Abstract
Background:
Alcohol use is on the rise among women in the United States which is especially concerning since women who drink have a higher risk of alcohol-related problems. Orexin (hypocretin) receptor antagonists may have some therapeutic value for alcohol-induced insomnia, however the use of this class of drugs following female adolescent binge drinking is limited. The current study will address whether adolescent intermittent ethanol (AIE) in female rats can result in lasting changes in sleep pathology and whether orexin-targeted treatment can alleviate these deficits.
Methods:
Following a 5-week AIE vapor model, young adult rats were evaluated on waking event-related oscillations (EROs) and EEG sleep. Subsequently, AIE rats were treated with orexin receptor 2 (OX2R) antagonist (MK-1064; 10, 20 mg/kg) to test for modifications in sleep pathology and waking ERO.
Results:
Female AIE rats exhibited lasting changes in sleep compared to controls. This was demonstrated by increased fragmentation of slow wave sleep (SWS) and rapid eye movement (REM) sleep, as well as reductions in delta and theta power during SWS. There was no impact of AIE on waking EROs. Acute MK-1064 hastened SWS onset and increased the number of SWS episodes, without increasing sleep fragmentation in AIE and controls. While treatment with MK-1064 did not impact sleep EEG spectra, waking ERO energy was increased in delta, theta and beta frequency bands.
Conclusions:
These results demonstrate that AIE can produce lasting changes in sleep in female rats, highly similar to what we previously found in males. Additionally, while the OX2R antagonist promoted sleep in both alcohol-exposed and unexposed rats, it did not reverse most of the alcohol-induced disruptions in sleep. Thus, OX2R antagonism may serve as a potential therapeutic strategy for the treatment of insomnia, but not the specific signs of alcohol-induced insomnia.
Keywords: alcohol, adolescence, sleep, orexin, hypocretin
Introduction
Alcohol use and misuse is on the rise among women in the United States. While the prevalence of alcohol use and related harms has historically been 2–12 times higher in men than women (Grant et al., 2004; Keyes et al., 2008; Khan et al., 2013), there is emerging evidence to suggest that this gap is significantly narrowing (Slade et al., 2016; White et al., 2015). Between 2002 and 2012, prevalence of binge drinking increased in women, but remained relatively stable among men. Further, the number of drinking days per month increased for women but decreased for men, further reducing this gender gap (White et al., 2015). This increase is especially concerning since women who drink have a higher risk of certain alcohol-related problems and are more susceptible to blackouts compared to men (Hommer, 2003; Nolen-Hoeksema, 2004). Further research has shown that the onset of alcohol-related problems occurs more quickly and at lower intoxication levels than in men (Erol and Karpyak, 2015). In addition, there is a growing body of evidence demonstrating that alcohol can impact normal adolescent brain development with specific difference between males and females (Feldstein Ewing et al., 2014). For instance, heavy drinking during adolescence has been associated with reductions in gray matter volume and behavioral risk factors for future heavy drinking (Seo et al., 2019). However, this lower gray matter volume associated with heavy drinking was stronger in females than in males. Adolescent females who reported binge drinking also demonstrated less BOLD response which was associated with poorer sustained attention and working memory as compared to peers who drank lightly or abstained, an effect not seen in age-matched males (Squeglia et al., 2009). These studies suggest a unique vulnerability of females to the neurotoxic effects of binge-levels of ethanol consumption during adolescence.
Alcohol use is known to cause disruptions in sleep which may lead to sleep disorders, such as insomnia, with prolonged use (Brower, 2015; Conroy et al., 2006; Clark et al., 1998 Brower and Prron, 2010; Stein and Friedmann, 2005; Thakkar, Sharma, and Sahota, 2015). Sleep disturbances have also been shown to play a critical role in the development of alcohol use disorder (AUD) (Thakkar et al., 2015). However, alcohol use seems to disrupt sleep differently depending on gender. Irrespective of family history of alcoholism, women who consumed moderate to high levels of alcohol showed overall reductions in sleep quality and quantity compared to men (Arnedt et al., 2011). Heavy binge drinking during adolescence is also associated with poorer sleep quality in young adulthood (Ogeil et al., 2019; Ehlers et al., 2019). Preclinical studies in rats have demonstrated that chronic binge-level ethanol exposure in adolescents can lead to persistent sleep disturbances, characterized by reduced duration of slow wave sleep (SWS) episodes as well as total time spent in SWS (Criado, et al., 2008; Ehlers et al.,2018a,2019). While there is substantial evidence demonstrating the relationship between alcohol abuse and sleep disturbances, there is very little known about the mechanisms underlying alcohol-induced insomnia which is critical for identifying therapeutic targets.
It has been hypothesized that ethanol might be impacting sleep by impacting the orexin (also known as hypocretin) system (Lawrence et al., 2006; Morganstern et al., 2010; Olney et al., 2015). The orexinergic system is fundamental for regulating sleep-wake cycle (Chow and Cao, 2016; De Lecea and Sutcliffe, 2005), with hallmark studies demonstrating depletion of orexin neurons or orexin receptor knock-down resulting in the inability to sustain wakefulness with frequent transitions between sleep/wake states(Chemelli et al., 1999; McCarley, 2007; Mieda et al., 2011). Chronic exposure to ethanol can also reduce hypothalamic orexin immunoreactivity and mRNA expression (Lawrence et al., 2006; Olney et al., 2015). Chronic ethanol intoxication during adolescence in female and male rats was not found to impact the expression of orexin receptor type 1 (OX1R) and type 2 (OX2R) mRNA expression in the frontal cortex during adulthood (Amodeo et al., 2018), although other brain areas were not evaluated.
Recent clinical trials have demonstrated that suvorexant (MK-4305), a dual orexin receptor antagonist, can reduce sleep latency and improve sleep maintenance in patients with insomnia (Herring et al., 2016), suggesting that it may be a potentially new target for alcohol-induced insomnia. The therapeutic effects of suvorexant occur through blocking the wake-promoting neuropeptides, orexin-A and orexin-B, from slightly selective binding to OX1R over OX2R, suppressing wakefulness (Betschart et al., 2013). Recently, our lab has demonstrated that after 8 weeks of ethanol administered for 14 hours a day, acute administration of suvorexant was effective in reducing the latency of SWS in adult rats. However, it also produced an increase in SWS fragmentation and an increase in beta waking event-related oscillations (EROs), similar to that seen during ethanol withdrawal (Sanchez-Alavez et al., 2019). These results suggest that while dual OX1R/2R antagonism can hasten SWS, it may also reduce the quality of sleep. We also tested 2 doses (10, 30 mg/kg PO) of a Dual Orexin Receptor Antagonist (DORA-12) to modify sleep in rats treated for 5 weeks of ethanol vapor during adolescence. Adolescent vapor exposure was found to result in a fragmentation of sleep, in male young adult rats, that was partially ameliorated by DORA-12. DORA-12 also produced deeper sleep as indexed by increases in delta and theta power in the sleep EEG in both ethanol and control rats. Rats given DORA-12 also fell asleep faster than vehicle treated rats as measured by a dose dependent reduction in the latency to both the first SWS and rapid eye movement (REM) sleep episodes (Ehlers et al., 2019). However, it is not clear whether these findings were the result of OX1R or OX2R blockade. Studies evaluating the selective blockade of OX1R have demonstrated mild increases or no impact on SWS and REM sleep (Dugovic et al., 2014, 2009; Morairty et al., 2012). Alternatively, selective blockade of OX2R appears to produce a quicker onset to SWS and increases in SWS duration (Dugovic et al., 2014; Morairty et al., 2012). These results suggest that OX2R antagonist might offer more beneficial effects for the treatment of alcohol-induced insomnia compared to OX1R or dual antagonists.
