Abstract
INTRODUCTION:
Treatment options for irritable bowel syndrome (IBS) are limited, causing many patients to remain symptomatic. This study assessed the potential of human milk oligosaccharides (HMOs) to normalize bowel habits. Secondary outcomes included IBS severity and health-related quality of life.
METHODS:
This multicenter, open-label trial recruited patients with IBS from 17 sites across the United States. Patients received daily orally administrated 5-g intervention of the HMOs 2'-fucosyllactose and lacto-N-neotetraose in a 4:1 mix. Bowel habits, IBS symptoms, and quality of life were assessed at baseline and every 4 weeks during the 12-week intervention.
RESULTS:
A total of 317 patients (70.7% women; mean age of 44.0 years, range 18–93 years) received the trial product, and 245 patients completed the trial according to protocol. Patients had a significant improvement from baseline to 12 weeks in total percentage of bowel movements with abnormal stool consistency (mean and [95% confidence interval]: 90.7 [88.9–92.9] vs 57.2% [53.9–60.5], P < 0.0001), overall IBS Symptom Severity Score (323 [314–332] vs 144 [133–155], P < 0.0001) and health-rela,ted quality of life (50.4 [48.0–52.8] vs 74.6 [72.3–76.9], P < 0.0001). Improvement was similar across IBS subtypes. Symptoms improved most in the first 4 weeks of intervention. The most common side effects were mild gastrointestinal symptoms such as flatulence, abdominal pain and discomfort, and distension.
DISCUSSION:
Supplementation with 2 selected HMOs improves IBS symptoms and quality of life without substantial side effects. These promising results suggest that this novel approach to IBS should be confirmed in a randomized, placebo-controlled trial.
INTRODUCTION
Irritable bowel syndrome (IBS) is a disorder of gut-brain interaction, characterized by recurrent abdominal pain associated with altered bowel habits, including abnormal stool consistency and frequency (1–3). IBS is one of the most common disorders of the digestive tract, with an estimated worldwide population prevalence of 5%–11% (4,5). Thirty percent of those suffering from IBS have symptoms severe enough to consult a physician (6). For these patients, the disease has a large impact on quality of life, commonly leading to the need for medical treatment, frequent health care seeking, significant impairment of daily life, and work absenteeism. This in turn leads to economic consequences for the individual and for the society (7–9).
Although IBS is prevalent, effective treatment of the condition is lacking. Moreover, medications approved for treating IBS are often limited to a specific subgroup of patients, i.e., IBS with predominant constipation (IBS-C) or diarrhea (IBS-D), leaving the large subgroup of patients who have a mixed bowel habits (IBS-M) without a designated therapy option. Furthermore, the therapeutic benefits of IBS drugs are modest, and they do not treat the underlying pathophysiology (10). For these reasons, many patients remain symptomatic long-term regardless of medical treatment. Hence, there is a clear unmet need for alternative effective strategies for patients to manage their IBS symptoms. It is also worth considering that patients may prefer a dietary approach to manage their condition on a daily basis instead of consuming drugs.
The pathogenesis of IBS is unknown, but the gut microbiota are being increasingly seen as a potential contributing factor (11). This has been prompted by a number of clinical observations, such as de novo development of IBS after enteric infections (12), compositional differences in the gut microbiota of patients with IBS relative to healthy individuals (13,14), e.g., a depletion of fecal and mucosal bifidobacteria (15), and clinical responses to interventions that modify the gut microbiota (16). The role of the gut microbiota in IBS pathogenesis may, in part, involve changes in the production by bacteria of several nutrients (17) that are crucial for maintaining gut health (18). Diet is, together with genetics and environmental factors, one of the main contributors to the composition of the human gut microbiota (19). Despite the potential contribution of the gut microbiota to IBS pathogenesis, there is currently no therapy available to normalize IBS-specific dysbiosis, and a dietary approach to manage symptoms is worth further investigation.
