Abstract
Lactation requires a series of adaptations in maternal calcium and bone metabolism to ensure a steady supply of calcium to the lactating mammary gland. The alterations in systemic metabolism are accompanied by alterations in the expression of calcium receptors, channels, binding proteins, pumps and transporters in mammary epithelial cells to increase the uptake of calcium from the extracellular fluid and to transport it into milk. Intracellular calcium regulates signaling pathways that mediate changes in cell proliferation, differentiation and death and many of the molecules involved in supporting and coordinating calcium secretion into milk are re-expressed and redeployed to support malignant behavior in breast cancer cells. In this article, we review adaptations of systemic calcium homeostasis during lactation, as well as the mechanisms of milk calcium transport. We then discuss how reactivation of these pathways contributes to the pathophysiology of breast cancer.
Keywords: Lactation, Breast Cancer, Bone, Calcium-sensing receptor, Parathyroid hormone-related protein, Mammary gland
INTRODUCTION
The breast makes milk to support reproduction. Breast tissue and maternal metabolism undergo profound and cyclical alterations to allow for the production and secretion of milk over multiple reproductive cycles (1). Breast cancer is one of the most common malignancies and causes of cancer-related mortality in women worldwide (2). It is not surprising that the biology of breast cancer is intimately related to the alterations in breast tissue and maternal metabolism that are driven by changes in reproductive hormones. This short review focuses specifically on alterations in maternal metabolism that supply calcium for milk production and how reactivation of these pathways contributes to the pathophysiology of breast cancer.
CALCIUM AND BONE METABOLISM DURING LACTATION
Calcium Delivery to the Lactating Mammary Gland
Milk provides all the calcium required to support rapid bone growth in neonates (1). Nursing mothers transfer approximately 200 mg calcium to their infants on a daily basis (3,4). Meeting the demand for milk calcium requires adaptations in maternal calcium and bone metabolism, including increased dietary calcium intake, increased gut calcium absorption, renal calcium conservation and the liberation of skeletal calcium reserves (3,5).
The diet is an important source of calcium for milk production in rodents and humans (5–7). Intestinal calcium absorption is increased during pregnancy and continues to be so throughout lactation in rodents (3,5–9). In humans, fractional calcium absorption in the intestine falls back to nonpregnant values in lactating women (3,7,10), but increased food intake offsets this change and raises the total amount of calcium absorbed from dietary sources (11,12). Renal calcium reabsorption increases in both lactating humans and rodents and, as a consequence, urine calcium concentrations fall to low levels (8,10). The increase in calcium intake coupled with the decrease in urinary calcium excretion accounts for a significant portion of milk calcium.
Another maternal adaptation is increased bone resorption. Woman lose between 5% to 10% of their bone mass over 6 months of breastfeeding, while rodents lose 25–35% over 3 weeks of lactation (3,12). Bone resorption occurs primarily within the trabecular and endocortical compartments and studies in rodents have documented a RANKL-dependent increase in marrow osteoclast progenitors and active osteoclasts on the bone surface (Fig. 1A) (6,13). In addition to increased surface bone turnover, lactation is also associated with an increase in osteocyte lacunar-canalicular remodeling, whereby osteocytes assume osteoclast-like chracteristics, allowing them to remove their perilacunar matrix and release free calcium into the circulation (Fig. 1A) (14,15). It is still not known whether this process of “osteocytic osteolysis” represents a quantitatively important contribution to milk calcium or whether it is simply part of a coordinated response regulating surface bone resorption (14). In rodents, bone resorption “backs up” the supply of dietary calcium, allowing for ongoing milk production in the face of limiting dietary calcium supplies. Inhibiting bone resorption does not change milk calcium content or milk production as long as dietary calcium is plentiful (6). However, inhibition of bone resorption in the face of dietary calcium restriction, causes severe hypocalcemia and death in lactating dams (6). Nonetheless, increasing dietary calcium does not prevent bone loss in either lactating rodents or humans (3,16,17). These data suggest that dietary intake and bone loss provide parallel sources of calcium to ensure that mammals have steady supply of calcium for milk production in the face of intermittent calcium availability in the environment.
Figure 1. CaSR and PTHrP feedback loop in lactation and in breast cancer.
