Abstract
Purpose:
To determine the impact of corneal cross-linking (CXL) performed over the LASIK flap (Standard CXL) or under the flap after flap lift (Flap-CXL) on regional corneal stiffness using Brillouin microscopy.
Setting:
University of Southern California Keck School of Medicine, Los Angeles, CA, and Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio, USA.
Design:
Laboratory ex vivo experiment
Methods:
After epithelium debridement, LASIK flaps were created on intact fresh porcine eyes with a mechanical microkeratome. Then, S-CXL (riboflavin applied to the corneal surface followed by 3 mW/cm2 UV exposure with the flap in place for 30 minutes) or Flap-CXL (riboflavin was applied to the stromal bed after reflecting the flap followed by the same UVA exposure with the flap replaced) was performed. Depth profile of stiffness variation and averaged elastic modulus of anterior, middle and posterior stroma were determined by analyzing Brillouin maps. Each eye served as its own control.
Results:
S-CXL had maximal stiffening impact at the corneal surface (8.40±0.04 GHz), while Flap-CXL had lower maximal stiffening impact (8.22±0.03 GHz, p<0.001), which occurred 249±34 μm under the corneal surface. S-CXL increased longitudinal modulus by 6.69% (anterior), 0.48% (middle), and −0.91% (posterior) as compared to Flap-CXL which increased longitudinal modulus by 3.43% (anterior, p<0.001), 1.23% (middle, p<0.1), and −0.78% (posterior, p=0.68).
Conclusion:
The S-CXL technique generated significantly greater stiffening effect in the anterior cornea than a modified protocol with riboflavin administration under the flap (Flap-CXL). Minimal stiffening occurred in the middle or posterior cornea with either protocol.
Keywords: Brillouin, microscopy, corneal cross-linking, LASIK, LASIK flap, Ectasia after LASIK, CXL
Corneal ectasia after LASIK leads to biomechanical instability followed by progressive corneal thinning and steepening, which can result in refractive aberrations and visual loss.1,2 To halt the progression of ectasia, corneal cross-linking (CXL) increases corneal biomechanical stability by creating interfibrillar connections through photoactivated interaction between riboflavin and ultraviolet-A (UVA).3 CXL for the treatment of corneal ectasia after refractive surgery was approved by the U.S. Food and Drug Administration (FDA) in 2016.4
The approved Standard CXL protocol (S-CXL) includes epithelium removal, riboflavin soaking on the corneal surface, over the LASIK flap, followed by UVA irradiation. Potential complications arising from epithelial removal can include sub-epithelial haze, ulcers and scarring.5,6 Moreover, epithelium debridement increases patients discomfort and can prolong visual rehabilitation.7
Performing CXL under the LASIK flap (flap-CXL) has been proposed to stabilize post-LASIK ectasia.8–10 Instead of corneal debridement as in Standard CXL, the existing flap is reflected, with riboflavin applied directly onto the stromal bed followed by flap repositioning and UVA irradiation. This technique bears similarities to LASIK with simultaneous prophylactic CXL under the flap, which has been purported to potentially reduce the risk of regression or the development of ectasia,11 but which has uncertain clinical and biomechanical benefit.12,13 The stiffening effect of flap-CXL may be limited due to decreasing fibril density from the anterior to the posterior of the cornea that may limit effective CXL at deeper corneal levels.14,15 This technique, if effective, could mitigate most of the complications associated with Standard CXL, improve patient comfort, and speed rate of recovery.
The purpose of this study was to compare the depth-dependent stiffness distribution of CXL performed over or under the flap in a laboratory model in porcine eyes using Brillouin microscopy.
METHODS
Specimen preparation
The study was performed on 24 fresh porcine eyes that were kept in BSS during shipment and were used within 24 hours after collection. These eyes were equally divided into two groups. In each group, LASIK flaps were created first and then CXL was performed over the flap using the Standard protocol (S-CXL) or under the flap (Flap-CXL). For all eyes, adherent muscle and adipose tissue was detached without damaging the whole globe to allow for stable fixation.
In both groups the epithelium was removed with a crescent knife before LASIK flap creation because the thick epithelium in the porcine eye limits the ability to create a stromal flap; the thickness of porcine epithelium was ~100 μm while the predicted flap thickness using the blades was ~120 μm.
LASIK Flap Creation:
Pachymetry (Pachette 4, DGH Technology) was performed on both groups immediately after removing the epithelium to confirm similar hydration status. Then, LASIK flaps were created using the Amadeus II microkeratome (Ziemer, Inc.) with a 140 μm microkeratome head, blade oscillation rate of 8000 rpm, translation speed of 2.5 mm/second, and a 9.0 mm suction ring diameter, with the ML7090 (+20) CLB blade (Med-Logics, Inc.) microkeratome blades. After LASIK flap creation, pachymetry was applied again on the residual stromal bed to evaluate flap thickness by subtracting thickness of the stroma bed from that of the debrided cornea.
