Abstract
Introduction:
Like other infectious diseases, it is expected that COVID-19 will mostly end with the development of neutralizing antibody immunity. This study aimed to evaluate the value of COVID-19 antibody rapid test assessment in emergency medical services (EMS) personnel.
Methods:
This cross-sectional study was conducted in Tehran, Iran from 20th March until 20th May 2020. The results of chest computed tomography (CT) scan, and antibody rapid test were compared in EMS personnel with confirmed COVID-19, as well as symptomatic and asymptomatic ones who had exposure to a probable/confirmed COVID-19 teammate. In symptomatic or asymptomatic individuals who were only IgM-positive, chest CT scan or RT-PCR was recommended.
Results:
A total of 243 EMS personnel with the mean age of 36.14±8.70 (range 21 to 59) years took part in this study (87.7% were males). Most of the participants (73.3%) had history of exposure. One hundred sixty-three EMS personnel were tested using either RT-PCR test or chest CT-scan or both, and 78 (47.9%) of them had at least one positive result. Among the participants who had undergone chest CT-scan and/or RT-PCR test (n=163), 78 had positive chest CT-scan and/or RT-PCR test; of these, 18 individuals had negative results for IgM and IgG. The rate of positive IgM and IgG in participants with positive chest CT-scan was 1.6 or 1.3 times more than those with negative chest CT-scan, respectively (p < 0.05). The percentage of positive results for both IgM and IgG in participants having positive RT-PCR test was 1.7 times more than those having negative RT-PCR test (p < 0.05).
Conclusion:
Rapid antibody test could help in diagnosis of COVID-19 in asymptomatic or symptomatic EMS personnel who did not undergo RT-PCR test or the test was reported as negative. However, its sensitivity could be enhanced through use along with other diagnostic methods.
Key Words: Antibodies, Clinical Laboratory Techniques, COVID-19, Reagent Kits, Diagnostic, Emergency Medical Services
Introduction
Coronavirus disease (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), became a global health concern. COVID-19 is a highly contagious and multifaceted disease, which has infected millions of people worldwide (1, 2). Reverse transcription polymerase chain reaction (RT-PCR) was mostly recommended and used in terms of diagnosis of COVID-19 (3-6). Some specific alterations in lung computed tomography (CT) scan was also highly frequent in COVID-19 patients but in some cases, normal lung CT-scan was reported along with positive RT-PCR (7). On the other hand, it should be mentioned that COVID-19 can cause widely different clinical manifestations, most of which are nonspecific, or be asymptomatic. Therefore, the prevalence of COVID-19 is mostly underestimated, which may increase the risk of exposure (8, 9).
Like other infectious diseases, it is expected that COVID-19 will mostly end with the development of neutralizing antibody immunity. Antibodies are produced within days to weeks after infection with the virus. However, negative results of antibodies in the patients with positive RT-PCR test have been reported for various reasons. The strength of the antibody response depends on various factors, including age, nutritional status, the severity of the disease, and certain medications or infections that suppress the immune system (8-12) .
The emergency medical services (EMS) personnel are at high risk of infection because of repeated exposures; so they are an appropriate population for the study on COVID-19 (13). It is vital to investigate the EMS personnel both in terms of their immune system and being a carrier for other health providers. Since due to the epidemic, it was not possible to perform definitive tests for all people, rapid antibody tests are the most appropriate option to investigate these cases, so this study was designed and conducted with the following four main objectives:
1. Assessing the immune system of emergency medical services personnel who were confirmed cases of COVID-19
2. Assessing the probability of infection and the immune system of emergency medical service personnel who were symptomatic but either had a negative result of COVID-19 test or were not tested
3. Assessing the probability of infection in asymptomatic personnel with a history of encountering a definite or probable COVID-19 case
4. Assessing the relationship between CT scan, RT-PCR, and epidemiological issues (symptoms, history of exposure) with the antibody test.
Methods
Study design and setting
This study was a cross-sectional study conducted in Tehran, Iran. The protocol of the study was approved by the ethics committee of Tehran University of Medical Science (Code: IR.TUMS.VCR.REC.1399.322) and the principles of confidentiality were adhered to. All information was analyzed and reported anonymously. Written informed consent was obtained from all patients prior to their participation in the study. This study did not impose any additional cost on participants or the healthcare system, and all costs were paid from the received grant.
