Supplemental Digital Content is available in the text.
Keywords: angiotensin II, hypertension, hypoxia, NMDA recetor, neural pathways
Abstract
Central infusion of Ang II (angiotensin II) has been associated with increased sympathetic outflow resulting in neurogenic hypertension. In the present study, we appraised whether the chronic increase in central Ang II activates the paraventricular nucleus of the hypothalamus (PVN) resulting in elevated sympathetic tone and altered baro- and chemoreflexes. Further, we evaluated the contribution of HIF-1α (hypoxia-inducible factor-1α), a transcription factor involved in enhancing the expression of N-methyl-D-aspartate receptors and thus glutamatergic-mediated sympathetic tone from the PVN. Ang II infusion (20 ng/minute, intracerebroventricular, 14 days) increased mean arterial pressure (126±9 versus 84±4 mm Hg), cardiac sympathetic tone (96±7 versus 75±6 bpm), and decreased cardiac parasympathetic tone (16±2 versus 36±3 versus bpm) compared with saline-infused controls in conscious rats. The Ang II-infused group also showed an impaired baroreflex control of heart rate (−1.50±0.1 versus −2.50±0.3 bpm/mm Hg), potentiation of the chemoreflex pressor response (53±7 versus 30±7 mm Hg) and increased number of FosB-labeled cells (53±3 versus 19±4) in the PVN. Concomitant with the activation of the PVN, there was an increased expression of HIF-1α and N-Methyl-D-Aspartate-type1 receptors in the PVN. Further, Ang II-infusion showed increased renal sympathetic nerve activity (20.5±2.3% versus 6.4±1.9% of Max) and 3-fold enhanced renal sympathetic nerve activity responses to microinjection of N-methyl-D-aspartate (200 pmol) into the PVN of anesthetized rats. Further, silencing of HIF-1α in NG108 cells abrogated the expression of N-methyl-D-aspartate-N-methyl-D-aspartate-type1 induced by Ang II. Taken together, our studies suggest a novel Ang II-HIF-1α-N-methyl-D-aspartate receptor-mediated activation of preautonomic neurons in the PVN, resulting in increased sympathetic outflow and alterations in baro- and chemoreflexes.
Hypertension is an independent risk factor for cardiovascular disease affecting ≈80% of the elderly population (65 years and older). As per the Heart Disease and Stroke Statistics, 2019, about ≈116.4 million American adults (accounts for ≈46%) have hypertension, which, if left untreated, may result in heart failure, renal failure, and/or stroke as well as multiple organ damage. The central nervous system plays an essential role in the onset and development of hypertension. More importantly, hypertensive patients and animal models of hypertension have increased sympathetic activity, accompanied by alterations in body fluid homeostasis.1,2 In the brain, the paraventricular nucleus of the hypothalamus (PVN) plays an essential role in regulating sympathetic drive, arterial blood pressure (BP), and body fluid homeostasis.3,4 The PVN plays an important role in integrating signals/inputs from circumventricular organs as well as other brain areas involved in the regulation of the cardiovascular system and generating an integrated output to the rostral ventrolateral medulla and the intermediolateral cell column in the spinal cord to influence overall sympathoexcitation.5 Studies have shown that the PVN is a critical brain region involved in the development and maintenance of hypertension in several animal models, including Ang II (angiotensin II)-induced hypertension,6,7 obesity-related hypertension,8 deoxycorticosterone acetate salt-sensitive hypertension,9 and spontaneously hypertensive rats.10–12 However, the underlying molecular mechanisms driving the preautonomic neurons in the PVN in hypertension are not fully elucidated.
Previous studies have demonstrated that activation of the central renin-angiotensin system and the subsequent increase in neuronal excitability in the PVN are intimately involved in the pathogenesis of cardiovascular diseases including hypertension and chronic heart failure (CHF).13–16 To identify novel therapeutic targets for hypertension, it is critical to understand the precise intraneuronal signaling mechanisms contributing to Ang II-induced neuronal excitability leading to increased sympathoexcitation. The glutamatergic input, especially NMDAR (N-methyl-D-aspartate [NMDA] receptor) in the PVN, is a significant source for the excitatory drive to the preautonomic neurons, which in turn increase sympathetic outflow.17,18 The NMDAR is composed of 2 GluN1 (structural subunit: NMDA-type1 [NR1]) and typically 2 GluN2 (regulatory subunit: NR2) subunits and are located at cell-cell contact sites and mediate a major component of excitatory neurotransmission in the central nervous system.19 Both presynaptic and postsynaptic NMDAR activity is potentiated in the PVN of spontaneously hypertensive rats and has been shown to contribute to the hyperactivity of preautonomic neurons in the PVN.18,20 Recently, we have shown a mechanistic link between HIF-1α (hypoxia-inducible factor-1α), a transcription factor, and NMDA-NR1 subunit in the PVN to elicit sympathoexcitation in rats with CHF.21 Further, it also showed that the silencing of HIF-1α within the PVN normalized the increased basal sympathetic tone and the sympathoexcitatory responses to NMDA microinjected into the PVN.21 However, the factor(s) that trigger and maintain HIF-1α activation in the PVN remain unknown. Although HIF-1α expression is tightly regulated by the oxygen tension,22 other nonhypoxic stimuli have also been implicated in elevating HIF-1α protein levels. It has been shown that Ang II induces HIF-1α in the absence of a hypoxic condition.23,24 Therefore, it is conceivable that increased levels of Ang II may contribute to the enhanced expression of HIF-1α. A large body of evidence strongly supports the notion of an important role for a central action of Ang II in the sympathoexcitatory process during hypertension7,25 and CHF.14,15,26,27 Further, the blockade of Ang II- type I receptor with losartan produced a greater reduction in neuronal activity in the PVN28 and in sympathetic nerve activity27 suggesting that enhanced Ang II-type I receptor expression in the PVN may be contributing to the altered sympathetic tone. Ang II and Ang II-type I receptor are also involved in the regulation of NMDA-NR1 levels since treatment with losartan normalizes the increased NMDA-NR1 expression in the PVN of rats with CHF.17 Similarly, NMDA-NR1 expression is increased in neuronal cell cultures treated with Ang II.17 Although the increased levels of Ang II27 and HIF-1α21 in the PVN has been associated with the increased NMDA-NR1 expression and sympathetic outflow, the direct relationship between Ang II and HIF-1α expression remains to be explored.
Therefore, the present study was conducted to evaluate the overall hypothesis that elevated central levels of Ang II would cause the augmentation of HIF-1α expression in the PVN, which in turn contributes to the exaggerated glutamatergic tone to the preautonomic neurons leading to enhanced sympathetic outflow, altered baro- and chemoreflexes, and subsequent hypertension.