Corneal Cross-Linking (CXL) Protocol
CXL protocols are shown in Figure 1. Riboflavin solution was prepared by diluting riboflavin and dextran to 0.1% and 10% separately with 1X PBS (Phosphate-buffered saline). For S-CXL, the flap was repositioned after LASIK flap creation, and intact porcine eyes were soaked by dropping the riboflavin solution on corneas directly every 2 minutes for 30 minutes. After soaking, eyes were placed under a UVA light (CCL-365 Vario, MLase AG) and were exposed to a power density of 3 mW/cm2 for 30 minutes. During exposure, riboflavin was added on corneas every 2 minutes.
Figure 1.
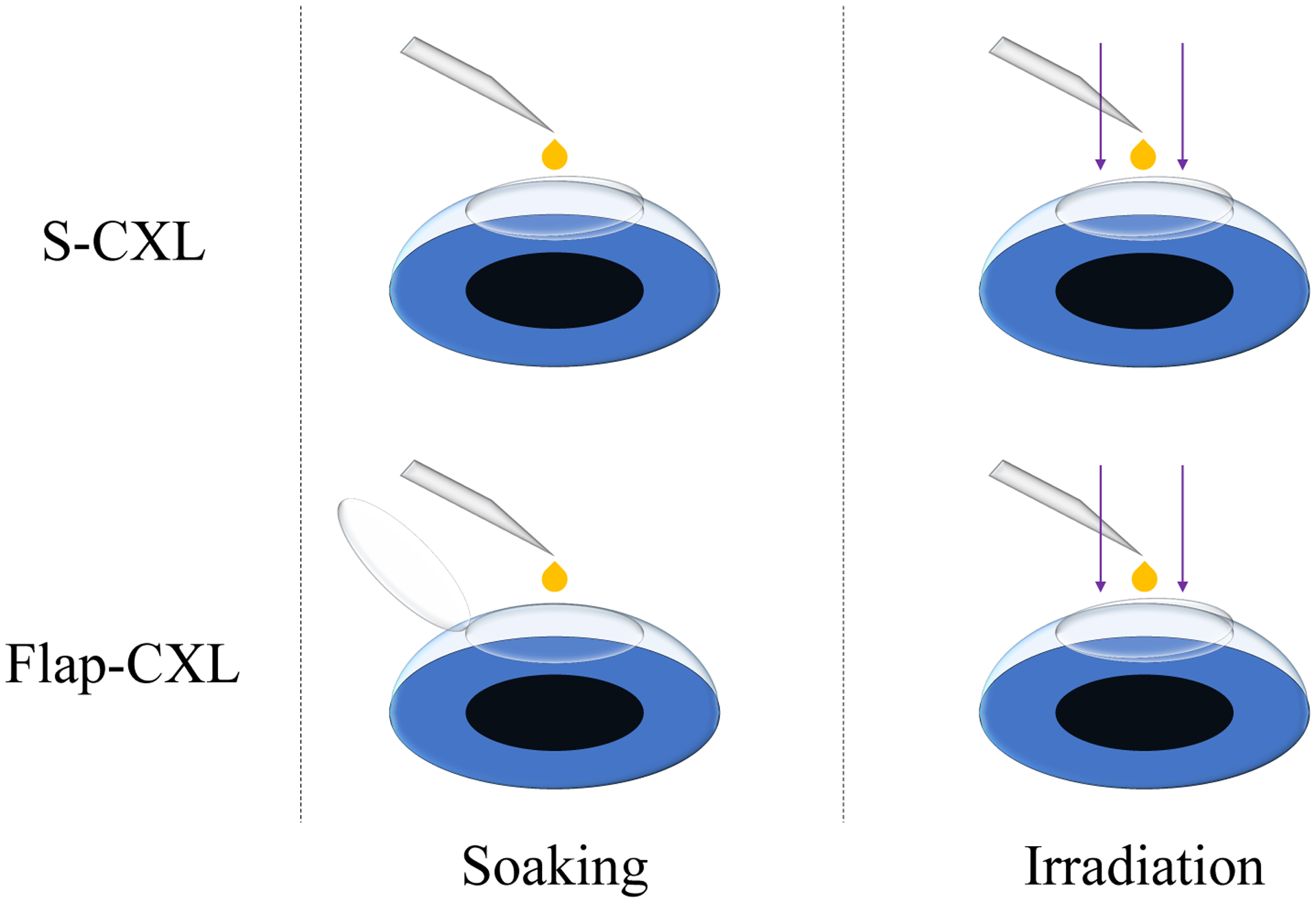
CXL protocols for S-CXL and Flap-CXL. When performing S-CXL, the flap kept being attached to the stromal bed during soaking and UVA irradiation. When performing Flap-CXL, the flap was reflected during soaking to avoid riboflavin contact. Then, it was repositioned during UVA irradiation.