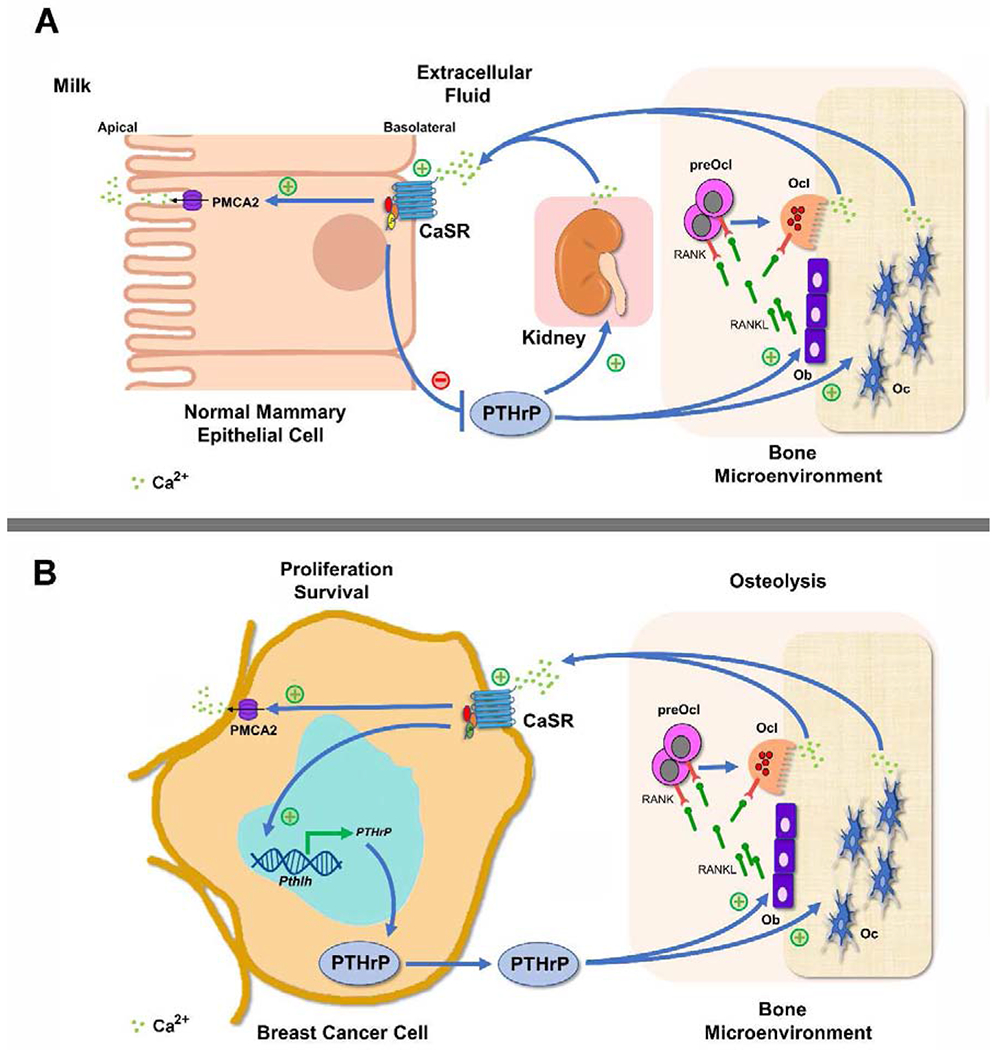
(A) Circulating PTHrP acts on the kidney and on the bone to stimulate calcium reabsorption and bone resorption, respectively. In the bone, PTHrP acts on osteoblasts (Ob) to produce RANKL that induces pre-osteoclast (preOcl) differentiation to osteoclast (Ocl) and Ocl bone resorption on one hand and osteocyte (Oc) osteolysis on the other. This, liberates skeletal calcium stores which are transported via the bloodstream to the lactating mammary gland, where calcium activates the CaSR on the basolateral surface to promote calcium entry into the cells, stimulate PMCA2 mediated calcium transport into milk and to inhibit PTHrP expression, therefore establishing a negative feedback loop. (B) In breast cancer cells, high local calcium concentrations activate the CaSR leading to increased PTHrP production. PTHrP secretion induces RANKL secretion by Ob, thereby driving more osteolysis and releasing calcium from the bone matrix that, in turn, stimulates the CaSR. Activation of the CaSR promotes breast cancer cell proliferation and enhances cell survival by stimulating intracrine and paracrine actions of PTHrP. The CaSR may also increase PMCA2 calcium pump activity to protect the breast cancer cells from calcium-mediated apoptosis. As a result, activation of the CaSR in breast cancer cells facilitates a feed-forward, vicious cycle of bone resorption, tumor growth and osteolysis.
Hormonal Regulation of Calcium and Bone Metabolism during Lactation
Adaptations in calcium and bone metabolism are regulated and coordinated by changes in systemic hormones and cytokines during lactation. Intestinal calcium absorption is stimulated during pregnancy by increased levels of 1,25 (OH)2-vitamin D, or calcitriol, the active metabolite of Vitamin D, which is produced by both the placenta and kidneys (9,18). Calcitriol acts on enterocytes to upregulate the expression of proteins involved in both transcellular and paracellular calcium transport (19). Although calcitriol levels remain elevated in lactating rodents, in breastfeeding women, they decline to nonpregnant values shortly after parturition (3,9,20). Prolactin also increases calcium absorption during lactation, both by upregulating CYP27B1 gene (1 alpha-hydroxylase) expression and calcitriol production in the kidney, and by directly acting on enterocytes to increase the expression of calcium transport proteins (21,22).
Several systemic changes contribute to increased bone resorption during lactation. First, suckling suppresses pulsatile GnRH release from the hypothalamus, causing hypogonadotropic hypogonadism and reducing circulating estrogens, a well-known trigger for increased bone resorption (23–25). In addition, parathyroid hormone-related protein (PTHrP) production is upregulated by mammary epithelial cells (MECs) during lactation and is secreted into the systemic circulation. PTHrP synergizes with estrogen deficiency to stimulate bone resorption and to increase osteocytic perilacunar canalicular remodeling (Fig. 1A) (15,25,26). PTHrP also stimulates renal tubular calcium reabsorption to lower urinary calcium excretion (26). Increased levels of prolactin, FGF21 and serotonin may also contribute to bone loss during lactation (3)although the effects of prolactin and serotonin could be largely mediated by PTHrP. Lactation-associated bone loss is modulated by the calcium-sensing receptor (CaSR), which is expressed on MECs (27,28).
