Abstract
Background:
Pulmonary vascular disease, pulmonary endothelial dysfunction, liver fibrosis, renal disease, and exercise intolerance are common in adults with Fontan physiology. Although the pathophysiologic mechanisms linking these phenomena have been studied, certain aspects are not well understood.
Objectives:
We hypothesized that impaired pulmonary vascular reserve (VR) plays a central role linking these abnormalities, and that patients with abnormal pulmonary VR with exercise would display poorer pulmonary endothelial function, greater liver stiffness, more renal dysfunction and poorer exercise capacity as compared to patients with normal VR.
Methods:
Symptomatic adults with the Fontan palliation (n=29) underwent invasive cardiopulmonary exercise testing, echocardiography, and assessment of microvascular function. Abnormal pulmonary VR was defined by the slope of increase in pulmonary pressure relative to cardiac output (mPAP/CO slope) with exercise >3mmHg/l/min. Pulmonary endothelial function was assessed using reactive hyperemia index (RHI). End-organ function was assessed using magnetic resonance elastography-derived liver stiffness, glomerular filtration rate (GFR), NT-proBNP, and peak oxygen consumption (VO2).
Results:
Compared to individuals with normal VR (n=8), those with abnormal VR (n=21) displayed higher central and pulmonary venous pressures, and more severely impaired cardiac output and stroke volume responses to exertion, but similar pulmonary vascular resistance at rest (PVRrest). Patients with abnormal VR displayed more severely impaired RHI, increased liver stiffness, lower GFR, higher NTproBNP, and lower peak VO2. As compared to PVRrest, mPAP/CO slope displayed stronger correlations with RHI (r=−0.63 vs r=−0.31, Meng’s test p=0.009), MRE-derived liver stiffness (r=0.47 vs r=0.29, Meng’s test p=0.07), GFR (r=−0.52 vs r=−0.24, Meng’s test p=0.03), NT-proBNP (r=0.56 vs r=0.17, Meng’s test p=0.02) and peak VO2 (r=−0.63 vs r=−0.26, Meng’s test p=0.02).
Conclusions:
Pulmonary vascular limitations in Fontan physiology are related to pulmonary endothelial and end-organ dysfunction, suggesting a mechanistic link between these commonly-observed findings, and these abnormalities are more apparent during exercise testing, with little relationship at rest.
Keywords: Fontan physiology, pulmonary vascular disease, pulmonary vascular reserve, cardiopulmonary exercise test, endothelial dysfunction
CONDENSED ABSTRACT:
Pulmonary vascular disease, pulmonary endothelial dysfunction, and end-organ dysfunction are common in adults with Fontan physiology, but the pathophysiologic mechanisms linking these phenomena are poorly understood. The current study showed that impaired pulmonary vascular reserve (VR) during exercise was common in patients with Fontan physiology, including those with normal pulmonary vascular resistance at rest. Pulmonary VR limitations were related to pulmonary endothelial and end-organ dysfunction suggesting a mechanistic link between impaired endothelium-dependent vasodilation and subsequent end-organ dysfunction. Pulmonary VR assessment can improve the detection of pulmonary vascular disease at an earlier stage, and allowing for targeting of novel therapies.
Introduction
The Fontan operation is the treatment of choice for a number of complex congenital heart diseases characterized by a single ventricle, and it involves diversion of systemic venous blood directly into the pulmonary circulation thereby creating a non-pulsatile pulmonary circulation.[1] Because there is no sub-pulmonary ventricle in the Fontan circulation, central venous pressure (CVP) becomes the driving force for pulmonary blood flow. Maintenance of adequate systemic ventricular preload and prevention of excessive venous congestion accordingly becomes heavily reliant on low pulmonary vascular resistance (PVR).[2, 3] PVR may be normal in the early years after a Fontan operation, but pulmonary vascular disease (PVD) commonly develops over time [4,5].
Reductions in blood flow due to PVD in patients with the Fontan circulation lead to systemic venous congestion and chronic decreased output, and in turn, end-organ dysfunction affecting the gut, liver, kidneys, and lymphatic systems.[2, 3] Over time, this contributes to reduced survival.[3, 6] The mechanisms driving PVD in patients with the Fontan circulation remain unclear, but are believed to be related to pulmonary endothelial dysfunction resulting from chronic exposure to non-pulsatile pulmonary blood flow.[4, 5] Diagnosis of PVD in Fontan physiology has traditionally been based on the assessment of PVR at rest (PVRrest), but this may lead to under-detection of clinically-significant PVD, especially given the lower cardiac output (CO) seen in Fontan patients as compared to those with biventricular circulation.[7] For instance a minimal increase in transpulmonary gradient at rest (which can easily be missed or unrecognized) will become exaggerated during exercise due to increased flow. Pulmonary vascular reserve (VR), defined by the change in mean pulmonary artery pressure (mPAP) as flow increases during exercise (mPAP/CO slope) provides a more robust assessment,[8–10] but has not been studied in patients with Fontan physiology. The purposes of this study were to determine the extent to which dynamic assessment of pulmonary VR using invasive exercise testing is related to pulmonary endothelial dysfunction and multiple measures of end-organ dysfunction in patients with the Fontan palliation.
Methods
Patient selection and study design
This is a prospective study assessing the role of invasive cardiopulmonary exercise testing in Fontan physiology. All consecutive symptomatic adult Fontan patients (age >18 years) referred for cardiac catheterization at Mayo Clinic Rochester, MN from June 1, 2017 to March 31, 2020 were approached for the study. The patients that were able and willing to undergo exercise testing were enrolled in the study. The Mayo Clinic Institutional Review Board approved the study, and written informed consent was obtained from all patients.
