Abstract
INTRODUCTION:
Although the Rome criteria were created primarily for research purposes, it was an important question whether the Rome criteria can distinguish organic dyspepsia from functional dyspepsia (FD). We evaluated the accuracy of the Rome IV criteria in identifying patients with FD and compared the differences between the Rome IV, Rome III, and potential Asia criteria in identifying patients with FD.
METHODS:
In this cross-sectional study, we analyzed data from patients who met the inclusion and exclusion criteria from March 2018 to January 2019 at 2 tertiary hospitals.
RESULTS:
A total of 600 patients were enrolled in this study, including 381 individuals met the Rome IV criteria for FD, 438 individuals met the Rome III criteria for FD, and 525 individuals met the potential Asia criteria for FD. The Rome IV criteria identified patients with FD with 67.3% sensitivity and 38.4% specificity, and the positive and negative likelihood ratios of FD identified by Rome IV criteria were 1.09 (95% confidence interval 0.97–1.24) and 0.85 (95% confidence interval 0.67–1.08), respectively. There was no significant difference in the area under Rome IV, Rome III, or potential Asia criteria receiver operating characteristic curves in identifying FD (P > 0.05).
DISCUSSION:
The Rome IV criteria were no better than the Rome III or potential Asia criteria in identifying FD and were not helpful in identifying patients with FD. Hence, although the Rome criteria remain useful for defining patients with FD for inclusion into clinical treatment trials, they should not be used for diagnosing FD.
INTRODUCTION
Dyspepsia is a complex of symptom originating from the upper gastrointestinal (GI) tract (1,2). Dyspepsia is present in approximately 20% of the global general population (3,4) and represents approximately one-third of those who seek health care (5). Dyspepsia can be subdivided into 2 subgroups: functional dyspepsia (FD) and organic dyspepsia. FD is defined as recurrent or chronic functional symptoms that are believed to originate from the gastroduodenal region without any structural abnormalities (6,7). Because of constant medical visits and continuous medication use, dyspeptic symptoms cause substantial socioeconomic burden (8,9), negatively affect the quality of daily life, and cause significant economic losses (10–13).
The Rome classification criteria for FD required a symptom duration of 6 months or more. However, for the Asian population, most experts involved in formulating the FD consensus in Asia believed that 6 months or more was too long as the FD classification criteria. A Japanese study found that most patients with dyspepsia seek their first medical care within 6 months (14). Among the experts participating in the formulation of the FD consensus in Asia, 68% believed that the duration of dyspepsia should be set at 3 months (15). For the diagnosis of FD in the Chinese population, it is unknown whether the duration of symptoms criterion set at 3 months is better than at 6 months as in the Rome criteria. There is still a lack of relevant research data in China, and further research is needed.
Although the Rome criteria were created primarily for research purposes, it was an important question whether the Rome criteria can distinguish organic dyspepsia from FD (16). Ford et al. (17) evaluated the accuracy of the Rome III criteria, Rome II criteria, and a broad definition in identifying patients with FD and found that the Rome III criteria were not significantly superior to the Rome II criteria and a broad definition. However, the accuracy of the Rome IV criteria in identifying patients with FD is still unclear; hence, further assessments on the Rome IV criteria are needed. The aim of this study was to evaluate the accuracy of the Rome IV criteria in identifying patients with FD. We also compared the differences between the Rome IV, Rome III, and potential Asia criteria in identifying FD.
METHODS
Study population
We conducted a multicenter, cross-sectional study of consecutive outpatients from 2 tertiary hospitals (the Second Affiliated Hospital of Xi'an Jiaotong University and Xi'an No. 3 Hospital) between March 2018 and January 2019 (see Supplementary study protocol, Supplementary Digital Content 2, http://links.lww.com/CTG/A457). The ethics committee of the Second Affiliated Hospital of Xi'an Jiaotong University approved this study. Oral informed consent was obtained from all the included patients. The Rome IV part of our study was registered at ClinicalTrials.gov (number NCT03479528). All authors had access to the study data and reviewed and approved the final manuscript see STROBE checklist, Supplementary Digital Content 3, http://links.lww.com/CTG/A458.
Inclusion criteria
Inclusion criteria were (i) aged 18 years and older; (ii) dyspeptic symptoms were present according to a broad definition (discomfort was characterized by the presence of 1 or more symptoms that included bothersome postprandial fullness at least several times per week, bothersome early satiation at least several times per week, bothersome epigastric pain ≥once per week, or bothersome epigastric burning ≥once per week; symptoms had to be present for at least 3 months); (iii) patients visited the gastroenterology clinics and completed upper GI endoscopy and epigastric ultrasounds at the same visit during the study period; and (iv) routine blood tests and liver function tests were conducted at any time after the onset of dyspeptic symptoms.
