Abstract
mRNA function is meticulously controlled. We provide an overview of the integral role that post-transcriptional controls play in the perception of painful stimuli by sensory neurons. These specialized cells, termed nociceptors, precisely regulate mRNA polarity, translation, and stability. A growing body of evidence has revealed that targeted disruption of mRNAs and RNA-binding proteins robustly diminishes pain-associated behaviours. We propose that the use of multiple independent regulatory paradigms facilitates robust temporal and spatial precision of protein expression in response to a range of pain-promoting stimuli.
Graphical Abstract
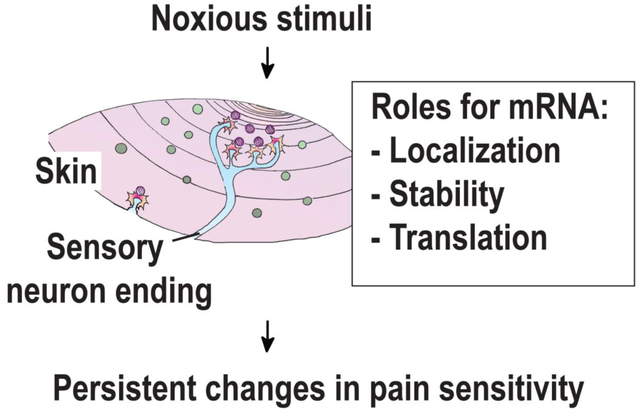
A key function of the peripheral nervous system is detection and amplification of pain promoting cues. Here we summarize multiple lines of evidence in support of the notion that mRNA localization, stability, and translation serve integral functions in pain signals originating from sensory neurons.
Introduction
Pain generates tremendous human suffering. Advances in our understanding of its origins have the potential to yield new targets for therapeutic development. The peripheral nervous system reacts to potentially harmful cues with appropriate behavioural responses through a process called nociception. This conserved process ensures survival through avoidance of additional tissue damage (Tracey, 2017). However, when pain transitions into a chronic phase, it greatly diminishes quality of life. A variety of cues promote pain including changes in temperature (e.g. heat or cold), certain chemicals (e.g. capsaicin), inflammatory mediators (e.g. nerve growth factor, interleukin 6), and mechanical stimuli (e.g. pressure, poke, etc…). Not all pain is nociceptive in origin. Damage to the nervous system can cause neuropathic pain, which unlike nociceptive pain, does not always have a clear stimulus (e.g. phantom limb pain). Here, we describe five lines of evidence supporting the notion that mRNA regulation is integral to pain signalling. They are: (1) identification of essential RNA-binding proteins for sustained changes is nociceptor activity, (2) pharmacology that implicates the structural features in mRNA as therapeutically relevant, (3) perturbations in cap-dependent mRNA translation result in reduced pain sensitivity, (4) discovery of miRNAs that regulate key mRNAs in nociceptors, and finally, (5) emerging evidence that targeted destabilization of mRNAs with therapeutic oligonucleotides can robustly diminish certain forms of pain.
A key role for nociceptors
Nociceptors are specialized sensory neurons tasked with receiving information from the environment and relaying it to the central nervous system (Figure 1). Nociceptors are not identical. Distinctive patterns of gene expression enable subspecialisation. Nociceptors vary with respect to conduction velocity, diameter, and responsiveness. These attributes form the basis of their classification (Table 1). The unusual anatomy of these neurons is intimately linked to their function. Their nuclei are housed in specialized tissues termed the trigeminal ganglion (TG) in the head or the dorsal root ganglion (DRG) along the spinal cord. Approximately half of the neurons in these tissues are nociceptors. The nociceptor cell body (also known as the soma) contains organelles and the nucleus. Projecting away from the cell body is an axon with two branches. One end project back to the spinal cord and synapses with interneurons which in turn relay pain signals to the brain. The opposing branch innervates the skin and detects noxious stimuli (e.g. heat, cold, mechanical stimuli, inflammatory cues, etc.). The axon is encased by Schwann cells which are shed as they penetrate the keratinocyte layer. In the skin, the axon forms complex branched networks and contacts numerous cell types including fibroblasts and keratinocytes. A growing body of evidence suggests that these cells also contribute to nociceptive behaviours (Moehring, Halder, Seal, & Stucky, 2018). Thus, axons play a critical role in both detection of noxious cues and as mediators of signal propagation through action potentials from the periphery to the central nervous system (CNS).
Figure 1 –
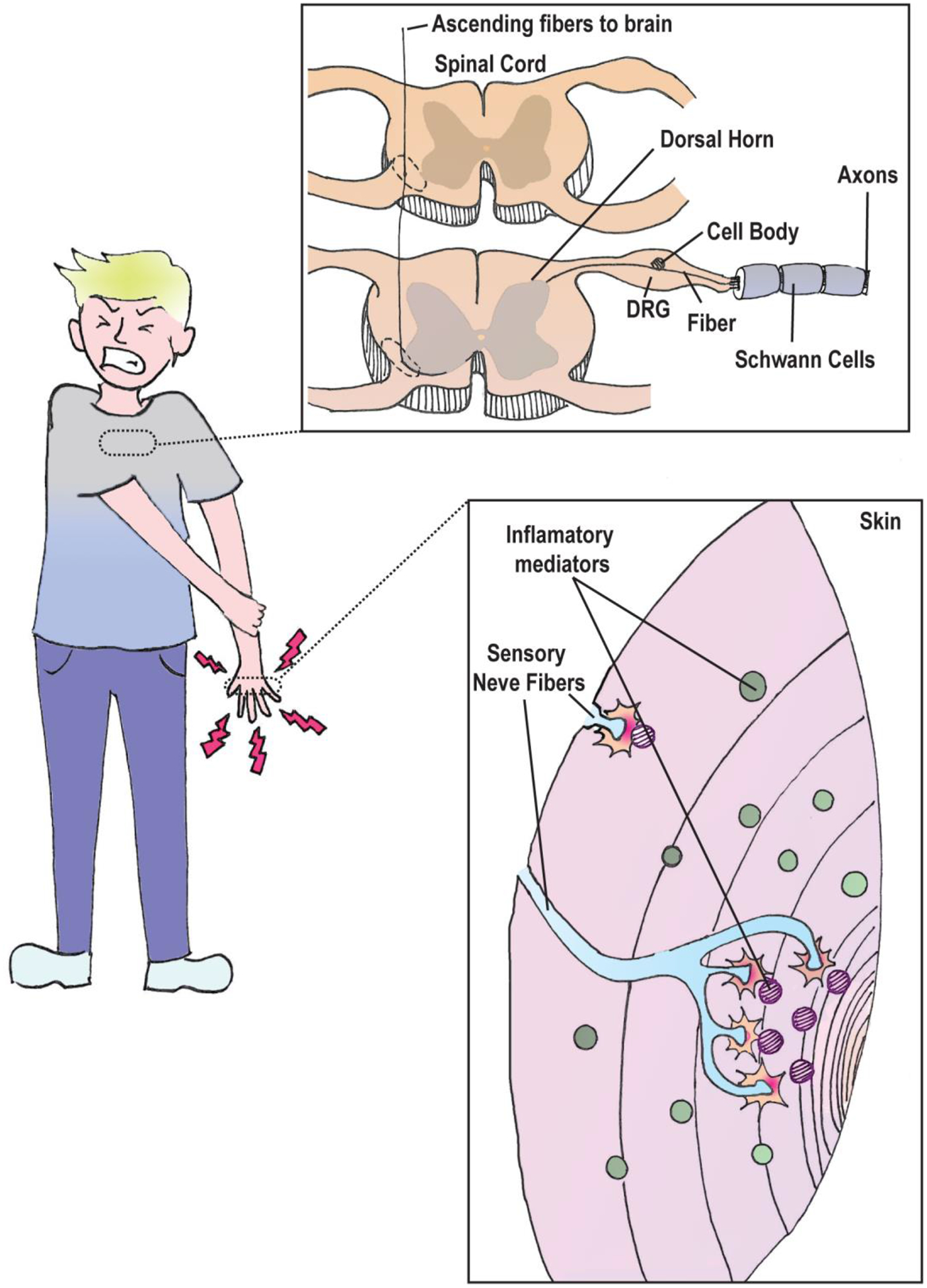
Nociceptor anatomy. Potential damaging stimuli are sensed by nerve endings in the skin that can detect inflammatory mediators (e.g. NGF, IL-6). Nociceptor axons are coated with Schwann cells which support its structure and conductance. The nociceptor cell body is situated in the trigeminal ganglion or dorsal root ganglion (DRG). Signals received in the skin are relayed by axons back to the dorsal horn of the spinal cord. Information is then forwarded to the central nervous system for further processing via ascending fibres along the spinal cord.
Table 1:
Classification of nociceptors
| Type | Activated by | Fiber type | Myelination | Diameter (μm) | Conduction velocity (m/sec) | Pain characteristic |
|---|---|---|---|---|---|---|
| Mechanical | Intense mechanical stimulus | Aδ | Myelinated | 1–5 | 5–40 | Fast, sharp, pricking pain. |
| Thermal | Intense thermal (hot or cold) stimulus | |||||
| Polymodal | Intense mechanical or thermal stimulus; specific chemicals | C | Unmyelinated | 0.2–1.5 | 0.5–2 | Burning, aching, sore pain. |
Beyond their roles in information relay from the periphery, nociceptors have emerged as key players in persistent pain (Barragan-Iglesias et al., 2018; Bogen, Alessandri-Haber, Chu, Gear, & Levine, 2012; Ferrari, Bogen, Chu, & Levine, 2013a; Ferrari, Bogen, & Levine, 2013; Inceoglu et al., 2015; Khoutorsky et al., 2016; Melemedjian et al., 2010; Moy et al., 2017; J. T. Xu et al., 2014). Generally, they are electrically silent and transmit all or none action potentials when stimulated (Dubin & Patapoutian, 2010). Injury changes the neurochemical and electrophysiological phenotypes of these neurons leading to persistent hyperexcitability that lasts for days or even weeks. These phenotypic changes outlive the healing process and contribute to abnormally increased sensitivity to noxious stimuli (referred to as hyperalgesia). Long-term changes in the electrophysiological activity of nociceptors, called plasticity, is thought to be a major driver of spontaneous pain (Khoutorsky & Price, 2018). What mediates the persistence of these signals? A potential mechanism that evokes the idea of synaptic plasticity is to control neurotransmitter release at the synapse between the nociceptor and the spinal cord in the dorsal horn (Todd, 2010). While this is an intuitive short-term solution, longer-term changes require sustained responses that appear to be driven largely by translation of mRNA (Alvarez, 2001; Alvarez, Giuditta, & Koenig, 2000; Khoutorsky et al., 2015; Price & Geranton, 2009). New insights into mRNA function within nociceptors suggests a plethora of mechanisms that contribute to nociceptor plasticity.