Human milk oligosaccharides (HMOs) are a heterogenous mix of glycans naturally occurring in high concentration in human breast milk (20). As HMOs are largely indigestible in the upper gastrointestinal (GI) tract (21–23), the majority of ingested HMOs reach the large intestine undigested. Here, they serve multiple roles, including modulation of the microbiota and the immune system, and prevention of epithelial adhesion of intestinal pathogens (20,24,25). Because of these characteristics, these HMOs potentially have a beneficial role in the management of IBS; particularly the symptoms relating to bowel function. Based on a recent, small proof-of-principle trial in patients with IBS (26), 2 HMOs, 2'-fucosyllactose (2'FL), and lacto-N-neotetraose (LNnT), can be expected to support normal bowel function in adults suffering from IBS without causing tolerance issues. They may also, through modulation of intestinal microbiota and associated metabolites, improve general gut health resulting in better management of IBS-related symptoms and thereby overall wellbeing.
The aim of this open-label trial was to assess the potential of a daily intake of 5 g of a 4:1 mix of 2'FL and LNnT to normalize bowel habits in a real-world setting in patients diagnosed with IBS. Furthermore, the impact on overall IBS symptom severity, other GI symptoms, and disease-specific health-related quality of life was also assessed.
METHODS
Trial design and conduct
This was a prospective, open-label, single-arm clinical trial to evaluate the efficacy and safety of daily oral intake of a 5-g combination of 2 HMOs, 2'FL, and LNnT in a 4:1 mix, in adults with IBS. Patients were recruited in medical clinics and clinical research centers at 17 sites across the United States. An e-pharmacy distributed the trial product to patients. The trial was conducted in accordance with the Declaration of Helsinki (27) and approved by the independent institutional review board IntegReview. The trial was registered at ClinicalTrials.gov with registration number NCT03550742.
Written informed consent was obtained from all participants at a screening visit. After inclusion in the trial, the patients completed surveys at baseline, and 4, 8, and 12 weeks after start of intervention to collect data on current symptoms, wellbeing, and medication use. All surveys were administered online through a password-protected patient web page. Validated questionnaires were used, including the Bristol Stool Form Scale (BSFS) (28), IBS Symptom Severity Score (IBS-SSS) (29), the IBS-specific version of the Gastrointestinal Symptom Rating Scale (GSRS-IBS) (30), and the IBS Quality of Life questionnaire (IBS-QOL) (31). Patients also answered questions related to health, medication usage, adherence to the intervention, perception of the trial product, and adverse events (AEs). A trial duration of 12 weeks was selected to provide sufficient time for stabilization of symptoms and decrease of the placebo effect, while retaining the exploratory nature of the trial.
Inclusion and exclusion criteria
Patients were eligible for participation in the trial if they were aged 18 years and older, had a diagnosis of IBS from a health care provider, and met the Rome IV diagnostic criteria for IBS (32). All IBS subtypes were included. No minimum IBS-SSS score was required. Lactose intolerant patients were eligible because of the low content of lactose in the trial product. In addition, patients had to be fluent in English and have access to the Internet through computer, tablet, or smartphone. Patients were excluded from participation if they had been diagnosed with celiac disease, diverticulitis, inflammatory bowel disease, or Clostridium difficile infection. Women who were pregnant or lactating were also excluded from participation.
Efficacy and safety evaluation
The primary endpoint in the trial was the mean change from baseline in the proportion of bowel movements with abnormal stool consistency as measured by BSFS (stool types 1 and 2 [hard stools], and types 6 and 7 [loose stools]), in all patients and in IBS subtypes. This endpoint was selected, rather than a combined pain and stool consistency endpoint as often used in current IBS clinical trials, because the aim of the trial was not to test the HMO blend as an overall IBS intervention but as nutritional support for normalizing bowel movements in patients with IBS. To assess stool consistency, patients were asked to report the percentage of all stools falling within the classification of hard stools and loose stools, respectively, during the previous 4 weeks. Secondary endpoints included change from baseline in the overall IBS-SSS score and the IBS-SSS parameters of abdominal pain severity and frequency, and bloating severity; severity of gastrointestinal symptoms measured by GSRS-IBS; and the patients' disease-specific health-related quality of life measured by IBS-QOL. Safety was assessed by collecting and monitoring AEs throughout the course of the trial.