Study population
From 20th March until 20th May 2020, two groups of EMS personnel working for Tehran EMS Center were invited for participation in this study. The EMS personnel with any of the below criteria were included:
Confirmed COVID-19 cases based on the results of RT-PCR test and/or non-enhanced chest CT-scan.
Those who had COVID-19 symptoms since the onset of the epidemic, and did not undergo any diagnostic test or whose test results were negative.
Asymptomatic ones who had exposure to suspected or confirmed COVID-19 teammates, and did not undergo diagnostic tests or whose test results were negative.
All EMS personnel who were unwilling to participate, refused to perform further required paraclinical investigation, and filled out the checklist incompletely, were excluded.
Definitions
Suspected, confirmed, and symptomatic cases were defined as follows (14-17):
Suspected case
A. A patient who has an acute respiratory illness (has fever and shows at least one sign and/or symptom of respiratory disease, such as cough and shortness of breath), and lives in or has traveled to a COVID-19 hotspot within 14 days before the onset of symptom.
OR
B. A patient who has an acute respiratory illness and has had a close encounter with a confirmed or suspected COVID-19 case within 14 days before the onset of symptom.
OR
C. A patient who has a severe acute respiratory illness (has fever and shows at least one sign and/or symptom of respiratory disease, such as cough and shortness of breath and needs to be hospitalized) when there are no alternative diagnoses that explain the clinical manifestations.
Confirmed case
A patient with positive result of laboratory test and/or chest CT-scan confirmed COVID-19, irrespective of clinical signs and symptoms.
Symptomatic case
A person with suspicious signs (based on physical examination) and symptoms of COVID-19 (defined as fever, chills, dry cough, shortness of breath, myalgia, diarrhea, loss of sense of smell and taste, chest pain, headache, weakness, or lethargy) who has not undergone any diagnostic tests yet.
Procedure
All participants underwent the COVID-19 IgM/IgG rapid test (manufactured by KarmaAzmaAndish Co. in Tehran, Iran). This rapid test is based on immune-chromatography, which is used to detect IgM and IgG antibodies in the blood and serum (total antibody). A drop of blood (about 20 microliters of serum), lancet, and fingertip are used for blood sampling and then two drops of the buffer are added to the blood. The result is determined in less than 20 minutes. Sampling and antibody testing were done outside the laboratory, by two of the investigators with a bachelor’s degree in nursing, in the Tehran EMS center.
For those who had only positive IgM, RT-PCR test and chest CT-scan were also performed, to investigate the probability of being an infectious carrier.
The COVID-19 RT-PCR test is a real-time test that can qualitatively assess the presence of nucleic acids associated with SARS-CoV-2 in samples (such as nasopharyngeal or oropharyngeal swabs, nasal swabs, or mid-turbinate swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate) obtained from both upper and lower respiratory systems of patients suspected to having COVID-19. This modality can be used both for all suspected patients, both those showing symptoms and those who do not have symptoms but have other reasons to suspect COVID-19 infection.
Data collection
All participants were asked to fill out a 3-part checklist. First part consisted of demographic data and baseline characteristics including age, gender, smoking status, recent history of weight loss, recent history of any infectious disease, history of any vaccination during last year, history of a disease in last five days so that patients were not able to eat anything.
Second part was about data related to COVID-19, including presentation of COVID-19 symptoms, history of exposure, having a contaminated teammate or roommate, undergoing related tests of COVID-19, number of exposures, time interval between symptom onset and positive RT-PCR test and/or chest CT-scan, and , time interval between symptom onset and positive result of antibody rapid test.
Third part was about the results of antibody rapid test (only IgM positive, only IgG positive, both positive, or both negative) and RT-PCR test or chest CT-scan results, if provided.
