Abstract
Background
Besides throat-nose swab polymerase chain reaction (PCR), unenhanced chest computed tomography (CT) is a recommended diagnostic tool for early detection and quantification of pulmonary changes in COVID-19 pneumonia caused by the novel corona virus. Demographic factors, especially age and comorbidities, are major determinants of the outcome in COVID-19 infection. This study examines the extra pulmonary parameter of bone mineral density (BMD) from an initial chest computed tomography as an associated variable of pre-existing comorbidities like chronic lung disease or demographic factors to determine the later patient's outcome, in particular whether treatment on an intensive care unit (ICU) was necessary in infected patients.
Methods
We analyzed 58 PCR-confirmed COVID-19 infections that received an unenhanced CT at admission at one of the included centers. In addition to the extent of pulmonary involvement, we performed a phantomless assessment of bone mineral density of thoracic vertebra 9–12.
Results
In a univariate regression analysis BMD was found to be a significant predictor of the necessity for intensive care unit treatment of COVID-19 patients. In the subgroup requiring intensive care treatment within the follow-up period a significantly lower BMD was found.
In a multivariate logistic regression model considering gender, age and CT measurements of bone mineral density, BMD was eliminated from the regression analysis as a significant predictor.
Conclusion
Phantomless assessed BMD provides prognostic information on the necessity for ICU treatment in course of COVID-19 pneumonia. We recommend using the measurement of BMD in an initial CT image to facilitate a potentially better prediction of severe patient outcomes within the 22 days after an initial CT scan.
Consequently, in the present sample, additional bone density analysis did not result in a prognostic advantage over simply considering age. Significantly larger patient cohorts with a more homogenous patient age should be performed in the future to illustrate potential effects.
Clinical relevance
While clinical capacities such as ICU beds and ventilators are more crucial than ever to help manage the current global corona pandemic, this work introduces an approach that can be used in a cost-effective way to help determine the amount of these rare clinical resources required in the near future.
Abbreviations: CT, unenhanced computed tomography; BMD, bone mineral density; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, real-time reverse-transcription polymerase chain reaction; VOI, volume of interest; SD, standard deviation; AIC, Akaike-Information-Criterion
Keywords: COVID-19, Bone mineral density, Severe acute respiratory syndrome coronavirus 2, Computed tomography
1. Introduction
Coronavirus disease (COVID-19) has been identified as an outbreak of severe acute respiratory syndrome in Wuhan, Hubei Province, China in the beginning of December 2019 caused by the novel coronavirus 2 (SARS-CoV-2) [1,2]. Real-time reverse-transcription polymerase chain reaction (RT-PCR) is considered to be the gold standard for the diagnosis of novel coronavirus disease. Additionally, unenhanced chest computed tomography is often performed fast and sensitive diagnostic tool [3]. Besides early detection and assessment of the severity of lung involvement in COVID-19 pneumonia, chest CT also offers the possibility to determine the individual stage of the disease [4]. For this reason, many guidelines recommend use of low dose chest CT for detection and quantification of the pulmonary involvement [5]. While pulmonary CT manifestations may be associated with the progression and prognosis of COVID-19, pulmonary involvement is additionally affected by a large variance regarding the individual stage of the disease as well as other clinical parameters [2,6].
Demographic factors, especially age, but also comorbidities including cardiovascular disease and chronic obstructive pulmonary disease, are major determinants of patients outcome in COVID-19 infection [7,8]. As COVID-19 patients are treated in centers for initial care and clinical triage, the full medical history is often not yet fully available. However, undiagnosed or unknown previous diseases comprise an important determinant of the later outcome [9].
Since there are - besides pulmonary involvement - other extrapulmonary parameters captured on chest CT which are associated with pre-existing comorbidities or demographic factors, this aims to assess bone mineral density. Characteristics like the degree of physical activity, body composition and lifestyle have been shown to have an influence on bone mineral density [10,11]. Smoking and, chronic lung diseases are proven to affect BMD negatively [[12], [13], [14]]. Therefore, we hypothesize that bone mineral density is a surrogate variable of many potentially outcome-relevant factors in a COVID-19 infection.
