Abstract
Aims/hypothesis
IL-2 injections are a promising therapy for autoimmune type 1 diabetes but the short half-life of this cytokine in vivo limits effective tissue exposure and necessitates frequent injections. Here we have investigated whether an injectable hydrogel could be used to promote prolonged IL-2 release in vivo.
Methods
Capitalising on the IL-2-binding capabilities of heparin, an injectable hydrogel incorporating clinical-grade heparin, collagen and hyaluronan polymers was used to deliver IL-2. The IL-2-release kinetics and in vivo stability of this material were examined. The ability of soluble IL-2 vs hydrogel-mediated IL-2 injections to prevent autoimmune diabetes in the NOD mouse model of type 1 diabetes were compared.
Results
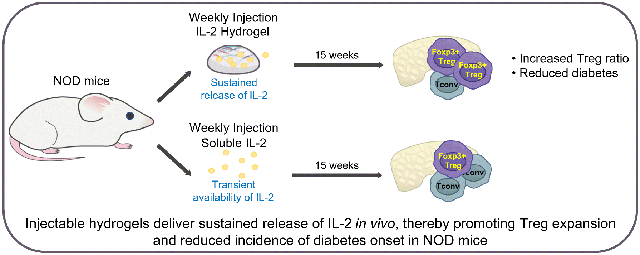
We observed in vitro that the hydrogel released IL-2 over a 12-day time frame and that injected hydrogel likewise persisted 12 days in vivo. Notably, heparin binding potentiates the activity of IL-2 and enhances IL-2- and TGFβ-mediated expansion of forkhead box P3-positive regulatory T cells (FOXP3+ Tregs). Finally, weekly administration of IL-2-containing hydrogel partially prevented autoimmune diabetes while injections of soluble IL-2 did not.
Conclusions/interpretation
Hydrogel delivery may reduce the number of injections required in IL-2 treatment protocols for autoimmune diabetes.
Keywords: Autoimmune, Controlled release, Diabetes, Heparin, Hyaluronan, Hydrogels, IL-2, Treg
Graphical Abstract
Graphical Abstract
Introduction
Type 1 diabetes is characterised by the progressive immune cell-mediated destruction of pancreatic beta cells and the failure of regulatory mechanisms that normally prevent this, including regulatory T cells (Tregs) (1).
One critical factor that governs Treg function is the cytokine IL-2 (2). The potential role of low-dose IL-2 in type 1 diabetes is a subject of active exploration and a frontier in the treatment and prevention of autoimmunity (3). Low-dose IL-2 affects two critical cell populations of the immune system: T cells and natural killer (NK) cells. However, IL-2 has a short half-life, depending on the route of administration, from 7 min for i.v. to several hours for i.p. and s.c. administration (4, 5). In addition, the dosing protocols vary depending on the disease. Cancer treatment usually requires daily dosing (6), whereas autoimmune disease protocols are variable, with some calling for administration of doses spaced at as much as 5 day intervals (6, 7). It would be desirable to extend the effective half-life of IL-2 to administer as few injections as possible.
One matrix component that has been used to bind and deliver cytokines in a sustained manner is heparin (8). Heparin binding also influences the bioactivity of many cytokines, including IL-2 (9-12), although effects on Tregs have not been previously examined.
We hypothesised that it would be possible to adapt a heparin-based hydrogel for the sustained and localised release of IL-2 to reduce autoimmune diabetes in the NOD mouse model of type 1 diabetes.
Methods
Mice
All animals were bred and maintained under specific pathogen-free conditions with free access to food and water in the vivarium at Stanford University (Stanford, CA). Female NOD and C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All experiments and animal use procedures were approved by the Animal Care & Use Committee of Stanford University. The animal assignment to experimental groups was performed randomly. Due to multiple different treatments during the course of the experiments, the experimenters were not blinded to group assignments, but experimenters were blinded for analysis.