Statistical analysis
The continuous variables were described using mean ± SD and categorical variables were described using frequency and percentage. The normality of distribution was assessed using Shapiro-Wilks test. The relationship between categorical variables, such as comparing the result of tests, was examined using Chi-square or Fisher’s exact test. Also, we used independent t-test for assessment of mean difference between two groups. P-value<0.05 was considered statistically significant. The sensitivity and specificity of rapid antibody test with 95% confidence interval (CI) was calculated based-on RT-PCR test and chest CT-scan diagnosis, as gold standards. Also, accuracy, positive likelihood ratio (PLR), negative likelihood ratio (NLR), positive predictive value (PPV), and negative predictive value (NPV) were calculated for the screening index. The data were analyzed using Stata statistical software: release 14 (College Station, TX: StataCorp LP).
Results
Baseline characteristics of participants
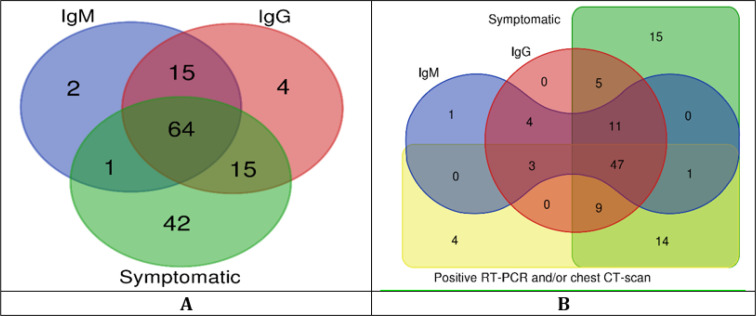
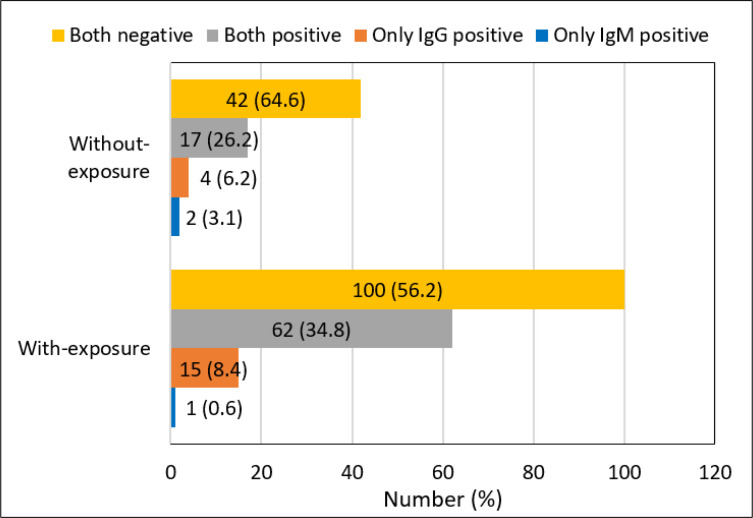
Two hundred forty-three EMS personnel with the mean age of 36.14±8.70 (range 21 to 59) years took part in this study. The baseline information is shown in table 1. The majority of the participants were male (87.7%) and most of the participants (73.3%) had history of exposure. Figure 1 and table 2 show the distribution of symptomatic and asymptomatic cases based on the results of different diagnostic tools. The frequency of positive result of rapid antibody test was higher in participants with history of exposure (43.8%) than those without history of exposure (35.4%) but the difference was not statistically significant (p=0.201) (Figure 2).
Table 1.
Baseline characteristics of participants (n=243)
| Variables | Values |
|---|---|
| Sex | |
| Male | 213 (87.7) |
| Female | 30 (12.3) |
| Body mass index | |
| Mean ± SD | 23.4±3.9 |
| History | |
| Smoking | 13 (5.3) |
| Recent weight loss | 51 (21.0) |
| Recent infectious disease | 21 (8.6) |
| Vaccination in the previous year | 48 (19.8) |
| Acute disease in the previous five days | 10 (4.1) |
| Exposure to COVID-19 patient | |
| Suspected | 151 (62.1) |
| Confirmed | 155 (63.8) |
| Having contaminated teammate or roommate | |
| Before getting infected | 15(6.2) |
| After getting infected | 16 (6.6) |
| Presentation | |
| Symptomatic | 122 (50.2) |
| Asymptomatic | 121 (49.8) |
| Chest CT-scan results | |
| Positive | 64 (42.9) |
| Negative | 85 (57.1) |
| RT-PCR test results (n=101) | |
| Positive | 38 (37.3) |
| Negative | 63 (63.7) |
| Rapid test results | |
| Only IgM positive | 3(1.2) |
| Only IgG positive | 19 (7.8) |
| IgM and IgG positive | 79 (32.5) |
| Time since symptom onset (day) | |
| Confirmation by the RT-PCR and/or chest CT-scan | 4.2±3.8 |
| Confirmation of COVID-19 by antibody rapid test | 50.6±18.4 |
Data are presented as mean ± standard deviation (SD) or frequency (%); CT: Computed Tomography; RT-PCR: Reverse Transcription Polymerase Chain Reaction; COVID-19: Coronavirus Disease.