Increased delivery of calcium to MECs activates the CaSR, causing a decrease in PTHrP production, an increase in milk calcium transport (see below) and a reduction in bone resorption (Fig. 1A). By contrast, inadequate delivery of calcium lowers CaSR activity, causing an increase in PTHrP production and an increase in bone resorption to liberate skeletal calcium for milk production. This classical endocrine feedback loop between breast and bone cells helps match calcium delivery to demand. Finally, calcitonin has been shown to protect against excessive bone resorption during lactation in mice by limiting bone resorption (29). Although it is not clear whether calcitonin deficiency increases bone loss in lactating women, calcitonin levels are elevated in nursing woman and calcitonin is secreted into human milk (3).
Calcium Transport Into Milk
All milk is rich in calcium although concentrations vary between species (30). Given the many signaling functions of ionized calcium in the cytosol, the lactating mammary gland must orchestrate a large throughput of calcium secretion into milk while maintaining free cytosolic calcium in the submicromolar range (31). Since there is little paracellular transport in the lactating mammary gland, calcium must pass from the extracellular fluid through the epithelial cell to enter milk (30,31). A working model of calcium transfer into milk involving four specific steps has been dubbed “Calcium movement by the calcium transporter (CALTRANS)” (Fig. 2) (31). It involves: 1) influx of calcium from the extracellular fluid/blood across the basolateral membrane of mammary epithelial cells, 2) intracellular sequestration of calcium in the endoplasmic reticulum (ER) to maintain low cytosolic free calcium, 3) transfer of calcium into the Golgi and secretory compartments where micelle formation and secretion occur, and/or 4) transfer of calcium from ER for direct transport across the apical membrane.
Figure 2. CALTRANS model of calcium transport across mammary epithelial cells into milk.
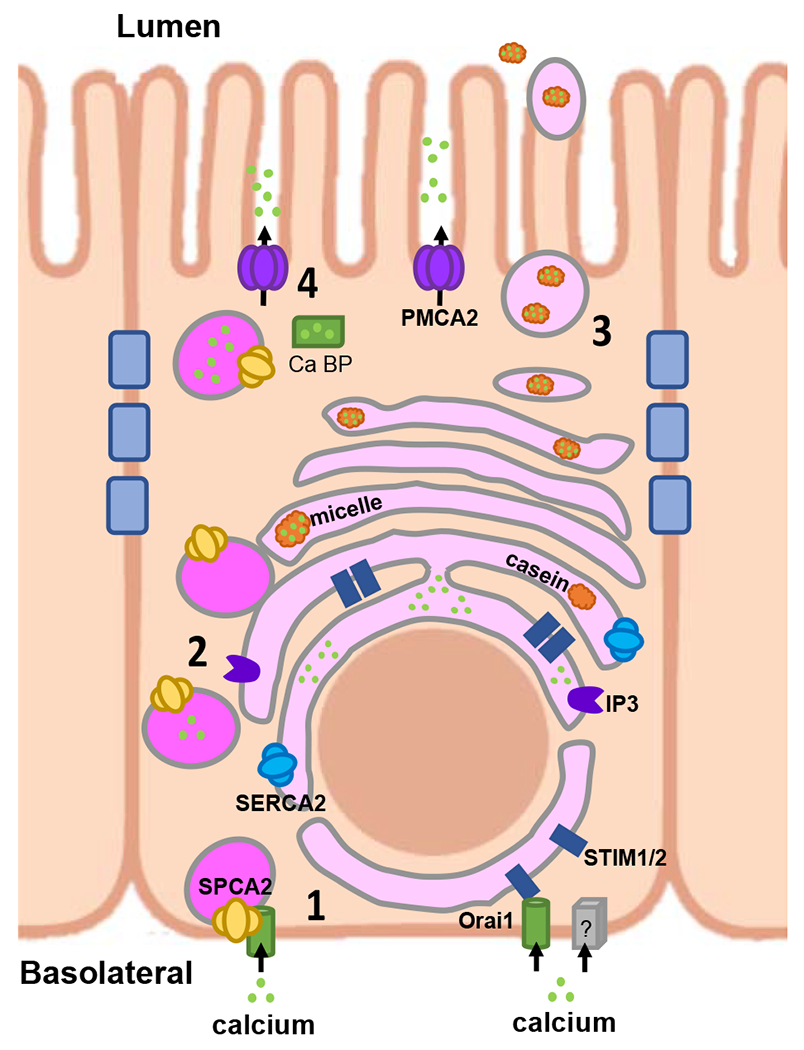
The 4 steps of the model 1) Calcium Entry, 2) Calcium Sequestration from cytosol, 3) Calcium packaging into casein micelles and 4) Caclium transport across the apical membrane are detailed in the text. Adapted from Cross et. Al, ref. 31.
