Abstract
Introduction
Progression of COVID-19 to severe disease and death is insufficiently understood.
Objective
Summarize the prevalence of risk factors and adverse outcomes and determine their associations in COVID-19 patients who were hospitalized.
Methods
We searched Medline, Embase and Web of Science for case-series and observational studies of hospitalized COVID-19 patients through August 31, 2020. Data were analyzed by fixed-effects meta-analysis using Shore’s adjusted confidence intervals to address heterogeneity.
Results
Seventy-seven studies comprising 38906 hospitalized patients met inclusion criteria; 21468 from the US-Europe and 9740 from China. Overall prevalence of death [% (95% CI)] from COVID-19 was 20% (18–23%); 23% (19–27%) in the US and Europe and 11% (7–16%) for China. Of those that died, 85% were aged≥60 years, 66% were males, and 66%, 44%, 39%, 37%, and 27% had hypertension, smoking history, diabetes, heart disease, and chronic kidney disease (CKD), respectively. The case fatality risk [%(95% CI)] were 52% (46–60) for heart disease, 51% (43–59) for COPD, 48% (37–63) for chronic kidney disease (CKD), 39% for chronic liver disease (CLD), 28% (23–36%) for hypertension, and 24% (17–33%) for diabetes. Summary relative risk (sRR) of death were higher for age≥60 years [sRR = 3.6; 95% CI: 3.0–4.4], males [1.3; 1.2–1.4], smoking history [1.3; 1.1–1.6], COPD [1.7; 1.4–2.0], hypertension [1.8; 1.6–2.0], diabetes [1.5; 1.4–1.7], heart disease [2.1; 1.8–2.4], CKD [2.5; 2.1–3.0]. The prevalence of hypertension (55%), diabetes (33%), smoking history (23%) and heart disease (17%) among the COVID-19 hospitalized patients in the US were substantially higher than that of the general US population, suggesting increased susceptibility to infection or disease progression for the individuals with comorbidities.
Conclusions
Public health screening for COVID-19 can be prioritized based on risk-groups. Appropriately addressing the modifiable risk factors such as smoking, hypertension, and diabetes could reduce morbidity and mortality due to COVID-19; public messaging can be accordingly adapted.
Introduction
Coronavirus disease-19 (COVID-19) caused by severe acute respiratory syndrome- coronavirus-2 (SARS-CoV-2) that first emerged in Wuhan, China in late December 2019 has spread with such rapidity and efficiency that in less than 10 months, it has caused more than 36 million cases and million deaths globally [1]. Driven by an urgency to solve the crisis, studies are being published at an unprecedented pace. However, across the publications, prevalence of death, severe disease and their association with epidemiological risk factors have greatly varied [2, 3], with studies showing conflicting results for association of key risk factors such as sex [4–8], smoking [9–12], hypertension [4, 7, 8, 13, 14] and diabetes [4, 7, 8, 13, 14] with COVID-19 disease severity and death. Whether or how cardiovascular risk factors, especially prior hypertension, diabetes and heart disease are associated with acquisition of SARS-CoV-2 and progression to severe disease or death is not understood well [15–18]. Meta-analyses conducted so far on prevalence of epidemiological risk factors and association with disease progression were mostly based on studies from China [9, 11, 18–20] and many of the analyses on prevalence estimates included studies focused on critically ill patients [9, 19], which can overestimate the prevalence and affect generalizability of results. To our knowledge, none of the analyses were restricted to hospitalized COVID-19 patients. Restricting our analysis to hospitalized patients provides an efficient sampling frame to investigate disease progression in relation to risk factors.
Therefore, we undertook a comprehensive systematic review and meta-analysis to investigate the association between key epidemiological factors–age, gender, smoking, hypertension, diabetes, heart disease, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD) and chronic liver disease (CLD)–and progression to death and severe disease in patients hospitalized due to COVID-19. We additionally compared the 1) the prevalence of risk factors and death in the US-Europe with that of China; 2) the prevalence of co-morbidities at baseline with the general population prevalence, and 3) prevalence of cardiovascular disease, COPD and CKD at baseline with corresponding organ injuries (acute cardiac injury, acute lung injury, and acute kidney injury) during hospital admission.
Methods
Literature search, study selection and data abstraction
We searched Medline, Embase, Web of Science and the WHO COVID-19 database to identify studies published through August 31, 2020 that investigated the risk of severe disease or death in hospitalized patients with confirmed COVID-19 disease. We used search terms, ‘coronavirus disease 19’, ‘COVID-19’, ‘severe acute respiratory syndrome coronavirus 2’ and ‘SARS-CoV-2’ for COVID-19 and the string ((characteristics) OR (risk factors) OR (epidemiology) OR (prevalence) OR (intensive care) OR (ventilator) OR (mechanical ventilator) OR (mortality) OR (survivor*) OR (smoking) OR (smoker*)) AND ((COVID-19) OR (COVID) OR (coronavirus)) for studies published between December 15, 2019 and August 31, 2020. We started the search on March 18, 2020 with biweekly search thereafter and final search on August 31, 2020. We included case series and observational studies that described the prevalence of death or severe disease in adult population stratified by risk factors: age, sex, hypertension, diabetes, heart disease, COPD, CKD and CLD. We excluded studies that included non-consecutive patients or exclusively focused on pregnant women, children, and elderly patients. We excluded studies that exclusively studied critically ill patients from calculation of prevalence of death but included them for calculating the association of risk factors with death. Screening of abstracts and full-text reviews were conducted using Covidence (Melbourne, Australia).
Risk factors and outcomes
Primary outcomes were prevalence of death and association of risk factors with death. We extracted data on death as recorded in the publications. We measured prevalence of severe disease and association with risk factors as secondary outcomes. We defined outcome as severe disease for any of 1) the study classified COVID-19 disease as severe or critical, 2) intensive care unit (ICU) admission, 3) acute respiratory distress syndrome, or 4) mechanical ventilation. Severe disease was defined by studies as respiratory rate≥30 per minute, oxygen saturation≤93%, and PaO2/FiO2<300 and/or lung infiltrates>50% within 24–48 hours [3]. Critical illness was defined as respiratory failure, shock and/or multiple organ dysfunction or failure [3]. Heart disease as a pre-existing condition was broadly defined by most studies as ‘cardiovascular disease’ (CVD). Additional outcomes were acute cardiac and kidney injury in the hospitalized patients that were defined as such by the studies.
Statistical analysis
We calculated and reported summary estimates from fixed-effects models [21]. We assessed heterogeneity across studies using Cochran’s Q-test (χ2 p value <0.10) [22] and I2 statistics (I2 >30%) [23]. In the presence of heterogeneity, we adjusted the confidence intervals for between-study heterogeneity using the method described by Shore et al. [24]. We presented the results from random effects meta-analysis as well. The meta-analysis was performed in Microsoft® Excel 2020 (Microsoft Corporation, Redmond, WA). We analyzed publication bias using funnel plots and Egger’s tests. Quality of each study was assessed using the Newcastle-Ottawa assessment scales using the PRISMA guidelines. We calculated the following as a part of our analyses: 1) prevalence of severe disease or death, 2) prevalence of risk factors, and 3) relative risk for the association of age, sex, and comorbidities with outcome. When not reported or when unadjusted odds ratio was presented, we calculated the relative risk (95% CI) using the frequencies provided. Adjusted estimates were used where available. Case fatality risk (and case severity risk) for a specific risk factor was calculated as number of deaths (or severe disease) in patients with a risk factor out of all patients possessing the risk factor.
Results
Study characteristics
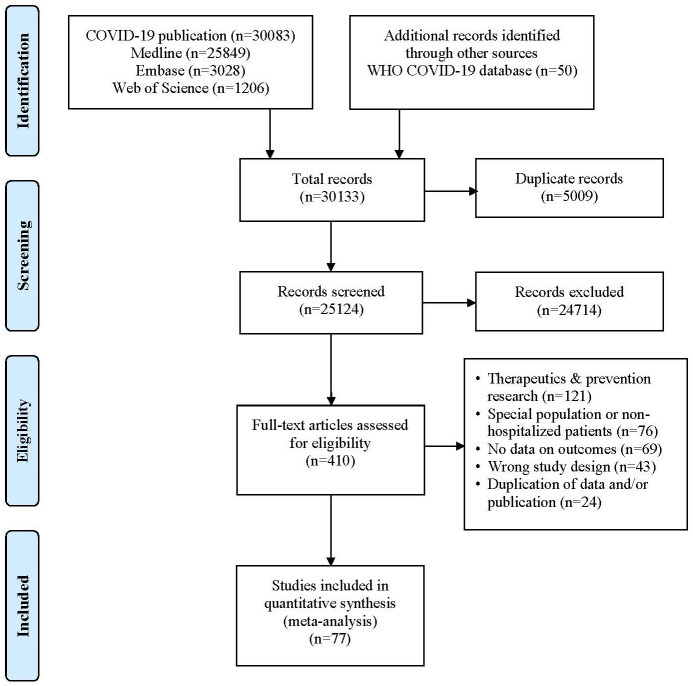
Initial search yielded 30133 citations. Articles were then screened (Fig 1). We identified 410 articles for full text review, of which 77 studies met inclusion criteria (Table 1) [4–8, 13, 14, 25–94]. The studies were conducted in: China (n = 35), USA (n = 18), Europe (n = 10), rest of Asia (n = 5) and Africa (n = 1). Two studies were prospective, five cross-sectional, and remaining retrospective in nature.
Fig 1. PRISMA flow diagram for selection of studies.
