,66]. Therefore, diagnosis and treatment in the early stage are essential for the prevention and control of SARS-CoV-2 infection. Moreover, the intensive review of the mechanism of infection is gaining attention as a way to understand existing drugs’ pathways for mitigating viral spread as illustrated in Fig. 2 and elucidated in the forthcoming sections.
Fig. 2.
Mechanisms of SARS-CoV-2 infection in human beings and target of potential agents.
Commonly used repurposed drugs
Many drug regulatory authorities have taken intrusive steps to hold the market of unproven therapies to account. Capitalists are investing money in research focusing on developing the best options either for the cure or de-escalation of COVID-19. Currently, several therapies possibly able to be repurposed for COVID-19 are available, but nothing finite is approved so far. The exceptional spread and severity of COVID-19 have increased the demand for alternative medications to treat symptoms and control the virulence. Examples include CQ, HCQ, remdesivir, favipiravir, ribavirin, umifenovir, lopinavir/ritonavir, vitamins, antipyretics, and herbal medications (i.e., TCM) (see Fig. 3 for more on antimalarial and antiviral drugs); these and other notable therapies are covered in Table 1 . More than 300 clinical trials have been registered to date worldwide to explore possible therapeutics for COVID-19 [67]. Repositioning existing clinical trials remains the most valid strategy by which to expedite the discovery of new effective drugs. The existing pharmaceutical supply chain of formulation and distribution could similarly be of help in this situation. Combinations of different drugs might be more effective than monotherapy [68]. Currently, scientists and physicians are putting some evident and aforesaid alternative drugs on trial for combating COVID-19. However, the following drugs are those considered most viable at this time. Importantly, these are tentative options and should not be given in practice freely for the treatment or prevention of COVID-19.
Fig. 3.
Chemical structure of potential repurposed drugs.
Table 1.
Recommendations, action mechanisms and adverse effects of potential drugs indicated the positive role in the treatment of COVID-19 patients.
| Drugs | Recommended use (Days/Dose) | EC50 value (μM) for SARS-CoV-2 | Action mechanism of drugs | Recommendation for COVID-19 treatment | Used before in other viral infections | Adversative effects | References |
|---|---|---|---|---|---|---|---|
| Chloroquine (CQ) | 500 mg twice per day | 1.13–5.47 | Inhibit viral replication process by increasing endosomal pH | Alternative of Hydroxychloroquine shortage | In the treatment of malaria and prophylaxis, | Electrolyte | [78,84] |
| For 10 days | Inhibits infection of cells by SARS-CoV-2 in vitro | imbalance | |||||
| - Fatal | |||||||
| dysrhythmias | |||||||
| Hydroxychloroquine (HCQ) | 400 mg orally per day for 7−10 days | 0.72 | Inhibit viral replication process by increasing endosomal pH | Recommended in with | In the treatment of malaria and prophylaxis, | Electrolyte | [11,78,84] |
| Azithromycin (500 mg for 1 day 1followed by 250 mg for next 2−5 days) for the treatment of moderate to severe disease | Inhibits infection of cells by SARS-CoV-2 in vitro, | imbalance | |||||
| Approved for | - Fatal | ||||||
| treatment of T2DM in India | dysrhythmias | ||||||
| Remdesivir (Nucleotide analog) | 200 mg IV within 30 min followed by 100 mg OD for 2–10 days | 0.77 | Terminate premature chain of RNA by combining with viral RNA chain | Recommended for sever patients and respiratory failure | Effective role against SARS and MERS viral infections | Gastrointestinal (GI) discomfort, elevated | [78,84] |
| transaminases, infusion site | |||||||
| reactions | |||||||
| Favipiravir (Nucleoside analog) | 1600−600 mg | 61.88 | inhibits viral RNA | Shown promising comparative results in inhibition of RNA viruses, but | influenza, arenavirus, bunyavirus and | Abnormal | [61,84,116] |
| For 1−6 days | polymerase | not recommended at this | filovirus infections | Transaminases, GI distress, serum uric acid increased, | |||
| time | Psychological symptoms | ||||||
| Ribavirin (Guanosine analog) | – | 109.5 | Also inhibits viral RNA replication | Has broad-spectrum antiviral activity In vitro activity against SARS-CoV-1, but not recommended at this time | No evidence in SARS and MERS diseases | Hemolytic anemia | [78,84] |
| Lopinavir/ritonavir (Protease inhibitors) | 400−100 mg | – | Inhibit the creation of new active viral peptides | Effective against SARS-CoV-1 both in vitro and human studies, not recommended at this | Approved for | GI pain, QT prolongation, drug–drug | [78,84] |
| Per day | time | HIV-1 treatment | interactions | ||||
| (ritonavir) | |||||||
| Corticosteroids | – | – | Inhibit the production of inflammatory mediators through binding with cytoplasmic | Low doses may be | – | Avascular necrosis, psychosis, hyperglycemia, adrenal suppression | [84] |
| receptors resulting to change the | beneficial by reducing harmful inflammatory responses in patients with severe COVID-19, while high dose steroid treatment may have | ||||||
| transcription of mRNA | deteriorated consequences in SARS, | ||||||
| Not recommended for routine use | |||||||
| Ibuprofen | – | – | Inhibiting production of | May be useful for its anti-inflammatory and antipyretic effects, no evidence in the contradiction of its use | – | GI ulcers/bleeding, may up regulate | [84] |
| prostaglandins by blocking COX-1 and COX-2 synthesis | ACE2 | ||||||
| Indomethacin | – | – | Inhibiting production of | May be useful for its anti-inflammatory | – | GI ulcers/bleeding, may up regulate | [84] |
| prostaglandins by blocking COX-1 and COX-2 synthesis | and antipyretic | ACE2 | |||||
| effects, no evidence of its antiviral | |||||||
| effects against SARS-CoV-2 | |||||||
| Tocilizumab Sarilumab | – | – | Monoclonal antibody | Recommended in patients with evidence of CRS and deteriorating respiratory function, Tocilizumab reduced fever and oxygen requirement in COVID-19 | Approved for rheumatoid arthritis | Abnormal | [78,84] |
| against the IL-6 receptor | No data on SARS or MERS | Transaminases, GI perforation, neutropenia, infusion reactions | |||||
| Convalescent plasma | – | – | Passive | May be considered in patients with deteriorating conditions | Recently used in SARS, historically has been used in | Hypersensitivity | [84] |
| immunization | refractory to other treatment, recommendations are | 1918 flu | Reactions, serum sickness | ||||
| using plasma from | debatable, while there is some evidence of benefit in COVID-19 | ||||||
| recovered patients |
(EC50- Half maximal effective concentration).
