Abstract
Background
Tamoxifen resistance in breast cancer is an unsolved problem in clinical practice. The aim of this study was to determine the potential mechanisms of tamoxifen resistance through bioinformatics analysis.
Methods
Gene expression profiles of tamoxifen-resistant MCF-7/TR and MCF-7 cells were acquired from the Gene Expression Omnibus dataset GSE26459, and differentially expressed genes (DEGs) were detected with R software. We conducted Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses using Database for Annotation, Visualization and Integrated Discovery. A protein–protein interaction (PPI) network was generated, and we analyzed hub genes in the network with the Search Tool for the Retrieval of Interacting Genes database. Finally, we used siRNAs to silence the target genes and conducted the MTS assay.
Results
We identified 865 DEGs, 399 of which were upregulated. GO analysis indicated that most genes are related to telomere organization, extracellular exosomes, and binding-related items for protein heterodimerization. PPI network construction revealed that the top 10 hub genes—ACLY, HSPD1, PFAS, GART, TXN, HSPH1, HSPE1, IRAS, TRAP1, and ATIC—might be associated with tamoxifen resistance. Consistently, RT-qPCR analysis indicated that the expression of these 10 genes was increased in MCF-7/TR cells comparing with MCF-7 cells. Four hub genes (TXN, HSPD1, HSPH1 and ATIC) were related to overall survival in patients who accepted tamoxifen. In addition, knockdown of HSPH1 by siRNA may lead to reduced growth of MCF-7/TR cell with a trend close to significance (P = 0.07), indicating that upregulation of HSPH1 may play a role in tamoxifen resistance.
Conclusion
This study revealed a number of critical hub genes that might serve as therapeutic targets in breast cancer resistant to tamoxifen and provided potential directions for uncovering the mechanisms of tamoxifen resistance.
Keywords: Breast cancer, Bioinformatics analysis, Tamoxifen, Drug resistance, Crucial genes
Introduction
About 70% of breast cancer cases worldwide are estrogen receptor (ER) positive. ER-positive breast cancer is an endocrine-dependent disease, and ER plays a vital role in the metastasis, occurrence and progression of this disease (Baum et al., 2002; Torre et al., 2015; Turner et al., 2017). Tamoxifen, a selective estrogen receptor modulator, binds to ERα and significantly suppresses the estrogen-induced growth of mammary epithelial cells (Early Breast Cancer Trialists’ Collaborative Group, 1998). However, approximately one-third of ER-positive breast cancers inevitably develop tamoxifen resistance, which leads to an unfavorable prognosis and remains a crucial clinical challenge to effective treatment (Ring & Dowsett, 2004). Thus, it is critical to elucidate the underlying factors that cause tamoxifen resistance, identify improved treatment strategies, and develop effective regimens for this disease (Shang & Brown, 2002; Gutierrez et al., 2005).
In recent years, many curative measures attempting to overcome tamoxifen resistance have been explored (Ring & Dowsett, 2004; Viedma-Rodriguez et al., 2014), and previous investigations have highlighted the use of specific molecular targeted drugs (Normanno et al., 2005). However, due to the unclear mechanisms, analyses of such approaches are still in the early stages and the treatment effects are not satisfactory (Nass & Kalinski, 2015; Zhao et al., 2017). Overall, the molecular mechanisms of tamoxifen resistance have not been determined. Exploration into the underlying factors of tamoxifen resistance would affect targeting strategies that might overcome tamoxifen resistance and enhance clinical results (Rondon-Lagos et al., 2016). Accordingly, identification of the genes related to tamoxifen resistance with powerful gene sequencing technologies, which may elucidate the mechanisms of tamoxifen resistance and help identify new curative strategies, is urgently needed (Katzenellenbogen & Frasor, 2004). However, few studies have been done on the key genes associated with tamoxifen resistance in breast cancer through bioinformatics analysis.
Gene microarray technology is an enhanced high-throughput method to effectively analyze gene expression profiles. Thus, gene microarrays have been comprehensively used to explore the underlying regulatory networks involved in different types of cancer and have important clinical applications in improving clinical diagnoses and discovering new drug targets (Zhang et al., 2017). This technology can also identify thousands of differentially expressed genes (DEGs) related to different biological processes (BPs), cellular components (CCs), molecular functions (MFs), and different signaling pathways of cancer. Moreover, bioinformatics methods allow for comprehensive analysis of large amounts of data derived from microarrays. Given the false positives and heterogeneity of different microarray results, we processed one microarray dataset, GSE26459, to obtain DEGs between the ER-positive breast cancer cell line MCF-7 (cell line sensitive to tamoxifen) and MCF-7/TR (cell line with acquired tamoxifen resistance). These findings combined with the bioinformatics results identified important signaling pathways and hub genes involved in breast cancer tamoxifen resistance. Through survival analysis, we found that ATIC, HSPD1, HSPH1 and TXN were related to survival. Further knockdown of target genes by siRNAs indicated that upregulation of HSPH1 is likely to play a role in tamoxifen resistance. Taken together, our results provide a valuable perspective into the mechanisms of tamoxifen resistance, identify possible candidate biomarkers, and suggest potential therapeutic drugs for overcoming tamoxifen resistance.