The study objective was to determine whether pulmonary VR, assessed using invasive hemodynamic exercise testing, was related to an underlying cause of PVD such as pulmonary endothelial dysfunction, as well as downstream effects of PVD, including end-organ dysfunction, and whether this would be superior to the current clinical standard metric of resting PVR alone (PVRrest).
Invasive hemodynamic cardiopulmonary exercise testing
Cardiac catheterization was performed on chronic medications in the fasting state and under mild sedation. As previously described, venous access was obtained via right internal jugular vein using an 8Fr or 9Fr sheath to allow for simultaneous superior vena cava pressure measurement.[11] Venous catheterization was performed using a 7Fr balloon wedge catheter. Radial access was obtained for measurement of arterial pressure and arterial oxygen saturation; in patients undergoing ventricular catheterization, a 4Fr or 5Fr pigtail catheter was utilized. Oxygen consumption (VO2) was directly measured using expired gas analysis (MedGraphics, St. Paul, MN). Pressures in the Fontan pathway (superior vena cava, inferior vena cava, Fontan conduit/right atrium, pulmonary artery) were directly measured to determine the CVP and pulmonary artery wedge pressure (PAWP). Rest and exercise pressure recordings reported herein represent a computer-generated average of ≥5 consecutive beats measured during spontaneous breathing.
Venous oxygen saturation was directly measured from samples drawn from the branch pulmonary arteries (mixed venous saturation) and pulmonary artery wedge position (pulmonary venous saturation). A saturation of 95% was used as the assumed pulmonary venous saturation in cases (n=3) where the pulmonary venous saturation was not measured directly. Arterial saturation was measured from radial artery or ventricular blood samples. If radial arterial access could not be obtained due to small vessel size or occlusion (n=2), arterial saturation was measured using pulse oximetry. Systemic blood flow and pulmonary blood flow were calculated using the direct Fick method, and Qp/Qs and PVR were calculated using standard formulas [6].
All participants underwent right and left heart catheterization with comprehensive hemodynamic assessment at rest. Next, the participants exercised on a supine cycle ergometry, starting at 20W workload, increasing in 20W increments every 2 minutes to volitional exhaustion, and this exercise protocol has been previously described.[11, 12] Hemodynamic assessments, expired gas analysis, and blood sampling was performed at every stage of exercise. CVP and pulmonary blood flow (Qp) values were plot at rest and at each stage of exercise, and the line of best fit for the CVP/Qp relationship during exercise was generated for each patient using linear regression.[9, 10] We used Qp instead of CO (Qs) for the assessment of pulmonary VR because some patients developed right-to-left shunt during exercise resulting in a Qp<Qs. The slope of the line of best fit of CVP/Qp (which is a surrogate for mPAP/CO slope), was used as the reference measure of pulmonary VR.
Exploratory analysis: Diagnostic criteria for PVD
Exploratory analysis was performed to determine the clinical implications of using pulmonary VR as the metric for PVD diagnosis as compared to the conventional method of using PVRrest.[7, 13, 14] Based on the conventional method for PVD diagnosis, patients with PVRrest >2 WU*m2 are classified as having PVD. However, if we apply the proposed diagnostic criteria[8–10] that is based on mPAP/CO slope, the patients with mPAP/CO slope >3 mmHg/l*min (including those with PVRrest <2 WU*m2) will be reclassified as having PVD.
Pulmonary endothelial function assessment
All assessments were performed in a quiet and darkened environment. Participants refrained from using tobacco, caffeine, or exercising for >8 hours prior to testing. Pulmonary endothelial function was measured using peripheral artery tonometry, EndoPAT 2000 (Itamar Medical, Caesarea, Israel), as previously described.[15, 16] The EndoPAT probes were placed on the index finger hand. The plethysmography probes record pulsatile volume signals. A 5-minute baseline recording was acquired and continued throughout cuff occlusion and the 5-minute period following cuff deflation. Reactive hyperemia index (RHI) was calculated as the ratio of the average amplitude of the signal for the second minute after cuff deflation divided by the average amplitude of the signal for 3.5 minutes of baseline, normalized to the control arm.
End-organ function
End-organ function was assessed across multiple domains: (1) liver congestion/fibrosis was assessed by liver stiffness using magnetic resonance elastography (MRE);[17] (2) kidney function was assessed using cystatin-C derived glomerular filtration rate; (3) volume status was assessed using N terminal pro hormone brain natriuretic peptide (NT-proBNP) and estimated plasma volume;[18] (4) aerobic capacity was assessed using peak VO2 from an upright treadmill exercise[19] (different from the supine exercise test performed in the cardiac catheterization laboratory); (5) quality of life was assessed using the Minnesota Living with Heart Failure Questionnaire.[20] All assessments of end-organ function were performed within 2 days prior to cardiac catheterization.
Statistical analysis
Data were presented as mean ± standard deviation, median (interquartile range), or count (%) as appropriate. Between-group comparisons were performed using Fisher’s exact test, unpaired t-test, and Wilcoxon rank sum test as appropriate. Linear regression analysis was used to assess the correlation between continuous variables, and Meng’s test was used for between-group comparison of the robustness of the regression models.[21] All statistical analyses were performed with JMP software (version 14.0; SAS Institute Inc, Cary NC). A p<0.05 was considered statistically significant. The “P values presented in this report have not been adjusted for multiplicity, and therefore inferences drawn from these statistics may not be reproducible
Results
A total 29 patients were enrolled in the study, the mean age at the time of enrollment was 29±6 years, mean age at the time of Fontan operation was 5±3 years, and 16 (55%) were males. The primary indications for cardiac catheterization were volume overload (n=8), arrhythmias (n=6), cyanosis (n=3), and liver disease (n=12). All patients were symptomatic (18 patients were in New York Heart Association functional class II while 11 patients were in New York Heart Association functional class III). Of the 8 patients with volume overload, 6 presented with pedal edema while 2 presented ascites and pedal edema. Of the 3 patients with cyanosis, 1 patient had a small Fontan conduit fenestration while 2 patients had veno-venous collateral that were subsequently coiled. All 12 patients with liver disease presented with evidence of chronic liver disease on liver ultrasound and magnetic resonance elastography.