Antimalarial drugs
Chloroquine and hydroxychloroquine
Antimalarial drugs are known to possess in vitro and clinical antiviral activities against SARS-CoV-1 and SARS-CoV-2. CQ and its derivative, HCQ, are well-recognized drugs for the treatment of malaria and certain autoimmune diseases and have been gaining worldwide attention for the treatment of COVID-19 [5]. CQ and HCQ are synthetic drugs and usually developed from the bark of the cinchona plant family Rubiaceae [5]. There are several in vivo mechanisms of action for these drugs against different viruses (including SARS-COV) that have been proposed [69]; however, in vivo data are still controversial [70,71]. The administration of CQ increases the endosomal pH, which creates an adverse environment for the virus. CQ interferes with the glycosylation of SARS-CoV cellular receptors and substantially curtails the viral infection potential [72]. At higher lysosomal pH levels, the administration of CQ lowers cathepsin levels, resulting in the formation of autophagosome, which is responsible for SARS-CoV-2 spike-protein cleavage (Fig. 2). CQ can also inhibit p38 mitogen-activated protein kinase (MAPK) via phosphorylation (activation) inside THP-1 cells as well as caspase-1 [73]. MAPK signaling activates virus cells to follow their replication cycle [74] alongside altering the virion assembly [72,75]. The mechanisms of action of CQ and HCQ are equal due to the structural resemblance of both molecules. Both molecules act as weak bases that can alter the pH of acidic intracellular organelles including endosomes/lysosomes, which are frequently required for membrane fusion. An extra hydroxyl group present in HCQ makes it less toxic, showing low permeability to the blood–retinal barrier and permitting rapid clearance from retinal pigment cells [76]. Additionally, in vitro testing indicated that HCQ sulfate is significantly superior (five days quicker) to the CQ phosphate in inhibiting SARS-CoV-2 [77,78]. Therefore, it is suggested that both agents are effective against SARS-CoV-1 and SARS-CoV-2 but with different degrees of potential [79,80]. Furthermore, an in vitro study revealed that HCQ is found significantly more potent than CQ against SARS-CoV-2 (EC50 values: 0.72 and 5.47 μM, respectively) and the Taiwan CDC on March 26, 2020 declared HCQ to be an important anti–SARS-CoV-2 agent [11,77,81]. Consequently, the United States Food and Drug Administration also granted emergency authorization for HCQ to treat COVID-19 infection [82]. The combination of HCQ with azithromycin was studied in a nonrandomized clinical trial of HCQ alone or in combination with azithromycin (which is active in vitro against the Zika and Ebola viruses) of 20 patients, where 14 patients were treated with HCQ alone and six patients were treated with a combination of both drugs. A substantial reduction of the viral load in infected patients was observed in the combination therapy (HCQ and azithromycin) group; however, most of the patients who were treated with HCQ alone showed upper or lower respiratory-tract infections [83]. Gautret et al. conducted a clinical trial for the use of the above combination in the treatment of COVID-19 patients, where they observed positive results in severe cases [83]. The combination of HCQ and azithromycin is recommended for the treatment of COVID-19 patients with moderate to severe disease in many countries [84]; however, there isn’t enough evidence specifically concerning its efficacy and safety in the treatment of COVID-19 (ASHP). Several ongoing clinical trials are investigating the efficacy of the therapeutic use and the safety of these medications against SARS-CoV-2 [77,85]. HCQ and CQ can lead to fatal dysrhythmias, electrolyte disturbances, retinopathy, deficiency of glucose-6-phosphatase, and corrected QT interval prolongation. Patients with a history of allergy to HCQ or who are pregnant or breastfeeding are contraindicated from using this medication [83].
Antiviral drugs
Remdesivir (nucleotide analog)
Remdesivir (nucleotide analog GS-5734), a monophosphate prodrug, has been reported as a potential agent against several RNA viruses, including Ebola, SARS, and MERS, both in vitro and in nonhuman primates (1, 4). It also exhibits SARS-CoV and MERS-CoV in vivo inhibition [72,86,87]. Notably, the nucleoside analog behavior of remdesivir facilitates the inhibition of RdRp, the protein complex of CoVs that controls RNA-based genome replication, thus potentially harming the viral genome replication process (Fig. 2). Once the host metabolizes remdesivir into active nucleoside triphosphate, the active metabolite develops the resistance toward adenosine triphosphate for its successful inclusion into the nascent RNA strand [88]. This inclusion of active substituents pauses RNA synthesis in an early stage and halts the growth of new RNA strands. While a proofreading tendency of CoVs that can detect and remove other nucleoside analogs and render them resistant against many of these drug agents exists, remdesivir outpaces this viral proofreading activity and generates antiviral activity [89]. Agostini et al. reported that a mutant murine hepatitis virus devoid of proofreading ability was abruptly sensitive to remdesivir [87]. However, the opposite is also possible; it can be assumed that mutations may also improve proofreading or increase the fidelity of the base-pairing process for remdesivir resistance [90]. However, some initial evidence suggests that remdesivir could have an additional mechanism of action that has not been ultimately discovered yet, which could be a new lead in favor of remdesivir still having partial antiviral activity despite viral mutations that enhance replication fidelity [87,91,92]. Recently, at a low micromolar concentration, remdesivir was found to be potentially active against SARS-CoV-2 in in vitro experiments and exhibited a high level of selectivity [72]. It has been reported to trigger a prompt reduction in fever and ensure greater improvement in clinical symptoms by the 12th day of illness; at this point, the chest roentgenography of the patient under assessment showed relatively mild infiltrations and the rRT-PCR assay of an oropharyngeal swab was negative [93]. Thereafter, remdesivir gained some attention globally due to its effect in the context of SARS-CoV-2 infection; however, more trials are needed to definitively prove its antiviral activity [94]. Some limited trials of remdesivir have yet to prove its efficacy [84,95]. Moreover, despite its strong in vitro potency against SARS-CoV-2, clinical success still must be proved [91,93] and randomized controlled trials are still required to confirm its efficacy and safety [19].
Favipiravir and ribavirin (nucleoside analog)
Favipiravir is one of the broad-spectrum nucleoside analogs antiviral drugs that has been approved against FLUAV in Japan (2014); this medication was found to act as a halting inhibitor for viral RNA polymerase and to suspend viral replication during the treatment of RNA viral infections as seen in case of Ebola and the influenza virus (Fig. 2) [61,96,97]. The Shenzhen Health Commission recently approved favipiravir for the treatment of COVID-19 patients [98]. Clinically, it has been observed that favipiravir had some effect against SARS-CoV-2 infection in Chinese patients [99]. Randomized clinical trials are still ongoing to evaluate the efficacy of favipiravir plus interferon-α (ChiCTR2000029600) and favipiravir plus baloxavir marboxil (ChiCTR2000029544) [11]. In one study, patients with moderate and severe COVID-19 given favipiravir (n = 120) and umifenovir (n = 120) were compared; however, no significant differences were recorded between the groups. As such, further randomized controlled trials are needed to prove the efficacy of favipiravir as a possible COVID-19 treatment [61].