Table 1. Characteristics of studies to determine prevalence of risk factors and death or severe disease and their associations in patients hospitalized for COVID-19 globally.
| Author, year of publication (journal) | Country | Region | Study Period | Study Design | Size | Epidemiological Risk Factor | Outcome | Measures of Association |
|---|---|---|---|---|---|---|---|---|
| Aggarwal S et al., 2020 (Diagnosis) | USA | Des Moines | 3-1-2020 to 4-4-2020 | Retrospective | 16 | Age, sex, smoking, substance use, obesity, HTN, DM, CVD, COPD, CKD, Cancer | Prevalence of death and primary end point (death, shock, or ICU admission). Association of risk factors with outcome | Unadjusted RR calculated |
| Argenziano M. G et al., 2020 (BMJ) | USA | New York City | 3-11-2020 to 4-6-2020 | Retrospective | 1,000 | Age, sex, ethnicity, obesity, smoking, HTN, DM, CVD, COPD, CKD, cancer, HIV, viral hepatitis, cirrhosis | Association of risk factors with disease severity and death. | Adjusted HR |
| Brill S. E et al., 2020 (BMC Medicine) | UK | Barnet | 3-10-2020 to 4-8-2020 | Retrospective | 450 | Age, race, sex, smoking, HTN, DM, CVD, immunosuppression | Prevalence of death. | Unadjusted RR calculated |
| Association of comorbidities with disease severity. | ||||||||
| Cao Z et al., 2020 (PLOS ONE) | China | Beijing | 1-21-2020 to 2-12-2020 | Retrospective | 80 | Sex, age, HTN, CVD, DM, COPD, smoking | Association of risk factors with disease severity. | Unadjusted RR calculated |
| CDC (MMWR) | USA | National | 2-12-2020 to 3-28-2020 | Retrospective | 5285 | Age, Current Smoker, DM, CVD, COPD, CKD, CLD | Prevalence of ICU admission. Association of risk factors with severe disease (ICU admission). | Unadjusted RR calculated |
| Chen G et al., 2020 (Journal of Clinical Investigation) | China | Wuhan | December 2019 to 01-27-2020 | Retrospective | 21 | Age, sex, Huanan sea food market exposure, HTN, DM | Prevalence of severe disease. Compared moderate and severe cases based on risk factors. | Unadjusted RR calculated |
| Chen J et al., 2020 (Journal of Infection) | China | Shanghai | 1-20-2020 to 2-6-2020 | Retrospective | 249 | Age, sex | Prevalence of ICU admission. Association of age and sex with ICU admission. | Adjusted OR reported for age and sex |
| Chen Q et al., 2020 (Infection) | China | Zhejiang province | 1-1-2020 to 3-11-2020 | Retrospective | 145 | Age, sex, smoking, exposure history, BMI, HTN, DM, COPD, CKD, Solid tumor, Heart disease, HIV infection | Prevalence of severe disease. Association of risk factors with severe disease. | Unadjusted RR calculated |
| Chen T et al., 2020 (BMJ) | China | Wuhan, Hubei | 1-13-2020 to 2-28-2020 | Retrospective | 274 | Age, sex, sea food market exposure, contact history, smoking HTN, DM, CVD, CHF, heart failure, cancer, HBV, HIV, CKD | Association of risk factors with death. | Unadjusted RR calculated |
| Compared death and recovered group. Presently hospitalized patients excluded from study. | ||||||||
| Chilimuri S et al., 2020 (West J Emerg Med) | USA | New York City | 3-9-2020 to 4-9-2020 | Retrospective | 375 | Age, sex, ethnicity, HTN, DM, CVD, COPD, CKD, HIV/AIDS, CLD | Association of risk factors with disease severity and death. | Adjusted OR |
| reported for age, sex and comorbidities | ||||||||
| Ciceri F et al., 2020 (Clinical Immunology) | Italy | Milan | 2-25-2020 to 5-1-2020 | Retrospective | 410 | Age, sex, ethnicity, BMI, HTN, CVD, DM, COPD, CKD, cancer | Prevalence of death. | Adjusted HR |
| Association of risk factors with disease severity. | ||||||||
| Cummings MJ et al., 2020 (The Lancet) | USA | New York City | 3-2-2020 to 4-1-2020 | Prospective | 257 | Age, sex, race, BMI, HTN, DM, chronic cardiac disease (CHD and CHF), CKD, smoking history, COPD, cancer, HIV, cirrhosis | Association of risk factors with death. | Adjusted HR |
| Deng Y et al., 2020 (Chin Med J) | China | Wuhan | 1-1-2020 to 2-21-2020 | Retrospective | 116 out of 964 | Age, sex, HTN, DM, Heart Disease, Cancer | Association of risk factors with death. | Unadjusted RR calculated |
| Compared death and recovered group. | ||||||||
| Presently hospitalized patients excluded from study. | ||||||||
| Du R-H et al., 2020 (ERJ) | China | Wuhan, Hubei | 1-25-2020 to 2-7-2020 | Retrospective | 179 | Age, sex, HTN, DM, CVD, TB, cancer, CKD or CLD | Prevalence of death. Association of risk factors with death. | Adjusted OR for age≥65 and CVD. Unadjusted RR calculated for other variables |
| Escalera-Antezana et al., 2020(Infez Med) | Bolivia | Nationwide | 3-2-2020 to 3-29-2020 | Retrospective | 107 | Age, HTN, CVD, DM, obesity, sex | Prevalence of death. | Adjusted OR |
| Association of risk factors with disease severity. | reported for age, sex and risk factors | |||||||
| Feng Y et al., 2020 (AJRCCM) | China | Wuhan, Shanghai, Anhui province | 1-1-2020 to 2-15-2020 | Retrospective | 476 | Age, age groups, sex, Wuhan exposure, smoking, alcohol, HTN, anti-hypertensives, CVD, DM, cancer, COPD, CKD | Prevalence of death. Association of risk factors with severe disease. | Adjusted HR for HTN, CVD, DM. Unadjusted RR calculated for other variables |
| Ferguson J et al., 2020 (EID) | USA | Northern California | 03-13-2020 to 04-11-2020 | Retrospective | 72 | Sex, race, smoking, HTN, DM, CKD, Heart Disease, COPD | Prevalence of ICU admission. Association of risk factors with severe disease (ICU admission). | Unadjusted RR calculated |
| Galloway J.B et al., 2020 (Journal of Infection) | UK | London | 3-1-2020 to 4-17-2020 | Retrospective | 1,157 | Age, sex, ethnicity, cancer, CKD, DM, HTN, CVD, COPD | Prevalence of death. | Adjusted HR reported for age and sex |
| Association of risk factors with disease severity. | ||||||||
| Garibaldi B et al., 2020 (Ann Intern Med) | USA | Maryland | 3-4-2020 to 6-27-2020 | Retrospective | 832 | Age, sex, alcohol, smoking, BMI, cancer, CVD, COPD, HTN, liver disease, CKD, HIV/AIDS DM | Association of risk factors with disease severity. | Adjusted HR |
| Washington DC | ||||||||
| reported for age, ethnicity and BMI | ||||||||
| Giacomelli A et al., 2020 (Pharmacol Res) | Italy | Milan | 2-21-2020 to 4-20-2020 | Prospective | 233 | Sex, age, smoking, obesity | Prevalence of death. | Adjusted HR |
| Association of risk factors with disease severity. | reported for sex, age, and obesity | |||||||
| Gold J et al, 2020 (MMWR) | USA | Georgia | 3-1-2020 to 3-30-2020 | Retrospective | 305 | Age, sex, race, HTN, DM, Heart Disease, COPD, CKD, Cancer | Prevalence of patient characteristics, death, and ICU. | Unadjusted RR calculated |
| Goyal P et al. 2020 (NEJM) | USA | New York City | 3-3-2020 to 3-27-2020 | Retrospective | 393 | Age, sex, race, smoking, HTN, DM, COPD, Heart Disease, Asthma | Prevalence of severe disease (mechanical ventilation). Association of risk factors with severe disease. | Unadjusted RR calculated |
| Gregoriano C et al.,2020 (Swiss Medical Weekly) | Switzerland | Aarau | 2-26-2020 to 4-30-2020 | Retrospective | 99 | Age, sex, smoking, HTN, cancer, CVD, COPD, obesity, DM, rheumatic disease, organ transplant recipient, obstructive sleep apnea | Prevalence of disease endpoints (transfer to ICU and in-hospital mortalities). | Unadjusted OR |
| Association of comorbidities with disease endpoints. | ||||||||
| Guan et al., 2020 (NEJM) | China | Nationwide | 12-11-2019 to 01-31-2020 | Retrospective | 1099 | Age, sex, smoking, exposure to transmission source, HTN, DM, CHD, CKD, COPD, Cancer, HBV, cerebrovascular disease, immunodeficiency | Prevalence of death, composite outcome, ((Death/MV/ICU) and severe disease. Association with severe disease and composite outcome. | Unadjusted RR calculated |
| Guan Wei-Jie, 2020(ERJ) | China | Nationwide | 12-11-2019 to 1-31-2020 | Retrospective | 1590 | Age, sex, smoking, CKD, COPD, HTN, DM, CVD, Cancer, HBV | Prevalence of patient characteristics, death and composite outcome (Death, ICU, MV). | Adjusted HR |
| Hewitt J et al., 2020 (Lancet) | UK | Nationwide (UK), | 2-27-2020 to 4-28-2020 | Prospective | 1,564 | Age, sex, smoking, DM, HTN, CVD, CKD | Prevalence of death. | Adjusted HR |
| Italy | Modena (Italy) | Association of risk factors with disease severity. | ||||||
| Hsu H. E et al., 2020 (Morbidity and Mortality Weekly Report) | USA | Boston | 3-1-2020 to 5-18-2020 | Retrospective | 2,729 | Age, sex, ethnicity, COPD, cancer, CKD, cirrhosis, CVD, DM, HIV/AIDS, HTN, obesity, substance use disorder | Association of risk factors with disease severity. | Unadjusted RR calculated |
| Hu L et al., 2020 (CID) | China | Wuhan | 1-8-2020 to 2-20-2020 | Retrospective | 323 | Age, sex, current smoker, HTN, DM, CVD, COPD, CKD, CLD, Cancer | Prevalence of severe (severe and critical) disease. Association of risk factors with disease severity. | Unadjusted RR calculated |