Figure 1.
(A) Veen diagram of positive symptoms related to COVID-19 and/or positive result of antibody rapid test in all participants (n=243); (B) Veen diagram of positive symptoms related to COVID-19 and/or positive result of antibody rapid test and/or positive RT-PCR and/or chest CT-scan in patients in participants who underwent chest CT-scan and/or RT-PCR test (n=163)
Table 2.
The results of performed diagnostic COVID-19 tests between symptomatic and asymptomatic EMS personnel
| Test |
Participants
|
p-value * | |
|---|---|---|---|
| Symptomatic | Asymptomatic | ||
| Chest CT-scan | |||
| Positive | 60 (61.9) | 4 (7.7) | <0.001 |
| Negative | 37 (38.1) | 45 (92.3) | |
| RT-PCR test | |||
| Positive | 34 (50.0) | 4 (12.1) | 0.001 |
| Negative | 34 (50.0) | 29 (87.9) | |
| Antibody test | |||
| Only IgM positive | 1 (0.8) | 2 (1.7) | <0.001 |
| Only IgG positive | 15 (12.3) | 4 (3.3) | |
| Both positive | 64 (52.5) | 15 (12.4) | |
| Both negative | 42 (34.4) | 100(82.6) | |
Data are presented as frequency (%). *EMS: Emergency Medical Services; CT: Computed Tomography; RT-PCR: Reverse Transcription Polymerase Chain Reaction.
Figure 2.
The results of rapid antibody test based on history of exposure with suspected and/or confirmed case
Symptom onset to performing rapid test
The time interval between symptom onset and performing antibody rapid test had a wide range (2 to 83 days). The relationship between the time interval between symptom onset and performing antibody rapid test and the result of chest CT-scan and/or RT-PCR test are shown in table 3.
Table 3.
The relationship between the time interval between symptom onset and performing antibody rapid test and the result of chest CT-scan and/or RT-PCR test
| Time interval (day) |
Chest CT-scan and/or
RT-PCR test |
|
|---|---|---|
| Positive (n=69) | Negative (n=31) | |
| IgM result | ||
| Positive | 49.8±16.8 | 58.0±16.6 |
| Negative | 45.1±17.8 | 53.4±18.5 |
| P-value | 0.291 | 0.504 |
| IgG result | ||
| Positive | 50.1±15.7 | 54.3±16.3 |
| Negative | 41.0±20.9 | 55.3±19.7 |
| P-value | 0.074 | 0.952 |
Data are presented as mean ± standard deviation. CT: Computed Tomography; RT-PCR: Reverse Transcription Polymerase Chain Reaction
Relationship between COVID-19 diagnostic tools
Table 4 shows the relationship between the results of performed diagnostic COVID-19 tests. The rate of positive IgM and IgG in participants with positive chest CT-scan were 1.6 or 1.3 times more than that of those with negative chest CT-scan, respectively. The rate of IgM and/ or IgG positive in participants with positive chest CT-scan was 2.5 times higher than those with negative chest CT-scan (p<0.001). The percentage of positive result of both IgM and IgG in participants having positive RT-PCR test was 1.7 times more than those having negative RT-PCR test (p=0.019). The sensitivity and specificity of rapid antibody test compared with chest CT-scan was 78.1% (95% CI: 66.0 to 87.5) and 68.2% (95% CI: 57.2 to 77.9), respectively. Also, the PPV, NPV, PLR, NLR, and accuracy of rapid antibody test compared with chest CT-scan were 64.9%, 80.6%, 2.5, 0.32, and 72.5%, respectively. The sensitivity and specificity of rapid antibody test compared with RT-PCR test were 71.1% (95% CI: 54.1 to 84.6) and 58.7% (95% CI: 45.6 to 71.0), respectively. Also, the PPV, NPV, PLR, NLR, and accuracy of rapid antibody test compared with RT-PCR were 50.9%, 77.1%, 1.7, 0.49, and 63.4%, respectively (Table 5).