Another possible synthetic nucleoside analog, ribavirin, which has shown potential antiviral activity against several viral infections including the hepatitis C virus, respiratory syncytial virus, some viral hemorrhagic fevers, and SARS in Hong Kong [[100], [101], [102]], may work in the context of COVID-19. Ribavirin inhibits both RNA and DNA viruses, stopping their replication by suppressing the activity of inosine monophosphate dehydrogenase, which is required for guanosine triphosphate formation (Fig. 2). Ribavirin in combination with interferon-α2b was recorded to be effective against MERS-CoV in a rhesus macaque model [103]. Additionally, the regimen of lopinavir/ritonavir plus ribavirin was found to be effective against SARS-CoV in tissue cultures [104]. Therefore, ribavirin could be considered as a possible candidate therapy for the treatment of COVID-19 patients [19]. However, its undesirable adverse effect of reducing hemoglobin concentrations is harmful to patients in respiratory distress [105]. As such, currently, the high risk of toxicity due to ribavirin therapy outweighs its potential benefits as a possible treatment option for SARS-CoV-2 [106].
Umifenovir
Umifenovir, sold under the trade name Arbidol, is often used as an antiviral drug against influenza and other viruses, including hepatitis C in Russia and China (“The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection - Full-Text View - ClinicalTrials.gov,” n.d. [107]). Umifenovir has the potency to inhibit the replication of SARS-CoV as reported in an in vitro study (Fig. 2) [108]. Currently, randomized clinical and controlled trials are being carried out in China to study the efficacy of umifenovir against COVID-19 [19]. However, no conclusive proof of its efficacy in COVID-19 has been reported to date, although a few shreds of evidence support that umifenovir alone or in combination with other antiviral drugs has some clinical success against COVID-19 [[109], [110], [111]].
Lopinavir/ritonavir (protease inhibitors)
Lopinavir in combination with ritonavir is used for the treatment of human immunodeficiency virus (HIV) by affecting protein synthesis, a major step in HIV replication, and is well known as a protease-inhibitor therapy. Moreover, the combination of these drugs has been used for the treatment of SARS and MERS patients by ameliorating acute respiratory distress syndrome [104,112,113]. However, despite showing a good effect against MERS-CoV-infected marmosets, the significant impact of lopinavir in a tissue culture model is still controversial [114]. Lopinavir/ritonavir may have the possibility to bind with endopeptidase C30 of the SARS-CoV-2 protease due to its protease-inhibition tendency and could exhibit an antiviral effect in COVID-19 given this virus’ resemblance to both the SARS-CoV-2 and SARS-CoV viruses (Fig. 2) [115]. Various clinical trials have tested the effect of the combination of lopinavir/ritonavir against COVID-19 but, unfortunately, promising results were not recorded [94,104,116,117]. Meanwhile, the administration of lopinavir/ritonavir or interferon-β1b for the treatment of MERS-CoV infection yielded better prognoses in a nonhuman primate model of common marmosets [114]. Sheahan et al. compared the efficacy of remdesivir as prophylaxis as well as a therapeutic with that of the combination of lopinavir/ritonavir and interferon-β combination therapy (ritonavir was used to prolong lopinavir’s half-life) in a humanized transgenic mouse MERS-CoV infection model and observed superior efficacy of remdesivir against MERS-CoV, with a reduced viral load and an extensive pathological improvement recorded in lung tissues [112]. Despite the discouraging results, it is intriguing that at higher concentrations, lopinavir/ritonavir inhibits pulmonary SARS-CoV-2 replication and researchers have claimed that slightly fewer deaths were observed in the group receiving lopinavir/ritonavir in the late stage of SARS-CoV-2 infection as compared with in the standard-care group [112,118]. In conclusion, lopinavir/ritonavir may not be effective for COVID-19 patients with mild pneumonia.
The above antimalarial (CQ and HCQ) and antiviral drugs (remdesivir) have emerged as promising treatment lead to the treatment of COVID-19. Moreover, synthetic recombinant interferon-α and interferon-β have also been considered in ongoing clinical trials because some evidence has been reported concerning the therapeutic benefit of synthetic recombinant interferon-α in SARS patients [119,120]. Separately, oseltamivir is an antiviral drug approved for influenza A and B treatment and has been recommended for the treatment of COVID-19 patients in China, either alone or in combination with antibiotics and corticosteroids (e.g., hydrocortisone) [11,31]. It is also under assessment in a clinical trial paired in multiple combinations with CQ and favipiravir (“Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID-19 : A Randomized Control Trial - Full-Text View - ClinicalTrials.gov,” n.d. [121]). The ASHP reviewed all of the abovementioned drugs and concluded that not enough evidence is available yet to prove the efficacy of any of them in the treatment of COVID-19 patients (ASHP website). Therefore, further evaluation of the efficacy and safety of the current antiviral drugs is still required [19]. The explanatory details about the clinical trials have been mention in the Section “Clinical Trials of Repurposed Drugs Against SARS-CoV-2”.
Vitamins
The adequate intake of vitamins could also be crucial in the treatment of COVID-19 patients. Many reports have suggested the importance of vitamins C and D in the prevention of lower respiratory-tract infections and suppression of viral replication rates, respectively [59,122]. The provision of a high dose of vitamin C (2–10 g/day given over 8–10 h) showed encouraging results in the treatment of 50 moderate to severe COVID-19 patients in China, triggering an improvement in the oxygenation index; notably, all of these patients were eventually cured and were discharged [123]. The simultaneous use of vitamin D was observed as worthy supplementation to reduce the chances of influenza [59]; evidence that may support this activity is the finding of reduced 25-hydroxyvitamin D (25(OH) D) concentrations in COVID-19 patients. Consequently, it is suggested that people at risk of influenza and/or COVID-19 should take 10,000 IU/day of vitamin D3 for a few weeks to rapidly raise their 25(OH) D concentrations, followed by 5000 IU/day. This regimen is safe to deploy either orally or intravenously in COVID-19 patients [59,124].
Antipyretics
The excessive use of nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, naproxen, and diclofenac has been associated with myocardial infarction, heart failure, and stroke, which is prevalent in acute respiratory-tract infection patients [[125], [126], [127]]. Furthermore, NSAIDs can cause nephrotoxicity, which is more likely to appear among patients severely affected by COVID-19 [128,129]. Hence, the role of NSAIDs in COVID-19 patients is still questionable in light of the drawbacks of ibuprofen in patients with COVID-19 that received wide publicity via international media coverage in France. In a case series report, four cases of children who took ibuprofen and experienced worsening symptoms of COVID-19 infection are detailed [130]. The reason for worsening symptoms was described by Fang et al.: coronaviruses bind to ACE2 and ibuprofen administration can increase the bioavailability of ACE2, therefore potentiating the infectious processes of the former [131]. One of the best replacement for NSAIDs is acetaminophen, which has been suggested as a reasonable option until more evidence is collected [130]. In summary, the current epidemiologic evidence is not strong enough to infer a causal link between the harmful effect of ibuprofen and COVID-19. Evidence from mechanistic studies alone should not be used to make strong judgments against the use of ibuprofen. Still, it is recommended to use acetaminophen or ibuprofen with acetaminophen for fever reduction in a patient with COVID-19 [132] and the risk of adding ibuprofen should continue to be assessed against its benefits [133,134].
