Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, children, endothelium, lung injury, pediatric, sepsis
Abstract
Objectives:
Acute respiratory distress syndrome occurring in the setting of direct versus indirect lung injury may reflect different pathobiologies amenable to different treatment strategies. We sought to test whether a panel of plasma biomarkers differed between children with sepsis-associated direct versus indirect acute respiratory distress syndrome. We hypothesized that a biomarker profile indicative of endothelial activation would be associated with indirect acute respiratory distress syndrome.
Design:
Observational cohort.
Setting:
Academic PICU.
Subjects:
Patients less than 18 years old with sepsis-associated direct (pneumonia, n = 52) or indirect (extrapulmonary sepsis, n = 46) acute respiratory distress syndrome.
Interventions:
None.
Measurements and Main Results:
Of 58 biomarkers examined, 33 differed by acute respiratory distress syndrome subtype. We used classification and regression tree methodology to examine associations between clinical and biochemical markers and acute respiratory distress syndrome subtype. The classification and regression tree model using only clinical variables (age, sex, race, oncologic comorbidity, and Pediatric Risk of Mortality-III score) performed worse than the classification and regression tree model using five clinical variables and 58 biomarkers. The best classification and regression tree model used only four endothelial biomarkers, including elevated angiopoietin-2/angiopoietin-1 ratio, vascular cell-adhesion molecule, and von Willebrand factor, to identify indirect acute respiratory distress syndrome. Test characteristics were 89% (80–97%) sensitivity, 80% (69–92%) specificity, positive predictive value 84% (74–93%), and negative predictive value 86% (76–96%).
Conclusions:
Indirect lung injury in children with acute respiratory distress syndrome is characterized by a biomarker profile indicative of endothelial activation, excess inflammation, and worse outcomes. A model using four biomarkers has the potential to be useful for more precisely identifying patients with acute respiratory distress syndrome whose pathobiology may respond to endothelial-targeted therapies in future trials.
Acute respiratory distress syndrome (ARDS) that occurs in the setting of infection can result from indirect (e.g., extrapulmonary sepsis) or direct (i.e., pneumonia) lung injury. Etiology of lung injury is not always considered in trials of ARDS, which may have contributed to negative trials of ARDS therapies. This issue is exacerbated in children, as pediatric ARDS management is often extrapolated from adults, despite possessing a distinct epidemiology (1). There are a paucity of studies examining the differences in biologic mechanism of pediatric ARDS, and improved understanding of the pathobiology of lung injury may allow an evaluation of differential response to therapies stratified by biologic subtypes.
Studies of adults with ARDS suggest differences in disease pathogenesis of ARDS arising from indirect versus direct lung injury (2), with indirect ARDS characterized by increased activation of the vascular endothelium and direct ARDS is characterized by epithelial injury (2–4). Our prior study suggested a potential role of endothelial activation in indirect ARDS occurring in children with extrapulmonary sepsis; however, this study excluded children with direct ARDS (5). Pneumonia, which reflects direct lung injury, is the most common cause of ARDS in children (1), and it remains unknown how its biomarker profile differs from that of indirect ARDS due to extrapulmonary infection.
Prior reports examining the pathobiologic mechanism of indirect ARDS in children, adults, and animal models have documented altered blood levels of biomarkers involved in regulation of vascular permeability (2, 4–7), neutrophil adhesion and chemotaxis (5, 8), platelet activation (5, 9, 10), and inflammation (3, 11). Prior reports examining the pathobiology of direct ARDS in adults (2) and mice (12) have documented increased circulating markers of lung epithelial injury. Few reports of indirect ARDS and no reports of direct ARDS included children, and the pathobiology of indirect versus direct ARDS has never been directly compared in children. Furthermore, these limited studies have interrogated relatively few biomarkers, thus providing only a partial view of endothelial activation and epithelial injury, and thus may miss an optimal combination of biomarkers to differentiate indirect from direct ARDS, and findings require external validation. Improved understanding of the pathobiology of lung injury in indirect versus direct ARDS could better inform our understanding of expected disease course (13) or response to therapies (14).
Focusing on children with pulmonary and extrapulmonary sepsis, the most common causes of ARDS in children (1), we sought to determine if alterations in a comprehensive panel of plasma biomarkers differed in children with sepsis-associated indirect versus direct ARDS. We hypothesized that alterations in specific biomarkers would differ by ARDS subtype. We further hypothesized that a biomarker profile consistent with endothelial activation would be associated with indirect ARDS.