The current study had two specific aims. First it addressed whether ethanol exposure in female Wistar rats during adolescence can have a persistent impact on sleep patterns, and during waking EROs that are similar to what we have reported in males (Ehlers et al., 2018a) Secondly, we investigated whether an acute OX2R antagonist, MK-1064, could reverse the effects on adolescent alcohol-induced sleep pathology and waking EROs.
Materials and Methods
Animals
Thirty female Wistar rats (Charles River, USA) arrived on postnatal day (PD) 21 and were housed in sets of 3–4 rats with same-sex littermates at the Scripps Research Institute animal facilities. Rats were group-housed in standard polycarbonate cages in a temperature- (20°C) and humidity-controlled room with a 12-hour light/dark cycle (lights on 08:00). Food and water were available ad libitum. Subjects were cared for in accordance with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80–23, revised 1996), and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Adolescent Ethanol Exposure
Adolescent rats were randomly divided into the adolescent intermittent ethanol exposure group (AIE; n = 14) or control (CONT; n = 16) group. The AIE rats were housed in sealed polycarbonate chambers, infused with vaporized 95% ethanol in a 14 hr-on/10 hr-off pattern (vapor beginning at 20:00) for 5 consecutive weeks (PD 22–57). This ethanol inhalation procedure has also been previously described (Ehlers et al., 2011; 2013; 2018a). To mimic levels of extreme binge drinking seen in adolescence, ethanol vapor chambers were calibrated to produce blood ethanol concentrations (BEC) between 175 and 225 mg/dL. Every 3 to 4 days during this 5-week exposure period, blood samples (200 μL) were collected from the tip of the tail to assess BECs. Blood samples were immediately centrifuged at 1500rpm for 15 minutes, with plasma extracted and stored at −80°C until further analysis. BECs were determined using the Analox microstatAM1 (Analox Instr. Ltd., Lunenberg, MA). Following 5-weeks of exposure, rats were transferred to standard polycarbonate cages for the duration of the experiment. Control animals were handled identically to ethanol rats, except they were not exposed to ethanol vapor and were maintained in standard cages throughout the experiment.
Surgical procedure
Surgical and electrophysiological recording procedures performed in this study have been previously described (Sanchez-Alavez et al., 2019). Following withdrawal from vapor exposure or control conditions, rats were surgically implanted (PD 64–73) with screw electrodes in the skull overlying the frontal cortex (FCTX, AP 1.5 mm, ML ± 3.0 mm, FR1) and parietal cortex (AP − 4.5 mm, ML ± 4.5 mm) with a corresponding reference electrode posterior to lambda in the skull overlying the cerebellum. Coordinates for these surgical sites were obtained from the Paxinos and Watson rat atlas (Paxinos and Watson, 1986). EEG electrodes were connected to a multi-pin PlasticsOne® connector which was anchored to the skull with dental acrylic and screws. Subsequently, rats were given at least 2 weeks of recovery before EEG recordings.
Electrophysiological recordings
After recovery from surgery and prior to EEG testing, rats were habituated to the polycarbonate testing chambers located in a large sound and electrically shielded recording chamber. Waking EROs were initially conducted to obtain a baseline (PD 78–86) difference between adolescent ethanol exposed animals and controls. For waking EROs, rats were individually placed in the testing chambers and presented with auditory stimuli through a small speaker centered approximately 70 cm above the rat’s head. Auditory stimuli and EROs were elicited using an oddball plus “noise” paradigm described previously (Ehlers et al., 2014; Ehlers et al., 2012). Each auditory ERO session lasted approximately 10 min and consisted of 312 trials. Individual trials consisted of one of three randomly presented tones: standard tone (84% probability, 75 dB, 1000 Hz), rare tone (10% probability, 85 dB, 2000 Hz), or noise tone (6% probability, 100 dB, white noise). These trials were 1000 ms in duration (200 ms prestimulus + 800 ms poststimulus) and the interval between tones varied from 750 to 1500 ms. About 5 min after the conclusion of the auditory ERO session, a 5 hr EEG sleep recording began. All testing chambers were lightly tapped to determine that the rats were in the same state of wakefulness prior to the start of each EEG session. This test served as the baseline EEG session. The EEG was recorded from two monopolar leads referenced to cerebellum ground (i.e. frontal cortex and parietal cortex) on a Sensorium preamplifier/amplifier unit (Shelburne, VT). Signals were transferred to a PC and digitized at a rate of 256 Hz. The EEG amplifier input range corresponding to the full range of the 12-bit analog-to-digital converter was about +/− 250 μV. Periodic calibration results were used to scale the digitized EEG to microvolts.
Following baseline (PD 78–86), three additional recording sessions were conducted for each rat to determine whether acute administration of OX2R antagonist, MK-1064 (obtained from PD 90–113), can significantly alter waking-ERO and sleep EEG recordings. Using a Latin square design, the order of drug administration was randomized for each rat: vehicle (20% vitamin E TGPS and sterile water), low-dose MK-1064 (10mg/kg), or high-dose MK-1064 (20 mg/kg). MK-1064 or vehicle was given via gavage (PO) at the onset of the light-cycle. Waking ERO session began approximately 60 mins post-gavage, subsequently followed by the 5hsleep recordings. The half-life of MK-1064 is 110 mins with an onset half-life of 21.1 mins (Gotter et al., 2016). These sessions were spaced out 7–10 days apart to avoid carry-over effects from the drug. Waking ERO and sleep EEG recordings were conducted in an identical manner as in the baseline sessions, except for drug administration.
Analysis of sleep patterns and sleep EEG
To quantify SWS, synchronized slow wave activity, which is depicted by low-amplitude, low-frequency waves (1–4 Hz), was visually identified during each of the 5-h EEG recording sessions (baseline, vehicle, MK-1064 10 mg/kg, and MK-1064 20 mg/kg). Episodes of SWS were determined by periods of at least twice the amplitude of waking EEG (low-amplitude, high-frequency waves) lasting longer than 8s. Quantification of REM sleep was depicted by synchronized theta activity (4–8 Hz) preceded by a SWS episode and in the absence of electromyography (EMG) activity. Patterns of SWS and REM included (1) onset latency of the first episode, (2) the duration of the first episode (3) the mean duration of SWS or REM episodes identified, and (4) the total number of SWS or REM episodes throughout the 5h session.
Spectral characteristics were also analyzed for the 5h EEG sleep sessions. Raw EEG signals were amplified (50% gain), band-pass filtered (0.53–70 Hz), digitized at a rate of 256 Hz, and then transferred to an IBM-compatible PC. A Fourier transform of 4 s epochs was used to create the power spectrum. Mean power density was quantified in micro V2/octave and peak frequency was calculated in hertz. Individual spectral epochs were averaged and compressed into frequency bands to calculate power for delta (1–4 Hz), theta (4–8 Hz), and beta (16–32 Hz) frequency ranges. Mean spectral power was calculated for all SWS and REM epochs over the entire 5-h recording session. EEG spectra were identified as containing artifact when average cortical power was > 2000 micro V2/octave. Artifact epochs were excluded only after visual analysis of the raw EEG and spectral distributions. These analysis procedures have been described previously (Ehlers and Havstad, 1982).