Calcium entry at the basolateral membrane occurs through the store-operated calcium influx protein, Orai1. Loss of Orai1 in vivo reduces milk calcium by 50%, demonstrating its importance, but suggesting other calcium channels may also function in this process (32,33). Orai1 is well described to mediate store-operated calcium entry (SOCE) in response to calcium depletion of the endoplasmic reticulum (ER). SOCE involves interactions between the ER protein, STIM1, and Orai1 at specialized locations where the ER and plasma membranes become closely opposed (34). However, during lactation, calcium influx through Orai1 occurs in a constitutive fashion by a process of store-independent calcium entry (SICE) mediated by the secretory pathway Ca2+-ATPase 2 (SPCA2) (31,35). Both Orai1 and SPCA2 are upregulated during lactation (35,36) and the C-terminus of SPCA2 opens Orai1 channels at the plasma membrane, independent of ER calcium stores or STIM sensors (31,35).
Once calcium enters MECs, it is thought to be sequestered into the Golgi by SPCA1 and/or SPCA2 (37), both of which are upregulated during lactation (33). In addition, calcium may be pumped into the ER/secretory pathway by the sarcoendoplasmic reticulum Ca2+-ATPase 2 (SERCA2), whose expression is also increased during lactation (38). Within the Golgi/secretory vesicles, calcium becomes bound to casein, phosphate and citrate to form protein and mineral micelles within vesicles which are transported to the apical membrane and secreted into milk (30,31,33). Thus, a significant amount of calcium enters milk via the exocytosis of casein micelles.
Calcium also enters milk by direct transport through the plasma membrane calcium/ATPase 2 (PMCA2) pump (39,40). The splice variant, PMCA2bw, is upregulated 200-fold at the peak of lactation and contains a 45 amino acid insert that directs it to the apical membrane. Milk from PMCA2-null mice contains 60–70% less calcium than controls, suggesting that PMCA2 is responsible for transporting a significant amount of milk calcium (39,40). Furthermore, loss of PMCA2 in MECs results in a sustained increase in intracellular calcium despite continued casein secretion (41). Exactly how calcium is delivered to PMCA2 remains unclear. Calcium binding proteins such as calbindin, calmodulin and annexins may be involved as calcium transport by PMCA2 is greatly stimulated by calcium/calmodulin (33). Alternatively, it is possible that calcium may be released from the ER via the IP3 or ryanodine receptors in the vicinity of PMCA2, which might explain how activation of the CaSR (a G-protein coupled receptor) activates PMCA2 calcium transport (33,40).
CALCIUM HANDLING IN BREAST CANCER
Molecules involved in supporting and coordinating calcium secretion into milk are often re-expressed and redeployed to support malignant behavior in breast cancer cells. We will review some of the better documented examples below although the reader is referred to comprehensive reviews for further information (31,42,43).
CaSR-PTHrP Axis and Bone Metastases
Normal MECs stimulate bone resorption to supply calcium for milk production, while breast cancer cells stimulate bone resorption to promote the growth of bone metastases and/or to cause systemic hypercalcemia (44). In both instances, PTHrP contributes to the increased osteoclast numbers/activity and, in both instances, PTHrP secretion is modulated by the CaSR. In the normal gland, activation of the CaSR inhibits MEC growth and decreases PTHrP production (27,28,45). In contrast, in breast cancer cells, activation of the CaSR stimulates MEC growth and increases PTHrP production (Fig. 1B) (45). This is due to a switch in G-protein activation by the CaSR: from Gai in normal breast cells to Gαs in breast cancer cells (44). As a result of this “rewiring” of the CaSR in breast cancer cells, the normal negative feedback loop between calcium and PTHrP is converted into a feedforward loop in which more PTHrP production induces increased calcium levels, either locally or systemically, which, in turn, stimulates even more PTHrP production. In turn, PTHrP increases cell proliferation and survival, increases peri-tumor bone destruction, and stimulates progressive growth of osteolytic bone metastases (45,46). Clinical series have supported the association between PTHrP production by tumors and increased incidence of bone metastases, although this association has not been uniformly observed (47–49).
Calcium Entry and Breast Cancers
In normal breast cells, intracellular free calcium concentrations are regulated by the integrated actions of a variety of different calcium channels, pumps, and exchangers, sometimes referred to collectively as the cellular “calcium toolkit” (31,33,42,43). The baseline calcium level is maintained around 100nM whereas extracellular calcium is more than 1mM. Transient increases in cytosolic calcium are an important aspect of many signaling events but persistent elevations in calcium trigger cell death (50). Many studies have identified adaptations in the “calcium toolkit” that contribute to the ability of cancer cells to maintain low cytosolic calcium in the face of chronic activation of oncogenic signaling pathways (31,42,43). However, details vary among different cancer types, which express different combinations of calcium channels and pumps (42). In breast cancers, entry of calcium across the plasma membrane occurs through members of the transient receptor potential (TRP) family and ORAI1&3. The resulting increased cytosolic calcium activates cell proliferation, invasion, and migration (31,42,43). There are over 20 different transient receptor potential (TRP) ion channels and different cell lines have been shown to overexpress different combinations (51). For example, MCF7 cells, an estrogen receptor-positive (ER+) luminal cell line, over-express TRPC1, TRPC6, TRPM8, and TRPV6, whereas T47D cells, also an ER+ luminal breast cancer line, overexpress TRPV4 and TRPV6. Silencing of different TRP genes in breast cancer cells has been shown to inhibit MAP kinase (ERK1/2) activity and to decrease cell proliferation and migration (31,42,51). Furthermore, the expression of TRPC1, TRPM7, TRPM8, and TRPV6 have been shown to correlate with the histological aggressiveness of breast cancers and TRPM7 predicts poor outcome in patients (31,42,51).