Exclusion criteria
Exclusion criteria were (i) history of diagnosed organic upper GI diseases which can explain dyspeptic symptoms, such as esophagitis, gastric ulcer, and duodenal ulcer; (ii) pregnant or lactating; (iii) history of major abdominal surgery; (iv) severe neuropsychiatric disease or severe liver, kidney, or respiratory disease; (v) pancreaticobiliary disease or metabolic disease (thyroid dysfunction and diabetes), liver dysfunction; (vi) steroids or nonsteroidal anti-inflammatory drugs were currently used; (vii) the main symptoms were related to reflux (acid regurgitation and posterior sternal burning); or (viii) declined to participate in this study.
Data collection
We obtained related data through clinic visits and telephone consultations. Patients were followed up by telephone to collect information if we were unable to perform outpatient consultations. The basic demographic data collected included name, age, height, weight, sex, and marital status. Dyspepsia data included dyspeptic symptoms, including duration and frequency per week. Lifestyle habit data included consumption spicy foods, smoking amount, drinking, sleep quality, and daily exercise duration. We also collected information about family history, outpatient cost (total outpatient cost of medical treatment due to dyspepsia), and examination results of routine blood tests, epigastric ultrasounds, and upper GI endoscopy, and a trained researcher imported all the related data into the database.
Definitions of FD
As in the study conducted by Ford et al. (17), the definitions of FD were all based on symptoms only, regardless of the results of upper GI endoscopy and other related examinations. Symptom-based Rome III-defined FD, Rome IV-defined FD, and Asia-defined FD were determined by the questionnaire according to the Rome III criteria, Rome IV criteria, and potential Asia criteria, respectively (17,18) (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A456). Potential Asia-defined FD was defined as dyspeptic symptoms meeting the Rome IV criteria and a dyspeptic duration ≥3 months (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A456).
Definition of organic upper GI disease
All included patients underwent upper GI endoscopy. The biopsy was obtained at the discretion of the endoscopists performing the upper GI endoscopy. Endoscopists and histopathologists were blinded to the patient's questionnaire data. As in the study conducted by Ford et al. (17), Barrett's esophagus, esophageal candidiasis, esophageal cancer, gastric ulcer, gastric erosion, gastric cancer, duodenal ulcer, and duodenal erosion were classified as organic upper GI diseases. Chronic gastritis and duodenitis as a result of endoscopy or biopsy are regarded as nonorganic upper GI diseases (18).
Reference standard
FD is a type of dyspepsia that has no organic, metabolic, or systemic disease to explain its symptoms, and only a few studies have rigorously diagnosed FD by laboratory examination, epigastric ultrasound, and upper GI endoscopy to exclude related diseases (19,20). Ford et al. (17) defined the reference standard for true FD as the presence of any epigastric pain or burning, postprandial fullness, or early satiety only with no evidence of organic upper GI disease and explained the reasons for not adding epigastric ultrasound and routine blood examinations. In our study, we rigorously diagnosed FD. The reference standard for defining true FD was the presence of any epigastric pain, epigastric burning, postprandial fullness, or early satiety with no evidence of abnormal upper GI endoscopy, abnormal routine blood tests, or abnormal epigastric ultrasounds that were likely to explain the symptoms (17,21,22).
Statistical analysis
EpiData3.1 software was used for data collection. SPSS 20.0 was used for statistical analysis. And, GraphPad Prism software was used for mapping the data. The results of categorical variables were expressed as counts and percentages, and χ2 tests or the Fisher exact test was used according to the analysis requirements. The results of continuous variables were expressed as the median ± SD, and the t test or Kruskal-Wallis test was used according to the analysis requirements. Among the patients who met the reference standard and those who did not meet the reference standard, the number of patients who met the Rome IV criteria, Rome III criteria, and the potential Asia criteria for FD was obtained respectively. The sensitivity (95% confidence interval [CI]), specificity (95% CI), positive predictive values (95% CI), negative predictive values (95% CI), positive likelihood ratio (LR) (95% CI), and negative LR (95% CI) were calculated within a Microsoft Excel spreadsheet. SPSS 20.0 statistical software was used to calculate the area under the receiver operating characteristic (ROC) curve and the 95% CI of these 3 definitions of FD, and 1-way ANOVA was used to compare them.