Analysis of waking ERO
Waking ERO analysis have been previously described (Ehlers et al., 2012; Sanchez-Alavez et al., 2019). Briefly, data from the individual ERP trials were entered into a time frequency analyses algorithm, S-transform (ST), a generalization of the Gabor transform (Stockwell et al., 1996). The S-transform resulted in a time–frequency representation of the data. The exact C language code used for the S-transform is available at the NIMH MEG Core Facility website (http://kurage.nimh.nih.gov/meglab/). The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time–frequency energy distributions.
S-transform magnitudes were quantified using region of interest (ROI) defined a priori by selecting a band frequency and time interval relative to the stimulus onset time, generating a rectangular ROI. Three time-frequency ROIs were defined (1) delta band, 1–4 Hz, 200–500 ms; (2) theta band, 4–7 Hz, 10–400 ms; (3) beta band, 13–30 Hz, 0–300 ms. These regions were chosen a priori to coincide with the major EEG frequencies and the latency windows of event-related potential components (e.g. N1, P3a, and P3b) in the rat (Ehlers et al., 2014, 2012). The maximum energy values were calculated for each ROI using the mean values over trials for each electrode location.
Identification of Stages of Estrous Cycle
Phase of estrous cycle were cytologically determined in adulthood through vaginal lavage 30m after habituation and 5h EEG sleep sessions. Rat vaginal secretions were collected by inserting a micropipette tip into the vagina, and gently flushing 200 μL of saline (0.9% NaCl). The aspirated vaginal fluid was placed onto a clean glass slide and smeared. Unstained smears were observed with a Fisher Scientific Micromaster II and photomicrographs of the estrous cycle stages taken with a Laxco 3MP digital camera. Smears were also stained with Cresyl Violet to further visualize differentiation of the stages. Estrus was determined by the presence of dense sheets of cornified epithelial cells, whereas non-estrus was identified by the presence of nucleated epithelial cells (proestrus) and/or the presence of leukocytes (diestrus) (Goldman et al., 2007).
Statistical Analysis
Data analyses were based on the three aims of the study which were to test how the effects adolescent alcohol and subsequent treatment with a OX2R antagonist (vehicle, 10 mg/kg, 20 mg/kg) significantly impacted (1) SWS and REM sleep patterns, (2) sleep EEG spectral characteristics and (3) waking EROs. For baseline differences in sleep patterns, Adolescent intermittent ethanol vapor (AIE) and control (CONT) animals were compared on SWS and REM for four measures (latency to first onset, number of episodes, mean duration of episodes, and fragmentation ratio) at baseline using one-way ANOVAs. Fragmentation ratio has been previously used as an overall measure of SWS or REM sleep quality by dividing the number of total episodes by the mean duration of episodes (Sanchez-Alavez et al., 2019). Subsequently, a 2 (AIE vs. CONT) X 3 (vehicle, MK-1064 low-dose 10 mg/kg, MK-1064 high-dose 20 mg/kg) ANOVA was used to determine therapeutic effect of the OX2R antagonist on the four sleep patterns for SWS and REM. Analysis of power in the EEG sleep frequency bands (delta, theta, beta) over all the SWS and REM sleep episodes in the frontal cortex (FCTX) and parietal cortex (PCTX) leads were evaluated for baseline differences between adolescent ethanol exposed and control animals (AIE vs. CONT; one-way ANOVA) and after OX2R antagonist treatment using a 2 (AIE vs. CONT) × 3 (vehicle, MK-1064 10 g/kg, MK-1064 20 mg/kg) ANOVA. For the ERO analyses, energy in the three time–frequency ROIs (delta, theta, beta) were compared in response to infrequent tone events, for the two leads (FCTX and PCTX) using 2 (AIE vs. CONT) × 3 (vehicle, MK-1064 10 mg/kg, MK-1064 20 mg/kg) ANOVAs. Post hoc analyses for group and/ or condition were used when significant main effects were found. Significance for all analyses was set at p < 0.05.
Results
Effects of adolescent ethanol exposure and OX2R antagonist treatment on sleep patterns
As depicted in Figure 1, adolescent ethanol vapor has a significant impact on SWS and REM sleep in female rats as measured by (1A) latency to first episode, (1B) number of episodes, (1C) mean duration of episodes, and (1D) fragmentation ratio (number of episodes/mean episode duration). During baseline testing, AIE rats exhibited significantly more episodes of SWS (77.71 ± 3.00) compared to CONT [62.75 ± 3.65; F(1, 29) = 9.701, p = 0.004] and significantly shorter mean duration of SWS episodes for AIE (93.54 ± 6.38 s) versus CONT [127.85 ± 8.38 s; F(1, 28) = 10.141, p = 0.004]. With decreases in duration of SWS episodes concomitant with an increase in the number of episodes resulted in a significant increase in SWS fragmentation for AIE rats (0.91 ± 0.10) compared to CONT [0.54 ± 0.06; F(1,29) = 11.07, p = 0.002]. While there were trends for shorter latency to first SWS episode in AIE (471.71 ± 104.16 s) compared to CONT (1166 ± 312.79 s), this difference was not significant [F (1, 29) = 3.959, p = 0.056]. For REM sleep patterns, AIE (48.00 ± 4.18) animals had significantly more episodes of REM compared to CONT [31.88 ± 3.41; F(1,28) = 9.091, p = 0.005]. This increase in REM episodes lead to an increase in REM sleep fragmentation for AIE (2.21 ± 0.21) compared to CONT [1.51 ± 0.14; F(1,29) = 8.06, p = 0.008]. There were no differences between groups on latency to first REM episode or mean duration of REM episodes.
Figure 1. Adolescent ethanol and OX2R antagonist treatment on sleep patterns.