Trial intervention
The HMOs were produced by Glycom Manufacturing A/S, Denmark, under good manufacturing practice conditions, using fermentation followed by separation and purification resulting in products of greater than 97% purity, and provided as white powder. Capstone Nutrition, UT, filled under good manufacturing practice conditions the 2'FL and LNnT in a 4:1 mass ratio in single-serve stick packs. Each stick pack contained the daily dose of 5 g of HMOs. The single-serve stick packs were distributed in boxes that each contained 30 stick packs. Compound mix ratio and daily dose were determined based on internal data. All patients received the HMO mix after entering the trial and completing the baseline survey. The intervention was open-label, so all patients were aware they were taking the active HMO mix. Patients were instructed to consume the content of 1 stick pack daily for 12 weeks, either mixed in food or beverage or on its own. The final product contained less than 1% lactose, corresponding to less than 50 mg of lactose per day.
Statistical methods
There were no previous data available to enable prediction of the size of the impact of 2'FL and LNnT on the primary outcome measure in this trial. In the absence of relevant pilot data, G*Power version 3.1.9.2 was used to calculate the sample size needed to achieve 95% power to detect a small effect size with the planned analysis method (assumptions: ANOVA: repeated measures, between factors; effect size f = 0.25; alpha = 0.05; number of groups = 3; number of measurement points = 2; correlation among measures = 0.5). This yielded a required total sample size of 189. Thus, the chosen sample size of 300 should be sufficient to detect even small intervention effects.
For both primary and secondary outcomes, analyses were conducted with a 2-factor mixed-design ANOVA, with the 3 IBS subtypes (corresponding to the number of groups) as the between-subject factor and 2 timepoints (baseline vs week 12) as within-subject factor. This was performed using an intention-to-treat (ITT) methodology, including data from all patients who received a shipment of the trial product regardless of whether they started the intervention, and carrying the last observations obtained forward to fill any missing data points from discontinued patients. Statistical analyses were performed using IBM SPSS Statistics Version 25.0.
RESULTS
Trial population
A total of 366 patients were screened for eligibility to participate in the trial between May 2018 and November 2018. Of these, 49 patients did not meet the inclusion criteria or met exclusion criteria after enrollment, or did not complete the baseline survey, leaving 317 patients to be included in the analyses. Thirteen of those subjects were discontinued after completing the baseline survey, as they did not start the intervention. Of the 304 subjects who started the intervention, 273 completed the trial and all assessments (Figure 1).
Figure 1.
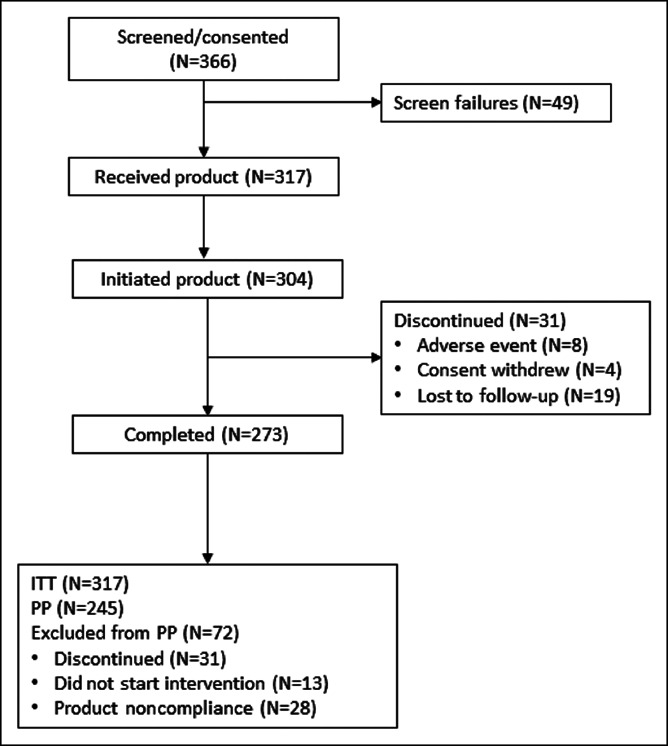
Patient flow. ITT, intention-to-treat; PP, per protocol.
The sample of 317 patients included in the analyses had a mean age of 44 years (range 18–93 years) and consisted predominantly of women (70.7%). The average IBS-SSS score was 323 (range 60–500), with 63.7% of the patients presenting with severe IBS (IBS-SSS >300). More than 65% of the patients in the analysis sample had experienced onset of IBS symptoms within the last 3 years before enrollment, and the distribution of IBS subtypes was 42.9% with IBS-C, 26.7% with IBS-D, 30.0% with IBS-M, and 0.3% with unspecified IBS. Most of the patients (88.3%) reported at baseline that they were taking medication regularly to manage their IBS symptoms, and they were allowed to continue to do so during the trial. Throughout the trial, 245 patients reported full adherence to the intervention. An overview of baseline demographics is shown in Table 1.
