Abstract
Autoimmune diseases are one of the dreadful group of human diseases that have always been of keen interest to researchers. Due to complex and broad-spectrum nature, scientists are not yet able to pinpoint the pathogenesis of and delineate effective therapy against this group of diseases. However, it is becoming clear that a decrease in number and function of T regulatory cells (Treg), an increase in autoreactive Th1/Th17 cells and associated immunomodulation and inflammation participate in the pathogenesis of many autoimmune diseases. Cinnamon (Cinnamonum verum or Cinnamonum cassia) is a widely used natural spice and flavoring ingredient and its metabolite sodium benzoate (NaB) is a food-additive and FDA-approved drug against nonketotic hyperglycinemia (NKH) and urea cycle disorders (UCD). Recent studies indicate that cinnamon either in powder or extract form and NaB are capable of modulating different autoimmune pathways as well as protecting animals from different autoimmune disorders. Here, we have made an honest attempt to delineate such pieces of evidence with available anti-autoimmune mechanisms and analyze whether cinnamon supplements could be used to control the fury of autoimmune disorders.
INTRODUCTION
Autoimmune disorders are a group of human disorders in which due to a faulty immune system, immune cells mistake the body’s normal cells and proteins as foreign ones to mount an immune against our organs to cause damage [1-4]. The most common symptoms of autoimmune disorders are usually fatigue, joint muscle pain and swelling, skin problems abdominal pain and digestive issues recurring fever swollen glands, numbness and tingling in the hands and feet, trouble concentrating, hair loss, etc. [5]. In some cases, patients become wheel chair bound and even die due to organ failure. At present, nearly 50 million Americans are suffering from different autoimmune diseases. There are about 80 different types of autoimmune diseases and the most common ones are rheumatic arthritis, type 1 diabetes, multiple sclerosis, Celiac disease, lupus, Psoriasis, Pernicious anemia, Myasthenia gravis, Addison’s disease, and Sjogren’s syndrome [1-4, 6-8]. In general, autoimmune disorders are gender-sensitive in which women are more sensitive than men [9, 10]. Although the exact cause of autoimmune diseases is unknown, it is believed that certain factors like viral infections, environment, hereditary components, diet, smoking habits, and an unhealthy life style may contribute to the onset of the disease.
Cinnamon, a brown inner bark of a cinnamon plant, has been being widely used to treat cough and sore throat since medieval times. It also has a popular history as a commonly used spice and flavoring material for desserts, candies, chocolate, etc. Chinese cinnamon (Cinnamonum cassia) and original Ceylon cinnamon (Cinnamonum verum or Cinnamonum zeylanicum) are two major types of cinnamon that are available in the US. It has been found that Cinnamonum cassia, but not Cinnamonum verum, contains small amount of toxic 1-benzopyran-2-one or coumarin [11]. However, cinnamaldehyde that is ultimately metabolized to sodium benzoate, the active component and an FDA-approved drug, is present as the major peak in both Cinnamonum cassia and Cinnamonum verum [11]. Recently, several studies from cell cultures to animal models to treatment of patients indicate anti-autoimmune properties of cinnamon and its components or metabolites [12-15], suggesting that cinnamon may be considered as a natural supplement to control autoimmune disorders.
CINNAMON IN RHEUMATOID ARTHRITIS
Rheumatic arthritis (RA), an inflammatory systemic auto-immune disorder, restricts the free mobility of the joints resulting in stiffness, pain and swelling. In rat models of inflammation and arthritis, oral treatment with a polyphenolic fraction of Ceylon cinnamon showed a strong and dose-dependent reduction in paw volume, weight loss reversal effects against carrageenan-induced paw edema [16]. Cinnamon treatment also demonstrated mild analgesic effects during acute treatment as evidenced by the reduction in the writhing and paw withdrawal threshold of the inflamed rat paw [16], indicating a possible anti-rheumatic effect of cinnamon. Cinnamaldehyde is the major component of cinnamon and Cheng et al [17] have shown that cinnamaldehyde treatment reduces swollen paw volume, lowers the severity of arthritis, decreases joint swelling, and attenuates bone erosion and destruction in collagen-induced arthritic rats. Accordingly, in a recent randomized double-blind clinical trial by Shishehbor et al in 36 women with RA, oral cinnamon (2000 mg/patient/day) treatment significantly reduced the disease activity score, visual analog scale, and tender and swollen joints counts [18]. Although cinnamon treatment also lowered diastolic blood pressure, the authors did not see any significant changes in lipid profile and erythrocyte sedimentation rate [18].