A total of 8 patients (29%) displayed normal mPAP/CO slope (28%) and 21 (72%) displayed abnormal mPAP/CO slope. Table 1 shows the baseline clinical characteristics of the cohort.
Table 1:
Baseline Characteristics
| All (n=29) | mPAP/CO slope >3 | |||
|---|---|---|---|---|
| No (n=8) | Yes (n=21) | p | ||
| Age at time catheterization, years | 29±6 | 27±4 | 30±5 | 0.09 |
| Age at Fontan operation, years | 5±3 | 4±1 | 5±3 | 0.3 |
| Male | 16 (55%) | 4 (50%) | 12 (57%) | 0.7 |
| Body surface area, m2 | 1.9±0.3 | 1.8±0.2 | 1.9±0.3 | 0.6 |
| Left ventricle | 18 (62%) | 4 (50%) | 14 (67%) | 0.7 |
| Atriopulmonary Fontan | 9 (31%) | 2 (25%) | 7 (33%) | 0.2 |
| History of atrial arrhythmia | 13 (45%) | 3 (38%) | 10 (48%) | 0.2 |
| Cirrhosis | 11 (38%) | 2 (25%) | 9 (42%) | 0.4 |
| Laboratory tests | ||||
| Hemoglobin, g/dl | 14.3 ±2.5 | 13.9±1.8 | 14.9±2.1 | 0.3 |
| Platelet, x109/l | 139±41 | 145±22 | 128±33 | 0.2 |
| Albumin, g/dl | 4.3±0.7 | 4.2±0.4 | 4.3±0.6 | 0.8 |
| Aspartate aminotransferase, U/l | 39±6 | 41±5 | 38±7 | 0.6 |
| Alanine aminotransferase, U/l | 47±8 | 46±4 | 47±8 | 0.7 |
| Therapies | ||||
| Paced rhythm | 3 (10%) | 1 (13%) | 2 (10%) | 0.4 |
| BB or CCB therapy | 6 (21%) | 1 (13%) | 5 (24%) | 0.5 |
| RAAS antagonist | 9 (31%) | 2 (26%) | 7 (33%) | 0.2 |
| Warfarin | 10 (34%) | 2 (26%) | 8 (38%) | 0.3 |
| Diuretics | 12 (41%) | 2 (25%) | 10 (48%) | 0.06 |
MRE: magnetic resonance elastography; GFR: glomerular filtration rate; NT proBNP: N-terminal pro hormone brain natriuretic peptide; VO2: oxygen consumption; FEV1: forced expiratory volume; FVC: forced vital capacity; BB: Beta-blocker; CCB: calcium channel blockers; RAAS: renin angiotensin aldosterone system; mPAP/CO slope: slope of mean pulmonary artery pressure versus cardiac output
Rest and exercise hemodynamics
Table 2 shows the hemodynamic indices at rest. As compared to the patients with normal mPAP/CO slope, those with abnormal mPAP/CO slope had higher resting CVP (16±3 vs 12±2 mmHg, p=0.002), higher PAWP (12±4 vs 8±2 mmHg, p=0.01), and lower stroke volume index (29±7 vs 35±5 mmHg, p=0.04), but similar PVRrest (1.8±0.6 vs 1.6±0.5, WU*m2, p=0.4). There was a poor correlation between PVRrest and mPAP/CO slope (r=0.23, p=0.1).
Table 2:
Rest Hemodynamics
| All (n=29) | mPAP/CO slope >3 | |||
|---|---|---|---|---|
| No (n=8) | Yes (n=21) | p | ||
| Mixed venous saturation, % | 63±7 | 65±6 | 62±5 | 0.2 |
| Pulmonary venous saturation, % | 94±2 | 95±2 | 94±3 | 0.6 |
| Systemic saturation, % | 94±3 | 94±2 | 93±2 | 0.6 |
| Heart rate, bpm | 72±9 | 71±7 | 77±8 | 0.07 |
| CVP, mmHg | 15±4 | 12±2 | 16±3 | 0.002 |
| PAWP, mmHg | 11±4 | 8±2 | 12±4 | 0.01 |
| TPG, mmHg | 4±2 | 4±1 | 4±2 | 0.6 |
| Systolic blood pressure, mmHg | 116±14 | 110±9 | 119±13 | 0.3 |
| Diastolic blood pressure, mmHg | 66±14 | 64±10 | 67±14 | 0.6 |
| Mean blood pressure, mmHg | 84±13 | 83±11 | 84±13 | 0.4 |
| Qp index, l/min/m2 | 2.3±0.9 | 2.5±0.6 | 2.2±0.7 | 0.3 |
| PVR index, WU*m2 | 1.7±0.6 | 1.6±0.5 | 1.8±0.6 | 0.4 |
| Qs index, l/min/m2 | 2.3±0.8 | 2.5±0.4 | 2.2±0.5 | 0.2 |
| Qp/Qs | 1.0±0.1 | 1. 0±0. 1 | 1.0±0.1 | 0.8 |
| SVI, ml/m2 | 32±8 | 35±5 | 29±7 | 0.04 |
| SVR index, WU*m2 | 30±11 | 28±10 | 31±8 | 0.5 |
PVR: pulmonary vascular resistance; CVP: central venous pressure; SVI: stroke volume index; SVR: systemic vascular resistance; PAWP: pulmonary artery wedge pressure; TPG: transpulmonary gradient; Qp: pulmonary blood flow; Qs: systemic blood flow; mPAP/CO slope: slope of mean pulmonary artery pressure versus cardiac output
Participants exercised to a peak workload of 73±25W, with exercise duration of 7.9±2.8 minutes. There were no hemodynamic or vascular complications during exercise. As expected, the CVP, PAWP, and CO increased with exercise. The between-group differences in CVP, PAWP and stroke volume index became more exaggerated at 20W and peak exercise (Table 3). Although both groups displayed similar PVRrest, those with abnormal mPAP/CO slope had higher PVR at 20W (2.8±0.6 vs 1.6±0.4, WU*m2, p<0.001) and peak exercise (3.6±0.7 vs 1.5±0.3, WU*m2, p<0.001).