Methods
The authors declare that all supporting data and detailed methods are available in the Data Supplement.
Animal Experiments
Adult male Sprague-Dawley rats were housed according to approved guidelines of the University of Nebraska Medical Center Institutional Animal Care and Use Committee (IACUC protocol No. 14-036-07-FC). Rats with intracerebroventricular infusion of Ang II (20 ng/minute, 0.5 µL/hour for 14 days) were used as a model of neurogenic hypertension as described.29 The dose of Ang II was based on previous studies showing the central action of Ang II.30–32 The baroreflex control of heart rate (HR) was evaluated by measuring the reflex changes in HR in response to transient increases or decreases in MAP (mm Hg) induced by bolus injections of phenylephrine (2.5–25 µg/mL in 0.1 mL, IV) or sodium nitroprusside (5–50 µg/mL in 0.1 mL, IV), respectively, as described previously.33 The peripheral chemoreflex was activated by intravenous injection of potassium cyanide (KCN, 200–800 µg/mL in 0.1 mL) in accordance with the procedures described previously by Franchini and Krieger.34 The cardiac autonomic tone was determined by pharmacological blockade of either the sympathetic or parasympathetic tone to the heart in 2 sequential days in a reversed order as described.35 The left femoral artery was cannulated to record MAP and HR and RSNA in response to NMDA microinjection in the PVN as extensively described previously36–38 (see Data Supplement for details).
Other Measurements and Analyses
Six serial coronal sections (100 µm) of the PVN were cut following the Palkovit and Brownstein39 and bilaterally punched as described previously.36,37 Methods for cell culture and molecular biology experiments were extensively used previously,15,21,29 and described in detail in the Data Supplement.
Statistical Analysis
All data are expressed as means±SEM. Statistical analysis was performed using the Prism 8; GraphPad Software. Statistical significance was assessed by t test (with and without Welch correction), 1- or 2-way ANOVA followed by Bonferroni test for post hoc analysis of significance, which is reported for each experiment in the figure legends. P<0.05 was considered to indicate statistical significance.
Results
Cardiovascular and Autonomic Effects of Chronic Central Infusion of Ang II
As expected, in conscious freely moving rats central infusion of Ang II induced an immediate and sustained increase in MAP with a peak increase of ≈40 mm Hg relative to the baseline or control, saline-infused group (Figure 1A) without significant changes in HR relative to baseline. However, HR was significantly increased relative to the saline-infused group in the late period (days 8–12). The initial starting basal MAP and HR were statistically not different between saline and Ang II groups on the first day of infusion. At the end of 14 days of infusion, there were significant increases in basal RSNA (24±7% versus 7±4% of max activity), MAP (101±3 versus 78±4 mm Hg), and HR (382±13 versus 271±12 bpm), in rats infused with Ang II compared with the saline-infused control group in anesthetized condition (Figure S1 in the Data Supplement). The number of cells in the PVN that stained positive for FosB, a marker of chronic neuronal activation, was increased in the Ang II group compared with the saline group (53±3 versus 19±4 cells; Figure 1B and 1C). Further, Ang II-infused rats also had higher cardiac sympathetic tone (97±6 beats/minute; Figure 1D) compared with the saline group (75±6 beats/minute) accompanied by a lower parasympathetic drive (22±5 versus 36±3 beats/minute in saline-infused rats; Figure 1E) to the heart. The intrinsic heart rate was considered to be the HR after the complete cardiac autonomic blockade and was calculated as the average of the values recorded on the first and second day. Intrinsic heart rate was not different between Saline and Ang II groups (335±4 versus 337±4 beats/minute). The balance between cardiac sympathetic and parasympathetic tone, evaluated by the ratio between these variables, was almost 2.5× higher in Ang II-infused rats (Figure 1F), showing that Ang II infusion leads to an overwhelming cardiac autonomic imbalance favoring tachycardia.
Figure 1.
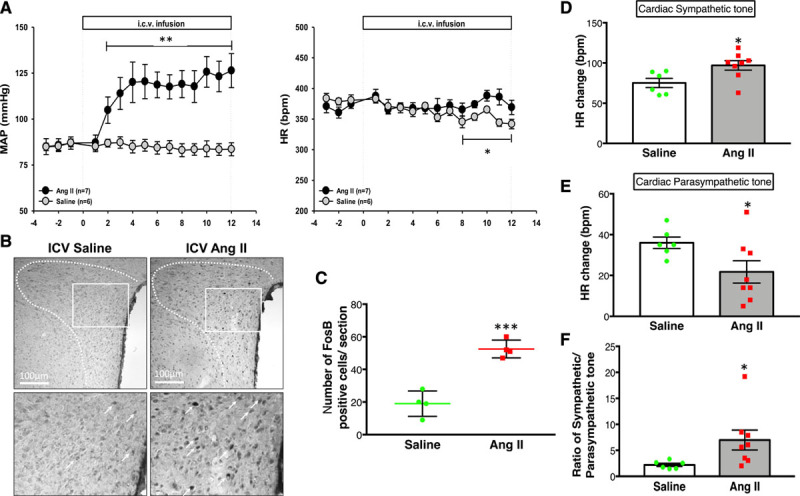
Hemodynamic, neural and autonomic changes to Ang II. A, Mean arterial pressure (MAP) and heart rate (HR) measured by telemetry throughout the experiment in intracerebroventricular (ICV), saline (n=6), and Ang II (angiotensin II; n=7) infused rats. B, Representative digital images of FosB (Proto-Oncogene) staining in saline- and Ang II-infused rats. Each pair of saline- and Ang II-infused images were adjusted for uniform brightness and contrast; scale bar=100 μm, (C) the mean number of FosB-positive cells in the paraventricular nucleus of the hypothalamus. n=3–4/group. D, Cardiac sympathetic tone and (E) cardiac parasympathetic tone calculated by the changes in HR responses to methylatropine and propranolol, and (F) the ratio between these variables in the Saline (n=6) and Ang II (n=7) infused rats. T-test (with Welch correction; A, C, D, E, and F). **P=0.004, *P=0.048 (A); ***P=0.0007 (C); *P=0.021 (D); *P<0.042 (E); *P=0.042 (F) vs saline control.