CINNAMON IN TYPE I DIABETES MELLITUS
Type I diabetes mellitus (TIDM) is the most common metabolic disease in children and adolescents. In TIDM, an overactive immune response is mounted against insulin-producing islets of Langerhans resulting in the loss of insulin production and an increase in blood sugar. Over the years, several in vitro and in vivo studies have demonstrated the efficacy of cinnamon in improving both insulin resistance and glucose metabolism [19-22]. According to Shen et al, a cinnamon extract is capable of enhancing glucose uptake in adipocytes/myocytes and stimulating glucose intolerance, but not insulin resistance, in type 2 diabetic rats [23]. In another study, authors have also verified the antidiabetic effects of Ceylon cinnamon in insulin-uncontrolled type 1 diabetic rats [24]. However, in a prospective, double-blind, placebo-controlled study in 72 adolescent TIDM patients, oral cinnamon treatment did not lead to significant differences in final A1C, change in A1C, total daily insulin intake, and the number of hypoglycemic episodes [25]. Upon meta-analysis of prospective randomized controlled trials, Baker et al [26] also did not find a significant difference in A1C, fasting blood glucose, or lipid parameters after cinnamon treatment. These findings are summarized in Table 1. Accordingly, it has been suggested that cinnamon should not be recommended for the improvement of glycemic control [27]. To find out a reason for positive animal studies and negative human studies, we carefully analyzed the design of animal and human studies and found that positive animal studies used cinnamon at doses of 100 and 200 mg/kg body weight/d. If these doses of cinnamon are translated to the clinic, depending on body weight, TIDM patients should be treated with 6 to 12 gm cinnamon per patient per day. In contrast, all negative human studies used cinnamon at doses of 1 and 2 gm per patient per day. Therefore, further human studies are necessary to delineate the anti-autoimmune efficacy of cinnamon in TIDM patients.
Table-1.
Cinnamon in autoimmune conditions
| Autoimmune conditions |
Authors | Finding | Reference number |
|---|---|---|---|
| Rheumatoid arthritis | Rathi, B., et al. | Oral polyphenolic fraction of Ceylon cinnamon protected arthritis in a rat model. | 16 |
| Rheumatoid arthritis | Cheng, W., X. et al. | Oral cinnamaldehyde protected rats from collagen-induced arthritic rats. | 17 |
| Rheumatoid arthritis | Shishehbor, F., et al. | Oral cinnamon reduced disease severity in women with rheumatoid arthritis | 18 |
| Type 1 diabetes | Shen, Y., et al. | Ceylon cinnamon exhibited anti-diabetic effect in insulin-uncontrolled type 1 diabetic rats | 24 |
| Type 1 diabetes | Altschuler, J. A., et al. | Oral cinnamon did not show significant anti-diabetic effect in adolescent TIDM patients. | 25 |
| Type 1 diabetes | Baker, W. L., et al. | Upon meta-analysis of prospective randomized controlled trials, cinnamon did not display anti-diabetic effect. | 26 |
| Multiple sclerosis | Brahmachari, S., et al. | Sodium benzoate, a metabolite of cinnamon, exhibited protection in a mouse model of MS. | 14 |
| Multiple sclerosis | Mondal, S., et al. | Oral cinnamon displayed protection in three different animal models of MS. | 12 |
| Inflammatory bowel disease | Kwon, H.K., et al. | Oral cinnamon extract protected mice from intestinal colitis. | 38 |
| Inflammatory bowel disease | Hagenlocher, Y., et al. | Cinnamaldehyde decreased fibrotic symptoms and markers in a mouse model of colitis. | 39 |
| Inflammatory bowel disease | Hagenlocher, Y., et al. | Oral cinnamon extract reduces murine colitis in IL-10−/− mice. | 40 |
| Inflammatory bowel disease | Li, A.L., et al. | Cinnamon essential oil protected mice from dextran sodium sulfate-induced colitis. | 41 |
CINNAMON IN EXPERIMENTAL AUTOIMMUNE ENCEPHALOMYELITIS AND MULTIPLE SCLEROSIS
Although the central nervous system (CNS) is traditionally considered as “immune privileged”, in certain conditions, immune responses generated in the periphery targets myelin-producing cells oligodendrocytes in the CNS resulting in oligodendrocyte death and demyelination. Multiple sclerosis (MS) is such an autoimmune disorder in which CNS myelin faces autoimmune insults to exhibit demyelination of axons and associated debilitating symptoms [6, 28, 29]. Experimental autoimmune encephalomyelitis (EAE) is a rodent model of MS and in this model, myelin antigen-specific T cells cross through the blood-brain barrier (BBB) to enter into the CNS to cause autoimmune demyelination [30-32]. Sodium benzoate (NaB) is a metabolite of cinnamon and when NaB was administered through drinking water at physiologically supportable doses, ameliorated clinical symptoms and disease progression of EAE in recipient mice and inhibited the production of encephalitogenic T cells in donor mice [14]. Accordingly, the oral cinnamon treatment improved locomotor activities and inhibited clinical symptoms of RR-EAE in female PLP-TCR transgenic mice and adoptively transferred female SJL/J mice [12]. Cinnamon also reduced clinical symptoms of chronic EAE in male C57/BL6 mice [12]. Since cinnamon has a long track record as a non-toxic natural product, these rodent results suggest cinnamon as a possible complementary and alternative medicine for MS. However, a clinical trial either as a monotherapy or an add-on with available MS medications is required to establish the fragrance of cinnamon in MS.