Materials and Methods
Cell culture
MCF-7 and tamoxifen-resistant MCF-7 (MCF-7/TR) cell lines were obtained from the Breast Tumor Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University (Zhu et al., 2018). Tamoxifen-resistant MCF-7 (MCF-7/TR) cells were established as formerly reported (Badia et al., 2000; Knowlden et al., 2003). MCF-7 cells were cultured in DMEM (Gibco, Gaithersburg, MD, USA) with 10% FBS (Gibco, Gaithersburg, MD, USA). Tamoxifen-resistant MCF-7 (MCF-7/TR) cells were cultured in DMEM (Gibco, Gaithersburg, MD, USA) supplemented with 10% FBS (Gibco, Gaithersburg, MD, USA) and one μM tamoxifen.
Identification of DEGs
To identify DEGs in tamoxifen-resistant MCF-7/TR cells and tamoxifen-sensitive MCF-7 cells, we obtained raw public gene expression data (GSE26459) from the Gene Expression Omnibus (GEO) database. This dataset contains information for MCF-7 and MCF-7/TR cells. These data were analyzed through R software with the affy and limma Bioconductor packages (Ritchie et al., 2015). To identify tamoxifen resistance-related DEGs between MCF-7/TR and MCF-7 cells, Student’s t test was carried out and statistical significance was set at P < 0.05 with a fold change ≥1.5.
DEG gene ontology and pathway enrichment analysis
To determine the biological functions and signaling pathways related to the DEGs, we submitted the data to Database for Annotation, Visualization and Integrated Discovery (DAVID), which is an online tool for gene annotation, function visualization and large volume data integration, including GO functional analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis (Schmid & Blaxter, 2008; Huang, Sherman & Lempicki, 2009). GO classifications include CC, BP and MF groups and P < 0.001 was considered significant.
Protein–protein interaction network construction by STRING
To evaluate protein–protein interaction (PPI) network information for the significant DEGs, we analyzed the 865 DEGs by the online database Search Tool for the Retrieval of Interacting Genes (STRING) to predict interactions among them (Szklarczyk et al., 2015). A combined score >0.7 was considered a high confidence score. These DEGs with many associations with other genes have critical relationships in the PPI interaction network. The top 15 upregulated DEGs in the PPI network were visualized through Cytoscape software (version 3.3.0) (Shannon et al., 2003). CytoHubba was employed to detect hub proteins according to their associations with other proteins by using Cytoscape software (version 3.3.0) (Chin et al., 2014).
RT-qPCR assay
The primer sequences used were as follows: ATP Citrate Lyase (ACLY) forward, 5′-TCGGCCAAGGCAATTTCAGAG-3′ and reverse, 5′-CGAGCATACTTGAACCGATTCT‑3′; Heat Shock Protein Family D (Hsp60) Member 1 (HSPD1) forward, 5′-ATGCTTCGGTTACCCACAGTC-3′ and reverse, 5′-AGCCCGAGTGAGATGAGGAG-3′; Phosphoribosylformylglycinamidine Synthase (PFAS) forward, 5′-CGAGCATACTTGAACCGATTCT-3′ and reverse, 5′-GTAGCACAGTTCAGTCTCGAC-3′; Phosphoribosylglycinamide Formyltransferase (GART) forward, 5′-GGAATCCCAACCGCACAATG-3′ and reverse, 5′-AGCAGGGAAGTCTGCACTCA-3′); Thioredoxin (TXN) forward, 5′-GTGAAGCAGATCGAGAGCAAG-3′ and reverse, 5′-CGTGGCTGAGAAGTCAACTACTA‑3′; Heat Shock Protein Family H (Hsp110) Member 1 (HSPH1) forward, 5′-ACAGCCATGTTGTTGACTAAGC-3′ and reverse, 5′-GCATCTAACACAGATCGCCTCT-3′; Heat Shock Protein Family E (Hsp10) Member 1 (HSPE1) forward, 5′-ATGGCAGGACAAGCGTTTAGA-3′ and reverse, 5′-TGGAAGCATAATGCCTCCTTTG-3′; TNF Receptor Associated Protein 1 (TRAP1) forward, 5′-AGGACGACTGTTCAGCACG-3′ and reverse, 5′-CCGGGCAACAATGTCCAAAAG‑3′; Nischarin (IRAS) forward, 5′-CAACCT ACGGATGACTCGTGCTT-3′ and reverse, 5′-TTTCTCCCTGACGGTCCCACTT-3′; 5-Aminoimidazole-4-Carboxamide Ribonucleotide (ATIC) forward, 5′-ACCTGACCGCTCTTGGTTTG-3′ and reverse, 5′-TACGAGCTAGGATTCCAGCAT-3′. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGGGTGCATCATTCTCATGG-3′.
Survival analysis of hub genes
We analyzed the overall survival of breast cancer patients stratified by the expression of 10 hub genes (high and low) using the Kaplan–Meier Plotter (http://www.kmplot.com), which is an online tool accumulating data on the gene expression and survival of breast cancer patients.
Cell viability assay
The breast cancer cell lines were plated in 96-well plates (2,000–5,000 cells/well) and treated with escalating doses of TAM as single agent. According to the manufacturer’s instructions, MTS assays (Promega, Madison, WI, USA) were conducted to detect the proliferation ability of tumor cells. The half maximal inhibitory concentrations (IC50) were calculated by GraphPad Prism version 7. Data were presented as the mean ± SD of three independent experiments.
siRNA transfection
MCF-7/TR cells were transfected with specific siRNA or negative control siRNA using RNAi MAX and the negative control were designed by ribo. These cells were cultured for 24–48 h in a CO2-containing chamber at 37 °C before being used for cell-based assays.
Statistical analysis
All data were statistically analyzed using Student’s t test with GraphPad Prism 7 software. The internal GAPDH gene was used for normalization of gene expression, at *P < 0.05; **P < 0.01; ***P < 0.001 compared with MCF-7 cells based on Student’s t test.
Results
Identification of DEGs
The research process of bioinformatics analysis is displayed in Fig. 1. We identified 865 significant DEGs, consisting of 399 upregulated and 466 downregulated DEGs, between MCF-7 cell lines and tamoxifen-resistant MCF-7/TR cell lines based on the public microarray dataset GSE26459. Among them, the most significantly upregulated gene was WNT2B, and the most significantly downregulated gene was ALCAM. The top ten downregulated and upregulated DEGs are presented in Table 1.
Figure 1. The flowchart of the bioinformatics analysis DEGs, differentially expressed genes; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PPI, protein-protein interaction; K–M Plotter, Kaplan–Meier Plotter; RT-qPCR, quantitative real-time polyme.
Table 1. The most significant up-regulated and down-regulated DEGs (top ten, Tamoxifen-resistant MCF-7/TR vs Tamoxifen-sensitive MCF-7).
| Gene | logFC | P Value | Gene full name | Gene ID number |
|---|---|---|---|---|
| Up-regulated | ||||
| WNT2B | 5.315 | 7.3 × 10−12 | Wnt Family Member 2B | 7482 |
| EVL | 5.292 | 4.1 × 10−11 | Enah/Vasp-Like | 51466 |
| TBC1D9 | 2.331 | 1.1 × 10−10 | TBC1 Domain Family Member 9 | 23158 |
| LMCD1 | 2.862 | 1.1 × 10−10 | LIM And Cysteine Rich Domains 1 | 29995 |
| CSTA | 3.675 | 1.5 × 10−10 | Cystatin A | 1475 |
| SLC6A14 | 3.391 | 5.9 × 10−10 | Solute Carrier Family 6 Member 14 | 11254 |
| PODXL | 2.196 | 7.1 × 10−10 | Podocalyxin Like | 5420 |
| COX6C | 2.163 | 1.2 × 10−09 | Cytochrome C Oxidase Subunit 6C | 1345 |
| TNS3 | 2.431 | 1.3 × 10−09 | Tensin 3 | 64759 |
| ACSL1 | 2.406 | 1.3 × 10−09 | Acyl-CoA Synthetase Long Chain Family Member 1 | 2180 |
| Down-regulated | ||||
| ALCAM | −5.393 | 3.7 × 10−12 | Activated Leukocyte Cell Adhesion Molecule | 214 |
| LXN | −4.032 | 7.3 × 10−12 | Latexin | 56925 |
| TARP | −5.160 | 2.1 × 10−11 | TCR Gamma Alternate Reading Frame Protein | 445347 |