Table 4.
The relationship between the results of rapid antibody test with performed diagnostic COVID-19 tests
| Rapid test |
Chest CT-scan
|
RT-PCR test
|
||||
|---|---|---|---|---|---|---|
| Positive (n=64) | Negative (n=85) | p | Positive (n=38) | Negative (n=63) | p | |
| IgM positive | 1(1.6) | 0 (0.0) | <0.001 | 0 (0.0) | 1 (1.6) | 0.019 |
| IgG positive | 7(10.9) | 7 (8.2) | 6 (15.8) | 4 (6.3) | ||
| Both positive | 42(65.6) | 20 (23.5) | 21 (55.3) | 21 (33.3) | ||
| Both negative | 14(21.9) | 58 (68.2) | 11 (28.9) | 37 (58.7) | ||
Note: Data are presented as frequency (%).
Table 5.
Screening performance characteristics of rapid test based on chest CT-scan and RT-PCR test as gold standards
| Variable |
Chest CT-scan
|
RT-PCR
|
|---|---|---|
| Value (95%CI) | Value (95%CI) | |
| Accuracy | 72.5 (65.2 - 79.7) | 63.4 (53.8 - 72.9) |
| Sensitivity | 78.1 (66.0 - 87.5) | 71.1 (54.1 - 84.6) |
| Specificity | 68.2 (57.2 - 77.9) | 58.7 (45.6 - 71.0) |
| Positive likelihood ratio | 2.5 (1.8 - 3.4) | 1.7 (1.2 - 2.5) |
| Negative likelihood ratio | 0.32 (0.2 - 0.5) | 0.49 (0.3 - 0.8) |
| Positive predictive value | 64.9 (53.2 - 75.5) | 50.9 (36.8 - 64.9) |
| Negative predictive value | 80.6 (69.5 - 88.9) | 77.1 (62.7 - 88.0) |
CT: Computed tomography; RT-PCR: Reverse Transcription Polymerase Chain Reaction; CI: Confidence interval.
Discussion
The present study investigated some aspects of paraclinical features of COVID-19 in EMS personnel. The majority of participants were symptomatic and had history of exposure. The positive result of all three tests was higher in symptomatic participants. Being symptomatic was most concordant with the positive result of chest CT-scan and then with the positive result of RT-PCR test or rapid antibody test. A few number of asymptomatic EMS personnel had positive rapid antibody test. The positive result of IgM and/or IgG was significantly higher in participants having positive chest CT-scan. The percentage of positive result of IgG or both IgM and IgG was significantly higher in participants having positive RT-PCR test. The result of rapid antibody test was more concordant with the result of chest CT-scan than with the result of RT-PCR test.
Various studies stated that measuring the level of antibodies is of great value in diagnosing COVID-19 (18, 19). The level of IgM and IgG were increased in the early and late phase of COVID-19, respectively. Antibody rapid test can be a useful tool for measuring the level of antibodies. Investigating the trend of changes in the level of antibodies can help in clinical evaluating of infection (8, 20, 21). In a study of 15 COVID-19 patients, the positive IgM and IgG increased from 50% to 81% and 81% to 100% of patients, respectively, within the first to the fifth day after symptom onset (22). Long et al. conducted a study on 285 patients, investigating the acute antibody responses to SARS-CoV-2, and showed that the prevalence of positive virus-specific IgG (within 17-19 days after symptom onset) and IgM (within 20-22 days after symptom onset) were 100% and 94.1%, respectively (20). Sun et al. conducted a study assessing the antibodies in 38 COVID-19 patients and the results showed that up to 75% of patients had increased levels of IgM and IgG, specific to SARS-CoV-2, in the first week after symptom onset (18). The positive results were higher compared to ours, which may be due to performing a more accurate antibody test in the study of Sun et al., which measured the level of IgM and IgG response against both SARS-CoV-2 nucleocapsid and spike protein.