CINNAMON IN INFLAMMATORY BOWEL DISEASE
Celiac disease is an autoimmune malady of the gastrointestinal system in which affected individuals suffer from diarrhea, abdominal pain, cramping, bloating, gas, etc. [33]. Celiac disease affects ~1% of the population in different parts of the world. Although celiac disease is not the same as inflammatory bowel disease, celiac disease is seen to be common in patients with inflammatory bowel disease (IBD) and ulcerative colitis [33]. Myosin IXB, a gene commonly associated with celiac disease, is also found to be mutated in IBD patients [34, 35]. Moreover, the chromosome 4q27 that is associated with celiac disease is also linked to IBD and ulcerative colitis [36, 37]. In a mouse model of colitis induced by 2,4,6 trinitrobenzenesulfonic acid, the oral cinnamon extract inhibited the induction and progression of intestinal colitis [38]. According to Hagenlocher et al [39], cinnamon extract or its main compound cinnamaldehyde is capable of decreasing fibrotic symptoms and markers in a mouse model of colitis. IL-10−/− mice present some of the symptoms of IBD. Interestingly, oral administration of cinnamon extract reduces symptoms of murine colitis, lowers infiltration of immune cells, decreases tissue damage, and normalizes bowel wall thickness in the colon tissue of IL-10−/− mice [40]. Moreover, in the dextran sodium sulfate-induced colitis mouse model, cinnamon essential oil enriched with cinnamaldehyde reduced the development of colitis and improved the diversity and richness of intestinal microbiota with a decrease in Helicobacter and Bacteroides and an increase in Bacteroidales_S24-7 family and short-chain fatty acid-producing bacteria [41]. Taken together, there are enough reasons to believe that cinnamon may be helpful for IBD and ulcerative colitis.
MECHANISMS BEHIND CINNAMON-MEDIATED ATTENUATION OF AUTOIMMUNE DISORDERS
How does cinnamon modulate autoimmune pathologies? Although the etiology of autoimmune disorders is poorly understood, several studies highlight activation of antigen-presenting cells (APCs), increase in inflammation, downregulation of anti-autoimmune regulatory T cells (Tregs) and Th2, upregulation of autoimmune Th1 and Th17, infiltration of mononuclear cells into target organs, and loss of protective molecules as critical components for the manifestation of different autoimmune pathologies [42]. Interestingly, cinnamon and its components/metabolites are capable of amending these autoimmune signaling pathways [13, 38].
Suppression of APCs by cinnamon:
Although other cells can present antigens under certain conditions, only macrophages, B cells, and dendritic cells are considered as professional APCs. In autoimmune signal transduction, APCs play an important role in the generation of autoreactive T cells via the presentation of specific antigen complexed with major histocompatibility complexes (MHCs) to T cells. Kwon et al [38] have described that treatment with cinnamon extract inhibited the maturation of MHCII-positive APCs or CD11c+ dendritic cells by suppressing the expression of co-stimulatory molecules (B7.1, B7.2, ICOS-L), MHCII, and cyclooxygenase (COX)-2. Microglia are considered as brain resident macrophages and it has been found that cinnamon metabolite NaB inhibits the expression of different surface markers (CD11b, CD11c, and CD68) in mouse microglia [13].
Decrease in pro-inflammatory molecules by cinnamon:
Chronic or acute inflammation plays an important role in the pathogenesis of many human disorders including autoimmune diseases [13, 31, 43-45]. However, oral cinnamon treatment is capable of reducing inflammation in vivo in the CNS of EAE mice [12]. Similarly, the administration of NaB through drinking water also inhibited the expression of pro-inflammatory molecules in the CNS of EAE mice [14]. According to Liu et al [46], cinnamaldehyde, the major component of cinnamon, suppresses IL-1β in rats with RA through the modulation of succinate/hypoxia-inducible factor-1α axis and inhibition of NLRP3. Conversely, cinnamic acid upregulated the level of suppressor of cytokine signaling 3 (SOCS3), an anti-inflammatory molecule, in brain cells [47]. In mouse models of colitis, either cinnamon extract or cinnamic acid reduces the level of IL-6 and different chemokines [39]. Similarly, oral cinnamon decreased serum levels of C-reactive protein (CRP) and TNF-α in RA patients as compared to the placebo group [18]. These results suggest cinnamon is capable of attenuating pro-inflammatory molecules in both humans and rodents with RA. Since the activation of NF-κB is essential for the transcription of most of the pro-inflammatory molecules, for a drug to exhibit an anti-inflammatory effect, it is almost compulsory to attenuate the activation of NF-κB. While investigating the mechanism, it is found that cinnamon metabolite NaB attenuates the activation of NF-κB and the expression of iNOS in glial cells via reducing the activation of small G protein p21ras [13].