In general, not least because of the increased risk of infection, it is not practical to measure bone mineral density in patients with recent SARS-CoV-2 infection. However, as many guidelines recommend an unenhanced CT examination of the thorax in cases of suspected infection, the information about bone mineral density is generated anyway and must ultimately only be evaluated opportunistically by phantomless assessment of the BMD [5,15]. Phantomless assessed BMD is - next to dual-energy X-ray - an established and reliable method for the non-invasive determination of bone mineral density (BMD) in vertebrae and other bones [16,17]. It allows the determination of BMD in contrast agent CTs as well as unenhanced CT, where degenerative changes such as partial calcification or osteophytes are not a relevant interfering factor [18,19]. We prognosticate that a statement concerning the later outcome, or the degree and necessity of an intensive care treatment can be derived from the bone mineral density measured in an initial chest CT scan.
2. Material and methods
The methodology used in this study involving human participants was in accordance with the ethical standards of the institutional and national research committee as well as the Declaration of Helsinki of 1964. This retrospective study was approved by the institutional review board (No. 20-1216). All imaging was performed in case of a clinical indication. No scan was conducted explicitly for the purpose of this study.
2.1. Patient enrollment and follow up
We screened our database for consecutive patients from the first confirmed case of COVID-19 at BLINDED FOR SUBMISSION (center 1) and BLINDED FOR SUBMISSION (center 2) in March and April of 2020. Inclusion criteria were a) admission due to a symptomatology associated with a COVID-19 infection and b) CT on admission and c) a positive RT-PCR SARS-CoV-2 test. All patients with suspected COVID 19 infection received a CT examination in the above mentioned observation period.
Exclusion criteria were:
-
a)
Other acute pathology at the time of admission responsible for the respiratory symptoms and fulminant clinical course such as pulmonary artery embolism (n = 1), acute coronary syndrome (n = 1) and aspiration (n = 1).
-
b)
Parallel, or shortly before performed contrast agent CT examination (n = 3).
-
c)
Children and adolescents under 18 years.
Under consideration of the inclusion and exclusion criteria, 58 patients were finally enrolled in the investigation.
The outcome and clinical development were evaluated on an ordinal scale from 1 to 5 for each patient with higher numbers representing higher severity of progression or disease associated complications (After the initial admission, no further hospitalization was necessary = 1/hospitalization (for several days, but at least one night) = 2/intensive care unit (ICU) = 3/tracheal intubation = 4/death = 5). The highest achieved status during the observation period of 22 days beginning with admission to the hospital was collected. 22 days was the maximum observation time of deceased patients recently published in The Lancet 4/2020 (median 18.5 days, 15.0–22.0) [20]. Patients with a rating of 3 or higher (see above) were considered to have “intensive care obligation”, which was the focus in the present investigation.
2.2. Scanning protocol LDCT and image reconstruction
Scans were performed on state of the art multidetector CT scanners (center 1: iCT 256, Philips, Amsterdam, Netherlands; center 2: and SOMATOM Definition AS+, SIEMENS, Erlangen, Germany). Patients were placed in a head-first supine position. No contrast agent was admitted.
Scan parameters for center 1: 29.4 ± 9.4 mAs, collimation 80 × 0.625 mm, pitch 0.763, tube voltage 120 kV, mean CTDIvol 2.1 ± 0.6 mGy, mean DLP 86.2 ± 26.8 mGy*cm. For center 2: 116.3 ± 41.3 mAs; collimation 38.4 × 0.6 mm; pitch 1,2; tube voltage 100 kV; CTDIvol 7.6 ± 2.5 mGy. Mean DLP 262 ± 90.2 mGy*cm.