Weight and diabetes monitoring
Beginning at 4 weeks of age, mice were weighed and bled weekly to measure their blood glucose levels. When two consecutive blood glucose readings of 13.87 mmol/l or greater were recorded, mice were considered diabetic.
Hydrogels
The hydrogel system is made up of thiolated hyaluronan/heparin/collagen components crosslinked with polyethylene glycol diacrylate. The heparin is a low-molecular-weight, clinical-grade product. The hyaluronan is a standardised, clinical-grade 500 kDa product. The collagen is type 1 collagen and is also clinical grade. These gel components together make up 1% weight/volume of the hydrogel. The Extracel and Extracel-HP are commercially available preparations that have been widely reported in the literature (13-15). Extracel (hyaluronan/collagen) and Extracel-HP (hyaluronan/heparin/collagen) hydrogels (BioTime, Alameda, CA, USA) were generated as per the manufacturer’s instructions. To assess the stability of these in vivo, 200 μl hydrogel incorporating an Alexa Fluor 790 fluorescent tag (Thermo Fisher, Waltham, MA, USA) were injected s.c. or i.p. into mice and allowed to polymerise in situ. Residual hydrogel mass was assessed at multiple days post injection using an IVIS 100 in vivo imaging system (Perkin Elmer, Waltham, MA, USA). A fibrin hydrogel was formulated by cleaving 5 mg/ml salmon fibrinogen (SeaRun Holdings, Freeport, ME, USA) with 2 U/ml salmon thrombin (SeaRun Holdings) in Extracel. A matrigel hydrogel (Corning, Corning, NY, USA) was prepared according to manufacturer’s instruction.
IL-2
The IL-2 used in this study was Proleukin (aldesleukin; Clinigen, PA, USA). It is a human recombinant IL-2 product, a highly purified protein with a molecular weight of approximately 15,300 Da. This is a clinical-grade product that has been used widely for over 20 years in numerous animal models and human clinical trials (6, 16).
IL-2 in vivo studies
For one set of experiments, 6-week-old NOD mice were treated with either 25,000 IU IL-2 once weekly or the same amount of IL-2 delivered in the context of a single hydrogel injection, or PBS injections as a negative control. After 1 month, mice were killed, mesenteric lymph nodes (LNs) were collected, and populations of lymphocytes were assessed by flow cytometry. For another set of experiments the above-mentioned treatment was administered to mice from 6 to 21 weeks of age. The mice were subsequently monitored for diabetes onset until 33 weeks of age.
Measurement of IL-2 release from hydrogels
Hydrogels were cast into a 96 well plate, 100 μl per well. After the hydrogels were formed, 200 μl of 1600 IU IL-2/ml PBS was added per well and incubated overnight. The loading solution was collected for later analysis. Release was conducted by incubating the samples in 200 μl of PBS, which was collected and replaced at the specified time points. IL-2 concentration was measured using an IL-2 ELISA (BioLegend, San Diego, CA, USA).
IL-2 proliferation assay
CTLL2 cells (ATCC, Manassas, VA, USA), were cultured for 48 h in the context of increasing concentrations of IL-2 with or without heparin. Proliferation was measured using a resazurin-based Reliablue Cell Viability Reagent (ATCC, Manassas, VA, USA).
Isolation and analysis of leucocyte populations
Total leukocytes were isolated from lymph nodes from 10-week-old NOD mice, as previously described (17). CD4+ T cells were isolated from the pooled cell suspensions using an EasySep Mouse CD4+ T Cell Isolation Kit (Stemcell, Vancouver, BC, Canada), following the manufacturer’s instructions. For T cell activation and forkhead box P3-positive (FOXP3+) induction studies, cell culture plates (96-well) were coated overnight with 5 μg/ml anti-CD3 antibody (catalogue no. 145-2C11; BD Biosciences, San Jose, CA, USA) and 2.5 μg/ml anti-CD28 antibody (catalogue no. 37.51; BD Biosciences). Subsequently, 1×105 cells were cultured with or without soluble 100 IU/ml IL-2 (Proleukin [aldesleukin]; Clinigen) and 50 ng/ml TGFβ (catalogue no. 21C11; BioLegend), as indicated using previously published protocols (18). Cells were stained and flow cytometry was performed as previously described (17). Analysis was done on an LSR II instrument (BD Biosciences) in the Stanford Shared FACS Facility (Stanford, CA, USA). Analysis was carried out on Flowjo v10 (Ashland, OR, USA).