| TRGC2 | −5.409 | 3.7 × 10−11 | T Cell Receptor Gamma Constant 2 | 6967 |
| PPP3CA | −2.883 | 1.8 × 10−10 | Protein Phosphatase 3 Catalytic Subunit Alpha | 5530 |
| CLEC7A | −2.609 | 2.8 × 10−10 | C-Type Lectin Domain Containing 7A | 64581 |
| SOWAHA | −2.190 | 5.8 × 10−10 | Sosondowah Ankyrin Repeat Domain Family Member A | 134548 |
| CD36 | −3.636 | 6.3 × 10−10 | CD36 Molecule | 948 |
| CLEC3A | −2.534 | 6.8 × 10−10 | C-Type Lectin Domain Family 3 Member A | 10143 |
| SDC2 | −2.583 | 8.2 × 10−10 | Syndecan 2 | 6383 |
DEG gene ontology and pathway enrichment analysis
We clustered the DEGs through GO and KEGG pathway analyses in DAVID to explore potential biological roles and functional enrichment. The enriched GO terms, including BP, CC and MF, are presented in Figs. 2A–2C, respectively. The most significantly enriched BP GO terms were “telomere organization” (GO:0032200), “nucleosome assembly” (GO:0006334) and “chromatin silencing at rDNA” (GO:0000183) (Fig. 2A). In the CC category (Fig. 2B), cell membrane components were the major enriched categories, which included extracellular exosomes (173 genes), protein complexes (41 genes) and extracellular matrices (34 genes). Other enriched GO terms consisted of membrane (124 genes) and cell-cell adherens junctions (33 genes, Fig. 2B). In the MF category (Fig. 2C), the most important GO categories were binding-related items for protein heterodimerization activity (60 genes) and histone binding (29 genes, Fig. 2C).
Figure 2. GO enrichment of DEGs.
(A) In biological process ontology; (B) in cellular component ontology; (C) in molecular function ontology.
Moreover, KEGG pathway analysis showed enrichment of five key pathways, including antigen processing and presentation signaling (8 genes), allograft rejection (6 genes) and cell adhesion molecules (CAMs) (11 genes, Table 2).
Table 2. The enriched KEGG pathway of DEGs.
| Term | Description | Count | P Value |
|---|---|---|---|
| hsa04612 | Antigen processing and presentation | 8 | 9.71E−04 |
| hsa05330 | Allograft rejection | 6 | 9.34E−04 |
| hsa04514 | Cell adhesion molecules (CAMs) | 11 | 7.19E−04 |
| hsa05332 | Graft-vs-host disease | 6 | 5.44E−04 |
| hsa04940 | Type I diabetes mellitus | 7 | 2.09E−04 |
Hub genes in STRING analysis of tamoxifen resistance were used to identify the PPI of the DEGs. PPI information for the 865 DEGs was evaluated in the STRING website, and we then used Cytoscape software to establish the PPI network for the top 15 DEGs, which contained 108 nodes and 226 edges.
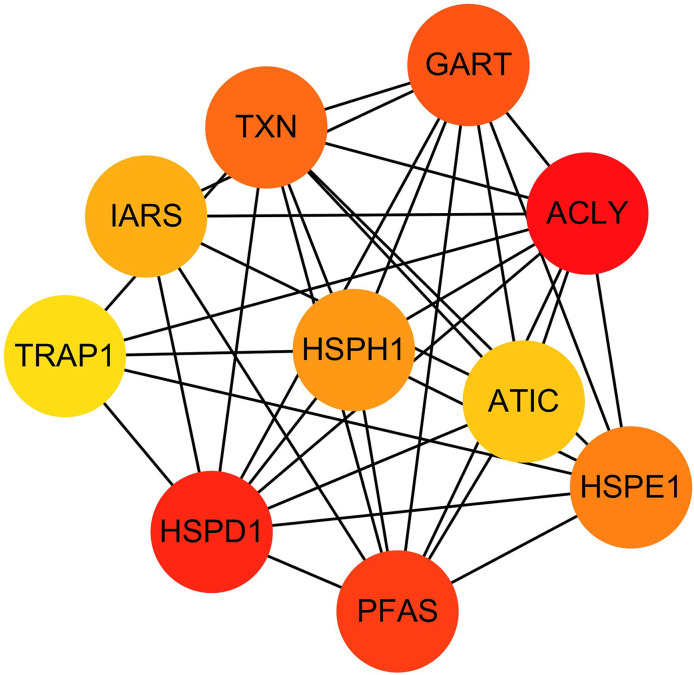
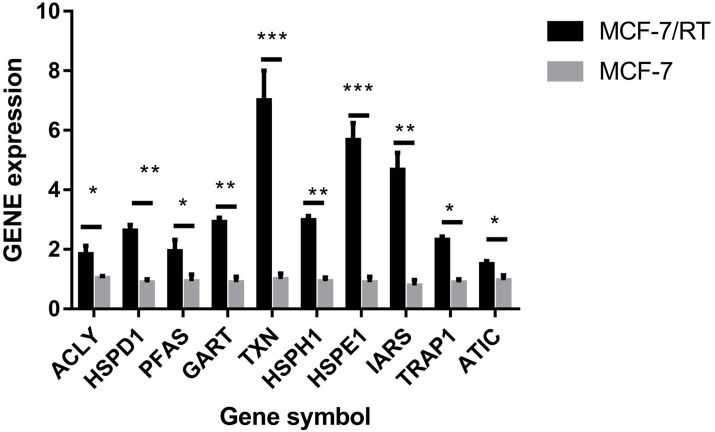
The hub genes were evaluated by CytoHubba. In the PPI networks, 10 node proteins—ACLY, HSPD1, PFAS, GART, TXN, HSPH1, HSPE1, IRAS, TRAP1 and ATIC—were associated strongly with other node proteins (more than 5), suggesting a high degree of connectivity (Fig. 3). Consistently, RT-qPCR analysis indicated that the expression level of these 10 genes was increased in MCF-7/TR (tamoxifen-resistant cell line) cells comparing with MCF-7 (tamoxifen-sensitive cell line) cells (Fig. 4). Collectively, it’s indicated that these hub genes and proteins may play a critical role in tamoxifen resistance.