Herbal medications
In most developing countries, given the scarcity of pharmaceuticals, about 80% of the population depends on traditional medicines for the treatment of various diseases [135]. Indian and Chinese traditional herbs have a wide range of applications in the treatment of infectious and noninfectious diseases [98,135]. Recently, TCM—mainly, Astragali Radix (Huangqi), Glycyrrhizae Radix Et Rhizoma (Gancao), Saposhnikoviae Radix (Fangfeng), Atractylodis Macrocephalae Rhizoma (Baizhu), Lonicerae Japonicae Flos and Fructus forsythia (Lianqiao)—have demonstrated a profound effect or role in the prevention and control of COVID-19 [135]. However, clinical evidence regarding these treatments in the prevention of this emerging viral infection remains inadequate and robust standard clinical trials should be organized to prove the potential preventive effects of these medicines [136,137]. Various researchers focusing on elucidating the activity of different Indian herbs and medicinal plants (Glycyrrhiza glabra, Allium sativum, Strobilanthes cusia, Acacia nilotica, Eugenia jambolana, Euphorbia granulate, Ocimum sanctum, Solanum nigrum, Vitex negundo, Ocimumkilim, and Scharicum) have observed their decisive role in the prevention of SARS-CoV, HCoV, and HIV [[138], [139], [140], [141], [142], [143]]. As such, these Indian medicinal plants and herbs may have favorable roles in the treatment of COVID-19 as well and research should focus on exploring these herbs and medicinal plants further in the context of SARS-CoV-2 [98].
Clinical trials of repurposed drugs against SARS-CoV-2
CQ and HCQ were found to be a blocker of virus cell entry by inhibiting autophagic flux and decreasing autophagosomelysosome fusion [144]. Gautret et al [83] reported the, nonrandomized clinical trial on 20 SARS-CoV-2-infected patients. The substantial results were found with reduction or disappearance of viral load using HCQ in COVID-19 patients. In this study, well know broad spectrum antibiotic azithromycin, were also given to the patients, and synergistic effect were observed to reinforce the antiviral effect of HCQ. After these finding HCQ get tremendous recognition for the treatment and prophylaxis of COVID-19. The off-label use of HCQ in the treatment of COVID-19 were announced by several health agencies [145]. Unfortunately, U.S. Veterans Health Administration medical centers in April 2020 were reported no evidence that the use of HCQ, either with or without azithromycin, reduced the risk of mechanical ventilation in patients hospitalized with COVID-19. They performed a retrospective analysis of data from 368 patients hospitalized with confirmed SARS-CoV-2 infection and did not find any benefit of the HCQ even raised the concern about its safety. Increased rate of overall mortality was identified on the treatment of HCQ alone [146]. Design Multicenter, in China, revealed efficacy and safety of HCQ with standard-of-care (SOC) compared with SOC alone in adult patients of COVID-19. In this randomized open-label, study 150 patients with confirmed COVID-19 infection were included. All patients were divided in to two groups each group has 75 patients. One group were treated with HCQ along with SOC and another group were on SOC alone. 200 mg HCQ was administrated daily for three days followed by a maintained dose of 800 mg daily for the remaining days up to 2 and 3 weeks for mild/moderate and severe patients, respectively. Overall data indicated that HCQ administration did not result in a significantly higher probability of negative conversion than SOC alone [147]. While in the safety population trial, adverse events were recorded in 7/80 (9%) non-HCQ-treated patients and in 21/70 (30%) HCQ recipients. Furthermore, diarrhoea was reported as the most common adverse event in the HCQ-treated group 7/70 (10%) patients; 2 HCQ recipients experienced serious adverse events. The toxicity displayed by HCQ also may preclude the use of this drug as a prophylactic against SARS-CoV-2. In Henri-Mondor Hospital, France, 181 patients aged 18–80 years with SARS-CoV-2, having pneumonia as secondary effect (required oxygen but not intensive care) were treated at a dose of 600 mg/day HCQ. Study compared the data of 84 patients who received HCQ within 48 h of admission to hospital (treatment group) with 89 patients who did not receive HCQ (control group) along with 8 additional patients received HCQ more than 48 h after admission. 8 patients in the treatment group (10%) experienced electrocardiographic modifications that required discontinuation of treatment. HCQ has received worldwide attention as a potential treatment for covid-19 because of positive results from small studies. However, the results of this study do not support its use in patients admitted to hospital with COVID-19 who require oxygen [148]. In New York City, hospitalised COVID-19 patients were treated with HCQ, was not associated with either a greatly lowered or increased risk of the composite endpoint of intubation or death [149]. A randomized, double-blind, placebo-controlled trial across the United States and parts of Canada testing HCQ as post exposure prophylaxis. In this trial 821 adults who had household or occupational exposure to someone with asymptomatic confirmed COVID-19 were included. Within 4 days after exposure, the patients were randomly assigned to receive either placebo or HCQ (800 mg once, followed by 600 mg in 6–8 h, then 600 mg daily for 4 additional days). Common HCQ side effects were reported than with placebo, but there were no serious adverse reactions reported. After high-risk or moderate-risk exposure to COVID-19, HCQ did not prevent illness compatible with COVID-19 or confirmed infection when used as post exposure prophylaxis within 4 days after exposure [150].