Statistical analysis
Data are expressed as means ± SEM of n independent measurements. A p value of <0.05 was considered significant. Significance of the difference between the means of two or three groups of data was evaluated using a two-tailed t test or one-way ANOVA with Šidák’s multiple comparisons post-test.
Results
Heparin potentiates IL-2 Treg induction
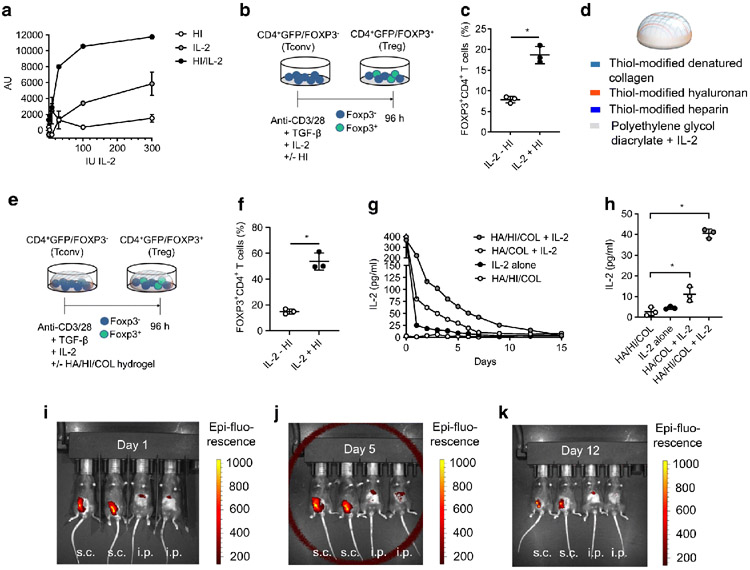
While heparin is reported to enhance the activity of IL-2, we investigated whether heparin-bound IL-2 likewise potentiates the effects of the cytokine on Tregs. In vitro we observed that proliferation with heparin complexed with IL-2 was more than twofold that with IL-2 alone (Fig. 1a). We then sought to determine whether heparin complexed with IL-2 likewise enhances the impact of IL-2 on Treg induction from CD4+GFP/FOXP3− conventional T cells (Tconv) precursors activated with anti-CD3 and anti-CD28 in the presence of TGFβ and IL-2 (Fig. 1b) (19). The presence of heparin and IL-2 greatly increased Treg induction in this assay (Fig. 1c).
Fig. 1.