Location, location, location
In neurons, translation can occur in the cell body, dendrites, or axons (Bramham & Wells, 2007; Brittis, Lu, & Flanagan, 2002). The latter two cases are examples of “local translation” (Medioni, Mowry, & Besse, 2012). De novo translation of newly transcribed transcripts in the cell body would require approximately 250 hours based on a transit distance of one meter (Buxbaum, Yoon, Singer, & Park, 2015). Neurons in the peripheral nervous system have the longest axons in the body. As a solution to generate new proteins, these neurons rely heavily on translation of pre-existing localized mRNA. The mechanism of local protein synthesis enables on-demand synthesis of polypeptides near or at the site where they are required and provides a means to rapidly modulate cellular phenotypes. The requirements for local translation include ribosomes, mRNA targets, and regulatory factors to ensure that the ribosomes are poised to translate the correct targets at the appropriate point in time. A variety of stimuli trigger local protein synthesis. For example, learning and memory promote translation of immediate early genes in dendrites (Jimenez-Diaz et al., 2008; Steward, Farris, Pirbhoy, Darnell, & Driesche, 2014; Steward & Levy, 1982). How these mechanisms contribute to pain is unclear. A growing body of evidence indicates that ribosomes present in axons catalyse protein synthesis which raises the question of how local translation is regulated in nociceptors and which mRNAs are the relevant targets (Barragan-Iglesias et al., 2018; Kar, Lee, & Twiss, 2018).
Anatomy of an mRNA
Regulatory information present in mRNA enables meticulous control. These controls can occur at the level of splicing, editing, translation, stability, and subcellular localization. In aggregate, they determine the amount, timing, and site of protein synthesis. What then dictates polarity in the distribution of mRNAs in the cytoplasm? Part of the answer can be found within the mRNA itself. mRNA is resplendent with regulatory information (Figure 2A). Virtually every portion of the mRNA is subject to reversible or irreversible modifications. For example, the presence of an m7G cap protects the mRNA from 5’ to 3’ exonucleolytic decay (Yanagiya et al., 2012). The cap also plays a central role in translation via the cytoplasmic cap-binding complex (referred to as eIF4F) consisting of the cap-binding protein (eIF4E), a DEAD-box RNA helicase (eIF4A), and a scaffold (eIF4G) (Gingras, Raught, & Sonenberg, 1999; Matsuo et al., 1997; Sonenberg, Morgan, Merrick, & Shatkin, 1978). One way eIF4F stimulates translation is through recruitment of the small ribosomal subunit. Removal of the cap by cytoplasmic decapping enzymes (e.g. dcp2) renders the 5’ end susceptible to exonucleases. Recently described cytoplasmic recapping enzymes may allow specific transcripts to resist decay (Otsuka, Kedersha, & Schoenberg, 2009). Immediately following the cap is the 5’ untranslated region (UTR). The 5’ UTR is a critical source of regulatory information encoded by sequences and structures. Examples include internal ribosomal entry sequences (IRES) that promote translation through direct binding to translation factors and/or ribosomes (Hellen & Sarnow, 2001). Upstream open reading frames (uORFs) have emerged as a major regulatory mechanism to downregulate the main open reading frame but also appear to have the potential to generate functional peptides or N-terminal extensions (Hellen & Sarnow, 2001; Starck et al., 2016; Y. Xu et al., 2019). In rare cases, such as during environmental stress, uORFs can enhance translation of the main reading frame (Hinnebusch, Ivanov, & Sonenberg, 2016). Secondary structures present in the 5’ UTR can also influence translation through an increased dependence on helicases such as eIF4A (Feoktistova, Tuvshintogs, Do, & Fraser, 2013; Garcia-Garcia, Frieda, Feoktistova, Fraser, & Block, 2015).
Figure 2 –
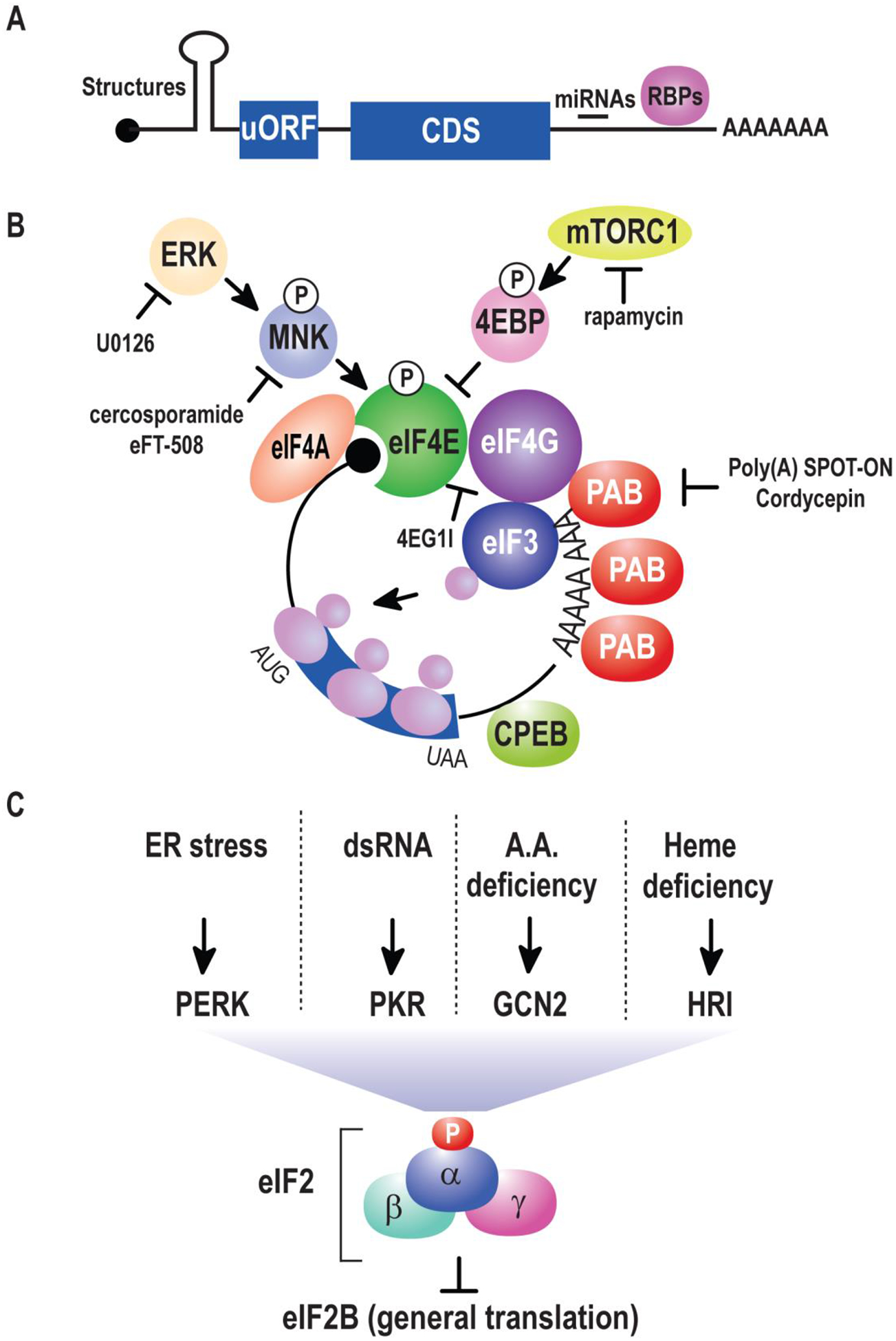
(A) Anatomy of an mRNA. mRNAs are typically capped (black ball). Immediately afterwards is a 5’ untranslated region (UTR) rendered here with a secondary structure that can modulate translation efficiency or mediate bypass of general translation factors. The coding sequences (CDS) contain the primary sequence of the polypeptide that results from translation. This is followed by a 3’ UTR rich in regulatory elements decoded by trans-acting RNAs (e.g. miRNAs) and RNA-binding proteins (RBPs). Most mRNA have a poly(A) tail at the 3’ end. (B) Regulation of cap-dependent translation initiation. The eIF4F complex containing eIF4A, eIF4E, and eIF4G recruits eIF3 and ribosomes to scan the mRNA for a suitable start codon. This process is controlled by MAPK kinases such as ERK that act via MNK to phosphorylate eIF4E. A second pathway controls eIF4E activity through mTORC1 which phosphorylates 4EBPs preventing their association and sequestration of eIF4E. Additionally, the association of eIF4G and the poly(A) binding protein (PAB) stimulates the efficiency of translation initiation. (C) An overview of eIF2α-dependent translation. Upstream kinases control the activity of eIF2. Phosphorylation of the α subunit inhibits the guanine nucleotide exchange factor eIF2B, resulting in bulk translational inhibition.
The coding region has emerged as a major player in mRNA stability (Hanson & Coller, 2018; Presnyak et al., 2015). In somatic cells, the distribution of optimal codons is directly proportional to mRNA stability which in turn is linked to protein output. The situation appears to be more complex in neurons where codon optimality is not directly linked to stability (Burow et al., 2018). This could provide a mechanism to amplify the regulatory effects of trans-acting factors over mRNA decay specifically in neurons. The coding region is controlled by additional pathways including RNA editing by ADAR enzymes that convert adenosine to inosine (Bass, 2002). Subsequent decoding by the ribosome results in recoding of inosine as guanine. Exceptionally efficient editing of the ionotropic AMPA glutamate receptor 2 in the brain renders the channels Ca2+-impermeable and is required for certain forms of learning and memory (Sommer, Kohler, Sprengel, & Seeburg, 1991). AMPA receptors play critical roles in spinal nociceptive transmission suggestive of a potential link between RNA editing and pain (Larsson & Broman, 2008; Tao, 2012).
The 3’ UTR is a key repository for regulatory information in the form of cis-acting elements. The 3’ UTR can contain elements that enhance or reduce RNA stability, translation, or localization. A prototypical example are miRNA binding elements. The 3’ ends of mRNA are also dynamic. Through a process conceptually similar to alternative splicing (though mechanistically distinct), alternative polyadenylation (APA) allows for rapid modulation of 3’ UTR length (Masamha et al., 2014; Mayr & Bartel, 2009). APA is critical to the survival of motor neurons and likely has major implications for which transcripts are destined to be translated locally (Gray et al., 2018).
Immediately following the 3’ UTR is the poly(A) tail. The number of adenosines in the poly(A) tail is subject to modulation through deadenylation and polyadenylation. Both processes can be controlled by cytoplasmic enzymes whose specificity appears to be dictated by recruitment through RNA-binding protein partners (Goldstrohm & Wickens, 2008). Loss of the poly(A) tail stimulates decapping and subsequent decay (Chen & Shyu, 2011). Additional modifications to the 3’ end include uridylation and guanylation (Lim et al., 2014; Lim et al., 2018). These newly discovered modifications have opposing effects on stability and may serve unexplored functions in sensory neurons. Knowledge of the corresponding polymerases provides the basis to probe their biological functions.
Cap-dependent translation
Nascent protein synthesis can be modulated in an activity-dependent fashion. One signalling cascade that links translation to extracellular factors is mTORC1 (Figure 2B). mTORC1 phosphorylates 4E-binding proteins (4EBPs) resulting in their sequestration and an increase in free eIF4E. eIF4E binds to the mRNA cap and nucleates the assembly of the eIF4F complex. Inhibition of mTORC1 with rapamycin (and related compounds) attenuates mechanical hypersensitivity in many models of inflammatory-, cancer-, and injury-induced pain (Geranton et al., 2009; Jimenez-Diaz et al., 2008; Price et al., 2007). eIF4E is also controlled by MAP kinase-interacting kinases (MNKs) which phosphorylate eIF4E and may enhance translation of a subset of transcripts (Altman et al., 2013; Gelinas et al., 2007; Konicek et al., 2011; Megat et al., 2019; Moy et al., 2017; Panja et al., 2009; Robichaud et al., 2018; Ueda et al., 2010).