2.3. Bone mineral measurements
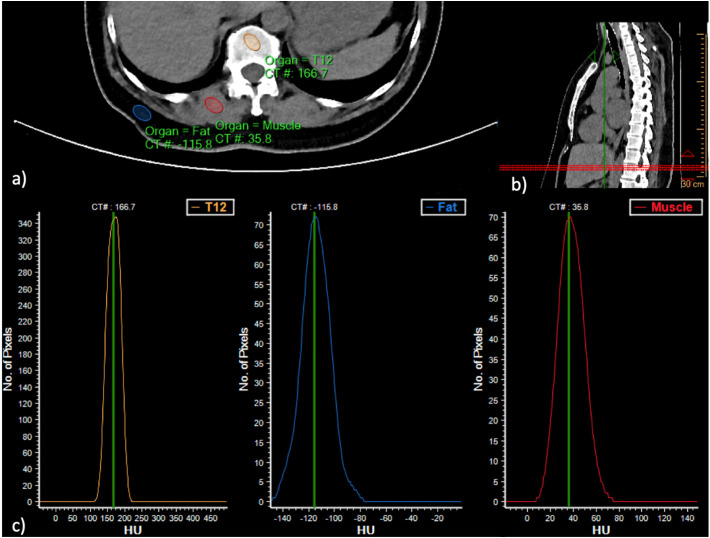
Measurement of vertebral bone density without the use of an external reference phantom was performed using an earlier described, standardized method using established software (IntelliSpace, Philips, Amsterdam, The Netherlands) [[21], [22], [23]]. The three most caudally registered vertebrae in the CT scans were primary objects for the bone mineral density measurements. In all patients these were the vertebral bodies TH 11–L 1. In the case of metastases, fractures or severe degeneration of any of those vertebrae, measurements were extended upwards to vertebrae TH 10 or 9 (n = 3). Sagittal reconstructions were used to angulate the transverse plane parallel to the end plate at each level. In axial reformations of the vertebral bodies an ellipsoid volume of interest (VOI) of 9-mm thickness was placed in the trabecular compartment of the vertebral body. Spectrometric calibration of Hounsfield units to the mineral scale was performed with density measurements in the paravertebral muscles (erector spinae muscle) and the subcutaneous fat tissue (see Fig. 1 ). The VOIs were placed in the anterior trabecular bone of the vertebral body, avoiding the internal vertebral venous plexus, the surrounding cortical bone, and any focal lytic or sclerotic lesion [17,22,23]. To determine inter- and intrarater reliability, 10 patients were randomly selected from the data set and measured twice by reader 1 (more than one year of experience in CT imaging). Furthermore, another 10 randomly selected patients were measured by a second radiologist (reader 2, more than one year of experience in CT imaging).
Fig. 1.
Exemplary measurement of the BMD one of three vertebrae in a 36-year-old male patient. No intensive care treatment was necessary for the patient. a) and b) Placement of the volumes of interest. c) density-controlled calibration of the reference tissue.
2.4. Statistics
Statistical data analysis was performed using R version 3.6.2 on Rstudio version 1.2.5033 [24]. Figures were plotted using the ggplot2 package [25]. To predict the categorical variable of intensive care treatment, a uni- and multivariate logistic regression analysis was used. The glm() function was used in “binomial” specification taking the independent variables of age, gender, and or only BMD into account. To illustrate the accuracy of fit, pseudo-R2 according to Nagelkerkes/Cragg & Uhlers was calculated using the descr package (0.2 > acceptable, 0.4 > good, 0.5 > very good) as well as the Akaike-Information-Criterion (AIC; low value indicates a higher informativeness of the model) [26]. Moderation and interaction effects between a) age, b) gender and c) BMD were formed using an interaction term from multiplication a) or b) by c) [27]. For the group comparisons, a two sample t-test was used after the normal distribution and variance homogeneity was confirmed by the Shapiro-Wilk- and Levene-test. Continuous variables were reported as mean ± standard deviation (SD). Intra- and interreader reliability was tested using the intraclass correlation coefficient in two-way random-effects model (<0.5 poor, >0.5 moderate, >0.75 good, >0.9 excellent) using the irr package. Statistical significance was defined as p ≤ .05.
3. Results
3.1. Patient characteristics, clinical outcome and bone mineral density
Of the 58 patients included, 37 were male and 21 female. The mean age was 59.3 (±16.2). All patients underwent a throat swab and CT on admission to the hospital due to suspected COVID-19 infection. In 51 (87.9%) of the patients, polymerase chain reaction tests were immediately positive for SARS-CoV-2, in 5 (8.2%) patients tests were positive within the next two days and 2 (3.5%) patients showed a positive sputum result after three days. Hence, all patients included in the study were tested positive for SARS-CoV-2. All patients demonstrated clinical symptoms such as malaise, fever, cough or shortness of breath upon admission to the hospital.
Among the patients included, 45 (78%) were admitted to the hospital, with 26 (45%) being administered to the intensive care unit. Of the latter, 12 (21%) were put on mechanical ventilation and 6 (10%) died (three without having been mechanically ventilated prior to their passing) within the observation period of 22 days. Detailed information on patient characteristics is listed in Table 1 .
Table 1.