F276L and V553L mutation were identified in the SARS-CoV viral RNA-dependent RNA polymerase (RdRp) gene at remdisiver presence, that conferred viral resistance to remdesivir [87] remdesivir significantly reduced lungs viral loads in mouse models of SARS-CoV [91] and MERS-CoV [112]. The remdesivir prophylactic and therapeutic efficacy was also tested in a nonhuman primate (rhesus macaque) model of MERS-CoV infection [86]. In light of these other human coronaviruses results, remdesivir got the attentions for possibly effective COVID-19 treatment. Remdisiver clinical trial have been done on 53 patients, 22 were in the United States, 22 in Europe or Canada, and 9 in Japan. During a median follow-up of 18 days, 36 patients (68%) had an improvement in oxygen-support class, while 13% mortality were recorded. But there were no control patients group were assigned for the study to examine the efficacy of remdisivir [151]. A randomised, double-blind, placebo-controlled, multicentre trial at ten hospitals in Hubei, China, has been performed on 237 patients. Randomly all patients were divided in to 2:1 ratio (158 to remdesivir and 79 to placebo) to intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) or the same volume of placebo infusions for 10 days. There was no statistically significant difference were obtained in the results of both the group, while, patients receiving remdesivir had a faster time to clinical improvement than those receiving placebo. The mortality 14% vs. 13% on 28th day of treatment which was almost equal in both the group. It is important to mention that adverse events were reported in 102 (66%) of 155 remdesivir recipients vs. 50 (64%) of 78 placebo recipients, and that remdesivir was stopped early because of adverse events in 18 (12%) patients vs. 4 (5%) patients who stopped placebo early. The overall conclusion from this study was that remdesivir not significantly associated with clinical benefits in COVID-19 patients; however, they also suggested that the reduction in time to clinical improvement in patients treated earlier begged confirmation in larger studies [152]. U.S. National Institute of Allergy and Infectious Diseases, sponsored a randomized, placebo-controlled trial of intravenous remdesivir in 1063 adults hospitalized with COVID-19 patients with evidence of lower respiratory tract involvement. Patients were randomly assigned to receive either remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) or placebo for up to 10 days, and noted that remdesivir was better than placebo with respect to time to recovery. The mortality rate was counted 7.1% for the group receiving remdesivir vs. 11.9% for the placebo group at the 14th day of treatment. The overall observation with these results suggests that remdisiver is helpful in COVID-19 but not to completely cure or stop its spread. There is some more combination of different antiviral agents has been recommended to improve COVID-19 patient outcomes. They also suggested that antiviral treatment should start before the pulmonary disease progresses to the point of requiring mechanical ventilation. However, the intravenous administration of remdesivir makes this drug difficult to prescribe following the first mild or moderate symptoms observed after the first few days of SARS-CoV-2 infection [153]. The effect of favipiravir vs. lopinavir /ritonavir for the treatment of COVID-19 has been observed in an open-label, nonrandomized trial of 80 patients in China. Patients with laboratory confirmed COVID-19 who received oral FPV (Day 1:1600 mg twice daily; Days 2−14: 600 mg twice daily) plus interferon (IFN)-α by aerosol inhalation (5 million U twice daily). An open-label, nonrandomized trial of 80 patients with COVID-19 in China, noticed, a fast viral load reduction in patients who were treated with favipiravir as compared with historical controls treated with lopinavir ritonavir. But there were no control group were set for efficacy examination and patients were co-treated with interferon-α1b (IFN-α1b) until viral clearance, which makes it difficult to estimate the antiviral activity of favipiravir. Further trials will be necessary to evaluate the efficacy of favipiravir against COVID-19 [154]. In China a total of 199 hospitalized adult patients with laboratory-confirmed SARS-CoV-2 infection underwent randomization; 99 were assigned to the lopinavir-ritonavir group, and 100 to the standard-care group for the evaluation of the efficacy and safety of oral lopinavir-ritonavir. There was no significate difference were found, in the group treated with lopinavir-ritonavir as compared with standard care [116]. In Hong Kong, a combination of lopinavir-ritonavir and IFN-β1b plus ribavirin were used for the treatment of COVID-19 patients. In this study, 127 patients were recruited; 86 were randomly assigned to the combination group, and 41 were assigned to the control group (lopinavir-ritonavir alone). The combination group had a significantly shorter median time from start of study treatment to negative nasopharyngeal swab (7 vs. 12 days; P = 0.0010). No differences in adverse events were reported. Assuming that lopinavir-ritonavir has poor antiviral activity, IFN-ribavirin might be responsible for the efficacy of the abovementioned triple combination [155]. The repurposed drugs were achieved the tremendous attention due to the rapid escalation of SARS-CoV-2 infections. These repurposed drugs were tried to control the morbidity, mortality, and spread of this new disease. There are so many clinical trials have been completed and many more are still running, but no repurposed drug has been claimed that could have high impact to the SARS-CoV-2 infection. Up to some extent remdesivir has shown the most promising results so far and reduce the mortality of COVID-19, but more potent and significantly specific, antivirals are required to curb the coronavirus pandemics.
Toxicities of repurposed drugs
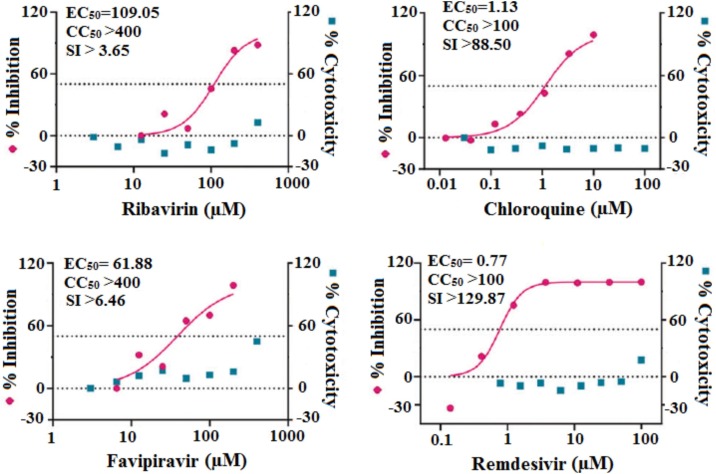
Despite the possible role of existing drugs in fighting SARS-CoV-2, the toxicities of these medications must be elucidated. Most of the recently used therapeutic drugs may show some adverse side effects such as cardiotoxic, hepatotoxic, nephrotoxic, hematologic toxicity consequences [61,156]. Therefore, the administration of available drugs either alone or in combination with one another remains under debate [97]. Sanders et al. reviewed the toxicities and adverse effects of most of these drugs [61]. CQ and HCQ can trigger retinopathy, neuromyopathy, and cardiomyopathy [85,157,158], although the half cytotoxic concentration (CC50) value of HCQ (249.50 μM) was lower than that of CQ (273.20 μM), suggesting the reduced toxicity of the former. Separately, statistical index (SI) values (CC50/EC50) of CQ (100.81, 71.71, 38.26, and 37.12) and HCQ (55.32, 61.45, 14.41, 19.25) at multiplicities of infection of 0.01, 0.02, 0.2, and 0.8, respectively, indicated the existence of relatively low SI values [81]. Wang et al. compared the in vitro (in Vero E6 cells) antiviral efficiency against 2019-nCoV and the cytotoxic effects of seven drugs including ribavirin, CQ, remdesivir (GS-5734), and favipiravir (T-705); the outcomes of this study are presented in Fig. 4 , which shows that ribavirin and favipiravir required high EC50 concentrations of 109.50 and 61.88 μM, respectively, with CC50 values of greater than 400 μM resulting in low SI values of greater than 3.65 and greater than 6.46 respectively, to inhibit 2019-nCoV. Elsewhere, CQ (EC50 = 1.13 μM; CC50 > 100 μM, SI > 88.50) and remdesivir (EC50 = 0.77 μM; CC50 > 100 μM; SI > 129.87) inhibited virus infection at lower micromolar concentrations, supporting that remdesivir and CQ might be effective in the controlling 2019-nCoV with lower levels of toxicity [72]. Some other antiviral drugs such as lopinavir and ritonavir also have toxic side effects together with poor SARS-CoV-2–inhibition efficacy [11,94]. NSAIDs can cause serious nephrotoxicity in severely affected COVID-19 patients [134]. The adequate use of all vitamins is a significant way to boost the immune system [159].
Fig. 4.
Comparison of cytotoxicity of various drugs used in the treatment of COVID-19 [72].