Two inflammatory mediators, nerve growth factor (NGF) and interleukin 6 (IL6) rapidly induce nascent protein synthesis in nociceptors (Melemedjian et al., 2010). They also increase phosphorylation of mTORC1, eIF4E, eIF4G, and 4EBP1. Blocking either mTORC1 or MNK1 (e.g. by Cercosporamide treatment) attenuates mechanical hypersensitivity resulting from injection of NGF or IL-6 (Melemedjian et al., 2010; Moy et al., 2017). Similarly, inhibition of the eIF4F assembly with a small molecule (4EGI1) that impairs binding of eIF4E to eIF4G reduces pain amplification behaviour. As all the compounds in these experiments were injected into the paw, these data suggest that local translation in afferent fibers is required for the pain amplification behaviour. This raises the question – what are the relevant targets of mTOR required for the maintenance of pain?
A major hindrance to translational profiling of tissues is cellular heterogeneity. One approach to overcome this problem is the use of translating ribosome affinity purification (TRAP) (Heiman et al., 2008). TRAP was recently applied to understand translational responses of sensory neurons in animals with neuropathic pain elected by a chemotherapeutic (Paclitaxel) (Megat et al., 2019). In this study, an epitope tagged ribosomal subunit (l10a) was expressed exclusively in nociceptors. Sequencing of ribosome-bound transcripts revealed that components of the mTOR pathway are preferential targets of translation. Similar to the IL6 and NGF model, chemotherapy-induced nociceptor plasticity is driven by sustained eIF4E phosphorylation. The pain-associated behavioural effects of paclitaxel were reversed upon injection of the MNK inhibitor, eFT508. This suggests that pharmacological disruption of cap-dependent translation may provide a means to reverse neuropathic pain states. Consistent with this notion, elimination of the sole phosphorylation site on eIF4E results in profound deficits in pain behavioural responses to inflammatory mediators (Moy et al., 2017). Two key questions remain. First, what is the relevant target of eIF4E driven translation? And second, does the target need to be translated locally or in the soma?
In addition to 4EBPs, mTORC1 targets p70S6K1 and p70S6K2 (S6K1/2). The role of S6K1/2 in pain does not appear to be as prominent as 4EBPs (Melemedjian et al., 2013). Deletion of S6K1/2 in mice does not impact thermal sensitivity but increases sensitivity to mechanical stimuli. A possible explanation for the sensitivity is increased Erk phosphorylation. Thus, animals with genetic loss of S6K1/2 appear to have hyperactive eIF4E phosphorylation which may obscure the role of S6Ks.
Sequence specific RNA-binding proteins
RNA-binding proteins (RBPs) collaborate to control mRNA function. Several RBPs have been associated with various pain conditions (Table 2; for in depth review see (de la Pena & Campbell, 2018)). Some of these RBPs have well characterized roles in activity-dependent mRNA trafficking in the CNS. For instance, Fragile X mental retardation protein (FMRP) binds and regulates the translation of plasticity-relevant transcripts in the synapse (Darnell & Klann, 2013). Loss of FMRP leads to Fragile X syndrome, a neurodevelopmental disorder characterized by autistic behaviour, cognitive impairment, and learning disabilities. Mice with genetic deletion of the FMRP gene exhibit altered nociceptive sensitization (Price et al., 2007). Additionally, mTOR inhibition fails to block inflammation-associated pain in these mice suggestive of major defects in plasticity. Mechanical hypersensitivity caused by IL-6 is also abrogated in FMRP-deficient animals. Why does loss of FMRP compromise pain sensitization? As FMRP promotes translation elongation (Darnell et al., 2011), one possibility is that the rate of protein synthesis in fibres is carefully regulated to ensure proper timing of protein synthesis following a noxious challenge.
Table 2:
RBPs implicated in pain
| RBP | Pain condition | Relevant findings | Reference |
|---|---|---|---|
| Fragile X mental retardation protein (FMRP) | Inflammation and neuropathic pain | Mice with genetic deletion of the FMR gene showed decreased nociceptive response. | (Price et al., 2007) |
| HuD or ELAV Like RNA Binding Protein 4 (Elavl4) | Antiretroviral toxic neuropathy | An ASO against HuD reverted pain hypersensitivity in mice. | (Sanna, Peroni, Quattrone, Ghelardini, & Galeotti, 2015) |
| HuR or ELAV Like RNA Binding Protein 1 (Elavl1) | Multiple Sclerosis related neuropathic pain | Spinal HuR silencing alleviated hypernociceptive behaviour in mice. | (Sanna, Quattrone, & Galeotti, 2017) |
| eukaryotic translation initiation factor 4E (eIF4E) | Inflammation and neuropathic pain | Phosphorylation of eIF4E, at serine 209, is a key factor in nociceptor sensitization and the development of chronic pain. | (Moy et al., 2017) |
| Cytoplasmic polyadenylation element binding protein (CPEB) | Inflammation and neuropathic pain | Blocking CPEB reduced hyperalgesia and mechanical allodynia. | (Bogen et al.,2012; Iida et al., 2016) |
| Poly(A)-binding protein (PABP) | Inflammation and injury | Local delivery of a PABP SPOT-ON blocked mechanical hyperalgesia induced by pro-inflammatory cytokines, capsaicin, or incision. | (Barragan-Iglesias et al.,2018) |
The Hu family of RBPs enhances the stability of transcripts by binding to adenylate-uridylate-rich elements (AU-rich elements) in the 3′ UTR (Antic, Lu, & Keene, 1999). Depletion of some members of the Hu family (HuR and HuD) attenuates neuropathic pain in mice, suggesting that these RBPs facilitate pain, likely at the level of RNA stability. A major unresolved question is the identity of the relevant targets and mechanisms of action responsible for attenuation of pain.
CPEBs are RNA-binding proteins that modulate poly(A) tail length. They bind AU-rich sequences found in the 3’UTR and can activate or repress polyadenylation as a direct result of post-translational modifications (Hodgman, Tay, Mendez, & Richter, 2001). Protein partners can also influence their binding specificity (Campbell, Bhimsaria, et al., 2012; Campbell, Menichelli, et al., 2012; Menichelli, Wu, Campbell, Wickens, & Williamson, 2013; J. Wu, Campbell, Menichelli, Wickens, & Williamson, 2013). Knockdown of CPEB through intrathecal injection of a CPEB ASO blocks nociception and mechanical hyperalgesia (Bogen et al., 2012). Deletion of CPEB3 results in thermal hypersensitivity (Fong, Lin, Wu, Chen, & Huang, 2016). Alpha calmodulin-dependent protein kinase II (αCaMKII) has been suggested as the downstream target of CPEB (Ferrari, Bogen, & Levine, 2013). The next logical question becomes: is αCaMKII the sole relevant target?
Poly(A)-binding proteins (PABPs) regulate mRNA stability, translation initiation, mRNA localization, mRNA export, nonsense mediated decay, and miRNA mediated translational repression (Gorgoni & Gray, 2004). The association of PABP with mRNA is linked to the length of the poly(A) tail and its translation (Gorgoni & Gray, 2004; Smith, Blee, & Gray, 2014). We have demonstrated that stabilized RNAs that mimic the poly(A) tail function as a competitive inhibitor of PABP, attenuate translation at initiation, and block mechanical hypersensitivity triggered by various inflammatory cues (Barragan-Iglesias et al., 2018). This inhibitor, termed specificity-derived competitive inhibitor oligonucleotides or SPOT-ON, is compact, taken up by neurons, and well-tolerated in vivo. The simplicity of its design is enabled by advances in unbiased assessment of RNA-protein interactions (Campbell, Valley, & Wickens, 2014; Campbell & Wickens, 2015; Zhou et al., 2018). The robust anti-hyperalgesic behavioural effects of the poly(A) decoy suggest that SPOT-ONs can be used to elucidate mechanisms underlying chronic pain and provide a new strategy for the identification of targets relevant to pain.
The poly(A) tail
Noxious stimuli can trigger long-term changes in nociceptor sensitivity. Intriguingly, certain forms of peripheral plasticity are blocked by peripheral administration of cordycepin, an inhibitor of polyadenylation (Ferrari, Araldi, & Levine, 2015; Ferrari, Bogen, Chu, & Levine, 2013b). Importantly, transcriptional inhibitors can prevent the initial onset of pain but fail to reverse hyposensitivity (Ferrari, Bogen, Reichling, & Levine, 2015). Taken together with the above-mentioned role of CPEB and PABP, these findings suggest that the poly(A) tail is important for the persistence of pain hypersensitivity. Advances in high throughput sequencing of poly(A) tails may provide a means to identify transcripts that undergo regulated polyadenylation, in axons and cell bodies, during the development of nociceptive sensitization (Lim et al., 2014).
eIF2α and the integrated stress response
Eukaryotic initiation factor 2 (eIF2) is an integral player in cellular stress response and nociception (Figure 2C) (Khoutorsky et al., 2016; Sidrauski, McGeachy, Ingolia, & Walter, 2015). Phosphorylation of the α subunit attenuates nascent translation specifically at the step of initiation (Holcik & Sonenberg, 2005). The net effect of eIF2α phosphorylation is inhibition of a second translation factor called eIF2B that mediates formation of the ribosomal pre-initiation complex. eIF2α resides at the heart of a regulatory network called the integrated stress response (ISR). Four upstream kinases control eIF2α phosphorylation. These include the viral sensor PKR (double-stranded RNA-dependent protein kinase); an ER stress responsive kinase called PERK (PKR-like ER kinase); a nutrient sensor GCN2 (general control non–derepressible-2); and the heme sensor HRI (heme-regulated inhibitor). Phosphorylation of eIF2α is increased in a model of chronic inflammatory pain (Khoutorsky et al., 2016). Heterozygous mice defective in eIF2α phosphorylation display enhanced thermal sensitivity. Intriguingly, these mice have no deficits with respect to mechanical perception but have reduced sensitivity to inflammatory pain. eIF2α phosphorylation has also been linked to neuropathic pain (Barragan-Iglesias et al., 2019). Methyl glyoxal, a reactive glycolytic metabolite associated with painful diabetic neuropathy, robustly increases eIF2α phosphorylation through PERK. The small molecule ISRIB is a robust initiator of PERK signalling that can reverse the effects of eIF2α phosphorylation and restore translational homeostasis through direct association with eIF2B (Tsai et al., 2018). Intriguingly, pain observed both in animals treated with streptozotocin, a chemical used for the destruction of insulin-producing cells that induces type I diabetes, and in animals given methyl glyoxal was reversed by ISRIB. Collectively, these experiments highlight the critical role of eIF2α phosphorylation in pain. Future efforts will require understanding the relevant regulatory targets. While eIF2α phosphorylation is generally inhibitory, translation of specific mRNAs with uORFs is increased. Alternatively, mRNAs with decreased reliance on cap-dependent translation are likely less impacted by eIF2α phosphorylation. Genome-wide assays of translation will be crucial to identify the relevant target(s) moving forward.