For Flap-CXL, the flap remained reflected while riboflavin solution was dropped onto the stroma bed directly every 2 minutes for 30 minutes. Care was taken to avoid riboflavin contact with the flap. When dropping riboflavin, the flap was brushed with a sponge infiltrated by BSS to maintain stable hydration. After drop completion, the flap was repositioned in place. The eye was then placed under the UVA light for CXL. During irradiation, riboflavin solution was dropped on the anterior surface of the flap every 2 minutes for 30 minutes.
Biomechanical properties measured by Brillouin microscopy
Spontaneous Brillouin scattering derives from the interaction between light and acoustic phonons generated by inherent density fluctuation, providing direct information on the longitudinal modulus and viscoelastic properties.16,17 The relation between the Brillouin shift Δf and the longitudinal modulus M’ can be expressed as
| (1) |
where ρ is the density, n is refractive index, λ is probing laser wavelength and M’ is the longitudinal modulus which can represent the stiffness of the specimen. Though refractive index and density varies spatially, the variation of these two parameters is synchronized in cornea and can be described through Gladstone-Dale relation.18 The ratio of ρ/n2 is found to be approximately constant with a value of 0.57 g/cm3 and this approximation introduces an uncertainty of less than 0.3% throughout the cornea.13,19
The Brillouin shifts were measured through a custom-built spectrometer using two virtually imaged phased arrays (VIPAs) as core dispersion components.20 12 mW continuous laser with a central wavelength of 532 nm (torus 532, Laser Quantum) was focused onto porcine corneas through a 40× objective lens with a numerical aperture of 0.6 (LUCPLFLN 40×, Olympus). The scattered light collected in epi-detection was spectrally analyzed via the home-built spectrometer and imaged by an EMCCD (iXon-L-897, Andor Technology) with an exposure time of 0.2 s. Raw data acquired by the EMCCD were fitted with Lorentzian function to determine Brillouin shifts. X-Z sectional images of the cornea were acquired by moving a translational stage (H117P1XD/D, Prior).
Brillouin image analysis
A cross-section of 200 μm (lateral) × 1200 μm (axial) in the center of a cornea was selected for each scan to image Brillouin shifts as a function of depth. The step sizes in both directions are 10 μm. After LASIK flap creation, corneas were imaged by Brillouin microscopy first to serve as the baseline for CXL evaluation. Then, CXL was performed on both groups, and corneas were re-imaged after S-CXL or Flap-CXL to compare stiffness variation.
To compare depth-dependent stiffness variation after different CXL protocols, depth profiles of Brillouin shifts were calculated by averaging over the lateral 200 μm. Then, the averaged cross-sections were equally divided into three segments (anterior, middle and posterior) and averaged percentage changes of longitudinal modulus in these 3 segments were calculated to indicate depth information.
RESULTS
After debridement, total corneal thickness was 961±37 μm in the S-CXL group and 970±52 μm in the Flap-CXL group (p=0.52). Flap thickness was 120±13 μm in the S-CXL group and 116±10 μm in the Flap-CXL group (p=0.38).
Brillouin Shift in S-CXL and Flap-CXL
Representative Brillouin shifts for S-CXL and Flap-CXL are shown in Figures 2 and 3. Figure 2(a) and Figure 3(a) present cross-sectional distribution of Brillouin shifts in two LASIK-only corneas (without CXL). In both figures, a region of weakness can be seen in the anterior section corresponding to the LASIK flap location. In Figure 2(b) and Figure 3(b), Brillouin shifts of the same eyes after S-CXL and Flap-CXL are depicted, showing the stiffening effect caused by CXL. For better visualization of depth-dependent stiffness change and easier comparison between S-CXL and Flap-CXL, depth profiles were calculated from these cross-sectional images by averaging over the transverse axis, as shown in Figure 2(c) and Figure 3(c). When plotting these two profiles, geometric thickness, measured by the pachymeter, was used as the abscissa. Horizontal shifts were added to depth profiles after CXL to make the posterior regions overlap for clearer comparison. In Figure 2(c), S-CXL led to a continuous decrease of the Brillouin shift from the anterior region to the posterior region, with the maximum Brillouin shift at the superficial surface of the cornea. Whereas, in Figure 3(c), the maximum shift occurs ~230 μm beneath the anterior surface and the shift is smaller than that in Figure 2(c).
Figure 2.