Table 3:
Exercise Hemodynamics
| 20W | Peak | |||||
|---|---|---|---|---|---|---|
| mPAP/CO slope >3 | mPAP/CO slope >3 | |||||
| No (n=8) | Yes (n=21) | p | No (n=8) | Yes (n=21) | p | |
| CVP, mmHg | 16±3 | 24±5 | <0.001 | 19±5 | 29±7 | <0.001 |
| PAWP, mmHg | 11 ±2 | 17±4 | <0.001 | 13±3 | 19±6 | 0.01 |
| TPG, mmHg | 5±1 | 7±3 | 0.08 | 6±2 | 10±3 | 0.002 |
| Qp index, l/min/m2 | 3.2±0.5 | 2.5±0.9 | 0.05 | 3.9±0.6 | 2.8±1.1 | 0.01 |
| PVR index, WU*m2 | 1.6±0.4 | 2.8±0.6 | <0.001 | 1.5 ±0.3 | 3.6±0.7 | <0.001 |
| Qs index, l/min/m2 | 3.2±0.4 | 2.5±0.5 | 0.002 | 3.9±0.5 | 3.0±0.9 | 0.01 |
| Qp/Qs | 1.0±0.1 | 1.0±0.1 | 0.8 | 1.0±0.1 | 0.9±0.1 | 0.5 |
| Heart rate, bpm | 83±8 | 86±9 | 0.4 | 122±9 | 119±14 | 0.6 |
| SVI, ml/m2 | 39±6 | 29±7 | 0.001 | 32±5 | 24±6 | 0.002 |
PVR: pulmonary vascular resistance; CVP: central venous pressure; SVI: stroke volume index; SVR: systemic vascular resistance; PAWP: pulmonary artery wedge pressure; TPG: transpulmonary gradient; Qp: pulmonary blood flow; Qs: systemic blood flow; mPAP/CO slope: slope of mean pulmonary artery pressure versus cardiac output
Pulmonary endothelial and end-organ function
Subjects with abnormal mPAP/CO slope displayed lower RHI (0.9 [0.2–2.5] vs 1.5 [0.6–4.2] indicating worse microvascular pulmonary endothelial function (Table 4). There was a strong correlation between mPAP/CO slope during exercise and RHI (r= −0.63, p<0.001), which was much stronger than the corresponding correlation between PVRrest and RHI (r= −0.31, p=0.04; Meng’s test p=0.009 between correlations) (Figure 1).
Table 4:
Comparison of Endothelial and End-organ Function Indices
| All (n=29) | mPAP/CO lope >3 | |||
|---|---|---|---|---|
| No (n=8) | Yes (n=12) | p | ||
| Endothelial function | ||||
| EndoPAT-derived RHI | 1.1 (0.2–4.2) | 1.5 (0.6–4.2) | 0.9 (0.2–2.5) | <0.001 |
| LnRHI | 0.22±0.17 | 0.27±0.11 | 0.20±0.11 | <0.001 |
| End-organ function | ||||
| MRE liver stiffness, kPa | 5.6±1.8 | 4.9±0.8 | 5.8±0.6 | 0.03 |