| Huang C et al., 2020 (The Lancet) | China | Wuhan | 12-16-2020 to 1-2-2020 | Prospective | 41 | Age, sex, Huanan seafood market exposure, smoking, HTN, DM, CKD, COPD, CVD, Cancer, CLD | Association of risk factors with severe disease (ICU care). | Unadjusted RR calculated |
| Hur K et al., 2020 (Otolaryngol Head Neck Surg) | USA | Chicago | 3-1-2020 to 4-8-2020 | Retrospective | 486 | Age, sex, BMI, smoking, HTN, DM, CVD, COPD, cancer, immunosuppression, CKD, | Association of risk factors with disease severity. | Adjusted HR (for age, sex, ethnicity BMI, HTN, smoking) |
| Iaccarino G et al., 2020 (Hypertension) | Italy | Nationwide | 3-9-2020 to 4-9-2020 | Cross-sectional | 1,591 | Age, sex, HTN, obesity, DM, COPD, CKD, CVD, cancer | Prevalence of death. | Adjusted OR |
| Association of risk factors with disease severity. | ||||||||
| Inciardi R et el., 2020 (Eur Heart J) | Italy | Lombardy | 3-4-2020 to 3-25-2020 | Retrospective | 99 | Sex, smoking, HTN, DM, coronary artery disease, COPD, CKD, cancer | Prevalence of death. Association of risk factors with death. | Unadjusted RR calculated |
| Jang J.G et al., 2020 (Journal of Korean Medical Science) | South Korea | Daegu | 2-19-2020 to 4-15-2020 | Retrospective | 110 | Age, sex, CVD, cerebrovascular disease, COPD, dementia, DM, HTN, connective tissue disease liver disease, malignancy, Parkinson’s disease | Association of risk factors with disease severity and death. | Adjusted OR |
| Javanian M et al., 2020 (Rom J Intern Med) | Iran | Mazandaran province | 2-25-2020 to 3-12-2020 | Retrospective | 100 | Age, sex, HTN, DM, CVD, CKD, cancer, CLD | Prevalence of death. Association of risk factors with death. | Unadjusted RR calculated |
| Kalligeros M et al., 2020 (Obesity Journal) | USA | Rhode Island | 2-17-2020 to 4-5-2020 | Retrospective | 103 | Age, sex, ethnicity, smoking, BMI (obesity), cancer, CKD, cirrhosis, DM, heart disease (CVD), HTN, lung disease (COPD), transplant | Association of risk factors with disease severity. | Adjusted OR |
| (for age, sex, ethnicity, BMI, DM, HTN, heart disease, lung disease) | ||||||||
| Khalil K et al., 2020 (Journal of Infection) | UK | London | 3-7-2020 to 4-7-2020 | Prospective | 220 | Age, sex, ethnicity, smoking, COPD, CVD, HTN, hyperlipidemia, DM, CKD, CVA, dementia, liver disease, cancer | Prevalence of death. | Unadjusted RR calculated |
| Association of risk factors with disease severity. | ||||||||
| Khamis F et al., 2020 (Journal of Infection and Public Health) | Oman | Muscat | 2-24- 2020 to 4-24-2020 | Retrospective | 63 | Age, sex, smoking, substance use, HTN, DM, CKD, CVD | Prevalence of severe disease and death. | Unadjusted RR calculated |
| Association of risk factors with disease severity. | ||||||||
| Lendorf M.E et al., 2020 (Danish Medical Journal) | Denmark | North Zealand | 3-1-2020 to 5-18-2020 | Retrospective | 111 | Age, sex, BMI, cancer, HTN, CVD, COPD, immunosuppression, CKD, DM, smoking | Association of risk factors with disease severity and death. | Unadjusted RR calculated |
| Li X et al., 2020 (J Allergy Clin Immunol) | China (Wuhan, Hubei) | Wuhan, Hubei | 1-26-2020 to 2-5-2020 | Retrospective | 548 | Age, sex, smoking, HTN, DM, Heart Disease, CKD, Cancer, COPD | Prevalence of death and severe disease. | Unadjusted RR calculated |
| Association of risk factors with severe disease. | ||||||||
| Liu S et al., 2020 (BMC Infectious Diseases) | China | Jiangsu Province | 1-10-2020 to 3-15-2020 | Retrospective | 625 | Sex, age, HTN, DM, CVD | Association of risk factors with disease severity. | Adjusted OR |
| (for age and HTN) | ||||||||
| Liu W et al. 2020 (Chin Med J) | China | Wuhan | 12-30-2019 to 01-15-2020 | Retrospective | 78 | Age, sex, smoking history, exposure to Huanan seafood market, HTN, diabetes, COPD, cancer | Compared progression group and stabilization group. Progression group defined by progression to severe or critical disease or death. | Unadjusted RR calculated |
| Nikpouraghdam M et al., 2020 (J Clin Virol) | Iran | Tehran | 2-19-2020 to 4-15-2020 | Retrospective | 2,964 | Age, sex, DM, COPD, HTN, CVD, CKD, cancer | Prevalence of death. | Adjusted OR |
| Association of risk factors with disease severity. | ||||||||
| Nowak B et al., 2020 (Pol Arch Intern Med) | Poland | Warsaw | 3-16-2020 to 4-7-2020 | Retrospective | 169 | Sex, smoking, HTN, DM, CVD, COPD, CKD, AKI, cancer | Prevalence of death. Association of risk factors with death. | Unadjusted RR calculated |
| Okoh A.K et al., 2020 (Int J Equity Health) | USA | Newark | 3-10-2020 to 4-20-2020 | Retrospective | 251 | Age, sex, ethnicity, BMI, HTN, DM, CVD, COPD, HIV, CKD, cancer | Prevalence of death. | Adjusted OR |
| Association of risk factors with disease severity and death. | ||||||||
| Palaiodimos L et al., 2020 (Metabolism) | USA | New York | 3-9-2020 to 3-22-2020 | Retrospective | 200 | Age, sex, race, smoking, HTN, DM, coronary artery disease, COPD, CKD, cancer | Prevalence of death. Association of risk factors with death. | Adjusted OR (provided by the study) |
| Pellaud C et al., 2020 (Swiss Medical Weekly) | Switzerland | Fribourg | 3-1-2020 to 5-10-2020 | Retrospective | 196 | Sex, age, HTN, DM, obesity, CVD, COPD, cancer, immunosuppression, smoking | Prevalence of death. | Unadjusted RR calculated |
| Association of risk factors with disease severity. | ||||||||
| Richardson S et al., 2020 (JAMA) | USA | New York | 3-1-2020 to 4-4-2020 | Retrospective | 5700 | Age, sex, race, smoking, HTN, DM, COPD, asthma, coronary artery disease, kidney disease, liver disease, obesity, cancer | Prevalence of ICU admission and death. | Unadjusted RR calculated |
| Association of risk factors with death. | ||||||||
| Rivera-Izquierdo M et al., 2020 (PLOS ONE) | Spain | Granada | 3-16-2020 to 4-10-2020 | Retrospective | 238 | Sex, age, smoking, HTN, DM, CVD, COPD, CKD, active neoplasia, medications | Prevalence of death. | Adjusted HR |
| Association of risk factors with disease severity. | ||||||||
| Shabrawishi M et al., 2020 (Plos One) | Saudi Arabia | Mecca | 3-12-2020 to 4-8-2020 | Retrospective | 150 | Age, sex, HTN, DM, CVD, CKD, hypothyroidism, cancer, CVA, COPD, CLD | Association of risk factors with disease severity and death. | Unadjusted RR calculated |
| Shahriarirad R et al., 2020 (BMC Infectious Diseases) | Iran | Fars Province | 2-20-2020 to 3-20-2020 | Multicenter Retrospective | 113 | Age, sex, HTN, DM, CVD, COPD, CKD, malignancy, other immunosuppressive diseases | Prevalence of death. | Unadjusted RR calculated |
| Association of risk factors with disease severity. | ||||||||
| Shekhar R et al., 2020 (Infectious Diseases) | USA | New Mexico | 1-19-2020 to 4-24-2020 | Cohort | 50 | Age, sex, HTN, DM, COPD, alcoholic cirrhosis, alcohol use, obesity | Association of risk factors with disease severity. | Unadjusted RR calculated |
| Shi Y et al., 2020 (Crit Care) | China | Zhejiang province | Not specified to 02-11-2020 | Retrospective | 487 | Age, sex, smoking, HTN, DM, CKD, CVD, CLD, cancer | Prevalence of and association of risk factors with severe disease | Unadjusted RR calculated |
| Suleyman G et al., 2020 (JAMA Network) | USA | Metropolitan Detroit | 3-9-2020 to 3-27-2020 | Retrospective | 463 | Age, sex, ethnicity, COPD, obstructive sleep apnea, DM, HTN, CVD, CKD, cancer, rheumatologic disease, organ transplant, obesity, smoking | Association of risk factors with disease severity. | Adjusted OR |
| Sun L et al., 2020 (Journal of Medical Virology) | China | Beijing | 1-20-2020 to 2-15-2020 | Retrospective | 55 | Age, sex, exposure, HTN, DM, CVD, Lung Disease, CKD, CLD | Prevalence of severe disease. Association of risk factors with severe disease. | Unadjusted RR calculated |
| Tambe M et al., 2020 (Indian J Public Health) | India | Pune | 3-31-2020 to 4-24-2020 | Cross-Sectional | 197 | Age, sex, HTN, DM, COPD, CVS, ALD, CKD | Association of risk factors with disease severity and death. | Unadjusted RR calculated |
| Tian S et al., 2020 (Journal of Infection) | China | Beijing | 1-20-2020 to 2-10-2020 | Retrospective | 262 | Age, sex, contact history, exposure to Wuhan. | Prevalence of death. Association of severe disease with risk factors. | Unadjusted RR calculated |
| Tomlins J et al., 2020 (Journal of Infection) | UK | Bristol | 3-10-2020 to 3-30-2020 | Retrospective | 95 | Age, sex, HTN, DM, COPD, CVD, cancer, renal disease, gastrointestinal disease, neurological disease | Prevalence of death. Association of risk factors with death. | Unadjusted RR calculated |
| Turcotte J.J et al., 2020 (PLOS ONE) | USA | Maryland | 3-1-2020 to 4-12-2020 | Retrospective | 117 | Age, BMI, sex, DM, obstructive sleep apnea, COPD, CVD, CKD, HTN, smoking, alcohol use, liver disease | Association of risk factors with disease severity and death. | Adjusted OR |
| Wan S et al., 2020 (Journal of Medical Virology) | China | Northeast Chongqing | 1-23-2020 to 2-8-2020 | Retrospective | 135 | Age, sex, smoking, CKD, COPD, HTN, DM, CVD, Cancer, CLD, exposure, travel history | Prevalence of severe disease. Association of risk factors with severe disease. | Unadjusted RR calculated |