RNA therapeutics directed against mRNA
miRNAs/siRNAs
MicroRNAs (miRNAs) are a class of small non-coding RNAs that destabilize mRNA and elicit translational repression. Compromised miRNA biogenesis via deletion of Dicer results in profound deficits in pain behavioural responses in an inflammatory pain model (J. Zhao et al., 2010). miRNAs have emerged as important players in post-transcriptional control of neuronal plasticity (Z. Hu & Li, 2017). miRNA modulation has also emerged as a new source of possible pain therapeutics (Table 3) (Dai et al., 2018; Lopez-Gonzalez, Landry, & Favereaux, 2017). For example, knockdown of miRNA 155 has anti-hyperalgesic effects in a neuropathic pain model (Liu, Zhu, Sun, & Xie, 2015). Specific miRNAs may possess deviant roles as mediators of intercellular messengers via binding to extracellular receptors (Park et al., 2014). Addition of the let-7b miRNA to DRG neurons results in inward action potentials. Injection of a let-7b mimic is sufficient to induce pain hypersensitivity. Conversely, administration of a let-7b inhibitor blocks pain associated behaviours in an inflammatory pain model. While it is unclear if micromolar concentrations of secreted let-7b can be attained in vivo, the sequence specificity of the interaction implies that this small RNA moonlights as a secreted pro-nociceptive mediator.
Table 3:
miRNAs associated with pain conditions in both preclinical and clinical studies.
| miRNA | Expression change | Pain condition/model | Subjects | Reference |
|---|---|---|---|---|
| miR-30c-5p | Up | Neuropathic pain | Human and mouse | (Tramullas et al., 2018) |
| miR-146a | Up | Diabetic neuropathy | Human | (Ciccacci et al., 2014) |
| Rat | (Yousefzadeh, Alipour, & Soufi, 2015) | |||
| miR-126 | Down | Neuropathic pain | Human | (Orlova et al., 2011) |
| Mouse | (Manners, Tian, Zhou, & Ajit, 2015) | |||
| miR-155 | Down | Inflammation and pain | Human | (Yao et al., 2011) |
| Up | Mouse | (Poh, Yeo, & Ong, 2011) | ||
| let-7 family | Down | Neuropathic pain | Human | (Orlova et al., 2011) |
| Up | Neuropathic pain | Rat, Mouse | (Brandenburger et al.,2012; Park et al., 2014) | |
| miR-200c | Up | Intervertebral disc degeneration | Human | (B. Zhao, Yu, Li, Guo, & He, 2014) |
| Spinal cord trauma | Mouse | (D. S. Yu et al., 2014) | ||
| miR-223 | Down | Fibromyalgia | Human | (Bjersing, Lundborg, Bokarewa, & Mannerkorpi, 2013) |
| Up | Inflammation and pain | Mouse | (Poh et al., 2011) | |
| miR-195 | Down | Fibromyalgia | Human | (Bjersing et al., 2013) |
| Up | Neuropathic pain | Rats | (Shi et al., 2013) |
Unlike miRNAs that base pair imperfectly to target mRNAs, siRNAs are typically exogenous dsRNAs that trigger cleavage of their targets and subsequent decrease in target protein levels. Early uses of siRNAs for pain include knockdown of the P2X3 ATP receptor resulting in a reduction in neuropathic pain (Dorn et al., 2004). A hindrance in this study is the need for large amounts of siRNA. Subsequent experiments have made use of improved delivery methods that increase knockdown efficiencies (e.g. polyethyleneimine/RNA complexes) by ~40% and require less RNA (Tan, Yang, Shih, Lan, & Cheng, 2005). siRNAs delivered as complexes directed against NR2B result in decreased spontaneous pain in an inflammatory model. More recently, knockdown of matrix metalloproteases (MMPs) has been shown to suppress mechanical sensitivity in a nerve injury model. Numerous advances in the area of siRNAs that modify preclinical models of pain are provided in Table 4. Perhaps the most exciting aspect of the preclinical uses of siRNAs is the recent FDA approval of Onpattro, an siRNA directed against transthyretin (TTR), a transport protein in the cerebrospinal fluid that shuttles the thyroid hormone thyroxine (T4) and retinol-binding protein bound to retinol (Solomon et al., 2019). Mutations in TTR can cause hereditary transthyretin-mediated amyloidosis (hATTR) in peripheral nerves resulting in nerve injury and neuropathic pain. With the successful demonstration of this type of pharmacology in humans, hopefully, Onpattro is a harbinger of improved treatment strategies for chronic pain in humans.
Table 4:
siRNA involved in various pain conditions
| Molecular target | Pain condition | Knockdown efficiency | Reference |
|---|---|---|---|
| P2X purinoceptor 3 (P2X3) | Neuropathic pain | 40% in DRG | (Dorn et al., 2004) |
| NMDA receptor, NR2B subunit | Inflammatory pain | 70–80% in SC | (Tan et al., 2005) |
| Bone cancer pain | ~50% in rACC | (Y. Xu, G. Wang, et al., 2016) | |
| NMDA receptor, NR1 subunit | Inflammatory pain | 60–75% in SC | (Garraway, Xu, & Inturrisi, 2007) |
| Tyrosine kinase receptor B (TrkB) | Inflammatory pain | 70% in medulla | (Guo et al., 2006) |
| Prostaglandin E2 receptor 4 (PGE2 EP4) | Inflammatory pain | 69% in DRG | (Lin et al., 2006) |
| Transient receptor potential cation channel subfamily V member 1 (TrpV1) | Inflammatory and neuropathic pain | 75% in DRG | (Christoph et al., 2008; Christoph et al., 2006) |
| ~20% in DRG | (Kasama et al., 2007) | ||
| Matrix metalloproteinase (MMP) −2 and −9 | Neuropathic pain | 50% in DRG and SC | (Kawasaki et al., 2008) |
| Inwardly rectifying potassium (Kir) 4.1 channel | Neuropathic pain | 40% in trigeminal ganglion | (Vit, Ohara, Bhargava, Kelley, & Jasmin, 2008) |
| Acid-sensing ion channel (ASIC-3) | Inflammatory pain | 50–90% in DRG | (Deval et al., 2008) |
| platelet-derived growth factor (PDGF) | Bone cancer pain | 60–80% SC | (Y. Xu, J. Liu, et al., 2016) |
| interferon regulatory factor 5 (IRF5) | Neuropathic pain | 50–60% microglia | (Terashima et al., 2018) |
| caspase-6 (CASP6) | Inflammatory pain | 30–40% DRG | (Berta et al., 2014) |
| Toll-like receptor 4 (TLR4) | Neuropathic pain Bone cancer pain |
~50% in SC ~60% in SC |
(F. X. Wu et al.,2010) (Pan et al., 2015) |
| YAP/TAZ | Neuropathic pain | ~50 – 60% in SC | (N. Xu et al.,2016) |
| Inflammatory and neuropathic pain | ~50% in DRG | (Yang et al., 2017) | |
| (CXCR-4) | Bone cancer pain | ~50% in SC | (X. M. Hu et al.,2017) |
| C-C chemokine receptor 2 (CCR2) | Inflammatory pain | ~50% in DRG | (Begin-Lavallee et al., 2016) |
| chemokine CXC motif ligand 12 (CXCL12) | Chemotherapy-induced peripheral neuropathy | ~50% in SC | (Li et al., 2017; T. Xu et al., 2017) |
| Phosphodiesterase 4 (PDE4) B | Neuropathic pain | ~40% in SC | (Ji et al., 2016) |
| FK506 binding protein 51 (FKBP51) | Neuropathic pain | ~80% in DRG | (H. M. Yu, Wang, & Sun, 2017) |
| NF-κB p65 | Chemotherapy-induced peripheral neuropathy | ~50% in DRG | (J. Wang et al.,2017) |
| NaV1.6 | Inflammatory and neuropathic pain | ~50–70% in DRG | (Xie, Strong, Ye, Mao, & Zhang, 2013; Xie, Strong, & Zhang, 2015) |
| growth-associated protein 43 (GAP43) | Neuropathic pain | ~50% in DRG and SN | (Xie, Strong, & Zhang, 2017) |
| Raf-1 | Opioid-induced hyperalgesia | ~80% in SC | (Tumati et al., 2008; Tumati et al.,2010) |
| Interferon regulatory factor-5 (IRF5) | Neuropathic pain | ~60% in SC | (Masuda et al.,2014) |
| CCR1 and CCR5 | Neuropathic pain | ~50% in SN | (Kiguchi, Maeda, Kobayashi, Fukazawa, & Kishioka, 2010) |
| TNF Receptor Associated Factor 6 (TRAF6) | Neuropathic pain | ~40% in cultured astrocytes | (Lu et al., 2014) |
| Pannexin-1 | Neuropathic pain | ~40% in DRG | (Zhang, Laumet, Chen, Hittelman, & Pan, 2015) |
| T-cell death-associated gene 8 (TDAG8) | Bone cancer pain | ~40% in SC | (Hang et al., 2012) |
| Sensory neuron-specific receptors (SNSRs) | Inflammatory pain | ~30% in DRG | (Ndong et al., 2009) |
| Brain-derived neurotrophic factor (BDNF) | Bone cancer pain | ~60% in SC | (L. N. Wang et al., 2012) |
| GTP cyclohydrolase I (GCH1) | Neuropathic pain | ~50% in DRG | (S. J. Kim et al., 2009) |
Antisense oligonucleotides (ASOs)
ASOs are exogenous single stranded nucleic acids that bind to mRNAs and modulate protein synthesis and RNA processing through a variety of mechanisms. Typically, ASOs suppress RNA stability through Watson-Crick base pairing to target mRNAs and subsequent cleavage by RNase H. Currently, there are ongoing clinical trials for the therapeutic use of ASOs in the nervous system for treatment of ALS (NCT02623699) and Huntington’s disease (NCT02519036). Additionally, an ASO therapy targeted to the nervous system, nusinersen, has been approved by the FDA for treatment of spinal muscular atrophy (Finkel et al., 2017; Rigo et al., 2014). ASOs delivered systemically or centrally can target transcripts in the spinal cord and DRG (Mohan et al., 2018). Remarkably, an ASO directed against the sodium channel Nav1.7 resulted in decreased mechanical sensitivity that persists for four weeks in rats. Additional studies have focused on p38, p65, CREB, and P2X receptors (Table 5). A limitation of ASOs are the modest effects on mRNA abundance ca. 50%. However, their ease of use and established safety and efficacy in humans suggests that mRNA destabilization is a viable and increasingly common strategy to attenuate pain.