Patient characteristics.
| Mean (SD) | Count (%) | |
|---|---|---|
| Age (y) | 59.3 (±16.2) | |
| Sexfemale | 21 (36.2%) | |
| BMD (mg/ml) | 131.4 (±33.2) | |
| T-scorea | −1.5 (±1.1) | |
| Z-scorea | −0.4 (±1) | |
| Outcome | ||
| Hospitalization | 45 (77.5%) | |
| ICU | 26 (44.8%) | |
| Mech. ventilation | 12 (20.7%) | |
| Death | 6b (10.3%) |
Patient characteristics given in mean value (SD) or count (%).
Based on patients aged between 18 and 80 years, the other data were excluded regarding T- and Z-Score.
Six patients died, three without having been mechanically ventilated prior to their passing.
The phantomless assessed BMD showed a mean bone density of 131.4 mg/ml (±33.2) in COVID-19 patients. The Z-score and T-score calculations provided by the manufacturer are reported but should be accompanied by a warning that they are not comparable to DXA-based result. For calculations a European reference group in the age between 18 and 80 years was used within the BMD tool to display the T- and Z-scores [28]. The mean T-score was −1.1 (±1.1) while the mean the Z-score was −0.4 (±1).
3.2. Prediction of the outcomes using the CT parameters
Considering the CT measurements of bone mineral density within the independent variable, we fit a logistic regression model to predict if the patient would require intensive care within a period of 22 days. In this univariate model, BMD is a significant predictor for the prognosis of intensive care obligation with a pseudo R2 to the Nagelkerke Index of 0.2 (p < .01), and an AIC of 80.2 (see Table 2 and Fig. 2 ). In comparison, a multivariate logistic regression model considering gender, age and CT measurements of bone mineral density, BMD was eliminated from the regression analysis as a significant predictor achieving a pseudo R2 to the Nagelkerke Index of 0.29 (p < .01) and an AIC of 76 (Table 3 ). None of the interaction terms described above proved to be a significant predictor of the intensive care obligation.
Table 2.
Parameters of the univariate logistic regression analysis.
Estimates for the univariate logistic regression model to predict intensive care obligation.
p < .001.
p < .01.
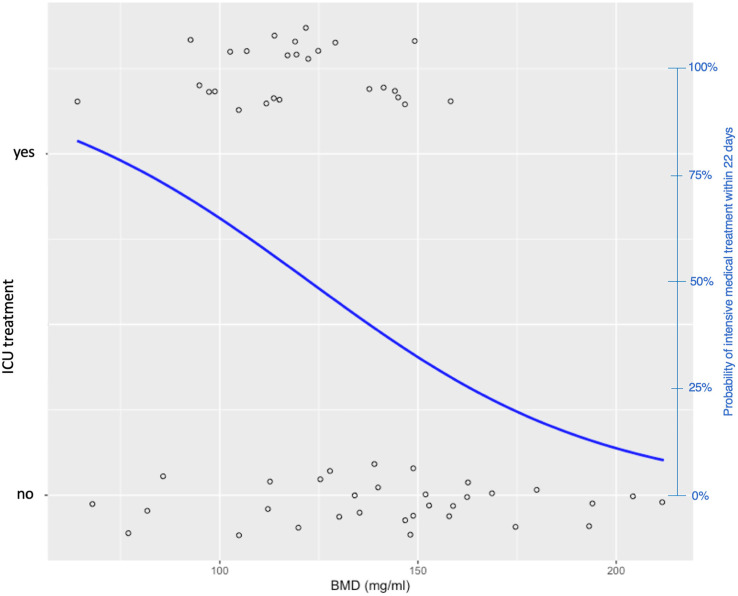
Fig. 2.
logistic regression model to predict whether intensive care treatment was necessary within the 22 days following LDCT. X-axis: BMD, left Y-axis: bidimensional representation of the need for intensive care treatment. Right-y-axis (blue) Probability (based on logistic regression model) of intensive care treatment being necessary depending on BMD. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Parameters of the multivariate logistic regression analysis.
| Estimate | Standard error | z-Value | |
|---|---|---|---|
| Intercept⁎ | −1.07 | 0.43 | 3.6 |
| Age⁎ | 0.01 | 0.03 | 0.02 |
| Sex | 0.6 | 0.12 | 0.18 |
| BMD (mg/ml) | −0.01 | 0.02 | 0.8 |
Estimates for the multivariate logistic regression model to predict intensive care obligation.
p < .05.
