Abstract
Cancer-related diseases represent the second overall cause of death worldwide. Human papilloma virus (HPV) is an infectious agent which is mainly sexually transmitted and may lead to HPV-associated cancers in both men and women. Almost all cervical cancers are HPV-associated, however, an increasing number of head and neck cancers (HNCs), especially oropharyngeal cancer, can be linked to HPV infection. Moreover, anogenital cancers, including vaginal, vulvar, penial, and anal cancers, represent a subset of HPV-related cancers. Whereas testing and prevention of cervical cancer have significantly improved over past decades, anogenital cancers remain more difficult to confirm. Current clinical trials including patients with HPV-related cancers focus on finding proper testing for all HPV-associated cancers as well as improve the currently applied treatments. The HPV viral oncoproteins, E6 and E7, lead to degradation of, respectively, p53 and pRb resulting in entering the S phase without G1 arrest. These high-risk HPV viral oncogenes alter numerous cellular processes, including DNA repair, angiogenesis, and/or apoptosis, which eventually result in carcinogenesis. Additionally, a comprehensive analysis of gene expression and alteration among a panel of DNA double strand breaks (DSB) repair genes in HPV-negative and HPV-positive HNC cancers reveals differences pointing to HPV-dependent modifications of DNA repair processes in these cancers. In this review, we discuss the current knowledge regarding HPV-related cancers, current screening, and treatment options as well as DNA damage response-related biological aspects of the HPV infection and clinical trials.
Keywords: Human papillomavirus (HPV), cervical cancer, HNC, DNA repair, clinical trials, cancer statistics
Cancer statistics worldwide and in the United States
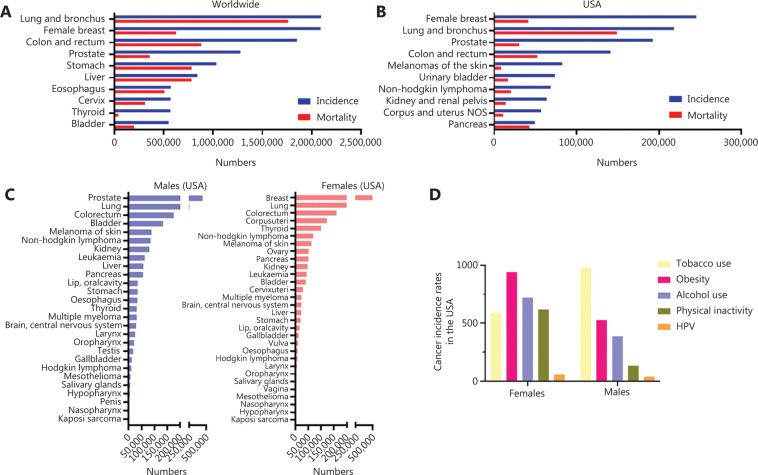
Cancer-related diseases represent the second leading cause of death worldwide and in the United States (US) behind cardiovascular diseases and stroke, and led to around 9.6 million deaths globally in 20181. As of 2016, the latest year for which incidence data are available, 1,658,716 new cases of cancer were reported, and 598,031 people died of cancer in the US. According to the American Cancer Society (ACS), more than 1.8 million new cases of cancer are estimated for 20203. Worldwide, lung, breast, and colorectum cancer show the highest numbers of new cases; on the other hand, the highest mortality is caused by lung, colorectum, stomach, and liver cancer1 (Figure 1A). In the US, the cancer type-related incidence rate resembles the global pattern with most new cases for breast and lung cancers and the highest mortality in patients suffering from lung, colorectum, and pancreas cancers2 (Figure 1B). Importantly, the high incidence of lung and colorectal cancers was very high in both men and women2 (Figure 1C). Fortunately, the overall cancer-associated death rate in the US has dropped by 29% since 1991 saving more than 2.9 million lives, as of 20173. This remarkable result is due to prevention and education, early detection, and the enormous improvement in cancer treatment. Although awareness across the population increased, the behavioral factors like alcohol use, unhealthy diet, and/or lack of physical activity, or tobacco use are still the most relevant cancer-associated risk factors4. Of note, tobacco use causes the majority of diverse cancer types with approximately 22% of all cancer deaths worldwide1. Interestingly, in the US the distribution of cancer-related risk factors is gender-dependent2 (Figure 1D). In men, the most cancer deaths were related to tobacco use, whereas in women most were related to obesity followed by alcohol use and poor physical activity2 (Figure 1D). Besides these lifestyle and diet-related risk factors, infection agents like the human papilloma virus (HPV) may lead to HPV-associated cancers in both men in women. In this review, we strive to give an overview of the available statistics, current testing and treatment options, clinical trials, and we also discuss the biological aspects of the HPV infection.
Figure 1.
Cancer incidence and mortality numbers according to the WHO and the Centers for Disease Control (CDC). (A) Incidence (blue) and mortality (red) numbers for 10 most prominent cancer types worldwide. (B) Incidence (blue) and mortality (red) numbers for 10 most prominent cancer types in the US. (C) Number of cancer cases in the US in men (left) and women (right) according to the CDC. (D) Rates for cancer associated with the most common five risk factors in women and men. Tobacco use-associated cancers include: oral cavity and pharynx, esophagus; stomach; colon and rectum; liver; pancreas; larynx; lung, bronchus, and trachea; cervix; kidney and renal pelvis; urinary bladder; and acute myeloid leukemia. Obesity-associated cancers include adenocarcinoma of the esophagus; cancers of the breast (in postmenopausal women), colon and rectum, endometrium (corpus uterus), gallbladder, gastric cardia, kidney (renal cell), liver, ovary, pancreas, and thyroid; meningioma, and multiple myeloma. Alcohol-associated cancers include: oral cavity and pharynx, esophagus, colon and rectum, liver, larynx, and female breast cancer. Physical inactivity-associated cancers include breast cancer in postmenopausal women, endometrium (corpus uterus) cancer, and colon cancer. HPV-associated cancers include microscopically confirmed carcinoma of the cervix and squamous cell carcinomas of the vagina, vulva, penis, anus, rectum, and oropharynx.
HPV-associated cancers and current statistics
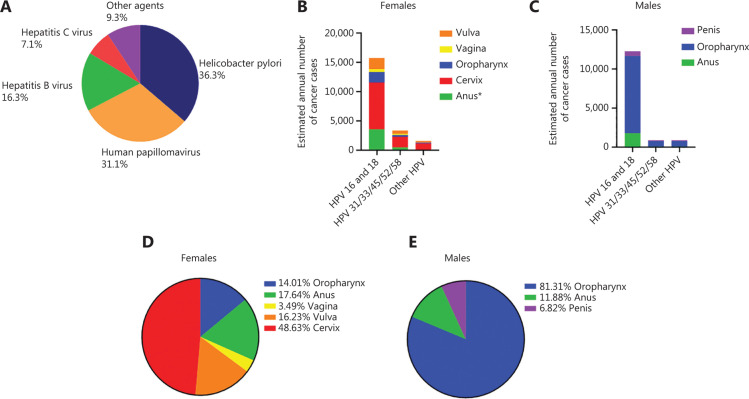
Despite the diversity of cancer-related risk factors, infections with bacteria and viruses are known risk factors for cancer. Worldwide, the most prominent infectious agents causing cancer are Helicobacter pylori (36.3%) and HPV (31.1%), followed by hepatitis B (16.3%) and C (7.1%)4 (Figure 2A). Despite lower numbers of cancer related to infection with other agents, including Epstein–Barr virus (EBV), human herpesvirus type 8 (HHV-8; also known as Kaposi sarcoma-associated herpesvirus), and human T-cell lymphotropic virus type 1 (HTLV-1) as well as three parasites: Opisthorchis viverrini, Clonorchis sinensis, and Schistosoma haematobium, are a cause of nearly 10% of all infection-mediated cancers (Figure 2A).
Figure 2.
HPV-associated cancer types in men and women according to the WHO and the CDC. (A) Distribution of infection-caused cancers according to infectious agents worldwide in men and women. (B) Numbers of cancers caused by distinct HPV subtypes in women. (C) Numbers of cancers caused by distinct HPV subtypes in men. (D) Percentage of different HPV-associated cancers in women. (E) Percentage of different HPV-associated cancers in men.