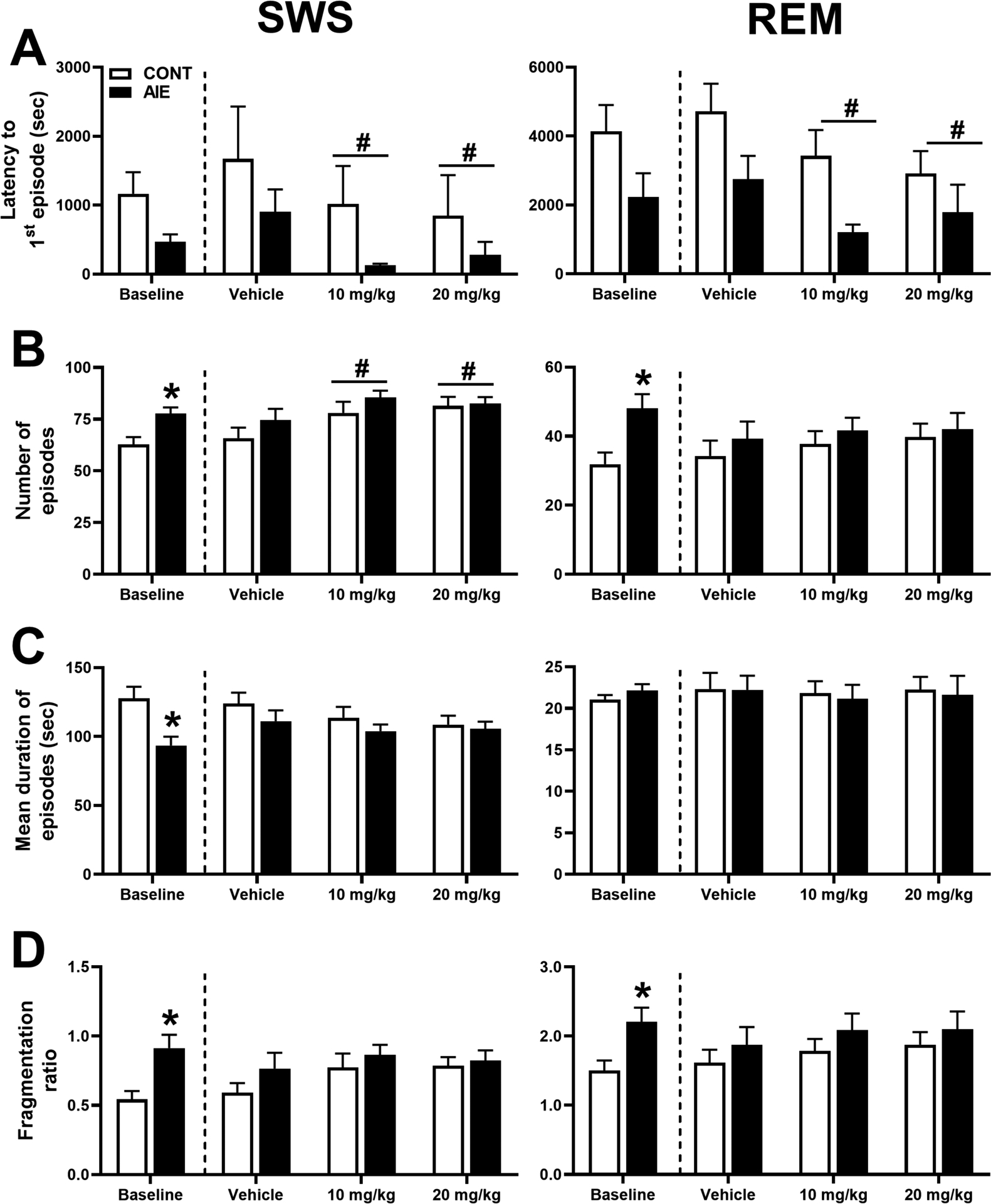
Adolescent ethanol vapor (AIE) had a significant impact on slow wave sleep (SWS; left column) and rapid eye movement sleep (REM; right column) in female rats as measured by (A) latency to first episode, (B) number of episodes, (C) mean duration of episodes, and (D) fragmentation ratio. During baseline testing, AIE rats (n=14) were compared to non-vapor control rats (CONT, n=16). Subsequently, AIE and CONT rats were acutely treated with OX-R2 antagonist (MK-1064; 10 mg/kg or 20 mg/kg) or vehicle prior to the 5-h sleep test. *p < 0.05 vs. CONT (group effect), #p < 0.05 vs. Vehicle (treatment effect). Error bars = SEM.
The OX2R antagonist, MK-1064, had a significant impact on latency to first SWS episode, [F(2, 27) = 6.915, p = 0.008], with quicker onset of SWS after MK-1064 10 mg/kg (571.50 ± 297.35 s) and 20 mg/kg (564.13 ± 328.12 s) treatment compared to vehicle controls (1233.55 ± 434.59 s). There was also a significant effect of treatment on the number of episodes [F(2,27) = 9.576, p = 0.001]. MK-1046 treatment resulted in more episodes after 10 mg/kg (81.75 ± 3.28) and 20 mg/kg (82.00 ± 2.75) compared to vehicle (70.26 ± 3.69). There were no differences in the mean duration of SWS episodes across treatments. Treatment also did not significantly impact SWS fragmentation ratio despite a significant difference in number of episodes. For REM sleep, there was a significant effect of onset to first REM episode [F(2,27) = 8.339, p = 0.001], with shorter latency after MK-1064 10 mg/kg (2316.39 ± 415.85 s) and 20 mg/kg (2351.55 ± 512.49 s) compared to vehicle (3732.25 ± 535.98 s). There were no other effects of OX2R treatment or ethanol exposure (AIE vs. CONT) on REM sleep patterns.
Effects of adolescent ethanol exposure and OX2R antagonist treatment on EEG sleep spectra
To determine whether adolescent ethanol exposure had a significant impact on EEG sleep spectral characteristics, baseline recordings for delta and theta power during SWS episodes (Figure 2) and theta and beta power during REM episodes (Figure 3) were analyzed in FCTX and PCTX leads. Evaluation of baseline SWS episodes revealed a significant effect of adolescent exposure (AIE vs. CONT) on power in delta [FCTX: F(1,28) = 10.776, p = 0.003; PCTX: F(1,28) = 24.324, p = 0.000] and theta frequencies [FCTX: F(1,28) = 7.326, p = 0.011; PCTX: F(1,28) = 14.872, p = 0.001],. AIE animals had lower delta (AIE: 704.32 ± 82.08; CONT: 1075 ± 77.54) and theta power (AIE: 735.08 ± 56.03; CONT: 993.91 ± 74.74) in the FCTX leads compared to controls, as well as lower delta (AIE: 985.88 ± 86.79; CONT: 1956.79 ± 167.47) and theta power(AIE: 667.38 ± 55.76; CONT: 1078.38 ± 86.81) in the PCTX leads compared to controls. Evaluation of baseline REM sleep episodes revealed a significant effect of adolescent exposure (AIE vs. CONT) on power of theta [FCTX: F(1,28) = 5.848, p = 0.022; PCTX: F(1,28) = 13.896, p = 0.001] and beta [PCTX: F(1,28) = 5.375, p = 0.028] frequencies. Specifically, lower theta power occurred in AIE rats during REM sleep in the FCTX (AIE: 704.32 ± 82.08; CONT: 1075 ± 77.54) and PCTX (AIE: 701.57 ± 59.08; CONT: 1099.50 ± 85.31) leads compared to CONT rats, as well as lower beta in the PCTX leads in AIE (243.99 ± 21.15) compared to CONT (342.63 ± 35.18).
Figure 2. Adolescent ethanol and OX2R antagonist treatment on EEG spectra during SWS episodes.