RESULTS
Patient characteristics
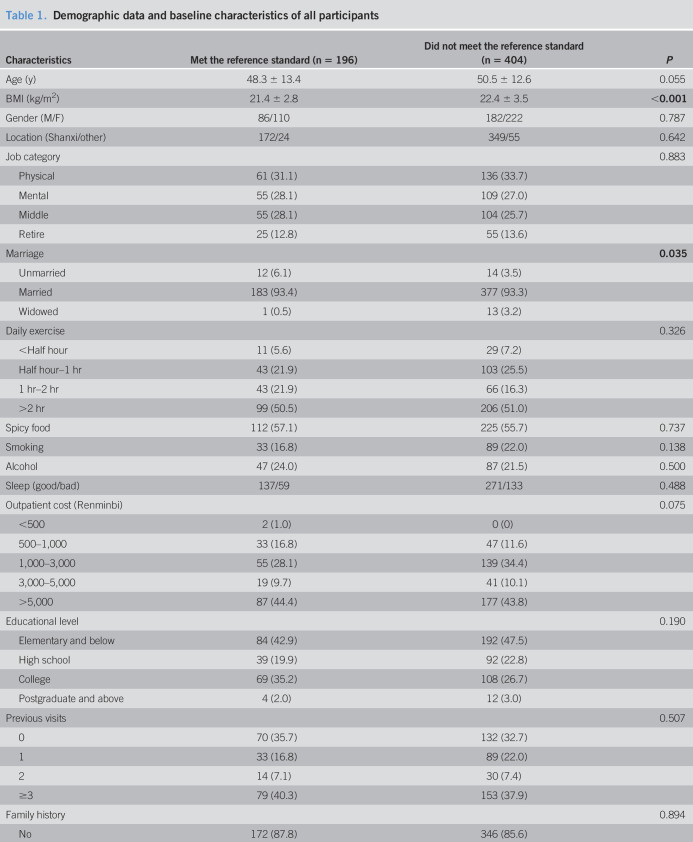
A total of 600 patients meeting the inclusion and exclusion criteria were enrolled in the study from March 2018 to January 2019; of these, 381 patients met the Rome IV criteria for FD, 438 patients met the Rome III criteria for FD, and 525 patients met the potential Asia criteria for FD. The mean age was 49.8 ± 12.9 years, and 332 (55.3%) patients were female. Among these patients, 196 met the reference standard, and 404 did not meet the reference standard, including 248 who had abnormal upper GI endoscopies, 39 who had abnormal routine blood tests, and 117 who had abnormal epigastric ultrasounds. The demographic data and baseline characteristics of all these patients are provided in Table 1. Those who met the reference standard had a lower body mass index and were more likely to be unmarried.
Table 1.
Demographic data and baseline characteristics of all participants
![]()
| Characteristics | Met the reference standard (n = 196) | Did not meet the reference standard (n = 404) | P |
| Age (y) | 48.3 ± 13.4 | 50.5 ± 12.6 | 0.055 |
| BMI (kg/m2) | 21.4 ± 2.8 | 22.4 ± 3.5 | <0.001 |
| Gender (M/F) | 86/110 | 182/222 | 0.787 |
| Location (Shanxi/other) | 172/24 | 349/55 | 0.642 |
| Job category | 0.883 | ||
| Physical | 61 (31.1) | 136 (33.7) | |
| Mental | 55 (28.1) | 109 (27.0) | |
| Middle | 55 (28.1) | 104 (25.7) | |
| Retire | 25 (12.8) | 55 (13.6) | |
| Marriage | 0.035 | ||
| Unmarried | 12 (6.1) | 14 (3.5) | |
| Married | 183 (93.4) | 377 (93.3) | |
| Widowed | 1 (0.5) | 13 (3.2) | |
| Daily exercise | 0.326 | ||
| <Half hour | 11 (5.6) | 29 (7.2) | |
| Half hour–1 hr | 43 (21.9) | 103 (25.5) | |
| 1 hr–2 hr | 43 (21.9) | 66 (16.3) | |
| >2 hr | 99 (50.5) | 206 (51.0) | |
| Spicy food | 112 (57.1) | 225 (55.7) | 0.737 |
| Smoking | 33 (16.8) | 89 (22.0) | 0.138 |
| Alcohol | 47 (24.0) | 87 (21.5) | 0.500 |
| Sleep (good/bad) | 137/59 | 271/133 | 0.488 |
| Outpatient cost (Renminbi) | 0.075 | ||
| <500 | 2 (1.0) | 0 (0) | |
| 500–1,000 | 33 (16.8) | 47 (11.6) | |
| 1,000–3,000 | 55 (28.1) | 139 (34.4) | |
| 3,000–5,000 | 19 (9.7) | 41 (10.1) | |
| >5,000 | 87 (44.4) | 177 (43.8) | |
| Educational level | 0.190 | ||
| Elementary and below | 84 (42.9) | 192 (47.5) | |
| High school | 39 (19.9) | 92 (22.8) | |
| College | 69 (35.2) | 108 (26.7) | |
| Postgraduate and above | 4 (2.0) | 12 (3.0) | |
| Previous visits | 0.507 | ||
| 0 | 70 (35.7) | 132 (32.7) | |
| 1 | 33 (16.8) | 89 (22.0) | |
| 2 | 14 (7.1) | 30 (7.4) | |
| ≥3 | 79 (40.3) | 153 (37.9) | |
| Family history | 0.894 | ||
| No | 172 (87.8) | 346 (85.6) | |
| Esophagus cancer | 6 (3.1) | 16 (4.0) | |
| Gastric cancer | 13 (6.6) | 29 (7.2) | |
| Other | 5 (2.6) | 13 (3.2) |
Values are expressed as the mean ± SD or n (%). Bold entries represent P < 0.05.