HPVs belong to the family Papillomaviridae. The virons are non-enveloped and contain a double-stranded DNA genome. The genetic material is enclosed by an icosahedral capsid composed of major and minor structural proteins, L1 and L2, respectively. These viruses are highly tissue-specific and infect both cutaneous and mucosal epithelium. Based on the genomic sequence of L1, the gene encoding the principal capsid protein, over 200 HPV types have been identified and characterized5, with at least 14 high risk types which may cause cancer6. Two HPV types (16 and 18) cause the most HPV-related cancers and about 70% of cervical cancers and pre-cancerous cervical lesions6 (Figure 2B, C). Importantly, HPV is mainly transmitted through sexual activity but people can also be infected by skin-to skin contact6. Notably, about 80% of people – both men and women – will get a HPV infection at some point in their lives. All sexually active persons can be infected with low- or high-risk HPV and usually become infected with HPV shortly after the onset of sexual activity. However, most often the immune system clears the virus, but for some, the infection persists and can lead to precancerous changes. In more detail, cells infected with high-risk HPV increase their proliferation rate and, over time, if not controlled by the immune system, they may form precancerous changes or a tumor. Factors like long-term usage of oral contraceptives, many pregnancies and smoking, a compromised immune system and coinfection with another sexually transmitted disease as well as the HPV type (high risk) may increase the risk of precancerous cervical cells leading to cancer2. Of note, nearly all cervical cancer cases are related to HPV infection. Nevertheless, HPV infection can be linked to other types of cancer, including anogenital cancer (vulvar, vaginal, anal, and penial), head and neck cancers (HNCs) or genital warts in both men and women. The estimated median annual incidence of new anogenital warts is 137 per 100,000 men and 121 per 100,000 women and the prevalence ranges from 0.15% to 0.18% in men and women, respectively7,8. In general, HPV is thought to be responsible for more than 90% of anal and cervical cancers, about 70% of vaginal and vulvar cancers, and more than 60% of penile cancers. In contrast, oropharyngeal cancers traditionally have been caused by tobacco and alcohol, but recent studies show that about 70% of cancers of the oropharynx may be linked to HPV. Many cancers of the oropharynx may be caused by a combination of tobacco, alcohol, and HPV infection2. Worldwide, high-risk HPVs cause about 5% of all cancer cases, with an estimated number of infections of 570,000 in women and 60,000 in men each year. As of 2018, approximately 311,000 women died from cervical cancer worldwide and more than 85% of these deaths occurred in low- and middle-income countries where cervical cancer represents the leading cancer-related death in women1. The reason being that in these parts of world there is very limited access to screening tests, which would highly increase chances of early detection and allow more timely application of treatment1. In the US, high-risk HPVs cause 3% of all cancers in women and 2% of all cancers in men, and about 44,000 new cases of HPV infection-associated cancers are found annually, and about 34,800 of these cancers are terminal2. Importantly, nearly 50% of all HPV-associated cancers in women are cervical cancers, whereas in men oropharyngeal cancer was mostly related to HPV infection9 (Figure 2D, E). In males, the highest prevalence of becoming infected with a HPV was observed in HIV-infected men who have sex with other men10,11. Across heterosexual men, the highest risk of infection was found in African men having at least three female sexual partners12. In addition, the average prevalence of infection with any type of HPV was significantly higher in HIV-positive vs HIV-negative men, 78.2% vs 49.4%, respectively13.
Current screening and treatment strategies for HPV-associated cancers
Prevention and screenings
Comprehensive cervical cancer control includes primary prevention (vaccination against HPV), secondary prevention (screening and treatment of pre-cancerous lesions), tertiary prevention (diagnosis and treatment of invasive cervical cancer), and palliative care. Vaccines that protect against HPV 16 and 18 are recommended by the World Health Organization (WHO) and have been approved for use in many countries. All males and females ages 9–26 years should get the HPV vaccine. It is most effective when given at ages 11–12 years. The vaccine is given in two doses for males and females ages 9–14 years. Beginning at age 15 through age 45, three doses are required for full immunity. The HPV vaccine prevents HPV-related genital warts, most cervical, anal, vaginal, and vulvar cancers and reduces the risk of most HPV-related cancers of the throat and the penis. These vaccines consist of recombinant viral vaccine proteins containing purified virus-like particles for each virus genotype, thus they protect against different HPV types. Importantly, they are free of viral DNA so they are inactive and cannot infect cells. Three vaccines, Gardasil 4v (Merck&Co, Kenilworth, NJ, US), Gardasil 9v (Merck&Co, Kenilworth, NJ, US), and Cervarix 2v (GlaxoSmithKline Biologicals, Rixensart, Belgium), are now being marketed in many countries throughout the world – a quadrivalent, a nonavalent, and a bivalent vaccine, respectively. Both Gardasil vaccines are produced in recombinant Saccharomyces cerevisiae, whereas for Cervarix’s production the recombinant baculovirus expression system is applied14. The nonavalent provides additional protection against HPV types 31, 33, 45, 52, and 58. Data from clinical trials and initial post-marketing surveillance conducted in several continents show all three vaccines to be safe. However, rare cases of anaphylaxis (1.7 cases in 1 million doses), a life threatening allergic reaction, have been reported14,15. HPV-related post-vaccination syndromes with focus on the adjuvants, which increase the immune response to a certain antigen, have been investigated. The autoimmune/inflammatory syndrome Induced by adjuvants (AIAS) have been reported in 3.6 cases per 100,000 vaccine doses16,17. Moreover, it remains controversial if the rare death cases after HPV vaccine could be linked to the adjuvant or the vaccine itself16,17. Despite the rare cases of post-vaccination syndromes, HPV vaccines are reported as being very safe and are the most effective preventive option. However, HPV vaccine is not recommended in pregnant women because of a lack of scientific proof from clinical studies. Nevertheless, previous clinical trials including in pregnant women showed no increase in spontaneous abortions if compared to non-pregnant women18.
Most importantly, HPV vaccine is not protective against all types of HPV and therefore women still need to be screened despite previous vaccinations. It is recommended for women between the ages of 21 and 29 years to be Papanicolaou (Pap) tested every 3 years. For women between the ages of 30 and 65 years, additional HPV testing or HPV/Pap testing every 5 years is recommended. Women older than 65 years who were frequently tested and diagnosed negative may be advised to no longer be screened for cervical cancer. However, continuing screening is recommended if recent tests were abnormal. Whereas Pap tests screen for precancerous and cancerous processes in the cervix, HPV tests check for the occurrence of the HPV in the tissue. It is important to consider that a positive test after years of negative testing is not necessarily caused by a new infection but can be a result of virus reactivation years later. Currently, it is not possible to distinguish between a new infection and viral reactivation, nor can we compare the risk of both to cause cancer. A different approach of testing for cervical cancer is currently under investigation within a clinical trial which proposes using self-collected menstrual blood for HPV DNA and HPV E6/E7 mRNA analysis19 (NCT03638427).
Currently, screening can only be performed in cervical tissue as the U.S. Food and Drug Administration (FDA)-approved tests for other types of HPV-related cancers are not available yet. Nevertheless, an ongoing study is testing anal Pap smears and conducting high resolution anoscopy (HRA) to find a proper testing method for anal cancer19 (NCT03061435). Additionally, a current clinical study measures the amount of HPV DNA in blood samples from patients who have recently been diagnosed with cervical cancer, trying to correlate the DNA amount of HPV with the stage of disease or the applied treatment19 (NCT03749720). This approach may help to uncover a potential recurrence earlier than is currently possible.
Patients with HPV-positive HNC were previously described as younger, White, non-smokers with a high number of sexual partners20,21. However, the incidence among older and non-White people has increased according to other studies22,23. Indeed, a recent comprehensive study investigated the differences in HPV genotype distribution in an age-dependent manner showing higher levels of HPV in younger HPV-positive oropharynx cancer patients in comparison to older patients6. Interestingly, 100% of the younger individuals were HPV-positive in comparison to 76% among the older tested patients. The investigators claim that the HPV-positivity in oropharynx cancer depends on the intensity of sexual exposure explaining the lower numbers of HPV-positive cancers in older patients. HNC can include oral cavity, larynx, pharynx, paranasal sinuses, and nasal cavity and salivary glands, so the symptoms can vary. Nevertheless, most of the HNCs cause a sore throat that does not heal, problems with swallowing and changes in the voice. Depending on the specific location, it may also include difficulties in breathing, pain in the neck area, headaches, earache or chronic sinus infections6. While nowadays the number of HNC cases has decreased due to reduced use of alcohol and/or tobacco, the HPV infection-related incidence has increased6. Since the symptoms may be very dependent on the localization of the cancerous changes, different diagnostic tests may be necessary. Additionally, to confirm a cancerous lesion, a tissue sample needs to be examined under a microscope. To confirm a HPV infection, the National Comprehensive Cancer Network (NCCN) guidelines note that p16 protein expression is highly correlated with HPV status and recommend assessment of p16 expression by immunohistochemistry (IHC) or detection of HPV DNA in tumor cell nuclei by in situ hybridization (ISH)6.
