Abstract
Placement of an epidural blood patch is the gold standard treatment for a postdural puncture headache when conservative measures have failed. If unsuccessful in relieving the symptoms, a second epidural blood patch may be warranted. However, when the accepted gold standard treatment has failed, alternative therapies may be pursued. A pterygopalatine ganglion block has been shown to be effective as an alternative to epidural blood patch placement. This case demonstrates the use of a suprazygomatic pterygopalatine ganglion block as a rescue technique for failed repeated epidural blood patch, with complete and permanent resolution of the headache.
Keywords: Epidural blood patch, postdural puncture headache, pterygopalatine ganglion block
Introduction
Epidural blood patch (EBP) is the gold standard for treating postdural puncture headache (PDPH) when conservative measures have failed (1). Transnasal pterygopalatine ganglion block (PPGB) has been described as a primary therapy in lieu of and in addition to EBP for the treatment of PDPH (2). While the transnasal approach to PPGB is technically simple and confers quick relief, its success is unpredictable, and it may need to be repeated multiple times for a lasting effect (3). In contrast, the suprazygomatic approach is safe, can be easily taught to any practitioner skilled in ultrasound, and confers lasting relief in a single treatment. This case describes a suprazygomatic approach to a PPGB as a rescue technique for repeated, failed EBP for the treatment of PDPH.
Case Presentation
A 25-year-old woman, American Society of Anaesthesiologists status 3, G1P0 at 41 weeks gestation, with a history of morbid obesity (body mass index, 45) and hypothyroidism, presented to the Labour and Delivery unit for planned vaginal delivery. The patient requested a labour epidural; however, due to unintended dural puncture, a continuous spinal catheter was placed for analgesia. She had an uneventful spontaneous vaginal delivery after which the continuous spinal catheter was removed. On postpartum day (PPD) 1, she developed a PDPH. The headache was not relieved with acetaminophen, fluid hydration, or intravenous cosyntropin or caffeine.
An EBP was uneventfully placed on PPD 3 in a standard fashion using 20 mL of autologous venous blood, and only partial relief of PDPH was obtained. On PPD 4, the patient requested a second EBP for persistent PDPH symptoms. Ultrasound was used to mark the targeted interspace and measure the distance to both the ligamentum flavum and dura. The loss of resistance equalled the measured distance on ultrasound exactly and blood was injected until the patient started to complain about the sensation of fullness in the back. The patient remained supine for 2 h after the second EBP but she reported no change in the pain score. Bilateral suprazygomatic PPGBs were offered to the patient and written informed consent was obtained.
The suprazygomatic approach to the PPGB was selected because of its low risk profile and high efficacy in delivering local anaesthetic to the ganglion. The patient was positioned supine with the head of the bed elevated to 30°. The patient’s head was turned to the contralateral side, and the zygomatic arch and posterior orbital rim were palpated. The needle entry was 1–1.5 cm superior to and posterior to the corner formed by the zygomatic arch and posterior orbital rim (Figure 1). A 13–6 MHz linear ultrasound probe (Sonosite HFL38, SonoSite Inc., Bothell, WA, USA) was positioned below the zygomatic arch and tilted to direct the ultrasound beam under the zygomatic arch and into the pterygopalatine fossa (Figure 2). On the ultrasound image, the maxilla can be identified anteriorly and the coronoid process of the mandible posteriorly with the pterygopalatine fossa located deeper in between the two bony structures. A 25-gauge spinal needle was advanced using the out-of-plane technique into the pterygopalatine fossa, and the local anaesthetic spread was observed (Figure 3). 4 mL of 0.5% ropivacaine were injected on each side.
Figure 1.

Image showing needle insertion location 1–1.5 cm superior to and posterior to the corner formed by the zygomatic arch and posterior orbital rim
Figure 2.