| Wang D et al., 2020 (JAMA) | China | Wuhan | 1-1-2020 to 1-28-2020 | Retrospective | 138 | Age, sex, Huanan Seafood Market Exposure, HTN, DM, CVD, COPD, Cancer, CKD, CLD, HIV | Prevalence of death and ICU admission. | Unadjusted RR calculated |
| Association of risk factors with severe disease (ICU care) | ||||||||
| Wang R et al., 2020 (Internal Journal of Infectious Diseases) | China | Fuyang | 1-20-2020 to 02-09-2020 | Retrospective | 125 | Age, sex, CVD, Cancer | Prevalence of critical disease. Association of age, sex, and smoking with critical disease. | Unadjusted RR calculated |
| Wang Z et al., 2020 (CID) | China | Wuhan | 1-16-2020 to 01-29-2020 | Retrospective | 69 | Age, sex, HTN, DM, CVD, COPD, Cancer, HBV, Asthma | Prevalence of death and severe disease (SpO2<90%). Association of risk factors with severe disease. | Unadjusted RR calculated |
| Wei Y et al., 2020 (BMC Infectious Diseases) | China | Hubei Province | 1-27-2020 to 3-22-2020 | Retrospective | 276 | Age, sex, smoking, obesity, HTN, COPD, CVD, DM, cerebrovascular disease, cancer | Association of risk factors with disease severity. | Unadjusted RR calculated |
| Wu C et al., 2020 (JAMA Intern Med) | China | Wuhan | 12-15-2019 to 01-26-2020 | Retrospective | 201 | Age, sex, HTN, DM, CVD, CKD, Chronic Lung Disease, Cancer, CLD, Sea Food Market Exposure. | Prevalence of ARDS, ICU admission and death. Association of risk factors with severe disease (ARDS) and death. | Unadjusted RR calculated |
| Yang X et al, 2020 (Lancet Respir Med) | China | Wuhan | 12-24-2019 to 1-26-2020 | Retrospective | 52 | Age, sex, exposure, COPD, diabetes, chronic cardiac disease, smoking, malnutrition | Association of risk factors with death. | Unadjusted RR calculated |
| Yao Q et al., 2020 (Pol Arch Intern) | China | Huanggang, Hubei | 1-30-2020 to 2-11-2020 | Retrospective | 108 | Age, sex, smoking, HTN, DM, CVD, CLD, cancer | Prevalence of severe disease and death. | Unadjusted RR calculated |
| Association of risk factors with severe disease and death. | ||||||||
| Young BE et al., 2020 (JAMA) | Singapore | Singapore | 1-23-2020 to 2-3-2020 | Retrospective | 18 | Age, sex | Prevalence of severe disease (receiving supplemental O2). Association of severe disease with age and sex. | Unadjusted RR calculated |
| Yu T et al., 2020 (Clinical Therapeutics) | China | Guangdong | January to February 2020 | Cross-sectional | 95 | Age, sex, current smoker | Prevalence of ARDS. | Unadjusted RR calculated |
| Association of age, sex, and smoking with ARDS. | ||||||||
| Yu X et al., 2020 (Transboundary and Emerging Diseases) | China | Shanghai | Up to 2-19-2020 | Retrospective | 333 | Age, sex, BMI, smoking, alcohol, exposure, HTN, DM, CVD | Prevalence of death and severe disease (Severe/critical pneumonia). Association of risk factors with severe disease. | Adjusted OR for age group, sex, CVD, DM, HTN. |
| Zhan T et al., 2020 (J Int Med Res) | China | Wuhan | 1-12-2020 to 3-8-2020 | Retrospective | 405 | Age, sex, smoking, alcohol history, CVD, gastrointestinal disease, COPD, CKD, CLD | Association of risk factors with disease severity. | Unadjusted RR calculated |
| Zhang G et al., 2020 BMC Respiratory Research) | China | Wuhan | 1-16-2020 to 2-25-2020 | Retrospective | 95 | Age, sex | Prevalence of severe disease, composite end point, and death. Association with severe disease. | Unadjusted RR calculated |
| Zhang J et al., 2020 (Clin Microbiol Infect) | China | Wuhan | 1-11-2020 to 2-6-2020 | Retrospective | 663 | Age, sex, COPD, CVD, gastrointestinal disease, CKD, cancer | Prevalence of death. | Adjusted OR |
| Association of risk factors with disease severity. | ||||||||
| Zhang JJ et al., 2020 (Allergy) | China | Wuhan | 1-16-2020 to 2-3-2020 | Retrospective | 140 | Age, sex, current smoker, past smoker, exposure history, HTN, DM, CVD, COPD, CKD, CLD | Prevalence of severe disease. Association of risk factors with severe disease (ICU admission). | Unadjusted RR calculated |
| Zhao X-Y et al., 2020 (BMC Inf Dis) | China | Hubei (Non-Wuhan) | 1-16-2020 to 2-10-2020 | Retrospective | 91 | Age, sex, DM, COPD, Cancer, Kidney disease | Prevalence of death. Association of risk factors with severe disease | Unadjusted RR calculated |
| Zheng S et al., 2020 (BMJ) | China | Zhejiang province | 1-19-2020 to 2-15-2020 | Retrospective | 96 | Age, sex, HTN, DM, CVD, lung disease, Liver disease, renal disease, malignancy, viral Load, immunocompromise | Prevalence of death and severe disease. | Unadjusted RR calculated |
| Association of risk factors with severe disease. | ||||||||
| Zheng Y et al., 2020 (Pharmacological Research) | China | Shiyan, Hubei | 1-16-2020 to 2-4-2020 | Retrospective | 73 | Age, sex, exposure, smoking history, DM, CVD | Prevalence of severe (severe/ critical) disease. Association of smoking and diabetes with severe disease. | Unadjusted RR calculated |
| Zhou F et al., 2020 (The Lancet) | China | Wuhan | 12-29-2019 to 1-31-2020 | Retrospective | 191 | Age, sex, current smoking, exposure history, HTN, DM, CVD, COPD, cancer, CKD | Prevalence of severe disease (ICU admission) and death. Association of risk factors with death. | Adjusted OR for age and CVD. Unadjusted RR calculated for other variables. |
CVD, cardiovascular disease; CKD, chronic kidney disease; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; HTN, hypertension; DM, diabetes mellitus; ICU, intensive care unit; BMI, body mass index; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; RR, relative risk; HR, hazard ratio; OR, odds ratio.
Population and demographics
There were 38,906 total COVID-19 hospitalized patients including 21468 patients from the US and Europe (87% from the US), and 9740 patients from China. Median age was 59 years [IQR: 57–62 years; I2 = 58%; n = 62 studies] and 48% [95% CI: 44–53%; I2 = 98%; n = 41] were aged≥60. Fifty-nine percent [95% CI: 57–60%; I2 = 98%; n = 75] of the patients were males.
Prevalence of death and severe disease
We calculated an overall prevalence of death of 20% [95% CI: 18–23%; I2 = 96%; n = 60], ranging from 1% to 38% across the studies, and of severe disease of 28% [95% CI: 24–33%; I2 = 98%; n = 60] for all patients hospitalized due to COVID-19 (Tables 2 and 3). Data on prevalence of death, severe disease, and risk factors (S1 Table), and association of the risk factors with death (S2 Table) and severe disease (S3 Table) for the individual studies are presented in the supplemental tables.
Table 2. Pooled prevalence of death stratified by epidemiological risk factors in COVID-19 patients hospitalized between December 2019-August 2020.
| Risk factor or Outcome | Overall prevalence of risk across studies | Pooled Prevalence of Death (Case Fatality Risk) and Risk Factor | Summary Relative Risk of Death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Pooled prevalence of risk factor and death, | No. of studies | *Case fatality risk (Prevalence of death in risk group), | #Prevalence of risk factor in persons who died, | No. of studies | Fixed Effects | Random Effects# | Heterogeneity | |
| Summary relative risk; 95% CI (Shore adjusted) | sRR; (95% CI) | I2; c2; p value | |||||||
| % (95% CI) | % (95% CI) | % (95% CI) | |||||||
| Death | 60 | 20 (18–23) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Age ≥ 60 years | 41 | 48 (44–53) | 18 | 35 (28–43) | 85 (80–89) | 24 | 3.61 (2.96–4.39) | 1.29 (1.03–1.62) | 77%; 99; p<0.01 |
| Male | 75 | 59 (57–60) | 31 | 26 (21–32) | 66 (64–69) | 36 | 1.31 (1.22–1.40) | 1.34 (1.24–1.45) | 18%; 43; p = 0.17 |
| Smoking history | 41 | 26 (22–31) | 11 | 27 (24–32) | 44 (38–50) | 13 | 1.28 (1.06–1.55) | 1.41 (1.12–1.78) | 68%; 37; p<0.01 |
| Current smoker | 21 | 10 (7–13) | 7 | 21 (14–29) | 13 (7–24) | 8 | 1.43 (91–2.26) | 1.53 (95–2.45) | 78%; 32; p<0.01 |
| COPD | 52 | 9 (8–11) | 20 | 51 (36–71) | 12 (7–19) | 22 | 1.70 (1.42–2.04) | 1.74 (1.43–2.13) | 66%; 61; p<0.01 |
| Hypertension | 64 | 50 (46–54) | 29 | 28 (23–36) | 66 (61–70) | 32 | 1.76 (1.58–1.96) | 1.88 (1.66–2.13) | 56%; 70; p<0.01 |
| Diabetes | 67 | 28 (25–31) | 29 | 24 (17–33) | 39 (35–44) | 33 | 1.50 (1.35–1.66) | 1.60 (1.42–1.79) | 58%; 77; p<0.01 |
| Cardiovascular disease | 65 | 17 (15–20) | 29 | 52 (46–60) | 37 (32–43) | 34 | 2.08 (1.81–2.39) | 2.25 (1.92–2.64) | 69%; 106; p<0.01 |
| Chronic kidney disease | 47 | 13 (11–16) | 18 | 48 (37–63) | 27 (21–34) | 23 | 2.52 (2.11–3.00) | 2.39 (1.91–2.99) | 72%; 79; p<0.01 |
| Chronic Liver Disease | 31 | 2(2–3) | 8 | 39(31–50) | 6 (4–8) | 9 | 2.65(1.88–3.75) | 1.99 (1.26–3.16) | 77%; 35; p<0.01 |
*Case fatality risk of represent total number of people that died in the specific risk group divided by total population in the risk group.
# Prevalence of risk group in dead represent total number of people having the risk group divided by total population that died.