Conclusion and future perspectives
The entire world continues to hope for the development of accurate treatments for COVID-19 in a timely fashion and hundreds of ongoing clinical trials aim to deescalate the pandemic. In this review, the potential roles of various existing drugs have been discussed and comparative information has been provided to help health care practitioners decide their effectiveness in the treatment of COVID-19. The mechanisms of these drugs have confirmed to promote the inhibition of SARS-CoV-2 virus replication, which suggests the ability of these medications to limit the viral load and prevent progression to severe disease [60,78]. Because of the potential rapid mutation of the SARS-CoV-2 virus, drug resistance needs to be explored too [78]. Some TCM and macronutrients and micronutrients investigated to date in clinical trials of COVID-19 also need to be further evaluated either in combination with Western medicines or alone to estimate their overall efficacy against viral infections [160,161]. More clinical studies are still required to develop standard protocols in COVID-19 treatment characterized by high efficacy and reduced toxicity. These promising findings could constitute an impactful blueprint for the future [60,111,162].
Apart from the potential roles of repurposed drugs, proper diagnosis remains the only viable tool to control and manage the current situation. Indeed, RT-PCR and serological ELISA assay are the only methods by which to establish the diagnosis of COVID-19, yet are tedious and time-consuming to use, so new techniques are needed to reduce the time to obtain a diagnosis so as to facilitate rapidly actionable results that could support the early identification of COVID-19 patients and discern the appropriate use of isolation resources, infection-control measures, and recruitment into clinical trials. Keeping these points in consideration, there is an urgent need for all relevant parties to join forces to develop new therapeutic drugs, vaccines, and diagnostic kits for COVID-19. Of note, other pharmaceutical companies are working on a gene-silencing approach, where the use of so-called small interfering RNA medicines could block the virus’ mutation by rendering some of its genes nonfunctional [163].
Funding
The authors acknowledge the generous support received from the Researchers Supporting Project (no. RSP-2020-122), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
None declared.
Acknowledgment
“The authors acknowledge the generous support received from the Researchers Supporting Project (no. RSP-2020-122), King Saud University, Riyadh, Saudi Arabia.” “The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.”
References
- 1.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol Sci. 2020;41:363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceribelli A., Motta F., De Santis M., Ansari A.A., Ridgway W.M., Gershwin M.E. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Situation Update Worldwide, as of 6 October 2020 n.d. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [Accessed 4 June 2020].
- 4.Advice for Public n.d. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public?gclid=Cj0KCQjwlN32BRCCARIsADZ-J4scnLfOt5TRY6NUf3VhGxhC_YPEgmDgch4o1Vo0VhfK7c5XbAv2xlsaAh5CEALw_wcB [Accessed 4 June 2020].
- 5.Pereira B.B. Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review. J Toxicol Environ Heal B. 2020;23:177–181. doi: 10.1080/10937404.2020.1752340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo D. Old weapon for new enemy: drug repurposing for treatment of newly emerging viral diseases. Virol Sin. 2020 doi: 10.1007/s12250-020-00204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean S.-S., Lee P.-I., Hsueh P.-R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C., Huang S., Zheng F., Dai Y. Controversial treatments: an updated understanding of the coronavirus disease 2019. J Med Virol. 2020 doi: 10.1002/jmv.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cases in the U.S. | CDC n.d. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-in-us.html [Accessed 4 June 2020].
- 14.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holstein B. Corona virus 101. J Nurse Pract. 2020 doi: 10.1016/j.nurpra.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakodkar P., Kaka N., Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Cureus. 2020 doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam T.T.-Y., Shum M.H.-H., Zhu H.-C., Tong Y.-G., Ni X.-B., Liao Y.-S. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 22.Jaimes J.A., Millet J.K., Stout A.E., André N.M., Whittaker G.R. A tale of two viruses: the distinct Spike Glycoproteins of feline coronaviruses. Viruses. 2020;12:83. doi: 10.3390/v12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020:94. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C., Liu X., Jia Z. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am J Respir Crit Care Med. 2020;201:7–8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 26.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection. J Med Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. BioRxiv. 2020 doi: 10.1101/2020.01.30.927806. 2020.01.30.927806. [DOI] [Google Scholar]
- 28.Kaul D. An overview of coronaviruses including the SARS-2 coronavirus–molecular biology, epidemiology and clinical implications. Curr Med Res Pract. 2020;10:54–64. doi: 10.1016/j.cmrp.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.How Coronavirus Spreads | CDC n.d. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreadshtml?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Ftransmission.html [Accessed 4 June 2020].
- 30.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X., Zhang B., Li P., Ma C., Gu J., Hou P. Cold Spring Harbor Laboratory Press; 2020. Incidence, Clinical Characteristics and Prognostic Factor of Patients with COVID-19: a Systematic Review and Meta-Analysis. [DOI] [Google Scholar]
- 34.Bai T., Tu S., Wei Y., Xiao L., Jin Y., Zhang L. Clinical and laboratory factors predicting the prognosis of patients with COVID-19: an analysis of 127 patients in Wuhan, China. SSRN Electron J. 2020 doi: 10.2139/ssrn.3546118. [DOI] [Google Scholar]
- 35.Li Q., Ding X., Xia G., Geng Z., Chen F., Wang L. A simple laboratory parameter facilitates early identification of COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.02.13.20022830. 2020.02.13.20022830. [DOI] [Google Scholar]
- 36.Niu S., Tian S., Lou J., Kang X., Zhang L., Lian H. Clinical characteristics of older patients infected with COVID-19: a descriptive study. Arch Gerontol Geriatr. 2020;89 doi: 10.1016/j.archger.2020.104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicola M., O’Neill N., Sohrabi C., Khan M., Agha M., Agha R. Evidence based management guideline for the COVID-19 pandemic-review article. Int J Surg. 2020;77:206–216. doi: 10.1016/j.ijsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang J., Yan M., Li H., Liu T., Lin C., Huang S. Evaluation of enzyme-linked immunoassay and colloidal gold- immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19) MedRxiv. 2020 doi: 10.1101/2020.02.27.20028787. 2020.02.27.20028787. [DOI] [Google Scholar]
- 41.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh A., Shaikh A., Singh R., Singh A.K. COVID-19: from bench to bed side. Diabetes Metab Syndr Clin Res Rev. 2020;14:277–281. doi: 10.1016/j.dsx.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao X., Zhou H., Wu C., Xiao Y., Ren L., Paranhos-Baccalà G. Antibody against nucleocapsid protein predicts susceptibility to human coronavirus infection. J Infect. 2015;71:599–602. doi: 10.1016/j.jinf.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang D., Lin M., Wei L., Xie L., Zhu G., Dela Cruz C.S. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295 doi: 10.1148/radiol.2020200236. 18–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin C., Ding Y., Xie B., Sun Z., Li X., Chen Z. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: the value of CT images in the course of the disease. Clin Imaging. 2020;63:7–9. doi: 10.1016/j.clinimag.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coronavirus Disease 2019 n.d. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Accessed 4 June 2020].