Alterations in Baro- and Chemoreflexes Induced by Central Infusion of Ang II
As shown in Figure 2A, Ang II-infused rats presented significant reduction in the sensitivity of the baroreflex tachycardia (−3.18±0.25 bpm/mm Hg) and baroreflex bradycardia (−1.50±0.15 bpm/mm Hg) compared with saline-infused group (−5.14±0.33 and −2.50±0.35 bpm/mm Hg). The best fit line that correlates the changes in HR and changes in MAP (Figure 2B) indicates a blunted slope during both tachycardia and bradycardia stages of the baroreflex, indicative of impairment in the baroreflex control of HR in the Ang II group. In contrast, the pressor response of the chemoreflex induced by KCN 80 µg was significantly enhanced in the Ang II group compared with the saline group (53±7 versus 30±9 mm Hg, respectively; Figure 2C). There was no significant difference in the bradycardic response of the chemoreflex between the Ang II and saline groups (Figure 2C).
Figure 2.
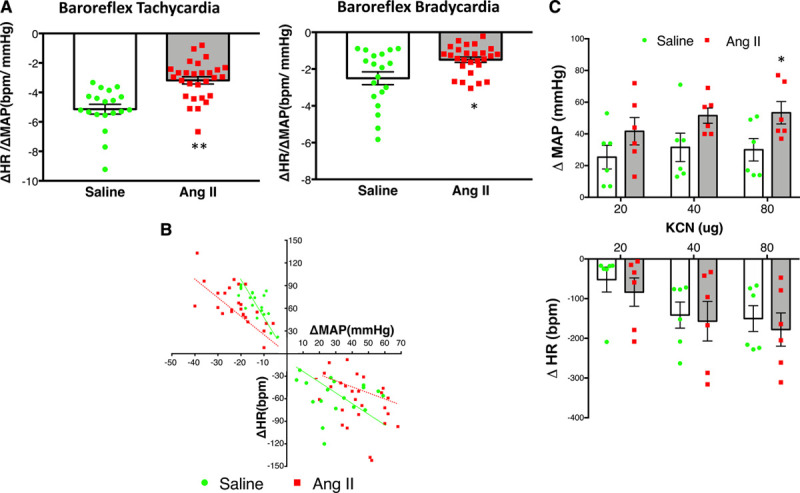
Changes in Baroreflex and chemoreflex evaluation after Ang II. A, Baroreflex sensitivity evaluated by tachycardiac and bradycardiac responses to changes in mean arterial pressure (MAP) induced by graded doses of sodium nitroprusside (0.5–5 µg, IV) or phenylephrine (0.25–2.5 µg, IE), respectively and expressed as the average of the ratio of the maximal changes in heart rate (HR) to a given change in MAP in saline (n=6) and Ang II (angiotensin II)-infused (n=8) rats. Statistical significance was assessed by t test (with Welch correction), **P=0.001,*P=0.014. B, Baroreflex sensitivity expressed as the best fit line that correlates change in HR vs change in MAP in Saline and Ang II-infused rats. Pearson correlation coefficient (r) were significant for tachycardia (saline, −0.74; Ang II, −0.54) and bradycardia (Ang II, −0.44) stages of the baroreflex. C, Changes in MAP and HR in response to potassium cyanide (KCN, 20–80 µg, IV) in saline and Ang II rats. Two-way ANOVA showed a significant effect of Ang II. *P=0.040 for ΔMAP, KCN 80 µg Ang II vs saline control (C).
Alteration in the Expression of HIF-1α in the PVN After Central Infusion of Ang II
Real-time polymerase chain reaction (RT-PCR) indicated that HIF-1α mRNA expression in the PVN from the Ang II group was significantly increased compared with the saline group (2.79±0.40 versus 1.01±0.09-fold changes; Figure 3A). Consistent with increased mRNA, HIF-1α protein levels were also significantly higher in the PVN from rats in Ang II compared with the saline group (0.32±0.01 versus 0.21±0.02; Figure 3C). There were no significant differences in HIF-1α mRNA or protein expression in the cerebral cortex obtained from the same brain sections used for PVN assessment between Ang II and saline groups (Figure 3B and 3D). These results were further confirmed by greater immunofluorescence staining in the PVN sections from Ang II-infused rats compared with saline-infused rats (Figure 3E and 3F). There were no significant differences in immunofluorescence staining in the cerebral cortex (from the same sections) between the Ang II and saline groups (data not shown).
Figure 3.
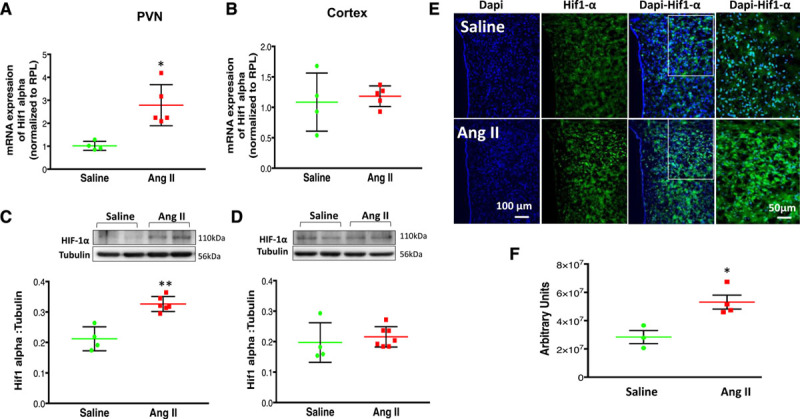
Changes in HIF-1α (hypoxia-inducible factor-1α) after Ang II. A, HIF-1α mRNA expression relative to rpl19 (ribosomal protein L 19) measured by real-time PCR in the paraventricular nucleus of the hypothalamus (PVN) and (B) cortex of vehicle and Ang II (angiotensin II)-infused group. The 2−ΔΔCT method was used to calculate relative changes in gene expression, which relates the expression of the target gene to the expression of a reference gene. Changes in HIF-1α mRNA are presented in folds with reference to the Saline group. C, Protein expression of HIF-1α expression in the PVN and (D) cortex of saline and Ang II-infused group top: a representative Western blot, bottom: densitometric analyses of HIF-1α level normalized to tubulin. E, Immunofluorescent staining of PVN sections from saline and Ang II-infused rats. HIF-1α (green) and nuclei (blue). F, Average values obtained by the reciprocal intensity method in each group were shown. Values are mean±SEM of analyses of 4 to 6 animals in each group. T-test (with Welch correction [A–D], and [F]). *P=0.010 (A); P=0.0721 (B); **P=0.004 (C); P=0.0626 (D); *P=0.016 (F) vs saline control.