Table 5:
ASOs used in preclinical models of pain
| Molecular target | Pain condition | Knockdown efficiency | Reference |
|---|---|---|---|
| Nav 1.8 | Neuropathic pain | ~40% in SN | (Gold et al., 2003) |
| ~40% in DRG | (Lai et al., 2002) | ||
| Inflammatory and neuropathic pain | ~50% in DRG | (Porreca et al., 1999) | |
| Inflammatory pain | ~30% in DRG | (Y. Q. Yu, Zhao, Guan, &Chen, 2011) | |
| Visceral pain | ~50% in DRG | (Yoshimura et al., 2001) | |
| cAMP response element binding protein (CREB) | Neuropathic pain | ~50% in SC | (Gu et al., 2013) |
| ~80% in SC | (Ma, Hatzis, & Eisenach, 2003) | ||
| ~50% in SC | (Y. Y. Wang et al., 2006) | ||
| P2X3 receptor | Inflammatory and neuropathic pain | ~30% in DRG | (Honore et al., 2002) |
| ~30% in SC | (Barclay et al., 2002) | ||
| metabotropic glutamate receptor 1 (mGluFM) | Neuropathic pain | ~50% in SC | (Fundytus et al., 2001) |
| Inflammation pain | ~50% in SC | (Fundytus, Osborne, Henry, Coderre, & Dray, 2002) | |
| p38α MAP kinase isoform | Neuropathic and postoperative pain | ~50% in SC | (Luo et al., 2018) |
| p38β MAP kinase isoform | Bone cancer pain | ~50% in SC | (Dong, Xiang, Ye, & Tian, 2014) |
| p65 subunit of NF-κB | Neuropathic pain | ~30% in SC | (T. Sun et al., 2006) |
| Nav1.7 | Inflammatory pain | ~50% in DRG | (Mohan et al., 2018) |
| TRPV1 | Neuropathic pain | ~80% in DRG, ~50% in SC |
(Christoph et al., 2007) |
| HuR | Multiple sclerosis associated pain | ~30% in SC | (Sanna et al., 2017) |
| Epac1 | Inflammatory pain | ~40% in DRG | (H. Wang et al., 2013) |
| extracellular signal-regulated protein kinase 5 (ERK5) | Neuropathic pain | ~60% in SC | (J. L Sun et al., 2013) |
| ERK | Neuropathic pain | ~70% in SC | (Song et al., 2005) |
| beta-arrestin | Neuropathic pain | ~60% in cultured cells | (Przewlocka et al., 2002) |
| Neurotrophin-3 | Neuropathic pain | ~45% in SC | (White, 2000) |
| acid-sensing ion channels (ASICs) | Inflammatory pain | ~60% in SC | (Duan et al., 2007) |
| NMDA-R1, NMDA-R2C, and NMDA-R2D | Inflammatory pain | 20–40% in SC | (Yukhananov, Guan, & Crosby, 2002) |
| T-subtype calcium channels (Cav3.2, and Cav3.3) | Neuropathic pain | ~70% in SC | (Duan et al., 2007) |
| glial fibrillary acidic protein (GFAP) | Neuropathic pain | ~60% in DRG ~30% in SC |
(D. S. Kim et al., 2009) |
Conclusion
Despite the clear genetic and pharmacologic evidence that post-transcriptional and translational controls permeate nociceptor function, in many cases it remains unclear which mRNAs must be properly repressed or activated to engender sustained excitability. Advances in high-throughput approaches to profiling nascent translation coupled to RNA directed interventions may provide a synergistic means to address this problem. Comprehensive identification of translational efficiencies across a range of pain states at different time points are a promising avenue to address this problem (Megat et al., 2019; Sonali Uttam, 2018). The identification of targets and new mechanisms afford remarkable advances in our understanding of pain molecular biology and hopefully improved strategies for blocking the plasticity that facilitates chronic pain. Therapeutics that act peripherally have tremendous clinical potential as they differ fundamentally from opiates that act on pain processing and have profound impacts on the reward centres of the brain. This work has highlighted a variety of approaches to disrupt nociceptive pain signalling by simply shooting the proverbial messenger (or otherwise disrupting its function). The major challenge moving forward is to define the most effective and safe target in humans.
Funding Information
This work was supported in part by an NIH grant R01NS100788.
Footnotes
No conflicts of interest
References
- Altman JK, Szilard A, Konicek BW, Iversen PW, Kroczynska B, Glaser H, … Platanias LC (2013). Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors. Blood, 121(18), 3675–3681. doi: 10.1182/blood-2013-01-477216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J (2001). The autonomous axon: a model based on local synthesis of proteins. Biol Res, 34(2), 103–109. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Giuditta A, & Koenig E (2000). Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol, 62(1), 1–62. [DOI] [PubMed] [Google Scholar]
- Antic D, Lu N, & Keene JD (1999). ELAV tumor antigen, Hel-N1, increases translation of neurofilament M mRNA and induces formation of neurites in human teratocarcinoma cells. Genes Dev, 13(4), 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, … Ganju P (2002). Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci, 22(18), 8139–8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan-Iglesias P, Kuhn J, Vidal-Cantu GC, Salinas-Abarca AB, Granados-Soto V, Dussor GO, … Price TJ (2019). Activation of the integrated stress response in nociceptors drives methylglyoxal-induced pain. Pain, 160(1), 160–171. doi: 10.1097/j.pain.0000000000001387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan-Iglesias P, Lou TF, Bhat VD, Megat S, Burton MD, Price TJ, & Campbell ZT (2018). Inhibition of Poly(A)-binding protein with a synthetic RNA mimic reduces pain sensitization in mice. Nat Commun, 9(1), 10. doi: 10.1038/s41467-017-02449-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass BL (2002). RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem, 71, 817–846. doi: 10.1146/annurev.biochem.71.110601.135501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begin-Lavallee V, Midavaine E, Dansereau MA, Tetreault P, Longpre JM, Jacobi AM, … Sarret P (2016). Functional inhibition of chemokine receptor CCR2 by dicer-substrate-siRNA prevents pain development. Mol Pain, 12. doi: 10.1177/1744806916653969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lu N, … Ji RR (2014). Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin Invest, 124(3), 1173–1186. doi: 10.1172/JCI72230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjersing JL, Lundborg C, Bokarewa MI, & Mannerkorpi K (2013). Profile of cerebrospinal microRNAs in fibromyalgia. PLoS One, 8(10), e78762. doi: 10.1371/journal.pone.0078762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Alessandri-Haber N, Chu C, Gear RW, & Levine JD (2012). Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci, 32(6), 2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, & Wells DG (2007). Dendritic mRNA: transport, translation and function. Nat Rev Neurosci, 8(10), 776–789. doi:nrn2150 [pii]10.1038/nrn2150 [DOI] [PubMed] [Google Scholar]
- Brandenburger T, Castoldi M, Brendel M, Grievink H, Schlosser L, Werdehausen R, … Hermanns H (2012). Expression of spinal cord microRNAs in a rat model of chronic neuropathic pain. Neurosci Lett, 506(2), 281–286. doi: 10.1016/j.neulet.2011.11.023 [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, & Flanagan JG (2002). Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell, 110(2), 223–235. [DOI] [PubMed] [Google Scholar]
- Burow DA, Martin S, Quail JF, Alhusaini N, Coller J, & Cleary MD (2018). Attenuated Codon Optimality Contributes to Neural-Specific mRNA Decay in Drosophila. Cell Rep, 24(7), 1704–1712. doi: 10.1016/j.celrep.2018.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum AR, Yoon YJ, Singer RH, & Park HY (2015). Single-molecule insights into mRNA dynamics in neurons. Trends Cell Biol, 25(8), 468–475. doi: 10.1016/j.tcb.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ZT, Bhimsaria D, Valley CT, Rodriguez-Martinez JA, Menichelli E, Williamson JR, … Wickens M (2012). Cooperativity in RNA-protein interactions: global analysis of RNA binding specificity. Cell Rep, 1(5), 570–581. doi: 10.1016/j.celrep.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ZT, Menichelli E, Friend K, Wu J, Kimble J, Williamson JR, & Wickens M (2012). Identification of a conserved interface between PUF and CPEB proteins. J Biol Chem, 287(22), 18854–18862. doi: 10.1074/jbc.M112.352815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ZT, Valley CT, & Wickens M (2014). A protein-RNA specificity code enables targeted activation of an endogenous human transcript. Nat Struct Mol Biol, 21(8), 732–738. doi: 10.1038/nsmb.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ZT, & Wickens M (2015). Probing RNA-protein networks: biochemistry meets genomics. Trends Biochem Sci, 40(3), 157–164. doi: 10.1016/j.tibs.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, & Shyu AB (2011). Mechanisms of deadenylation-dependent decay. Wiley Interdiscip Rev RNA, 2(2), 167–183. doi: 10.1002/wrna.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph T, Bahrenberg G, De Vry J, Englberger W, Erdmann VA, Frech M, … Kurreck J (2008). Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Mol Cell Neurosci, 37(3), 579–589. doi: 10.1016/j.mcn.2007.12.006 [DOI] [PubMed] [Google Scholar]
- Christoph T, Gillen C, Mika J, Grunweller A, Schafer MK, Schiene K, … Kurreck J (2007). Antinociceptive effect of antisense oligonucleotides against the vanilloid receptor VR1/TRPV1. Neurochem Int, 50(1), 281–290. doi: 10.1016/j.neuint.2006.08.017 [DOI] [PubMed] [Google Scholar]
- Christoph T, Grunweller A, Mika J, Schafer MK, Wade EJ, Weihe E, … Kurreck J (2006). Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun, 350(1), 238–243. doi: 10.1016/j.bbrc.2006.09.037 [DOI] [PubMed] [Google Scholar]
- Ciccacci C, Morganti R, Di Fusco D, D’Amato C, Cacciotti L, Greco C, … Spallone V (2014). Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol, 51(4), 663–671. doi: 10.1007/s00592-014-0582-2 [DOI] [PubMed] [Google Scholar]
- Dai Z, Chu H, Ma J, Yan Y, Zhang X, & Liang Y (2018). The Regulatory Mechanisms and Therapeutic Potential of MicroRNAs: From Chronic Pain to Morphine Tolerance. Front Mol Neurosci, 11, 80. doi: 10.3389/fnmol.2018.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, & Klann E (2013). The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci, 16(11), 1530–1536. doi: 10.1038/nn.3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, … Darnell RB (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell, 146(2), 247–261. doi: 10.1016/j.cell.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Pena JB, & Campbell ZT (2018). RNA-binding proteins as targets for pain therapeutics. Neurobiol Pain, 4, 2–7. doi: 10.1016/j.ynpai.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, … Lingueglia E (2008). ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J, 27(22), 3047–3055. doi: 10.1038/emboj.2008.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Xiang HB, Ye DW, & Tian XB (2014). Inhibitory effects of intrathecal p38beta antisense oligonucleotide on bone cancer pain in rats. Int J Clin Exp Pathol, 7(11), 7690–7698. [PMC free article] [PubMed] [Google Scholar]
- Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, … Hall J (2004). siRNA relieves chronic neuropathic pain. Nucleic Acids Res, 32(5), e49. doi: 10.1093/nar/gnh044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu L, … Xu TL (2007). Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci, 27(41), 11139–11148. doi: 10.1523/JNEUROSCI.3364-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, & Patapoutian A (2010). Nociceptors: the sensors of the pain pathway. J Clin Invest, 120(11), 3760–3772. doi: 10.1172/jci42843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistova K, Tuvshintogs E, Do A, & Fraser CS (2013). Human eIF4E promotes mRNA restructuring by stimulating eIF4A helicase activity. Proc Natl Acad Sci U S A, 110(33), 13339–13344. doi: 10.1073/pnas.1303781110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Araldi D, & Levine JD (2015). Distinct terminal and cell body mechanisms in the nociceptor mediate hyperalgesic priming. J Neurosci, 35(15), 6107–6116. doi: 10.1523/JNEUROSCI.5085-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Chu C, & Levine JD (2013a). Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain, 14(7), 731–738. doi: 10.1016/j.jpain.2013.01.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Chu C, & Levine JD (2013b). Peripheral Administration of Translation Inhibitors Reverses Increased Hyperalgesia in a Model of Chronic Pain in the Rat. J Pain. doi: 10.1016/j.jpain.2013.01.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, & Levine JD (2013). Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci, 33(27), 11002–11011. doi: 10.1523/JNEUROSCI.1785-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Reichling DB, & Levine JD (2015). Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci, 35(2), 495–507. doi: 10.1523/JNEUROSCI.5147-13.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, … De Vivo DC (2017). Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N Engl J Med, 377(18), 1723–1732. doi: 10.1056/NEJMoa1702752 [DOI] [PubMed] [Google Scholar]
- Fong SW, Lin HC, Wu MF, Chen CC, & Huang YS (2016). CPEB3 Deficiency Elevates TRPV1 Expression in Dorsal Root Ganglia Neurons to Potentiate Thermosensation. PLoS One, 11(2), e0148491. doi: 10.1371/journal.pone.0148491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Osborne MG, Henry JL, Coderre TJ, & Dray A (2002). Antisense oligonucleotide knockdown of mGluR1 alleviates hyperalgesia and allodynia associated with chronic inflammation. Pharmacol Biochem Behav, 73(2), 401–410. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Yashpal K, Chabot JG, Osborne MG, Lefebvre CD, Dray A, … Coderre TJ (2001). Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol, 132(1), 354–367. doi: 10.1038/sj.bjp.0703810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia C, Frieda KL, Feoktistova K, Fraser CS, & Block SM (2015). RNA BIOCHEMISTRY. Factor-dependent processivity in human eIF4A DEAD-box helicase. Science, 348(6242), 1486–1488. doi: 10.1126/science.aaa5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway SM, Xu Q, & Inturrisi CE (2007). Design and evaluation of small interfering RNAs that target expression of the N-methyl-D-aspartate receptor NR1 subunit gene in the spinal cord dorsal horn. J Pharmacol Exp Ther, 322(3), 982–988. doi: 10.1124/jpet.107.123125 [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, & Nguyen PV (2007). ERK and mTOR signaling couple beta -adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent LTP. J Biol Chem. doi: 10.1074/jbc.M701077200 [DOI] [PubMed] [Google Scholar]
- Geranton SM, Jimenez-Diaz L, Torsney C, Tochiki KK, Stuart SA, Leith JL, … Hunt SP (2009). A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states. J Neurosci, 29(47), 15017–15027. doi:29/47/15017 [pii]10.1523/JNEUROSCI.3451–09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, & Sonenberg N (1999). eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem, 68, 913–963. doi: 10.1146/annurev.biochem.68.1.913 [DOI] [PubMed] [Google Scholar]
- Gold MS, Weinreich D, Kim CS, Wang R, Treanor J, Porreca F, & Lai J (2003). Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci, 23(1), 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm AC, & Wickens M (2008). Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol, 9(4), 337–344. doi: 10.1038/nrm2370 [DOI] [PubMed] [Google Scholar]
- Gorgoni B, & Gray NK (2004). The roles of cytoplasmic poly(A)-binding proteins in regulating gene expression: a developmental perspective. Brief Funct Genomic Proteomic, 3(2), 125–141. [DOI] [PubMed] [Google Scholar]
- Gray KM, Kaifer KA, Baillat D, Wen Y, Bonacci TR, Ebert AD, … Matera AG (2018). Self-oligomerization regulates stability of survival motor neuron protein isoforms by sequestering an SCF(Slmb) degron. Mol Biol Cell, 29(2), 96–110. doi: 10.1091/mbc.E17-11-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Bo J, Zhang W, Sun X, Zhang J, Yang Y, & Ma Z (2013). Intrathecal administration of cyclic AMP response element-binding protein-antisense oligonucleotide attenuates neuropathic pain after peripheral nerve injury and decreases the expression of N-methyl-D-aspartic receptors in mice. Oncol Rep, 30(1), 391–398. doi: 10.3892/or.2013.2437 [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, & Ren K (2006). Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci, 26(1), 126–137. doi: 10.1523/JNEUROSCI.3686-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang LH, Yang JP, Yin W, Wang LN, Guo F, Ji FH, … Zuo JL (2012). Activation of spinal TDAG8 and its downstream PKA signaling pathway contribute to bone cancer pain in rats. Eur J Neurosci, 36(1), 2107–2117. doi: 10.1111/j.1460-9568.2012.08087.x [DOI] [PubMed] [Google Scholar]
- Hanson G, & Coller J (2018). Codon optimality, bias and usage in translation and mRNA decay. Nat Rev Mol Cell Biol, 19(1), 20–30. doi: 10.1038/nrm.2017.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, … Heintz N (2008). A translational profiling approach for the molecular characterization of CNS cell types. Cell, 135(4), 738–748. doi: 10.1016/j.cell.2008.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen CU, & Sarnow P (2001). Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev, 15(13), 1593–1612. doi: 10.1101/gad.891101 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG, Ivanov IP, & Sonenberg N (2016). Translational control by 5’-untranslated regions of eukaryotic mRNAs. Science, 352(6292), 1413–1416. doi: 10.1126/science.aad9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgman R, Tay J, Mendez R, & Richter JD (2001). CPEB phosphorylation and cytoplasmic polyadenylation are catalyzed by the kinase IAK1/Eg2 in maturing mouse oocytes. Development, 128(14), 2815–2822. [DOI] [PubMed] [Google Scholar]
- Holcik M, & Sonenberg N (2005). Translational control in stress and apoptosis. Nat Rev Mol Cell Biol, 6(4), 318–327. doi:nrm1618 [pii] 10.1038/nrm1618 [DOI] [PubMed] [Google Scholar]
- Honore P, Kage K, Mikusa J, Watt AT, Johnston JF, Wyatt JR, … Lynch K (2002). Analgesic profile of intrathecal P2X(3) antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain, 99(1–2), 11–19. [DOI] [PubMed] [Google Scholar]
- Hu XM, Zhang H, Xu H, Zhang HL, Chen LP, Cui WQ, … Shen W (2017). Chemokine receptor CXCR4 regulates CaMKII/CREB pathway in spinal neurons that underlies cancer-induced bone pain. Sci Rep, 7(1), 4005. doi: 10.1038/s41598-017-04198-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, & Li Z (2017). miRNAs in synapse development and synaptic plasticity. Curr Opin Neurobiol, 45, 24–31. doi: 10.1016/j.conb.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T, Yi H, Liu S, Huang W, Kanda H, Lubarsky DA, & Hao S (2016). Spinal CPEB-mtROS-CBP signaling pathway contributes to perineural HIV gp120 with ddC-related neuropathic pain in rats. Exp Neurol, 281, 17–27. doi: 10.1016/j.expneurol.2016.04.012 [DOI] [PubMed] [Google Scholar]
- Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, & Hammock BD (2015). Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci U S A, 112(29), 9082–9087. doi: 10.1073/pnas.1510137112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Di Y, He X, Liu Q, Liu J, Li W, & Zhang L (2016). Intrathecal injection of phosphodiesterase 4B-specific siRNA attenuates neuropathic pain in rats with L5 spinal nerve ligation. Mol Med Rep, 13(2), 1914–1922. doi: 10.3892/mmr.2015.4713 [DOI] [PubMed] [Google Scholar]
- Jimenez-Diaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, … Hunt SP (2008). Local translation in primary afferent fibers regulates nociception. PLoS One, 3(4), e1961. doi: 10.1371/journal.pone.0001961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar AN, Lee SJ, & Twiss JL (2018). Expanding Axonal Transcriptome Brings New Functions for Axonally Synthesized Proteins in Health and Disease. Neuroscientist, 24(2), 111–129. doi: 10.1177/1073858417712668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasama S, Kawakubo M, Suzuki T, Nishizawa T, Ishida A, & Nakayama J (2007). RNA interference-mediated knock-down of transient receptor potential vanilloid 1 prevents forepaw inflammatory hyperalgesia in rat. Eur J Neurosci, 25(10), 2956–2963. doi: 10.1111/j.1460-9568.2007.05584.x [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, … Ji RR (2008). Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med, 14(3), 331–336. doi: 10.1038/nm1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoutorsky A, Bonin RP, Sorge RE, Gkogkas CG, Pawlowski SA, Jafarnejad SM, … Sonenberg N (2015). Translational control of nociception via 4E-binding protein 1. Elife, 4. doi: 10.7554/eLife.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoutorsky A, & Price TJ (2018). Translational Control Mechanisms in Persistent Pain. Trends Neurosci, 41(2), 100–114. doi: 10.1016/j.tins.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoutorsky A, Sorge RE, Prager-Khoutorsky M, Pawlowski SA, Longo G, Jafarnejad SM, … Sonenberg N (2016). eIF2alpha phosphorylation controls thermal nociception. Proc Natl Acad Sci U S A, 113(42), 11949–11954. doi: 10.1073/pnas.1614047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, & Kishioka S (2010). Macrophage inflammatory protein-1alpha mediates the development of neuropathic pain following peripheral nerve injury through interleukin-1beta up-regulation. Pain, 149(2), 305–315. doi: 10.1016/j.pain.2010.02.025 [DOI] [PubMed] [Google Scholar]
- Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, & Luo ZD (2009). Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acidic protein in maintenance of pain behaviors [corrected]. Pain, 143(1–2), 114–122. doi: 10.1016/j.pain.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee WI, Lee YS, Kim DH, Chang JW, Kim SW, & Lee H (2009). Effective relief of neuropathic pain by adeno-associated virus-mediated expression of a small hairpin RNA against GTP cyclohydrolase 1. Mol Pain, 5, 67. doi: 10.1186/1744-8069-5-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konicek BW, Stephens JR, McNulty AM, Robichaud N, Peery RB, Dumstorf CA, … Graff JR (2011). Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res, 71(5), 1849–1857. doi: 10.1158/0008-5472.CAN-10-3298 [DOI] [PubMed] [Google Scholar]
- Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, & Porreca F (2002). Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain, 95(1–2), 143–152. [DOI] [PubMed] [Google Scholar]