As noted in the previous section, ORAI1 is involved in both SOCE in all cells as well as SICE, in MECs. It appears that both the classical SOCE and lactation-associated SICE pathways of calcium entry are active in breast cancer cells (31,42,43). SOCE mediated by ORAI1/STIM1 interactions has been shown to be important for proliferation, cell migration and metastasis in basal-type breast cancer cells and patients whose breast cancers demonstrate elevated expression of both ORAI1 and STIM1 have a poor prognosis (42,52). SICE and SPCA2/ORAI1 interactions are elevated in luminal-type breast cancers, including HER2-positive cancers (42,53). Disruption of this pathway inhibits proliferation and tumor growth in immunocompromised mice, in part, by inhibiting Ras/ERK/MAPK signaling (53). Therefore, while calcium entry appear to stimulate malignant behaviors, the details and molecules involved differ by molecular sub-type of breast cancer.
PMCA2 and Breast Cancer
PMCAs constitute an important pathway for calcium efflux from cells and typically reduce cytosolic calcium levels back to baseline after calcium transients (54). There are four PMCA genes, each of which gives rise to multiple different isoforms. As reviewed above, PMCA2wb is highly expressed in mammary epithelial cells during lactation and is important for milk calcium transport. PMCA2 expression is rapidly downregulated upon weaning, but it become re-expressed in many breast cancers, where it is associated with HER2 expression, more aggressive tumor characteristics and increased mortality, especially in younger women (41,55). Our laboratory has demonstrated that PMCA2 becomes an integral part of a multi-protein signaling complex that includes HER2, EGFR or HER3, NHERF1, Ezrin and HSP90, and that is located in specific lipid-raft- and actin-rich plasma membrane domains (Fig. 3) (55,56). Both the scaffolding and ATPase activity of PMCA2 are important to this complex and loss of PMCA2 or its activity results in a calcium and PKC-alpha dependent internalization, ubiquitinylation and degradation of HER2 (55,56). Disrupting PMCA2 expression decreases tumor cell proliferation, increases tumor cell apoptosis and profoundly inhibits tumor formation and growth in MMTV-Neu mice, a transgenic model of HER2-positive cancer (55). Interestingly HER2-positive tumors upregulate both SPCA2 and SICE as well as PMCA2 and calcium extrusion, both key aspects of calcium handling during lactation (42,53,55,56). Whether the activities of SPCA2 and PMCA2 are coordinated in HER2-positive cancer cells is not known but is an interesting question for further research.
Figure 3. HER2 signaling complex in breast cancer cells.
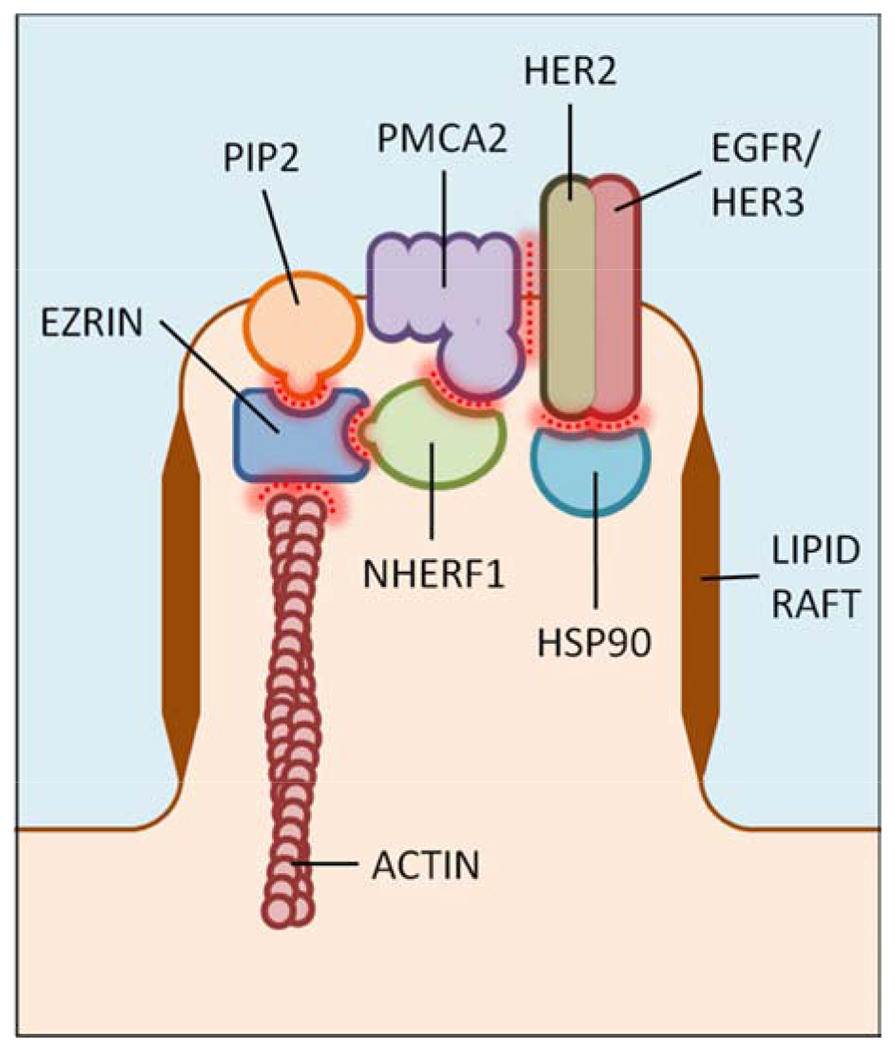
ATP2b2 (PMCA2) gene expression is reactivated in HER2-positive breast cancer cells and PMCA2 becomes an integral part of a multiprotein and lipid signaling complex that is required for the membrane retention of actively signaling HER2. Adapted from Jeong et. al., ref 56.