Alteration in the Expression of NMDA-NR1 in the PVN After Central Infusion of Ang II
The chronic infusion of Ang II also increased NMDA-NR1 mRNA levels (2.78±0.40-fold change) when compared with saline-infused rats (1.01±0.10-fold change; Figure 4A). Corroborating these data, Figure 4B shows that protein expression of NMDA-NR1 was also significantly higher in the PVN from rats infused with Ang II (0.79±0.03-fold change) compared with saline-infused rats (0.53±0.08-fold change). In contrast to these results, NMDA-NR1 mRNA and protein levels within the cerebral cortex obtained from the same coronal sections from which the PVN was punched showed no significant differences between Ang II and Saline groups (Figure 4C and 4D).
Figure 4.
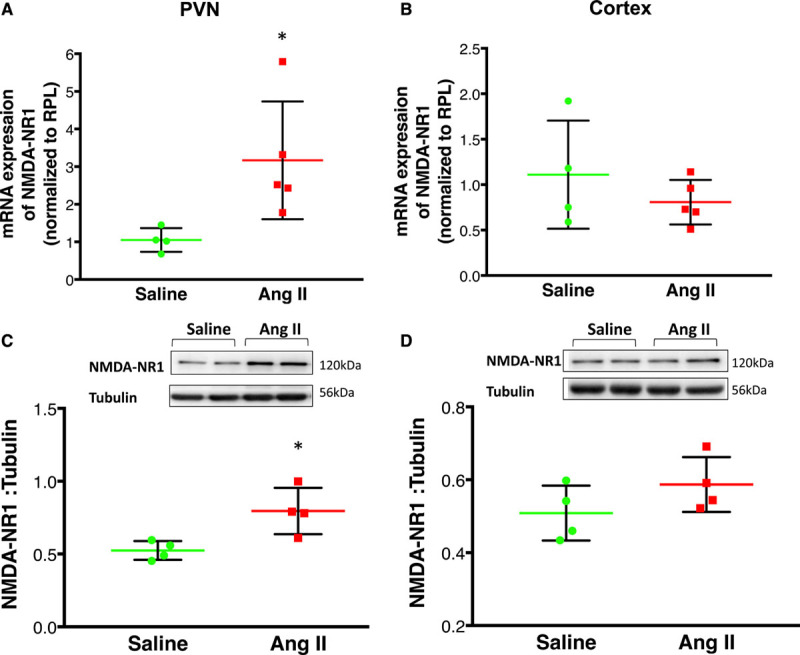
Changes in expression of NMDA-NR1 (N-methyl-D-aspartate NR1 receptor) after Ang II infusion. A, NMDA-NR1 mRNA expression relative to rpl19 (ribosomal protein L 19) measured by real-time polymerase chain reaction in the (A) paraventricular nucleus of the hypothalamus (PVN) and (B) cortex of saline and Ang II-infused group. Changes in NMDA-NR1 mRNA are presented in folds with reference to the Saline group. C, Protein expression of NMDA-NR1 expression in the PVN (C) and (D) cortex of saline and Ang II-infused group top: a representative Western blot, bottom: densitometric analyses of NMDA-NR1 level normalized to tubulin. Values are mean±SEM (n=4–6 rats per group). T-test with Welch correction (A–D). *P=0.037 (A); P=0.396 (B); *P=0.035 (C); P=0.189 (D) vs saline control.
Alteration of MAP, HR, and RSNA Responses to NMDA Administration Into the PVN After Central Infusion of Ang II
Raw tracings of the RSNA, MAP, and HR responses to the administration of NMDA (200 pmol) into the PVN in the 2 groups of rats (saline and Ang II) are shown in Figure 5A. As shown in Figure 5B, microinjections of NMDA (50, 100, and 200 pmol) elicited increases in RSNA, MAP, and HR in a dose-dependent manner. The vehicle control (100 nL artificial cerebrospinal fluid) microinjected into the PVN had no effects on RSNA, MAP, and HR. The RSNA for the highest dose of NMDA (200 pmol) was significantly elevated in the Ang II-infused rats compared with saline-infused rats (73±16% versus 22±4%) suggesting potentiated glutamatergic activation within the PVN of rats infused with Ang II.
Figure 5.
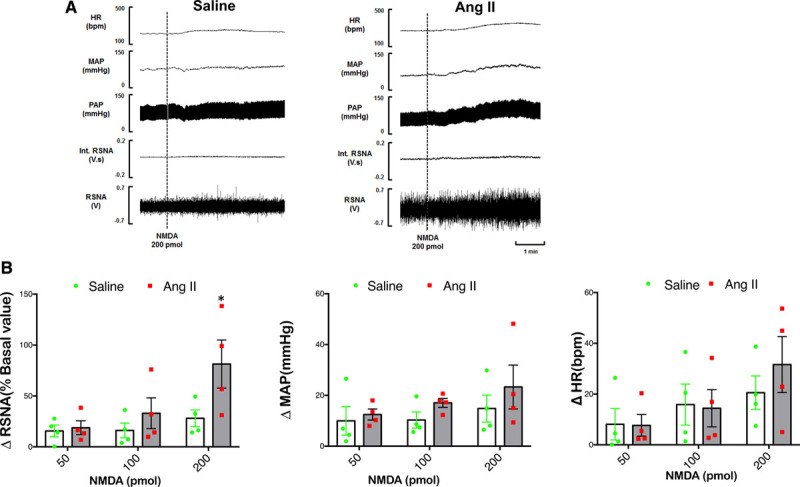
Reponses to NMDA (N-methyl-D-aspartate) in the PVN. Renal sympathetic nerve activity (RSNA), mean arterial pressure (MAP), and heart rate (HR) responses to N-methyl-D-aspartate (NMDA) injected into the PVN. A, A segment of an original recording from an individual rat demonstrating the baseline parameters and peak changes in RSNA, integrated RSNA, MAP, and HR by the administration of 200 pmol of NMDA into the PVN in the 2 groups. B, Mean data of changes in RSNA, MAP, and HR following different doses of NMDA (50–200 pmol) of NMDA into the PVN of Saline and Ang II (angiotensin II) infused rats. Two-way ANOVA revealed significant interaction between effect of Ang II and dose of NMDA (P=0.031). *P=0.026 for ΔRSNA following 200 pmol of NMDA into the PVN vs saline control.