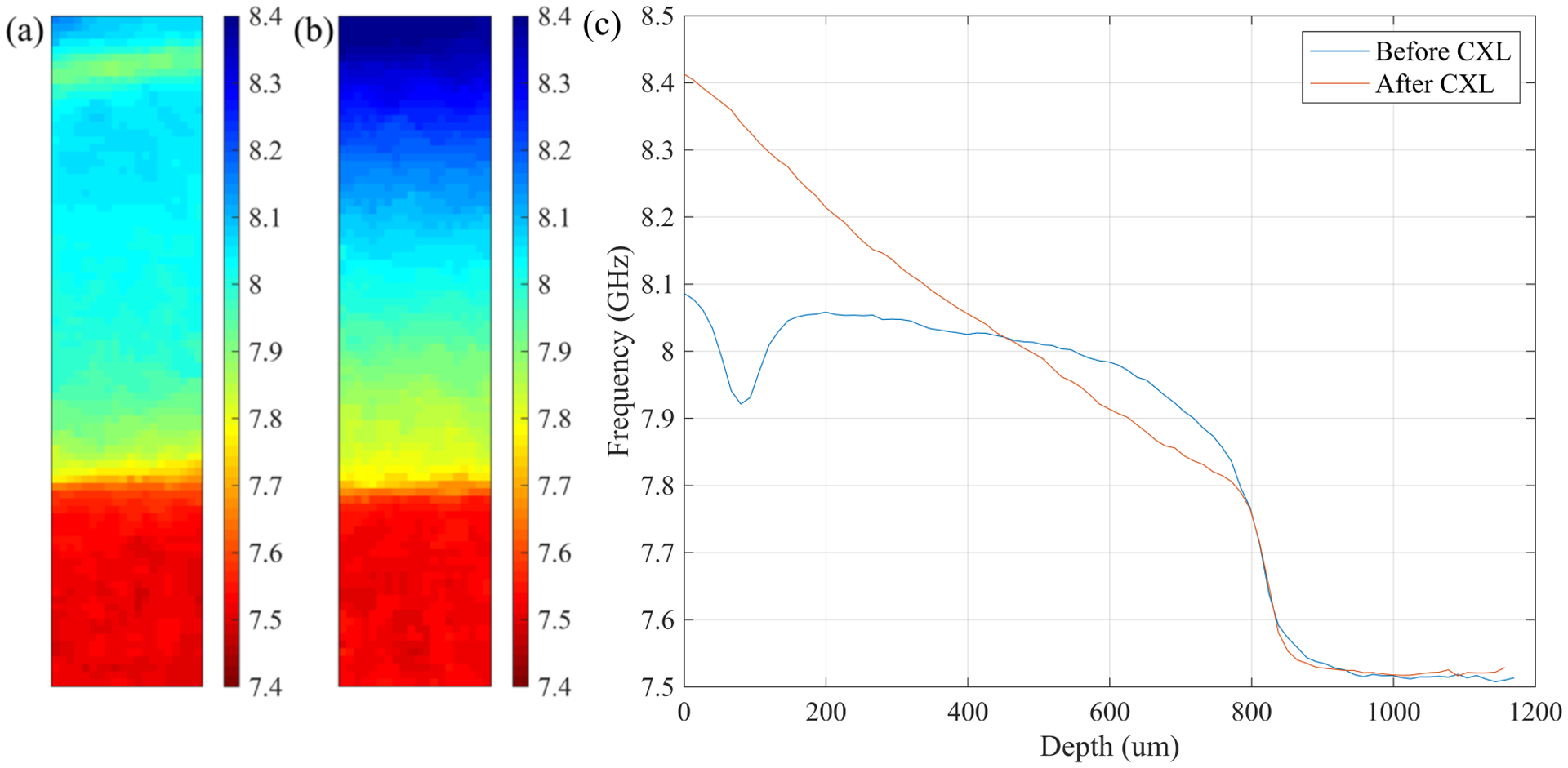
Representative Brillouin results for the S-CXL group. (a) Distribution of Brillouin shifts in a LASIK-only eye. The distribution is depicted top down from the anterior to the posterior. (b) Distribution of Brillouin shifts in the same eye after CXL over the flap. According to Eq. (1), a higher Brillouin shift correlates to a larger longitudinal modulus. Increase of longitudinal modulus is elucidated with different colors. (c) Lateral averaged depth profiles of Brillouin shifts in (a) and (b).
Figure 3.