Our study showed a long interval between symptom onset and positive result of IgM, which was higher than other studies (20, 23). The reason may be due to the higher number of exposures in EMS personnel.
Our study showed that all three diagnostic tests had high efficacy in detecting COVID-19 infection. In a study by Zhao et al. conducted on 173 COVID-19 patients, the sensitivity of IgM, IgG, and RT-PCR test were 73.3%, 54.1%, and 54%, respectively during 15 days after symptom onset (21).
Heydari et al., showed a high proportion (83%) of positive chest CT-scan in diagnosing COVID-19 in symptomatic patients (24). However, some studies recommended performing the combination of PCR and antibody test for diagnosing the COVID-19 patients (19, 21, 22, 25) Guo et al. stated that the rate of diagnosis of COVID-19 with combination of IgM and PCR test (98.6%) was higher than PCR test alone (51.9%) (19).
Our study stated that adding rapid antibody test decreased false negative cases, especially in asymptomatic ones. In this regard, a study in Italy showed that 44% of confirmed cases of COVID-19 based on laboratory tests, were asymptomatic (26). Some studies had also added serological tests, especially in asymptomatic patients or those with negative RT-PCR tests to increase the accuracy of COVID-19 detection (19, 20, 22). In this regard, Guo et al. conducted a study on 82 confirmed cases (based on positive quantitative PCR) and 58 probable cases (symptomatic patients with negative quantitative PCR) of COVID-19. They measured the level of IgM and IgG and showed that IgM and IgG were positive in 93.3% and 77.9% of samples, respectively. The positive result of IgM was observed in 75.6% and 93.1% of confirmed and probable cases, respectively (19). The lower positive results of antibodies in our study may be due to the shorter period of our study.
The stronger association between the results of rapid antibody test and CT-scan compared to the association between rapid antibody test and RT-PCR test may be due to the incorrect sampling of RT-PCR test, different sample types, using low quality and low consistent diagnostic tools, or untimely performance of the tests because the tests are mostly time sensitive (9, 21). Early diagnosis of COVID-19 in EMS personnel is of paramount importance to avoid spread of disease, especially to high-risk patients who receive the most common services of EMS. Therefore, it is highly recommended to use symptoms and signs, rapid antibody test and other diagnostic methods for ruling out COVID-19 and proper training of the EMS personnel is also of great value. In this study, eighteen cases whose COVID-19 had been confirmed in the previous two months had negative antibody test results. In this regard, the possibility of reinfection with COVID-19 should be considered.
Limitations
Given that the antibody assessment tests of the participants in this study were performed at different time intervals from the onset of their disease, this can affect the accuracy of the tests. Some of the participants with positive result of IgM on their antibody rapid test did not undergo RT-PCR test or ELISA test against medical advice. The interval between performing chest CT-scan and other tests was not consistent among the cases. Considering the impossibility of performing serial antibody tests from the first days of the onset of symptoms or exposure, the time since which immunological tests became positive was not clear. The kit of antibody rapid test was not officially approved. To date, there is no approved rapid antibody test in Iran and all kits are in the testing phase. However, the rapid tests used in this project have been reviewed in several reputable centers and its sensitivity and specificity have been evaluated as acceptable; but there was no official authorization for these tests. It is better to carry out a study with a large sample size on different populations and also with approved tests and kits in future studies.
Conclusions
Rapid antibody test could help in diagnosis of COVID-19 in asymptomatic EMS personnel. The positive result of rapid antibody test was more concordant with positive result of chest CT-scan than with positive result of RT-PCR test. Our study showed that being symptomatic was most concordant with the positive result of chest CT-scan and then with the positive result of RT-PCR test or rapid antibody test.
Acknowledgments
We would like to express our gratitude to the Pre-hospital and Hospital Emergency Research Center affiliated to Tehran University of Medical Sciences.
Authors’ contribution
The conception and design of the work by PS, PHS, SMM and AB; Data acquisition by PHS and MJ; Analysis and interpretation of data by PHS, SB, EA and AB; Drafting the work by SB, EA and MJ; Revising it critically for important intellectual content by PS, PHS, SMM and AB; All the authors approved the final version to be published; AND agree to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work will be answered.