BMI, body mass index; F, female; M, male.
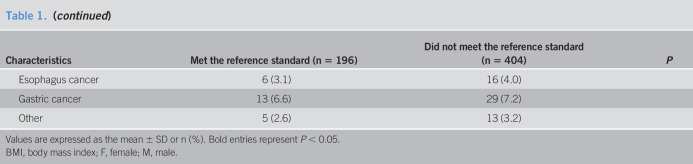
In the aggregate, of the 600 patients who underwent upper GI endoscopy, there were 349 patients with chronic gastritis, 185 patients with gastric erosion, 16 patients with gastric ulcer, 13 patients with duodenal ulcer, 12 patients with Barrett's esophagus, 9 patients with gastric cancer, 7 patients with esophageal cancer, and others (Figure 1). Among the 600 patients, 381 (63.5%) met the Rome IV criteria for FD. The average age of these 381 patients was 49.9 years, 231 (60.6%) were female, and 154 (40.4%) had organic lesions on the upper GI endoscopy. Gastric erosion was the most common, accounting for 31.5%. Of the 219 patients who did not meet the Rome IV criteria for FD, 94 (42.9%) had organic lesions on the upper GI endoscopy. The incidence of organic lesions in patients who met the Rome IV criteria for FD and those who did not is shown in Table 2.
Figure 1.
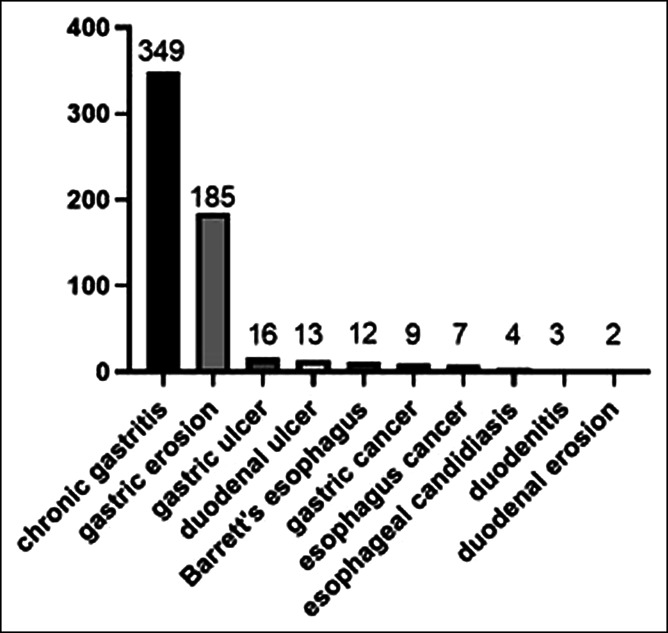
Endoscopy results for all participants. Among the 600 patients who underwent upper gastrointestinal endoscopy, there were 349 patients with chronic gastritis, 185 patients with gastric erosion, 16 patients with gastric ulcer, 13 patients with duodenal ulcer, 12 patients with Barrett's esophagus, 9 patients with gastric cancer, 7 patients with esophageal cancer, and others.
Table 2.
Incidence of organic lesions in patients who met the Rome IV criteria for FD and those who did not
| Met Rome IV criteria for FD (n = 381) | Did not meet Rome IV criteria for FD (n = 219) | |
| Barrett's esophagus (%) | 8 (2.1) | 4 (1.8) |
| Esophageal candidiasis (%) | 0 (0) | 4 (1.8) |
| Esophagus cancer (%) | 2 (0.5) | 5 (2.3) |
| Gastric ulcer (%) | 9 (2.4) | 7 (3.2) |
| Gastric erosion (%) | 120 (31.5) | 65 (29.7) |
| Gastric cancer (%) | 6 (1.6) | 3 (1.4) |
| Duodenal ulcer (%) | 7 (1.8) | 6 (2.7) |
| Duodenal erosion (%) | 2 (0.5) | 0 (0) |
Values are expressed as n (%).
FD, functional dyspepsia.