- Larsson M, & Broman J (2008). Translocation of GluR1-containing AMPA receptors to a spinal nociceptive synapse during acute noxious stimulation. J Neurosci, 28(28), 7084–7090. doi:28/28/7084 [pii] 10.1523/JNEUROSCI.5749–07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Li H, Liu ZL, Li Q, Qiu HW, Zeng LJ, … Li ZY (2017). Activation of STAT3-mediated CXCL12 up-regulation in the dorsal root ganglion contributes to oxaliplatin-induced chronic pain. Mol Pain, 13, 1744806917747425. doi: 10.1177/1744806917747425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Ha M, Chang H, Kwon SC, Simanshu DK, Patel DJ, & Kim VN (2014). Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell, 159(6), 1365–1376. doi: 10.1016/j.cell.2014.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Kim D, Lee YS, Ha M, Lee M, Yeo J, … Kim VN (2018). Mixed tailing by TENT4A and TENT4B shields mRNA from rapid deadenylation. Science, 361(6403), 701–704. doi: 10.1126/science.aam5794 [DOI] [PubMed] [Google Scholar]
- Lin CR, Amaya F, Barrett L, Wang H, Takada J, Samad TA, & Woolf CJ (2006). Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther, 319(3), 1096–1103. doi: 10.1124/jpet.106.105569 [DOI] [PubMed] [Google Scholar]
- Liu S, Zhu B, Sun Y, & Xie X (2015). MiR-155 modulates the progression of neuropathic pain through targeting SGK3. Int J Clin Exp Pathol, 8(11), 14374–14382. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gonzalez MJ, Landry M, & Favereaux A (2017). MicroRNA and chronic pain: From mechanisms to therapeutic potential. Pharmacol Ther, 180, 1–15. doi: 10.1016/j.pharmthera.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Lu Y, Jiang BC, Cao DL, Zhang ZJ, Zhang X, Ji RR, & Gao YJ (2014). TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-alpha and IL-1beta signaling. Pain, 155(12), 2618–2629. doi: 10.1016/j.pain.2014.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Fitzsimmons B, Mohan A, Zhang L, Terrando N, Kordasiewicz H, & Ji RR (2018). Intrathecal administration of antisense oligonucleotide against p38alpha but not p38beta MAP kinase isoform reduces neuropathic and postoperative pain and TLR4-induced pain in male mice. Brain Behav Immun, 72, 34–44. doi: 10.1016/j.bbi.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Hatzis C, & Eisenach JC (2003). Intrathecal injection of cAMP response element binding protein (CREB) antisense oligonucleotide attenuates tactile allodynia caused by partial sciatic nerve ligation. Brain Res, 988(1–2), 97–104. [DOI] [PubMed] [Google Scholar]
- Manners MT, Tian Y, Zhou Z, & Ajit SK (2015). MicroRNAs downregulated in neuropathic pain regulate MeCP2 and BDNF related to pain sensitivity. FEBS Open Bio, 5, 733–740. doi: 10.1016/j.fob.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamha CP, Xia Z, Yang J, Albrecht TR, Li M, Shyu AB, … Wagner EJ (2014). CFIm25 links alternative polyadenylation to glioblastoma tumour suppression. Nature, 510(7505), 412–416. doi: 10.1038/nature13261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, Mak TW, … Inoue K (2014). Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun, 5, 3771. doi: 10.1038/ncomms4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H, Li H, McGuire AM, Fletcher CM, Gingras AC, Sonenberg N, & Wagner G (1997). Structure of translation factor eIF4E bound to m7GDP and interaction with 4E-binding protein. Nat Struct Biol, 4(9), 717–724. [DOI] [PubMed] [Google Scholar]
- Mayr C, & Bartel DP (2009). Widespread shortening of 3’UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell, 138(4), 673–684. doi: 10.1016/j.cell.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni C, Mowry K, & Besse F (2012). Principles and roles of mRNA localization in animal development. Development, 139(18), 3263–3276. doi: 10.1242/dev.078626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megat S, Ray PR, Moy JK, Lou TF, Barragan-Iglesias P, Li Y, … Price TJ (2019). Nociceptor Translational Profiling Reveals the Ragulator-Rag GTPase Complex as a Critical Generator of Neuropathic Pain. J Neurosci, 39(3), 393–411. doi: 10.1523/jneurosci.2661-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, … Price TJ (2010). IL-6- and NGF-Induced Rapid Control of Protein Synthesis and Nociceptive Plasticity via Convergent Signaling to the eIF4F Complex. J Neurosci, 30(45), 15113–15123. doi:30/45/15113 [pii] 10.1523/JNEUROSCI.3947–10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Khoutorsky A, Sorge RE, Yan J, Asiedu MN, Valdez A, … Price TJ (2013). mTORC1 inhibition induces pain via IRS-1-dependent feedback activation of ERK. Pain. doi: 10.1016/j.pain.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichelli E, Wu J, Campbell ZT, Wickens M, & Williamson JR (2013). Biochemical characterization of the Caenorhabditis elegans FBF.CPB-1 translational regulation complex identifies conserved protein interaction hotspots. J Mol Biol, 425(4), 725–737. doi: 10.1016/j.jmb.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring F, Halder P, Seal RP, & Stucky CL (2018). Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron, 100(2), 349–360. doi: 10.1016/j.neuron.2018.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Fitzsimmons B, Zhao HT, Jiang Y, Mazur C, Swayze EE, & Kordasiewicz HB (2018). Antisense oligonucleotides selectively suppress target RNA in nociceptive neurons of the pain system and can ameliorate mechanical pain. Pain, 159(1), 139–149. doi: 10.1097/j.pain.0000000000001074 [DOI] [PubMed] [Google Scholar]
- Moy JK, Khoutorsky A, Asiedu MN, Black BJ, Kuhn JL, Barragan-Iglesias P, … Price TJ (2017). The MNK-eIF4E Signaling Axis Contributes to Injury-Induced Nociceptive Plasticity and the Development of Chronic Pain. J Neurosci, 37(31), 7481–7499. doi: 10.1523/jneurosci.0220-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndong C, Pradhan A, Puma C, Morello JP, Hoffert C, Groblewski T, … Laird JM (2009). Role of rat sensory neuron-specific receptor (rSNSR1) in inflammatory pain: contribution of TRPV1 to SNSR signaling in the pain pathway. Pain, 143(1–2), 130–137. doi: 10.1016/j.pain.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Orlova IA, Alexander GM, Qureshi RA, Sacan A, Graziano A, Barrett JE, … Ajit SK (2011). MicroRNA modulation in complex regional pain syndrome. J Transl Med, 9, 195. doi: 10.1186/1479-5876-9-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka Y, Kedersha NL, & Schoenberg DR (2009). Identification of a cytoplasmic complex that adds a cap onto 5’-monophosphate RNA. Mol Cell Biol, 29(8), 2155–2167. doi: 10.1128/MCB.01325-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R, Di H, Zhang J, Huang Z, Sun Y, Yu W, & Wu F (2015). Inducible Lentivirus-Mediated siRNA against TLR4 Reduces Nociception in a Rat Model of Bone Cancer Pain. Mediators Inflamm, 2015, 523896. doi: 10.1155/2015/523896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panja D, Dagyte G, Bidinosti M, Wibrand K, Kristiansen AM, Sonenberg N, & Bramham CR (2009). Novel translational control in Arc-dependent long term potentiation consolidation in vivo. J Biol Chem, 284(46), 31498–31511. doi: 10.1074/jbc.M109.056077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, & Ji RR (2014). Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron, 82(1), 47–54. doi: 10.1016/j.neuron.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh KW, Yeo JF, & Ong WY (2011). MicroRNA changes in the mouse prefrontal cortex after inflammatory pain. Eur J Pain, 15(8), 801 e801–812. doi: 10.1016/j.ejpain.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Porreca F, Lai J, Bian D, Wegert S, Ossipov MH, Eglen RM, … Hunter JC (1999). A comparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc Natl Acad Sci U S A, 96(14), 7640–7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presnyak V, Alhusaini N, Chen YH, Martin S, Morris N, Kline N, … Coller J (2015). Codon optimality is a major determinant of mRNA stability. Cell, 160(6), 1111–1124. doi: 10.1016/j.cell.2015.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, & Geranton SM (2009). Translating nociceptor sensitivity: the role of axonal protein synthesis in nociceptor physiology. Eur J Neurosci, 29(12), 2253–2263. doi:EJN6786 [pii] 10.1111/j.1460–9568.2009.06786.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, & Cervero F (2007). Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci, 27(51), 13958–13967. doi:27/51/13958 [pii] 10.1523/JNEUROSCI.4383–07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewlocka B, Sieja A, Starowicz K, Maj M, Bilecki W, & Przewlocki R (2002). Knockdown of spinal opioid receptors by antisense targeting beta-arrestin reduces morphine tolerance and allodynia in rat. Neurosci Lett, 325(2), 107–110. [DOI] [PubMed] [Google Scholar]
- Rigo F, Chun SJ, Norris DA, Hung G, Lee S, Matson J, … Bennett CF (2014). Pharmacology of a central nervous system delivered 2’-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J Pharmacol Exp Ther, 350(1), 46–55. doi: 10.1124/jpet.113.212407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud N, Hsu BE, Istomine R, Alvarez F, Blagih J, Ma EH, … Sonenberg N (2018). Translational control in the tumor microenvironment promotes lung metastasis: Phosphorylation of eIF4E in neutrophils. Proc Natl Acad Sci U S A, 115(10), E2202–E2209. doi: 10.1073/pnas.1717439115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna MD, Peroni D, Quattrone A, Ghelardini C, & Galeotti N (2015). Spinal RyR2 pathway regulated by the RNA-binding protein HuD induces pain hypersensitivity in antiretroviral neuropathy. Exp Neurol, 267, 53–63. doi: 10.1016/j.expneurol.2015.02.036 [DOI] [PubMed] [Google Scholar]
- Sanna MD, Quattrone A, & Galeotti N (2017). Silencing of the RNA-binding protein HuR attenuates hyperalgesia and motor disability in experimental autoimmune encephalomyelitis. Neuropharmacology, 123, 116–125. doi: 10.1016/j.neuropharm.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z, … Yuan W (2013). Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia, 61(4), 504–512. doi: 10.1002/glia.22451 [DOI] [PubMed] [Google Scholar]
- Sidrauski C, McGeachy AM, Ingolia NT, & Walter P (2015). The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. Elife, 4. doi: 10.7554/eLife.05033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RW, Blee TK, & Gray NK (2014). Poly(A)-binding proteins are required for diverse biological processes in metazoans. Biochem Soc Trans, 42(4), 1229–1237. doi: 10.1042/BST20140111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SD, Adams D, Kristen A, Grogan M, Gonzalez-Duarte A, Maurer MS, … Suhr O (2019). Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients With Hereditary Transthyretin-Mediated Amyloidosis. Circulation, 139(4), 431–443. doi: 10.1161/circulationaha.118.035831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, & Seeburg PH (1991). RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell, 67(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Uttam Sonali, W. C, Amorimb Inês S., Jafarnejad Seyed Mehdi, Tansley Shannon N., Yang Jieyi, Prager-Khoutorsky Masha, Mogil Jeffrey S., Gkogkas Christos G., Khoutorsky Arkady. (2018). Translational profiling of dorsal root ganglia and spinal cord in a mouse model of neuropathic pain. Neurobiology of Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Morgan MA, Merrick WC, & Shatkin AJ (1978). A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5’-terminal cap in mRNA. Proc Natl Acad Sci U S A, 75(10), 4843–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XS, Cao JL, Xu YB, He JH, Zhang LC, & Zeng YM (2005). Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol Sin, 26(7), 789–798. doi: 10.1111/j.1745-7254.2005.00123.x [DOI] [PubMed] [Google Scholar]