MATERIALS AND METHODS
Study Design and Population
This study was approved with a waiver of informed consent by the Institutional Review Board at the Children’s Hospital of Philadelphia (CHOP). We conducted a secondary analysis of prospectively collected plasma from a cohort of pediatric patients with ARDS admitted to the PICU of a single academic children’s hospital (CHOP). Participants had been enrolled in one of two prospective observational parent studies of pediatric sepsis (15) and ARDS (4, 16) between May 2014 and January 2018.
The parent sepsis study included patients less than 18 years old with severe sepsis or septic shock defined as: 1) greater than or equal to two systemic inflammatory response syndrome criteria, 2) suspected or confirmed systemic infection, and 3) greater than or equal to two organ system dysfunctions or cardiovascular dysfunction (17), and it excluded patients with WBC count less than 0.5 × 103/μL, known mitochondrial disorder, or unrepaired cyanotic congenital heart disease. The parent ARDS study included intubated patients greater than 1 month and less than 18 years old with ARDS defined by Berlin criteria, which were in use when the first patient was enrolled, including: 1) acute respiratory failure within 7 days of known risk factor requiring mechanical ventilation, 2) Pao2/Fio2 ≤ 300 in two consecutive arterial blood gas samples drawn at least 2 hours apart with the patient receiving invasive positive end-expiratory pressure at least 5 cm H2O, and 3) bilateral infiltrates on chest x-ray (18), and it excluded patients with chronic invasive mechanical ventilation, respiratory failure from primarily cardiac failure, or unrepaired cyanotic congenital heart disease.
Patients eligible for the current study had blood collected as part of their participation in the parent study, had sufficient volume of residual blood to permit biomarker analysis, and had consented for residual blood to be used for future research. This analysis was limited to patients who met criteria for sepsis (17) within 72 hours of meeting criteria for ARDS (18).
The primary site of infection was determined to be pulmonary (i.e., pneumonia) or extrapulmonary using established criteria (19, 20) with three-person adjudication of cases in which the site of infection was ambiguous after initial review. Patients with “direct ARDS” had infectious pneumonia and patients with “indirect ARDS” had extrapulmonary sepsis.
Biomarkers
Blood was collected in an EDTA or lithium heparin tube less than or equal to 72 hours of meeting criteria for ARDS. Samples were centrifuged within 30 minutes of collection at 3,000 × g for 10 minutes and aliquoted plasma was stored at –80°C. Plasma was thawed once at the time of biomarker measurement, which was performed at the Penn Center for Cellular Immunotherapies.
Biomarker panel components were defined a priori based on prior reports suggesting utility in sepsis, ARDS, or pathways distinguishing these syndromes including inflammatory biomarkers (C-reactive protein, endocan, interleukins, interferons, monocyte chemoattractant protein, monocyte induced by interferon γ, macrophage inflammatory protein-1α/β, serum glycoprotein 130, soluble receptor for advanced glycation end-products [sRAGE], and tumor necrosis factor [TNF]-α/-β [4, 21–23]) and endothelial biomarkers, including: angiopoietin family biomarkers (Ang-1, Ang-2, Ang-2/-1 ratio, and Tie-2 [5, 6, 24]), cell adhesion molecules (intracellular adhesion molecule, vascular cell-adhesion molecule [VCAM], E-selectin, and endocan [25, 26]), vascular endothelial growth factor (VEGF) family biomarkers (VEGF, soluble fms-like tyrosine kinase, VEGF-R1, VEGF-R2, and VEGF-R3 [5, 27]), prothrombotic (von Willebrand factor [vWF] and thrombomodulin [9, 28]), chemotactic proteins (eotaxin, interferon gamma-induced protein-10, cluster of differentiation [CD]-14, CD-30, and CD-163 [29]), and growth factors (epidermal growth factor, fibroblast growth factor, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and hepatocyte growth factor [1, 30]). Biomarkers were measured using a combination of commercially available assays (Life Technologies, Carlsbad, CA; EMD Millipore; Darmstadt, Germany), custom multiplex panels (R&D Systems, Minneapolis, MN), and enzyme-linked immunosorbent assay (ELISA) with human-specific reagents. Samples were run in duplicate, and standard curves for each analyte were required to have R2 > 95%.
Data Collection
Clinical data including demographics, comorbidities, and primary site of infection were collected using the Research Data Capture system (31) by one investigator (J.E.W.), who reviewed all patient charts and was blinded to biomarker measurements. Oxygenation index (OI [Fio2 × mean airway pressure]/Pao2) and Pao2/Fio2 were calculated to reflect ARDS severity at the time of biomarker measurement. In the absence of arterial blood gas data, we computed oxygen saturation index (Fio2 × mean airway pressure/Spo2), and Spo2/Fio2 ratio then converted these values to OI and Pao2/Fio2 ratios (32), using accepted cutoffs (1). Illness severity less than 12 hours of PICU admission was summarized using the Pediatric Risk of Mortality (PRISM)-III score (33).