| GFR, ml/min/1.73 m2 | 86±11 | 98±8 | 77±6 | 0.02 |
| NT-proBNP, pg/ml | 461±128 | 314±66 | 502±102 | 0.01 |
| Estimated plasma volume, ml | 3,181 ±613 | 2,284±207 | 3,301±422 | 0.07 |
| Peak VO2 ,% predicted | 56±18 | 67±11 | 49±15 | <0.001 |
| MLHFQ | 39±22 | 24±15 | 42±13 | 0.004 |
RHI: reactive hyperemia index; LnRHI: natural log of reactive hyperemia index; MRE: magnetic resonance elastography; GFR: glomerular filtration rate; NT proBNP: N-terminal pro hormone brain natriuretic peptide; VO2: oxygen consumption; MLHFQ: Minnesota living with heart failure questionnaire; mPAP/CO slope: slope of mean pulmonary artery pressure versus cardiac output
Figure 1:
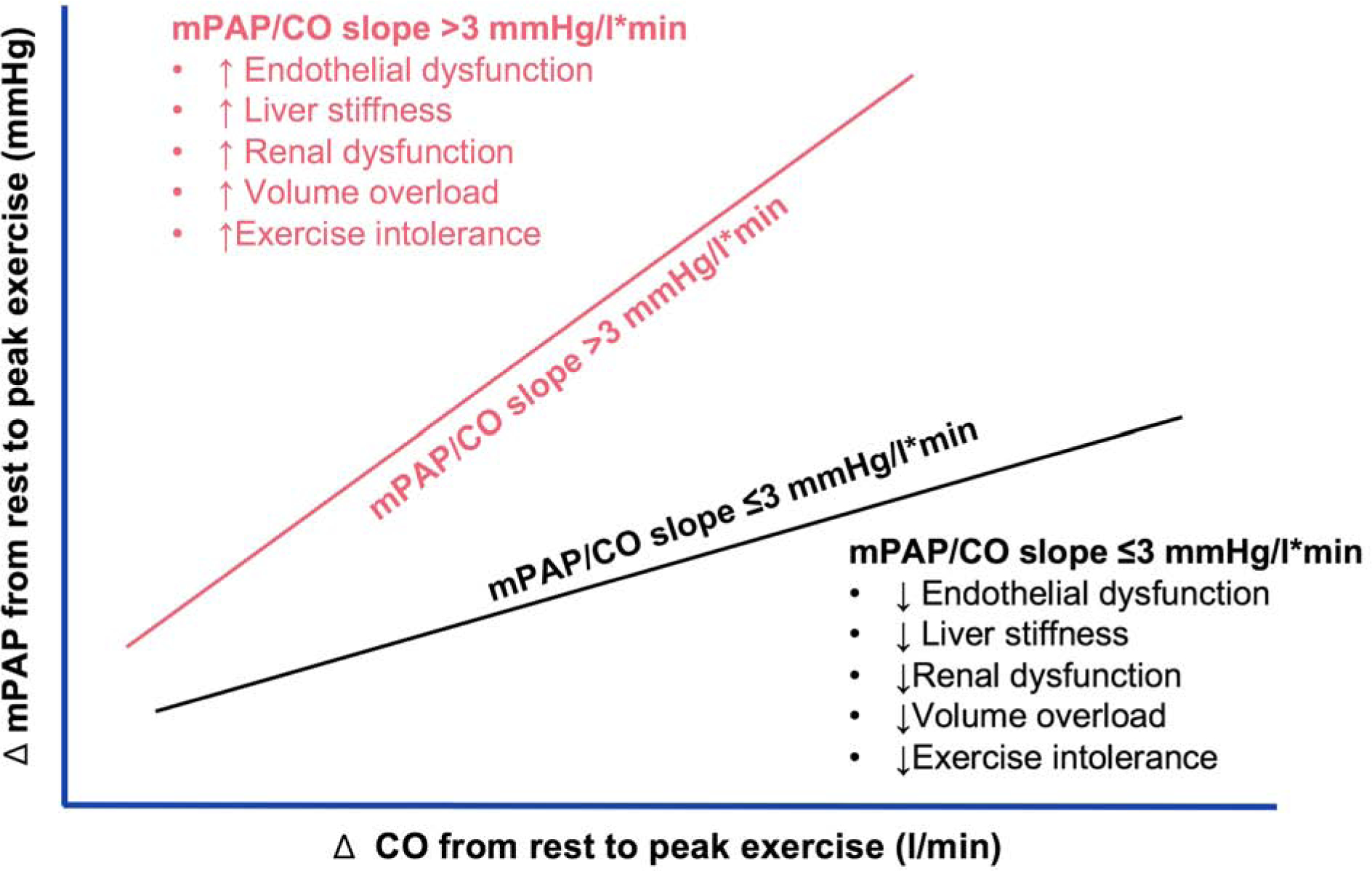
Comparison of the correlations between pulmonary vascular function and endothelial function. As compared to PVRrest (black), mPAP/CO slope (red) had a stronger correlation with RHI (Meng’s test p=0.009). PVRrest: pulmonary vascular resistance at rest; mPAP/CO slope: change in mean pulmonary artery pressure per unit change in cardiac output; RHI: reactive hyperemia index.
End-organ function was assessed across multiple domains (Table 4). As compared to participants with normal mPAP/CO slope, those with abnormal mPAP/CO slope displayed more severe renal dysfunction (lower GFR), increased liver stiffness (MRE-derived liver stiffness), more volume overload (higher NT-proBNP and estimated plasma volume), reduced aerobic capacity (lower peak VO2), and poorer quality of life outcomes (Table 4, Central Illustration). As compared to PVRrest, mPAP/CO slope displayed stronger correlations with MRE-derived liver stiffness (r=0.47 vs r=0.29, Meng’s test p=0.07), GFR (r= −0.52 vs r= −0.24, Meng’s test p=0.03), NT-proBNP (r=0.56 vs r=0.17, Meng’s test p=0.02) and peak VO2 (r= −0.63 vs r= −0.26, Meng’s test p=0.02) (Table 5). The regression coefficients with slopes and y-intercepts are shown in Supplemental Table 1.
Central illustration.