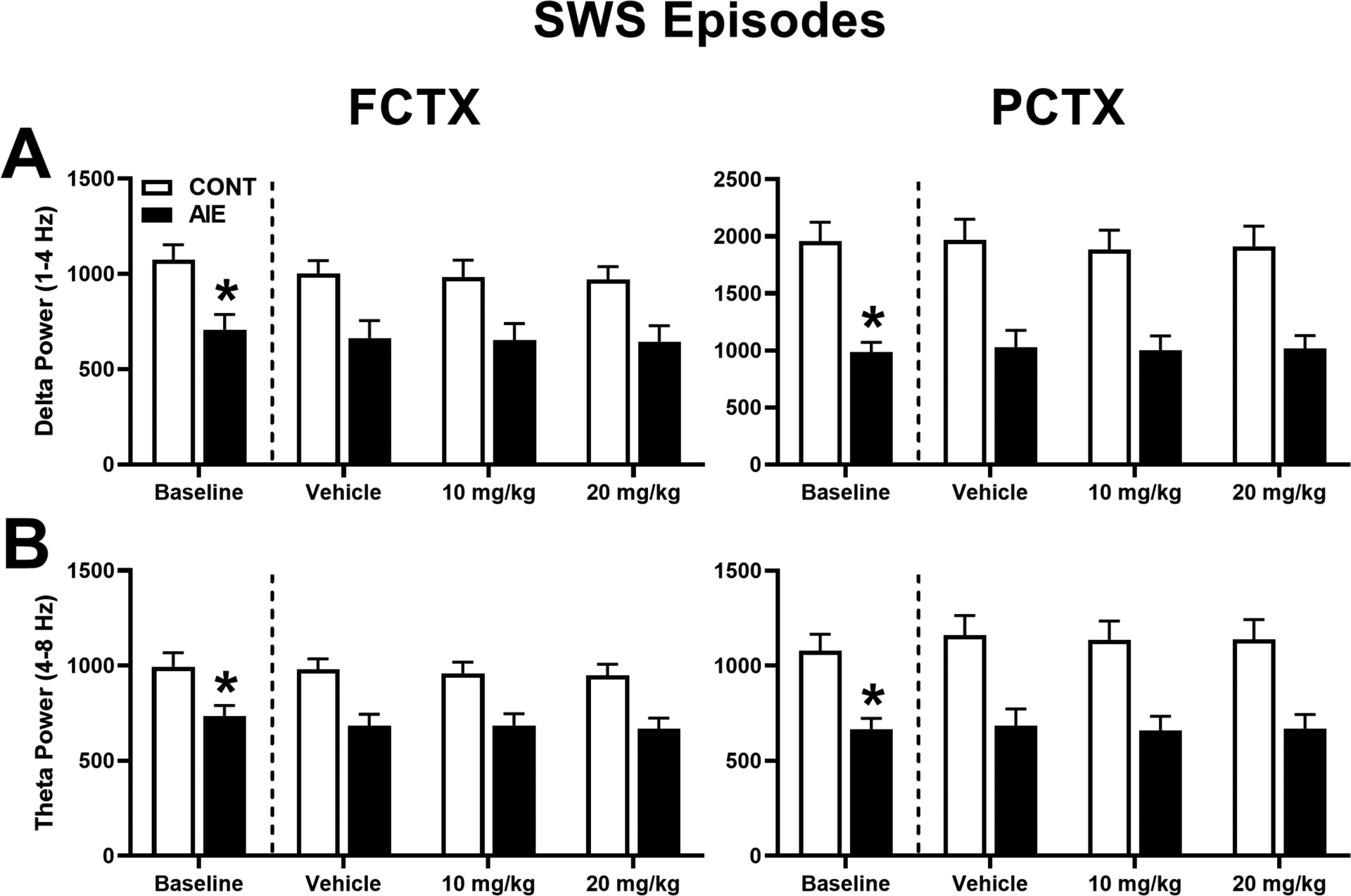
(A) Delta and (B) theta EEG power for all SWS episodes in frontal cortex (FCTX; left column) and parietal cortex (PCTX; right column). During baseline testing, AIE rats were compared to non-vapor control rats (CONT) for each of the bands. Subsequently, AIE and CONT rats were acutely treated with OX2R antagonist (MK-1064; 10 mg/kg or 20 mg/kg) or vehicle prior to the 5-h EEG sleep recordings. *p < 0.05 vs. CONT (group effect), #p < 0.05 vs. Vehicle (treatment effect). Error bars = SEM.
Figure 3. Adolescent ethanol and OX2R antagonist treatment on EEG spectra during REM episodes.
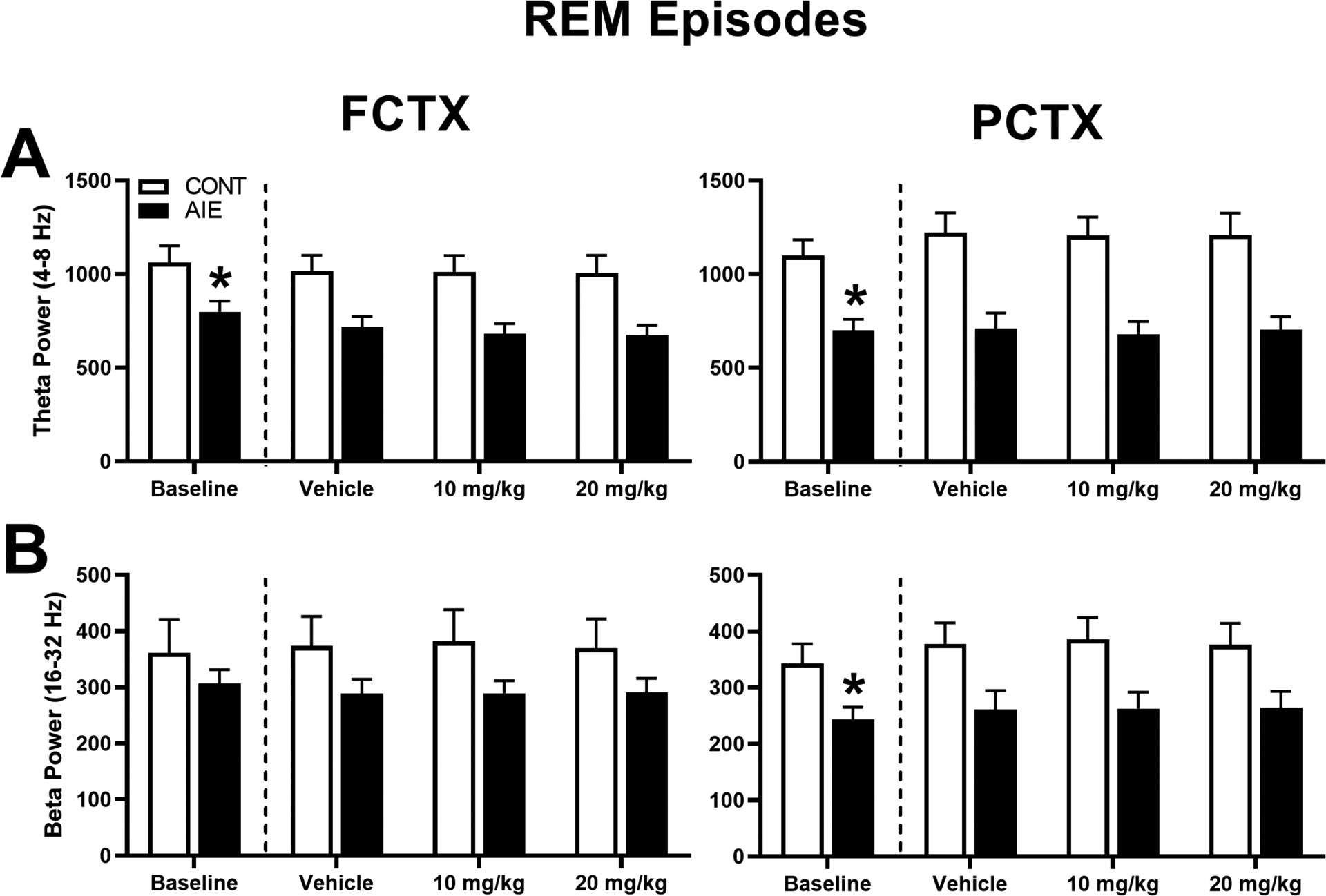
(A) Theta and (B) beta EEG power for all REM sleep episodes in frontal cortex (FCTX; left column) and parietal cortex (PCTX; right column). During baseline testing, AIE rats were compared to non-vapor control rats (CONT) for each of the bands. Subsequently, AIE and CONT rats were acutely treated with OX2R antagonist (MK-1064; 10 mg/kg or 20 mg/kg) or vehicle prior to the 5-h EEG sleep recordings. *p < 0.05 vs. CONT (group effect), #p < 0.05 vs. Vehicle (treatment effect). Error bars = SEM.