Outcomes
The main outcomes were biomarker concentrations by ARDS subtypes.
Confounders
Clinical variables were chosen a priori as potential confounders based on biologic plausibility of an association with the exposure or outcome, providing they were absent in the causal pathway linking exposure and outcome (34). We considered five confounders: age, sex, race, oncologic comorbidity, and PRISM-III score.
Statistical Analyses
Analyses were performed using Stata version 14 (StataCorp, College Station, TX) and SAS (SAS Institute, Cary, NC). We reported median and interquartile range for continuous variables and proportions for categorical variables. We compared continuous variables using Wilcoxon rank sum and proportions using chi-squared or Fisher exact test.
First, we determined the association between biomarkers and ARDS subtype (indirect/direct) using univariate logistic regression. When used as predictors, biomarkers were expressed as log-transformed units of standard deviation to facilitate comparison with other arrays. Next, we generated two models to determine the association between clinical or biochemical predictor variables and ARDS subtype using the classification and regression tree (CART [35]). Compared with traditional regression approaches, CART provides flexibility to allow for nonlinear effects and interaction effects of predictors, and it generates optimal cutoffs for continuous predictors (35).
We also performed a supplementary analysis using elastic net (EN) as a comparator method, since it takes into account variable grouping, in order to account for potential biomarker clusters that naturally associated. We used CART rather than EN as the primary analytic method, because we did not know if the relationship between biomarkers and ARDS type would be linear, CART does not require selection of tuning parameters, and the optimal cutoff values required for EN, which represent the probability of ARDS type per patient, are subject to bias.
The first CART model used only the five clinical variables (age, sex, race, oncologic comorbidity, and PRISM-III score) as predictors, and the second model used all 58 biomarkers and the five clinical variables. Each model resulted in a decision tree in which each branchpoint was determined by a predictor variable and a cutoff value, and each end node contains a predictor for the outcome variable. We used five-fold cross-validation to mitigate overfitting. We reported the test characteristics for each decision tree, and the models were compared using a paired likelihood regression method (36).
The EN model used 10-fold cross validation to select optimal tuning parameters α and λ for penalized logistic regression models. We applied EN using the selected α and λ to select a set of predictors from all biomarkers. The final models were refitted using the selected variables. We used individual predicted probabilities to generate the receiver-operating-characteristic curve (ROC) and reported the area under the ROC curve (AUC). We chose a probability cutoff optimized for combined sensitivity and specificity.
RESULTS
Among 332 potentially eligible patients, 98 met all inclusion criteria, including 46 with indirect ARDS and 52 with direct ARDS (Supplemental Fig. 1, http://links.lww.com/CCX/A443). Fifteen cases (15%) required three-person adjudication to determine the primary site of infection. Patient characteristics are shown in Table 1. Patients with direct ARDS had a higher prevalence of pulmonary comorbidity (p = 0.006). PRISM-III score was higher in patients with indirect ARDS (p = 0.007). Fifteen patients (15%) died prior to hospital discharge, including 11 with indirect and four with direct ARDS (p = 0.046).
TABLE 1.