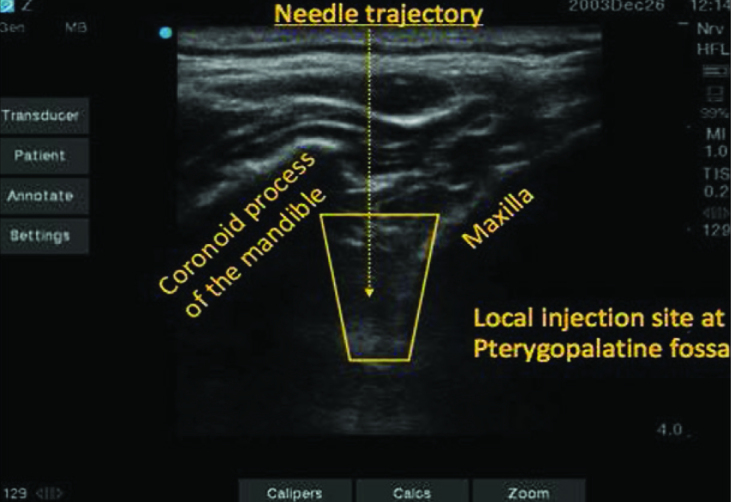
Ultrasound image showing the needle trajectory between the maxilla anteriorly and coronoid process of the mandible posteriorly to the pterygopalatine fossa located deeper in between the two bony structures
Figure 3.
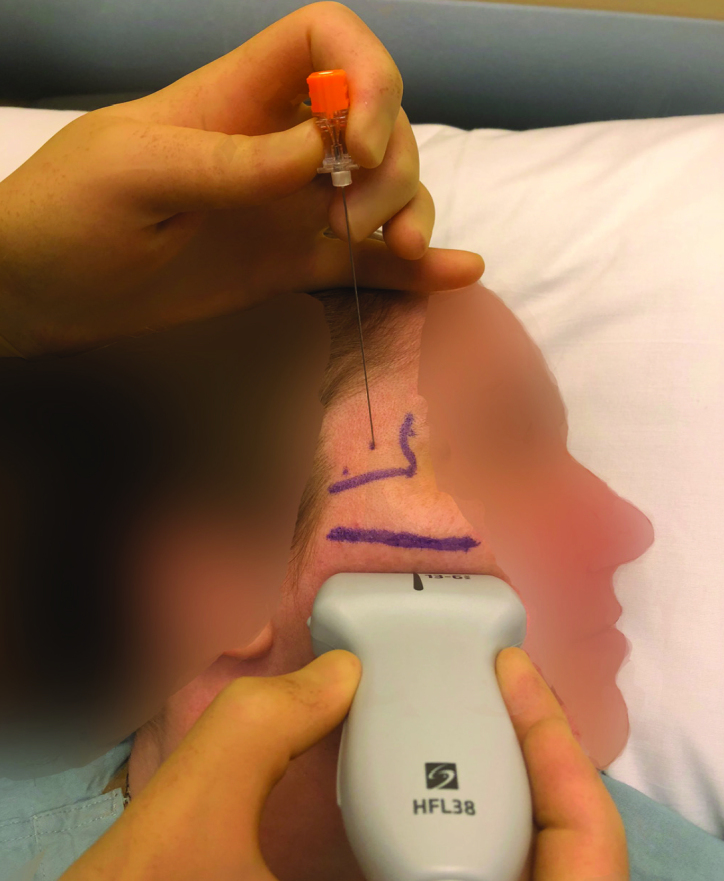
A 25-gauge spinal needle placed at the needle insertion site and advanced using an out-of-plane technique into the pterygopalatine fossa via a 13–6 MHz Sonosite HFL38 linear ultrasound probe
The patient experienced complete relief of her headache in the supine and sitting positions within 10 min of completion of the bilateral PPGB. The patient was discharged home and the follow-up was performed at 24 h and 72 h after discharge; she reported no recurrence of the symptoms.