Animals were subsequently treated with OX2R antagonist, MK-1064 (10, 20 mg/kg) or vehicle at three different time points to determine therapeutic benefit on EEG spectra during SWS. Repeated measures ANOVA revealed a significant effect of adolescent exposure (AIE vs. CONT) on SWS spectral power for delta [FCTX: F (1,28) = 8.869, p = 0.006; PCTX: F(1,28) = 16.761, p = 0.000] and theta [FCTX: F (1,28) = 12.518, p = 0.001; PCTX: F(1,28) = 13.068, p = 0.001]. Post-hoc analyses revealed that ethanol-exposed rats had overall lower delta power in the FCTX (AIE: 653.60 ± 81.32; CONT: 985.22 ± 76.07) and lower theta power in the FCTX (AIE: 679.67 ± 58.47; CONT: 962.92 ± 54.69) and PCTX (AIE: 804.16 ± 117.30; CONT: 1222.46 ± 109.72) compared to controls. There was no significant effect of MK-1064 treatment or interaction (AIE x MK-1064 treatment) across any of the frequency bands for the two leads analyzed.
Spectral changes in REM sleep as a function of AIE and MK-1064 administration (vehicle, 10 mg/kg, or 20 mg/kg) were also determined for theta and beta frequency bands in the FCTX and PCTX leads. Repeated measures ANOVA revealed a significant effect of adolescent exposure (AIE vs. CONT) on REM spectral power for theta [FCTX: F (1,28) = 9.428, p = 0.005; PCTX: F(1,28) = 14.556, p = 0.001], and beta power [PCTX: F(1,28) = 5.270, p = 0.029]. Post-hoc analyses revealed that AIE rats had overall lower theta power in the FCTX (AIE: 509.41 ± 63.54; CONT: 731.27 ± 59.44) and PCTX (AIE: 856.17 ± 129.25; CONT: 1531.40 ± 120.90) compared to controls and lower beta power compared to CONT in the PCTX (AIE: 309.08 ± 45.59; CONT: 452.39 ± 42.65). There was no significant effect of MK-1064 treatment or interaction (AIE x MK-1064 treatment) across any of the frequency bands for the two leads analyzed.
Effects of adolescent ethanol exposure and OX2R antagonist treatment on waking EROs
To determine whether AIE had a significant impact on waking EEG as indexed by ERO energy, data were evaluated across adolescent exposure (AIE vs. CONT) in the FCTX and PCTX leads, for delta, theta, and beta time–frequency ROIs (Figure 4). During baseline sessions, there was no difference between adolescent exposure (AIE vs. CONT) groups in ERO energy for delta, theta, or beta frequency band, to the infrequent tone, in FCTX or PCTX.
Figure 4. Adolescent ethanol and OX2R antagonist treatment on waking EROs.

Event-related oscillation (ERO) energy in the (A) delta, (B) theta, and (C) beta time-frequency regions of interest (ROI) during waking in frontal cortex (FCTX; left column) and parietal cortex (PCTX; right column). Energy EROs were compared at baseline between AIE and CONT rats. Delta, theta, and beta EROs for AIE and CONT rats were compared at three different treatment timepoints for the two regions of interest: vehicle, MK-1064 10 mg/kg, or MK-1064 20 mg/kg. *p < 0.05 vs. CONT (group effect), #p < 0.05 vs. Vehicle (treatment effect). Error bars = SEM.
Subsequently, AIE and CONT animals were treated with OX2R antagonist (MK-1064 10mg/kg or 20 mg/kg) or vehicle and tested for changes in ERO activity. Repeated measures ANOVA revealed a significant effect of MK-1064 treatment for infrequent tones in delta frequency bands [F(2,27) = 8.994, p = 0.002], theta frequency bands [F(2,27) = 8.334, p = 0.002], and beta frequency bands [F(2,27) = 5.143, p = 0.015] for leads in the FCTX. Post hoc revealed significantly more delta energy in the FCTX after MK-1064 10 mg/kg (171.02 ± 16.87) and 20 mg/kg (214.54 ± 27.99) treatments when compared to vehicle (120.31 ± 7.68). There was also significantly more theta energy in the FCTX after MK-1064 10 mg/kg (325.47 ± 28.19) and 20 mg/kg (351.65 ± 42.01) treatments compared to vehicle (226.32 ± 13.66). Lastly, there was significantly more beta energy in the FCTX only after the higher dose of MK-1064 (20 mg/kg; 249.87 ± 38.37) compared to vehicle (151.61 ± 16.11).
For the PCTX, repeated measures ANOVA revealed a significant effect of treatment for infrequent tones in delta frequency bands [F(2,27) = 8.013, p = 0.002] and beta frequency bands [F(2,27) = 4.546, p = 0.021]. Post hoc revealed significantly more delta energy after MK-1064 10 mg/kg (202.27 ± 22.88) and 20 mg/kg (238.43 ± 26.63) treatment compared to vehicle (133.31 ± 12.76). There was also a significant increase in beta energy after only the higher dose of MK-1064 (20 mg/kg; 229.96 ± 26.18) treatment compared to vehicle (169.37 ± 19.08).
Effects of estrous cycle on sleep EEG and waking EROs
After each session, rats were examined for whether the estrous cycle (estrus vs. non-estrus) might significantly impact EEG recordings or sleep patterns. During baseline recordings sessions, rats determined to be in estrus (n= 8) took significantly longer to transition into SWS (1057.82 ± 232.96 s) compared to rats not in estrus (n= 22) [248.50 ± 50.01 s; F(1,28) = 4.268, p = 0.048]. Estrous cycle did not significantly impact any other measures of sleep in SWS or REM. Estrus also did not significantly impact any of the EEG spectral power frequencies for SWS or REM and had no impact on waking EROs as indexed by ERO energy. Overall, these data suggest that estrus has minimal effects on sleep patterns and no impact on the EEG sleep and waking ERO recordings as analyzed in this study.