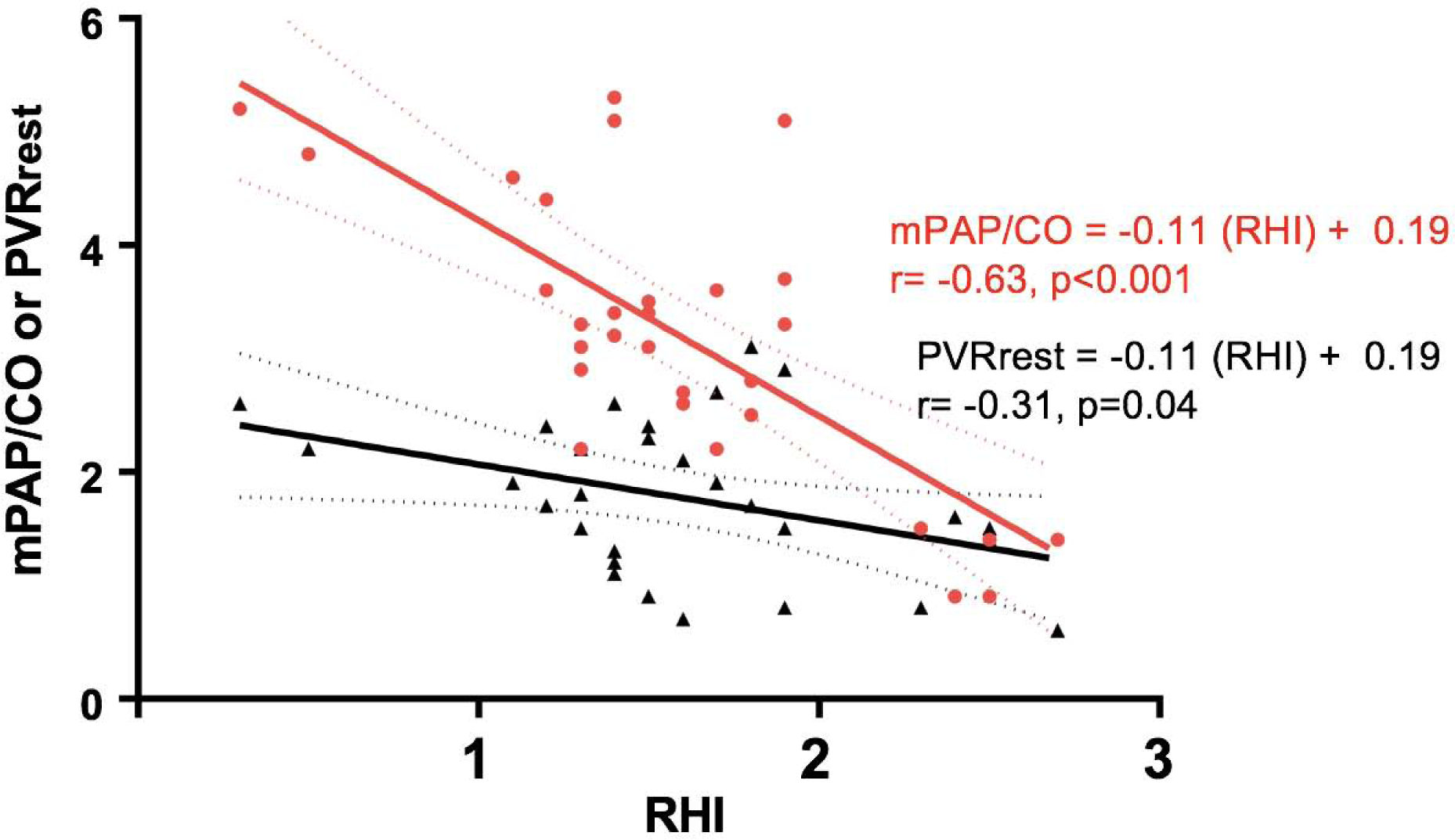
Schematic showing the relationship between pulmonary vascular reserve and end-organ function. Pressure-Flow relationship showing change in mean pulmonary artery pressure (Fontan pressure) per unit change in cardiac output during exercise. Abnormal pulmonary vascular reserve defined as mPAP/CO slope >3 (red) is associated with worse endothelial dysfunction and end-organ dysfunction (more liver stiffness, renal dysfunction, volume overload and exercise intolerance) as compared to normal pulmonary vascular reserve defined as mPAP/CO slope ≤3 (black). mPAP/CO slope: change in mean pulmonary artery pressure per unit change in cardiac output
Table 5:
Comparison of Correlation coefficients (r) and Slopes (β coefficient) Between Models Based on PVRrest vs Models Based on mPAP/CO slope
| End-organ function | PVRrest r (p) | mPAP/CO slope r (p) | Meng test p | PVRrest β (95%CI) | mPAP/CO slope β (95%CI) | p |
|---|---|---|---|---|---|---|
| Endothelial function | ||||||
| EndoPAT-derived RHI | −0.31 (0.04) | −0.63 (<0.001) | 0.02 | −0.06 (−0.12 – −0.01) | −0.11 (−0.18 – −0.0) | <0.001 |
| End-organ function | ||||||
| MRE liver stiffness | 0.29 (0.04) | 0.47 (0.008) | 0.07 | 0.08 (0.02 – 1.98) | 0.21 (2.84 – 5.57) | <0.001 |
| GFR | −0.24 (0.07) | −0.52 (<0.001) | 0.03 | −0.03 (−0.07 – 0.01) | −0.09 (−0.13 – −0.04) | 0.008 |
| NT-proBNP | 0.17 (0.2) | 0.56 (<0.001) | 0.02 | 0.05 (−0.13 – 0.19) | 0.26 (0.14 – 0.39) | <0.001 |
| Est plasma volume | 0.08 (0.5) | 0.24 (0.1) | 0.4 | 0.01 (−0.16 – 0.14) | 0.06 (−0.09 – 0.21) | 0.2 |
| Peak VO2 | −0.26 (0.09) | −0.63 (<0.001) | 0.02 | −0.06 (−0.11 – 0.02) | −0.19 (−0.30 – −0.04) | 0.01 |
| MLHFQ | 0.22 (0.1) | 0.28 (0.08) | 0.4 | 0.01 (−0.18 – 0.20) | 0.07 (−0.03 – 0.15) | 0.5 |
PVR: pulmonary vascular resistance; mPAP/CO slope: slope of mean pulmonary artery pressure versus cardiac output; RHI: reactive hyperemia index; MRE: magnetic resonance elastography; GFR: glomerular filtration rate; NT proBNP: N-terminal pro hormone brain natriuretic peptide; VO2: oxygen consumption; MLHFQ: Minnesota living with heart failure questionnaire; CI: confidence interval
Clinical implications of using mPAP/CO slope for PVD Diagnosis
Applying the conventional criteria for PVD diagnosis (PVRrest >2 WU*m2), 9 of the 29 patients (31%) would be classified as having PVD and would qualify for initiating pulmonary vasodilator therapy, while 20 (69%) patients would be classified as not having PVD and hence not qualify for pulmonary vasodilators. However, if PVD diagnosis was based on mPAP/CO slope, 12 of 20 (60%) patients (i.e. 41% of the entire cohort), initially classified have been no PVD, will be reclassified as PVD.