- Starck SR, Tsai JC, Chen K, Shodiya M, Wang L, Yahiro K, … Walter P (2016). Translation from the 5’ untranslated region shapes the integrated stress response. Science, 351(6272), aad3867. doi: 10.1126/science.aad3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Farris S, Pirbhoy PS, Darnell J, & Driesche SJ (2014). Localization and local translation of Arc/Arg3.1 mRNA at synapses: some observations and paradoxes. Front Mol Neurosci, 7, 101. doi: 10.3389/fnmol.2014.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, & Levy WB (1982). Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci, 2(3), 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, … Yan M (2013). CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J Neurosci Res, 91(4), 545–553. doi: 10.1002/jnr.23168 [DOI] [PubMed] [Google Scholar]
- Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM, & Yao SL (2006). Alleviation of neuropathic pain by intrathecal injection of antisense oligonucleotides to p65 subunit of NF-kappaB. Br J Anaesth, 97(4), 553–558. doi: 10.1093/bja/ael209 [DOI] [PubMed] [Google Scholar]
- Tan PH, Yang LC, Shih HC, Lan KC, & Cheng JT (2005). Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther, 12(1), 59–66. doi: 10.1038/sj.gt.3302376 [DOI] [PubMed] [Google Scholar]
- Tao YX (2012). AMPA receptor trafficking in inflammation-induced dorsal horn central sensitization. Neurosci Bull, 28(2), 111–120. doi: 10.1007/s12264-012-1204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima T, Ogawa N, Nakae Y, Sato T, Katagi M, Okano J, … Kojima H (2018). Gene Therapy for Neuropathic Pain through siRNA-IRF5 Gene Delivery with Homing Peptides to Microglia. Mol Ther Nucleic Acids, 11, 203–215. doi: 10.1016/j.omtn.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ (2010). Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci, 11(12), 823–836. doi: 10.1038/nrn2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD Jr. (2017). Nociception. Curr Biol, 27(4), R129–R133. doi: 10.1016/j.cub.2017.01.037 [DOI] [PubMed] [Google Scholar]
- Tramullas M, Frances R, de la Fuente R, Velategui S, Carcelen M, Garcia R, … Hurle MA (2018). MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci Transl Med, 10(453). doi: 10.1126/scitranslmed.aao6299 [DOI] [PubMed] [Google Scholar]
- Tsai JC, Miller-Vedam LE, Anand AA, Jaishankar P, Nguyen HC, Renslo AR, … Walter P (2018). Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Science, 359(6383). doi: 10.1126/science.aaq0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumati S, Milnes TL, Yamamura HI, Vanderah TW, Roeske WR, & Varga EV (2008). Intrathecal Raf-1-selective siRNA attenuates sustained morphine-mediated thermal hyperalgesia. Eur J Pharmacol, 601(1–3), 207–208. doi: 10.1016/j.ejphar.2008.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumati S, Roeske WR, Largent-Milnes T, Wang R, Vanderah TW, & Varga EV (2010). Sustained morphine-mediated pain sensitization and antinociceptive tolerance are blocked by intrathecal treatment with Raf-1-selective siRNA. Br J Pharmacol, 161(1), 51–64. doi: 10.1111/j.1476-5381.2010.00869.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Sasaki M, Elia AJ, Chio II, Hamada K, Fukunaga R, & Mak TW (2010). Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci U S A, 107(32), 13984–13990. doi: 10.1073/pnas.1008136107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Bhargava A, Kelley K, & Jasmin L (2008). Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J Neurosci, 28(16), 4161–4171. doi: 10.1523/JNEUROSCI.5053-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Heijnen CJ, van Velthoven CT, Willemen HL, Ishikawa Y, Zhang X, … Kavelaars A (2013). Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest, 123(12), 5023–5034. doi: 10.1172/jci66241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang XS, Tao R, Zhang J, Liu L, Jiang YH, … Xia LJ (2017). Upregulation of CX3CL1 mediated by NF-kappaB activation in dorsal root ganglion contributes to peripheral sensitization and chronic pain induced by oxaliplatin administration. Mol Pain, 13, 1744806917726256. doi: 10.1177/1744806917726256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LN, Yang JP, Ji FH, Zhan Y, Jin XH, Xu QN, … Zuo JL (2012). Brain-derived neurotrophic factor modulates N-methyl-D-aspartate receptor activation in a rat model of cancer-induced bone pain. J Neurosci Res, 90(6), 1249–1260. doi: 10.1002/jnr.22815 [DOI] [PubMed] [Google Scholar]
- Wang YY, Wu SX, Zhou L, Huang J, Wang W, Liu XY, & Li YQ (2006). Dose-related antiallodynic effects of cyclic AMP response element-binding protein-antisense oligonucleotide in the spared nerve injury model of neuropathic pain. Neuroscience, 139(3), 1083–1093. doi: 10.1016/j.neuroscience.2006.01.011 [DOI] [PubMed] [Google Scholar]
- White DM (2000). Neurotrophin-3 antisense oligonucleotide attenuates nerve injury-induced Abeta-fibre sprouting. Brain Res, 885(1), 79–86. [DOI] [PubMed] [Google Scholar]
- Wu FX, Bian JJ, Miao XR, Huang SD, Xu XW, Gong DJ, … Yu WF (2010). Intrathecal siRNA against Toll-like receptor 4 reduces nociception in a rat model of neuropathic pain. Int J Med Sci, 7(5), 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Campbell ZT, Menichelli E, Wickens M, & Williamson JR (2013). A protein.protein interaction platform involved in recruitment of GLD-3 to the FBF.fem-3 mRNA complex. J Mol Biol, 425(4), 738–754. doi: 10.1016/j.jmb.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, Ye L, Mao JX, & Zhang JM (2013). Knockdown of sodium channel NaV1.6 blocks mechanical pain and abnormal bursting activity of afferent neurons in inflamed sensory ganglia. Pain, 154(8), 1170–1180. doi: 10.1016/j.pain.2013.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, & Zhang JM (2015). Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience, 291, 317–330. doi: 10.1016/j.neuroscience.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, & Zhang JM (2017). Active Nerve Regeneration with Failed Target Reinnervation Drives Persistent Neuropathic Pain. eNeuro, 4(1). doi: 10.1523/ENEURO.0008-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JT, Zhao JY, Zhao X, Ligons D, Tiwari V, Atianjoh FE, … Tao YX (2014). Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest, 124(2), 592–603. doi: 10.1172/JCI70236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Wu MZ, Deng XT, Ma PC, Li ZH, Liang L, … Song XJ (2016). Inhibition of YAP/TAZ Activity in Spinal Cord Suppresses Neuropathic Pain. J Neurosci, 36(39), 10128–10140. doi: 10.1523/JNEUROSCI.0800-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Zhang XL, Ou-Yang HD, Li ZY, Liu CC, Huang ZZ, … Xin WJ (2017). Epigenetic upregulation of CXCL12 expression mediates antitubulin chemotherapeutics-induced neuropathic pain. Pain, 158(4), 637–648. doi: 10.1097/j.pain.0000000000000805 [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu J, He M, Liu R, Belegu V, Dai P, … Wang TH (2016). Mechanisms of PDGF siRNA-mediated inhibition of bone cancer pain in the spinal cord. Sci Rep, 6, 27512. doi: 10.1038/srep27512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Poggio M, Jin HY, Shi Z, Forester CM, Wang Y, … Ruggero D (2019). Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat Med. doi: 10.1038/s41591-018-0321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang G, Zou X, Yang Z, Wang Q, Feng H, & Zhang M (2016). siRNA-mediated downregulation of GluN2B in the rostral anterior cingulate cortex attenuates mechanical allodynia and thermal hyperalgesia in a rat model of pain associated with bone cancer. Exp Ther Med, 11(1), 221–229. doi: 10.3892/etm.2015.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagiya A, Suyama E, Adachi H, Svitkin YV, Aza-Blanc P, Imataka H, … Sonenberg N (2012). Translational homeostasis via the mRNA cap-binding protein, eIF4E. Mol Cell, 46(6), 847–858. doi: 10.1016/j.molcel.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Sun W, Luo WJ, Yang Y, Yang F, Wang XL, & Chen J (2017). SDF1-CXCR4 Signaling Contributes to the Transition from Acute to Chronic Pain State. Mol Neurobiol, 54(4), 2763–2775. doi: 10.1007/s12035-016-9875-5 [DOI] [PubMed] [Google Scholar]
- Yao R, Ma Y, Du Y, Liao M, Li H, Liang W, … Liao Y (2011). The altered expression of inflammation-related microRNAs with microRNA-155 expression correlates with Th17 differentiation in patients with acute coronary syndrome. Cell Mol Immunol, 8(6), 486–495. doi: 10.1038/cmi.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Novakovic SD, Tzoumaka E, Erickson VL, Erickson KA, … de Groat WC (2001). The involvement of the tetrodotoxin-resistant sodium channel Na(v)1.8 (PN3/SNS) in a rat model of visceral pain. J Neurosci, 21(21), 8690–8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh N, Alipour MR, & Soufi FG (2015). Deregulation of NF-small ka, CyrillicB-miR-146a negative feedback loop may be involved in the pathogenesis of diabetic neuropathy. J Physiol Biochem, 71(1), 51–58. doi: 10.1007/s13105-014-0378-4 [DOI] [PubMed] [Google Scholar]
- Yu DS, Lv G, Mei XF, Cao Y, Wang YF, Wang YS, & Bi YL (2014). MiR-200c regulates ROS-induced apoptosis in murine BV-2 cells by targeting FAP-1. Spinal Cord. doi: 10.1038/sc.2014.185 [DOI] [PubMed] [Google Scholar]
- Yu HM, Wang Q, & Sun WB (2017). Silencing of FKBP51 alleviates the mechanical pain threshold, inhibits DRG inflammatory factors and pain mediators through the NF-kappaB signaling pathway. Gene, 627, 169–175. doi: 10.1016/j.gene.2017.06.029 [DOI] [PubMed] [Google Scholar]
- Yu YQ, Zhao F, Guan SM, & Chen J (2011). Antisense-mediated knockdown of Na(V)1.8, but not Na(V)1.9, generates inhibitory effects on complete Freund’s adjuvant-induced inflammatory pain in rat. PLoS One, 6(5), e19865. doi: 10.1371/journal.pone.0019865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukhananov R, Guan J, & Crosby G (2002). Antisense oligonucleotides to N-methyl-D-aspartate receptor subunits attenuate formalin-induced nociception in the rat. Brain Res, 930(1–2), 163–169. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Laumet G, Chen SR, Hittelman WN, & Pan HL (2015). Pannexin-1 Up-regulation in the Dorsal Root Ganglion Contributes to Neuropathic Pain Development. J Biol Chem, 290(23), 14647–14655. doi: 10.1074/jbc.M115.650218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Yu Q, Li H, Guo X, & He X (2014). Characterization of microRNA expression profiles in patients with intervertebral disc degeneration. Int J Mol Med, 33(1), 43–50. doi: 10.3892/ijmm.2013.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Lee MC, Momin A, Cendan CM, Shepherd ST, Baker MD, … Wood JN (2010). Small RNAs control sodium channel expression, nociceptor excitability, and pain thresholds. J Neurosci, 30(32), 10860–10871. doi: 10.1523/jneurosci.1980-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Kunder N, De la Paz JA, Lasley AE, Bhat VD, Morcos F, & Campbell ZT (2018). Global pairwise RNA interaction landscapes reveal core features of protein recognition. Nat Commun, 9(1), 2511. doi: 10.1038/s41467-018-04729-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