Alterations in the Expression of HIF-1α and NMDA-NR1 in Neuronal Cells (In Vitro) to Direct Application of Ang II
To evaluate if Ang II-induced changes in the expression of NMDA-NR1 is mediated through HIF-1α, we first treated NG108-15 neuronal cells40 with different concentrations of Ang II and then assessed the changes in HIF-1α expression by Western Blot analysis after 8 hours. As shown in Figure 6A, treatment with Ang II (1 µmol/L) resulted in significantly increased levels of HIF-1α protein (0.53±0.04 versus 0.81±0.03 control). This augmentation was confirmed by immunofluorescence data (Figure 6B) since Ang II-treated cells exhibited an intense HIF-1α immunoreactivity when compared with nontreated control cells. The treatment of the NG108-15 cells with Ang II, besides increasing the HIF-1α expression, also induces an increase in the expression of the NMDA-NR1 as shown previously.17 Confirming these previous findings, we also demonstrate that the immunofluorescence staining for NMDA-NR1 in NG108-15 cells was higher after 8 hours of Ang II treatment (Figure 6B). Considering that (1) Ang II treatment induced an increase in both HIF-1α and NMDA-NR1 expression and (2) our previous data showing an upregulation of NMDA-NR1 induced by hypoxia is mediated by HIF-1α,21 we tested the hypothesis that expression of HIF-1α contributes to the upregulation of NMDA-NR1 induced by Ang II-dependent mechanism, using siRNA-mediated targeting of HIF-1α. Silencing of HIF-1α (≈60%) with siRNA in NG108-15 cells (Figure 6C and 6D) leads to a significant decrease in expression of NMDA-NR1 induced by Ang II compared with scrambled siRNA control (0.61±0.06 versus 0.80±0.04; Figure 6E).
Figure 6.
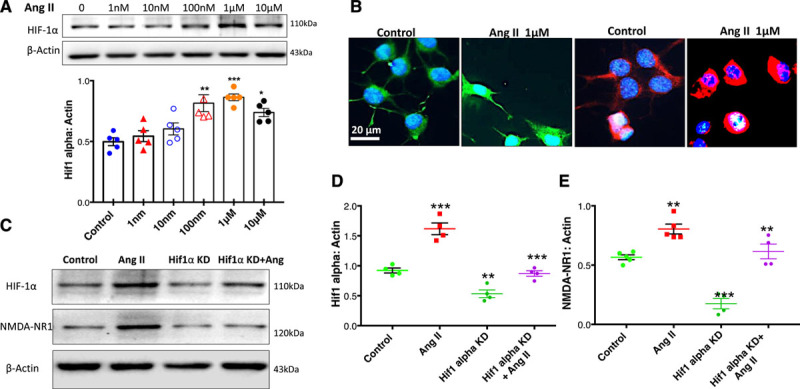
Changes in expression of HIF-1α (hypoxia-inducible factor-1α) and NMDA-NR1 (N-methyl-D-aspartate NR1 receptor) after Ang II (angiotensin II) in NG108 cells. A, Western blot to assess levels of HIF-1α protein after treatment with different doses of Ang II. Top: a representative Western blot, bottom: densitometry analyses of HIF-1α level normalized to Actin, T-test (with Welch correction). **P=0.006 vs Control, ***P<0.0001 vs Control, *P=0.010 vs Control. B, Immunofluorescent staining for HIF-1α and NMDAR (N-methyl-D-aspartate [NMDA] receptor)-NMDA-type1 (NR1) after 8 h of Ang II treatment in NG108 cells. NMDA-NR1 (red), HIF-1α (green), and Nuclei (blue). C, Western blot of HIF-1α and NMDAR-NR1 expression after siRNA HIF-1α (8 pmol) knockdown (KD) and Ang II, 1 μM stimulation. D, Cumulative data of HIF-1α expression. Two-way ANOVA revealed significant interaction between effect of Ang II and HIF-1α KD (P=0.019). ***P=0.0001 Ang II vs Control, **P=0.008 HIF-1α KD vs Control, ***P=0.0001 Ang II+HIF-1α KD vs Ang II and (E) cumulative data of NMDA-NR1 expression. Two-way ANOVA revealed significant interaction between effect of Ang II and HIF-1α KD (P=0.032). **P=0.005 Ang II vs Control, ***P=0.0001 HIF-1α KD vs Control, **P=0.004 Ang II+ HIF-1α KD vs Ang II. Values were mean±SE from 4 to 6 independent experiments.
Discussion
The sympathetic nervous system and renin-angiotensin system are critically involved in the development and maintenance of hypertension. In the present study, central Ang II infusion produced a blunted baroreflex with a concomitant exaggerated chemoreflex suggesting a centrally mediated autonomic imbalance, leading to enhanced basal RSNA and an increase in arterial BP. Concurrently, central Ang II infusion increased the number of FosB-labeled cells in the PVN, suggesting activation of preautonomic neurons. In tandem, there was an increased expression of HIF-1α, and NMDA-NR1 subunit in the PVN of rats infused with central Ang II. These molecular changes within the PVN are likely intimately related to the autonomic imbalance. As a corollary, the direct application of Ang II induced an increase in the expression of HIF-1α and NMDA-NR1 proteins via AT1 receptors in a neuronal cell line (NG108-15), in vitro. Further, silencing HIF-1α decreases the expression of NMDA-NR1 induced by Ang II in NG108 cells. Taken together, our studies indicate that increased levels of Ang II centrally contribute to the upregulation of HIF-1α expression leading to stabilization of the HIF1 transcription complex. The HIF1 transcription factors bind to hypoxia response elements (HREs) to increase the expression of NMDA-NR1, which leads to potentiation of glutamate activated of preautonomic neurons in the PVN, resulting in a hyper sympathetic tone leading to neurogenic hypertension.
We acknowledge that there are several technical limitations associated with punching the PVN specifically, and semiquantitative immunofluorescence staining including signal fading and cross-reactivity of secondary antibodies, among others. We have standardized all procedures overtime to specifically punch areas within the PVN as evident from our previous publications.15,21,29,36,37 It is also recognized that the PVN has a myriad of different neuronal phenotypes but the specific focus of our interest was on the preautonomic neurons activated by glutamate. Future studies, targeting glutamatergic neurons specifically in the PVN using optogenetics technique would strengthen our conclusions. For immunofluorescence and immunohistochemistry, we aimed at minimizing, to the best of our capabilities, limitations by experimenting with control and experimental group at the same time, validation of antibody specificity, and the use of sequential laser scanning a to avoid cross talk of fluorescent signals. Still, we cannot completely rule out that any of these factors affected our analysis. Further, the present study was completed using only male Ang II-infused rats. However, growing evidence indicates that the pathophysiology of hypertension and its development differs significantly between the sexes.41,42 Whether similar mechanisms within the PVN are involved in female rats infused with Ang II remain to be explored.43 Last, it is well recognized that basal level of RSNA measurement between different animals is wrought with multiple concerns as well outlined previously.44 In the present study, we attempted to normalize basal RSNA by expressing it as percentage of maximum activity elicited by temporary occlusion of the trachea. It should be noted that there is no significant difference between the maximum RSNA between the Ang II and saline groups. Furthermore, it should be noted that our interpretations and conclusions are not based on a single technique but rather on the combination of complementary approaches including immunohistochemistry, Western blotting, RT-PCR, and whole animal and in vitro studies.
