Systemic large arteries normally exert a powerful cushioning function, allowing for nearly steady flow and pressure in the microvasculature, despite the intermittent left ventricular ejection. Large artery stiffening (LASt) leads to loss of this cushioning function, with multiple deleterious consequences, including excessive transmission of pulsatility to the microvasculature of peripheral organs, excessive pulsatile afterload for the left ventricle, and a rapid diastolic pressure decay that can contribute to impaired coronary perfusion and flow reserve.1,2
In the presence of LASt, the transmission of pulsatility to the microvasculature is likely to be particularly prominent in low-resistance vascular beds. Peripheral vascular resistance, largely imposed by small arteries, governs the amount of mean capillary flow for any given mean arteriovenous pressure gradient (which is turn, depends mostly on mean arterial pressure). Given that mean pressure varies minimally throughout the arterial system, differences in vascular resistance determine the vastly different flow rates seen in various vascular beds. Since resistance is highly correlated with arteriolar tone, it is thought that in low-resistance, high-flow vascular beds, a low pre-capillary arteriolar tone allows for greater transmission of proximal pulsatility into the capillaries, resulting in increased pulsatile pressure (barotrauma) and pulsatile flow (with excessive pulsatile shear forces). In contrast, the high arteriolar tone in high-resistance vascular beds uncouples the proximal pulsations from the distal capillaries, which are more effectively protected from excessive pulsatility.
Interestingly, although LASt has often been regarded as a marker of early vascular disease imposed by various risk factors (such as hypertension, diabetes, obesity, etc), arterial stiffening actually precedes isolated systolic hypertension.1 Similarly, it has long been known that individuals with established diabetes mellitus (DM) exhibit higher LASt compared to their non-diabetic counterparts,3 even in the setting of established chronic kidney disease4,5 or heart failure with preserved ejection fraction.6 However, recent data also suggests that increased LASt precedes new onset DM and correlates with its future onset. Among 2,450 older adults without diabetes at baseline enrolled in a large cohort study in southern Sweden,7 carotid-femoral PWV was reported to predict the future development of DM over ~4.4 years, with an approximate doubling and tripling of risk in the mid and highest PWV tertiles, respectively, compared to the lowest tertile. This association was independent of potential confounders. This study was consistent with a previous large study that demonstrated that pulse pressure independently predicted new-onset DM among 2,685 hypertensive patients enrolled in the Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) trial.8
In this issue of the journal, Zheng et al9 report on a large study including 14,159 adult participants who were free of DM, cardiovascular, cerebrovascular and chronic kidney disease, and underwent brachial-ankle pulse wave velocity (baPWV) and fasting blood glucose (FBG) measurements at baseline. Among 8,956 participants, repeated assessments of both baPWV and FBG were available. The authors demonstrate that, in analyses that adjusted for a comprehensive list of potential confounders, the risk of new-onset DM over a mean follow-up of 3.72 years more than doubled (Hazard Ratio=2.28) in people with frankly elevated ba-PWV (≥18 m/s) compared with those with low (“ideal”) values (<14 m/s). Moreover, the authors used cross-lagged regression, which estimates directional relationships from one variable to another and vice versa (hence the term “crossed”) using longitudinal data recorded at multiple time points (hence the term “lagged”). In these analyses, baseline baPWV was associated with follow-up FBG, but there was no significant relationship between baseline FBG and follow-up baPWV.