Table 3. Pooled prevalence of severe disease stratified by epidemiological risk factors in COVID-19 patients.
| Risk group or outcome | Prevalence of Severe Disease (Case Severity Risk) and Risk Factors | Summary Relative Risk of Severe Disease | |||||
|---|---|---|---|---|---|---|---|
| No. of studies | Prevalence of severe disease and case severity risk*, % (95% CI) | *Prevalence of risk factor in people with severe disease, % (95% CI) | No. of studies | Fixed Effects | Random Effects# | Heterogeneity | |
| sRR; 95% CI (Shore adjusted) | sRR; (95% CI) | I2; c2; p value | |||||
| Severe disease | 25 | 20 (16–25) | N/A | N/A | N/A | N/A | N/A |
| Age ≥ 60 years | 26 | 48 (39–59) | 56 (52–61) | 29 | 1.57 (1.36–1.80) | 1.76 (1.50–2.07) | 85%; 184; p<0.01 |
| Male | 45 | 40 (34–47) | 63 (61–66) | 47 | 1.26 (1.18–1.35) | 1.33 (1.22–1.44) | 38%; 75; p<0.01 |
| Smoking history | 27 | 39 (34–46) | 26 (21–32) | 27 | 1.29 (1.18–1.42) | 1.32 (1.18–1.47) | 33%; 39; p = 0.05 |
| Current smoker | 13 | 38 (28–53) | 13 (9–20) | 15 | 1.52 (1.21–1.91) | 1.25 (94–1.66) | 75%;56; p<0.01 |
| COPD | 24 | 43 (35–52) | 14 (12–17) | 29 | 1.71 (1.49–1.97) | 1.83 (1.54–2.18) | 84%;179; p<0.01 |
| Hypertension | 39 | 44 (37–53) | 55 (50–61) | 40 | 1.46 (1.28,1.65) | 1.54 (1.33,1.78) | 77%;168; p<0.01 |
| Diabetes | 43 | 43 (38–49) | 33 (30–38) | 44 | 1.48 (1.35–1.63) | 1.64 (1.47–1.82) | 59%;104; p<0.01 |
| Cardiovascular disease | 37 | 56 (48–65) | 28 (24–33) | 38 | 1.54 (1.39–1.72) | 1.74 (1.52–1.98) | 77%;164; p<0.01 |
| Chronic kidney disease | 22 | 36 (33–40) | 26 (19–37) | 27 | 1.56 (1.31–1.86) | 1.42 (1.15–1.76) | 85%; 176; p<0.01 |
| Chronic Liver Disease | 12 | 43(32–57) | 5 (3–7) | 15 | 1.63 (1.23–2.15) | 1.66 (1.16–2.36) | 82%; 76; p<0.01 |
*Case severity risk represent total number of people developing severe disease in the specific risk group divided by total population in that risk group.
# Prevalence of risk factor in severe disease represent total number of people with the risk factor divided by total population with severe disease.
Predictors of death and severe disease (Tables 2 and 3)
Age and sex
Median age for people who died was 79 years [IQR: 77–80; I2 = 89%; n = 28] and who had severe disease was 61 years [IQR: 59–63; I2 = 48%; n = 26]. Eighty-five percent [95% CI: 80–89; I2 = 76%; n = 18] of the deaths were in people aged ≥ 60 years and 66% [95% CI: 64–69; n = 34] were in males. The CFR (95% CI) was 35% (28–43%) for age≥60 years and 26% (21–32%) for males. Patients aged≥60 years [summary relative risk (sRR): 3.61; 95% CI: 2.96–4.39; I2 = 77%; n = 24] and males [sRR: 1.34; 95% CI: 1.22–1.40; I2 = 18%; n = 36] had higher risk of death. The risk of severe disease was similarly higher for age>60 [sRR: 1.57; 95% CI: 1.36–1.80; I2 = 85%; n = 29] and males [sRR: 1.26; 95% CI: 1.18–1.35; I2 = 38%; n = 47].
Hypertension
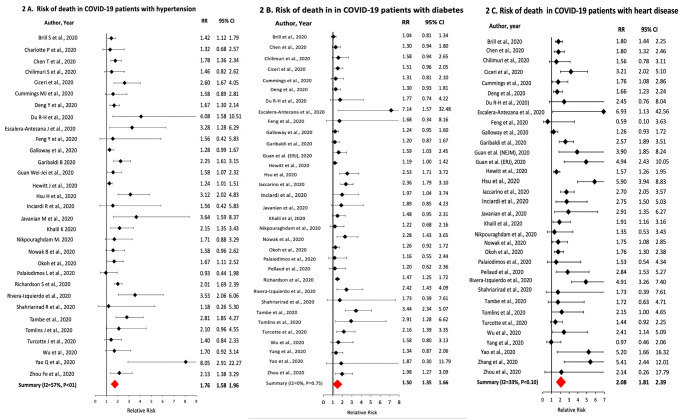
The prevalence of hypertension in the COVID-19 patients was 50% [95% CI: 46–54% I2 = 98%; n = 64], with a CFR in hypertensive patients of 28% [95% CI: 23–36%; I2 = 97%; n = 29] and a CSR of 44% [95% CI: 37–53%; I2 = 95%; n = 39]. Of the COVID-19 patients that died, 66% [95% CI: 61–70%; I2 = 83%; n = 29] had hypertension. Hypertensives had higher relative risk of death [sRR: 1.76; 95% CI: 1.58–1.96; I2 = 56%; n = 32] and severe disease [sRR: 1.46; 95% CI: 1.28–1.65; I2 = 77%; n = 40] compared to non-hypertensives (Fig 2A).
Fig 2. Association of hypertension, diabetes and heart disease with death in COVID-19 patients.
Diabetes
The prevalence of diabetes was 28% [95% CI: 25–31%; I2 = 97%; n = 67] with a CFR of 24% [95% CI: 17–33%; I2 = 98%; n = 29] and CSR of 43% [95% CI: 38–49%; I2 = 99%; n = 30] in the diabetics. Of the COVID-19 patients that died, 33% [95% CI: 32–44%; I2 = 83%; n = 29] were diabetics. Diabetics had higher relative risk of death [sRR: 1.50; 95% CI: 1.35–1.66; I2 = 58%; n = 33] and severe disease [sRR: 1.48; 95% CI: 1.35–1.63; I2 = 59%; n = 44] compared to non-diabetics (Fig 2B).
Cardiovascular disease
The pooled prevalence of CVD was 17% [95% CI: 15–20%; I2 = 96%; n = 65] with a CFR of 52% [95% CI: 46–60%; I2 = 81%; n = 29] and CSR of 56% [95% CI: 48–65%; I2 = 91%; n = 37] among cardiac patients. Of the patients that died, 37% [95% CI: 32–43%; I2 = 83%; n = 29] had CVD. Patients with CVD had higher relative risk of death [sRR: 2.08; 95% CI: 1.81–2.39; I2 = 69%; n = 34] and severe disease [sRR: 1.54; 95% CI: 1.39–1.72; I2 = 77%; n = 38] compared to patients without CVD (Fig 2C).
Smoking and COPD
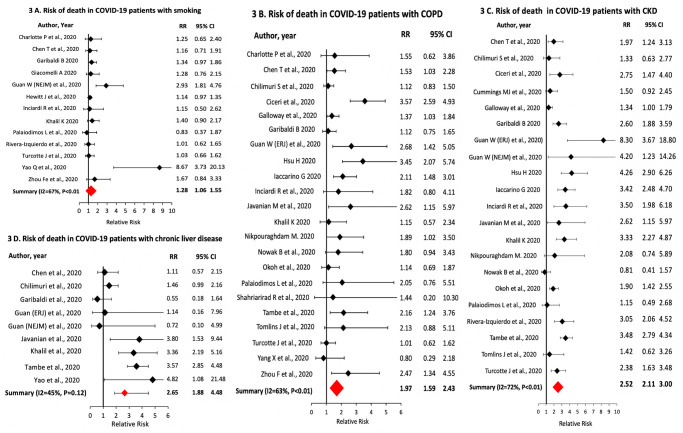
The prevalence of any history of smoking in the patients was 26% [95% CI: 22–31%; I2 = 98%; n = 41]. For patients with smoking history, the CFR was 27% [95% CI: 24–32%; I2 = 61%; n = 11] and CSR was 39% [95% CI: 34–46; I2 = 78%; n = 27]. Compared to never smokers, patients with smoking history had higher relative risk of death [sRR: 1.28; 95% CI: 1.06–1.55; I2 = 68%; n = 13] and severe COVID-19 disease [sRR: 1.29; 95% CI: 1.18–1.42; I2 = 33%; n = 27] (Fig 3A). The prevalence of COPD was 9% [95% CI: 8–11%; I2 = 94%; n = 52]. Patients with COPD had a CFR of 51% [95% CI: 43–59%; I2 = 0%; n = 21]; CSR of 43% [95% CI: 35–52%; I2 = 84%; n = 24]; a sRR of death of 1.70 [95% CI: 1.42–2.04; I2 = 66%; n = 22] and of severe disease of 1.71 [95% CI: 1.49–1.97; I2 = 84%; n = 29] (Fig 3B).
Fig 3. Association of smoking, chronic obstructive pulmonary disease, chronic kidney disease and chronic liver disease with death in COVID-19 patients.
Chronic kidney disease
The prevalence of CKD was 13% [95% CI: 11–16%; I2 = 96%; n = 47] with a CFR of 48% [95% CI: 37–63%; I2 = 89%; n = 18] and CSR of 36% [95% CI: 33–40%; I2 = 56%; n = 22] in CKD patients. CKD was present in 27% [95% CI: 21–34%; I2 = 79%; n = 18] of all COVID-19 patients that died. CKD patients had higher relative risk of death [sRR: 2.52; 95% CI: 2.11–3.00; I2 = 72%; n = 23] and severe disease [sRR: 1.56; 95% CI: 1.31–1.86; I2 = 85%; n = 27] compared to non-CKD patients (Fig 3C).
Chronic liver disease
The prevalence of CLD was 2% [95% CI: 2–3%; I2 = 72%; n = 31] with a CFR of 39% [95% CI: 31–50%; I2 = 0%; n = 8] and CSR of 43% [95% CI: 32–57%; I2 = 5%; n = 12] in CLD patients. CLD was present in 6% [95% CI: 4–8%; I2 = 0%; n = 8] of the COVID-19 patients who died. Patients with CLD had higher relative risk of death [sRR: 2.65; 95% CI: 1.88–3.75; I2 = 77%; n = 9] and severe disease [sRR: 1.63; 95% CI: 1.23–2.15; I2 = 82%; n = 15] compared to non-CKD patients (Fig 3D).
COVID-19 related organ system injury
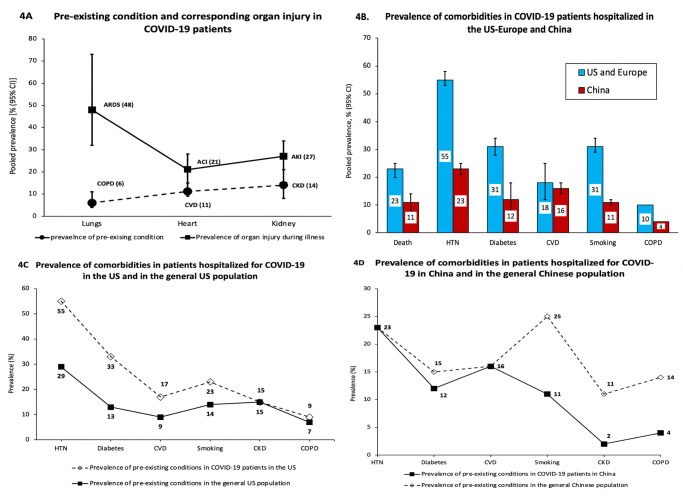
To understand how pre-existing health conditions may be correlated with the risk of specific organ injury, we calculated the prevalence of acute injury to lung, heart and kidney for studies that reported prevalence of both the pre-existing condition(s) and corresponding organ injury (Fig 4A). Pooled across 12 studies [14, 25, 32, 45, 48, 49, 52, 54, 60, 62, 79], the prevalence of COPD at baseline was 6% [95% CI: 4–11%] and the proportion of patients developing ARDS during hospitalization was 48% [32–73%]. The pooled prevalence of baseline CVD (n = 13 studies) was 11% [95% CI: 9–15%] and that of acute cardiac injury (ACI) during hospitalization was 21% [95% CI: 15–28%] [6, 14, 25, 32, 35, 43, 48, 49, 54, 79, 84]. The prevalence of CKD (n = 12 studies) was 14% [95% CI: 8–26%] and that of acute kidney injury during hospitalization (AKI) was 27% [95% CI: 21–34%] [6, 14, 25, 32, 45, 48, 65, 79].