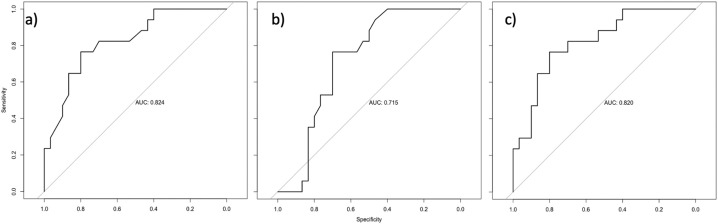
The comparison of the area under the curve with respect to the respective ROC curves for the models a) only old in the regression model (0.824), b) only BMD (0.715) in the regression model and c) only and BMD (0.820) combined in the regression model showed no advantage for the addition of BMD (see Fig. 4).
Fig. 4.
The comparison of the area under the curve with respect to the respective ROC curves for the models a) only age in the regression model (0.824), b) only BMD (0.715) in the regression model and c) only and BMD (0.820).
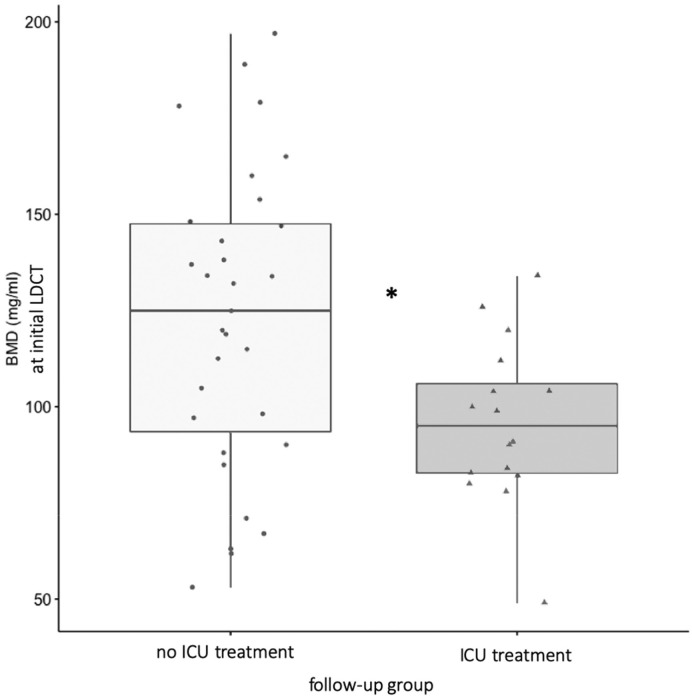
A significantly lower BMD was found in the subgroup requiring ICU treatment (mean: 98.2; ±22.1) as compared to the subgroup requiring no ICU treatment (mean 139.3; ±31.3) treatment within the follow-up period (p < .01; see Fig. 3 ). Intra- and interreader reliability achieved values in the range of “good” (Table 4 ).
Fig. 3.
Initially measured significantly different BMD of the two groups that either required no (light gray) or ICU treatment (dark gray) in the course of time of 22 days.
Table 4.
Intraclass correlation coefficient to determine the inter- and intrarater reliability.
| Intraraterreliability | Interraterreliability | |
|---|---|---|
| BMD (mg/ml) | 0.81 | 0.76 |
Intraclass correlation coefficient to determine the inter- and intrarater reliability, (<0.5 poor, >0.5 moderate, >0.75 good, >0.9 excellent).
4. Discussion
The course and severity of a SARS-CoV-2 infection depends on various factors such as the patient's age, basic physical condition and possible comorbidities [7,8]. It was shown that these, as well as physical activity and body composition impact bone mineral density [[29], [30], [31]]. Additionally, it has been shown that smoking, chronic lung diseases have a negative effect on bone mineral density [[12], [13], [14]].
Thus, the present study was concerned with the question whether reduced BMD as a surrogate variable for possible pulmonary comorbidities, lifestyle factors or general physical condition can provide a statement about an increased risk for complications associated with COVID-19 pneumonia. The central issue was to identify cases with severe progressions and to predict whether a patient had to be treated in an ICU within the observation period. Using BMD, it was possible to predict whether a patient would need intensive care treatment in the period following admission. It was shown that in a logistic regression model, BMD acts as a significant predictor for a potential intensive care treatment. Adding age and gender in a multivariate regression model or within the range of interaction terms, BMD as a significant predictor is omitted - primarily due to a high collinearity between age and BMD. Thus, it can be observed that when asking whether intensive care treatment is necessary in the following period, the bone density in this group of patients does not reveal any additional variance compared to patient age alone.