Table 1.
Baseline characteristics and demographics of the trial population

| Age (yr) | 44.0 (range 18–93) |
| Sex, n (%) | |
| Women | 224 (70.7) |
| Men | 93 (29.3) |
| BMI (kg/m2) | 28 (range 17–56) |
| Race/ethnicity, n (%) | |
| Non-Hispanic white | 61 (19.2) |
| Non-Hispanic black | 30 (9.5) |
| Hispanic | 212 (66.9) |
| Other | 10 (3.2) |
| Did not wish to disclose | 4 (1.3) |
| State of residence, n (%) | |
| Alabama | 31 (9.8) |
| California | 57 (18.0) |
| Florida | 187 (59.0) |
| Michigan | 7 (2.2) |
| New York | 20 (6.3) |
| North Carolina | 5 (1.6) |
| Oklahoma | 4 (1.4) |
| Pennsylvania | 3 (0.9) |
| Other | 3 (0.9) |
| IBS subtype, n (%) | |
| IBS-C | 136 (42.9) |
| IBS-D | 85 (26.7) |
| IBS-M | 95 (30.0) |
| IBS-U | 1 (0.3) |
| IBS severity (IBS-SSS), n (%) | |
| Severe IBS (300+) | 202 (63.7) |
| Moderate IBS (≥175–299) | 103 (32.5) |
| Mild IBS (<175) | 12 (3.8) |
| Time since onset of IBS symptoms, n (%) | |
| <6 mo | 1 (0.3) |
| 6–12 mo | 77 (24.3) |
| 1–3 yr | 130 (41.0) |
| 3–5 yr | 27 (8.5) |
| >5 yr | 82 (25.9) |
BMI, body mass index; IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, alternating/mixed-pattern IBS; IBS-U, unspecified IBS; IBS-SSS, IBS Symptom Severity Scale.
Efficacy
Overall, the total proportion of bowel movements with abnormal stool consistency (BSFS 1, 2, 6 and 7) improved from baseline to week 12 (mean and [95% confidence interval]: 90.7 [88.9–92.9] vs 57.2% [53.9–60.5], P < 0.0001) (Figure 2), with no differences in improvement between IBS subtypes. The proportion of loose stools (BSFS 6 or 7) was reduced from baseline to week 12 in the total sample (Table 2). This change was mainly driven by the significant change in the IBS-D subgroup, followed by a smaller change in the patients with IBS-M, whereas no change was seen in the patients with IBS-C. The proportion of hard stools (BSFS 1 and 2) was also reduced from baseline to week 12 in the ITT population. This was mainly driven by the IBS-C subgroup, followed by the patients with IBS-M, whereas the patients with IBS-D showed an increase in the proportion of hard stools from baseline to 12 weeks. There were no differences in the change in the proportion of bowel movements with abnormal stool consistency between men and women (−32.6% vs −33.9%, P = 0.78), but younger patients (younger than 40 years) had a greater reduction in bowel movements with abnormal stool consistency than patients older than 40 years (37.6% vs 30.5%, P = 0.04).
Figure 2.

Changes in total % of abnormal consistency stools (Bristol Stool Form Scale) during 12 weeks of daily supplementation of a 5-g mix of 2'-fucosyllactose and lacto-N-neotetraose. *Significantly reduced total % of abnormal stools (diarrhea + constipation) compared with baseline at P < 0.0001.
Table 2.
Percentage of bowel movements with hard stools (BSFS 1 and 2) and loose stools (BSFS 6 and 7) (mean [95% confidence interval]) at baseline and after 12 weeks of intervention
| ITT population | IBS-C | IBS-D | IBS-M | |
| Hard stools (%) | ||||
| Baseline | 50.2 (47.1–53.4) | 72.7 (70.0–75.5) | 14.4 (11.5–17.2) | 49.4 (46.3–53.5) |
| Week 12 | 30.7 (28.4–33.1)a | 34.8 (31.2–38.3)a | 21.1 (16.6–25.5)b | 33.4 (29.3–37.4)a |
| Loose stools (%) | ||||
| Baseline | 40.4 (37.1–43.7) | 14.4 (11.5–17.2) | 74.4 (71.1–77.6) | 47.9 (44.3–51.5) |
| Week 12 | 26.5 (23.8–29.1)a | 15.4 (11.8–19.1) | 38.9 (34.6–43.3)a | 31.1 (26.4–35.7)a |
BSFS, Bristol Stool Form Scale; IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, alternating/mixed-pattern IBS; ITT, intention-to-treat.