Protection and/or upregulation of Tregs by cinnamon:
Regulatory T cells (Tregs), capable of modulating the immune system, maintaining the tolerance to self-antigens, and preventing autoimmune disease are considered as the master regulator of immune responses. These specialized cells are characterized by transcription factor Foxp3 and surface markers CD25, CD39, CD73, cytotoxic T-lymphocyte-associated protein 4 (CTLA4), etc. Huan et al [48] have described that the expression of Foxp3 and the numbers of peripheral Tregs are significantly reduced in patients with relapsing-remitting MS as compared to age-matched healthy subjects. According to Morita et al [49], the proportion of Tregs defined by both Foxp3 and CD25 is lower in patients with RA than control subjects. Accordingly, the suppressive abilities of Tregs in TIDM patients are diminished compared to Tregs in healthy controls [50]. Xufre et al [51] have shown that Tregs found in PBMCs of TIDM patients have low expression of Treg marker glucocorticoid-induced tumor necrosis factor-related receptor (GITR). Therefore, protection, up-regulation and/or maintenance of healthy Tregs may be beneficial for autoimmune disorders.
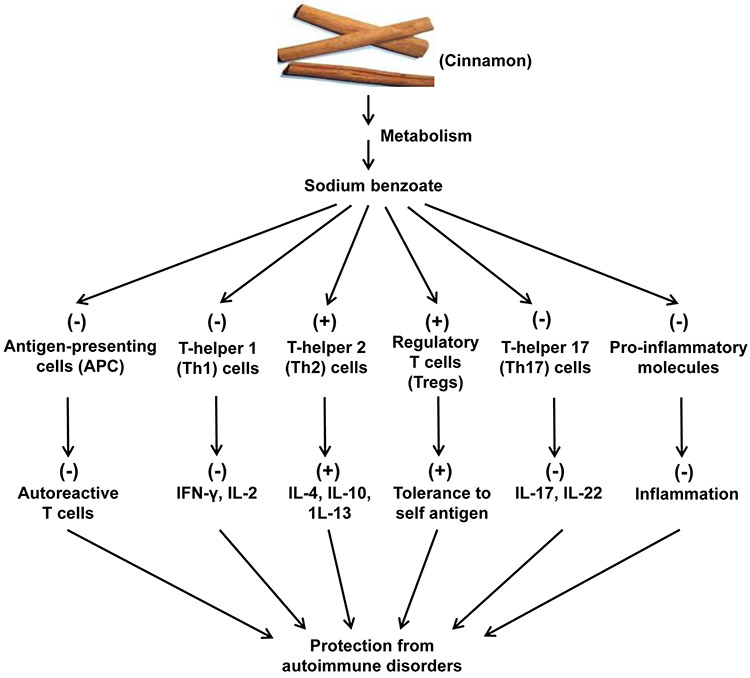
It has been found that oral feeding of cinnamon powder enriches Foxp3+ Tregs and inhibits the loss of CD25, CD62L, CTLA4, and GITR in vivo in EAE mice [12]. Cinnamon-induced Tregs are also capable of inhibiting the level of IFN-γ in autoreactive T cells, suggesting that cinnamon-induced Tregs have suppressive activity [12]. Accordingly, cinnamon metabolite NaB also protects Tregs in mice [14]. Interestingly, NaB attenuates the production of NO [13, 14] and restores Foxp3 in myelin basic protein (MBP)-primed splenocytes via suppression of NO [15], suggesting a critical role of NO in Treg homeostasis. According to Kundu et al [15], NaB enriches Tregs via STAT6-mediated upregulation of transforming growth factor β. Taken together, these preclinical studies indicate that cinnamon could be a perfect natural option to protect Tregs (Fig. 1) under autoimmune conditions.
Figure 1. Schematic diagram representing cinnamon-induced anti-autoimmune pathways.
Cinnamon is metabolized into sodium benzoate (NaB) that inhibits the activation of antigen-presenting cells (APCs), suppresses Th1 cells and associated autoimmune cytokines, stimulates Th2 cells and related anti-autoimmune cytokines, upregulates regulatory T cells (Tregs), attenuates Th17 cells and connected cytokines, and reduces inflammation, ultimately exhibiting protection against different autoimmune conditions.
Modulation of Th1-Th2 balance by cinnamon:
Th1 cells capable of producing pro-inflammatory cytokines like IFN-γ, IL-2, TNFα, etc. induce and/or aggravate inflammation and thereby play an important role in the pathogenesis of different autoimmune disorders [52]. For example, Li et al [53] have reported an increased level of IL-2 in the serum of patients with active RA. On the other hand, Th2 cells that secrete cytokines like IL-4, IL-10, and IL-13 are anti-inflammatory and known to protect against inflammatory autoimmune conditions [54, 55]. Therefore, a proper balance between Th1 and Th2 cells is necessary for a normal healthy immune system. While GATA3-dependent production of IL-10 and IL-4 is a characteristic of Th2 cells, Th1 cells display a T-bet-dependent IFN-γ release [7, 31]. Interestingly, oral treatment with cinnamon led to the decrease in IFN-γ and T-bet mRNAs as well as the CD4+IFN-γ+ Th1 cells, while increasing the CD4+IL-4+ Th2 response in mice with EAE [12]. Accordingly, it is seen that NaB treatment markedly inhibits the production of IFN-γ while stimulating the release of IL-4 and IL-10 from MBP-immunized splenocytes [14]. Therefore, cinnamon and NaB are capable of attenuating the differentiation of Th1 cells and stimulating the differentiation of Th2 cells (Fig. 1). It is shown that NO plays a critical role in Th1 to Th2 switching [56]. While scavenging of NO favored the induction of GATA3 expression and the production of IL-10 from MBP-primed splenocytes, excess NO stimulated the expression of T-bet and the release of IFN-γ [56]. Since NaB is capable of suppressing the production of NO [13, 14], NaB may employ this mechanism for switching the differentiation from Th1 to Th2.