Treatment and its effectiveness
Presumably, access to Pap tests and HPV vaccine contributed to a decline in cervical cancer cases over decades which was also associated with a lower mortality6. According to the ACS6, the 5-year overall survival for cervical cancer is relatively high. Of note, HPV infection is not treatable but therapies for HPV-related precancerous cellular alternations and cancers are available. In most women with precancerous lesions, the loop electrosurgical excision procedure (LEEP) is applied6. During LEEP, the abnormal cells can be removed using an electrical current. Moreover, laser therapy, cryotherapy – freezing the abnormal tissue, and cold knife conization are the treatment options for precancerous lesions. External genital warts caused by HPV infection can be treated by topical medicine7.
As the testing for HNC depends on the type and location of the HNC, the treatment is also not unified for all HNCs, but it may include surgery, radiotherapy, chemotherapy, targeted therapy, or a combination of those6. A new type of surgery, transoral robotic surgery, is supported by three-dimensional (3D)-imaging which helps to remove tumors at places that are difficult to reach. Stereotactic body radiation applies high doses of radiation into the tumor by positioning the patient in the same way in each session which reduces the chance of damaging surrounding healthy tissue. Intensity-modulated radiation therapy (IMRT) uses 3D-imaging to precisely irradiate the tumor, which also reduces damage to healthy tissue. For oropharyngeal cancer, cetuximab, a monoclonal antibody for epidermal growth factor receptor (EGFR), which stops the tumor cell proliferation, is applied as a targeted therapy for HNC patients6. However, patients with HPV-related cancers receive the same treatment as patients with HPV-unrelated cancers at the same anatomical site. An FDA-approved anti-PD-1 antibody, pembrolizumab, became a first-line treatment option for all PD-L1 expressing tumors including HPV-positive and -negative HNC24,25. It can be used as a monotherapy or in combination with chemotherapy.
Because HPV-associated HNC respond vastly better to treatment than the HPV-negative ones, they also show a better survival rate. Using data obtained from the Surveillance, Epidemiology, and End Results HPV Status Database, a recent study reported that from 2013 to 2014, the US incidence of HPV-positive oropharyngeal squamous cell carcinoma (OPSCC) was 4.62 [95% confidence interval (CI), 4.51–4.73] vs 1.82 (95% CI, 1.75–1.89) per 100,000 persons for HPV-negative OPSCC and the incidence of HPV-positive vs negative non-OPSCC of the head and neck was 0.62 (95% CI, 0.58–0.66) vs 1.38 (95% CI, 1.32–1.44)26. Current treatments for both cervical cancer and HNC cover a wide spectrum of options, which not only kill the tumor but also consider the protection of healthy tissue.
Biology of HPV-associated cancers and potentially underlying mechanisms in HPV infection
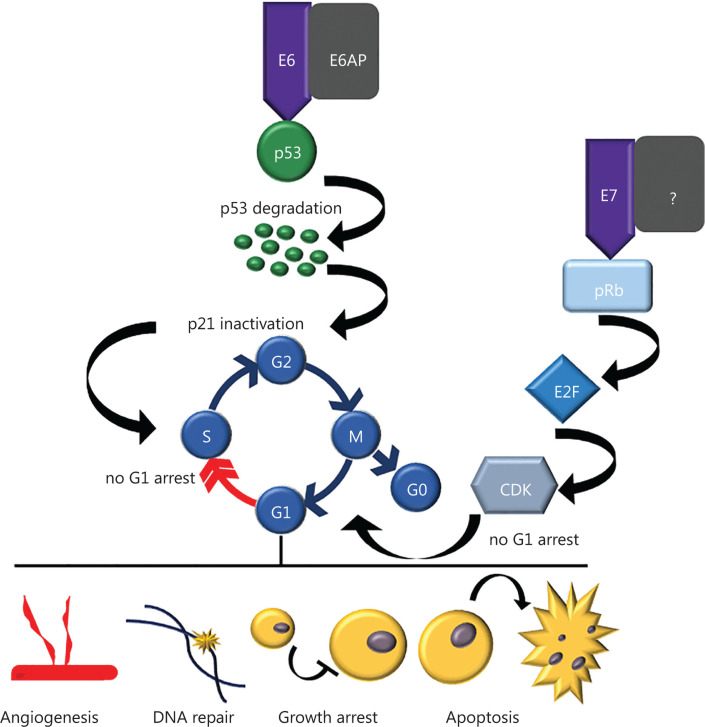
Persistent cervical HPV infections may progress towards premalignant glandular or squamous intra-epithelial lesions, classified histopathologically as cervical intraepithelial neoplasia (CIN), and to cancer. CIN is further classified as: CIN 1: mild dysplasia; CIN 2: moderate to marked dysplasia; and CIN 3: severe dysplasia to carcinoma in situ. Most CIN lesions regress spontaneously, although over a number of years, lesions on the cervix can slowly become cancerous27,28. A diagnosis of CIN is based primarily on the presence of nuclear atypia and loss of normal squamous maturation (polarity). Accurate grading of CIN lesions is essential as the treatment and clinical follow-up algorithms are very different for the low-grade (CIN 1) and high-grade lesions (CIN 2 and 3)29. Although the likelihood of progression clearly increases with the increasing grade of cervical intraepithelial neoplasia, a proportion of high-grade lesions could still regress. Infection with high-risk HPV subtypes has been shown to be a risk factor for persistent and/or progressive cervical dysplasia. It has also been proposed that HPV DNA integration into host DNA is critical in cervical carcinogenesis30,31 mediated by disruption of the E1/E2 open reading frames of the HPV genome and subsequent loss of the E2-controlled regulation of E6 and E731. This leads to strongly decreased expression levels of p53 in HPV-associated cancer cells32. The inactivation of the host p53 and retinoblastoma protein (pRb) by E6 and E7, respectively, results in suppressed cell cycle checkpoints and uncontrolled cell proliferation33–36 (Figure 3). In detail, E6 binds p53 leading to its degradation via E6-associated protein (E6AP)-mediated ubiquitination33–37. Interestingly, E6 first needs to bind to the protein E3-ligase E6AP as neither E6 or E6AP can bind to p53 alone34,38. Additionally, only high-risk HPV E6 and E7 can bind to p53 or pRb39 confirming that HPV-related cancers originate only from high-risk HPV types. Degradation of p53 leads to inactivation of one of its targets – p21 (also known as p21WAF1/Cip1)40, a cyclin-dependent kinase inhibitor, which prevents cells from entering the S phase by promoting cell cycle arrest in the G1 phase in response to many stimuli41–44 (Figure 3). E7 targets pRb for ubiquitination, leading to the release of E2F transcription factors, which transcribe cyclin E, cyclin A, and p16INK4A, an inhibitor of CDK4/6, forcing the cells through premature S phase entry45 (Figure 3). To date, it remains unclear how E7 mediates the pRb ubiquitination. However, it has been proposed that Cullin2 can bind to E7 and be responsible for the ubiquitination of pRb46. Of note, E7 was found to be ubiquitinated by the complex Cullin 1/Skp247. Although pRb also interacts with Skp2, it is not involved in its ubiquitination47. Overall, degradation of both p53 and pRb mediated by high-risk HPV results in cellular immortalization which presumably leads to the development of high-grade dysplasia (CIN 2 and 3), with a potential to progress to invasive carcinoma27,48.
Figure 3.
Effect of HPV E6 and E7 viral oncoproteins on cellular processes. E6 and E7 viral oncoproteins can affect angiogenesis, DNA repair, growth arrest and apoptosis by impairing G1 arrest. E6 binds to E6AP, and subsequently binds to p53 and promotes its degradation by ubiquitination. Thus, the function of p53 as a tumor suppressor is diminished and cell cycle is not properly controlled by p21, a cyclin-dependent kinase inhibitor that ensures G1/S checkpoint. E7 leads to degradation of pRb, the retinoblastoma protein that functions as a tumor suppressor. Because of RB inhibition by E7, cells can enter S phase without a G1 arrest.