Patient Demographics and Clinical Characteristics by Group Are Shown
| Patient Characteristic | Indirect ARDS (n = 46) | Direct ARDS (n = 52) | pa |
|---|---|---|---|
| Age (yr) | 5.1 (3.6–13.5) | 5.9 (2.5–13.2) | 0.83 |
| Sex, n (%) | |||
| Female | 21 (46) | 23 (44) | 0.53 |
| Male | 25 (54) | 29 (56) | |
| Race | |||
| Black/African American | 10 (22) | 15 (29) | 0.04a |
| Other | 8 (17) | 18 (35) | |
| White | 28 (61) | 19 (37) | |
| Comorbiditiesb | |||
| None | 9 (20) | 10 (19) | 0.58 |
| Cardiac | 9 (20) | 10 (19) | 0.58 |
| Pulmonary | 10 (22) | 26 (50) | 0.006a |
| Gastrointestinal | 19 (41) | 18 (35) | 0.54 |
| Endocrine | 7 (15) | 10 (19) | 0.40 |
| Renal | 1 (2) | 0 (0) | 0.47 |
| Rheumatologic | 3 (7) | 0 (0) | 0.10 |
| Immunologic | 3 (7) | 4 (8) | 0.57 |
| Hematologic | 4 (9) | 2 (4) | 0.28 |
| Oncologic | 11 (24) | 4 (8) | 0.03a |
| Musculoskeletal | 7 (15) | 6 (12) | 0.41 |
| Dermatologic | 1 (2) | 1 (2) | 0.72 |
| Neurologic | 18 (39) | 28 (54) | 0.11 |
| Immunosuppressedc | 16 (35) | 9 (17) | 0.40 |
| Oxygenation index | 7.6 (5.1–11) | 8 (5.1–13.4) | 0.52 |
| Pao2/Fio2 ratio | 241 (166–291) | 225 (153–291) | 0.50 |
| Pediatric Risk of Mortality-III score | 15 (10–24) | 9 (6–15) | 0.007a |
| Sepsis source | |||
| Respiratory | 0 (0) | 52 (100) | |
| Blood | 19 (41) | 0 (0) | |
| Gastrointestinal | 12 (26) | 0 (0) | |
| Urinary | 5 (11) | 0 (0) | |
| CNS | 1 (2) | 0 (0) | |
| Skin | 2 (4) | 0 (0) | |
| Musculoskeletal | 1 (2) | 0 (0) | |
| Unknown | 6 (13) | 0 (0) | |
ARDS = acute respiratory distress syndrome.
ap value reflects difference between indirect versus direct ARDS. Significance is defined as p < 0.05.
bCategories are not mutually exclusive as some patients had more than one baseline comorbidity.
cImmunosuppressed patients included those with immune deficiencies and those receiving immunosuppressive therapies.
Data are presented as median (interquartile range) or n (%). Boldface values indicate race, pulmonary and oncologic comorbidity, and Pediatric Risk of Mortality-III score differed by ARDS type.
Individual Biomarkers and ARDS Subtype
Figure 1 shows the unadjusted odds of indirect ARDS for each biomarker. Supplemental Table 1 (http://links.lww.com/CCX/A445) shows biomarker values by ARDS subtype. Thirty-three biomarkers differed by ARDS subtype; 31 were higher in indirect ARDS, whereas Ang-1 and regulated on activation, normal T cell expressed and secreted (RANTES) were higher in direct ARDS.
Figure 1.
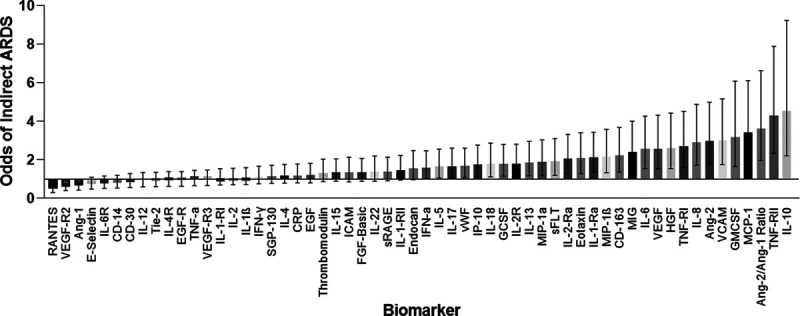
Odds of indirect acute respiratory distress syndrome (ARDS) by biomarker. Logistic regression was used to determine the association between biomarker and ARDS subtype. Odds ratio (OR) < 1 indicates an association with direct ARDS and OR > 1 indicates an association with indirect ARDS. Whiskers represent 95% CI. Ang = angiopoietin, ARDS = acute respiratory distress syndrome, CD = cluster of differentiation, CRP = C-reactive protein, EGF = epidermal growth factor, FGF = fibroblast growth factor, GCSF = granulocyte colony-stimulating factor, GMCSF = granulocyte-macrophage colony-stimulating factor, HGF = hepatocyte growth factor, ICAM = intracellular adhesion molecule, IFN = interferon, IL = interleukin, IP = interferon gamma-induced protein, MCP = monocyte chemoattractant protein, MIG = monocyte induced by interferon γ, MIP = macrophage inflammatory protein, –R = –receptor, RANTES = regulated on activation, normal T cell expressed and secreted, sFLT = soluble fms-like tyrosine kinase, SGP = serum glycoprotein, sRAGE = soluble receptor for advanced glycation end-products, Tie-2 = tyrosine kinase with immunoglobulin-like loop epidermal growth factor homology domain 2, TNF = tumor necrosis factor, VCAM = vascular adhesion molecule, VEGF = vascular endothelial growth factor, vWF = von Willebrand factor.