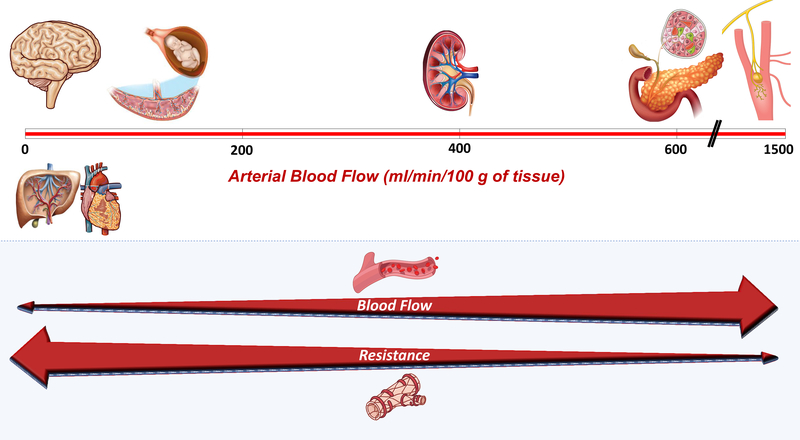
The association between arterial stiffness and future new-onset DM could be the result of residual confounding or may have a cause-effect relationship. Residual confounding by factors that may contribute to both LAS and DM may have persisted despite the careful effort by the investigators to adjust for risk factors in statistical models. However, a potential causal relationship must also be carefully considered. What are the possible mechanisms by which arterial stiffening could contribute to DM? As stated previously, LASt has ill-effects in various organs, particularly those that require torrential blood flow and must operate at very low vascular resistance, such as the kidney. Interestingly, not only is average pancreatic flow amongst the highest in the body, but the endocrine pancreas, which comprises only ~1–2% of the pancreatic mass, receives ~10–20% of its blood supply. Although measurements of bulk pancreatic blood flow in humans vary in the literature,10 available studies imply that islet blood flow is actually higher than renal blood flow and second only to the carotid body when normalized for tissue mass (Figure 1A). Therefore, it is likely that LASt impacts microvascular health in pancreatic islets, which may lead to dysfunctional or dysregulated endocrine function.
Figure.
A. Arterial blood flow relative to tissue mass in low-resistance, high-flow organs. Organs are placed along a vertical axis in which higher flow rates per 100 g of tissue increase from left to right red bar). The lower panel demonstrates the inverse relationship between local flow and local resistance, which is largely a microvascular property. We note that although there is a wide range of resistance and blood flow as shown, organs in the figure are all considered to exhibit “low resistance-high flow” hemodynamics, compared to most other organs (such as the gut, the skin, subcutaneous tissue, or skeletal muscle at rest). Of note, measurements of bulk pancreatic blood flow in humans (used to estimate islet flow rates) vary in the literature. It should also be noted that liver blood flow is much higher to what is depicted in the figure, which only considers arterial hepatic flow.
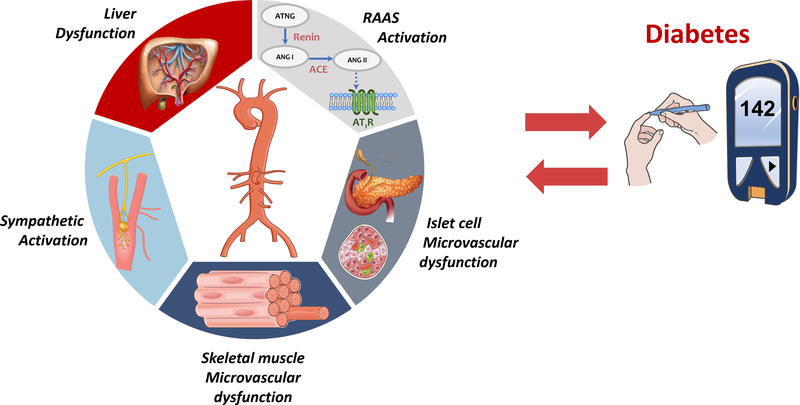
B. Mechanisms that underlie the relationship between large artery stiffness and DM. RAAS=renin-angiotensin-aldosterone system.