CONCLUSIONS
The ability of normal breast cells to regulate systemic and cellular calcium homeostasis during lactation is remarkable. Various proteins in the calcium signaling “tool kit” are upregulated during lactation and tightly regulate intracellular calcium levels to allow for a large transcellular flux of calcium into milk while maintaining cytoplasmic calcium signaling and MEC survival. The fact that many of the same molecules are dysregulated and contribute to the malignant phenotype in breast cancer cells, highlights the importance of gaining a more detailed understanding of the changes in calcium regulation in both normal MECs and breast cancer cells. A more comprehensive understanding of calcium homeostasis during lactation should help to highlight new therapeutic opportunities for the treatment of breast cancers.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health [R21 AR073146, R01 HD100468 and R01 HD076248]; and the Department of Defense/Breast Cancer Research Program [BC151665].
ABREVIATIONS:
- GnRH
Gonadotropin-releasing hormone
- PTHrP
Parathyroid hormone-related protein
- MECs
mammary epithelial cells
- FGF21
Fibroblast growth factor 21
- CaSR
Calcium-sensing receptor
- CALTRANS
Calcium movement by the calcium transporter
- Orai1
Calcium release-activated calcium channel protein 1
- SOCE
store-operated calcium entry
- SICE
store-independent calcium entry
- STIM1
Stromal Interaction Molecule 1
- SPCA2
Secretory pathway Ca2+-ATPase 2
- SERCA2
sarcoendoplasmic reticulum Ca2+-ATPase 2
- PMCA2
Plasma membrane calcium/ATPase 2
- TRP
transient receptor potential
- NHERF1
Na+/H+ exchanger regulatory factor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sadovnikova A, Wysolmerski John J, Hovey Russell C. The onset and maintenance of human lactation and its endocrine regulation. In: Kovacs C, Deal C, eds. 1st Edition ed: Academic Press; 2019:189–200. [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- **3.Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiological Reviews. 2016;96(2):449–547. [DOI] [PubMed] [Google Scholar]; This is an extensive review of the maternal adaptations in mineral and bone physiology during pregnancy, lactation and post-weaning recovery
- 4.Ross AC, Manson JAE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. Vol 96: Endocrine Society; 2011:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres DA, Freitas MB, Gonçalves RV. Changes in bone turnover and calcium homeostasis during pregnancy and lactation in mammals: a meta-analysis. Reproduction, Fertility and Development. 2018;30(5):681–681. [DOI] [PubMed] [Google Scholar]
- 6.Ardeshirpour L, Dumitru C, Dann P, Sterpka J, VanHouten J, Kim W, Kostenuik P, Wysolmerski J. OPG Treatment Prevents Bone Loss During Lactation But Does Not Affect Milk Production or Maternal Calcium Metabolism. Endocrinology. 2015; 156(8):2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalkwarf HJ, Specker BL, Heubi JE, Vieira NE, Yergey AL. Intestinal calcium absorption of women during lactation and after weaning. The American Journal of Clinical Nutrition. 1996;63(4):526–531. [DOI] [PubMed] [Google Scholar]
- 8.Beggs MR, Appel I, Svenningsen P, Skjcdt K, Alexander RT, Dimke H. Expression of transcellular and paracellular calcium and magnesium transport proteins in renal and intestinal epithelia during lactation. American Journal of Physiology-Renal Physiology. 2017;313(3):F629–F640. [DOI] [PubMed] [Google Scholar]
- 9.Kirby BJ, Ma Y, Martin HM, Buckle Favaro KL, Karaplis AC, Kovacs CS. Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone. Journal of Bone and Mineral Research. 2013;28(9):1987–2000. [DOI] [PubMed] [Google Scholar]
- 10.Moser-Veillon PB, Mangels AR, Vieira NE, Yergey AL, Patterson KY, Hill AD, Veillon C. Calcium Fractional Absorption and Metabolism Assessed Using Stable Isotopes Differ between Postpartum and Never Pregnant Women. The Journal of Nutrition. 2001;131(9):2295–2299. [DOI] [PubMed] [Google Scholar]
- 11.Crowley WR. Neuroendocrine regulation of lactation and milk production. Compr Physiol. 2015;5(1):255–291. [DOI] [PubMed] [Google Scholar]
- 12.Wysolmerski JJ. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Annals of the New York Academy of Sciences. 2010;1192(1):161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ardeshirpour L, Dann P, Adams DJ, Nelson T, Vanhouten J, Horowitz MC, Wysolmerski JJ. Weaning triggers a decrease in receptor activator of nuclear factor-KB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology. 2007;148(8):3875–3886. [DOI] [PubMed] [Google Scholar]
- *14.Lotinun S, Ishihara Y, Nagano K, Kiviranta R, Carpentier VT, Neff L, Parkman V, Ide N, Hu D, Dann P, Brooks D, Bouxsein ML, Wysolmerski J, Gori F, Baron R. Cathepsin K-deficient osteocytes prevent lactation-induced bone loss and parathyroid hormone suppression. J Clin Invest. 2019;129(8):3058–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a mouse model of target cathepsin K deletion in osteocytes, the authors show that ablation of this protein blunted osteocytic osteolysis and further prevented of the normal osteoclast function.