Biomarker Clusters and ARDS Subtype
In the first CART model (model 1), which used five clinical variables (age, sex, nonwhite/white race, oncologic comorbidity, and PRISM-III) as predictors and ARDS subtype as the outcome, a tree using age and PRISM-III score discriminated indirect from direct ARDS (Supplemental Fig. 2, http://links.lww.com/CCX/A444).
In the second CART model (model 2), which used all biomarkers plus five clinical variables as predictors, elevated Ang-2/Ang-1 ratio, VCAM, and vWF concentration identified indirect ARDS (Fig. 2).
Figure 2.
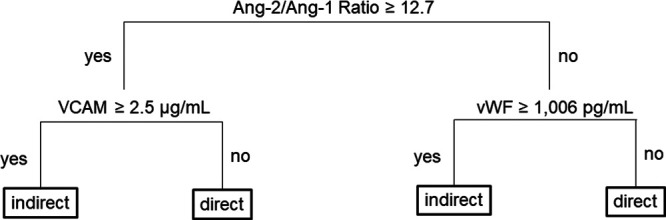
Decision tree from classification and regression analysis (CART) analysis using biomarkers and clinical variables. CART analysis using all 58 biomarkers and five clinical variables (age, sex, non-White/White race, oncologic comorbidity, and Pediatric Risk of Mortality-III score), only angiopoietin (Ang)-2/Ang-1 ratio, vascular adhesion molecule (VCAM), and von Willebrand factor (vWF) concentration-discriminated acute respiratory distress syndrome (ARDS) subtype. The decision tree shows branchpoints based on raw biomarker concentration determined by the model. In the first generation, the branchpoint is determined by Ang-2/Ang-1 ratio ≥ 12.7 (high) vs < 12.7 (low). In the second generation, branchpoints are determined by VCAM for patients with high Ang-2/Ang-1 ratio and by vWF for patients with low Ang-2/Ang-1 ratio. Indirect ARDS is associated with VCAM ≥ 2.5 µg/mL and high Ang-2/Ang-1 ratio, and with vWF ≥ 1,006 pg/mL and low Ang-2/Ang-1 ratio.
The positive likelihood ratio (LR+) of model 1 was 2.03, the LR+ of model 2 was 4.52, and the ratio of the two was 0.45 (95% CI, 0.25–0.80, p = 0.007). The negative likelihood ratio (LR–) of model 1 was 0.20, the LR– for model 2 was 0.14, the ratio of the two was 1.42 (95% CI, 0.55–3.70, p = 0.5). Both ratios suggest that model 2 is significantly better for discriminating ARDS subtype than model 1. Diagnostics for both models are presented in Table 2. Compared with model 1, model 2 had similar sensitivity (89% [80–97%] vs 89% [77–78%]), superior specificity (80% [69–92%] vs 57% [41–71%]), positive predictive value (84% [74–93%] vs 70% [57–80%]), and negative predictive value (86% [76–96%] vs 81% [64–93%]).
TABLE 2.
Diagnostic Characteristics of Two Classification and Regression Analysis Models
| Model | Indirect ARDS (n = 46) | Direct ARDS (n = 52) | Sensitivity % (95% CI) | Specificity % (95% CI) | Positive Predictive Value % (95% CI) | Negative Predictive Value % (95% CI) |
|---|---|---|---|---|---|---|
| Model 1 (clinical variables) | 89 (77–96) | 57 (41–71) | 70 (57–80) | 81 (64–93) | ||
| Predicted indirect | 26 | 6 | ||||
| Predicted direct | 20 | 46 | ||||
| Model 2 (biomarkers and clinical variables) | 89 (80–97) | 80 (69–92) | 84 (74–93) | 86 (76–96) | ||
| Predicted indirect | 37 | 6 | ||||
| Predicted direct | 9 | 46 | ||||
ARDS = acute respiratory distress syndrome.
In the EN model, which used all biomarkers, high Ang-2, interleukin (IL)-8, TNF-RII, and low RANTES identified indirect ARDS. Compared with CART model 2, EN had inferior sensitivity (65% [51–78%] vs 86% [80–97%]), similar specificity (83% [69–92%] vs 80% [69–92%]), positive predictive value (81% [66–91%] vs 84% [74–93%]), and inferior negative predictive value (68% [54–80%] vs 86% [76–96%]). The AUC was 0.84 and the optimal cutoff probability was 68%. Diagnostics are summarized in Supplemental Table 2 (http://links.lww.com/CCX/A446).