- 52.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 53.Gasmi A., Noor S., Tippairote T., Dadar M., Menzel A., Bjørklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Heal. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell E.P. Corona virus: global pandemic causing world-wide shutdown. J Natl Med Assoc. 2020;112:113–114. doi: 10.1016/j.jnma.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balachandar V., Mahalaxmi I., Kaavya J., Vivekanandhan G., Ajithkumar S., Arul N. COVID-19: emerging protective measures. Eur Rev Med Pharmacol Sci. 2020;24:3422–3425. doi: 10.26355/eurrev_202003_20713. [DOI] [PubMed] [Google Scholar]
- 58.Fao. Maintaining a Healthy Diet During the COVID-19 Pandemic n.d. 10.4060/ca8380en. [DOI]
- 59.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L. Evidence that vitamin d supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao Y., Deng Q., Dai S. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19) JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 62.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fehr A.R., Perlman S. Coronaviruses methods protoc. Springer; New York: 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front Microbiol. 2014;5:1–23. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan C., Li K., Ding Y., Lu W.L., Wang J. ACE2 expression in kidney and testis may cause kidney and testis damage after 2019-nCoV infection. MedRxiv. 2020 doi: 10.1101/2020.02.12.20022418. 2020.02.12.20022418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.COVID-19 Clinical Trials Worldwide by Region May 28, 2020 | Statista n.d. https://www.statista.com/statistics/1106306/coronavirus-clinical-trials-worldwide/ [Accessed 4 June 2020].
- 68.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Pública. 2020;44:1. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keyaerts E., Vijgen L., Maes P., Neyts J., Ranst M. Van. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paton N.I., Lee L., Xu Y., Ooi E.E., Cheung Y.B., Archuleta S. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis. 2011;11:677–683. doi: 10.1016/S1473-3099(11)70065-2. [DOI] [PubMed] [Google Scholar]
- 71.Roques P., Thiberville S.-D., Dupuis-Maguiraga L., Lum F.-M., Labadie K., Martinon F. Paradoxical effect of chloroquine treatment in enhancing Chikungunya virus infection. Viruses. 2018;10:268. doi: 10.3390/v10050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seitz M., Valbracht J., Quach J., Lotz M. Gold sodium thiomalate and chloroquine inhibit cytokine production in monocytic THP-1 cells through distinct transcriptional and posttranslational mechanisms. J Clin Immunol. 2003;23:477–484. doi: 10.1023/B:JOCI.0000010424.41475.17. [DOI] [PubMed] [Google Scholar]
- 74.The Protein Tyrosine Kinase p56lck is Required for Triggering NF-kappaB Activation Upon Interaction of Human Immunodeficiency Virus Type 1 Envelope Glycoprotein gp120 With Cell Surface CD4-PubMed n.d. https://pubmed.ncbi.nlm.nih.gov/9621091/ [Accessed 4 June 2020]. [DOI] [PMC free article] [PubMed]
- 75.Colson P., Rolain J.-M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marmor M.F., Kellner U., Lai T.Y.Y., Melles R.B., Mieler W.F. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 77.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr Clin Res Rev. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biot C., Daher W., Chavain N., Fandeur T., Khalife J., Dive D. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49:2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- 81.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bright R. 2020. DHHS Letterhead. [Google Scholar]
- 83.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Mehta N., Mazer-Amirshahi M., Alkindi N., Pourmand A. Pharmacotherapy in COVID-19; a narrative review for emergency providers. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Olano J., Howland M.A., Su M.K., Hoffman R.S., Biary R. Toxicokinetics of hydroxychloroquine following a massive overdose. Am J Emerg Med. 2019;37 doi: 10.1016/j.ajem.2019.158387. 2264.e5-2264.e8. [DOI] [PubMed] [Google Scholar]
- 86.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018:9. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kirchdoerfer R.N., Ward A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat Commun. 2019;10:2342. doi: 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., Sims A.C., Feng J.Y. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169 doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheng C.-Y., Lee Y.-L., Chen C.-P., Lin Y.-C., Liu C.-E., Liao C.-H. Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J Microbiol Immunol Infect. 2020;53:488–492. doi: 10.1016/j.jmii.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanville B., Corbett R., Pidcock W., Hardin K., Sebat C., Nguyen M.-V. A community transmitted case of severe acute respiratory distress syndrome due to SARS CoV2 in the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Japan Acad Ser B. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersen P.I., Ianevski A., Lysvand H., Vitkauskiene A., Oksenych V., Bjørås M. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int J Infect Dis. 2020;93:268–276. doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B. COVID-19: a promising cure for the global panic. Sci Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Favipiravir Shows Good Clinical Efficacy in Treating COVID-19: Official-Xinhua | English.news.cn n.d. http://www.xinhuanet.com/english/2020-03/17/c_138888226.htm [Accessed 4 June 2020].
- 100.Wenzel R.P., Edmond M.B. Managing SARS amidst uncertainty. N Engl J Med. 2003;348:1947–1948. doi: 10.1056/NEJMp030072. [DOI] [PubMed] [Google Scholar]
- 101.Jones B.M., Ma E.S.K., Peiris J.S.M., Wong P.C., Ho J.C.M., Lam B. Prolonged disturbances of in vitro cytokine production in patients with severe acute respiratory syndrome (SARS) treated with ribavirin and steroids. Clin Exp Immunol. 2004;135:467–473. doi: 10.1111/j.1365-2249.2003.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peiris J., Lai S., Poon L., Guan Y., Yam L., Lim W. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV–infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020:64. doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mount Sinai Health System Treatment Guidelines for SARS-CoV-2 Infection (COVID-19) 1,2. n.d.
- 107.The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection-Full Text View-ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT04252885 [Accessed 4 June 2020].
- 108.Antiviral Activity of Arbidol and Its Derivatives Against the Pathogen of Severe Acute Respiratory Syndrome in the Cell Cultures] - PubMed n.d. https://pubmed.ncbi.nlm.nih.gov/18756809/ [Accessed 4 June 2020]. [PubMed]
- 109.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 110.Zhang J., Zhou L., Yang Y., Peng W., Wang W., Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A., Hershan A.A., Kamal M.A. Therapeutic management of patients with COVID-19: a systematic review. Infect Prev Pract. 2020;2 doi: 10.1016/j.infpip.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chan J.F.-W., Yao Y., Yeung M.-L., Deng W., Bao L., Jia L. Treatment with Lopinavir/Ritonavir or Interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin S., Shen R., He J., Li X., Guo X. Molecular modeling evaluation of the binding effect of ritonavir, lopinavir and darunavir to severe acute respiratory syndrome coronavirus 2 proteases. BioRxiv. 2020 doi: 10.1101/2020.01.31.929695. 2020.01.31.929695. [DOI] [Google Scholar]
- 116.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Treatment of Severe Acute Respiratory Syndrome with Lopinavir/Ritonavir: A Multicentre Retrospective Matched Cohort Study - PubMed n.d. https://pubmed.ncbi.nlm.nih.gov/14660806/ [Accessed 4 June 2020].
- 118.Baden L.R., Rubin E.J. Covid-19—the search for effective therapy. N Engl J Med. 2020;382:1851–1852. doi: 10.1056/NEJMe2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H. Interferon Alfacon-1 plus corticosteroids in severe acute respiratory syndrome. JAMA. 2003;290:3222. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 120.Therapeutic Options for COVID-19 Patients | CDC n.d. https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html [Accessed 4 June 2020].