Figure 3. The PPI network diagram of the node proteins showed many associations with other node proteins, indicating that they have high degrees of connectivity.
The darker the color was, the more proteins associated with the node protein.
Figure 4. RT-qPCR validation of the DEGs between MCF-7 and MCF-7/TR.
Relative expression of ACLY, HSPD1, PFAS, GART, TXN, HSPH1, HSPE1, IRAS, TRAP1, and ATIC. *P < 0.05; **P < 0.01; ***P < 0.001.
Tamoxifen resistance-associated hub genes predict poor prognosis
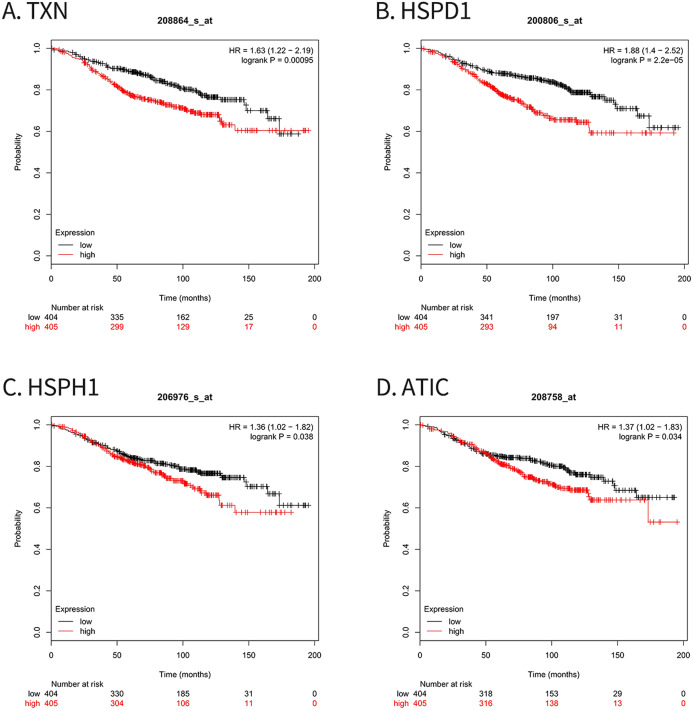
For survival analysis, we evaluated associations between expression of the 10 hub genes and patient survival with the Kaplan–Meier Plotter database. Four hub genes (TXN, HSPD1, HSPH1 and ATIC) were found to be related to poor overall survival in breast cancer patients who received tamoxifen (Fig. 5).
Figure 5. Kaplan–Meier Plotter analysis of four genes involved in breast cancer.
(A) TXN; (B) HSPD1; (C) HSPH1; (D) ATIC. HR, Hazard ratio; CI, confidence interval.
Knockdown of target genes by siRNAs
MTS assay verified that MCF-7/TR cell line (IC50 17.21μM) was less sensitive to tamoxifen than MCF-7 cell line (IC50 7.46μM) (P < 0.05) (Fig. 6A). RT–qPCR experiments confirmed that small interfering RNA (siRNA) resulted in over 60% reduction in expression of 10 hub genes (Fig. 6B). MTS assay showed that knockdown of HSPH1 may lead to reduced growth of MCF-7/TR cell (P = 0.07), whereas knockdown of other genes did not significantly affect cell growth (Fig. 6C). It’s indicated that upregulation of HSPH1 is likely to play an important role in tamoxifen resistance.