Hydrogels release IL-2 in a sustained manner and potentiate Treg induction in the presence of heparin (HI). (a) CTLL2 cells were cultured in the presence of increasing concentrations of IL-2 with or without HI. Proliferation was measured by resazurin incorporation and expressed as arbitrary units (AU). Data are shown for triplicate wells per condition. (b) Schematic of CD4+GFP/FOXP3− T cells isolated from healthy (non-diabetic) mice, cultured in the setting of anti-CD3 and anti-CD28, TGFβ and IL-2 in the absence or presence of HI. (c) Percentage of CD4+ T cells that were FOXP3+ from experiment described in (b). Quantification of n=3 identical experiments. Data represent mean ± SEM; *p<0.05 as determined by a two-sided t test. (d) Schematic of hydrogel composition. (e) Schematic of CD4+GFP/FOXP3− T cells, isolated from healthy (non-diabetic) mice cultured in the setting of anti-CD3/-CD28, TGFβ and IL-2 in the absence or presence of HI and hydrogel. COL, collagen; HA, hyaluronan. (f) Percentage of CD4+ T cells that were FOXP3+ from experiment described in (e). Quantification of n=3 identical experiments. Data represent mean ± SEM; *p<0.05 as determined by a two-sided t test. (g) Hydrogel IL-2 release curve for different hydrogel compositions (HA, HI, COL and/or IL-2). (h) IL-2 release from hydrogels measured by IL-2 ELISA at day 7. Data represent mean ± SEM; *p<0.05 as determined by ANOVA followed by Šidák’s multiple comparisons test. Data in (g) and (h) are shown for the mean of triplicate wells per condition. (i–k) Hydrogels of 50 μl volume incorporating an Alexa Fluor 790 fluorescent tag were injected into mice s.c. and i.p. and allowed to polymerise in situ. Residual hydrogel mass was then assessed at 1 (i), 5 (j) and 12 days (k) post injection using an IVIS in vivo imaging system. Data are representative of three independent experiments
Heparin- and IL-2 containing hydrogels potentiates Treg induction
We next studied whether heparin in the context of a hydrogel also potentiates the effects of IL-2 on Treg induction. To this end, we coated tissue culture plates with a commercially available hyaluronan/heparin/collagen hydrogel preparation (Fig. 1d) and used the same induction protocol (Fig. 1e). In the presence of IL-2, the hyaluronan/heparin/collagen hydrogel significantly increased Treg induction vs the non-hydrogel-containing condition (Fig. 1f). Notably, repeating the experiment with a hyaluronan/collagen hydrogel lacking the heparin component, a fibrin hydrogel or a matrigel hydrogel did not potentiate Treg induction (data not shown).
Together, these data indicate that heparin potentiates the effects of IL-2 on Treg expansion, and that this stimulus can be delivered as a hydrogel.
Hydrogels release IL-2 in a sustained manner
We next sought to quantify the capacity of hydrogels to bind and slowly release IL-2 over time. The hyaluronan/heparin/collagen hydrogel eluted IL-2 through day 12, while the hyaluronan/collagen hydrogel eluted IL-2 through day 7 (Fig. 1g, h). These data indicate that hydrogels retain IL-2, releasing it over time, and that heparin enhances this.
Hydrogel persists in vivo
To determine whether the hyaluronan/heparin/collagen hydrogel is stable when injected in vivo, the hydrogel was delivered via s.c. and i.p. injection and allowed to polymerise in situ. Residual hydrogel mass in the mice was then assessed using the IVIS in vivo imaging system at 1, 5 and 12 days post hydrogel injection (Fig. 1i-k). Injection of the hydrogel s.c. resulted in longer persistence, which was still clearly seen at day 12 post injection (Fig. 1k). In general, hydrogel introduced via i.p. injection did not show a strong signal using the IVIS in vivo imaging (Fig. 1i-k). These data demonstrate that a hyaluronan/heparin/collagen hydrogel polymerises in vivo and is stable for up to 12 days.
Hydrogel-mediated IL-2 delivery is associated with an in vivo Treg increase
To investigate whether hydrogel-mediated IL-2 delivery could be used to promote Treg expansion in vivo, NOD mice were treated by once weekly i.p. injection of 25,000 IU IL-2 either in its soluble form or in a hyaluronan/heparin/collagen hydrogel. After 1 month of treatment, the mice were killed and T cell populations in the mesenteric LNs were then assessed via flow cytometry. We observed that the percentage of CD3+CD4+ T cells and CD3+CD8+ T cells in the LNs was significantly decreased in the IL-2 hydrogel treatment group (Fig. 2a, b). The percentage of CD3+CD4+FOXP3+ Tregs was significantly increased in the setting of the IL-2 hydrogel group (Fig. 2c), while the FOXP3 mean fluorescence intensity (MFI) was unchanged by either treatment (Fig. 2d). Together, these data indicate that hydrogel-mediated IL-2 delivery increases Tregs in vivo.
Fig. 2.