Regarding the graph of logistic regression to predict the risk of intensive care treatment based on bone density, it can be stated that the risk for an intensive care treatment increases to over 75% at a BMD of under 80 mg/ml, whereas the risk with a bone density measured by CT of over 160 mg/ml is associated with an risk of under 25% for the need of an ICU treatment in the follow-up period (see Fig. 2).
The strength of the present study lies in the fact that a relatively large number of virologically confirmed COVID-19 cases were evaluated by an initial unenhanced CT of the thorax. With regard to the clinical outcome, an observation period of the recommended 22 days could be maintained. Here, the bone-density was determined in phantomless CT using validated and established algorithms [22,23]. The possibility of phantomless determination of the BMD offers the advantage that the bone density can be assessed on the basis of the CT scans made anyway, without the need for further diagnostic procedures. In summary, the methodological approach and the results are consistent with other observational studies and support the hypothesis that multimorbid and elderly people suffer from a more severe and complicated course of COVID-19 infection [7,20]. The analyses showed that the measurements of BMD provided a good inter- and intrareader reliability and can be considered as robust and well reproducible.
The most relevant limitation of the present study is, the high correlation between age and bone density. It should be noted that a larger sample size would have allowed a focus on exclusively older patients. Thus, it might be possible to determine in a selected group of patients whether an additional decrease in bone density would clarify further variance. In addition, it must be stated that the BMD measurement couldn't be performed at the heights of the lumbar spine used in relevant preliminary studies and had to be performed in the thoracic region. The present study considers only a small, locally targeted patient population within the context of a retrospective design. Furthermore, a fully automated determination of BMD for the evaluation of possible COVID-19 associated complications should be considered. Future studies should also clarify the extent to which other parameters (e.g. obesity) determined in an initial CT scan show possible interaction effects in order to enable a more precise prognosis of the outcome and possible disease-associated complications. In addition, the extent of pulmonary opacities was not taken into account, which should be considered in the context of larger analyses. Additional studies should verify our hypotheses on the basis of a larger prospective patient collective. In this sense, our pilot study is suggesting that it might be reasonable to add the BMD measurement into AI tools for automatic characterization of lung infiltrations in COVID-19 patients and additional perform a risk classification for upcoming ICU treatment.
This multicentric study emphasizes the prognostic relevance of low BMD as a risk factor for SARS-CoV-2 infected patients. We present a method using an initial unenhanced CT scan to screen opportunistically for patients with low BMD who have an increased risk for the need of intensive care treatment. While there is a high correlation between age and BMD in this patient sample and much of the variance in outcome is explained by this, BMD alone is a significant predictor of whether intensive care treatment is necessary. But in the present sample, additional bone density analysis did not result in a prognostic advantage over simply considering age. Significantly larger patient cohorts with a more homogenous patient age should be performed in the future to illustrate potential effects.
While clinical capacities such as ICU beds and ventilators are more crucial than ever to help manage the current global corona pandemic, this work introduces an approach that can be used in a cost-effective way to help determine the amount of these rare clinical resources required in the near future.
CRediT authorship contribution statement
Jonathan Kottlors: Conceptualization, Methodology, Writing - original draft. Nils Große Hokamp: Conceptualization. Philipp Fervers: Data curation. Johannes Bremm: Data curation. Florian Fichter: Data curation. Thorsten Persigehl: Writing - review & editing. Orkhan Safarov: Data curation. David Maintz: Supervision. Stephanie Tritt: Supervision. Nuran Abdullayev: Writing - review & editing.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
The present study was conducted without any support from third parties.
Footnotes
The article is not under consideration for publication elsewhere.