Supplemental Table 2 shows a comparison of the 3 PVD subgroups. The ‘no PVD’ group represents the patients with normal PVRrest and normal mPAP/CO slope while the ‘overt PVD’ group represents the patients with abnormal PVRrest and abnormal mPAP/CO slope. The ‘occult PVD’ group represents the patients with normal PVRrest and abnormal mPAP/CO slope, and these are the patients that will be reclassified if mPAP/CO slope were used for PVD diagnosis. As compared to the no PVD group, the occult PVD and overt PVD groups displayed worse hemodynamics, more severe pulmonary endothelial dysfunction, and greater burden of end-organ dysfunction. Notably, there was no difference between the occult PVD and overt PVD groups in hemodynamic severity, pulmonary endothelial dysfunction and end-organ dysfunction, suggesting that both subgroups share a common pathophysiologic signature in the disease spectrum.
Discussion
In this prospective study we evaluated rest and exercise hemodynamics, and the association between hemodynamic abnormalities and measures of non-cardiac organ function, including endothelium-dependent vasodilation as well as indicators of liver and kidney function, volume status, and aerobic capacity in patients with the Fontan palliation. We demonstrate that patients with reduced pulmonary VR (elevated mPAP/CO slope) displayed more severe pulmonary endothelial and end-organ dysfunction, and that the observed abnormalities in pulmonary VR were more tightly correlated to measures of pulmonary endothelial and end-organ functions than was apparent from assessing PVR at rest. These data provide new insight into the pathophysiology of PVD in the Fontan circulation, and indicating how pulmonary VR limitation may potentially impact end-organ function.
Pathophysiologic implications
In health, pulmonary blood flow increases during exercise with minimal increase in pulmonary arterial pressure, as PVR normally decreases and distensibility increases.[22, 23] This is due to the vasodilation and increased capillary recruitment in the pulmonary vascular bed. Pulmonary VR is mediated via different mechanisms, of which the endothelium-dependent nitric oxide system plays a prominent role.[4, 5, 24, 25] Endothelial dysfunction is common in patients with Fontan palliation,[26–28] and we show for the first time that the extent of pulmonary endothelial dysfunction is directly associated with pulmonary VR limitations that develop during exercise, suggesting that there may be a mechanistic relationship. The present data also reveal how the hemodynamic impact of pulmonary endothelial dysfunction in pulmonary blood vessels may be subtle, often not evident at rest, but becoming apparent during exercise, unmasked by the increase in pulmonary blood flow. A clinical correlate of this concept is seen in Fontan patients listed for transplant with normal PVR, who then ‘develop’ significant increases in PVR post-transplant because of the increased CO provided by the cardiac allograft.[7]
The present data show that pulmonary VR, defined by the change in pulmonary arterial pressure per unit change in pulmonary blood (CO), provides a more robust assessment of pulmonary vascular function as compared to hemodynamic indices obtained at rest.[8–10] The prognostic role of mPAP/CO slope has been demonstrated in patients with acquired heart disease, in which patients with abnormal mPAP/CO slope display a 2-fold increase in risk of all-cause mortality as compared to those with normal mPAP/CO slope.[10] The increased risk of mortality due to abnormal mPAP/CO slope remained significant after adjustment for PVRrest suggesting that both indices assess different components of pulmonary vascular function.[10] Consistent with this finding, our data showed a dissociation of PVRrest and mPAP/CO slope as demonstrated by the poor correlation between both indices.
Clinical implications
The use of mPAP/CO slope for PVD diagnosis instead of the conventional approach of using PVRrest has important clinical implications because it leads to re-classification of 60% (12 of 20 patients) of the patients that would have been considered as having no PVD. Additionally, we observed that the patients that were re-classified (occult PVD) had significantly different pathophysiologic characteristics as compared to those with normal mPAP/CO slope (no PVD), but had similar severity of pulmonary endothelial and end-organ dysfunction as the overt PVD group (Supplemental Table 2). This suggests that mPAP/CO slope improves disease detection by unmasking abnormal pulmonary vascular function that would have been missed using the conventional approach of PVD diagnosis. This may help refine identification of patients likely to respond favorably to pulmonary vasodilators or other novel therapies in the future.
Limitations
These results were based on data derived from symptomatic Fontan patients requiring cardiac catheterization, and hence may not be not be generalizable to other cohorts of asymptomatic ambulatory Fontan patients. However, this is the ideal patient sample to study PVD given the limited sample size, since PVD is more prevalent in in symptomatic patients. These studies were carried out using supine exercise, which is associated with greater venous return as compared to upright exercise. As such, the increase in pulmonary driving pressure is greater, and that further study would be required to evaluate VR responses with upright exercise. Additionally, the current study did not provide data regarding the efficacy of pulmonary vasodilator therapy based treatments selection using pulmonary VR.
Conclusions
Impaired pulmonary VR during exercise was common in patients with Fontan physiology, including those with normal PVR at rest. Pulmonary VR limitations were related to pulmonary endothelial and end-organ dysfunction suggesting a mechanistic link between impaired endothelium-dependent vasodilation and subsequent end-organ dysfunction. Invasive hemodynamic cardiopulmonary exercise test was feasible and safe in patients with Fontan physiology, and can unmask pulmonary vascular function abnormalities that were not apparent at rest. This has important clinical implications, because it can improve the detection of pulmonary vascular disease at an earlier stage, allowing for targeting of novel therapies.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge:
Pulmonary vascular disease (PVD), pulmonary endothelial dysfunction, and end-organ dysfunction are common in adults with Fontan physiology, and are associated with increased mortality. Impaired pulmonary vascular reserve (PVR) as assessed during exercise is common in patients with Fontan physiology and related to pulmonary endothelial and end-organ dysfunction, suggesting a mechanistic link between impaired endothelium-dependent vasodilation and end-organ dysfunction in this condition.