A recent in vitro report suggested applying antisense HPV RNA transcripts in combination with cisplatin for HPV-positive HNC cells49. Here, the investigators infected a HPV-positive HNC cell line, UM-SCC-47 harboring HPV 16, and the HPV-negative HNC cancer cell line YCU-T892, with antisense RNA transcripts of HPV 16 E6/E7 using a recombinant adenoviral vector Ad-E6/E7-AS. They observed a significantly suppressed cell growth in the HPV-positive but not in the HPV-negative cells which correlated with increased expression of p53 and pRb in the HPV-positive cell line. Finally, a combinatory treatment with cisplatin evoked a cell growth reduction in vitro and suppressed tumor growth significantly if compared to monotherapy of either cisplatin or transfection with an Ad-E6/E7-AS construct. Another study also reported suppressed cell growth in HPV-related cervical cancer cells after downregulating HPV 16 E6 and E7 using an adenovirus-mediated transfection which correlated with higher expression of p53 and/or pRb proteins50,51. Additionally, a similar effect of increased sensitization to cisplatin treatment has been shown in cervical cancer cells after treatment with siRNA against the HPV 16 and HPV 18 E6 and E752. Furthermore, downregulation of HPV 16 and HPV 18 E6 and E7 led to radiosensitizing effects and suppressed growth in cervical cancer cells and xenograft mouse tumors53. Targeting HPV 16 E6/E7 by a gRNA induced apoptosis in HPV-positive cervical cancer cells SiHa and an additional treatment with a gRNA against PD1 significantly reduced the growth of SiHa cell-xenografted humanized SCID mice54. Taken together, downregulation of HPV E6 and E7 significantly suppresses cell growth and reconstitutes p53 and pRb expression. Moreover, downregulation of HPV makes tumor cells more susceptible to chemotherapy.
Ongoing clinical trials on treatment for HPV-related cancers
Immunotherapy is nowadays listed as a treatment option for cancer patients. On the one hand, immunotherapy can be used as a booster in the form of checkpoint inhibitors or vaccines to trigger a patient’s immune system. On the other hand, immunotherapy uses monoclonal engineered T-cells or antibodies as substances mimicking the compounds of the immune system. Of note, the FDA has approved 14 different immunomodulators including seven checkpoint inhibitors disturbing the PD-1/PD-L1 interaction or blocking the CTLA-4 which can support the immune system to recognize the cancer cells and to induce cell death55. Moreover, four cytokines, small molecular messengers between cells, are approved for the treatment of patients with kidney cancer, leukemia, lymphoma, melanoma, and sarcoma. Since 1997 the FDA has approved multiple monoclonal antibodies for the treatment of several solid tumors and hematological cancers56. For HPV-related cancers, cervical and HNC, an anti-CTLA4 monoclonal antibody ipilimumab is being tested within phase II clinical studies alone or in combination with radiotherapy19 (NCT03799445, NCT01693783). CUE-101 is a novel E7-pHLA-IL-2-Fc fusion protein and its Fc domain is linked to the protein of interest. Thus, it is designed to activate and expand a population of tumor specific T cells to eradicate HPV-driven malignancies. It is currently being tested in a new phase I clinical study recruiting patients to verify the toxicity and estimate the optimal dosage19 (NCT03978689). Avelumab is a monoclonal antibody which targets protein programmed death-ligand 1 (PDL-1) and has been recently approved by the FDA for a specific type of skin cancer – Merkel cell carcinoma, renal, and bladder cancer. Currently, over 200 clinical trials are testing avelumab’s effect on different types of cancer. One of these trials combines avelumab with a newly developed vaccine TG4001 with HPV 16 antigens in HPV-related cancers19 (NCT03260023). Durvalumab is another FDA-approved human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody which blocks the interaction of PD-L1 with the PD-1 and is used in treatment of different types of lung and bladder cancers. Durvalumab is now being tested with a vaccine MEDI0457 in HPV-positive OPSCC in a phase 2 clinical trial19 (NCT04001413). MEDI0457 is composed of DNA plasmids encoding the E6/E7 genes of HPV 16 and HPV 18 (VGX-3100) and DNA plasmid encoding the human interleukin-12 (IL-12), INO-9012, which triggers the immune system6. A recent paper revealed that MEDI0457 was well-tolerated by patients with cervical cancer after receiving chemoradiation and raised the idea of combining tumor-specific vaccines with radiotherapy57. MEDI0457 was also well tolerated by patients suffering from HPV-associated HNC cancer showing elevated antigen-specific T cell activity58. However, despite the promising safety studies, the final efficacy of a vaccine can only be evaluated after phase 2 and 3 clinical trials. Moreover, durvalumab is also being tested in combination with radiation in patients with HPV-associated OPSCC19 (NCT03623646).
Many kinase inhibitors have now entered cancer treatment. A phosphoinositide-3-kinase gamma (PI3Kγ)-inhibitor, IPI-549, is currently under investigation in a phase II clinical study including HNC cancer patients either as a monotherapy19 (NCT03795610) or in combination with PD-1 blocking antibody nivolumab in a panel of HPV-related cancers19 (NCT02379520). The underlying working hypothesis is that mRNA signatures of immune response will be increased in IPI-549-treated patients and therefore this combination may enhance anti-PD-1 therapy.
Interestingly, a novel treatment involving HPVST cells, HPV-specific T cells, is currently under investigation in one clinical study including patients with recurring HPV-related cancers19 (NCT02379520). HPVST cells originate from the patients who previously suffered from HPV-related cancer. The goal is to validate whether these cells can affect the tumors and whether they can develop resistance against transforming growth factor-beta (TGF-β) produced by HPV-mediated tumors. After the optimal dose of HPVST cells is established, an FDA-approved human IgG4 monoclonal antibody, nivolumab, that blocks PD-1, will be applied in combination with HPVST. The overall purpose of this study is to potentially increase the effectiveness of HPVST cells using the nivolumab in patients suffering from HPV-related cancers. A 6-month application of nivolumab is a final step of a phase II study including patients diagnosed with HPV-positive OPSCC who will first receive chemotherapy (carboplatin, nab-paclitaxel and nivolumab) followed by radiotherapy or surgery19 (NCT03107182, NCT03829722).
Another phase I clinical study uses magnetic resonance imaging (MRI)-guided brachytherapy (internal radiation therapy) to verify whether this approach can improve the high-risk clinical target volume (HR-CTV) D90 (dose to 90% of the high-risk clinical target volume) rate compared to conventional guidance using ultrasound and a freehand technique in stage IB2-IV cervical or stage II-IVA vaginal cancer19 (NCT03634267). Furthermore, proton therapy has attracted attention and is now being considered as treatment option for many cancer patients and can be applied in a variety of tumor types. An ongoing clinical trial is using the traditional radiotherapy and/or proton therapy in patients with HPV-related OPSCC to achieve a reduction of toxicity by maintaining the efficiency of the treatment19 (NCT03621696). The limitation of radiotherapy-induced side effects in patients suffering from HPV-associated HNC is a focus of a phase II studies applying IMRT and chemotherapy19 (NCT02281955, NCT01932697).
CRISPR/Cas9 technology is now widely used for in vitro and in vivo studies. The usage of this genome editing tool is currently under investigation in a phase I clinical trial19 (NCT03747965). In more detail, the clinical study includes patients with different solid tumors, especially pancreatic cancer, cholangiocarcinoma cancer, and ovarian cancer, and monitors the safety of CRISPR-Cas9-mediated PD-1 gene-knocked out chimeric antigen receptor (CAR) T cells. Another study is addressing the safety and efficacy of CRISPR/Cas9-HPV E6/E7, which can disrupt the DNA of HPV 16 and HPV 18, in patients with HPV-related cervical intra-epithelial neoplasia (CIN)19 (NCT03057912). Zinc finger nucleases, called ZFN-603 and ZFN-758, cleave HPV 16 and HPV 18 E7 oncogene and are currently under investigation including in patients suffering from CIN19 (NCT02800369).
Altogether, there are many ongoing clinical trials including patients suffering from HPV-related cancers which address different aspects and methods of treatment, but these trials mainly focus on unique HPV antigens for vaccination as well as in combination with immune checkpoint agents.