References
- 1.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han X., Cao Y., Jiang N., et al. Novel coronavirus pneumonia (COVID-19) progression course in 17 discharged patients: comparison of clinical and thin-section CT features during recovery. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan F., Ye T., Sun P., et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin D., Ryerson C., Haramati L., Sverzellati N., Kanne Jeffrey P., Raoof Suhail, Schluger Neil W., Volpi Annalisa, Yim Jae-Joon, Martin Ian B.K., Anderson Deverick J., Kong Christina, Altes Talissa, Bush Andrew, Sujal R., ANL The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Radiology. 2020 doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L., He W., Yu X., et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J. Inf. Secur. 2020 doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerkin K.J., Fried J.A., Raikhelkar J., et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020 doi: 10.1161/circulationaha.120.046941. [DOI] [PubMed] [Google Scholar]
- 9.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maïmoun L., Sultan C. Effects of physical activity on bone remodeling. Metabolism. 2011 doi: 10.1016/j.metabol.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Kohrt W.M., Bloomfield S.A., Little K.D., Nelson M.E., Yingling V.R. Physical activity and bone health. Med. Sci. Sports Exerc. 2004 doi: 10.1249/01.MSS.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 12.Alghadir A.H., Gabr S.A., Al-Eisa E. Physical activity and lifestyle effects on bone mineral density among young adults: sociodemographic and biochemical analysis. J. Phys. Ther. Sci. 2015 doi: 10.1589/jpts.27.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pompe E., de Jong P.A., van Rikxoort E.M., et al. Smokers with emphysema and small airway disease on computed tomography have lower bone density. Int. J. COPD. 2016 doi: 10.2147/COPD.S103680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jong W.U., De Jong P.A., Vliegenthart R., et al. Association of chronic obstructive pulmonary disease and smoking status with bone density and vertebral fractures in male lung cancer screening participants. J. Bone Miner. Res. 2014 doi: 10.1002/jbmr.2248. [DOI] [PubMed] [Google Scholar]
- 15.Zopfs D., Lennartz S., Zaeske C., et al. Phantomless assessment of volumetric bone mineral density using virtual non-contrast images from spectral detector computed tomography. Br. J. Radiol. 2020 doi: 10.1259/bjr.20190992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genant H.K., Block J.E., Steiger P., Glueer C.C., Smith R. Quantitative computed tomography in assessment of osteoporosis. Semin. Nucl. Med. 1987 doi: 10.1016/S0001-2998(87)80024-7. [DOI] [PubMed] [Google Scholar]
- 17.Engelke K., Adams J.E., Armbrecht G., et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD official positions. J. Clin. Densitom. 2008 doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Cann C.E. Quantitative CT for determination of bone mineral density: a review. Radiology. 1988 doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 19.Adams J.E. Quantitative computed tomography. Eur. J. Radiol. 2009 doi: 10.1016/j.ejrad.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boden S.D., Goodenough D.J., Stockham C.D., Jacobs E., Dina T., Allman R.M. Precise measurement of vertebral bone density using computed tomography without the use of an external reference phantom. J. Digit. Imaging. 1989 doi: 10.1007/BF03168013. [DOI] [PubMed] [Google Scholar]
- 22.Neuhaus V., Abdullayev N., Hellmich M., et al. Association of quality and quantity of bone metastases and computed tomography volumetric bone mineral density with prevalence of vertebral fractures in breast cancer patients. Clin. Breast Cancer. 2016 doi: 10.1016/j.clbc.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Mueller D.K., Kutscherenko A., Bartel H., Vlassenbroek A., Ourednicek P., Erckenbrecht J. Phantom-less QCT BMD system as screening tool for osteoporosis without additional radiation. Eur. J. Radiol. 2011 doi: 10.1016/j.ejrad.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Team RC . 2019. R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- 25.Wickham H. ggplot2: elegant graphics for data analysis. J. Stat. Softw. 2010;35(1) [Google Scholar]
- 26.Nagelkerke N.J.D. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–692. doi: 10.1093/biomet/78.3.691. [DOI] [Google Scholar]
- 27.Coulton C., Chow J. Interaction effects in multiple regression. J. Soc. Serv. Res. 1993 doi: 10.1300/J079v16n01_09. [DOI] [Google Scholar]
- 28.Bauer J.S., Virmani S., Mueller D.K. Quantitative CT to assess bone mineral density as a diagnostic tool for osteoporosis and related fractures. Medicamundi. 2010;54(2):31–37. [Google Scholar]
- 29.Barr R., Nayiager T., Gordon C., Marriott C., Athale U. Body composition and bone health in long-term survivors of acute lymphoblastic leukaemia in childhood and adolescence: the protocol for a cross-sectional cohort study. BMJ Open. 2015 doi: 10.1136/bmjopen-2014-006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roschger P., Paschalis E.P., Fratzl P., Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008 doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Marques E.A., Mota J., Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Omaha) 2012 doi: 10.1007/s11357-011-9311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]