Abstract
Introduction
The nature of nocturnal cough is largely unknown. It might be a valid marker for asthma control but very few studies characterized it as a basis for better defining its role and its use as clinical marker. This study investigated prevalence and characteristics of nocturnal cough in asthmatics over the course of four weeks.
Methods
In two centers, 94 adult patients with physician-diagnosed asthma were recruited. Patient-reported outcomes and nocturnal sensor data were collected by a smartphone with a chat-based study app.
Results
Patients coughed in 53% of 2212 nights (range: 0–345 coughs/night). Median coughs per hour were 0 (IQR 0–1). Nocturnal cough rates showed considerable inter-individual variance. The highest counts were measured in the first 30 min in bed (4.5-fold higher than rest of night). Eighty-six percent of coughs were part of a cough cluster. Clusters consisted of a median of two coughs (IQR 2–4). Nocturnal cough was persistent within patient.
Conclusion
To the best of the authors’ knowledge, this study is the first to describe prevalence and characteristics of nocturnal cough in asthma over a period of one month, demonstrating that it was a prevalent symptom with large variance between patients and high persistence within patients. Cough events in asthmatics were 4.5 times more frequent within the first 30 min in bed indicating a potential role of positional change, and not more frequent during the early morning hours. An important next step will investigate the association between nocturnal cough and asthma control.
Keywords: asthma, nocturnal cough, passive monitoring
Introduction
Asthma is a highly prevalent, heterogeneous, chronic respiratory disease, characterized by chronic airway inflammation and a variable expiratory airflow limitation. Key symptoms are wheezing, shortness of breath, chest tightness and cough, which typically vary over time and in intensity.1
Cough is commonly perceived as a troublesome symptom2 and is associated with asthma severity3 and a worse prognosis.4 In asthma, symptoms tend to worsen at night and often cause awakenings impacting on quality-of-life.1
The role of nocturnal cough is not well-defined. Due to practical reasons, current measures for asthma control do not objectively capture nocturnal cough as a parameter of asthma control and potential treatment target. Marsden et al found that the rate of nocturnal cough might be a valid marker for asthma control.5 Due to the cross-sectional design, however, information regarding longitudinal trends and cause–effect relationships of nocturnal cough in asthma is lacking. In a recent study, cough in general and even more nocturnal cough improved after appropriate treatment,6 indicating the potential to serve as a useful parameter for disease self-monitoring.
The aim of this study was to investigate the prevalence and characteristics of nocturnal cough in asthmatic individuals over the course of four weeks by means of a smartphone.
Methods
A detailed study protocol of this two-center, longitudinal, observational study has been published before.7 The study has been reviewed and approved by the ethics committee responsible for research involving humans in eastern Switzerland (“Ethikkommission Ostschweiz”, ID: 2017–01872). The trial registration number on clinicaltrials.gov is NCT03635710. All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki. In this work, we report results of the first study stage, focused on the description of nocturnal cough. The results regarding the interaction of nocturnal cough and asthma control (ie, the second study stage) will be published in a separate paper.
Study Setting
The study was conducted in two centers in Switzerland between February 2018 and June 2019: the Lung Center of the Cantonal Hospital of St Gallen and the mediX Group Practice Zurich in cooperation with the University of Zurich. The study duration per participant was 29 days. On the first (d1) and the last (d29) study days, participants had an in-person appointment at one center. In between, patient-reported outcomes and nocturnal sensor data were collected by a smartphone. In total, 94 patients were recruited. For the recruitment of study participants multiple recruitment channels, personal or impersonal, were employed.7
Eligibility Criteria
Eligible for inclusion were adult patients with physician-diagnosed asthma, who could give written informed consent and were capable of operating a smartphone. Exclusion criteria were cognitive impairment, severe insomnia, shift work and sharing the bedroom with a person from the same sex. The latter criteria would have made a solely acoustic-based allocation of nocturnal coughs to the correct person difficult.