In silico analysis-based correlations within HPV-associated cancers
A comprehensive analysis of 228 primary cervical cancers revealed an upregulation of SHKBP1, ERBB3, CASP8, HLA-A, and TGFBR2 as novel significantly mutated genes in cervical cancer and confirmed mutations in PIK3CA, EP300, FBXW7, HLA-B, PTEN, NFE2L2, ARID1A, KRAS, and MAPK1 in these patients59. In contrast, HPV-negative cancers, mostly including HNC and a few endometrial-like cervical cancers, showed an increased mutation rate in ARID1A, KRAS, and PTEN. Moreover, amplifications in PD-L1 and PD-L2, and the BCAR4 long non-coding RNA which has previously been linked to respond to the chemotherapy drug lapatinib, an EGFR/HER2 inhibitor, have also been observed59. Importantly, over 70% of cervical cancers exhibited genomic alterations in either one or both of PI3K–MAPK and TGF-β signaling pathways, suggesting compounds of these pathways may be targets for anticancer therapy. Moreover, a cross-cancer analysis in this study highlighted the similarity among HPV-positive squamous cancers [cervical and HPV-related head and neck squamous cell carcinoma (HNSCC)] independent of the anatomical site. Interestingly, HPV 18-associated tumors exhibited a significantly higher ratio of unspliced vs spliced versions of the E6 protein than the HPV 16-related cancers showing the diversity between different HPV types. This suggests different functional aspects of E6 and E7 in tumors infected with different HPV types. Taken together, this study provides a comprehensive analysis of gene deregulations in cervical cancers including the dependence on HPV subtypes which underlines the importance of targeted therapy for certain cancer associated with particular HPV subtypes.
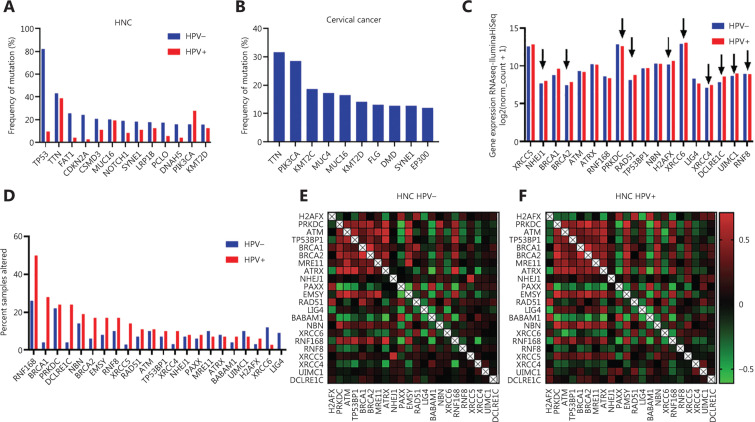
Our own in silico analysis including HPV-positive and HPV-negative HNC revealed that TP53 was the most frequently mutated (over 80% of samples) gene in HPV-negative HNC60 (Figure 4A). In contrast, HPV-positive HNC samples were mostly harboring a mutation in TTN which encodes the protein Titin responsible for the passive elasticity of muscles. The second highly mutated gene was PIK3CA which correlates with findings of Burk59. Additionally, we performed the same analysis with cervical cancer samples and found that TTN and PIK3CA were also highly mutated in these samples (Figure 4A, B).
Figure 4.
Different frequencies in gene mutations in HPV-positive and negatives cancers. (A) Frequency of mutations in HPV-positive and HPV-negative HNC cancer patients based on TCGA bioportal in silico analysis. (B) Frequency of mutations in cervical cancer patients based on TCGA bioportal in silico analysis. (C, D) Co-expression of DNA DSB repair genes in HPV-negative (C) and HPV-positive (D) HNC patients based on TCGA bioportal in silico analysis.
As DNA repair processes in tumor cells are affected, we performed an additional mRNA expression analysis including DNA repair genes within HPV-positive (n = 38) and HPV-negative (n = 75) HNC61. Our data reveals significant mRNA expression differences in NHEJ1, BRCA2, PRKDC, RAD51, H2AFX, XRCC6, XRCC4, DCLRE1C, UIMC1, and RNF8 (Figure 4C). Interestingly, most of the genes, except for PRKDC and RNF168, showed higher expression in HPV-positive HNC. However, 50% of all HPV-positive HNC samples were altered in the RNF168 gene which overlaps with a study from Sitz and colleagues62 (Figure 4D). Our analysis uncovered additionally high rates of alterations in the BRCA1 gene in HPV-positive HNC samples (Figure 4D). As BRCA1 and RNF168 both promote homologous recombination repair (HRR)63,64, the high alteration rate of both suggests a HPV-mediated facilitated HRR in HPV-associated HNC cancers. In contrast, HPV-negative HNC samples showed alterations in the LIG4 and XRCC6 genes involved in non-homologous end joining (NHEJ), suggesting an affected DNA double strand breaks (DSB) repair by NHEJ (Figure 4D). However, comparing the co-expression of DNA DSB repair genes in HPV-positive and HPV-negative HNC has not shown striking differences60 (Figure 4E, F), indicating that co-expression between those genes cannot be significantly affected by the virus, despite the affected DNA repair processes described.
Role of DNA repair in the treatment of HPV-mediated cancers
DNA damaging agents leading to generation of diverse lesions, for example, DNA DSBs, induce DNA damage response and DNA repair. DSBs represent the most severe type of DNA lesions repaired by NHEJ or HRR, which enforce the maintenance of genomic stability. NHEJ is a fast but error-prone DNA repair pathway with DNA-dependent protein kinase (DNAPKcs) as a key protein kinase important for recruitment of downstream factors necessary to join and ligate the broken DNA ends65. Proper DNA repair ensures cell survival and finally the health of an individual. However, different diseases, especially cancer, can result in imbalanced DNA damage response and repair, but also HPV infections result in the deregulations of DNA repair processes. DNAPKcs has been found to be downregulated in HPV-positive OPSCC if compared to HPV-negative carcinomas66 which we also observed in our in silico analysis (Figure 4C). Moreover, the decreased expression of DNAPKcs inversely correlated with the increased expression of the viral oncoproteins E6 and E7, and was associated with higher radiosensitivity66. Furthermore, cervical cancer cell lines and HPV-positive HNSCC, but not the HPV-negative HNSCC, were associated with higher amount of persistent infrared (IR)-induced γH2AX, a marker for DNA DSBs indicating a HPV-dependent impaired removal of DNA DSBs66–68. In line with this observation, it has been suggested that the lack of DNAPK and BRCA2 in HPV-positive HNSCC has led to a reduced DNA repair capacity by NHEJ and/or HRR, despite sustained activity of 53BP1 and BRCA1, which are independent of HPV status66. Moreover, it has been proposed that HPV decreases the efficacy of HRR by hindering RAD51 to localize to DNA DSBs69. A new report presented that E6-transduced U2OS cells failed to show colocalization of the Fanconi anemia complementation group D2 (FancD2),a protein involved in interstrand crosslink repair, with DNA DSBs, which subsequently hindered the recruitment of the downstream repair protein RAD51 to DSBs70. Further, E6 expression caused delayed FancD2 de-ubiquitination, an important process for effective interstrand crosslink repair, resulting in its increased chromatin retention. Finally, E6 could suppress USP1 de-ubiquitinating activity, and led to persistent activation of ATR/CHK-1/pS565 FancI signaling70–72. Thus, a prediction to sensitivity to DNA crosslinking agents in HPV-negative HNSCC based on an interstrand crosslink repair gene expression has been introduced and confirmed that defects in these DNA repair genes correlated with a poor prognosis71. Indeed, ATR or CHK1 inhibitors suppress both amplification and late viral gene expression in differentiated cells and reduce stable copy numbers of the viral genome in undifferentiated cells73. Moreover, application of the ATR inhibitor in both HPV- and HPV+ HNSCC cell lines led to enhanced sensitivity to cisplatin treatment74. Interestingly, ATM- and ATR-mediated signaling is constitutively active in HPV-associated cells unexposed to any DNA damaging agent71,75,76, suggesting that application of ATR and ATM inhibitors could represent a promising approach in the treatment of HPV-related carcinoma.