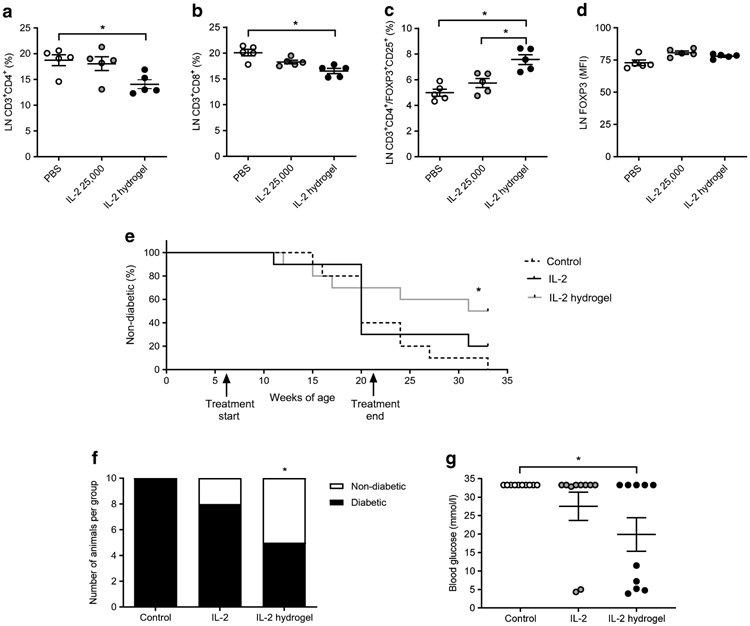
In vivo heparin (HI) hydrogels are associated with an increase in FoxP3+ Tregs and reduce diabetes onset in NOD mice. (a–d) Six-week-old NOD mice were treated with either 25,000 IU IL-2 once weekly or the same amount of IL-2 delivered in the context of a single hydrogel injection, or PBS injections as a negative control. After 1 month, mice were killed, mesenteric LNs were collected, and populations of lymphocytes were assessed by flow cytometry. In particular, the percentage of CD3+CD4+ T cells (a), the percentage of CD3+CD8+ T cells (b), CD3+CD4+FOXP3+ Tregs (c) and FOXP3 mean fluorescence intensity (MFI) (d) were assessed. Data are representative of two independent experiments; n=5 mice per group. Data represent mean ± SEM; *p<0.05 as determined by ANOVA followed by Šidák’s multiple comparisons test. (e) Starting at 6 weeks of age, NOD mice received either 25,000 IU IL-2 once weekly, or the same amount of IL-2 delivered in the context of a once a week hydrogel injection. PBS injections served as negative controls. Mice were then monitored weekly for diabetes onset. This treatment was administered to mice from 6 to 21 weeks of age and mice were subsequently monitored until 33 weeks of age. (f, g) Number of diabetic mice per group (f) and blood glucose (g) at 33 weeks of age, 3 months after treatment ended. Data represent mean ± SEM, n=10 mice per group. *p<0.05 as determined by ANOVA followed by Šiák’s multiple comparisons test
IL-2 delivery via hydrogel reduces the incidence of diabetes onset in NOD mice
In parallel to studying the effects of the IL-2 hydrogel on Treg expansion, we also investigated whether this treatment regimen could prevent autoimmune diabetes in NOD mice.
NOD mice were i.p. injected once weekly with 25,000 IU IL-2 either in its soluble form or in a hyaluronan/heparin/collagen hydrogel. This treatment was administered to 6-week-old mice for 15 weeks, a Kaplan–Meier curve shows the percentage of non-diabetic mice in the different treatment groups over time (Fig. 2e). Three months later, at 33 weeks of age, all mice in the control group, eight out of ten in the IL-2 group, and five out of ten in the IL-2 hydrogel group were diabetic (Fig. 2f). At 33 weeks of age, the blood glucose values of the control mice were >33.33 mmol/1, ~27.75 mmol/l for the IL-2 treatment group and ~20.53 mmol/l for the IL-2 hydrogel group (Fig. 2g). These data suggest that once weekly hydrogel delivery of IL-2 could partially prevent autoimmunity in this animal model.