- 15.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross NA, Hillman LS, Allen SH, Krause GF. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. Journal of Bone and Mineral Research. 1995; 10(9): 1312–1320. [DOI] [PubMed] [Google Scholar]
- 17.Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The Effect of Calcium Supplementation on Bone Density during Lactation and after Weaning. New England Journal of Medicine. 1997;337(8):523–528. [DOI] [PubMed] [Google Scholar]
- 18.Gillies BR, Ryan BA, Tonkin BA, Poulton IJ, Ma Y, Kirby BJ, St-Arnaud R, Sims NA, Kovacs Cs. Absence of Calcitriol Causes Increased Lactational Bone Loss and Lower Milk Calcium but Does Not Impair Post-lactation Bone Recovery in Cyp27b1 Null Mice. J Bone Miner Res. 2018;33(1):16–26. [DOI] [PubMed] [Google Scholar]
- 19.Diaz De Barboza G, Guizzardi S, Tolosa De Talamoni N. Molecular aspects of intestinal calcium absorption. World Journal of Gastroenterology. 2015;21(23):7142–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson SG, Retallack RW, Kent JC, Worth GK, Gutteridge DH. Serum Free 1,25-dihydroxyvitamin D and the Free 1,25-dihydroxyvitamin D Index During a Longitudinal Study of Human Pregnancy and Lactation. Clinical Endocrinology. 1990;32(5):613–622. [DOI] [PubMed] [Google Scholar]
- 21.Ajibade DV, Dhawan P, Fechner AJ, Meyer MB, Pike JW, Christakos S. Evidence for a role of prolactin in calcium homeostasis: Regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D3 1α hydroxylase gene by prolactin. Endocrinology. 2010;151 (7):2974–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spanos E, Brown DJ, Stevenson JC, MacIntyre I. Stimulation of 1,25-dihydroxycholecalciferol production by prolactin and related peptides in intact renal cell preparations in vitro. Biochim Biophys Acta. 1981;672(1):7–15. [DOI] [PubMed] [Google Scholar]
- 23.McNeilly AS, Tay CC, Glasier A. Physiological Mechanisms Underlying Lactational Amenorrhea. Annals of the New York Academy of Sciences. 1994;709(1):145–155. [DOI] [PubMed] [Google Scholar]
- 24.Riggs BL. The mechanisms of estrogen regulation of bone resorption. Vol 106: The American Society for Clinical Investigation; 2000:1203–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology. 2003;144(12):5521–5529. [DOI] [PubMed] [Google Scholar]
- 26.VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. Journal of Clinical Investigation. 2003;112(9):1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamillapalli R, VanHouten J, Dann P, Bikle D, Chang W, Brown E, Wysolmerski J. Mammary-Specific Ablation of the Calcium-Sensing Receptor During Lactation Alters Maternal Calcium Metabolism, Milk Calcium Transport, and Neonatal Calcium Accrual. Endocrinology. 2013;154(9):3031–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanHouten J, Dann P, McGeoch G, Brown EM, Krapcho K, Neville M, Wysolmerski JJ. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. Journal of Clinical Investigation. 2004;113(4):598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodrow JP, Sharpe CJ, Fudge NJ, Hoff AO, Gagel RF, Kovacs CS. Calcitonin Plays a Critical Role in Regulating Skeletal Mineral Metabolism during Lactation. Endocrinology. 2006; 147(9):4010–4021. [DOI] [PubMed] [Google Scholar]
- 30.Neville MC. Calcium secretion into milk. J Mammary Gland Biol Neoplasia. 2005;10(2):119–128. [DOI] [PubMed] [Google Scholar]
- 31.Cross BM, Breitwieser GE, Reinhardt TA, Rao R. Cellular calcium dynamics in lactation and breast cancer: from physiology to pathology. Am J Physiol Cell Physiol. 2014;306(6):C515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis FM, Janoshazi A, Janardhan KS, Steinckwich N, D’Agostin DM, Petranka JG, Desai PN, Roberts-Thomson SJ, Bird GS, Tucker DK, Fenton SE, Feske S, Monteith GR, Putney JW Jr. Essential role of Orai1 store-operated calcium channels in lactation. Proc Natl Acad Sci U S A. 2015;112(18):5827–5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanHouten JN, Wysolmerski JJ. Transcellular calcium transport in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2007;12(4):223–235. [DOI] [PubMed] [Google Scholar]
- 34.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev. 2015;95(4):1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cross BM , Hack A, Reinhardt TA, Rao R. SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS One. 2013;8(6):e67348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faddy HM , Smart CE, Xu R, Lee GY, Kenny PA, Feng M, Rao R, Brown MA, Bissell MJ, Roberts-Thomson SJ, Monteith GR. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem Biophys Res Commun. 2008;369(3):977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wuytack F, Raeymaekers L, Missiaen L. PMR1/SPCA Ca2+ pumps and the role of the Golgi apparatus as a Ca2+ store. Pflugers Arch. 2003;446(2):148–153. [DOI] [PubMed] [Google Scholar]