As the viral oncoproteins, E6 and E7, are crucial in HPV-mediated tumorigenesis, researchers are focusing on their mechanistic functions, partially in DNA repair, in promoting cancer. A recent study proposed E7 as the oncoprotein which hampers the DNA damage response to DSBs by interacting with the E3 ligase RNF168 involved in DNA DSB repair62. It has also been observed that expression of an exonuclease with a proofreading function, Three prime repair exonuclease 1 (TREX1), is upregulated exclusively in HPV-transformed primary keratinocytes expressing HPV 16 E6 and E777. Downregulation of TREX1 resulted in inhibition of cell growth, which was associated with p53 upregulation.
Remarkably, HPV infection can block TGF-β resulting in more prominent activation of alternative end joining (altEJ) instead of HRR, enhancing the cellular sensitivity to cisplatin or PARP inhibitors78–80 suggesting that HPV-positive cancer cells are more sensitive to therapy by interfering with DNA DSB repair. In line with this observation, HPV-positive OPSCCs are more sensitive to radiotherapy than the HPV-negative ones, correlating with the reduced DNA DSB repair capacity in HPV-positive cancers81. Several studies showed that HPV-positive HNSCC cells treated with ionizing radiation show a significantly higher cell death than the HPV-negative HNSCC82–85. Importantly, a higher radiosensitizing effect of olaparib was observed in HPV-negative OPSCCs than in HPV-positive OPSCC, suggesting this combined treatment to be applied in relatively more radioresistant HPV-negative cancers85. In addition, roscovitine, a cyclin-dependent kinase inhibitor targeting CDK2, CDK7, and CDK9, has been proposed as an olaparib-enhancer towards radiosensitization for HPV-negative HNSCC86. In contrast, a study using a panel of HPV-positive and HPV-negative OPSCC revealed that only a subset of HPV-positive OPSCCs were susceptible for olaparib treatment at a therapeutic concentration (0.1–0.5 μM), whereas other HPV-positive and HPV-negative OPSCCs were either sensitive to the PARP inhibition at higher doses or not at all87.
The wide spectrum of cancer treatment includes proton beam irradiation which uses particles to irradiate tumors. Despite a relatively low number of studies including proton irradiation and HPV-related cancers, most reports claim an advantage of proton beam therapy especially for relatively radioresistant HPV-negative cancers. For instance, proton irradiation-induced reduction of cell growth in HPV-negative HNC cells was significantly higher than the standard photon-based radiation88,89. Furthermore, a comprehensive analysis of protein expression in HPV-positive and HPV-negative HNC cell lines revealed different expression patterns between proton and photon irradiation90. In brief, they found differences in expression of IL-6, GSK3, Src, AMPK, cyclooxygenase (COX)-2, vascular endothelial growth factor (VEGF) pathways in HNC cells treated with proton or photon irradiation90, suggesting varying benefit from both radiotherapy modulations in combination with targeted therapy or immunotherapy.
On the other hand, HPV infection can activate different DNA repair proteins involved in response to ultraviolet (UV)-mediated DNA damage, including nucleotide end resection (NER), the Fanconi anemia (FA) pathway, and translesion synthesis (TLS) because HPV requires these DNA repair mechanisms to properly replicate its genome71,75,76,91,92. However, it has also been shown that HPV 31 needs an accumulation of ATM and HR-related factors, for example, MRN complex composed of RAD50-MRE11-NBS1 proteins, to its replication sites75,76,92. Furthermore, HPV abolishes the apoptosis upon agents inducing UV-mediated lesions93–95 suggesting that HPV can induce resistance to DNA crosslinking agents, for example, cisplatin. To predict a potential cisplatin resistance in cervical cancer, different proteins have been identified, for example, p5396–98, excision repair cross-complementation group 1 (ERCC1)99, an apoptosis regulator B-cell lymphoma 2 (Bcl-2), p2197,98,100. This approach supports screenings and personalized treatment for HPV-related cancers to avoid resistances and to apply the best suitable treatment following diagnosis.
Acknowledgments
We thank all our colleagues from Dr. Chen’s laboratory. This work was supported by the Pamela and Wayne Garrison Distinguished Chair in Cancer Research to J.C. J.C. also received support from the CPRIT awards (Grant Nos RP160667 and RP180813) and NIH grants (Grant Nos P01CA193124, R01CA210929, R01CA216911, and R01CA216437).
Footnotes
Conflicts of interest statement No potential conflicts of interest are disclosed.
References
- 1.EGDAHL A. WHO: World Health Organization. Ill Med J. 1954;105:280–2. [PubMed] [Google Scholar]
- 2.Singh Jatinder, Olabode Reference Centers for Disease Control and Prevention. Indian J Pharmacol. 2004;36:268–9. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 4.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–16. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 5.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:F55–70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 6.Comprehensive Cancer Information – National Cancer Institute. https://www.cancer.gov/
- 7.Patel RV, Yanofsky VR, Goldenberg G. Genital warts: a comprehensive review. J Clin Aesthet Dermatol. 2012;5:25–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13:39. doi: 10.1186/1471-2334-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. Cancers Attributable to Infections. International Agency for Research on Cancer; 2020. https://www.iarc.fr/ [Google Scholar]
- 10.Smith JS, Gilbert PA, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of human papillomavirus infection in males: a global review. J Adolesc Health. 2011;48:540–52. doi: 10.1016/j.jadohealth.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Schim van der Loeff MF, Mooij SH, Richel O, de Vries HJC, Prins JM. HPV and anal cancer in HIV-infected individuals: a review. Curr HIV/AIDS Rep. 2014;11:250–62. doi: 10.1007/s11904-014-0224-x. [DOI] [PubMed] [Google Scholar]
- 12.Vardas E, Giuliano AR, Goldstone S, Palefsky JM, Moreira ED, Penny ME, et al. External genital human papillomavirus prevalence and associated factors among heterosexual men on 5 continents. J Infect Dis. 2011;203:58–65. doi: 10.1093/infdis/jiq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olesen TB, Munk C, Christensen J, Andersen KK, Kjaer SK. Human papillomavirus prevalence among men in sub-Saharan Africa: a systematic review and meta-analysis. Sex Transm Infect. 2014;90:455–62. doi: 10.1136/sextrans-2013-051456. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO Safety update of HPV vaccines. Geneva: World Health Organization; 2017. Report form GACVS meeting of 7-8 June 2017. [Google Scholar]
- 15.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–78. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmieri B, Poddighe D, Vadalà M, Laurino C, Carnovale C, Clementi E. Severe somatoform and dysautonomic syndromes after HPV vaccination: case series and review of literature. Immunol Res. 2017;65:106–16. doi: 10.1007/s12026-016-8820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cervantes JL, Doan AH. Discrepancies in the evaluation of the safety of the human papillomavirus vaccine. Mem Inst Oswaldo Cruz. 2018;113:e180063. doi: 10.1590/0074-02760180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Information Sheet: Observed rate of vaccine reactions Human Papilloma Virus Vaccine. 2012. https://www.who.int/vaccine_safety/initiative/tools/HPV_Vaccine_rates_information_sheet.pdf?ua=1.