P < 0.0001 compared with baseline.
P = 0.007 compared with baseline.
The overall severity of IBS symptoms on the IBS-SSS was reduced by 54.2% (323 [314–332] vs 144 [133–155], P < 0.0001) on average from baseline to week 12, and a similar pattern was seen in all 3 IBS subtypes with no differences between the subtypes (Figure 3). A reduction of at least 50 points in the total IBS-SSS score is considered a clinically significant improvement (29). By that standard, 260 (82.0%) of the patients had a clinically significant improvement in overall IBS severity. Abdominal pain and bloating severity were reduced by 59.4% (62.5 [60.1–64.9] vs 25.4 [22.6–28.2], P < 0.0001) and 59.2% (56.8 [53.8–59.8] vs 23.2 [20.5–25.8], P < 0.0001), respectively, with no differences between IBS subtypes (Figure 4a, c). Furthermore, significant reductions in number of days with abdominal pain during the last 10 days were observed in the total patient sample and in each of the IBS subtypes, with no differences between subtypes (Figure 4b). The GSRS-IBS score from baseline to week 12 also changed from 54.9 [53.6–56.1] to 34.0 [32.5–35.4] (P < 0.0001) in the overall sample, with no differences between subtypes (see Supplementary Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A453).
Figure 3.
Changes in the overall IBS Symptom Severity Score during 12 weeks of daily supplementation with a 5-g mix of 2'-fucosyllactose and lacto-N-neotetraose in the different IBS subtypes. *Significantly different from baseline at P < 0.0001. Error bars: 95% confidence interval. IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, alternating/mixed-pattern IBS.
Figure 4.
Changes in (a) abdominal pain severity (0–100 scale), (b) number of days with abdominal pain (out of 10 days), and (c) bloating severity (0–100 scale) during 12 weeks of daily supplementation with a 5-g mix of 2'-fucosyllactose and lacto-N-neotetraose in the different IBS subtypes. All subtypes and the overall sample were significantly improved on all these parameters at 12 weeks. *Significantly different from baseline at P < 0.0001. Error bars: 95% confidence interval. IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, alternating/mixed-pattern IBS.
Health-related quality of life (IBS-QOL) was significantly improved at week 12 compared with baseline, with scores rising from 50.4 [48.0–52.8] to 74.6 [72.3–76.9] (P < 0.0001). There were no differences among the 3 IBS subtypes in change in IBS-QOL scores (see Supplementary Figure 2, Supplementary Digital Content 2, http://links.lww.com/CTG/A454).
Safety
Overall, there were no incidents causing safety concerns in this trial, and the patients generally reported that the intervention was well tolerated. The number of reported AEs was relatively low; 46 patients (14.5% of the ITT sample) reported any AEs during the trial, with a combined total of 87 AEs. Of these, 65 AEs reported by 33 patients were considered to be possibly or probably related to the intervention on review. Most AEs (n = 61) were related to the gastrointestinal tract, and the most common side effect of the intervention was passing gas, followed by abdominal distension and abdominal pain (see Supplementary Figure 3, Supplementary Digital Content 3, http://links.lww.com/CTG/A455). Only 8 patients (2.5%) discontinued their participation in the trial prematurely due to AEs. One serious AE (brief hospitalization due to colitis) occurred during the trial, but this was considered on review by the study's medical safety officer of the available information to be unrelated to the intervention.
DISCUSSION
In this prospective, open-label, single-arm clinical trial, daily ingestion of a 5-g mix of 2 HMOs, 2'FL and LNnT, in a 4:1 mass ratio reduced abnormal stool consistency, improved abdominal pain, bloating and overall IBS severity, and increased IBS-related quality of life. These improvements were seen in both the entire IBS group and in all IBS subtypes, with a similar degree of beneficial changes in all IBS subtypes. Most of the observed improvement in symptoms occurred within the first 4 weeks of intervention, and it then remained stable during the remainder of the 12-week intervention period. Furthermore, the low rate of reported AEs demonstrated that the HMOs are safe in adult patients with IBS.