DISCUSSION
In this cohort of children with sepsis-associated ARDS, 33 of 58 biomarkers differed by ARDS subtype, and a biomarker profile indicative of endothelial activation and inflammation was associated with indirect ARDS. Using CART analysis, a model using four endothelial biomarkers discriminated ARDS subtype better than a model using clinical variables. Using EN analysis, a model using four biomarkers (one endothelial and three inflammatory) discriminated ARDS subtype. Elevated Ang-2 was identified by both models to be associated with indirect ARDS. This result supports a role for endothelial activation and inflammation in the pathogenesis of indirect, but not direct, ARDS in children. Discrimination of disease subphenotypes according to pathobiology may permit investigation of therapies most likely to ameliorate sequelae of inflammation or endothelial activation (predictive enrichment) and may improve identification of patients at risk for worse outcomes (prognostic enrichment).
Elevated Ang-2 identified indirect ARDS by CART and EN. Ang-2 antagonism of the Tie-2 receptor causes weakened endothelial cell junctions, increased expression of leukocyte adhesion molecules (e.g., VCAM), and increased prothrombotic proteins at the endothelial surface (e.g., vWF) (37). Ang-1 promotes vascular quiescence (37), so increased Ang-2/Ang-1 ratio indicates a state of endothelial activation, which has been associated with poor outcomes in patients with sepsis and acute lung injury (5). VCAM promotes migration of leukocytes and endothelial progenitor cells to the source of infection and vascular damage (38). vWF causes platelet aggregation on the vessel wall (39) and is associated with microangiopathy in patients with sepsis (40, 41) and worse clinical outcomes in patients with ARDS (42).
Our finding that increased Ang-2/Ang-1 ratio is associated with indirect ARDS by CART is consistent with prior literature, including our prior finding of increased Ang-2 in patients with ARDS and indirect lung injury (4). Calfee et al (24) previously reported that low Ang-2 was associated with direct ARDS in septic adults, and Reilly et al (7) used causal inference methods to implicate Ang-2 in the development of ARDS in septic adults. Our finding that increased vWF is associated with indirect ARDS by CART is consistent with prior reports by Rubin (9), who documented high vWF in adults with ARDS secondary to indirect lung injury, and Calfee et al (24), who documented lower vWF in adults with direct compared with indirect ARDS. To our knowledge, VCAM has not previously been studied in sepsis-associated ARDS.
Several biomarkers measured in our cohort, which did not discriminate ARDS subtype, performed differently from those in prior reports. Ware et al (3) found sRAGE, IL-6, and IL-8 to be associated with development of ARDS in adults with sepsis. IL-6 and IL-8 were higher in our patients with indirect compared with direct ARDS (Supplemental Table 1, http://links.lww.com/CCX/A445), and elevated IL-8 was associated with indirect ARDS by EN. IL-6 and IL-8 levels were lower in our cohort than Ware et al’s (3), possibly due to differences in timing of measurements, measurement method (i.e., multiplex versus singleplex ELISA), or pediatric versus adult physiology. Despite being suggested as a marker of alveolar epithelial damage (12), we found no difference in sRAGE between indirect and direct ARDS. However, sRAGE is also expressed in the endothelium and is associated with the strength of the immune response (43).
Supplementary EN analysis identified an association between high TNF-RII, consistent with prior literature showing elevated TNF-RII in adult ARDS (44). TNF-RII is an inflammatory biomarker but may indicate endothelial activation as it is expressed on endothelial cells (45). Few studies have demonstrated elevated RANTES in rodent models of ARDS (46), in contrast with our finding that low RANTES associated with indirect ARDS. One possible interpretation of our finding is that elevation in biomarkers associated with endothelial activation is more prominent than those associated with inflammation in indirect ARDS.
Our study has limitations. First, the retrospective nature of this work introduces the potential for misclassification bias. ARDS subtype was determined by a single author’s review of the electronic medical record and a minority (15%) of cases required three-person adjudication to determine the primary site of infection. Second, our cohort was composed of patients who had been enrolled in either of two prospective parent studies with overlapping but distinct enrollment criteria, which may have contributed to selection bias and may limit generalizability. Third, our sample size provided adequate power to investigate our aims but precluded subgroup analyses, including the ability to investigate thoroughly the possibility of differences in endothelial activation associated with race or chemotherapy exposure given the relatively high proportion of oncologic comorbidity in the indirect ARDS group. Finally, although our selection of biomarkers was guided by prior literature, it was not exhaustive, and endothelial biomarkers were overrepresented. Results from CART analysis were supported but completely replicated by supplementary EN analysis. Future studies are needed to validate prospectively our findings.