- 19. https://www.clinicaltrials.gov/
- 20.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 21.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zumsteg ZS, Cook-Wiens G, Yoshida E, Shiao SL, Lee NY, Mita A, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2016;2:1617–23. doi: 10.1001/jamaoncol.2016.1804. [DOI] [PubMed] [Google Scholar]
- 23.D’Souza G, Westra WH, Wang SJ, Van Zante A, Wentz A, Kluz N, et al. Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol. 2017;3:169–77. doi: 10.1001/jamaoncol.2016.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazer C, Price KA. Management of immune-related dermatitis and mucositis associated with pembrolizumab in metastatic human papillomavirus-associated squamous cell carcinoma of the oropharynx. JCO Oncol Pract. 2020;16:20s–4s. doi: 10.1200/JOP.19.00648. [DOI] [PubMed] [Google Scholar]
- 25.Cohen AC, Roane BM, Leath CA. Novel therapeutics for recurrent cervical cancer: moving towards personalized therapy. Drugs. 2020;80:217–27. doi: 10.1007/s40265-019-01249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahal BA, Catalano PJ, Haddad RI, Hanna GJ, Kass JI, Schoenfeld JD, et al. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28:1660–7. doi: 10.1158/1055-9965.EPI-19-0038. [DOI] [PubMed] [Google Scholar]
- 27.Kalof AN, Cooper K. Our approach to squamous intraepithelial lesions of the uterine cervix. J Clin Pathol. 2007;60:449–55. doi: 10.1136/jcp.2005.036426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mello V, Sundstrom RK. Cancer, Cervical Intraepithelial Neoplasia (CIN) Treasure Island, FL: StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 29.Adams AK, Wise-Draper TM, Wells SI. Human papillomavirus induced transformation in cervical and head and neck cancers. Cancers. 2014;6:1793–820. doi: 10.3390/cancers6031793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon S, Allen-Hoffmann BL, Lambert PF. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J Virol. 1995;69:2989–97. doi: 10.1128/jvi.69.5.2989-2997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peitsaro P, Johansson B, Syrjänen S. Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique. J Clin Microbiol. 2002;40:886–91. doi: 10.1128/JCM.40.3.886-891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheffner M, Munger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991;88:5523–7. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 34.Scheffner M, Huibregtse JM, Howley PM. Identification of a human ubiquitin-conjugating enzyme that mediates the E6-AP-dependent ubiquitination of p53. Proc Natl Acad Sci U S A. 1994;91:8797–801. doi: 10.1073/pnas.91.19.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talis AL, Huibregtse JM, Howley PM. The role of E6AP in the regulation of p53 protein levels in human papillomavirus (HPV)-positive and HPV-negative cells. J Biol Chem. 1998;273:6439–45. doi: 10.1074/jbc.273.11.6439. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Hong X, Wei Z, Xie M, Li W, Liu G, et al. Ubiquitination of the HPV oncoprotein E6 is critical for E6/E6AP-mediated p53 degradation. Front Microbiol. 2019;10:2483. doi: 10.3389/fmicb.2019.02483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mietz JA, Unger T, Huibregtse JM, Howley PM. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO J. 1992;11:5013–20. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13:775–84. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–9. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 40.El-Deiry WS. p21(WAF1) mediates cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res. 2016;76:5189–91. doi: 10.1158/0008-5472.CAN-16-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barr AR, Cooper S, Heldt FS, Butera F, Stoy H, Mansfeld J, et al. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat Commun. 2017;8:1–17. doi: 10.1038/ncomms14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. P21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 43.Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbas T, Dutta A. P21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4. [PubMed] [Google Scholar]
- 46.Huh K, Zhou X, Hayakawa H, Cho J-Y, Libermann TA, Jin J, et al. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol. 2007;81:9737–47. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh K-J, Kalinina A, Wang J, Nakayama K, Nakayama KI, Bagchi S. The papillomavirus E7 oncoprotein is ubiquitinated by UbcH7 and cullin 1- and Skp2-containing E3 ligase. J Virol. 2004;78:5338–46. doi: 10.1128/JVI.78.10.5338-5346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho GYF, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–71. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- 49.Kojima Y, Otsuki N, Kubo M, Kitamoto J, Takata E, Saito H, et al. Adenovirus-mediated transfer of HPV 16 E6/E7 antisense RNA combined with cisplatin inhibits cellular growth and induces apoptosis in HPV-positive head and neck cancer cells. Cancer Gene Ther. 2018;25:274–83. doi: 10.1038/s41417-018-0024-3. [DOI] [PubMed] [Google Scholar]
- 50.Hamada K, Sakaue M, Alemany R, Zhang WW, Horio Y, Roth JA, et al. Adenovirus-mediated transfer of HPV 16 E6/E7 antisense RNA to human cervical cancer cells. Gynecol Oncol. 1996;63:219–27. doi: 10.1006/gyno.1996.0310. [DOI] [PubMed] [Google Scholar]
- 51.Hamada K, Shirakawa T, Gotoh A, Roth JA, Follen M. Adenovirus-mediated transfer of human papillomavirus 16 E6/E7 antisense RNA and induction of apoptosis in cervical cancer. Gynecol Oncol. 2006;103:820–30. doi: 10.1016/j.ygyno.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 52.Rajasekaran N, Jung HS, Bae SH, Chelakkot C, Hong S, Choi JS, et al. Effect of HPV E6/E7 siRNA with chemotherapeutic agents on the regulation of TP53/E2F dynamic behavior for cell fate decisions. Neoplasia (United States) 2017;19:735–49. doi: 10.1016/j.neo.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung HS, Rajasekaran N, Song SY, Kim YD, Hong S, Choi HJ, et al. Human papillomavirus E6/E7-specific siRNA potentiates the effect of radiotherapy for cervical cancer in vitro and in vivo. Int J Mol Sci. 2015;16:12243–60. doi: 10.3390/ijms160612243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhen S, Lu J, Liu YH, Chen W, Li X. Synergistic antitumor effect on cervical cancer by rational combination of PD1 blockade and CRISPR-Cas9-mediated HPV knockout. Cancer Gene Ther. 2019;27(3–4):168–78. doi: 10.1038/s41417-019-0131-9. [DOI] [PubMed] [Google Scholar]
- 55.Cancer Research Institute – Advancing Immunotherapy Research. https://www.cancerresearch.org/?gclid=CjwKCAjw0On8BRAgEiwAincsHO8IarepQbHw-6OVM6VAVwAE-Sn6eDN5d6fx7v0Nk9ucQ3l9UsVwNxoCMSQQAvD_BwE.
- 56.Cuesta-Mateos C, Alcaraz-Serna A, Somovilla-Crespo B, Muñoz-Calleja C. Monoclonal antibody therapies for hematological malignancies: Not just lineage-specific targets. Frontiers in Immunology. 2018;8:1936. doi: 10.3389/fimmu.2017.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasan Y, Furtado L, Tergas A, Lee N, Brooks R, McCall A, et al. A Phase 1 trial assessing the safety and tolerability of a therapeutic DNA vaccination against HPV16 and HPV18 E6/E7 oncogenes after chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys. 2020;107:487–98. doi: 10.1016/j.ijrobp.2020.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aggarwal C, Cohen RB, Morrow MP, Kraynyak KA, Sylvester AJ, Knoblock DM, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res. 2019;25:110–24. doi: 10.1158/1078-0432.CCR-18-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burk RD, Chen Z, Saller C, Tarvin K, Carvalho AL, Scapulatempo-Neto C, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–84. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.cBioPortal for Cancer Genomics. https://www.rochefoundationmedicine.com/cancertesting.html.