- 38.Reinhardt TA, Horst RL. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am J Physiol. 1999;276(4):C796–802. [DOI] [PubMed] [Google Scholar]
- 39.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem. 2004;279(41):42369–42373. [DOI] [PubMed] [Google Scholar]
- 40.VanHouten JN, Neville MC , Wysolmerski JJ. The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: a mechanism for calcium-regulated calcium transport into milk. Endocrinology. 2007;148(12):5943–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanHouten J, Sullivan C, Bazinet C, Ryoo T, Camp R, Rimm DL, Chung G, Wysolmerski J. PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer. Proc Natl Acad Sci U S A. 2010;107(25):11405–11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makena MR, Rao R. Subtype specific targeting of calcium signaling in breast cancer. Cell Calcium. 2020;85:102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.So CL, Saunus JM, Roberts-Thomson SJ, Monteith GR. Calcium signalling and breast cancer. Semin Cell Dev Biol. 2019;94:74–83. [DOI] [PubMed] [Google Scholar]
- 44.Kim W, Wysolmerski JJ. Calcium-Sensing Receptor in Breast Physiology and Cancer. Front Physiol. 2016;7:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Kim W, Takyar FM, Swan K, Jeong J, VanHouten J, Sullivan C, Dann P, Yu H, Fiaschi-Taesch N, Chang W, Wysolmerski J. Calcium-Sensing Receptor Promotes Breast Cancer by Stimulating Intracrine Actions of Parathyroid Hormone-Related Protein. Cancer Res. 2016;76(18):5348–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that ablation of the CaSR in a mouse model of breast cancer inhibited tumor cell proliferation and tumor outgrowth and reduced tumor PTHrP expression.
- 46.Akhtari M, Mansuri J, Newman KA, Guise TM, Seth P. Biology of breast cancer bone metastasis. Cancer Biol Ther. 2008;7(1):3–9. [DOI] [PubMed] [Google Scholar]
- 47.Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PF, Spillane JB, Hopper JL, Martin TJ. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006;66(4):2250–2256. [DOI] [PubMed] [Google Scholar]
- 48.Takagaki K, Takashima T, Onoda N, Tezuka K, Noda E, Kawajiri H, Ishikawa T, Hirakawa K. Parathyroid hormone-related protein expression, in combination with nodal status, predicts bone metastasis and prognosis of breast cancer patients. Exp Ther Med. 2012;3(6):963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu C, Wang Z, Cui R, He H, Lin X, Sheng Y, Zhang H. Co-expression of parathyroid hormone related protein and TGF-beta in breast cancer predicts poor survival outcome. BMC Cancer. 2015;15:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niki I, Yokokura H, Sudo T, Kato M, Hidaka H. Ca2+ signaling and intracellular Ca2+ binding proteins. J Biochem. 1996;120(4):685–698. [DOI] [PubMed] [Google Scholar]
- 51.Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP channels: diagnostic markers and therapeutic targets for breast cancer? Trends Mol Med. 2013;19(2):117–124. [DOI] [PubMed] [Google Scholar]
- 52.Yang Y, Jiang Z, Wang B, Chang L, Liu J, Zhang L, Gu L. Expression of STIM1 is associated with tumor aggressiveness and poor prognosis in breast cancer. Pathol Res Pract. 2017;213(9):1043–1047. [DOI] [PubMed] [Google Scholar]
- 53.Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143(1):84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strehler EE. Plasma membrane calcium ATPases as novel candidates for therapeutic agent development. J Pharm Pharm Sci. 2013;16(2):190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Jeong J, VanHouten JN, Dann P, Kim W, Sullivan C, Yu H, Liotta L, Espina V, Stern DF, Friedman PA, Wysolmerski JJ. PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer. Proc Natl Acad Sci U S A. 2016;113(3):E282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that PMCA2 interacts with HER2 in membrane domains to block HER2 endocytosis and maintain oncogenic signaling.
- 56.Jeong J, Choi J, Kim W, Dann P, Takyar F, Gefter JV, Friedman PA, Wysolmerski JJ. Inhibition of ezrin causes PKCalpha-mediated internalization of erbb2/HER2 tyrosine kinase in breast cancer cells. J Biol Chem. 2019;294(3):887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]