Pancreatic islets are supplied by 1–5 arterioles per islet, which branch into a dense glomerular capillary network. Endocrine cells reside within this network and receive chemical signals across the capillary endothelium. The islets of Langerhans are made up of 5 major endocrine cell types: β-cells (which secrete insulin), α-cells (which secrete glucagon), δ-cells (which secrete somatostatin), PP-cells (which produce pancreatic polypeptide) an ε-cells (which produce ghrelin). Although type 2 DM is primarily associated with insulin resistance, insufficient insulin secretion and an imbalance in insulin vs. glucagon secretion play a key role in its pathogenesis. Islet blood flow is a highly regulated process under metabolic, autonomic and paracrine dynamic control. Interestingly, in the endocrine pancreas, regional blood flow appears to be not only regulated by pre-capillary arterioles, but also by pericapillary contractile pericytes (which extensively cover the capillaries of the endocrine pancreas). In pancreatic islets, hyperglycemia and the associated beta cell activation result in arteriolar dilation, capillary dilation and increased islet capillary pressure.11 Moreover, vascular integrity may be essential to maintain an order of sequential perfusion of β, δ and α cells, which appears to be allow for adequate inhibition of glucagon secretion by insulin.12 Given the key role of the pancreatic microvasculature, pancreatic islet microvascular dysfunction is thought to contribute to the pathogenesis of type 2 DM, which could represent a mechanistic causal link between LASt and the risk of DM. Further research is required for a better understanding of the regulation of islet microvascular hemodynamics, its relationship with endocrine function, its relationship with proximal arterial hemodynamics, and its role in the pathogenesis of DM.
In addition to intrinsic pancreatic microvascular processes, various other potential mechanisms could be involved in the relationship between LAS and new-onset DM (Figure 1B). Increased sympathetic tone can lead to renin-angiotensin-aldosterone system (RAAS) activation, which influences insulin sensitivity via multiple mechanisms in skeletal muscle, adipocytes, and pancreas. Moreover, the liver (which receives ~25% of the cardiac output) also exhibits low-resistance arterial hemodynamics since the hepatic artery provides approximately one-third of the liver blood flow.2 The microvasculature downstream of the hepatic artery constantly regulates arterial flow to maintain total liver blood flow constant, regardless of changes in portal vein flow. This process, called hepatic arterial buffer response, appears to be important for metabolic homeostasis. Interestingly, there also appears to be a relationship between LAS and non-alcoholic fatty liver disease (NAFLD), a highly prevalent condition strongly associated with the metabolic syndrome and DM.2 In skeletal muscle, microvascular dysfunction and remodeling may contribute to insulin resistance.13 However, it is unknown whether LASt causes skeletal muscle microvascular damage. Bulk skeletal muscle microvascular flow is low due to high arteriolar resistance at rest, although even modest exercise can substantially reduce resistance in order to increase local flow rates. Nevertheless, these hyperemic transients are relatively brief and seem unlikely to lead to LASt-induced microvascular alterations. However, as described above, LASt may contribute to alterations in insulin/glucagon secretion from pancreatic islet microvascular damage, or liver metabolic dysfunction, which could in turn induce secondary abnormalities in metabolism and insulin signaling in skeletal muscle. The potential consequences of LASt on the microvasculature of specific organs involved in glucose metabolism require further study.
It is important to note some limitations of the study by Zhang et al. The metric of LAS utilized by the authors was baPWV, which incorporates muscular arterial segments.14 The authors only included participants who underwent at least one baPWV measurement and at least one follow-up survey. Out of 26,360 potentially eligible participants, 5,528 were excluded due to lack of follow-up examinations and out of 14,159 included in the study, only 8,956 participants had repeated baPWV and FBG data (thus being included in the cross-lagged regression analyses). It is possible that the baseline baPWV value (which was presumably known to study participants) might have influenced with the decision to return for follow-up testing (due to, for instance, concerns regarding lifestyle or other unmeasured factors); patients who were newly diagnosed with DM in the interim might have also been more likely to return for follow-up vascular testing, resulting in collider bias and/or complex confounding. Moreover, the lack of a relationship between FBG and subsequent baPWV should not be regarded as indicative of lack of an effect of DM on LASt, since the impact of hyperglycemia on LASt likely exhibits a threshold effect and participants were, by design, not diabetic at baseline.