Validation of FD diagnosis by the Rome IV criteria
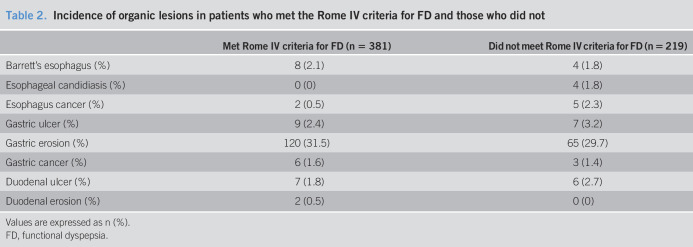
In the 196 patients diagnosed with true FD according to the reference standard, 132 met the Rome IV criteria for FD, and the sensitivity was 67.3%. Among 404 patients who were not diagnosed with true FD according to the reference standard, 155 did not meet the Rome IV criteria, and the specificity was 38.4% (Figure 2). Therefore, the positive and negative LRs of FD diagnosed by Rome IV criteria were 1.09 (95% CI 0.97–1.24) and 0.85 (95% CI 0.67–1.08), respectively (Table 3). The area under the ROC curve of the Rome IV criteria was 0.53 (95% CI 0.48–0.58).
Figure 2.
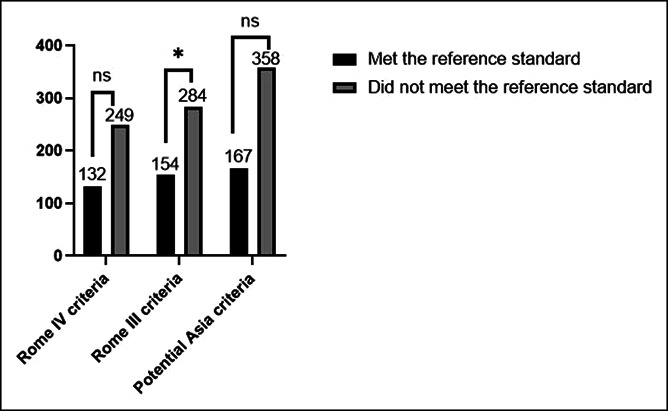
Functional dyspepsia (FD) according to Rome IV, Rome III, and potential Asia criteria. In the 196 patients diagnosed with true FD according to the reference standard, 132 met the Rome IV criteria for FD, 154 met the Rome III criteria for FD, and 167 met the potential Asia criteria for FD; among 404 patients who were not diagnosed with true FD according to the reference standard, 249 met the Rome IV criteria for FD, 284 met the Rome III criteria for FD, and 358 met the potential Asia criteria for FD. Ns, no significance; *P < 0.05.
Table 3.
Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios for the Rome IV, Rome III, and potential Asia criteria
| Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Positive predictive value (%) (95% CI) | Negative predictive value (%) (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | |
| Rome IV FD (n = 381) | 67.3 (60.7–74.0) | 38.4 (33.6–43.1) | 34.6 (29.9–39.5) | 70.8 (64.7–76.9) | 1.09 (0.97–1.24) | 0.85 (0.67–1.08) |
| Rome III FD (n = 438) | 78.6 (72.8–84.4) | 29.7 (25.2–34.2) | 35.2 (30.7–39.7) | 74.1 (67.3–80.9) | 1.12 (1.01–1.23) | 0.72 (0.50–1.04) |
| Asia FD (n = 525) | 85.2 (80.2–90.2) | 11.4 (8.3–14.5) | 31.8 (27.8–35.8) | 61.3 (50.1–72.6) | 0.96 (0.90–1.03) | 1.30 (0.84–2.00) |
CI, confidence interval; FD, functional dyspepsia.
Validation of FD diagnosis by the Rome III criteria
In the 196 patients diagnosed with true FD according to the reference standard, 154 met the Rome III criteria for FD, and the sensitivity was 78.6%. Among 404 patients who were not diagnosed with true FD according to the reference standard, 120 did not meet the Rome III criteria, and the specificity was 29.7% (Figure 2). The positive LR of FD diagnosed by the Rome III criteria was 1.12 (95% CI 1.01–1.23), and the negative LR of FD diagnosed by the Rome III criteria was 0.72 (95% CI 0.50–1.04) (Table 3). The area under the ROC curve of the Rome III criteria was 0.54 (95% CI 0.49–0.59).
Validation of FD diagnosis by the potential Asia criteria
Among the 196 patients diagnosed with true FD according to the reference standard, 167 met the potential Asia criteria for FD, and the sensitivity was 85.2%. Among 404 subjects who were not diagnosed with true FD according to the reference standard, 46 did not meet the potential Asia criteria, and the specificity was 11.4% (Figure 2). The positive LR of FD diagnosed by the potential Asia criteria was therefore 0.96 (95% CI 0.90–1.03), while the negative LR of FD diagnosed by the potential Asia criteria was 1.30 (95% CI 0.84–2.00) (Table 3). The area under the ROC curve of the potential Asia criteria was 0.52 (95% CI 0.47–0.57).