Discussion
We report that once weekly injections of a hydrogel containing heparin and IL-2 promote Treg expansion and partially prevent autoimmunity in the NOD mouse model of type 1 diabetes. In contrast, the same IL-2 regimen injected without hydrogel did not. Other groups have used protocols in which IL-2 was administered daily or several times a week (20-23); these different treatment regimen make it difficult to compare data between studies. Our data suggest that it may be possible to reduce the likelihood of onset of autoimmune diabetes with less frequent injections using IL-2-releasing hydrogel preparations. Currently, IL-2 use alone cannot be used in humans to prevent autoimmune diabetes; we used an exploratory approach in our heparin- and IL-2-containing hydrogel study.
We have demonstrated that heparin delivered in diverse ways, alone or in the form of a hydrogel, amplifies the impact of IL-2 on multiple cell types. We report that a hydrogel containing heparin can release IL-2 over time using an assay performed in the absence of cells that does not therefore involve cellular responses to IL-2. Thus, we conclude that heparin has effects both on the potency of IL-2 as well as its sustained release from a hydrogel over time. We believe that enhanced IL-2 activity, as well as the extended release of IL-2 through very slow diffusion and degradation of the hydrogel itself, contribute to the effects reported here.
This hydrogel may have potential in treating other autoimmune disorders that are responsive to IL-2 (24), perhaps particularly autoimmune diseases of the skin. However, the safety and tolerability of hydrogel materials in the context of IL-2 will need to be examined (25, 26). Moreover, it would be important to evaluate the performance of these hydrogel materials over a range of IL-2 concentrations and formulations. Based on our findings, we conclude that hydrogel-mediated IL-2 delivery may be a useful approach for delivering IL-2 therapeutically.
Acknowledgements
We thank N. L. Haddock (Department of Medicine, Stanford University, Stanford, CA, USA) for her assistance with drafting the revised manuscript and for generating the graphical abstract.
Funding This work was supported in part by the National Institutes of Health (NIH) grants R01 DK096087-01, R01 HL113294-01A1, R01 DK116782-01A1 and U01 AI101984 to PLB. KY was supported by the Swiss National Science Foundation early postdoctoral mobility grant and the Stanford Child Health Research Institute and the Stanford NIH-NCATS-CTSA (grant no. UL1 TR001085). This work was also supported by grants from the JDRF 3-PDF-2014-224-A-N to NN and 1-SRA-2018-518-S-B Innovation Award to PLB and by grants from the Harrington Institute, Stanford SPARK, the Stanford Child Health Research Institute, all to PLB.
Abbreviations
- FOXP3
Forkhead box P3
- HA
Hyaluronan
- HI
Heparin
- LN
Lymph node
- Tconv
Conventional T cell
- Treg
FOXP3+ regulatory T cell
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data availability
The data are available from the corresponding author.