Endpoints
The primary endpoint was nocturnal cough rate, obtained through smartphone audio recordings, manually labelled by trained human annotators. In concordance with recommendations of the European Respiratory Society, cough was defined as a three-phase expulsive motor act characterized by an inspiratory effort, followed by a forced expiratory effort against a closed glottis, followed by an opening of the glottis and rapid expiratory airflow.8 The secondary endpoint was cough severity (measured daily by a visual analog scale).9
A detailed sample size calculation, participant timeline, tasks, and the study flow chart are described in detail in the study protocol.7
Data Collection
Medical assessments on d1 and d29 were performed by physicians in the study centers. For the measurements between physician appointments, patients were equipped with a smartphone (Samsung Galaxy A3 2017, SM-A320FL) with an installed chat-based study app named “Clara”, which is a study-specific adaptation of MobileCoach (www.mobile-coach.eu), an open-source platform for ecological momentary assessments and behavioral health interventions.10,11 It recorded the participants’ sensor data at night (audio data) and delivered the daily questionnaires (see also Figure 1 in the published protocol).7 A description of all measurements including temporal framing and the measures to promote participant adherence were described in detail in the published protocol.7 Nocturnal cough was labelled only by means of audio data. To quantify the labeling quality, two approaches were employed. First, human annotators were instructed to tag an acoustic event if they were uncertain whether it was a cough. Out of a total of 704,697 acoustic events, 30,260 were classified unambiguously as coughs. In 0.11% of all acoustic events, annotators were uncertain (ie, 767 cases). These events were not considered in the analysis. Second, interrater reliability was estimated for two of the annotators, which together have labelled 90.2% of all nights. The two labelers had an intraclass correlation (ICC) of 95.8% (mean absolute error: 0.44 c/n) and 85.4% (mean absolute error: 0.76 c/n) for cough in general and coughs correctly assigned to the participant’s sex, respectively. These ICCs can be interpreted as excellent and good reliabilities, respectively, and were calculated based on 65 nights using a zero-inflated generalized mixed-effects model with a Poisson response distribution. To prepare data for analysis, missing values for nocturnal cough were imputed. Different imputation methods were benchmarked by comparing imputations to deleted observations. For nocturnal cough, imputation was performed by means of a Kalman smoother (based on an auto-regressive moving average model fitted across all patients). Imputations with negative values were set to zero.
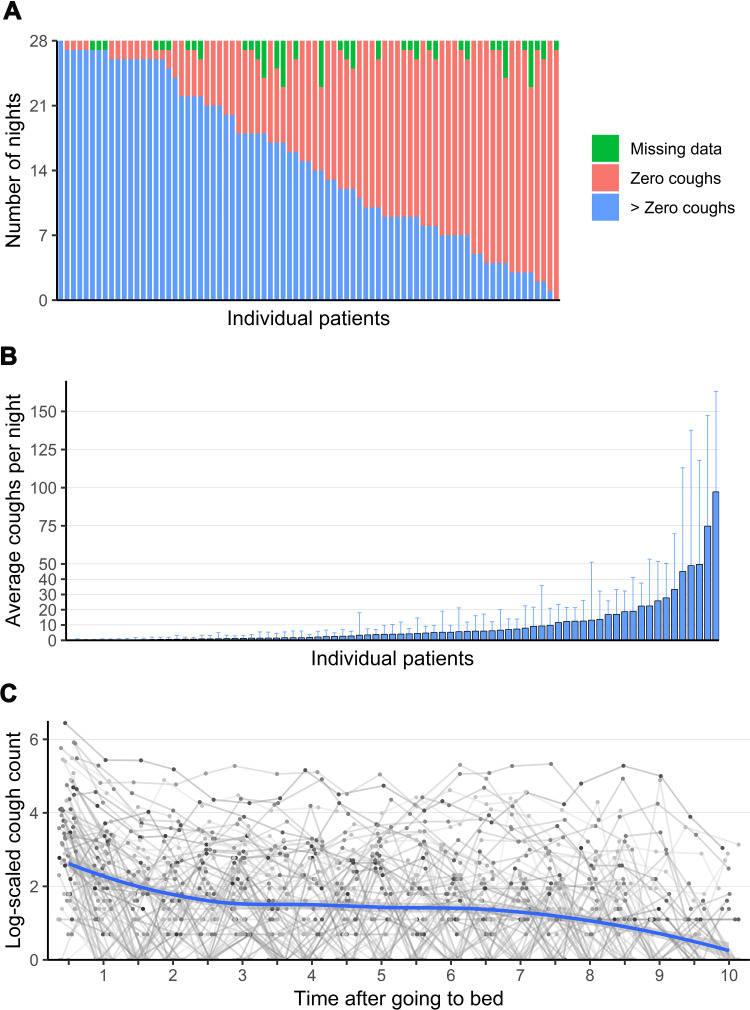
Figure 1.
(A) Distribution of nocturnal cough data. Each bar represents one patient; (B) Nocturnal cough counts—each bar represents one patient. Error bars indicate one standard deviation. One patient has not coughed at all; (C) Distribution of cough events in relation to bedtime over all 28 nights (log-scaled). Each dot represents the mean number of coughs of a single patient at a given time after going to bed.
Statistical Analysis
All data transformation and analyses were performed with R 3.5.1. Bivariate relationships were estimated using Pearson’s product-moment correlation (for two metric variables) and Spearman’s rho (if one variable was on an ordinal scale). Differences between groups were tested for statistical significance with pairwise Wilcoxon rank-sum tests. Holm’s p-value correction was employed to account for multiple comparisons. The following R packages were used: “dplyr” (version 0.8.0.1) for data transformation, “ggplot2” for plotting (version 3.1.0), “Hmisc” (version 4.2) for estimating the autocorrelation function, and “stats” (default R) for significance testing.