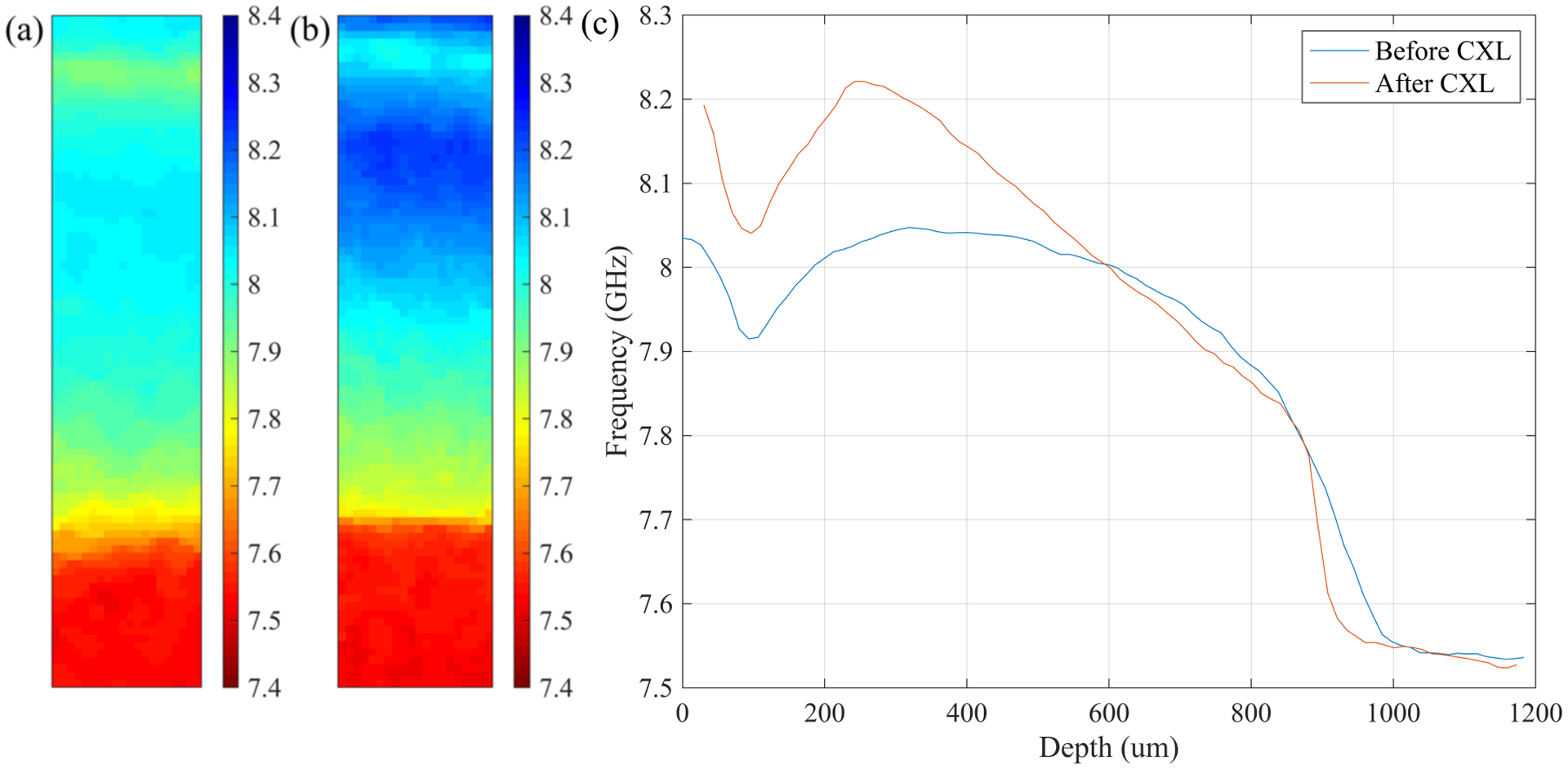
Representative Brillouin results for the Flap-CXL group. (a) Distribution of Brillouin shifts for a LASIK-only eye. (b) Distribution of Brillouin shifts for the same eye after Flap-CXL. (c) Lateral averaged depth profiles of Brillouin shifts in (a) and (b).
For better understanding of the difference between S-CXL and Flap-CXL, depth-dependent statistical analysis was performed. As LASIK-only corneas served as the baseline for CXL comparison, it was necessary to confirm that these corneas in both groups had similar Brillouin distribution after the mechanical impact of LASIK flap creation. Based on our results, there was no significant difference between the two groups. For the anterior region, S-CXL had a Brillouin shift of 8.03±0.04 GHz, while the Brillouin shift for Flap-CXL was 8.03±0.03 GHz (p=0.82). For the middle region, the Brillouin shift for S-CXL was 8.03±0.05 GHz and it was 8.02±0.04 GHz for Flap-CXL (p=0.08). For the posterior region, we had 7.85±0.05 GHz for S-CXL and 7.85±0.05 GHz for Flap-CXL (p=0.98). This data showed that LASIK introduced similar mechanical influence in both groups.