This is the first large-scale trial to show that adult patients with IBS can achieve an improvement of IBS symptoms with supplementation of HMOs. A recent randomized, placebo-controlled proof-of-principle and safety trial including 61 adults diagnosed with IBS found that the same 4:1 mix of 2'FL and LNnT increased the abundance of fecal bifidobacteria without worsening GI symptoms (26), increasing the abundance of a bacterium that has been found to be depleted in individuals with IBS (15). The increase in Bifidobacterium abundance was driven by Bifidobacterium adolescentis and to a lesser degree Bifidobacterium longum (33).
There are several different effects of HMOs that can explain the beneficial effects on GI symptoms. Supplementation with a mix of 2'FL and LNnT has previously been shown to increase the abundance of fecal bifidobacteria, in healthy infants (34), children (data on file), healthy adults (35), and in adults with IBS (26). Bifidobacteria have been found to be depleted in individuals with IBS (15), which may influence the metabolites produced by the gut microbiota. In particular, bifidobacteria produce the short-chain fatty acid acetate (36). In addition to maintaining a beneficial environment by lowering the colonic pH, acetate is a substrate in the synthesis of butyrate, an important energy source for the colonocytes (18). Increasing the concentration of butyrate in the colon might support gut barrier integrity which has been reported to be compromised in patients with IBS (37). Supplementation with bifidobacteria has also previously been shown to have a beneficial impact on IBS symptoms (38). However, as supplementation with probiotics introduces a single or few bacterial strains, potentially foreign to the recipient, supplementation with prebiotics such as HMOs supports the growth of beneficial bacteria already present in the recipient's gut. These potential mechanisms posed through gut microbial modulation and function may, at least in part, explain why the intervention had a significant impact on bowel habits and IBS symptoms, irrespective of subtype, and it may further be speculated that the great symptom improvement leads to the observed improvement in health-related quality of life. Furthermore, it has been reported that patients with IBS may have an increased number of activated mast cells (39) and a low-grade mucosal inflammation (40), which in turn may affect enteric nerve function and the gut-brain interactions, thereby influencing GI motility and sensitivity (41). Fucosylated HMOs, such as 2'FL, have been shown to moderate nociceptive stimuli associated with activated mast cells and may therefore modulate gut motility and visceral pain (42). The observed improvement may also, at least in part, be explained by the statistical phenomenon of regression to the mean.
Although the large symptom improvement observed in this trial is very encouraging, the present trial has limitations. These include the open-label nature of the trial and absence of a placebo or comparator group, which make it impossible to determine how much of the impact can be ascribed placebo effect. However, the substantial effects of the HMO mix on bowel habits, other IBS symptoms, and health-related QOL, as well as the sustained nature of these effects during the entire 12-week intervention period, indicate that intervention with 2'FL and LNnT has a promising potential in the management of IBS. As the beneficial impacts of the intervention were seen across age, sex, race/ethnicity, and IBS subtypes, it seems likely that a broad range of patients with IBS will benefit from ingesting the 2'FL and LNnT mix.
In conclusion, daily oral administration of the 2 HMOs, 2'FL and LNnT in a 4:1 mix, for 12 weeks significantly improves bowel habits, other IBS symptoms, and health-related quality of life in adults who have clinical IBS diagnosis and fulfill the Rome IV criteria for IBS. Furthermore, our results indicate that the HMO mix tested is safe for use in adult patients with IBS. However, the encouraging findings from this open-label trial need to be confirmed in a randomized, placebo-controlled trial.
CONFLICTS OF INTEREST
Guarantor of the article: Olafur S. Palsson, PsyD.
Specific author contributions: O.S.P., M.S., and B.M.: contributed in designing the trial. O.S.P.: responsible for data collection and database cleaning and analyzed the data. O.P. and I.D.A.: prepared the manuscript. All authors had full access to the study data, had input to the content of the manuscript, contributed in finalizing the manuscript, and approved the final version for submission.
Financial support: The trial was sponsored by Glycom, Inc., LA. The trial sponsor was involved in designing the trial and writing of the manuscript.