Data Sharing Statement
It is not intended to share individual participant data.
Results
The baseline characteristics of all 94 included patients are summarized in Table 1.
Table 1.
Baseline Characteristics
| Demographics and Body Composition | ||
|---|---|---|
| Age (years) | 45 (30–59) | |
| Male, n (%) | 41 (44%) | |
| Female, n (%) | 53 (56%) | |
| Height, cm | 170 (163–176) | |
| Weight, kg | 71 (63–83) | |
| Body mass index, kg/m2 | 25 (22–28) | |
| Smoking status, n (%) | ||
| Current | 3 (3%) | |
| Former | 27 (29%) | |
| Never | 64 (68%) | |
| For current or former smokers | ||
| Years smoked | 15 (9–20) | |
| Cigarettes per day | 14 (10–20) | |
| Pack years | 10 (4–15) | |
| Lung function | ||
| FEV1, liters | 2.9 (2.3–3.4) | |
| FEV1, % predicted | 88 (77–101) | |
| FeNO, ppb | 20 (12–34) | |
| GINA-stage, n (%) | ||
| 1 | 15 (16%) | |
| 2 | 20 (21%) | |
| 3 | 44 (47%) | |
| 4 | 13 (14%) | |
| 5 | 2 (2%) | |
| Asthma severity, n (%) | ||
| Intermittent | 13 (14%) | |
| Mild | 42 (45%) | |
| Moderate | 35 (37%) | |
| Severe | 4 (4%) | |
| Asthma control test at baseline, points | 21 (19–23) | |
| Asthma control at baseline, n (%) | ||
| Controlled | 66 (70%) | |
| Partially controlled | 18 (19%) | |
| Uncontrolled (Incomplete measurements) | 9 (10%) 1 (1%) | |
| Exacerbations within last 12 months, n (%) | ||
| No | 34 (36%) | |
| Yes | 60 (64%) | |
|
2 (1–2) | |
|
11 (13%) | |
|
4 (4%) | |
| Asthma medication, n (%) | Prescribed | Used |
| SAMA | 5 (5%) | 3 (3%) |
| SABA | 52 (55%) | 41 (44%) |
| ICS | 86 (91%) | 77 (82%) |
| LABA | 78 (83%) | 71 (76%) |
| LAMA | 9 (10%) | 8 (9%) |
| Systemic corticosteroids | 1 (1%) | 0 (0%) |
| LTRA | 10 (11%) | 10 (11%) |
| Anti-IL5 | 2 (2%) | 2 (2%) |
| Comorbidities, n (%) | ||
| Allergic and/or chronic rhinosinusitis | 58 (62%) | |
| Arterial hypertension | 14 (15%) | |
| Coronary heart disease | 4 (4%) | |
| History of myocardial infarction | 2 (2%) | |
| Active cancer | 2 (2%) | |
| Diabetes | 4 (4%) | |
| Psychiatric disease GERD |
1 (1%) 0 (0%) |
|
| Other | 23 (25%) |
Note: Data are expressed as median (interquartile range) unless stated otherwise.
Abbreviations: FEV1, forced expiratory volume in 1 second; FeNO, fraction of nitric oxide in exhaled air; Ppb, parts per billion; GINA, global initiative for asthma; SAMA, short-acting muscarinic antagonist; SABA, short-acting beta-agonist; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene-receptor-antagonist; IgE, immunoglobulin E; IL5, interleukin-5; ED, emergency department; GERD, gastroesophageal reflux disease.
In total, 15 of 94 patients had to be excluded from analysis (=dropouts). Dropout reasons were the following: two patients withdrew by themselves, three patients were nonadherent to the study protocol and in 10 patients we missed audio recordings for more than five nights. The reasons for missed audio recordings can be technology-related (eg, app crash) or patient-related (eg, turned off the smartphone at night).
Descriptive Characteristics of Nocturnal Cough
Patients coughed in 53% of 2212 nights. Three percent of night recordings were missing (67 nights, Figure 1A). Median coughs per night were one (IQR 0–8, range: 0–345). Nocturnal cough counts per patient showed considerable variance between patients. Furthermore, the standard deviations per patient indicate that nocturnal cough was a time-variant symptom (Figure 1B). The median number of coughs per hour was 0 (IQR 0–1, range: 0–136).
The highest cough counts were measured in the first 30 min in bed with a rather homogeneous distribution afterward (first 30 min: 4751 coughs, rest of night: mean 1050 coughs/30 min; ratio 4.53, Figure 1C).