After performing CXL, S-CXL and Flap-CXL showed different stiffening effects. After S-CXL there was a significant increase in Brillouin shift in the anterior region (8.29±0.04 GHz vs 8.03±0.04 GHz, p<0.001), while there was no significant stiffening induced in the middle (8.05±0.05 GHz vs 8.03±0.05 GHz, p=0.09) or posterior (7.84±0.03 GHz vs 7.85±0.05 GHz, p=0.27) regions. After Flap-CXL, there was a significant increase in Brillouin shift in the anterior region (8.17±0.03 GHz vs 8.03±0.03 GHz, p<0.001). A less but still significant increase in Brillouin shift could be seen in the middle region (8.06±0.04 GHz vs 8.02±0.04 GHz, p<0.01). In the posterior region, there was no significant stiffening effect (7.83±0.06 GHz vs 7.85±0.05 GHz, p=0.12). The maximum Brillouin shift was 8.40±0.04 GHz in S-CXL appearing at the anterior surface and 8.22±0.03 GHz in Flap-CXL (p<0.001) which occurred 249±34 μm under the anterior surface. As shown in Figure 4, longitudinal modulus, calculated through Eq. 1, was used in this comparison. Each cornea served as its own control when calculating percentage change. For the anterior region, compared to corneas before CXL, the increase of mean modulus in S-CXL was 6.69% while that in Flap-CXL was 3.43% (p<0.001). For the middle region, Flap-CXL led to a larger but not statistically significant increase of modulus than that caused by S-CXL (1.23% vs 0.48%, p<0.1). For the posterior region, S-CXL and Flap-CXL had comparable stiffness variation (−0.91% vs. −0.78%, p=0.68). This softer posterior region was mainly caused by the use of hypo-osmolar riboflavin solution with 10% dextran and corresponding corneal hydration in this region.
Figure 4.
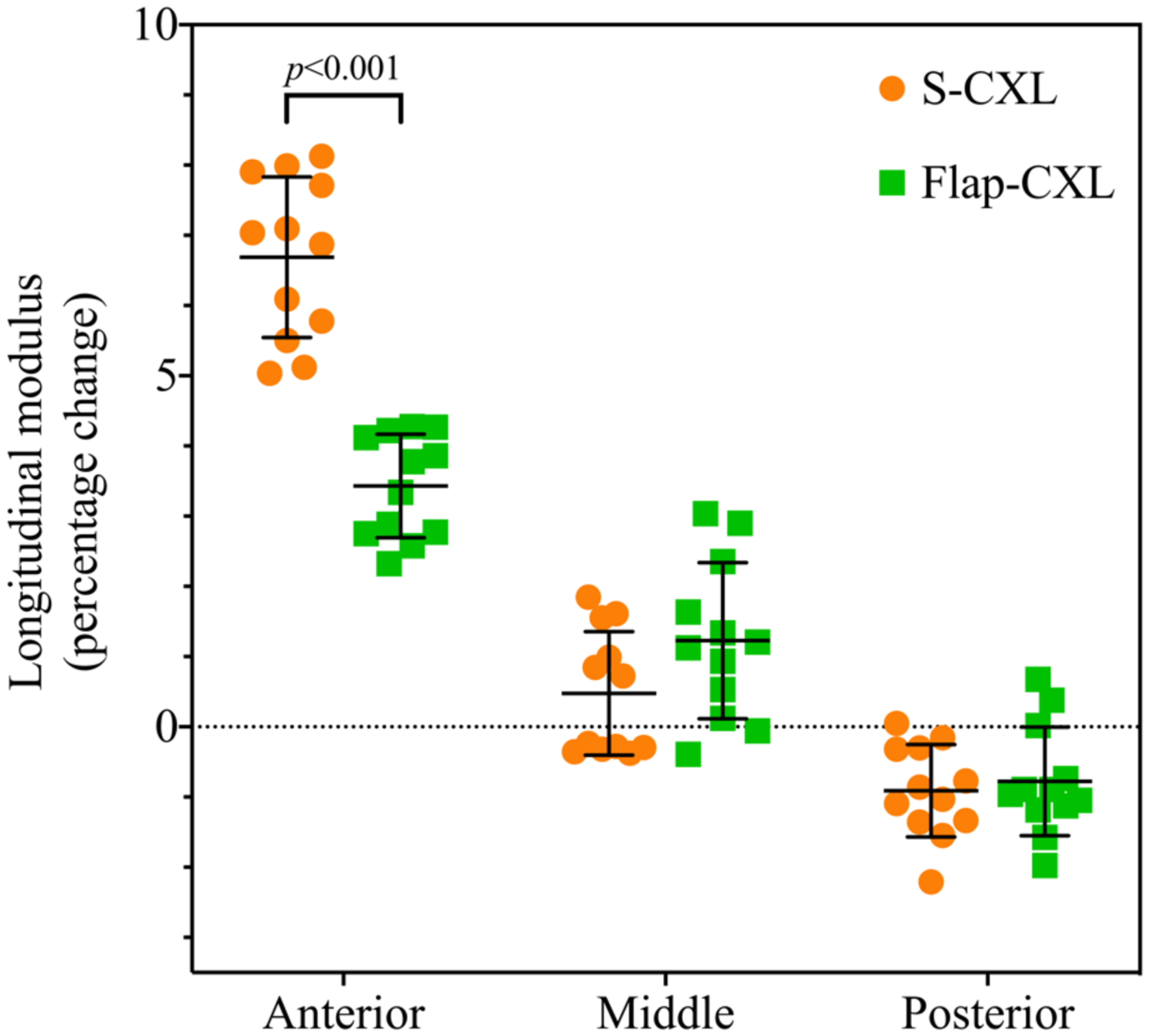
Percentage change of mean longitudinal modulus of the anterior, middle and posterior for S-CXL (N=12) versus Flap-CXL (N=12).