Attenuation of Th17 response by cinnamon:
Nowadays, Th17 cells capable of secreting IL-17A, IL-17F, IL-21, and IL-22 are considered to play a more active role than Th1 cells in the pathogenesis of autoimmune disorders [52, 57]. Ghaffari et al [58] have seen that the IL-17 concentrations are significantly higher in patients with RRMS and PRMS compared with healthy individuals. According to Babaloo et al [59], levels of IL-17A and IL-17F are significantly higher in the serum of MS patients than healthy controls, and that there is a significant positive correlation between serum IL-17F with the number of relapses. Similarly, plasma levels of IL-17 and IL-22 are significantly higher in RA patients than those in healthy controls [60]. Kim et al [61] have shown that the level of Th17 cells in peripheral blood is associated with disease activity in RA. In Sjogren’s syndrome, IL-17F is correlated with increased autoantibody levels [62]. These results suggest that controlling and/or normalizing Th17 response may be beneficial for many autoimmune disorders.
Similar to that found in different autoimmune disorders, the expression of IL-17 mRNA and the level of CD4+IL-17+ T cells increase in EAE mice as compared to control mice. However, cinnamon treatment suppresses EAE-induced upregulation of IL-17 mRNA as well as the CD4+IL-17+ T cell population. Th17 cells are characterized by transcription factor RORγT and it has been found that cinnamon also reduces RORγT mRNA and CD4+RORγT+ T cells in EAE mice. Therefore, we may expect a reduction of Th17 response by cinnamon treatment in patients with autoimmune disorders.
Decrease in inflammatory infiltration by cinnamon:
Autoreactive T cells targeting a broad repertoire of antigens and epitopes are considered the main drivers of the destruction of target tissues and organs during most autoimmune disorders [63]. This aspect of autoimmune disorders has been probably best characterized in the case of MS and its animal model EAE [64]. It has been found that CD4 and CD8 T cells reactive to myelin antigens [e.g., myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG)] are present in elevated proportion in serum and cerebrospinal fluid (CSF) of patients with MS as compared to healthy controls [64-66]. Accordingly, the infiltration of autoreactive T cells and associated mononuclear cells is also a hallmark of EAE [30, 31, 67]. Similarly, in TIDM, pancreatic beta cells are destroyed by T cells of the immune system [68]. According to Noble et al [69], 50%–60% of the genetic risk for TIDM derives from HLA alleles encoding molecules involved in the presentation of antigen peptides to T cells. It has been found that CD8+ cytotoxic T cells are the most abundant population during insulitis followed by CD68+ macrophages, indicating an important contribution of both CD8+ cytotoxic cells and macrophages during early insulitis [64]. Similarly, T cell infiltration is also seen in rheumatoid arthritis [70], Sjogren’s syndrome [71], Guillain-Barre syndrome [72], and psoriasis [73]. Therefore, controlling inflammatory infiltration is an important mechanism to mitigate the damage of autoimmune disorders. It has been found that oral cinnamon treatment from the onset of the acute phase markedly reduces the infiltration of inflammatory cells into the CNS of mice with relapsing-remitting (RR) EAE. Quantitation shows that cinnamon is capable of reducing infiltration and the appearance of cuffed vessels in the spinal cord of RR-EAE mice. Therefore cinnamon may reduce inflammatory infiltration in autoimmune diseases.
CONCLUSION
In spite of extensive research, there are no effective treatments or cures for autoimmune conditions. Available NSAIDs and immunosuppressants only control the symptoms of autoimmune diseases temporarily. Some monoclonal antibodies (e.g. Natalizumab, Vedolizumab, Alemtuzumab, Rituximab, etc.) have also been approved for controlling some autoimmune disorders. Steroids are used to attenuate the flare of different autoimmune conditions when other treatments fail. However, in general, these treatments exhibit a number of side effects including fatigue, nausea, headache, joint/muscle pain, gastrointestinal disorders, immunosuppression, lung infection, breathing problems, wheezing, urinary tract infection, vaginitis, opportunistic viral infections like progressive multifocal leukoencephalopathy, etc. Therefore, it is important to identify a safe, effective and economical therapeutic option for autoimmune disorders.