Figure 6. (A) MTS assay verified that MCF-7/TR cell line (IC50 17.21μM) was less sensitive to tamoxifen than MCF-7 cell line (IC50 7.46 μM) (P < 0.05). (B) RT–qPCR experiments confirmed that small interfering RNA (siRNA) resulted in over 60% reduction in expression of 10 hub genes. (C) MTS assay showed that knockdown of HSPH1 may lead to reduced growth of MCF-7/TR cell with a trend close to significance (P = 0.07), whereas knockdown of other genes did not significantly affect cell growth.
Discussion
Tamoxifen resistance is an unsolved problem in breast cancer treatment, hence it is particularly important to identify critical genes and signaling pathways related to tamoxifen resistance to determine its complex mechanism. In the present study, we identified 865 DEGs, including 399 upregulated and 466 downregulated DEGs, between the MCF-7 and MCF-7/TR cell lines. These DEGs possibly mediated tamoxifen resistance. Previous findings suggested that several DEGs might be responsible for resistance in diverse cancers. For example, WNT2B, among the top ten upregulated DEGs, is important in chemotherapy resistance and tumorigenesis in head and neck squamous cell carcinoma and may be a treatment target for oral cancers (Li, Yang & Qian, 2015). The present study identified SLC6A14 as a new treatment target for ER-positive breast cancer. It’s indicated that SLC6A14 may play a role in tamoxifen resistance (Karunakaran et al., 2011). COX6C, listed in the top ten upregulated DEGs, affects mitochondrial genes and function and induces ABCG2-overexpressing cells to pump mitoxantrone from the cell matrix (Chang et al., 2017). ALCAM, among the top ten downregulated DEGs, is a new serum marker correlating with cell growth, adhesion and chemotherapy resistance in pancreatic cancer (Hong et al., 2010). In addition, the miR-20a-5p/SDC2 axis is a possible biomarker for diagnosis and treatment targets (Zhao et al., 2017). In conclusion, these DEGs are likely to play vital roles in tamoxifen resistance via various mechanisms.
We explored the biological functions of the DEGs with GO analysis. In the BP category, the DEGs were mostly enriched in telomere organization; other DEGs were enriched in nonmembrane-related items, including nucleosome assembly and chromatin silencing at rDNA. Regarding the CC category, stimulus-related items, including extracellular exosomes, were most significant. Other enriched categories included protein complexes and extracellular matrix. These data showed that the molecular mechanisms of tamoxifen resistance might be related to both membrane and nonmembrane structures. Binding-related items for protein heterodimerization activity were most significant in the MF category, and other enriched MF terms included histone binding and nucleosomal DNA binding.
Analysis of pathways may uncover more exact biological functions of DEGs than those obtained through Gene Ontology analysis. We identified three enriched pathways: antigen processing and presentation signaling pathways, pathways in allograft rejection and CAMs. Cancer pathways may participate in cancer evolution, including drug resistance.
Our PPI network included ACLY, HSPD1, PFAS, GART, TXN, HSPH1, HSPE1, IRAS, TRAP1, and ATIC, which were confirmed by RT-qPCR, and several of them are associated with drug resistance. Four hub genes (TXN, HSPD1, HSPH1 and ATIC) were related to OS in patients who accepted tamoxifen treatment according to Kaplan–Meier Plotter. It’s indicated that the overexpression of TXN proteins was one of the atypical multidrug resistance mechanisms associated with overexpression of P-glycoprotein (Mieszala et al., 2018). TXN modulate the expression of estrogen responsive genes in breast cancer cells, and reducing oxidized TXN restore tamoxifen sensitivity to previously resistant breast cancer cells (Rosalind & Deodutta, 2013). A previous study demonstrated that the expression level of the HSPD1 mRNA was closely related to the emergence of cisplatin resistance in head and neck cancer cell lines (Nakata et al., 1994). In addition, high transcript level of HSPD1 was associated with the resistance to hormonal therapy, and a short 4-gene signature including HSPD1 might effectively predict tamoxifen-resistance (Sotgia, Fiorillo & Lisanti, 2017). The function of HSPH1 in DNA repair may partially explain the higher resistance to genotoxic drugs of colorectal cancers expressing higher levels of HSPH1 (Causse et al., 2018). ATIC was associated with resistance to methotrexate (You et al., 2013). We further knocked down 10 hub genes by siRNAs and found silence of HSPH1 led to reduced growth of MCF-7/TR cell with a tendency toward statistical significance (P = 0.07). Upregulation of HSPH1 may be important in the mechanism of tamoxifen resistance.