DISCUSSION
Depth-dependent Brillouin results showed that S-CXL and Flap-CXL led to different stiffness distributions. For S-CXL, maximal stiffening effect occurred in the anterior most region, including the LASIK flap itself, while maximal stiffening for the Flap-CXL group occurred just posterior to the flap. As seen in Figure 4, there was no comparatively greater increase in stiffening for the Flap-CXL group as compared to S-CXL, merely a different location for maximal stiffening. Difference of stiffness increase between S-CXL and Flap-CXL existed primarily in the anterior third of the cornea (Figure 4), with Flap-CXL achieving 51% of the stiffening effect of S-CXL in this region. In the middle and posterior regions there were no significant differences between techniques, although the middle stromal region appeared slightly stiffer after Flap-CXL the true difference was minimal (<5%) and within the margin of error of frequency accuracy. Further, when taking into account the total corneal thickness differences between porcine and human corneas, the possible difference found would be even less significant in clinical practice. The stiffness difference in the anterior region could derive from a lower fibril density under the flap. It was also possible that the un-soaked flap absorbed a part of UVA power because it was repositioned with riboflavin adding on it during UVA irradiation. However, as the flap was not fully soaked, the UVA power it could absorb was also limited.
Besides the difference of stiffness increase, there was another difference in the anterior region at the location of the flap wound. As shown in Figure 2 and Figure 3, the sudden decrease in stiffness was obvious after Flap-CXL, while it disappeared after S-CXL. Moreover, the Brillouin shift of S-CXL was larger than that of Flap-CXL in the flap region, which suggested that stronger interface bonding was created by S-CXL. The possible reason for this phenomenon could be that the two sides of the interface were both indirectly saturated in S-CXL, while in Flap-CXL only one side was soaked/saturated. Thus, interfibrillar connection between the flap and the stromal bed was easier to create with S-CXL, so that better adhesion of flaps was expected when using S-CXL.
Although it is not surprising to see no significant CXL in the posterior region due to its ultrastructural differences as compared to the anterior and middle corneal regions,15 it is of interest to determine the reason for softer posterior regions observed in both S-CXL and Flap-CXL. As the endothelium of the porcine eye was dysfunctional, hypo-osmolar riboflavin solution could not be pumped out of the stroma. Moreover, sparse fibrils in the posterior region provided space for accumulation of the hypo-osmolar riboflavin solution. Thus, averaged Brillouin shifts from the cornea and the riboflavin solution were measured in this region. When filling the posterior region with the riboflavin solution, a decrease in the Brillouin shift was expected. The softer posterior region indicated that corneas in this study were hydrated after CXL. As the hydration status were similar in S-CXL and Flap-CXL (Figure 4), it was still reasonable to compare the ratio between the two groups. Decreasing the frequency of riboflavin application and/or increasing dextran concentration might increase the Brillouin shift in the posterior region. However, it might dehydrate the anterior region and exaggerate CXL efficacy.
There are limitations to our study. As an ex-vivo experiment, only the immediate effects of CXL could be measured, without taking into consideration any remodeling over time. Further, while we set the experimental protocol to control for hydration, it is challenging to do ex vivo. In both groups the epithelium was removed with a crescent knife before LASIK flap creation because the thick epithelium in the porcine eye limits the ability to create a stromal flap. This is not standard practice in humans but necessary for this experiment. If this impacted the results, epithelial removal should have benefited the Flap-CXL technique by removing the epithelial barrier to oxygen and UV light. Finally, as only LASIK flaps were created in this experiment, it was not a true LASIK ectasia model. An ectatic cornea may behave differently given the collagen disruption/alteration that occurs in the residual stromal bed.
In conclusion, although Flap-CXL induced some stiffening effect in the anterior most residual stromal bed region, stiffening difference between S-CXL and Flap-CXL primarily existed in the anterior region, where stiffening effect of S-CXL is almost twice of that of Flap-CXL. Furthermore, stronger bonding between the LASIK flap and the stromal bed was seen in S-CXL. Given no improvement in efficacy and other concerns for flap lift, including epithelial ingrowth at the interface, there does not seem to be any advantage to a flap-lift CXL technique.
WHAT WAS KNOWN
The Standard corneal cross-linking (CXL) technique is an effective treatment to stabilize post-LASIK ectasia.
The effect of CXL appears to be primarily impactful within the anterior region of the corneal stroma due to stomal microarchitecture
Potential benefit of CXL performed under the LASIK flap has been investigated in limited case series, but the biomechanical impact remains unknown
WHAT THIS PAPER ADDS
The Standard CXL technique generated significantly greater stiffening effect in the anterior corneal stroma than a modified protocol with riboflavin administration under the flap (flap-CXL).
Minimal stiffening occurred in the middle or posterior corneal stoma with either protocol.