Comparison of the 3 criteria
The Rome IV criteria, Rome III criteria, and potential Asia criteria were compared. There was no significant difference in area under the ROC curve of the Rome IV, Rome III, or potential Asia criteria for the diagnosis of FD (P > 0.05, 1-way ANOVA).
DISCUSSION
To the best of our knowledge, this study was not only the first to compare the accuracy of the Rome IV and Rome III criteria in identifying patients with FD but also the first to test the accuracy of the potential Asia criteria in identifying FD. FD is one of the most common clinical diseases due to its high incidence and its chronic recurrence nature (7,23,24). The Rome IV criteria were published in 2016; previous studies comparing Rome IV with Rome III standards were mostly conducted in children (25–27) and were mostly about irritable bowel syndrome (28–30). In this cross-sectional study, we showed that the Rome IV criteria had a sensitivity of 67.3% and a specificity of 38.4% for the diagnosis of FD. The area under the ROC curve of the Rome IV criteria was 0.53 (95% CI 0.48–0.58). And, there was no significant difference in area under the ROC curve of the Rome IV, Rome III, or potential Asia criteria for the diagnosis of FD.
In this study, the duration of dyspepsia was adjusted to 3 months on the basis of the Rome IV criteria, and the results showed that there was no significant difference in area under the ROC curves of Rome IV, Rome III, or potential Asia criteria in the diagnosis of FD. There was no difference between the dyspepsia period of 3 months and the Rome dyspepsia standard of 6 months in the Asian population, which may be because the Asian population was more likely to be hospitalized for consultation and it was convenient for Asian populations to undergo endoscopic examination. However, because of the limited number of patients, further studies are needed to confirm this hypothesis.
The main aim of this study was to evaluate the accuracy of the Rome IV criteria in identifying patients with FD. The results demonstrated that the Rome IV criteria were of limited value in predicting the diagnosis of FD in patients with upper GI symptoms, with a positive LR of FD of 1.09 and a negative LR of FD of 0.85. In terms of the positive LR and negative LR, the performance of the Rome IV criteria was similar to that of the Rome III criteria. However, the positive LR of the Rome IV and Rome III criteria for FD was higher than that of the potential Asia criteria, and the negative LR of the potential Asia criteria for FD was higher. The results of the area under the ROC curve of the 3 classification criteria were very similar, and the 95% CIs overlapped, showing no significant difference in performance.
This study had several limitations. First, the study population we included was selected based on the presence of dyspeptic symptoms according to a broad definition, but did not include all the people with upper GI symptoms. Therefore, the true sensitivity and negative predictive value of the Rome IV criteria, Rome III criteria, or potential Asia criteria may have been artificially increased, which might have led to an overestimation of the accuracy. In addition, although our study was conducted at 2 tertiary hospitals, the study population mainly came from Northwest China, and there may be regional differences. Larger sample data from all over China will be needed in the future.
The reference standard for defining true FD was no evidence of organic, systemic, or metabolic disease that was likely to explain the dyspeptic symptom. In our study, the reference standard for true FD included the evaluation normal upper GI endoscopy, normal routine blood tests, normal epigastric ultrasounds, and normal liver function tests, which was a strength of this study. In our study, among individuals meeting the Rome IV criteria for FD, the proportion of organic diseases diagnosed by endoscopy was 39.6% (154/381), and in individuals meeting the Rome III criteria for FD, the proportion of organic diseases was 39.3% (172/438). A previous cross-sectional study of functional and organic dyspepsia showed that in 783 patients who met the Rome III criteria for dyspepsia, 29.5% (231/783) had organic dyspepsia following upper GI endoscopy (31). A study of dyspepsia in South China demonstrated that after screening 1,304 patients using Rome III criteria, 165 patients had organic dyspepsia, and 203 patients were diagnosed with FD. The proportion of patients who met the Rome III criteria for FD with organic dyspepsia by endoscopy was 44.8 (165/368) (32). In other studies of FD vs organic dyspepsia, the proportion of patients who met the Rome III criteria for FD with organic dyspepsia was between 23% and 34% (33–36). Overall, the proportion of organic dyspepsia in our study was slightly higher than in most other studies. The reason may be that all patients included in this study required epigastric ultrasounds and routine blood tests, and partial FD patients who did not have epigastric ultrasounds or routine blood tests were excluded. Among these patients, the number of FD diagnoses was relatively high, while the number of organic dyspepsia diagnoses was relatively low. Another possible reason was that the Asian population had a slightly higher proportion of patients with organic dyspepsia.