References
- 1.Bollyky PL, Falk BA, Long SA, et al. (2009) CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J Immunol. 183(4):2232–41. 10.4049/jimmunol.0900191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer AL, Yu A, Malek TR (2007) Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 178(7):4062–71. 10.4049/jimmunol.178.7.4062 [DOI] [PubMed] [Google Scholar]
- 3.Rosenzwajg M, Churlaud G, Mallone R, et al. (2015) Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. 58:48–58. 10.1016/j.jaut.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA (1985) In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol. 134(1):157–66 [PubMed] [Google Scholar]
- 5.Cheever MA, Thompson JA, Kern DE, Greenberg PD (1985) Interleukin 2 (IL 2) administered in vivo: Influence of IL 2 route and timing on T cell growth. J Immunol 134:3895–3900. https://www.jimmunol.org/content/134/6/3895 [PubMed] [Google Scholar]
- 6.Jeal W, Goa KL (1997) Aldesleukin (recombinant interleukin-2): a review of its pharmacological properties, clinical efficacy and tolerability in patients with renal cell carcinoma. BioDrugs 7(4):285–317. 10.2165/00063030-199707040-00005 [DOI] [PubMed] [Google Scholar]
- 7.Tahvildari M, Dana R (2019) Low-dose IL-2 therapy in transplantation, autoimmunity, and inflammatory diseases. J Immunol 203 (11) 2749–2755. 10.4049/jimmunol.1900733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D, Esko JD (2014) Demystifying heparan sulfate-protein interactions. Annu Rev Biochem. 83(1): 129–57. 10.1146/annurev-biochem-060713-035314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM (1991) Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 22;64(4):841–848. 10.1016/0092-8674(91)90512-w [DOI] [PubMed] [Google Scholar]
- 10.Wrenshall LE, Carlson A, Cerra FB, Platt JL (1994) Modulation of cytolytic T cell responses by heparan sulfate. Transplantation. 57(7):1087–1094 [PubMed] [Google Scholar]
- 11.Najjam S, Mulloy B, Theze J, Gordon M, Gibbs R, Rider CC (1998) Further characterization of the binding of human recombinant interleukin 2 to heparin and identification of putative binding sites. Glycobiology 8(5):509–516 10.1093/glycob/8.5.509 [DOI] [PubMed] [Google Scholar]
- 12.Ramsden L, Rider CC (1992) Selective and differential binding of interleukin (IL)-1a, IL-1b, IL-2 and IL-6 to glycosaminoglycans. Eur J Immunol 22:3027–3031 10.1002/eji.1830221139 [DOI] [PubMed] [Google Scholar]
- 13.Bollyky PL, Wu RP, Falk BA et al. (2011) ECM components guide IL-10 producing regulatory T-cell (TR1) introduction from effector memory T-cell precursors. PNAS 108(19):7938–7943 10.1073/pnas.1017360108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA (2010) Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials 31(17):4630–8 10.1016/j.biomaterials.2010.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Advanced BioMatrix (2020) HyStem: Thiol-Modified Hyaluronan Hydrogel Kit. Available from https://advancedbiomatrix.com/hystem.html. Accessed 20 July 2020
- 16.Proleukin (2020) Proleukin (aldesleukin) recombinant IL-2. Available from: www.proleukin.com. Accessed 20 July 2020
- 17.Ruppert SM, Falk BA, Long SA, Bollyky PL (2015) Regulatory T cells resist cyclosporine-induced cell death via CD44-mediated signaling pathways. Int J Cell Biol. 2015(4):614297–10. 10.1155/2015/614297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy N, Kaber G, Johnson PY, et al. (2015) Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J Clin Invest. 125(10):3928–40 10.1172/JCI79271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker MR, Kasprowicz DJ, Gersuk VH, et al. (2003) Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 112(9) 1437–43. 10.1172/JCI19441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serreze DV, Hamaguchi K, Leiter EH (1989) Immunostimulation circumvents diabetes in NODLt mice. J Autoimmun 2 (6) 759–776 10.1016/0896-8411(89)90003-6 [DOI] [PubMed] [Google Scholar]
- 21.Rabinovitch A, Suarez-Pinzon WL, Shapiro AMJ, Rajotte RV, Power R (2002) Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes 51(3)638–45 10.2337/diabetes.51.3.638 [DOI] [PubMed] [Google Scholar]
- 22.Tang Q, Adams JY, Penaranda C et al. (2008) Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28(5):687–697. 10.1016/j.immuni.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinberg-Bleyer Y, Baeyens A, You S et al. (2010) IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 207(9):1871–1878. 10.1084/jem.20100209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castela E, Le Duff F, Butori C, et al. (2014) Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 150(7):748–51. 10.1001/jamadermatol.2014.504 [DOI] [PubMed] [Google Scholar]
- 25.Long SA, Buckner JH, Greenbaum CJ (2013) IL-2 therapy in type 1 diabetes: “Trials” and tribulations. Clin Immunol. 149(3):324–31. 10.1016/j.clim.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 26.Long SA, Rieck M, Sanda S, et al. (2012) Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 61(9):2340–8. 10.2337/db12-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author.