Our study also has several strengths. This was the first study to use plasma biomarkers to compare indirect and direct ARDS in pediatric patients. We used a large panel of biomarkers, assembled based on previous adult, pediatric, and animal studies, to examine pathobiologic differences in pediatric ARDS subphenotypes. The model of four biomarkers that identified indirect ARDS demonstrated both biologic plausibility and good discriminative performance.
CONCLUSIONS
In a cohort of pediatric ARDS, biomarkers indicative of endothelial activation and inflammation were associated with indirect ARDS. Models incorporating endothelial and inflammatory biomarkers identified indirect ARDS with good sensitivity, specificity, and positive and negative predictive values. The association of biomarkers indicative of endothelial activation and inflammation with indirect, but not direct, ARDS suggests differences in underlying pathobiology. External validation is required. Prospective studies are warranted to investigate further biological differences in ARDS subtypes. Plasma biomarkers, as a tool for predictive enrichment, may aid in identification of patients with ARDS whose physiology may respond more favorably to targeted therapies in future studies.
Supplementary Material
Footnotes
Previous addresses for Dr. Whitney: 333 Longwood Avenue, LO 008, Medical Critical Care, Boston MA 02115.
This study was performed at the Children’s Hospital of Philadelphia.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Supported, in part, by the National Institutes of Health: National Heart, Lung, and Blood Institute (NHLBI) T32 HL-00891 (to Dr. Whitney trainee), Loan Repayment Program 2674-1 (to Dr. Whitney), National Institute of Child Health and Human Development K12HD047349 (to Dr. Weiss), National Institute of General Medical Sciences K23GM110496 (to Dr. Weiss), NHLBI K23HL136688 (to Dr. Yehya), and the Department of Anesthesiology & Critical Care at the Children’s Hospital of Philadelphia.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Khemani RG, Smith L, Lopez-Fernandez YM, et al. ; Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) Investigators; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): An international, observational study. Lancet Respir Med. 2019; 7:115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calfee CS, Janz DR, Bernard GR, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015; 147:1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ware LB, Koyama T, Zhao Z, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013; 17:R253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yehya N, Thomas NJ, Meyer NJ, et al. Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Intensive Care Med. 2016; 42:1137–1145 [DOI] [PubMed] [Google Scholar]
- 5.Whitney JE, Zhang B, Koterba N, et al. Systemic endothelial activation is associated with early acute respiratory distress syndrome in children with extrapulmonary sepsis. Crit Care Med. 2020; 48:344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006; 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reilly JP, Wang F, Jones TK, et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: Evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018; 44:1849–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calfee CS, Ware LB, Glidden DV, et al. ; National Heart, Blood, and Lung Institute Acute Respiratory Distress Syndrome Network. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med. 2011; 39:711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin DB, Wiener-Kronish JP, Murray JF, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990; 86:474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware LB, Eisner MD, Thompson BT, et al. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004; 170:766–772 [DOI] [PubMed] [Google Scholar]
- 11.Yehya N, Thomas NJ, Wong HR. Evidence of endotypes in pediatric acute hypoxemic respiratory failure caused by sepsis. Pediatr Crit Care Med. 2019; 20:110–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabaudon M, Blondonnet R, Roszyk L, et al. Soluble receptor for advanced glycation end-products predicts impaired alveolar fluid clearance in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2015; 192:191–199 [DOI] [PubMed] [Google Scholar]
- 13.Prescott HC, Calfee CS, Thompson BT, et al. Toward smarter lumping and smarter splitting: Rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016; 194:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwashyna TJ, Burke JF, Sussman JB, et al. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015; 192:1045–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss SL, Zhang D, Bush J, et al. Persistent mitochondrial dysfunction linked to prolonged organ dysfunction in pediatric sepsis. Crit Care Med. 2019; 47:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yehya N, Thomas NJ, Margulies SS. Circulating nucleosomes are associated with mortality in pediatric acute respiratory distress syndrome. Am J Physiol Cell Mol Physiol. 2016; 42:1137–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 18.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 19.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36:309–332 [DOI] [PubMed] [Google Scholar]