Fig 4. Prevalence of acute organ injuries during hospital stay and regional difference in prevalence of death and comorbidities in patients hospitalized for COVID-19.
ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; ACI, acute cardiac injury; CVD, cardiovascular disease; AKI, acute kidney injury; CKD, chronic kidney disease; HTN, hypertension.
Regional difference in prevalence of death and risk factors
Upon sub-group analysis, we noted significantly higher prevalence of death and risk factors among COVID-19 patients in the US and Europe than in China (Fig 4B). The prevalence of death was 23% [95% CI: 19–27%; I2 = 97%; n = 29] in the US and Europe, and 11% [95% CI: 7–16%; I2 = 94%; n = 24] in China. Prevalence of severe disease was 20% [95% CI: 16–25%; I2 = 98%; n = 25] for US and Europe, and 39% [95% CI: 32–47%; I2 = 97%; n = 30] for China. Median age of patients was 65 years [IQR: 63–67 years; I2 = 0%; n = 24] for the US and Europe and 55 years [IQR: 52–58 years; I2 = 57%; n = 27] for China. Fifty-two percent [95% CI: 46–59%; I2 = 98%; n = 16] of the patients hospitalized were aged ≥60 years in the US and Europe as compared to 36% [95% CI: 30–43%; I2 = 96%; n = 22] for China. The prevalence of co-morbidities between US-Europe and China differed as follows: 1) US-Europe: HTN = 55% [95% CI: 52–57%]; diabetes = 31% [95% CI: 29–34]; CVD = 18% [95% CI: 15–21%]; smoking history = 15% [95% CI: 11–21%]; COPD = 9% [95% CI: 6–13%] and 2) China: HTN = 23% [95% CI: 20–26%]; diabetes = 12% [95% CI: 10–14%]; CVD = 16% [95% CI: 12–22%]; smoking history = 11% [95% CI: 9–13%]; CKD = 2.3% [95 CI: 1.6–3.4%] and COPD = 4% [95 CI: 3–5%].
Comorbidities in COVID-19 patients and the general populations in the US and China
In order to gain some understanding of whether patients with comorbidities are at higher risk of COVID-19 infection or hospitalization, we compared the prevalence of comorbidities between COVID-19 patients hospitalized in the US and the prevalence of comorbidities in the general US population. We observed that the prevalence of hypertension (55%), diabetes (33%), CVD (17%), and smoking history (23%) were substantially higher in the COVID-19 patients than in the general US population (Fig 4C). For the Chinese population, the overall prevalence of hypertension (23%) and diabetes (12%) in the COVID-19 patients were similar to that of the general Chinese population. However, the prevalence of smoking history (11%), COPD (4%), CKD (2%), and heart disease (16%) in the COVID-19 patients hospitalized in China were unexpectedly lower as compared to their corresponding prevalence in the general Chinese population (Fig 4D).
Sensitivity analyses
The positive associations of age≥65 years, male sex, smoking history, COPD, hypertension and diabetes with the risk of death in the COVID-19 patients were relatively homogenous (I2<70%). However, we carried out sensitivity analyses to assess the effects of outliers. For the risk of death for hypertension and smoking history, we removed the study by Yao et al. [86] which showed significantly higher risk compared to other studies; the results for both hypertension [sRR = 1.74; 95% CI: 1.58–1.94] and smoking [sRR:1.24; 95% CI: 1.08–1.42] remained significant. Guan et al. [13] had published a second study with additional patients and reported adjusted estimates for COPD, diabetes and hypertension. We used the adjusted risk estimates for the analyses. For the risk of death with other risk factors (CVD, CKD, and CLD) for Guan et al. [45], we conducted sensitivity analyses by using the counts only from the original study. The results [sRR (95% CI)] were similar as: CVD = 2.06 [95 CI: 1.80–2.36], CKD = 2.48 [95% CI: 2.09–2.94] and CLD = 2.67 [95% CI: 1.85–3.85].
Small study effects and quality assessment
We observed asymmetry in the funnel plot for studies that reported prevalence of death in COVID-19 patients (Egger’s test p = 0.001) (S1 Fig). On further analysis, the plot remained asymmetrical when restricted to studies from China (Egger’s p = 0.003) but was symmetrical for studies from US-Europe (Egger’s p = 0.160). We observed symmetrical funnel plots with no bias for pooled prevalence severe disease (Egger’s p = 0.128). On average, prospective or retrospective studies scored a score of 6 out of 9 and cross-sectional studies scored 6 out of 10. Many studies did not get a full score because they did not adjust for confounders (age, sex, or other risk factors) or patients remained hospitalized even after the follow-up ended, suggesting inadequate follow-up period (S4 Table).
Discussion
We carried out a comprehensive systematic review and meta-analysis of 77 studies that included 38906 hospitalized patients to investigate the prevalence and risk factors for death and severe disease in COVID-19 patients. We calculated an overall prevalence of death of 20% and severe disease of 28%. Nearly 50% of the patients admitted to hospitals due to COVID-19 were ≥60 years of age and 59% were males. We observed high prevalence of hypertension and diabetes of 50% and 28%, respectively, for the patients. The risk factors were more prevalent in patients who died and were distributed as: age ≥60 years: 85%; males: 66%; hypertension: 66%; diabetes: 39%; heart disease: 37%; CKD: 27%; smoking history: 44%; COPD: 12%, and CLD: 9%. In comparison with the overall prevalence of death of 20% for all COVID-19 hospitalized patients, the CFR was higher for male patients (26%) and for patients having the following risk factors: age≥60 years (35%), heart disease (52%), COPD (51%), CKD (48%), CLD (39%), hypertension (28%), diabetes (24%), and smoking history (27%). The elevation in the risk of death was statistically significant for age ≥60 (sRR = 3.6; 95% CI: 3.0–4.4), male sex 1.3 (95% CI: 1.2–1.4), smoking history (sRR = 1.3; 95% CI: 1.1–1.6), COPD (sRR = 1.7; 95% CI: 1.4–2.0), heart disease (sRR = 2.1; 95% CI: 1.8–2.4), CKD (sRR = 2.5; 95% CI: 2.1–3.0), hypertension (sRR = 1.8; 95% CI: 1.7–2.1), and diabetes (sRR = 1.5; 95% CI: 1.4–1.7). All of the risk factors we analyzed were positively associated with progression to severe disease as well. The results suggest that older age, male sex and the co-morbidities increase the risk of progression to severe disease and death in COVID-19 patients.
We observed significant difference in the prevalence of death between US-Europe (23%) and China (11%). This lower risk of death from COVID-19 for the hospitalized patients in China may be explained by the lower median age as well as lower prevalence of co-morbidities for COVID-19 patients in China. However, this >200% lower prevalence of death in China is incommensurate with our finding of a higher prevalence of severe disease observed for patients in China (39%) as compared to patients in the US-Europe (20%). Notably, we observed asymmetry in the funnel plot and a statistically significant tests for publication bias or small study effects for the prevalence of death for studies from China that could suggest selective outcome reporting. As such, while the lower median age and prevalence of co-morbidities for COVID-19 patients in China may explain the lower prevalence of death, it is also possible that a selective under-reporting of death had occurred for studies from China. The death toll in China was initially under-reported and later updated on April 17, 2020 [95].
Whether or not cigarette smoking has been associated with SARS-CoV-2 acquisition or progression to severe disease has been strongly debated with studies showing both positive, null, and inverse association between smoking and COVID-19 [10, 11, 96–98]. We found that patients with any history of smoking have both a higher risk of death (RR: 1.28; 95% CI: 1.06–1.55) and severe disease (1.29; 95% CI: 1.18–1.42). The case fatality risk for those with smoking history (27%) was also higher than the overall CFR of 20%. Whereas a higher COVID-19 mortality and morbidity among smokers may be due its causal association with COPD and CVD, Cai et al. [99] has also observed upregulation of pulmonary Angiotensin Converting Enzyme 2 (ACE2) gene expression and hence, pulmonary ACE2 receptors in smokers suggesting a direct effect of smoking on COVID-19 susceptibility and disease progression. ACE2 receptors are used by SARS-CoV-2 to translocate intracellularly [15, 100–104].