Discussion
PDPH is a known complication of any procedure that intentionally or unintentionally violates the spinal dura (1). The proposed mechanism of action includes leakage of the cerebrospinal fluid (CSF) from the dural puncture site, which results in downward traction on the meninges and reflex intracranial vasodilation mediated by the parasympathetic nervous system (3). EBP is proposed to act as a physical barrier to any further CSF leakage and temporarily increases the intrathecal pressure to eliminate the PDPH symptoms. Although this technique targets the CSF leakage, it may not have a significant effect on the parasympathetic-mediated vasodilation. Although PPGB is extremely effective at counteracting the parasympathetic-mediated vasodilation, we are not proposing that it replaces EBP because of its inability to form a physical barrier to prevent further CSF leakage, which is the triggering event for PDPH.
The use of PPGB for the treatment of PDPH was first described by Cohen et al. in 2001, (4) and later in 2009 (5). In their study, PPGB was the primary treatment for PDPH, whereas EBP served as the rescue. Herein, we have described the opposite. We selected not to use a less invasive nasal topical application technique, as described by Cohen et al., and instead selected a transcutaneous approach, in part because the topical transnasal technique does not deliver medication directly to the parasympathetic ganglion to produce the cerebral vasoconstriction. It depends on the penetration of the local anaesthetic through a mucosal membrane and connective tissue, whereas a transcutaneous technique allowed us to deliver local anaesthetic directly into the ganglion, providing a quick and reliable onset of action. Furthermore, the transnasal technique is frequently short-lived, requiring repeated applications until there is a complete resolution of the symptoms. Ropivacaine is a significantly longer-acting local anaesthetic when compared with lidocaine, which is why we elected to inject ropivacaine instead of using a transnasal cotton swab for lidocaine application.
Conclusion
The suprazygomatic PPGB is performed bilaterally to achieve complete resolution of the headache. The risk profile is low because the needle is directed away from the orbit and cranial foramen. Delivery of local anaesthetic directly around the nerve provides higher efficacy than relying on diffusion through a membrane such as in the oral or transnasal approaches. The suprazygomatic PPGB is an efficacious, long-lasting, safe, and easily taught rescue technique for a failed EBP.
Main Points.
EBP is the gold standard for treating PDPH when conservative measures have failed.
A case wherein the suprazygomatic PPGB is demonstrated as a rescue technique for failed repeated EBP placement is reported.
Complete and permanent resolution of the headache resulted.
Footnotes
Informed Consent: Written informed consent was obtained from the patient in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – K.R.O., M.A.C., Y.Z.; Design – K.R.O., M.A.C., Y.Z.; Supervision – K.R.O., M.A.C., Y.Z.; Resources – K.R.O., M.A.C., Y.Z.; Materials – K.R.O., M.A.C., Y.Z.; Data Collection and/or Processing – K.R.O., M.A.C., Y.Z.; Analysis and/or Interpretation – K.R.O., M.A.C., Y.Z.; Literature Search – K.R.O., M.A.C., Y.Z.; Writing Manuscript – K.R.O., M.A.C., Y.Z.; Critical Review – K.R.O., M.A.C., Y.Z.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Peralta F, Devroe S. Any news on the postdural puncture headache front? Best Pract Res Clin Anaesthesiol. 2017;31:35–47. doi: 10.1016/j.bpa.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Kent S, Mehaffey G. Transnasal sphenopalatine ganglion block for the treatment of postdural puncture headache in the ED. Am J Emerg Med. 2015;33:1714.e1–2. doi: 10.1016/j.ajem.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–29. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Trnovski S, Zada Y. A new interest in an old remedy for headache and backache for our obstetric patients: a sphenopalatine ganglion block. Anaesthesia. 2001;56:585–610. doi: 10.1111/j.1365-2044.2001.2094-34.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Sakr A, Katyal S, Chopra D. Sphenopalatine ganglion block for postdural puncture headache. Anaesthesia. 2009;64:574–5. doi: 10.1111/j.1365-2044.2009.05925.x. [DOI] [PubMed] [Google Scholar]