In summary, there was no significant difference between the Rome IV, Rome III, or potential Asia criteria in diagnosing FD. The Rome IV criteria were no better than the Rome III or potential Asia criteria in identifying FD and were not helpful in identifying patients with FD. Hence, although the Rome criteria remain useful for defining patients with FD for inclusion into clinical treatment trials, they should not be used for diagnosing FD.
CONFLICTS OF INTEREST
Guarantor of the article: Zhongcao Wei, MD.
Specific author contributions: Zhongcao Wei, MD, and Qian Yang, MD, contributed equally to this work. Z.W., Y.P., J.W., and N.L.: designed the research. Z.W., Qian Yang, Qi Yang, and J.Y.: performed the research. X.T., X.X., and C.X.: contributed analytic tools. Z.W. and Qian Yang: analyzed the data. Z.W., Y.P., J.W., and N.L.: wrote the paper. All authors finally approved the manuscript.
Financial support: None to report.
Potential competing interests: None to report.
Trial registration: The Rome IV part of our study was registered at ClinicalTrials.gov (number NCT03479528).
Study Highlights.
WHAT IS KNOWN
✓ The accuracy of the Rome IV criteria in identifying patients with functional dyspepsia (FD) was unclear.
✓ No studies have compared the differences between the Rome IV, Rome III, and potential Asia criteria in identifying patients with FD.
WHAT IS NEW HERE
✓ In this cross-sectional study, we found that there was no significant difference in area under the receiver operating characteristic curve of Rome IV, Rome III, or potential Asia criteria in identifying FD.
TRANSLATIONAL IMPACT
✓ The Rome IV criteria were not helpful in identifying patients with FD. Hence, they should not be used for this purpose.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A456; http://links.lww.com/CTG/A457; and http://links.lww.com/CTG/A458.
REFERENCES
- 1.ASGE Standards of Practice Committee; Shaukat A, Wang A, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc 2015;82(2):227–32. [DOI] [PubMed] [Google Scholar]
- 2.Stanghellini V, Tosetti C, Barbara G, et al. Management of dyspeptic patients by general practitioners and specialists. Gut 1998;43(Suppl 1):S21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feld L, Cifu AS. Management of dyspepsia. JAMA 2018;319(17):1816–7. [DOI] [PubMed] [Google Scholar]
- 4.Aziz I, Palsson OS, Törnblom H, et al. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: A cross-sectional population-based study. Lancet Gastroenterol Hepatol 2018;3(4):252–62. [DOI] [PubMed] [Google Scholar]
- 5.Chen SL, Gwee KA, Lee JS, et al. Systematic review with meta-analysis: Prompt endoscopy as the initial management strategy for uninvestigated dyspepsia in Asia. Aliment Pharmacol Ther 2015;41(3):239–52. [DOI] [PubMed] [Google Scholar]
- 6.Masuy I, Carbone F, Holvoet L, et al. The effect of rikkunshito on gastrointestinal symptoms and gastric motor function: The first study in a Belgian functional dyspepsia population. Neurogastroenterol Motil 2019;32:e13739. [DOI] [PubMed] [Google Scholar]
- 7.Tack J, Masuy I, Van Den Houte K, et al. Drugs under development for the treatment of functional dyspepsia and related disorders. Expert Opin Investig Drugs 2019;28(10):871–89. [DOI] [PubMed] [Google Scholar]
- 8.Kang SJ, Park B, Shin CM. Helicobacter pylori eradication therapy for functional dyspepsia: A meta-analysis by region and H. pylori prevalence. J Clin Med 2019;8:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talley NJ, Goodsall T, Potter M. Functional dyspepsia. Aust Prescr 2017;40(6):209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford AC, Forman D, Bailey AG, et al. Initial poor quality of life and new onset of dyspepsia: Results from a longitudinal 10-year follow-up study. Gut 2007;56(3):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Zanten SV, Wahlqvist P, Talley NJ, et al. Randomised clinical trial: The burden of illness of uninvestigated dyspepsia before and after treatment with esomeprazole: Results from the STARS II study. Aliment Pharmacol Ther 2011;34(7):714–23. [DOI] [PubMed] [Google Scholar]
- 12.Lacy BE, Weiser KT, Kennedy AT, et al. Functional dyspepsia: The economic impact to patients. Aliment Pharmacol Ther 2013;38(2):170–7. [DOI] [PubMed] [Google Scholar]
- 13.Moayyedi P, Lacy BE, Aadrews CN, et al. ACG and CAG clinical guideline: Management of dyspepsia. Am J Gastroenterol 2017;112(7):988–1013. [DOI] [PubMed] [Google Scholar]