Our results of higher risk of death and severe disease associated with hypertension, diabetes and CVD in COVID-19 patients concurred with most studies conducted to date including studies that specifically investigated these associations [14, 65, 105, 106]. However, it is unclear if cardiovascular risk factors including smoking, hypertension, diabetes, heart disease and CKD increases the susceptibility toward SARS-CoV-2 infection in the population [15, 100, 101, 107]. On one hand, angiotensin-converting enzyme 2 (ACE2)–by blocking the renin angiotensin aldosterone system (RAAS) and decreasing or countering the vasoconstrictive, proinflammatory and profibrotic properties of angiotensin-II through catalysis of angiotensin-II to angiotensin-(1–7)–have been shown to exert cardiovascular protective effect and prevent acute lung injury from SARS-CoV-2 [15, 100, 101]. However, on the other hand, a possible greater expression of ACE2, the functional receptor mediating cellular entry of SARS-CoV-2 in humans, in patients with cardiovascular disease and other comorbidities can lead to increased susceptibility towards infection with SARS-CoV-2 [108, 109]. In this context, it would be reasonable to posit that a substantially higher prevalence of cardiovascular comorbidities in the hospitalized patients compared to the prevalence in the general population may suggest elevated risk of acquisition of SARS-CoV-2 for patients with cardiovascular risk factors. To this end, we found that the prevalence of smoking history (23%), hypertension (55%), diabetes (33%) and heart disease (17%) in the hospitalized COVID-19 patients in the US were substantially higher than the corresponding prevalence of smoking (14%) [110], hypertension (29%) [111], diabetes (13%) [112] and heart disease (9%) [113] in the general US population that could suggest an association between these comorbidities and risk of SARS-CoV-2 infection or disease progression. However, we note that if the prevalence of these comorbidities in the asymptomatic individuals with COVID-19 in the general population is similar to that of their prevalence in the non-COVID-19 general population, then this difference–the higher prevalence of comorbidities in the hospitalized patients compared to the general population–could simply imply a higher risk of symptomatic infection or hospitalization for individuals having SARS-CoV-2 infection. The prevalence of other risk factors i.e. COPD (9%) and CKD (15%) in the COVID-19 patients in the US was similar to the overall prevalence of COPD (7%) [114] and CKD (15%) [115] in the country. Generally, we noted a lower prevalence of comorbidities for patients in China. The prevalence of hypertension (23%) and diabetes (12%) in the hospitalized patients in China, which were lower than that of the US, approximate the respective prevalence of hypertension (23%) [116] and diabetes (15%) [117] in the general population of China. A previous meta-analysis also noted this observation [19]. Surprisingly, the prevalence of smoking (11%) in the COVID-19 patients hospitalized in China are inexplicably lower than the corresponding prevalence of smoking (23%) among COVID-19 patients in the US despite a higher prevalence of smoking (47% in Chinese males) [118] in the general Chinese population is significantly higher than that of the US. The prevalence of CVD (16%), COPD (4%) and CKD (2%) among COVID-19 patients in China are substantially lower than the corresponding prevalence of CVD (21%) [119], COPD (14%) [120], and CKD (11%) [121] in the general Chinese population. Given these discrepancies, we are unsure whether the lower prevalence of comorbidities noted for the COVID-19 patients in China are representative of the true prevalence. There was a great sense of urgency and a race to publish data in the early phase of the outbreak. As such, there exists the possibility of substantial under-recording of data on covariables. Had there been under-reporting, the implication would be a higher true prevalence estimate. We do not see reason for any systematic difference in reporting of risk factors based on outcome, or vice-versa, and hence, our summary relative risk estimates for association of risk factors with death or severe disease should not have been affected.
We assessed if patients with specific co-morbidities at baseline had higher risk of specific organ injury from SARS-CoV-2 during hospitalization. While the available data did not allow direct assessment of this relation, we compared the prevalence of comorbidities with the prevalence of corresponding organ system injury for studies that reported both baseline comorbidity and corresponding organ injury. We observed that the risk of acute lung injury/ARDS (48%), ACI (21%), and AKI (27%) were substantially higher than the baseline prevalence of COPD (6%), heart disease (11%) and CKD (14%), respectively. The higher prevalence of acute organ injury than the prevalence of baseline comorbidity simply indicates that ARDS, ACI and AKI were also occurring in patients who did not have a corresponding comorbidity at baseline in addition to people having the comorbidities.
Most studies reported only frequencies of risk factors and did not present adjusted measures for disease severity or death. Given this limitation, the risk ratio we calculated from the frequencies are largely unadjusted estimates. Future studies could additionally present, at the least, age- and sex-adjusted measures for association of risk of comorbidities with death or severe disease. Many studies reported odds ratio for the measure of association between pre-existing conditions and risk of severe disease or death. Odds ratio poorly approximates risk ratio when the disease prevalence is high at baseline. For example, Zhou et al. [14] calculated an odds ratio of 5.4 (95% CI: 0.96–30.4) for risk of death from COPD in COVID-19 patients whereas the risk ratio we calculated from the frequencies presented is RR = 2.47 (95% CI: 1.34–4.55). Prevalence of severe disease or death in COVID-19 patients was high in several studies. Similarly, several meta-analyses calculated odds ratios instead of risk ratios to summarize the risk of disease severity or death in association with risk factors such as smoking, diabetes, hypertension and cardiovascular disease [10, 11, 18], often to be interpreted by media and even by researchers as a measure of relative risk. Lack of rigor in research design, analysis and interpretation could generate inconsistent and ungeneralizable results across studies leading to controversy and confusion around serious public health issues such as that existing for association (or not) of smoking with COVID-19 disease acquisition, severity or death. As publications evolve at a pace that could be overwhelming for researchers and practitioners, we attempted to present a meaningful summary and inference for association of risk factors with death or severe disease from literatures published globally. Additionally, we provide an epidemiological framework for the risk of infection by SARS-CoV-2 based on presence of cardiovascular risk factors. This analysis can inform public health measures for COVID-19 screening and prevention, risk stratification and management of patients in clinical practice, analysis and presentation strategies for research data and inspire etiological investigations.
Conclusion
Epidemiological risk factors for progression of COVID-19 to severe disease and death and for acquisition of SARS-CoV-2, the causal agent for COVID-19, based on presence of pre-existing conditions have been insufficiently understood. Meta-analysis of 77 studies including 39023 COVID-19 patients hospitalized globally revealed case fatality risk of 52% for those having heart disease, 51% for COPD, 48% for CKD, 39% for CLD, 28% for hypertension, 27% for smoking history, 24% for diabetes, 35% for age≥60 years, and 26% for males. Of all the patients who died, an overwhelming majority (85%) were in people aged≥60 years. Also, of the people who died, 66% were males, 66% had hypertension, 44% had history of smoking, 39% had diabetes, 37% had CVD, 27% had CKD, and 6% had CLD. All of the above risk factors were significantly associated with death and severe disease in the patients hospitalized for COVID-19. The prevalence of ARDS was 48%, ACI 21%, and AKI 28% in the hospitalized patients. A higher prevalence of hypertension, diabetes, smoking and heart disease in the COVID-19 inpatients as compared to that of the general population could imply a higher risk of SARS-CoV-2 infection or disease progression for patients having these risk factors. These findings could inform public health strategies for targeted screening and appropriate control of modifiable risk factors such as smoking, hypertension, and diabetes to reduce morbidity and mortality. Finally, based on the published literature, there were vast differences in the prevalence of death and risk factors for the populations in China and in US-Europe that should be further investigated.
Supporting information
(DOCX)
(DOCX)
(DOCX)
#Award of Points: Selection: points were awarded based on representativeness of the exposed group and unexposed group (2 points), ascertainment of exposures (1 point), and demonstration that outcome of interest was not present at the start of the study (1 point). Comparability (2 points): points were awarded based on whether the analyses were adjusted for age, sex, and other risk factors (2 points for adjustment to age and sex). Outcome (3points): points were awarded based on ascertainment of outcome through record linkage or independent blind assessment (1 points); duration of follow-up (1 point) (hospitalization till discharge); and adequacy of follow up for study population (complete follow up for the patients (vs whether patients were currently under treatment at the time of study report) (1 point), or if the patients currently under admission are excluded from outcome assessment (1 point).
(DOCX)
(TIF)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
Dr. Dorjee is supported by grants from the US National Institute of Allergy and Infectious Diseases of the National Institute of Health (Grant # K01AI148583); Johns Hopkins Alliance for a Healthier World (Grant # 80045453); STOP TB PARTNERSHIP TB REACH (Grant # 134126); the Pittsfield Anti-TB Association and dedicated private philanthropists.
References
- 1.Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html. Published 2020. Accessed October 8, 2020.
- 2.Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262 10.1016/j.metabol.2020.154262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS One. 2020;15(5):e0233147 10.1371/journal.pone.0233147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–108. 10.1016/j.ejim.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patanavanich R, Glantz SA. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. 10.1093/ntr/ntaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossato M, Russo L, Mazzocut S, Di Vincenzo A, Fioretto P, Vettor R. Current smoking is not associated with COVID-19. Eur Respir J. 2020;55(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5). 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akhmerov A, Marban E. COVID-19 and the Heart. Circ Res. 2020;126(10):1443–1455. 10.1161/CIRCRESAHA.120.317055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33(5):373–374. 10.1093/ajh/hpaa057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Guan B, Su T, et al. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106(15):1142–1147. 10.1136/heartjnl-2020-317062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 22.Cochran WG. The Combination of Estimates From Different Experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 24.Shore RE, Gardner MJ, Pannett B. Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. Br J Ind Med. 1993;50(11):971–997. 10.1136/oem.50.11.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis. 2020;7(2):91–96. 10.1515/dx-2020-0046 [DOI] [PubMed] [Google Scholar]
- 26.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. Bmj. 2020;369:m1996 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brill SE, Jarvis HC, Ozcan E, et al. COVID-19: a retrospective cohort study with focus on the over-80s and hospital-onset disease. BMC Med. 2020;18(1):194 10.1186/s12916-020-01665-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Z, Li T, Liang L, et al. Clinical characteristics of Coronavirus Disease 2019 patients in Beijing, China. PLoS One. 2020;15(6):e0234764 10.1371/journal.pone.0234764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6. 10.1016/j.jinf.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48(4):543–551. 10.1007/s15010-020-01432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091–m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chilimuri S, Sun H, Alemam A, et al. Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study from New York City. West J Emerg Med. 2020;21(4):779–784. 10.5811/westjem.2020.6.47919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509 10.1016/j.clim.2020.108509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133(11):1261–1267. 10.1097/CM9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escalera-Antezana JP, Lizon-Ferrufino NF, Maldonado-Alanoca A, et al. Risk factors for mortality in patients with Coronavirus Disease 2019 (COVID-19) in Bolivia: An analysis of the first 107 confirmed cases. Infez Med. 2020;28(2):238–242. [PubMed] [Google Scholar]