The hypothalamic PVN is identified and well documented to be an essential site for cardiovascular regulation.5,45 An imbalance between excitatory (Ang II and glutamate) and inhibitory (nitric oxide and gamma aminobutyric acid) mechanisms within the PVN neurons can alter the sympathetic tone in pathological conditions associated with increased sympathoexcitation such as hypertension7,25 and CHF.14,26,27,46 Further, the increased activation of glutamate receptors in the PVN has been demonstrated in multiple models of hypertension resulting in an increased sympathetic drive in neurogenic hypertension.47 Recently, the contribution of the glutamatergic neuronal population in the PVN during the development and maintenance of hypertension has been demonstrated by photoactivation of PVN neurons in conscious mice leading to a frequency-dependent increase in arterial pressure while lesioning of glutamatergic neurons in the PVN shows attenuated hypertension under deoxycorticosterone acetate-salt treatment.9 Altered activity of the PVN has been documented to alter baroreflex48,49 as well as chemoreflex50,51 functions. In this study, we observed that activation of the PVN, evinced by an increase in FosB, was correlated to altered baro-and chemoreflexes. There also seems to be an increase overall sympathetic tone but a reduction of parasympathetic tone indicative of an overall autonomic imbalance toward a sympathoexcitatory state of central origin leading to hypertension.
The NMDA receptor plasticity in PVN neurons significantly contributes to the elevated arterial BP mediated by central Ang II since GluN1 deletion in PVN neurons attenuates the Ang II-induced increases in arterial BP. This further suggests the importance of NMDARs within the PVN involved in regulating the overall level of arterial BP.52 Previously, we demonstrated an increase in glutamatergic activity via upregulation of the NMDA-NR1 subunit of the NMDA receptor, to play an essential role in the enhanced sympathoexcitation in another hyper-sympathetic state, the congestive heart failure condition.36 Further, chronic intermittent hypoxia leads to an increase in cytoplasmic density of NMDA-NR1 and a reduction in the surface/synaptic targeting of NMDA-NR1 with decreases in NMDAR-mediated currents in the PVN before the development of hypertension.53 The molecular mechanism responsible for these alterations of NMDA-NR1 in these different pathologies is not entirely unclear. Being an obligatory subunit of NMDAR, the molecular mechanisms of NMDA-NR1 subunit regulation are fundamental to receptor function. Therefore, the present study was undertaken to investigate the Ang II-mediated regulatory mechanism of NMDA-NR1 expression.
Herein, we show that there was an increased expression of HIF-1α, a transcriptional regulator of NMDA-NR1 within the PVN of centrally Ang II-infused rats. This increase in NMDA-NR1 expression was shown to be functionally effective as there was an increase in NMDA-mediated-sympathoexcitation in Ang II-infused rats compared with saline-infused rats. At the cellular level, in vitro studies showed that stimulation of neuronal cells with Ang II caused an upregulation of HIF-1α as well as NMDA-NR1 subunit. Further, silencing HIF-1α in neuronal cells decreased the expression of the NMDA-NR1 subunit after Ang II treatment suggesting that NMDA-NR1 upregulation is in part mediated by HIF-1α. Consistent with these observations, we have previously observed that knockdown of HIF-1α with microinjection of HIF-1α siRNA within the PVN abrogated the enhanced glutamatergic sympathetic tone in rats with CHF,21 a pathological state associated with activation of renin-angiotensin system and enhanced NMDA-NR1 expression in the PVN. This suggests that perhaps minimal subtle changes in blood flow to areas of the brain that are highly vascularized, such as the PVN, during the pathological condition (CHF) could induce activation of renin-angiotensin system and hence Ang II in the PVN leading to enhanced tonic effects of glutamatergic activation of preautonomic neurons within the PVN via HIF-1 to cause enhanced overall sympathetic activity.
The molecular mechanism proposed in this study implies that the HIF-1 transcription complex may function as a central signal in the upregulation of the NMDA-NR1 in the PVN in Ang II-mediated hypertension. The NMDA-NR1 promoter sequence (NCBI accession no. NM017010) has 2 HREs elements, designated as HRE1 and HRE254 containing the core sequence 5′-RCGTG-3′ located between −473 and −356 bp upstream of the translation start site of the GluN1 gene and function as putative HIF-1 transcription factor-binding sites. Our previous studies using chromatin immunoprecipitation assay confirmed HIF-1 binding to HREs elements of GluN1,21 contributing to transcriptional upregulation of NMDA-NR1. In the present studies, we observed significant increases in the HIF-1α expression after central Ang II infusion along with an increase in both sympathoexcitation and arterial BP, which leads us to speculate that perhaps the increase in central Ang II during pathological conditions plays an important role in HIF-1α-mediated increased sympathetic outflow. Ang II has been shown to induce expression of HIF-1α23 and NMDA-NR1 subunit17 in cultured NG108 cells similar to what we observed in present studies. Perhaps increased levels of Ang II in cardiovascular diseases such as hypertension and heart failure may be a contributory factor for the stabilization of HIF-1 and hence increased NMDAR-mediated sympathoexcitation. However, in vitro studies using Ang II stimulation after HIF-1α silencing was not able to restore the NMDAR-NR1 levels to the basal control level, which suggests that perhaps Ang II-HIF-1α is not the only mechanism for the regulation of NMDA-NRI. The in silico analysis of NMDA-NR1 promoter along with DNA-protein binding assay identified binding sites of Activator protein 1, Sp1 Transcription Factor, nuclear factor-κB, Early growth response protein 1, and cAMP response element-binding protein.55 The role of these transcriptional factors downstream of Ang II mediated signaling mechanisms regulating NMDA-NR1 transcription remains to be determined.
Perspectives
In conclusion, our study provides new information that augmented glutamatergic activation via stabilization HIF-1α in the PVN presympathetic neurons plays a pivotal role in the elevated sympathetic vasomotor tone in neurogenic hypertension induced by central Ang II. Pharmacological and gene therapy strategies designed to modulate HIF-1α activity may represent a novel and effective therapeutic approach to alleviate the sympathoexcitation. Further studies are necessary to determine the specific contribution of the Ang II-HIF-1 axis to the overall sympathoexcitation observed in various cardiovascular diseases including other models of hypertension, such as spontaneously hypertensive, obesity-related hypertension, and salt-sensitive hypertension as well as congestive heart failure.