Several limitations of the present study merit discussion, including the fact that only the GEO26459 dataset of microarray data was used and that the sample size was relatively small. The aforementioned results, including the DEGs and their functions, should be confirmed by in vivo or in vitro experiments, which will be carried out in future studies. Nonetheless, the preliminary findings of our study provide comprehensive molecular insight and potential directions for further elucidating the underlying mechanisms of tamoxifen resistance.
Conclusions
Our study revealed a number of critical hub genes which might play an important role in the mechanism of tamoxifen resistance. These hub genes, including TXN, HSPD1, HSPH1, and ATIC, serve as potential therapeutic targets in breast cancer resistant to tamoxifen and suggest directions for uncovering the mechanisms of tamoxifen resistance. The study results have provided a theoretical basis for future translational research, which may eventually solve the clinical problem of tamoxifen resistance.
Supplemental Information
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Kai Zhang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Kuikui Jiang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Ruoxi Hong conceived and designed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Fei Xu conceived and designed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Wen Xia conceived and designed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Ge Qin performed the experiments, prepared figures and/or tables, and approved the final draft.
Kaping Lee performed the experiments, prepared figures and/or tables, and approved the final draft.
Qiufan Zheng performed the experiments, prepared figures and/or tables, and approved the final draft.
Qianyi Lu performed the experiments, prepared figures and/or tables, and approved the final draft.
Qinglian Zhai performed the experiments, prepared figures and/or tables, and approved the final draft.
Shusen Wang conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.
References
- Badia et al. (2000).Badia E, Duchesne MJ, Semlali A, Fuentes M, Giamarchi C, Richard-Foy H, Nicolas JC, Pons M. Long-term hydroxytamoxifen treatment of an MCF-7-derived breast cancer cell line irreversibly inhibits the expression of estrogenic genes through chromatin remodeling. Cancer Research. 2000;60:4130–4138. doi: 10.1097/00002820-200008000-00010. [DOI] [PubMed] [Google Scholar]
- Baum et al. (2002).Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. doi: 10.1016/S0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- Causse et al. (2018).Causse SZ, Marcion G, Chanteloup G, Uyanik B, Boudesco CH, Grigorash BB, Douhard R, Dias AMM, Dumetier B, Dondaine L, Gozzi GJ, Moussay E, Paggetti J, Mirjolet C, De Thonel A, Dubrez L, Demidov ON, Gobbo J, Garrido C. HSP110 translocates to the nucleus upon genotoxic chemotherapy and promotes DNA repair in colorectal cancer cells. Oncogene. 2018;38:2767–2777. doi: 10.1038/s41388-018-0616-2. [DOI] [PubMed] [Google Scholar]
- Chang et al. (2017).Chang FW, Fan HC, Liu JM, Fan TP, Jing J, Yang CL, Hsu RJ. Estrogen enhances the expression of the multidrug transporter gene ABCG2-increasing drug resistance of breast cancer cells through estrogen receptors. International Journal of Molecular Sciences. 2017;18:E163. doi: 10.3390/ijms18010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin et al. (2014).Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. CytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Systems Biology. 2014;8(Suppl. 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group (1998).Early Breast Cancer Trialists’ Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467. doi: 10.1016/S0140-6736(97)11423-4. [DOI] [PubMed] [Google Scholar]
- Gutierrez et al. (2005).Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Dowsett M. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. Journal of Clinical Oncology. 2005;23(11):2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- Hong et al. (2010).Hong X, Michalski CW, Kong B, Zhang W, Raggi MC, Sauliunaite D, De Oliveira T, Friess H, Kleeff J. ALCAM is associated with chemoresistance and tumor cell adhesion in pancreatic cancer. Journal of Surgical Oncology. 2010;101(7):564–569. doi: 10.1002/jso.21538. [DOI] [PubMed] [Google Scholar]
- Huang, Sherman & Lempicki (2009).Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Karunakaran et al. (2011).Karunakaran S, Ramachandran S, Coothankandaswamy V, Elangovan S, Babu E, Periyasamy-Thandavan S, Gurav A, Gnanaprakasam JP, Singh N, Schoenlein PV, Prasad PD, Thangaraju M, Ganapathy V. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. Journal of Biological Chemistry. 2011;286(36):31830–31838. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen & Frasor (2004).Katzenellenbogen BS, Frasor J. Therapeutic targeting in the estrogen receptor hormonal pathway. Seminars in Oncology. 2004;31:28–38. doi: 10.1053/j.seminoncol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Knowlden et al. (2003).Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144(3):1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- Li, Yang & Qian (2015).Li SJ, Yang X, Qian HY. Antitumor effects of WNT2B silencing in GLUT1 overexpressing cisplatin resistant head and neck squamous cell carcinoma. American Journal of Cancer Research. 2015;5:300–308. [PMC free article] [PubMed] [Google Scholar]