- 37.Feng Y, Ling Y, Bai T, et al. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. 10.1164/rccm.202002-0445OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson J, Rosser JI, Quintero O, et al. Characteristics and Outcomes of Coronavirus Disease Patients under Nonsurge Conditions, Northern California, USA, March-April 2020. Emerg Infect Dis. 2020;26(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galloway JB, Norton S, Barker RD, et al. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: An observational cohort study. J Infect. 2020;81(2):282–288. 10.1016/j.jinf.2020.05.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garibaldi BT, Fiksel J, Muschelli J, et al. Patient Trajectories Among Persons Hospitalized for COVID-19: A Cohort Study. Ann Intern Med. 2020. 10.7326/M20-3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giacomelli A, Ridolfo AL, Milazzo L, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: A prospective cohort study. Pharmacol Res. 2020;158:104931 10.1016/j.phrs.2020.104931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19—Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):545–550. 10.15585/mmwr.mm6918e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregoriano C, Koch D, Haubitz S, et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: an observational analysis. Swiss Med Wkly. 2020;150:w20316 10.4414/smw.2020.20316 [DOI] [PubMed] [Google Scholar]
- 45.Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. 10.1016/S2468-2667(20)30146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu HE, Ashe EM, Silverstein M, et al. Race/Ethnicity, Underlying Medical Conditions, Homelessness, and Hospitalization Status of Adult Patients with COVID-19 at an Urban Safety-Net Medical Center—Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(27):864–869. 10.15585/mmwr.mm6927a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu L, Chen S, Fu Y, et al. Risk Factors Associated with Clinical Outcomes in 323 COVID-19 Hospitalized Patients in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hur K, Price CPE, Gray EL, et al. Factors Associated With Intubation and Prolonged Intubation in Hospitalized Patients With COVID-19. Otolaryngol Head Neck Surg. 2020;163(1):170–178. 10.1177/0194599820929640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension. 2020;76(2):366–372. 10.1161/HYPERTENSIONAHA.120.15324 [DOI] [PubMed] [Google Scholar]
- 52.Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821–1829. 10.1093/eurheartj/ehaa388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang JG, Hur J, Choi EY, Hong KS, Lee W, Ahn JH. Prognostic Factors for Severe Coronavirus Disease 2019 in Daegu, Korea. J Korean Med Sci. 2020;35(23):e209 10.3346/jkms.2020.35.e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Javanian M, Bayani M, Shokri M, et al. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: a retrospective cohort study. Rom J Intern Med. 2020;58(3):161–167. 10.2478/rjim-2020-0013 [DOI] [PubMed] [Google Scholar]
- 55.Kalligeros M, Shehadeh F, Mylona EK, et al. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity (Silver Spring). 2020;28(7):1200–1204. 10.1002/oby.22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khalil K, Agbontaen K, McNally D, et al. Clinical characteristics and 28-day mortality of medical patients admitted with COVID-19 to a central London teaching hospital. J Infect. 2020;81(3):e85–e89. 10.1016/j.jinf.2020.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khamis F, Al-Zakwani I, Al Naamani H, et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: An experience from Oman. J Infect Public Health. 2020;13(7):906–913. 10.1016/j.jiph.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lendorf ME, Boisen MK, Kristensen PL, et al. Characteristics and early outcomes of patients hospitalised for COVID-19 in North Zealand, Denmark. Dan Med J. 2020;67(9). [PubMed] [Google Scholar]
- 59.Liu S, Luo H, Wang Y, et al. Clinical characteristics and risk factors of patients with severe COVID-19 in Jiangsu province, China: a retrospective multicentre cohort study. BMC Infect Dis. 2020;20(1):584 10.1186/s12879-020-05314-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133(9):1032–1038. 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikpouraghdam M, Jalali Farahani A, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study. J Clin Virol. 2020;127:104378 10.1016/j.jcv.2020.104378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nowak B, Szymanski P, Pankowski I, et al. Clinical characteristics and short-term outcomes of patients with coronavirus disease 2019: a retrospective single-center experience of a designated hospital in Poland. Pol Arch Intern Med. 2020;130(5):407–411. 10.20452/pamw.15361 [DOI] [PubMed] [Google Scholar]
- 63.Okoh AK, Sossou C, Dangayach NS, et al. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health. 2020;19(1):93 10.1186/s12939-020-01208-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pellaud C, Grandmaison G, Pham Huu Thien HP, et al. Characteristics, comorbidities, 30-day outcome and in-hospital mortality of patients hospitalised with COVID-19 in a Swiss area—a retrospective cohort study. Swiss Med Wkly. 2020;150:w20314 10.4414/smw.2020.20314 [DOI] [PubMed] [Google Scholar]
- 65.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rivera-Izquierdo M, Del Carmen Valero-Ubierna M, JL Rd, et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: A retrospective observational study. PLoS One. 2020;15(6):e0235107 10.1371/journal.pone.0235107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shabrawishi M, Al-Gethamy MM, Naser AY, et al. Clinical, radiological and therapeutic characteristics of patients with COVID-19 in Saudi Arabia. PLoS One. 2020;15(8):e0237130 10.1371/journal.pone.0237130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shahriarirad R, Khodamoradi Z, Erfani A, et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect Dis. 2020;20(1):427 10.1186/s12879-020-05128-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shekhar R, Sheikh AB, Upadhyay S, Atencio J, Kapuria D. Early experience with COVID-19 patients at academic hospital in Southwestern United States. Infect Dis (Lond). 2020;52(8):596–599. 10.1080/23744235.2020.1774645 [DOI] [PubMed] [Google Scholar]
- 70.Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108 10.1186/s13054-020-2833-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suleyman G, Fadel RA, Malette KM, et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270 10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J Med Virol. 2020. 10.1002/jmv.25966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tambe MP, Parande MA, Tapare VS, Borle PS, Lakde RN, Shelke SC. An epidemiological study of laboratory confirmed COVID-19 cases admitted in a tertiary care hospital of Pune, Maharashtra. Indian J Public Health. 2020;64(Supplement):S183–s187. 10.4103/ijph.IJPH_522_20 [DOI] [PubMed] [Google Scholar]
- 74.Team CC-R. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019—United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. 10.15585/mmwr.mm6913e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tian S, Hu N, Lou J, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. 10.1016/j.jinf.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomlins J, Hamilton F, Gunning S, Sheehy C, Moran E, MacGowan A. Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID-19), the first UK cohort. J Infect. 2020;81(2):e59–e61. 10.1016/j.jinf.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turcotte JJ, Meisenberg BR, MacDonald JH, et al. Risk factors for severe illness in hospitalized Covid-19 patients at a regional hospital. PLoS One. 2020;15(8):e0237558 10.1371/journal.pone.0237558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. 10.1002/jmv.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 Hospitalized Patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421–428. 10.1016/j.ijid.2020.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–777. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei Y, Zeng W, Huang X, et al. Clinical characteristics of 276 hospitalized patients with coronavirus disease 2019 in Zengdu District, Hubei Province: a single-center descriptive study. BMC Infect Dis. 2020;20(1):549 10.1186/s12879-020-05252-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao Q, Wang P, Wang X, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130(5):390–399. 10.20452/pamw.15312 [DOI] [PubMed] [Google Scholar]
- 85.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu T, Cai S, Zheng Z, et al. Association Between Clinical Manifestations and Prognosis in Patients with COVID-19. Clin Ther. 2020;42(6):964–972. 10.1016/j.clinthera.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu X, Sun X, Cui P, et al. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai, China. Transbound Emerg Dis. 2020;67(4):1697–1707. 10.1111/tbed.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhan T, Liu M, Tang Y, et al. Retrospective analysis of clinical characteristics of 405 patients with COVID-19. J Int Med Res. 2020;48(8):300060520949039 10.1177/0300060520949039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respiratory Research. 2020;21(1):74–74. 10.1186/s12931-020-01338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–772. 10.1016/j.cmi.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 2020: 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 92.Zhao XY, Xu XX, Yin HS, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20(1):311 10.1186/s12879-020-05010-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443 10.1136/bmj.m1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng Y, Xiong C, Liu Y, et al. Epidemiological and Clinical Characteristics Analysis of COVID-19 in the Surrounding Areas of Wuhan, Hubei Province in 2020. Pharmacological Research. 2020:104821 10.1016/j.phrs.2020.104821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin A. China Raises Coronavirus Death Toll by 50% in Wuhan. The New York Times. April 17, 2020, 2020. [Google Scholar]
- 96.National Smoking Rates Correlate Inversely with COVID-19 Mortality. 2020. https://www.medrxiv.org/content/10.1101/2020.06.12.20129825v1. Accessed Oct 6, 2020.
- 97.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol. 2020. 10.1002/jmv.25889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cai G, Bosse Y, Xiao F, Kheradmand F, Amos CI. Tobacco Smoking Increases the Lung Gene Expression of ACE2, the Receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;201(12):1557–1559. 10.1164/rccm.202003-0693LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and Cardiovascular Disease. Circulation. 2020;141(20):1648–1655. 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 101.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–H1090. 10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan W, Zhang J, Wang M, et al. Clinical Features of COVID-19 in Patients With Essential Hypertension and the Impacts of Renin-angiotensin-aldosterone System Inhibitors on the Prognosis of COVID-19 Patients. Hypertension. 2020;76(3):732–741. 10.1161/HYPERTENSIONAHA.120.15289 [DOI] [PubMed] [Google Scholar]
- 106.Zhu L, She ZG, Cheng X, et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31(6):1068–1077 e1063. 10.1016/j.cmet.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kreutz R, Algharably EAE, Azizi M, et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. 2020;116(10):1688–1699. 10.1093/cvr/cvaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pinto BGG, Oliveira AER, Singh Y, et al. ACE2 Expression Is Increased in the Lungs of Patients With Comorbidities Associated With Severe COVID-19. J Infect Dis. 2020;222(4):556–563. 10.1093/infdis/jiaa332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Creamer MR, Wang TW, Babb S, et al. Tobacco Product Use and Cessation Indicators Among Adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013–1019. 10.15585/mmwr.mm6845a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1–8. [PubMed] [Google Scholar]
- 112.United States Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, GA: 2020. [Google Scholar]
- 113.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 114.Biener AI, Decker SL, Rohde F. Prevalence and Treatment of Chronic Obstructive Pulmonary Disease (COPD) in the United States. JAMA. 2019;322(7):602 10.1001/jama.2019.10241 [DOI] [PubMed] [Google Scholar]
- 115.United States Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019. https://www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf. Published 2019. Accessed June 7, 2020, 2020. [Google Scholar]
- 116.Wang Z, Chen Z, Zhang L, et al. Status of Hypertension in China: Results From the China Hypertension Survey, 2012–2015. Circulation. 2018;137(22):2344–2356. 10.1161/CIRCULATIONAHA.117.032380 [DOI] [PubMed] [Google Scholar]
- 117.Hu C, Jia W. Diabetes in China: Epidemiology and Genetic Risk Factors and Their Clinical Utility in Personalized Medication. Diabetes. 2018;67(1):3–11. 10.2337/dbi17-0013 [DOI] [PubMed] [Google Scholar]
- 118.Wang M, Luo X, Xu S, et al. Trends in smoking prevalence and implication for chronic diseases in China: serial national cross-sectional surveys from 2003 to 2013. Lancet Respir Med. 2019;7(1):35–45. 10.1016/S2213-2600(18)30432-6 [DOI] [PubMed] [Google Scholar]
- 119.Ma LY, Chen WW, Gao RL, et al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. 2020;17(1):1–8. 10.11909/j.issn.1671-5411.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. 10.1016/S2213-2600(18)30103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. 10.1016/S0140-6736(12)60033-6 [DOI] [PubMed] [Google Scholar]