Cinnamon, a natural herb commonly used to spice up the delicacy of different cuisine, has a long track record of human use without any toxic incidence. It can be taken orally, the least painful route, and after oral intake, it is metabolized into NaB, the active compound and the FDA-approved drug for UCD and NKH. Similar to cinnamon, NaB can be also taken through food and drinking water or milk. Since cinnamon and its metabolite NaB upregulate anti-autoimmune Tregs and Th2, suppress autoimmune Th17 and Th1, inhibit inflammatory infiltration, and reduce the expression of pro-inflammatory molecules, cinnamon and NaB may have therapeutic importance in different autoimmune disorders (Fig. 1). Although cinnamon has been tested in human rheumatoid arthritis and TIDM and different rodent models (Table-1), further clinical trials at appropriate doses are required to understand the beneficial effect of this complementary and alternate option in different autoimmune disorders.
Acknowledgements:
This study was supported by merit awards (1I01BX003033 and 1I01BX005002) from US Department of Veterans Affairs and a grant from NIH (AT10980). Moreover, Dr. Pahan is the recipient of a Research Career Scientist Award (1IK6 BX004982) from the Department of Veterans Affairs.
Footnotes
Conflicts of Interest: None
Data Availability:
This is a review and the readers can access all the published article supporting the conclusions of this study through PubMed.
References
- 1.Edner NM, et al. , Targeting co-stimulatory molecules in autoimmune disease. Nat Rev Drug Discov, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC, Alexopoulos H, and Spaeth PJ, Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsokos GC, Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol, 2020. 21(6): p. 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi Y, Hirota K, and Sakaguchi S, Impaired T cell receptor signaling and development of T cell-mediated autoimmune arthritis. Immunol Rev, 2020. 294(1): p. 164–176. [DOI] [PubMed] [Google Scholar]
- 5.Farley A and Hendry C, Autoimmune disorders. Nurs Stand, 2002. 16(41): p. 38–40. [DOI] [PubMed] [Google Scholar]
- 6.Franklin RJ and Ffrench-Constant C, Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci, 2008. 9(11): p. 839–55. [DOI] [PubMed] [Google Scholar]
- 7.Pahan K, Multiple Sclerosis and Experimental Allergic Encephalomyelitis. J Clin Cell Immunol, 2013. 4. [PMC free article] [PubMed] [Google Scholar]
- 8.Pisetsky DS and Lipsky PE, New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol, 2020. 16(10): p. 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein SL and Flanagan KL, Sex differences in immune responses. Nat Rev Immunol, 2016. 16(10): p. 626–38. [DOI] [PubMed] [Google Scholar]
- 10.Brahmachari S and Pahan K, Gender-specific expression of beta1 integrin of VLA-4 in myelin basic protein-primed T cells: implications for gender bias in multiple sclerosis. J Immunol, 2010. 184(11): p. 6103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jana A, et al. , Up-regulation of neurotrophic factors by cinnamon and its metabolite sodium benzoate: therapeutic implications for neurodegenerative disorders. J Neuroimmune Pharmacol, 2013. 8(3): p. 739–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mondal S and Pahan K, Cinnamon ameliorates experimental allergic encephalomyelitis in mice via regulatory T cells: implications for multiple sclerosis therapy. PLoS One, 2015. 10(1): p. e0116566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmachari S, Jana A, and Pahan K, Sodium benzoate, a metabolite of cinnamon and a food additive, reduces microglial and astroglial inflammatory responses. J Immunol, 2009. 183(9): p. 5917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmachari S and Pahan K, Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis. J Immunol, 2007. 179(1): p. 275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brahmachari S and Pahan K, Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. J Immunol, 2010. 184(4): p. 1799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathi B, et al. , Ameliorative Effects of a Polyphenolic Fraction of Cinnamomum zeylanicum L. Bark in Animal Models of Inflammation and Arthritis. Sci Pharm, 2013. 81(2): p. 567–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng WX, et al. , Cinnamaldehyde Inhibits Inflammation of Human Synoviocyte Cells Through Regulation of Jak/Stat Pathway and Ameliorates Collagen-Induced Arthritis in Rats. J Pharmacol Exp Ther, 2020. 373(2): p. 302–310. [DOI] [PubMed] [Google Scholar]
- 18.Shishehbor F, et al. , Cinnamon Consumption Improves Clinical Symptoms and Inflammatory Markers in Women With Rheumatoid Arthritis. J Am Coll Nutr, 2018: p. 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Onderoglu S, et al. , The evaluation of long-term effects of cinnamon bark and olive leaf on toxicity induced by streptozotocin administration to rats. J Pharm Pharmacol, 1999. 51(11): p. 1305–12. [DOI] [PubMed] [Google Scholar]