- Mieszala et al. (2018).Mieszala K, Rudewicz M, Gomulkiewicz A, Ratajczak‐Wielgomas K, Grzegrzolka J, Dziegiel P, Borska S. Expression of genes and proteins of multidrug resistance in gastric cancer cells treated with resveratrol. Oncology Letters. 2018;15:5825–5832. doi: 10.3892/ol.2018.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata et al. (1994).Nakata B, Barton RM, Robbins KT, Howell SB, Los G. Association between hsp60 messenger-RNA levels and Cisplatin resistance in human head and neck-cancer cell-lines. International Journal of Oncology. 1994;5:1425–1432. doi: 10.3892/ijo.5.6.1425. [DOI] [PubMed] [Google Scholar]
- Nass & Kalinski (2015).Nass N, Kalinski T. Tamoxifen resistance: from cell culture experiments towards novel biomarkers. Pathology, Research and Practice. 2015;211(3):189–197. doi: 10.1016/j.prp.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Normanno et al. (2005).Normanno N, Di Maio M, De Maio E, De Luca A, De Matteis A, Giordano A, Perrone F. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocrine-Related Cancer. 2005;12(4):721–747. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- Ring & Dowsett (2004).Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocrine-Related Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- Ritchie et al. (2015).Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon-Lagos et al. (2016).Rondon-Lagos M, Villegas VE, Rangel N, Sánchez MC, Zaphiropoulos PG. Tamoxifen resistance: emerging molecular targets. International Journal of Molecular Sciences. 2016;17(8):E1357. doi: 10.3390/ijms17081357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosalind & Deodutta (2013).Rosalind BP, Deodutta R. Thioredoxin-mediated redox regulation of resistance to endocrine therapy in breast cancer. Biochimica et Biophysica Acta. 2013;1836:60–79. doi: 10.1016/j.bbcan.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Schmid & Blaxter (2008).Schmid R, Blaxter ML. Annot8r: GO, EC and KEGG annotation of EST datasets. BMC Bioinformatics. 2008;9(1):180. doi: 10.1186/1471-2105-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang & Brown (2002).Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295(5564):2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- Shannon et al. (2003).Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgia, Fiorillo & Lisanti (2017).Sotgia F, Fiorillo M, Lisanti MP. Mitochondrial markers predict recurrence, metastasis and tamoxifen-resistance in breast cancer patients: early detection of treatment failure with companion diagnostics. Oncotarget. 2017;8(40):68730–68745. doi: 10.18632/oncotarget.19612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk et al. (2015).Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, Von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Research. 2015;43(D1):D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre et al. (2015).Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Turner et al. (2017).Turner NC, Neven P, Loibl S, Andre F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet. 2017;389(10087):2403–2414. doi: 10.1016/S0140-6736(16)32419-9. [DOI] [PubMed] [Google Scholar]
- Viedma-Rodriguez et al. (2014).Viedma-Rodriguez R, Baiza-Gutman L, Salamanca-Gomez F, Diaz-Zaragoza M, Martínez-Hernández G, Ruiz Esparza-Garrido R, Velázquez-Flores MA, Arenas-Aranda D. Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review) Oncology Reports. 2014;32(1):3–15. doi: 10.3892/or.2014.3190. [DOI] [PubMed] [Google Scholar]
- You et al. (2013).You X, Williams A, Dervieux T, He WJ, Cronstein BN. Fibroblasts from methotrexate-sensitive mice accumulate methotrexate polyglutamates but those from methotrexate-resistant mice do not. Clinical & Experimental Rheumatology. 2013;31(3):433–435. doi: 10.1016/j.jbspin.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang L, Huang Y, Zhuo W, Zhu Y, Zhu B, Chen Z. Identification and characterization of biomarkers and their functions for Lapatinib-resistant breast cancer. Medical Oncology. 2017;34(5):89. doi: 10.1007/s12032-017-0953-y. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2017).Zhao F, Pu Y, Cui M, Wang H, Cai S. MiR-20a-5p represses the multi-drug resistance of osteosarcoma by targeting the SDC2 gene. Cancer Cell International. 2017;17(1):100. doi: 10.1186/s12935-017-0470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2018).Zhu Y, Liu Y, Zhang C, Chu J, Wu Y, Li Y, Liu J, Li Q, Li S, Shi Q, Jin L, Zhao J, Yin D, Efroni S, Su F, Yao H, Song E, Liu Q. Tamoxifen-resistant breast cancer cells are resistant to DNA-damaging chemotherapy because of upregulated BARD1 and BRCA1. Nature Communications. 2018;9(1):1595. doi: 10.1038/s41467-018-03951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.