Potential competing interests: O. S. Palsson has received research support from Glycom and the Rome Foundation and served as Consultant/Advisory Board Member for Ironwood and metaMe Health. A. Peery declares no conflict of interest. D. Seitzberg, I. D. Amundsen, and B. McConnell are employed at Glycom A/S, Denmark. M. Simrén has received unrestricted research grants from Danone and Ferring Pharmaceuticals and served as a Consultant/Advisory Board member for AstraZeneca, Danone Nutricia Research, Nestlé, Almirall, Allergan, Albireo, Genetic Analysis AS, Biocodex, Glycom, Arena, and Shire and as a speaker for Tillotts, Takeda, Menarini, Kyowa Kirin, Allergan, Shire, Biocodex, Alimentary Health, AlfaSigma, and Almirall.
ClinicalTrials.gov identifier: NCT03550742.
Study Highlights.
WHAT IS KNOWN
✓ Human milk oligosaccharides (HMOs) promote growth of Bifidobacterium spp., which is depleted in patients with irritable bowel syndrome (IBS).
✓ A mix of 2 HMOs, 2'-fucosyllactose (2'FL) and lacto-N-neotetraose (LNnT), increases the abundance of fecal Bifidobacterium spp. in patients with IBS without inducing gastrointestinal symptoms.
✓ The impact of HMOs on bowel habits and IBS symptoms in patients with IBS is unknown.
WHAT IS NEW HERE
✓ Daily supplementation with a mix of 2'FL and LNnT normalizes bowel habits in adult patients with IBS by decreasing the proportion of both hard and loose stools.
✓ Patients reported improvement of IBS symptoms and increased quality of life after ingestion of the HMO mix.
TRANSLATIONAL IMPACT
✓ The study findings suggest that HMOs may have value as nutritional support to improve bowel symptoms and wellbeing of clinical patients with IBS.
Supplementary Material
ACKNOWLEDGMENT
We thank the participating patients and investigators for their contribution to the trial.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A453; http://links.lww.com/CTG/A454; and http://links.lww.com/CTG/A455.
REFERENCES
- 1.Ford AC, Talley NJ. Irritable bowel syndrome. BMJ 2012;345:e5836. [DOI] [PubMed] [Google Scholar]
- 2.Spinelli A. Irritable bowel syndrome. Clin Drug Investig 2007;27(1):15–33. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130(5):1480–91. [DOI] [PubMed] [Google Scholar]
- 4.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol 2012;10(7):712–21.e4. [DOI] [PubMed] [Google Scholar]
- 5.Palsson OS, Whitehead W, Törnblom H, et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020;158(5):1262–73.e3. [DOI] [PubMed] [Google Scholar]
- 6.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014;6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choung RS, Locke GR., III Epidemiology of IBS. Gastroenterol Clin North Am 2011;40(1):1–10. [DOI] [PubMed] [Google Scholar]
- 8.Lea R, Whorwell PJ. Quality of life in irritable bowel syndrome. Pharmacoeconomics 2001;19(6):643–53. [DOI] [PubMed] [Google Scholar]
- 9.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: Systematic review and meta-analysis. Am J Gastroenterol 2012;107(7):991–1000. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Ford AC. Pharmacotherapy for irritable bowel syndrome. J Clin Med 2017;6(11):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyland NP, Quigley EM, Brint E. Microbiota-host interactions in irritable bowel syndrome: Epithelial barrier, immune regulation and brain-gut interactions. World J Gastroenterol 2014;20(27):8859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thabane M, Simunovic M, Akhtar-Danesh N, et al. An outbreak of acute bacterial gastroenteritis is associated with an increased incidence of irritable bowel syndrome in children. Am J Gastroenterol 2010;105(4):933–9. [DOI] [PubMed] [Google Scholar]
- 13.Casen C, Vebø H, Sekelja M, et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther 2015;42(1):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffery IB, O'Toole PW, Öhman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012;61(7):997–1006. [DOI] [PubMed] [Google Scholar]
- 15.Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—A systematic review. Gastroenterology 2019;157(1):97–108. [DOI] [PubMed] [Google Scholar]
- 16.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006;101(7):1581–90. [DOI] [PubMed] [Google Scholar]
- 17.Vigsnæs LK, Salomonsson E, McConnell B. Human milk oligosaccharides; now as powerful, specific modulators of the adult gut microbial community. The 11th Vahouny Fiber Symposium, 2017. June 15, 2017, Bethesda, MD.