Discussion
Adolescent ethanol exposure in female rats resulted in lasting changes in sleep. This was demonstrated by increased fragmentation of SWS and REM sleep, as well as reductions in delta and theta power during SWS and reduced beta and theta during REM sleep. There was no impact of adolescent ethanol on waking EROs for the frequency bands analyzed. Further, we investigated whether an acute OX2R antagonist, MK-1064, could reverse or alter the effect of adolescent alcohol exposure on sleep pathology and waking EROs. The results demonstrated that MK-1064 could reduce the latency to the first SWS episode and increase the number of SWS episodes without increasing fragmentation of sleep. Additionally, MK-1064 reduced the latency to first REM episode, however this might be a by-product of SWS shifting earlier in the sleep session. While treatment with MK-1064 did not impact sleep EEG spectra, waking ERO energy was increased in the delta, theta and beta frequency bands.
Chronic high-dose ethanol during adolescence was found to result in compromised sleep in adulthood. Female AIE rats exhibited substantially more fragmented sleep, with reductions in SWS duration together with an increase in the number of SWS episodes. Adolescent exposure also led to an increase in the number of REM occurrences and an increase in sleep fragmentation during REM. Previous research from our laboratory has demonstrated that 5-week AIE vapor in male rats produces a decrease in mean duration of SWS, an increase in the number of episodes, and an increase in total time in SWS (Criado et al., 2008; Ehlers et al., 2019). Poor sleep quality has also been exhibited in the clinical setting with young adults who report heavy binge drinking in adolescence (Ehlers et al., 2018b). While both men and women in the aforementioned study reported ethanol-induced sleep disturbances, women described more problems related to intermittently waking at night and habitually later bedtimes. It’s important to note that differences in sleep quality (increased number of episodes leading to fragmented sleep) in AIE compared to controls during baseline, were no longer visible during the vehicle treatment. We suspect that this behavioral effect is sensitive to repeated testing periods resulting from the within-subject design. Additionally, this could suggest that the effects of AIE on sleep are not permanent and with extended abstinence (> 1 month) we may begin to see recovery of normal sleep patterns. Further research is needed to better determine the impact of AIE on prolonged abstinence.
The current study also demonstrated a reduction in delta and theta power during SWS episodes in adulthood after 5-week AIE exposure in female rats. Similarly, male AIE rats showed the same reduction in SWS spectra power (Ehlers et al., 2018a). Reduced delta and theta power has also been repeatedly demonstrated in a clinical population with AUD (Colrain et al., 2009; Irwin et al., 2000). Specifically, women show the same general pattern of ethanol-induced change in SWS as men (Colrain et al., 2009). Further, our lab has consistently demonstrated that ethanol exposure in male adult rats alters SWS (Sanchez-Alavez, et al., 2018;2019) and reduces SWS spectral characteristics (Ehlers and Slawecki 2000) in a similar pattern to adolescent exposure. This demonstrates that alcohol can alter sleep both in adolescents and adults. Our results also reflected lower beta and theta power during REM sleep in AIE compared to controls which is consistent with reduced depth of sleep also seen in SWS.
The OX2R antagonist increased some measures of sleep by hastening SWS onset and increasing the number of episodes, without adversely impacting sleep fragmentation. However, blockade of OX2R did not ameliorate reductions in sleep EEG spectra as a result of ethanol exposure in adolescence. While dual OX receptor antagonist treatment after chronic ethanol vapor in rats has consistently demonstrated shortened latencies to SWS and REM sleep (Ehlers et al., 2019; Sanchez-Alavez et al., 2019), results are mixed when it comes to impact on sleep fragmentation. The newly developed DORA-12 has been shown to ameliorate SWS fragmentation resulting from 5-weeks of vapor ethanol in adolescence, whereas suvorexant exacerbated the sleep fragmentation in both controls and vapor ethanol exposed rats (Ehlers et al., 2019; Sanchez-Alavez et al., 2019). The affinity ratio for OX2R over OX1R is stronger in DORA-12 compared to suvorexant which may be leading to a more efficient treatment of ethanol-induced sleep pathology in preclinical trials (Dugovic et al., 2009; Winrow and Renger, 2014). Others have also demonstrated that OX2R antagonism (JNJ-10397049) can decrease the latency for persistent sleep and increase sleep duration in rats (Dugovic et al., 2009). Further, co-administration of the OX1R antagonist (SB-408124) with the OX2R antagonist greatly attenuated the sleep-promoting effects exhibited by the OX2R antagonist. Taken together, these results indicate that OX2R blockade is imperative for initiation and maintenance of sleep in preclinical models. In waking ERO activity, OX2R antagonist MK-1064 was found to increase energy in the delta and theta bands. This increases in ERO energy is an indication of increased arousal and has been repeatedly shown with other drugs approved or undergoing clinical trials for the treatment of ethanol-induced insomnia (Ehlers et al., 2019; Sanchez-Alavez et al., 2019, 2018).
Chronic ethanol exposure in adolescent rats can result in persistent changes in sleep pathology. Consistent with males, female rats also exhibit increases in sleep fragmentation and EEG spectral characteristics (Ehlers et al., 2019). These results do not seem to be mediated by estrous cycle as exhibited by minimal effects on sleep patterns and no impact on the EEG sleep or waking EROs. Despite our results, other have shown changes in SWS and REM sleep power spectra across the hormonal cycle (Swift et al., 2019). Thus, further work is needed to better elucidate the impact gonadal hormones might have on ethanol-induced insomnia.
This study demonstrates that AIE can produce lasting changes in female sleep patterns, highly similar to what we previously found in males. Additionally, while the OX2R antagonist promoted sleep in both AIE and control rats, it did not reverse the alcohol-induced disruptions in sleep. Thus, OX2R antagonism may serve as a potential therapeutic strategy for the treatment of insomnia, but not the specific signs of alcohol-induced insomnia. Acute MK-1064 has also been shown to promotes sleep onset and maintenance similarly in clinical populations by increasing both NREM and REM sleep (Gotter et al., 2016). Together, MK-1064 is sufficient to block the arousing effects of OX across mammalian species and may serve as a potential therapeutic strategy for the treatment of insomnia. However, selective OX2R antagonism alone does not appear to offer a measurable advantage for adolescent ethanol-induced insomnia over the dual orexin antagonist, DORA-12 (Ehlers et al., 2019).
Acknowledgments
This work was supported by National Institute of Health (NIH) grants, U01 AA019969; R01 AA006059 to Cindy L. Ehlers from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The authors thank Phil Lau for help in statistical analyses. James Havstad developed the software used for EEG and EROs assessments.