- 20.Qin B, et al. , Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract, 2003. 62(3): p. 139–48. [DOI] [PubMed] [Google Scholar]
- 21.Qin B, et al. , Cinnamon extract prevents the insulin resistance induced by a high-fructose diet. Horm Metab Res, 2004. 36(2): p. 119–25. [DOI] [PubMed] [Google Scholar]
- 22.Verspohl EJ, Bauer K, and Neddermann E, Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother Res, 2005. 19(3): p. 203–6. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y, et al. , Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS One, 2014. 9(2): p. e87894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen Y, et al. , Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci Biotechnol Biochem, 2010. 74(12): p. 2418–25. [DOI] [PubMed] [Google Scholar]
- 25.Altschuler JA, et al. , The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care, 2007. 30(4): p. 813–6. [DOI] [PubMed] [Google Scholar]
- 26.Baker WL, et al. , Effect of cinnamon on glucose control and lipid parameters. Diabetes Care, 2008. 31(1): p. 41–3. [DOI] [PubMed] [Google Scholar]
- 27.Kleefstra N, et al. , [Cinnamon: not suitable for the treatment of diabetes mellitus]. Ned Tijdschr Geneeskd, 2007. 151(51): p. 2833–7. [PubMed] [Google Scholar]
- 28.Noseworthy JH, Progress in determining the causes and treatment of multiple sclerosis. Nature, 1999. 399(6738 Suppl): p. A40–7. [DOI] [PubMed] [Google Scholar]
- 29.Noseworthy JH, et al. , Multiple sclerosis. N Engl J Med, 2000. 343(13): p. 938–52. [DOI] [PubMed] [Google Scholar]
- 30.Becher B, Bechmann I, and Greter M, Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med (Berl), 2006. 84(7): p. 532–43. [DOI] [PubMed] [Google Scholar]
- 31.Pahan K, Neuroimmune pharmacological control of EAE. J Neuroimmune Pharmacol, 2010. 5(2): p. 165–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pahan S and Pahan K, Mode of Action of Aspirin in Experimental Autoimmune Encephalomyelitis. DNA Cell Biol, 2019. 38(7): p. 593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly CP, et al. , Advances in diagnosis and management of celiac disease. Gastroenterology, 2015. 148(6): p. 1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monsuur AJ, et al. , Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet, 2005. 37(12): p. 1341–4. [DOI] [PubMed] [Google Scholar]
- 35.Hegan PS, et al. , Mice lacking myosin IXb, an inflammatory bowel disease susceptibility gene, have impaired intestinal barrier function and superficial ulceration in the ileum. Cytoskeleton (Hoboken), 2016. 73(4): p. 163–79. [DOI] [PubMed] [Google Scholar]
- 36.Marquez A, et al. , Novel association of the interleukin 2-interleukin 21 region with inflammatory bowel disease. Am J Gastroenterol, 2009. 104(8): p. 1968–75. [DOI] [PubMed] [Google Scholar]
- 37.Janse M, et al. , Three ulcerative colitis susceptibility loci are associated with primary sclerosing cholangitis and indicate a role for IL2, REL, and CARD9. Hepatology, 2011. 53(6): p. 1977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon HK, et al. , Cinnamon extract suppresses experimental colitis through modulation of antigen-presenting cells. World J Gastroenterol, 2011. 17(8): p. 976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagenlocher Y, et al. , Cinnamon reduces inflammatory response in intestinal fibroblasts in vitro and in colitis in vivo leading to decreased fibrosis. Mol Nutr Food Res, 2017. 61(9). [DOI] [PubMed] [Google Scholar]
- 40.Hagenlocher Y, et al. , Cinnamon extract reduces symptoms, inflammatory mediators and mast cell markers in murine IL-10(−/−) colitis. J Nutr Biochem, 2016. 30: p. 85–92. [DOI] [PubMed] [Google Scholar]
- 41.Li AL, et al. , Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol Immunol, 2020. 64(1): p. 23–32. [DOI] [PubMed] [Google Scholar]
- 42.Havnaer A and Han G, Autoinflammatory Disorders: A Review and Update on Pathogenesis and Treatment. Am J Clin Dermatol, 2019. 20(4): p. 539–564. [DOI] [PubMed] [Google Scholar]
- 43.Brahmachari S, Fung YK, and Pahan K, Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci, 2006. 26(18): p. 4930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brahmachari S and Pahan K, Role of cytokine p40 family in multiple sclerosis. Minerva Med, 2008. 99(2): p. 105–18. [PMC free article] [PubMed] [Google Scholar]
- 45.Parkinson JF, Mitrovic B, and Merrill JE, The role of nitric oxide in multiple sclerosis. J Mol Med, 1997. 75(3): p. 174–86. [DOI] [PubMed] [Google Scholar]
- 46.Liu P, et al. , Cinnamaldehyde suppresses NLRP3 derived IL-1beta via activating succinate/HIF-1 in rheumatoid arthritis rats. Int Immunopharmacol, 2020. 84: p. 106570. [DOI] [PubMed] [Google Scholar]
- 47.Chakrabarti S, et al. , Upregulation of Suppressor of Cytokine Signaling 3 in Microglia by Cinnamic Acid. Curr Alzheimer Res, 2018. 15(10): p. 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huan J, et al. , Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res, 2005. 81(1): p. 45–52. [DOI] [PubMed] [Google Scholar]