- 121.Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID-19 : A Randomized Control Trial - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT04303299 [Accessed 4 June 2020].
- 122.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020;5:100028. doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shanghai 2019 Expert Coronary Disease Comprehensive Treatment Consensus n.d. https://mp.weixin.qq.com/s?__biz=MzA3Nzk5Mzc5MQ==&mid=2653620168&idx=1&sn=2352823b79a3cc42e48229a0c38f65e0&chksm=84962598b3e1ac8effb763e3ddb4858435dc7aa947a8f41790e8df2bca34c20e6ffea64cd191#rd [Accessed 4 June 2020].
- 124.High-Dose Vitamin C. (PDQ®)–Health Professional Version - National Cancer Institute n.d. https://www.cancer.gov/about-cancer/treatment/cam/hp/vitamin-c-pdq [Accessed 4 June 2020].
- 125.Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wen Y.-C., Hsiao F.-Y., Lin Z.-F., Fang C.-C., Shen L.-J. Risk of stroke associated with use of nonsteroidal anti-inflammatory drugs during acute respiratory infection episode. Pharmacoepidemiol Drug Saf. 2018;27:645–651. doi: 10.1002/pds.4428. [DOI] [PubMed] [Google Scholar]
- 127.Wen Y.-C., Hsiao F.-Y., Chan K.A., Lin Z.-F., Shen L.-J., Fang C.-C. Acute respiratory infection and use of nonsteroidal anti-inflammatory drugs on risk of acute myocardial infarction: a nationwide case-crossover study. J Infect Dis. 2017;215:503–509. doi: 10.1093/infdis/jiw603. [DOI] [PubMed] [Google Scholar]
- 128.Clavé S., Rousset-Rouvière C., Daniel L., Tsimaratos M. The invisible threat of non-steroidal anti-inflammatory drugs for kidneys. Front Pediatr. 2019:7. doi: 10.3389/fped.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang X., Donnan P.T., Bell S., Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2017;18:256. doi: 10.1186/s12882-017-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020 doi: 10.1136/bmj.m1086. m1086. [DOI] [PubMed] [Google Scholar]
- 131.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.WHO Clarifies Guidance on Ibuprofen, Says There’s No Evidence It Can Worsen COVID-19 | CBC News n.d. https://www.cbc.ca/news/health/ibuprofen-covid-19-novel-coronavirus-1.5501496 [Accessed 4 June 2020].
- 133.Sodhi M., Etminan M. Safety of ibuprofen in patients with COVID-19. Chest. 2020 doi: 10.1016/j.chest.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020 doi: 10.1136/bmj.m1185. m1185. [DOI] [PubMed] [Google Scholar]
- 135.Liu C. Pay attention to situation of SARS-CoV-2 and TCM advantages in treatment of novel coronavirus infection. Chinese Herb Med. 2020;12:97–103. doi: 10.1016/j.chmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cunningham A.C., Goh H.P., Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24:91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luo H., Tang Q., Shang Y., Liang S., Yang M., Robinson N. Can chinese medicine Be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. 2020;26:243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nourazarian S.M., Nourazarian A., Majidinia M., Roshaniasl E. Effect of root extracts of medicinal Herb Glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 Colon Cancer cell line. Asian Pac J Cancer Prev. 2016;16:8563–8566. doi: 10.7314/APJCP.2015.16.18.8563. [DOI] [PubMed] [Google Scholar]
- 139.Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Amber R., Adnan M., Tariq A., Mussarat S. A review on antiviral activity of the Himalayan medicinal plants traditionally used to treat bronchitis and related symptoms. J Pharm Pharmacol. 2017;69:109–122. doi: 10.1111/jphp.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Otake T., Mori H., Morimoto M., Ueba N., Sutardjo S., Kusumoto I.T. Screening of Indonesian plant extracts for anti-human immunodeficiency virus—type 1 (HIV-1) activity. Phyther Res. 1995;9:6–10. doi: 10.1002/ptr.2650090103. [DOI] [Google Scholar]
- 142.Evaluation of Ocimum sanctum and Tinospora cordifolia as Probable HIV-Protease inhibitors | Request PDF n.d. https://www.researchgate.net/publication/284715195_Evaluation_of_Ocimum_sanctum_and_Tinospora_cordifolia_as_Probable_HIV-Protease_inhibitors [Accessed 4 June 2020].
- 143.(PDF) HIV-1 reverse transcriptase inhibition by Vitex negundo L. leaf extract and quantification of flavonoids in relation to anti-HIV activity n.d. https://www.researchgate.net/publication/236605044_HIV-1_reverse_transcriptase_inhibition_by_Vitex_negundo_L_leaf_extract_and_quantification_of_flavonoids_in_relation_to_anti-HIV_activity [Accessed 4 June 2020].
- 144.Mauthe M., Orhon I., Rocchi C., Zhou X., Luhr M., Hijlkema K.-J. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.US Food and Drug Administration. 28 March 2020. Emergency use authorization, hydroxychloroquine sulfate health care provider fact sheet. U.S. Food and Drug Administration, Washington, DC. n.d. https://www.fda.gov/media/136534/download [Accessed 6 October 2020].
- 146.Magagnoli J., Narendran S., Pereira F., Cummings T.H., Hardin J.W., Sutton S.S. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med. 2020 doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020 doi: 10.1136/bmj.m1849. m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mahévas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020 doi: 10.1136/bmj.m1844. m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 154.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.HiNet Life n.d. https://times.hinet.net/mobile/ [Accessed 4 June 2020].
- 157.Ocular Toxicity of Hydroxychloroquine - PubMed n.d. https://pubmed.ncbi.nlm.nih.gov/16912357/ [Accessed 4 June 2020].
- 158.Song Z., Hu Y., Zheng S., Yang L., Zhao R. Hospital pharmacists’ pharmaceutical care for hospitalized patients with COVID-19: recommendations and guidance from clinical experience. Res Social Adm Pharm. 2020 doi: 10.1016/j.sapharm.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vitamin C deployed in big doses to help treat coronavirus patients | South China Morning Post n.d. https://www.scmp.com/news/china/society/article/3077341/vitamin-c-deployed-big-doses-help-treat-coronavirus-patients [Accessed 4 June 2020].
- 160.Wang S., Wang Y., Lu Y., Li J., Song Y., Nyamgerelt M. Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine. J Integr Med. 2020 doi: 10.1016/j.joim.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system–Working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Study of High-Dose Chloroquine For COVID-19 Stopped Early Due to Patient Deaths n.d. https://www.sciencealert.com/clinical-trial-for-high-dose-of-chloroquine-stopped-early-due-to-safety-concerns [Accessed 4 June 2020].
- 163.Medicines against Coronavirus - research overview | vfa n.d. https://www.vfa.de/de/englische-inhalte/therapeutic-medicines-coronavirus-covid-19 [Accessed 4 June 2020].