Footnotes
Conflicts of Interest
None
References
- Amodeo LR, Wills DN, Sanchez-Alavez M, Nguyen W, Conti B, Ehlers CL (2018) Intermittent voluntary ethanol consumption combined with ethanol vapor exposure during adolescence increases drinking and alters other behaviors in adulthood in female and male rats. Alcohol 73:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Rohsenow DJ, Almeida AB, Hunt SK, Gokhale M, Gottlieb DJ, Howland J (2011) Sleep Following Alcohol Intoxication in Healthy, Young Adults: Effects of Sex and Family History of Alcoholism. Alcohol Clin Exp Res 35:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschart C, Hintermann S, Behnke D, Cotesta S, Fendt M, Gee CE, Jacobson LH, Laue G, Ofner S, Chaudhari V, Badiger S, Pandit C, Wagner J, Hoyer D (2013) Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem 56:7590–7607. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Clay Williams S, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M (1999) Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 98:437–451. [DOI] [PubMed] [Google Scholar]
- Chow M, Cao M (2016) The hypocretin/orexin system in sleep disorders: Preclinical insights and clinical progress. Nat Sci Sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Turlington S, Baker FC (2009) Impact of alcoholism on sleep architecture and EEG power spectra in men and women. Sleep 32:1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL (2008) Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol 42:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lecea L, Sutcliffe JG (2005) The hypocretins and sleep. FEBS J. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI, Lovenberg TW (2009) Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther 330:142–51. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Yun S, Bonaventure P, Shireman BT, Lovenberg TW (2014) Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers C, Liu W, Wills DN, Crews FT (2013) Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience 244:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers C, Sanchez-Alavez M, Wills D (2018a) Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology (Berl) 235:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Benedict J, Wills D, Sanchez-Alavez M (2019) PSPH-D-18–00526: Effect of a dual orexin receptor antagonist (DORA-12) on sleep and event-related oscillations in rats exposed to ethanol vapor during adolescence. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT (2011) Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: Correlation with behavioral pathology. Neuroscience 199:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Desikan A, Wills DN (2014) Event-related potential responses to the acute and chronic effects of alcohol in adolescent and adult Wistar rats. Alcohol Clin Exp Res 38:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Havstad JW (1982) Characterization of drug effects on the EEG by power spectral band time series analysis. Psychopharmacol Bull 18:43–47. [Google Scholar]
- Ehlers CL, Wills D, Gilder DA (2018b) A history of binge drinking during adolescence is associated with poorer sleep quality in young adult Mexican Americans and American Indians. Psychopharmacology (Berl) 235:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Havstad J (2012) Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res 1450:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A, Karpyak VM (2015) Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend 156:1–13. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Sakhardande A, Blakemore S-J (2014) The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage Clin 5:420–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL (2007) The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res Part B - Dev Reprod Toxicol. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Forman MS, Harrell CM, Stevens J, Svetnik V, Yee KL, Li X, Roecker AJ, Fox SV., Tannenbaum PL, Garson SL, De Lepeleire I, Calder N, Rosen L, Struyk A, Coleman PJ, Herring WJ, Renger JJ, Winrow CJ (2016) Orexin 2 Receptor Antagonism is Sufficient to Promote NREM and REM Sleep from Mouse to Man. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP (2004) The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend 74:223–34. [DOI] [PubMed] [Google Scholar]
- Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, Krystal AD, Walsh JK, Benca RM, Rosenberg R, Sangal RB, Budd K, Hutzelmann J, Leibensperger H, Froman S, Lines C, Roth T, Michelson D (2016) Suvorexant in Patients with Insomnia: Results from Two 3-Month Randomized Controlled Clinical Trials. Biol Psychiatry 79:136–148. [DOI] [PubMed] [Google Scholar]
- Hommer DW (2003) Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health 27:181–5. [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Miller C, Christian Gillin J, Demodena A, Ehlers CL (2000) Polysomnographic and spectral sleep EEG in primary alcoholics: An interaction between alcohol dependence and African-American ethnicity. Alcohol Clin Exp Res 24:1376–1384. [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS (2008) Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend 93:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Okuda M, Hasin DS, Secades-Villa R, Keyes K, Lin K-H, Grant B, Blanco C (2013) Gender differences in lifetime alcohol dependence: results from the national epidemiologic survey on alcohol and related conditions. Alcohol Clin Exp Res 37:1696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang H-J, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW (2007) Neurobiology of REM and NREM sleep. Sleep Med 8:302–330. [DOI] [PubMed] [Google Scholar]
- Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T (2011) Differential roles of orexin receptor-1 and −2 in the regulation of non-REM and REM sleep. J Neurosci 31:6518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morairty SR, Revel FG, Malherbe P, Moreau JL, Valladao D, Wettstein JG, Kilduff TS, Borroni E (2012) Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang G-Q, Barson JR, Ye Z, Karatayev O, Leibowitz SF (2010) Differential Effects of Acute and Chronic Ethanol Exposure on Orexin Expression in the Perifornical Lateral Hypothalamus. Alcohol Clin Exp Res 34:886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2004) Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev 24:981–1010. [DOI] [PubMed] [Google Scholar]
- Ogeil RP, Cheetham A, Mooney A, Allen NB, Schwartz O, Byrne ML, Simmons JG, Whittle S, Lubman DI (2019) Early adolescent drinking and cannabis use predicts later sleep-quality problems. Psychol Addict Behav 33:266–273. [DOI] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE (2015) Binge-Like Consumption of Ethanol and Other Salient Reinforcers is Blocked by Orexin-1 Receptor Inhibition and Leads to a Reduction of Hypothalamic Orexin Immunoreactivity. Alcohol Clin Exp Res 39:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The Rat Brain: In Stereotaxic Coordinates (2nd edition). Sydney, Orlando: Academic Press. [Google Scholar]
- Sanchez-Alavez M, Benedict J, Wills DN, Ehlers CL (2019) Effect of suvorexant on event-related oscillations and EEG sleep in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal. Sleep 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Wills DN, Amodeo L, Ehlers CL (2018) Effect of Gabapentin on Sleep and Event-Related Oscillations (EROs) in Rats Exposed to Chronic Intermittent Ethanol Vapor and Protracted Withdrawal. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Beck A, Matthis C, Genauck A, Banaschewski T, Bokde ALW, Bromberg U, Büchel C, Quinlan EB, Flor H, Frouin V, Garavan H, Gowland P, Ittermann B, Martinot J-L, Paillère Martinot M-L, Nees F, Papadopoulos Orfanos D, Poustka L, Hohmann S, Fröhner JH, Smolka MN, Walter H, Whelan R, Desrivières S, Heinz A, Schumann G, Obermayer K (2019) Risk profiles for heavy drinking in adolescence: differential effects of gender. Addict Biol 24:787–801. [DOI] [PubMed] [Google Scholar]
- Slade T, Chapman C, Swift W, Keyes K, Tonks Z, Teesson M (2016) Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: Systematic review and metaregression. BMJ Open 6:e011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Ehlers CL (2005) Enhanced Prepulse Inhibition Following Adolescent Ethanol Exposure in Sprague-Dawley Rats. Alcohol Clin Exp Res 29:1829–1836. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF (2009) Initiating Moderate to Heavy Alcohol Use Predicts Changes in Neuropsychological Functioning for Adolescent Girls and Boys. Psychol Addict Behav 23:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Friedmann PD (2005) Disturbed sleep and its relationship to alcohol use. Subst Abus 26:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP (1996) Localization of the complex spectrum: The S transform. IEEE Trans Signal Process 44:998–1001. [Google Scholar]
- Swift KM, Keus K, Echeverria CG, Cabrera Y, Jimenez J, Holloway J, Clawson BC, Poe GR, Sex differences within sleep in gonadally intact rats. Sleep zsz289.Thakkar MM, Sharma R, Sahota P (2015) Alcohol disrupts sleep homeostasis. Alcohol 49:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Castle IJP, Chen CM, Shirley M, Roach D, Hingson R (2015) Converging Patterns of Alcohol Use and Related Outcomes Among Females and Males in the United States, 2002 to 2012. Alcohol Clin Exp Res 39:1712–1726. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Renger JJ (2014) Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]