- 49.Morita T, et al. , The Proportion of Regulatory T Cells in Patients with Rheumatoid Arthritis: A Meta-Analysis. PLoS One, 2016. 11(9): p. e0162306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visperas A and Vignali DA, Are Regulatory T Cells Defective in Type 1 Diabetes and Can We Fix Them? J Immunol, 2016. 197(10): p. 3762–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xufre C, et al. , Low frequency of GITR+ T cells in ex vivo and in vitro expanded Treg cells from type 1 diabetic patients. Int Immunol, 2013. 25(10): p. 563–74. [DOI] [PubMed] [Google Scholar]
- 52.Pope RM and Shahrara S, Possible roles of IL-12-family cytokines in rheumatoid arthritis. Nat Rev Rheumatol, 2013. 9(4): p. 252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, et al. , Increased Serum Interleukin-2 Levels Are Associated with Abnormal Peripheral Blood Natural Killer Cell Levels in Patients with Active Rheumatoid Arthritis. Mediators Inflamm, 2020. 2020: p. 6108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z, et al. , Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol, 2019. 15(1): p. 9–17. [DOI] [PubMed] [Google Scholar]
- 55.Fang D and Zhu J, Molecular switches for regulating the differentiation of inflammatory and IL-10-producing anti-inflammatory T-helper cells. Cell Mol Life Sci, 2020. 77(2): p. 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dasgupta S, et al. , Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-alpha. Mol Pharmacol, 2007. 72(4): p. 934–46. [DOI] [PubMed] [Google Scholar]
- 57.van den Berg WB and Miossec P, IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol, 2009. 5(10): p. 549–53. [DOI] [PubMed] [Google Scholar]
- 58.Ghaffari SA, et al. , Circulating concentrations of interleukin (IL)-17 in patients with multiple sclerosis: Evaluation of the effects of gender, treatment, disease patterns and IL-23 receptor gene polymorphisms. Iran J Neurol, 2017. 16(1): p. 15–25. [PMC free article] [PubMed] [Google Scholar]
- 59.Babaloo Z, et al. , The role of Th17 cells in patients with relapsing-remitting multiple sclerosis: interleukin-17A and interleukin-17F serum levels. Immunol Lett, 2015. 164(2): p. 76–80. [DOI] [PubMed] [Google Scholar]
- 60.Zhao L, et al. , IL-22+ CD4+ T cells in patients with rheumatoid arthritis. Int J Rheum Dis, 2013. 16(5): p. 518–26. [DOI] [PubMed] [Google Scholar]
- 61.Kim J, et al. , Elevated levels of T helper 17 cells are associated with disease activity in patients with rheumatoid arthritis. Ann Lab Med, 2013. 33(1): p. 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gan Y, et al. , Increased Interleukin-17F is Associated with Elevated Autoantibody Levels and More Clinically Relevant Than Interleukin-17A in Primary Sjogren's Syndrome. J Immunol Res, 2017. 2017: p. 4768408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pugliese A, Autoreactive T cells in type 1 diabetes. J Clin Invest, 2017. 127(8): p. 2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin R, McFarland HF, and McFarlin DE, Immunological aspects of demyelinating diseases. Annu Rev Immunol, 1992. 10: p. 153–87. [DOI] [PubMed] [Google Scholar]
- 65.Ota K, et al. , T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature, 1990. 346(6280): p. 183–7. [DOI] [PubMed] [Google Scholar]
- 66.Liblau R, et al. , T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol, 1991. 21(6): p. 1391–5. [DOI] [PubMed] [Google Scholar]
- 67.Kuerten S and Lehmann PV, The immune pathogenesis of experimental autoimmune encephalomyelitis: lessons learned for multiple sclerosis? J Interferon Cytokine Res, 2011. 31(12): p. 907–16. [DOI] [PubMed] [Google Scholar]
- 68.Burrack AL, Martinov T, and Fife BT, T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front Endocrinol (Lausanne), 2017. 8: p. 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noble JA, et al. , HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes, 2010. 59(11): p. 2972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nevius E, Gomes AC, and Pereira JP, Inflammatory Cell Migration in Rheumatoid Arthritis: A Comprehensive Review. Clin Rev Allergy Immunol, 2016. 51(1): p. 59–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh N and Cohen PL, The T cell in Sjogren's syndrome: force majeure, not spectateur. J Autoimmun, 2012. 39(3): p. 229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCombe PA and Csurhes PA, T cells from patients with Guillain-Barre syndrome produce interferon-gamma in response to stimulation with the ganglioside GM1. J Clin Neurosci, 2010. 17(4): p. 537–8. [DOI] [PubMed] [Google Scholar]
- 73.Prinz JC, Human Leukocyte Antigen-Class I Alleles and the Autoreactive T Cell Response in Psoriasis Pathogenesis. Front Immunol, 2018. 9: p. 954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review and the readers can access all the published article supporting the conclusions of this study through PubMed.