Clearly, much remains to be learned regarding the mechanisms that link LASt with the risk of DM. Zheng et al. should be congratulated for their important contribution, which should stimulate additional translational and clinical studies to elucidate the causality of the relationship, potential mechanisms involved, and the degree to which hemodynamic vs. non-hemodynamic factors are involved in this association. Given the central and increasingly appreciated role of LASt human health, developing strategies to prevent or reduce it should remain a top priority in cardiovascular disease prevention.
Acknowledgments
Disclosures: J.A.C. is supported by NIH grants R01-HL 121510, R33-HL-146390, R01HL153646, R01-AG058969, 1R01-HL104106, P01-HL094307, R03-HL146874, and R56-HL136730. He has recently consulted for Bayer, Sanifit, Fukuda-Denshi, Bristol-Myers Squibb, JNJ, Edwards Life Sciences, Merck and the Galway-Mayo Institute of Technology. He received University of Pennsylvania research grants from the NIH, Fukuda-Denshi, Bristol-Myers Squibb and Microsoft. He is named as inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites in Heart Failure with Preserved Ejection Fraction. He has received research device loans from Atcor Medical, Fukuda-Denshi, Uscom, NDD Medical Technologies, Microsoft and MicroVision Medical.
References
- 1.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. Jama. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: Jacc state-of-the-art review. J Am Coll Cardiol. 2019;74:1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015;238:370–379. [DOI] [PubMed] [Google Scholar]
- 4.Townsend RR, Wimmer NJ, Chirinos JA, et al. Aortic pwv in chronic kidney disease: A cric ancillary study. Am.J.Hypertens. 2010;23:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in ckd and esrd. Kidney Int. 2012;82:388–400. [DOI] [PubMed] [Google Scholar]
- 6.Chirinos JA, Bhattacharya P, Kumar A, Proto E, Konda P, Segers P, Akers SR, Townsend RR, Zamani P. Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2019;8:e011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhammad IF, Borne Y, Ostling G, Kennback C, Gottsater M, Persson M, Nilsson PM, Engstrom G. Arterial stiffness and incidence of diabetes: A population-based cohort study. Diabetes Care. 2017;40:1739–1745. [DOI] [PubMed] [Google Scholar]
- 8.Yasuno S, Ueshima K, Oba K, Fujimoto A, Hirata M, Ogihara T, Saruta T, Nakao K. Is pulse pressure a predictor of new-onset diabetes in high-risk hypertensive patients?: A subanalysis of the candesartan antihypertensive survival evaluation in japan (case-j) trial. Diabetes Care. 2010;33:1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng M, Zhang X, Chen S, Song Y, Zhao Q, Gao X, Wu S.. Arterial stiffness preceding diabetes: A longitudinal study. Circ Res. 2020; 127: xx–xxx. [DOI] [PubMed] [Google Scholar]
- 10.Tsushima Y, Miyazaki M, Taketomi-Takahashi A, Endo K. Feasibility of measuring human pancreatic perfusion in vivo using imaging techniques. Pancreas. 2011;40:747–752. [DOI] [PubMed] [Google Scholar]
- 11.Almaca J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab. 2018;27:630–644 e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagner JI, Samols E. The vascular order of islet cellular perfusion in the human pancreas. Diabetes. 1992;41:93–97. [DOI] [PubMed] [Google Scholar]
- 13.Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic microvascular disease: An endocrine society scientific statement. J Clin Endocrinol Metab. 2017;102:4343–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segers P, Rietzschel ER, Chirinos JA. How to measure arterial stiffness in humans. Arterioscler Thromb Vasc Biol. 2020;40:1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]