Financial Disclosures:
FH - Shareholder/investor for EMAGine AG (Zug, Switzerland), consultant for GroupAdvance Consulting GmbH (Zug, Switzerland), exclusive patent owner for PCT patent/application (corneal apparatus used for CXL and chromophore for CXL application), recipient of travel funds from Light for Sight Foundation (Zurich, Switzerland), directed research funds from Light for Sight Foundation (Zurich, Switzerland), Schwind Eye Tech Solutions (Kleinostheim, Germany), VELUX Foundation (Søborg, Denmark), Gelbert Foundation (Geneva, Switzerland), and in-kind financial contribution for research materials from SOOFT Italia (Montegiorgio, Italy).
The remaining authors have no financial disclosures
Funding: Supported in part by National Institutes of Health Grant R01 EY028666 (J.B.R., G.S.) and an unrestricted departmental grant to the Cole Eye Institute, Cleveland Clinic, from Research to Prevent Blindness (New York, NY, USA).
References
- 1.Randleman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology 2003;110(2):267–75. [DOI] [PubMed] [Google Scholar]
- 2.Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology 2008;115(1):37–50. [DOI] [PubMed] [Google Scholar]
- 3.Wollensak G, Spoerl E, Seiler T, Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 135, 620–627 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Hersh PS, Stulting RD, Muller D, et al. US Multicenter Clinical Trial of Corneal Collagen Crosslinking for Treatment of Corneal Ectasia after Refractive Surgery. Ophthalmology 2017;124(10):1475–84. [DOI] [PubMed] [Google Scholar]
- 5.Pollhammer M, Cursiefen C. Bacterial keratitis early after corneal crosslinking with riboflavin and ultraviolet-A. J Cataract Refract Surg 2009;35(3):588–9. [DOI] [PubMed] [Google Scholar]
- 6.Mazzotta C, Balestrazzi A, Baiocchi S, et al. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clin Exp Ophthalmol 2007;35(6):580–2. [DOI] [PubMed] [Google Scholar]
- 7.Spadea L, Salvatore S, Paroli MP, Vingolo EM. Recovery of corneal sensitivity after collagen crosslinking with and without epithelial debridement in eyes with keratoconus. Journal of Cataract and Refractive Surgery 2015;41(3):527–32. [DOI] [PubMed] [Google Scholar]
- 8.Moscovici BK, Campos M. Intrastromal crosslinking in post-LASIK ectasia. Arq Bras Oftalmol 2014;77(3):191–2. [DOI] [PubMed] [Google Scholar]
- 9.Wallerstein A, Adiguzel E, Gauvin M, et al. Under-flap stromal bed CXL for early post-LASIK ectasia: a novel treatment technique. Clin Ophthalmol 2017;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Khoury S, Abdelmassih Y, Amro M, et al. Under-the-Flap Crosslinking and LASIK in Early Ectasia with Hyperopic Refractive Error. J Ophthalmol 2018;2018:4342984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanellopoulos AJ, Binder PS. Management of corneal ectasia after LASIK with combined, same-day, topography-guided partial transepithelial PRK and collagen cross-linking: the athens protocol. J Refract Surg 2011;27(5):323–31. [DOI] [PubMed] [Google Scholar]
- 12.Chan TC, Yu MC, Ng AL, et al. Short-term variance of refractive outcomes after simultaneous LASIK and high-fluence cross-linking in high myopic correction. J Refract Surg. 2016;32:664–670. [DOI] [PubMed] [Google Scholar]
- 13.Randleman JB, Su JP, Scarcelli G. Biomechanical Changes After LASIK Flap Creation Combined With Rapid Cross-Linking Measured With Brillouin Microscopy. J Refract Surg 2017;33(6):408–14. [DOI] [PubMed] [Google Scholar]
- 14.Winkler M, Shoa G, Xie Y, et al. Three-dimensional distribution of transverse collagen fibers in the anterior human corneal stroma. Invest Ophthalmol Vis Sci 2013;54(12):7293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatami-Marbini H Influence of Microstructure on Stiffening Effects of Corneal Cross-linking Treatment. J Refract Surg 2018;34(9):622–7. [DOI] [PubMed] [Google Scholar]
- 16.Scarcelli G, Yun SH. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nature Photonics 2008;2(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarcelli G, Polacheck WJ, Nia HT, et al. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat Methods 2015;12(12):1132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard DW, Meek KM. Refractive indices of the collagen fibrils and extrafibrillar material of the corneal stroma. Biophys J 1997;72(3):1382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webb JN, Su JP, Scarcelli G. Mechanical outcome of accelerated corneal crosslinking evaluated by Brillouin microscopy. J Cataract Refract Surg 2017;43(11):1458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarcelli G, Yun SH. Multistage VIPA etalons for high-extinction parallel Brillouin spectroscopy. Opt Express 2011;19(11):10913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