Translational Outlook:
Future studies should explore the utility of pulmonary VR measured during invasive hemodynamic cardiopulmonary exercise testing in patients with Fontan physiology to facilitate earlier detection of pulmonary vascular disease and guide management.
Acknowledgement:
We acknowledge the staff in the Earl Wood Cardiac Catheterization Laboratory and the patients who volunteered to participate in this study.
Funding: Dr. Egbe is supported by National Heart, Lung, and Blood Institute (NHLBI) grant K23 HL141448. Dr. Borlaug is supported by RO1 HL128526 and U10 HL110262.
Abbreviations:
- CVP
Central venous pressure
- PVR
Pulmonary vascular resistance
- VR
vascular reserve
- PVD
Pulmonary vascular disease
- mPAP/CO slope
Change in mean pulmonary artery pressure per unit change in cardiac output during exercise
- VO2
Oxygen consumption
- PAWP
Pulmonary artery wedge pressure
- MRE
Magnetic resonance elastography
- NT-proBNP
N-terminal pro brain natriuretic peptide
- RHI
Reactive hyperemic index
- GFR
Glomerular filtration rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102:1081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egbe AC, Reddy YNV, Khan AR, Al-Otaibi M, Akintoye E, Obokata M and Borlaug BA. Venous congestion and pulmonary vascular function in Fontan circulation: Implications for prognosis and treatment. Int J Cardiol. 2018;271:312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zongtao Y, Huishan W, Zengwei W, et al. Experimental study of nonpulsatile flow perfusion and structural remodeling of pulmonary microcirculation vessels. Thorac Cardiovasc Surg. 2010;58:468–72. [DOI] [PubMed] [Google Scholar]
- 5.Binotto MA, Maeda NY, Lopes AA. Altered endothelial function following the Fontan procedure. Cardiol Young. 2008;18:70–4. [DOI] [PubMed] [Google Scholar]
- 6.Egbe AC, Connolly HM, Miranda WR, Ammash NM, Hagler DJ, Veldtman GR, Borlaug BA. Hemodynamics of Fontan Failure: The Role of Pulmonary Vascular Disease. Circ Heart Fail. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell MB, Campbell DN, Ivy D, et al. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg. 2004;128:693–702. [DOI] [PubMed] [Google Scholar]
- 8.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 9.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–9. [DOI] [PubMed] [Google Scholar]
- 10.Ho JE, Zern EK, Lau ES, et al. Exercise Pulmonary Hypertension Predicts Clinical Outcomes in Patients With Dyspnea on Effort. J Am Coll Cardiol. 2020;75:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obokata M, Reddy YNV, Borlaug BA. The Role of Echocardiography in Heart Failure with Preserved Ejection Fraction: What Do We Want from Imaging? Heart Fail Clin. 2019;15:241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–8. [DOI] [PubMed] [Google Scholar]
- 14.Agnoletti G, Gala S, Ferroni F, Bordese R, Appendini L, Pace Napoleone C, Bergamasco L. Endothelin inhibitors lower pulmonary vascular resistance and improve functional capacity in patients with Fontan circulation. J Thorac Cardiovasc Surg. 2017;153:1468–1475. [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD and Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borlaug BA, Lewis GD, McNulty SE,et al. Effects of sildenafil on ventricular and vascular function in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egbe A, Miranda WR, Connolly HM, et al. Temporal changes in liver stiffness after Fontan operation: Results of serial magnetic resonance elastography. Int J Cardiol. 2018;258:299–304. [DOI] [PubMed] [Google Scholar]
- 18.Duarte K, Monnez JM, Albuisson E, Pitt B, Zannad F and Rossignol P. Prognostic Value of Estimated Plasma Volume in Heart Failure. JACC Heart Fail. 2015;3:886–93. [DOI] [PubMed] [Google Scholar]
- 19.Egbe A, Khan AR, Miranda WR, et al. Mechanism for temporal changes in exercise capacity after Fontan palliation: Role of Doppler echocardiography. Am Heart J. 2018;196:144–152. [DOI] [PubMed] [Google Scholar]
- 20.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–25. [DOI] [PubMed] [Google Scholar]
- 21.Meng test. https://www.psychometrica.de/correlation.html. Updated June 4, 2010. Accessed April 27, 2020.
- 22.Julius S, Amery A, Whitlock LS, Conway J. Influence of age on the hemodynamic response to exercise. Circulation. 1967;36:222–30. [DOI] [PubMed] [Google Scholar]
- 23.van Empel VP, Kaye DM, Borlaug BA. Effects of healthy aging on the cardiopulmonary hemodynamic response to exercise. Am J Cardiol. 2014;114:131–5. [DOI] [PubMed] [Google Scholar]
- 24.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. [DOI] [PubMed] [Google Scholar]
- 25.Ghalayini IF. Nitric oxide-cyclic GMP pathway with some emphasis on cavernosal contractility. Int J Impot Res. 2004;16:459–69. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein BH, Golbus JR, Sandelin AM, et al. Usefulness of peripheral vascular function to predict functional health status in patients with Fontan circulation. Am J Cardiol. 2011;108:428–34. [DOI] [PubMed] [Google Scholar]
- 27.Mahle WT, Todd K, Fyfe DA. Endothelial function following the Fontan operation. Am J Cardiol. 2003;91:1286–8. [DOI] [PubMed] [Google Scholar]
- 28.Jin SM, Noh CI, Bae EJ, Choi JY and Yun YS. Impaired vascular function in patients with Fontan circulation. Int J Cardiol. 2007;120:221–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