Sources of Funding
This article received funding from American Heart Association National Center grant 14SDG19980007 (N.M. Sharma), National Institutes of Health, Heart, Lung, & Blood Institute, Grant R01-DK-114663, HL62222 and McIntyre Professorship (K.P. Patel) supported this work. A.S. Haibara was the recipient of a postdoctoral fellowship from CNPq-Brazil, 234520/2014-0.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- Ang II
- angiotensin II
- BP
- blood pressure
- CHF
- chronic heart failure
- DOCA
- deoxycorticosterone acetate
- HIF-1α
- hypoxia-inducible factor-1α
- HR
- heart rate
- HRE
- hypoxia response element
- NMDA
- N-methyl-D-aspartate
- NMDAR
- N-methyl-D-aspartate receptor
- NR1
- NMDA-type1
- PVN
- paraventricular nucleus of the hypothalamus
- RSNA
- renal sympathetic nerve activity
These authors contributed equally to this work.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.16002.
For Sources of Funding and Disclosures, see page 156.
Contributor Information
Neeru M. Sharma, Email: nsharma@unmc.edu.
Andréa S. Haibara, Email: haibara@icb.ufmg.br.
Kenichi Katsurada, Email: katsurada@jichi.ac.jp.
Shyam S. Nandi, Email: shyam.nandi@unmc.edu.
Xuefei Liu, Email: xuefei.liu@usd.edu.
Hong Zheng, Email: hong.zheng@usd.edu.
Novelty and Significance
What Is New?
N-methyl-D-aspartate-NR1 expression is upregulated in the paraventricular nucleus of the hypothalamus through HIF-1α (hypoxia-inducible factor-1α)-mediated mechanism induced by Ang II (angiotensin II) in neurogenic hypertension.
What Is Relevant?
Understanding the underlying molecular mechanism by which there is increased expression of N-methyl-D-aspartate-NR1 in pathological conditions leading to increased sympathoexcitation may provide the basis for the development of new therapeutic agents with enhanced specificity.
Summary
We have identified a novel mechanism linking Ang II-HIF-1α-N-methyl-D-aspartate receptor in preautonomic neurons in the paraventricular nucleus of the hypothalamus, resulting in increased sympathetic outflow and consequent autonomic alterations in baro- and chemoreflexes contributing to increased sympathoexcitation commonly observed in pathologies associated with increased central Ang II levels.
References
- 1.Esler M. Sympathetic nervous system: contribution to human hypertension and related cardiovascular diseases. J Cardiovasc Pharmacol. 1995;26Suppl 2S24–S28 [PubMed] [Google Scholar]
- 2.DiBona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. doi: 10.1161/HYPERTENSIONAHA.111.00633 [DOI] [PubMed] [Google Scholar]
- 3.Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst. 1994;50:1–11. doi: 10.1016/0165-1838(94)90117-1 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Liu XF, Cornish KG, Zucker IH, Patel KP. Effects of nNOS antisense in the paraventricular nucleus on blood pressure and heart rate in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H205–H213. doi: 10.1152/ajpheart.00497.2004 [DOI] [PubMed] [Google Scholar]
- 5.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111 [DOI] [PubMed] [Google Scholar]
- 6.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κBin the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–408. doi: 10.1093/cvr/cvr242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou JJ, Yuan F, Zhang Y, Li DP. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology. 2015;99:481–490. doi: 10.1016/j.neuropharm.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basting T, Xu J, Mukerjee S, Epling J, Fuchs R, Sriramula S, Lazartigues E. Glutamatergic neurons of the paraventricular nucleus are critical contributors to the development of neurogenic hypertension. J Physiol. 2018;596:6235–6248. doi: 10.1113/JP276229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciriello J, Kline RL, Zhang TX, Caverson MM. Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain Res. 1984;310:355–359. doi: 10.1016/0006-8993(84)90159-8 [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, Hayashi J, Oguro M, Sasaki S, Nakagawa M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res. 1991;543:296–300. doi: 10.1016/0006-8993(91)90040-3 [DOI] [PubMed] [Google Scholar]
- 12.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272 [DOI] [PubMed] [Google Scholar]
- 13.Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23:45–55. doi: 10.1081/ceh-100001196 [DOI] [PubMed] [Google Scholar]
- 14.Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann N Y Acad Sci. 2001;940:431–443 [PubMed] [Google Scholar]
- 15.Sharma NM, Llewellyn TL, Zheng H, Patel KP. Angiotensin II-mediated posttranslational modification of nNOS in the PVN of rats with CHF: role for PIN. Am J Physiol Heart Circ Physiol. 2013;305:H843–H855. doi: 10.1152/ajpheart.00170.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fyhrquist F, Metsärinne K, Tikkanen I. Role of angiotensin II in blood pressure regulation and in the pathophysiology of cardiovascular disorders. J Hum Hypertens. 1995;9Suppl 5S19–S24 [PubMed] [Google Scholar]
- 17.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure. Am J Physiol Heart Circ Physiol. 2010;1152:1546–1555. doi: 10.1152/ajpheart.01006.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–1647. doi: 10.1113/jphysiol.2007.149732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen KB, Yi F, Perszyk RE, Menniti FS, Traynelis SF. NMDA receptors in the central nervous system. Methods Mol Biol. 2017;1677:1–80. doi: 10.1007/978-1-4939-7321-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li DP, Zhou JJ, Zhang J, Pan HL. CAMK II regulates synaptic NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J. Neurosci. 2017;37:10690–10699. doi: 10.1523/JNEUROSCI.2141-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma NM, Cunningham CJ, Zheng H, Liu X, Patel KP. Hypoxia-inducible factor-1 alpha mediates increased sympathoexcitation via glutamatergic n-methyl-d-aspartate receptors in the paraventricular nucleus of rats with chronic heart failure. Circ Heart Fail. 2016;9:e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safran M, Kaelin WG., Jr. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf G, Schroeder R, Stahl RA. Angiotensin II induces hypoxia-inducible factor-1 alpha in PC 12 cells through a posttranscriptional mechanism: role of AT2 receptors. Am J Nephrol. 2004;24:415–421. doi: 10.1159/000080086 [DOI] [PubMed] [Google Scholar]