- 20.Yehya N, Keim G, Thomas NJ. Subtypes of pediatric acute respiratory distress syndrome have different predictors of mortality. Intensive Care Med. 2018; 44:1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones TK, Feng R, Kerchberger VE, et al. Plasma sRAGE acts as a genetically regulated causal intermediate in sepsis-associated acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020; 201:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YT, Wang YC, Lee HL, et al. Monocyte chemoattractant protein-1, a possible biomarker of multiorgan failure and mortality in ventilator-associated pneumonia. Int J Mol Sci. 2019; 20:2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matera G, Puccio R, Giancotti A, et al. Impact of interleukin-10, soluble CD25 and interferon-γ on the prognosis and early diagnosis of bacteremic systemic inflammatory response syndrome: A prospective observational study. Crit Care. 2013; 17:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calfee CS, Gallagher D, Abbott J, et al. ; NHLBI ARDS Network. Plasma angiopoietin-2 in clinical acute lung injury: Prognostic and pathogenetic significance. Crit Care Med. 2012; 40:1731–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orbegozo D, Rahmania L, Irazabal M, et al. Endocan as an early biomarker of severity in patients with acute respiratory distress syndrome. Ann Intensive Care. 2017; 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whalen MJ, Doughty LA, Carlos TM, et al. Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 are increased in the plasma of children with sepsis-induced multiple organ failure. Crit Care Med. 2000; 28:2600–2607 [DOI] [PubMed] [Google Scholar]
- 27.Whitney JE, Silverman M, Norton JS, et al. Vascular endothelial growth factor and soluble vascular endothelial growth factor receptor as novel biomarkers for poor outcomes in children with severe sepsis and septic shock. Pediatr Emerg Care. 2018; 00:1–5 [DOI] [PubMed] [Google Scholar]
- 28.Orwoll BE, Spicer AC, Zinter MS, et al. Elevated soluble thrombomodulin is associated with organ failure and mortality in children with acute respiratory distress syndrome (ARDS): A prospective observational cohort study. Crit Care. 2015; 19:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punyadeera C, Schneider EM, Schaffer D, et al. A biomarker panel to discriminate between systemic inflammatory response syndrome and sepsis and sepsis severity. J Emerg Trauma Shock. 2010; 3:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faiotto VB, Franci D, Enz Hubert RM, et al. Circulating levels of the angiogenesis mediators endoglin, HB-EGF, BMP-9 and FGF-2 in patients with severe sepsis and septic shock. J Crit Care. 2017; 42:162–167 [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khemani RG, Patel NR, Bart RD, 3rd, et al. Comparison of the pulse oximetric saturation/fraction of inspired oxygen ratio and the PaO2/fraction of inspired oxygen ratio in children. Chest. 2009; 135:662–668 [DOI] [PubMed] [Google Scholar]
- 33.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996; 24:743–752 [DOI] [PubMed] [Google Scholar]
- 34.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019; 16:22–28 [DOI] [PubMed] [Google Scholar]
- 35.Breiman L, Friedman J, Olshen R, et al. Classification and Regression Trees. 1984. Belmont, CA: Wadsworth [Google Scholar]
- 36.Gu W, Pepe MS. Estimating the capacity for improvement in risk prediction with a marker. Biostatistics. 2009; 10:172–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sack KD, Kellum JA, Parikh SM. The angiopoietin-Tie2 pathway in critical illness. Crit Care Clin. 2020; 36:201–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafat N, Tönshoff B, Bierhaus A, et al. Endothelial progenitor cells in regeneration after acute lung injury: Do they play a role? Am J Respir Cell Mol Biol. 2013; 48:399–405 [DOI] [PubMed] [Google Scholar]
- 39.Methia N, André P, Denis CV, et al. Localized reduction of atherosclerosis in von Willebrand factor-deficient mice. Blood. 2001; 98:1424–1428 [DOI] [PubMed] [Google Scholar]
- 40.Claus RA, Bockmeyer CL, Sossdorf M, et al. The balance between von-Willebrand factor and its cleaving protease ADAMTS13: Biomarker in systemic inflammation and development of organ failure? Curr Mol Med. 2010; 10:236–248 [DOI] [PubMed] [Google Scholar]
- 41.Claus RA, Bockmeyer CL, Budde U, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost. 2009; 101:239–247 [PubMed] [Google Scholar]
- 42.Zinter MS, Delucchi KL, Kong MY, et al. Early plasma matrix metalloproteinase profiles. A novel pathway in pediatric acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019; 199:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt AM, Yan SD, Yan SF, et al. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001; 108:949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsons PE, Matthay MA, Ware LB, et al. ; National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005; 288:L426–L431 [DOI] [PubMed] [Google Scholar]
- 45.Sinha P, Ware LB. Selective tumour necrosis factor receptor-1 inhibition in acute lung injury: A new hope or a false dawn? Thorax. 2018; 73:699–701 [DOI] [PubMed] [Google Scholar]
- 46.Chen CH, Chen YL, Sung PH, et al. Effective protection against acute respiratory distress syndrome/sepsis injury by combined adipose-derived mesenchymal stem cells and preactivated disaggregated platelets. Oncotarget. 2017; 8:82415–82429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