Competing Interests
None declared.
Funding
This study was funded with a grant received from Tehran EMS Center.
References
- 1.Kalkeri R, Goebel S, Sharma GD. SARS-CoV-2 Shedding from Asymptomatic Patients: Contribution of Potential Extrapulmonary Tissue Reservoirs. The American journal of tropical medicine and hygiene. 2020;103(1):18–21. doi: 10.4269/ajtmh.20-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vahidi E, Jalili M. Why COVID-19? Advanced Journal of Emergency Medicine. 2020;4(2s) [Google Scholar]
- 3.Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Annals of clinical microbiology and antimicrobials. 2020;19(1):18. doi: 10.1186/s12941-020-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, Bergwelt-Baildon MV, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. The Journal of allergy and clinical immunology. 2020;146(1):128–36. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Annals of laboratory medicine. 2020;40(5):351–60. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou H, Zhang B, Huang H, Luo Y, Wu S, Tang G, et al. Using IL-2R/lymphocytes for predicting the clinical progression of patients with COVID-19. Clinical and experimental immunology. 2020;201(1):76–84. doi: 10.1111/cei.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Zhang Z, Chen Y, Long Q, Tian W, Deng H, et al. The clinical and immunological features of pediatric COVID-19 patients in China. Genes & diseases. 2020 doi: 10.1016/j.gendis.2020.03.008. doi: 10.1016/j.gendis.2020.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azkur A, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen M, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020 doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García LF. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Frontiers in immunology. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal transduction and targeted therapy. 2020;5(1):84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong D, de Man S, Lindeboom F, Koeleman J. Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected coronavirus disease 2019 presenting to the hospital. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020;26(8):1094. doi: 10.1016/j.cmi.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paces J, Strizova Z, Smrz D, Cerny J. COVID-19 and the immune system. Physiological research. 2020;69(3):379–88. doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalili M. How Should Emergency Medical Services Personnel Protect Themselves and the Patients During COVID-19 Pandemic? Adv J Emerg Med. 2020;4(2s):e37. [Google Scholar]
- 14.Ontario. Case Definition – Novel Coronavirus (COVID-19) 2020. [Available from: http://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_case_definition.pdf]
- 15.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 61 2020. [Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200321-sitrep-61-covid-19.pdf?sfvrsn=ce5ca11c_2]
- 16.European Centre for Disease Prevention and Control. Case definition for coronavirus disease 2019 (COVID-19), as of 29 May 2020 2020. [Available from: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition]
- 17.Centers for Disease Control and Prevention. Public Health Guidance for Community-Related Exposure 2020. [Available from: https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html]
- 18.Sun B, Feng Y, Mo X, Zheng P, Wang Q, Li P, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerging microbes & infections. 2020;9(1):940–8. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19) Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020:ciaa310. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long Q, Liu B, Deng H, Wu G, Deng K, Chen Y, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine. 2020;26(6):845–8. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020:ciaa344. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerging microbes & infections. 2020;9(1):386–9. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman T, Nissen K, Krambrich J, Rönnberg B, Akaberi D, Esmaeilzadeh M, et al. Evaluation of a COVID-19 IgM and IgG rapid test; an efficient tool for assessment of past exposure to SARS-CoV-2. Infection Ecology & Epidemiology. 2020;10(1):1754538. doi: 10.1080/20008686.2020.1754538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heydari K, Rismantab S, Shamshirian A, Lotfi P, Shadmehri N, Houshmand P, et al. Clinical and Paraclinical Characteristics of COVID-19 patients: A systematic review and meta-analysis. medRxiv : the preprint server for health sciences. 2020 [Google Scholar]
- 25.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. The Lancet Infectious diseases. 2020;20(5):565–74. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.european Centre for Disease Prevention and Control. Novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – sixth update 2020 [Available from: European Centre for Disease Prevention and Control (ECDC) Novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – sixth update [Internet] Stockholm, Sweden; 2020. [Available from: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-sixth-pdateOutbreak-of-novel-coronavirus-disease-2019-COVID-19.pdf]