- 18.Astbury SM, Corfe BM. Uptake and metabolism of the short-chain fatty acid butyrate, a critical review of the literature. Curr Drug Metab 2012;13(6):815–21. [DOI] [PubMed] [Google Scholar]
- 19.Turroni F, Peano C, Pass DA, et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS One 2012;7(5):e36957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bode L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012;22(9):1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand-Miller JC, McVeagh P, McNeil Y, et al. Digestion of human milk oligosaccharides by healthy infants evaluated by the lactulose hydrogen breath test. J Pediatr 1998;133(1):95–8. [DOI] [PubMed] [Google Scholar]
- 22.Engfer MB, Stahl B, Finke B, et al. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr 2000;71(6):1589–96. [DOI] [PubMed] [Google Scholar]
- 23.Gnoth MJ, Kunz C, Kinne-Saffran E, et al. Human milk oligosaccharides are minimally digested in vitro. J Nutr 2000;130(12):3014–20. [DOI] [PubMed] [Google Scholar]
- 24.Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev 2015;91(11):619–22. [DOI] [PubMed] [Google Scholar]
- 25.Bode L. Human milk oligosaccharides and their beneficial effects. In: Zibadi S, Watson RR, Preedy VR. (eds). Handbook of Dietary and Nutritional Aspects of Human Breast Milk. Wageningen Academic Publishers: The Netherlands, 2013, pp 515–31. [Google Scholar]
- 26.Iribarren C, Törnblom H, Aziz I, et al. Human milk oligosaccharide supplementation in irritable bowel syndrome patients: A parallel, randomized, double‐blind, placebo‐controlled study. Neurogastroenterol Motil 2020;32(10):e13920. [DOI] [PubMed] [Google Scholar]
- 27.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310(20):2191–4. [DOI] [PubMed] [Google Scholar]
- 28.Lewis S, Heaton K. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32(9):920–4. [DOI] [PubMed] [Google Scholar]
- 29.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11(2):395–402. [DOI] [PubMed] [Google Scholar]
- 30.Dimenäs E, Glise H, Hallerbäck B, et al. Well-being and gastrointestinal symptoms among patients referred to endoscopy owing to suspected duodenal ulcer. Scand J Gastroenterol 1995;30(11):1046–52. [DOI] [PubMed] [Google Scholar]
- 31.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome (development and validation of a new measure). Dig Dis Sci 1998;43(2):400–11. [DOI] [PubMed] [Google Scholar]
- 32.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150(6):1393–407.e5. [DOI] [PubMed] [Google Scholar]
- 33.Iribarren C, Magnusson MK, Törnblom H, et al. Efficacy of human milk oligosaccharides on fecal microbiota in patients with IBS. Gastroenterology 2019;156(6):S-2–35.. [Google Scholar]
- 34.Steenhout P, Sperisen P, Martin FP, et al. Term infant formula supplemented with human milk oligosaccharides (2′ fucosyllactose and lacto-N-neo tetraose) shifts stool microbiota and metabolic signatures closer to that of breastfed infants. FASEB J 2016;30(1_Suppl):275–7. [Google Scholar]
- 35.Elison E, Vigsnaes LK, Rindom Krogsgaard L, et al. Oral supplementation of healthy adults with 2'-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br J Nutr 2016;116(8):1356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 2016;7:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006;101(6):1288–94. [DOI] [PubMed] [Google Scholar]
- 38.Staudacher HM, Whelan K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc Nut Soc 2016;75(3):306–18. [DOI] [PubMed] [Google Scholar]
- 39.Powell N, Walker MM, Talley NJ. Gastrointestinal eosinophils in health, disease and functional disorders. Nat Rev Gastroenterol Hepatol 2010;7(3):146–56. [DOI] [PubMed] [Google Scholar]
- 40.Shulman RJ, Eakin MN, Czyzewski DI, et al. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr 2008;153(5):646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002;123(6):2108–31. [DOI] [PubMed] [Google Scholar]
- 42.Bienenstock J, Buck RH, Linke H, et al. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One 2013;8(10):e76236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