- 24.Araki-Taguchi M, Nomura S, Ino K, Sumigama S, Yamamoto E, Kotani-Ito T, Hayakawa H, Kajiyama H, Shibata K, Itakura A, et al. Angiotensin II mimics the hypoxic effect on regulating trophoblast proliferation and differentiation in human placental explant cultures. Life Sci. 2008;82:59–67. doi: 10.1016/j.lfs.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 25.Braga VA, Medeiros IA, Ribeiro TP, França-Silva MS, Botelho-Ono MS, Guimarães DD. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: implications in neurogenic hypertension. Braz J Med Biol Res. 2011;44:871–876. doi: 10.1590/s0100-879x2011007500088 [DOI] [PubMed] [Google Scholar]
- 26.Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats. Hypertension. 2008;52:342–350. doi: 10.1161/HYPERTENSIONAHA.108.110445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1364–R1374. doi: 10.1152/ajpregu.00149.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H423–H433. doi: 10.1152/ajpheart.00685.2001 [DOI] [PubMed] [Google Scholar]
- 29.Sharma NM, Haibara AS, Katsurada K, Liu X, Patel KP. Central angiotensin II-Protein inhibitor of neuronal nitric oxide synthase (PIN) axis contribute to neurogenic hypertension. Nitric Oxide. 2020;94:54–62. doi: 10.1016/j.niox.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camara AK, Osborn JL. AT1 receptors mediate chronic central nervous system AII hypertension in rats fed high sodium chloride diet from weaning. J Auton Nerv Syst. 1998;72:16–23. doi: 10.1016/s0165-1838(98)00080-0 [DOI] [PubMed] [Google Scholar]
- 31.Clayton SC, Zhang Z, Beltz T, Xue B, Johnson AK. CNS neuroplasticity and salt-sensitive hypertension induced by prior treatment with subpressor doses of ANG II or aldosterone. Am J Physiol Regul Integr Comp Physiol. 2014;306:R908–R917. doi: 10.1152/ajpregu.00010.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker BK, Wang H, Zucker IH. Central TrkB blockade attenuates ICV angiotensin II-hypertension and sympathetic nerve activity in male Sprague-Dawley rats. Auton Neurosci. 2017;205:77–86. doi: 10.1016/j.autneu.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol. 1992;2631 Pt 2R89–R94. doi: 10.1152/ajpregu.1992.263.1.R89 [DOI] [PubMed] [Google Scholar]
- 34.Franchini KG, Krieger EM. Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. J Auton Nerv Syst. 1993;42:63–69. doi: 10.1016/0165-1838(93)90342-r [DOI] [PubMed] [Google Scholar]
- 35.Caligiorne SM, Silva AQ, Fontes MA, Silva JR, Baltatu O, Bader M, Santos RA, Campagnole-Santos MJ. Baroreflex control of heart rate and renal sympathetic nerve activity in rats with low brain angiotensinogen. Neuropeptides. 2008;42:159–168. doi: 10.1016/j.npep.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 36.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–997. doi: 10.1161/01.RES.0000102865.60437.55 [DOI] [PubMed] [Google Scholar]
- 37.Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1035–R1043. doi: 10.1152/ajpregu.00338.2004 [DOI] [PubMed] [Google Scholar]
- 38.Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005 [DOI] [PubMed] [Google Scholar]
- 39.Palkovits M, Brownstein M. Cuello AE, ed. Brain microdissection techniques. In: Brain microdissection techniques. 1983. Chichester: John Wiley & Sons [Google Scholar]
- 40.Fishman MC, Zimmerman EA, Slater EE. Renin and angiotensin: the complete system within the neuroblastoma x glioma cell. Science. 1981;214:921–923. doi: 10.1126/science.6272392 [DOI] [PubMed] [Google Scholar]
- 41.Sandberg K, Ji H, Hay M. Sex-specific immune modulation of primary hypertension. Cell Immunol. 2015;294:95–101. doi: 10.1016/j.cellimm.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabbatini AR, Kararigas G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage. Biol Sex Differ. 2020;11:31 doi: 10.1186/s13293-020-00306-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marques-Lopes J, Tesfaye E, Israilov S, Van Kempen TA, Wang G, Glass MJ, Pickel VM, Iadecola C, Waters EM, Milner TA. Redistribution of NMDA receptors in estrogen-receptor-β-containing paraventricular hypothalamic neurons following slow-pressor angiotensin II hypertension in female mice with accelerated ovarian failure. Neuroendocrinology. 2017;104:239–256. doi: 10.1159/000446073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol. 2017;312:H1031–H1051. doi: 10.1152/ajpheart.00703.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802 [DOI] [PubMed] [Google Scholar]
- 46.Zheng H, Sharma NM, Liu X, Patel KP. Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: role of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2012;303:R387–R394. doi: 10.1152/ajpregu.00046.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li DP, Pan HL. Glutamatergic Regulation of Hypothalamic Presympathetic Neurons in Hypertension. Curr Hypertens Rep. 2017;19:78 doi: 10.1007/s11906-017-0776-4 [DOI] [PubMed] [Google Scholar]
- 48.Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst. 1988;22:211–219. doi: 10.1016/0165-1838(88)90109-9 [DOI] [PubMed] [Google Scholar]
- 49.Patel KP, Salgado HC, Liu X, Zheng H. Exercise training normalizes the blunted central component of the baroreflex in rats with heart failure: role of the PVN. Am J Physiol Heart Circ Physiol. 2013;305:H173–H181. doi: 10.1152/ajpheart.00009.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol. 2005;289:R789–R797. doi: 10.1152/ajpregu.00222.2005 [DOI] [PubMed] [Google Scholar]
- 51.Reddy MK, Schultz HD, Zheng H, Patel KP. Altered nitric oxide mechanism within the paraventricular nucleus contributes to the augmented carotid body chemoreflex in heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H149–H157. doi: 10.1152/ajpheart.00117.2006 [DOI] [PubMed] [Google Scholar]
- 52.Glass MJ, Wang G, Coleman CG, Chan J, Ogorodnik E, Van Kempen TA, Milner TA, Butler SD, Young CN, Davisson RL, et al. NMDA receptor plasticity in the hypothalamic paraventricular nucleus contributes to the elevated blood pressure produced by angiotensin II. J Neurosci. 2015;35:9558–9567. doi: 10.1523/JNEUROSCI.2301-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci. 2010;30:12103–12112. doi: 10.1006/meth.2001.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeh SH, Hung JJ, Gean PW, Chang WC. Hypoxia-inducible factor-1alpha protects cultured cortical neurons from lipopolysaccharide-induced cell death via regulation of NR1 expression. J Neurosci. 2008;28:14259–14270. doi: 10.1523/JNEUROSCI.4258-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai G, Hoffman PW. Transcriptional regulation of NMDA receptor expression. 2009. Boca Raton (FL): CRC Press/Taylor & Francis; [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