- 14.Manabe N, Haruma K, Hata J, et al. Clinical characteristics of Japanese dyspeptic patients: Is the Rome III classification applicable? Scand J Gastroenterol 2010;45(5):567–72. [DOI] [PubMed] [Google Scholar]
- 15.Miwa H, Ghoshal UC, Fock KM, et al. Asian consensus report on functional dyspepsia. J Gastroenterol Hepatol 2012;27(4):626–41. [DOI] [PubMed] [Google Scholar]
- 16.Holtmann G, Talley NJ. Functional dyspepsia. Curr Opin Gastroenterol 2015;31(6):492–8. [DOI] [PubMed] [Google Scholar]
- 17.Ford AC, Bercik P, Morgan DG, et al. The Rome III criteria for the diagnosis of functional dyspepsia in secondary care are not superior to previous definitions. Gastroenterology 2014;146(4):932–40; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 18.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology 2016;150(6):1380–92. [DOI] [PubMed] [Google Scholar]
- 19.Holtmann G, Talley NJ, Liebregts T, et al. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med 2006;354(8):832–40. [DOI] [PubMed] [Google Scholar]
- 20.Tack J, Ly HG, Carbone F, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol 2016;14(3):385–92.e4. [DOI] [PubMed] [Google Scholar]
- 21.Enck P, Azpiroz F, Boeckxstaens G, et al. Functional dyspepsia. Nat Rev Dis Primers 2017;3:17081. [DOI] [PubMed] [Google Scholar]
- 22.Tack J, Talley NJ. Functional dyspepsia: Symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol 2013;10(3):134–41. [DOI] [PubMed] [Google Scholar]
- 23.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006;130(5):1466–79. [DOI] [PubMed] [Google Scholar]
- 24.Lacy BE, Saito YA, Camilleri M, et al. Effects of antidepressants on gastric function in patients with functional dyspepsia. Am J Gastroenterol 2018;113(2):216–24. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Chen M, Peng S, et al. The Rome IV versus Rome III criteria for heartburn diagnosis: A comparative study. United European Gastroenterol J 2018;6(3):358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saps M, Velasco-Benitez CA, Langshaw AH, et al. Prevalence of functional gastrointestinal disorders in children and adolescents: Comparison between Rome III and Rome IV criteria. J Pediatr 2018;199:212–6. [DOI] [PubMed] [Google Scholar]
- 27.Edwards T, Friesen C, Schurman JV. Classification of pediatric functional gastrointestinal disorders related to abdominal pain using Rome III vs. Rome IV criterions. BMC Gastroenterol 2018;18(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin L, Chang L. Benefits and pitfalls of change from Rome III to Rome IV criteria for irritable bowel syndrome and fecal incontinence. Clin Gastroenterol Hepatol 2020;18:297–9. [DOI] [PubMed] [Google Scholar]
- 29.Black CJ, Yiannakou Y, Houghton LA, et al. Epidemiological, clinical, and psychological characteristics of individuals with self-reported irritable bowel syndrome based on the Rome IV vs Rome III criteria. Clin Gastroenterol Hepatol 2020;18:392–8.e2. [DOI] [PubMed] [Google Scholar]
- 30.Aziz I, Törnblom H, Palsson OS, et al. How the change in IBS criteria from Rome III to Rome IV impacts on clinical characteristics and key pathophysiological factors. Am J Gastroenterol 2018;113(7):1017–25. [DOI] [PubMed] [Google Scholar]
- 31.Gracie DJ, Bercik P, Morgan DG, et al. No increase in prevalence of somatization in functional vs organic dyspepsia: A cross-sectional survey. Neurogastroenterol Motil 2015;27(7):1024–31. [DOI] [PubMed] [Google Scholar]
- 32.Xu JH, Lai Y, Zhuang LP, et al. Certain dietary habits contribute to the functional dyspepsia in south China rural area. Med Sci Monit 2017;23:3942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XB, Liu WZ, Ge ZZ, et al. Analysis of clinical characteristics of dyspeptic symptoms in Shanghai patients. Chin J Dig Dis 2005;6(2):62–7. [DOI] [PubMed] [Google Scholar]
- 34.Faintuch JJ, Silva FM, Navarro-Rodriguez T, et al. Endoscopic findings in uninvestigated dyspepsia. BMC Gastroenterol 2014;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford AC, Marwaha A, Lim A, et al. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2010;8(10):830–7, 837.e1–2. [DOI] [PubMed] [Google Scholar]
- 36.Vakil N, Talley N, Van Zanten SV, et al. Cost of detecting malignant lesions by endoscopy in 2741 primary care dyspeptic patients without alarm symptoms. Clin Gastroenterol Hepatol 2009;7(7):756–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.