- 61.Goldman M, Craft B, Zhu J, Swatloski T, Cline M, Haussler D. UCSC Xena [Google Scholar]
- 62.Sitz J, Blanchet SA, Gameiro SF, Biquand E, Morgan TM, Galloy M, et al. Human papillomavirus E7 oncoprotein targets RNF168 to hijack the host DNA damage response. Proc Natl Acad Sci U S A. 2019;116:19552–62. doi: 10.1073/pnas.1906102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosen EM. BRCA1 in the DNA damage response and at telomeres. Front Genet. 2013;4:85. doi: 10.3389/fgene.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zong D, Adam S, Wang Y, Sasanuma H, Callén E, Murga M, et al. BRCA1 Haploinsufficiency is masked by RNF168-mediated chromatin ubiquitylation. Mol Cell. 2019;73(6):1267–81.e7. doi: 10.1016/j.molcel.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schipler A, Iliakis G. DNA double-strand-break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice. Nucleic Acids Res. 2013;41:7589–605. doi: 10.1093/nar/gkt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weaver AN, Cooper TS, Wei S, Carroll WR, Rosenthal EL, Yang ES. DNA-Pk(CS) expression in oropharyngeal squamous cell carcinoma: Correlations with human papillomavirus status and recurrence after transoral robotic surgery. Head Neck. 2017;39:206–14. doi: 10.1002/hed.24562. [DOI] [PubMed] [Google Scholar]
- 67.Park JW, Nickel KP, Torres AD, Lee D, Lambert PF, Kimple RJ. Human papillomavirus type 16 E7 oncoprotein causes a delay in repair of DNA damage. Radiother Oncol. 2014;113:337–44. doi: 10.1016/j.radonc.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banáth JP, MacPhail SH, Olive PL. Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res. 2004;64:7144–9. doi: 10.1158/0008-5472.CAN-04-1433. [DOI] [PubMed] [Google Scholar]
- 69.Wallace NA, Khanal S, Robinson KL, Wendel SO, Messer JJ, Galloway DA. High-risk alphapapillomavirus oncogenes impair the homologous recombination pathway. J Virol. 2017;91:e01084–17. doi: 10.1128/JVI.01084-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khanal S, Galloway DA. High-risk human papillomavirus oncogenes disrupt the Fanconi anemia DNA repair pathway by impairing localization and de-ubiquitination of FancD2. PLoS Pathog. 2019;15:e1007442. doi: 10.1371/journal.ppat.1007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Essers PBM, Van Der Heijden M, Verhagen CVM, Ploeg EM, De Roest RH, Leemans CR, et al. Drug sensitivity prediction models reveal a link between DNA repair defects and poor prognosis in HNSCC. Cancer Res. 2019;79:5597–611. doi: 10.1158/0008-5472.CAN-18-3388. [DOI] [PubMed] [Google Scholar]
- 72.Mehta K, Laimins L. Human papillomaviruses preferentially recruit DNA repair factors to viral genomes for rapid repair and amplification. MBio. 2018;9:e00064–18. doi: 10.1128/mBio.00064-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong S, Cheng S, Iovane A, Laimins LA. STAT-5 Regulates transcription of the topoisomerase IIβ-binding protein 1 (TopBP1) gene to activate the ATR pathway and promote human papillomavirus replication. MBio. 2015;6:e02006. doi: 10.1128/mBio.02006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leonard BC, Lee ED, Bhola NE, Li H, Sogaard KK, Bakkenist CJ, et al. ATR inhibition sensitizes HPV− and HPV+ head and neck squamous cell carcinoma to cisplatin. Oral Oncol. 2019;95:35–42. doi: 10.1016/j.oraloncology.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gillespie KA, Mehta KP, Laimins LA, Moody CA. Human papillomaviruses recruit cellular DNA repair and homologous recombination factors to viral replication centers. J Virol. 2012;86:9520–6. doi: 10.1128/JVI.00247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anacker DC, Gautam D, Gillespie KA, Chappell WH, Moody CA. Productive replication of human papillomavirus 31 requires DNA repair factor Nbs1. J Virol. 2014;88:8528–44. doi: 10.1128/JVI.00517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prati B, da Silva Abjaude W, Termini L, Morale M, Herbster S, Longatto-Filho A, et al. Three Prime Repair Exonuclease 1 (TREX1) expression correlates with cervical cancer cells growth in vitro and disease progression in vivo. Sci Rep. 2019;9:351. doi: 10.1038/s41598-018-37064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Q, Lopez K, Murnane J, Humphrey T, Barcellos-Hoff MH. Misrepair in context: TGFβ regulation of DNA repair. Front Oncol. 2019;9:799. doi: 10.3389/fonc.2019.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Q, Ma L, Jones T, Palomero L, Pujana MA, Martinez-Ruiz H, et al. Subjugation of TGFb signaling by human papilloma virus in head and neck squamous cell carcinoma shifts DNA repair from homologous recombination to alternative end joining. Clin Cancer Res. 2018;24:6001–14. doi: 10.1158/1078-0432.CCR-18-1346. [DOI] [PubMed] [Google Scholar]
- 80.Weaver AN, Cooper TS, Rodriguez M, Trummell HQ, Bonner JA, Rosenthal EL, et al. DNA double strand break repair defect and sensitivity to poly ADP-ribose polymerase (PARP) inhibition in human papillomavirus 16-positive head and neck squamous cell carcinoma. Oncotarget. 2015;6:26995–7007. doi: 10.18632/oncotarget.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta AK, Lee JH, Wilke WW, Quon H, Smith G, Maity A, et al. Radiation response in two HPV-infected head-and-neck cancer cell lines in comparison to a non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys. 2009;74:928–33. doi: 10.1016/j.ijrobp.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rieckmann T, Tribius S, Grob TJ, Meyer F, Busch CJ, Petersen C, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107:242–6. doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Gubanova E, Brown B, Ivanov SV, Helleday T, Mills GB, Yarbroug WG, et al. Downregulation of SMG-1 in HPV-positive head and neck squamous cell carcinoma due to promoter hypermethylation correlates with improved survival. Clin Cancer Res. 2012;18:1257–67. doi: 10.1158/1078-0432.CCR-11-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nickson CM, Moori P, Carter RJ, Rubbi CP, Parsons JL. Misregulation of DNA damage repair pathways in HPV-positive head and neck squamous cell carcinoma contributes to cellular radiosensitivity. Oncotarget. 2017;8:29963–75. doi: 10.18632/oncotarget.16265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ziemann F, Seltzsam S, Dreffke K, Preising S, Arenz A, Subtil FSB, et al. Roscovitine strongly enhances the effect of olaparib on radiosensitivity for HPV neg. but not for HPV pos. HNSCC cell lines. Oncotarget. 2017;8:105170–83. doi: 10.18632/oncotarget.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pirotte EF, Holzhauser S, Owens D, Quine S, Al-Hussaini A, Christian AD, et al. Sensitivity to inhibition of DNA repair by Olaparib in novel oropharyngeal cancer cell lines infected with Human Papillomavirus. PLoS One. 2018;13:e0207934. doi: 10.1371/journal.pone.0207934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L, Wang X, Li Y, Han S, Zhu J, Wang X, et al. Human papillomavirus status and the relative biological effectiveness of proton radiotherapy in head and neck cancer cells. Head Neck. 2017;39:708–15. doi: 10.1002/hed.24673. [DOI] [PubMed] [Google Scholar]
- 89.Wang L, Han S, Zhu J, Wang X, Li Y, Wang Z, et al. Proton versus photon radiation-induced cell death in head and neck cancer cells. Head Neck. 2019;41:46–55. doi: 10.1002/hed.25357. [DOI] [PubMed] [Google Scholar]
- 90.Wang L, Yang L, Han S, Zhu J, Li Y, Wang Z, et al. Patterns of protein expression in human head and neck cancer cell lines differ after proton vs photon radiotherapy. Head Neck. 2020;42:289–301. doi: 10.1002/hed.26005. [DOI] [PubMed] [Google Scholar]
- 91.Gu W, Sun S, Kahn A, Dacus D, Wendel SO, McMillan N, et al. Cervical cancer cell lines are sensitive to sub-erythemal UV exposure. Gene. 2019;688:44–53. doi: 10.1016/j.gene.2018.11.079. [DOI] [PubMed] [Google Scholar]
- 92.Moody CA, Laimins LA. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. In: Galloway D, editor. PLoS Patho. Vol. 5. 2009. e1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garnett TO, Duerksen-Hughes PJ. Modulation of apoptosis by human papillomavirus (HPV) oncoproteins. Arch Virol. 2006;151:2321–35. doi: 10.1007/s00705-006-0821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14:3065–73. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leverrier S, Bergamaschi D, Ghali L, Ola A, Warnes G, Akgül B, et al. Role of HPV E6 proteins in preventing UVB-induced release of pro-apoptotic factors from the mitochondria. Apoptosis. 2007;12:549–60. doi: 10.1007/s10495-006-0004-1. [DOI] [PubMed] [Google Scholar]
- 96.Garzetti GG, Ciavattini A, Provinciali M, Di Stefano G, Lucarini G, Goteri G, Biagini G. Expression of p53 and apoptosis of tumor cells in locally advanced cervical carcinoma after cisplatin based neoadjuvant chemotherapy. Anticancer Res. 1996;16:3229–34. [PubMed] [Google Scholar]
- 97.Saito T, Takehara M, Tanaka R, Lee R, Horie M, Wataba K, et al. Correlation between responsiveness of neoadjuvant chemotherapy and apoptosis-associated proteins for cervical adenocarcinoma. Gynecol Oncol. 2004;92:284–92. doi: 10.1016/j.ygyno.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 98.Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther. 2016;10:1885–95. doi: 10.2147/DDDT.S106412. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hasegawa K, Kato R, Torii Y, Ichikawa R, Oe S, Udagawa Y. The relationship between ERCC1 expression and clinical outcome in patients with FIGO stage I to stage II uterine cervical adenocarcinoma. Int J Gynecol Cancer. 2011;21:1479–85. doi: 10.1097/IGC.0b013e31822265e7. [DOI] [PubMed] [Google Scholar]
- 100.Henríquez-Hernández LA, Lloret M, Pinar B, Bordón E, Rey A, Lubrano A, et al. BCL-2, in combination with MVP and IGF-1R expression, improves prediction of clinical outcome in complete response cervical carcinoma patients treated by radiochemotherapy. Gynecol Oncol. 2011;122:585–9. doi: 10.1016/j.ygyno.2011.05.037. [DOI] [PubMed] [Google Scholar]