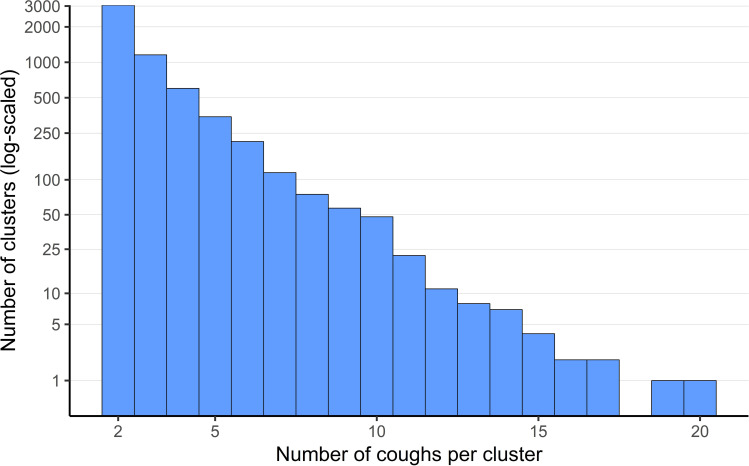
The following analyses are based on all nights with at least one cough. The term “cough cluster” is defined as a series of at least two coughs with a maximum time of two seconds in between their expulsive phases. Most coughs (85.6%) were not isolated, but part of a cough cluster. Clusters consisted of a median of two coughs (IQR 2–4; Figure 2 and Table s1 and s2).
Figure 2.
Distribution of coughs per cluster.
To explore the temporal stability of nocturnal cough, we estimated the auto-correlation function. We found that nocturnal cough was persistent within patient up to all 28 nights (figure s1).
We did not find significant differences regarding nocturnal cough counts depending on seasons.
Correlation of Measured Nocturnal Cough with Subjective Cough Perception
The subjective cough intensity and the predominant daytimes subjectively affected by coughing at baseline are described in Table 2.
Table 2.
Cough Perception at Baseline
| Subjective Cough Intensity at Baseline | n (%) |
|---|---|
| No cough | 7 (7) |
| Very weak | 10 (11) |
| Weak | 27 (29) |
| Moderate | 21 (22) |
| Strong | 9 (10) |
| Very strong | 20 (21) |
| Predominant Daytime With Cough at Baseline, (multiple choices possible) | n (%) |
| Night | 42 (45) |
| Morning | 55 (59) |
| Mid-morning | 34 (36) |
| Afternoon | 31 (33) |
| Evening | 51 (54) |
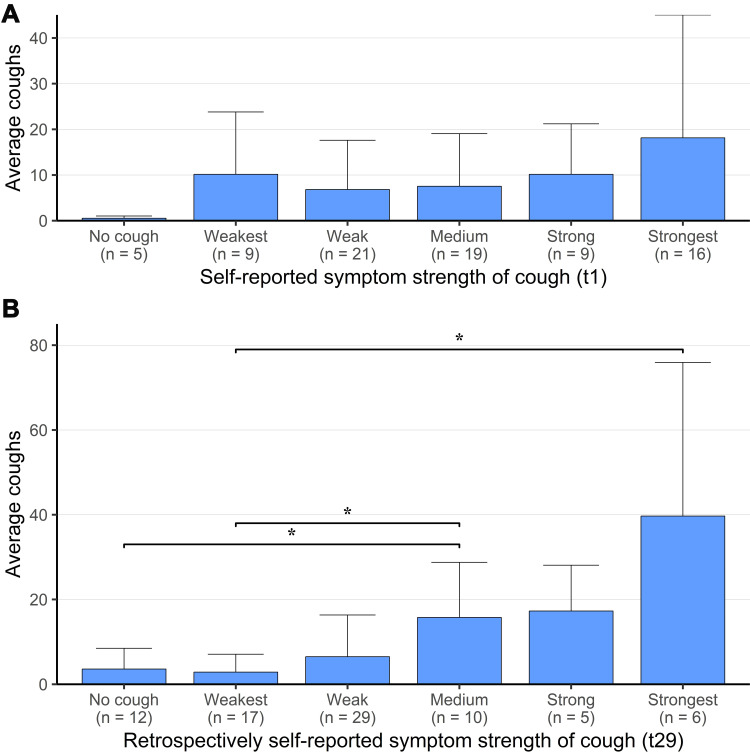
In Figure 3A, we demonstrate the correlation between the self-reported intensity of cough at the first study day with the average cough rate over the study period, which was statistically significant overall (Spearman’s rank correlation rho=0.297375; S=57728; p=0.008), but showed no statistically significant differences between the groups with different self-reported cough intensities. The correlation between the self-reported intensity of cough at the last study day with the average cough rate over the study period was highly statistically significant (Spearman’s rank correlation rho=0.5401344; S=37783; p=2.784e-07) and also statistically significant differences between groups were apparent, meaning that at the end of the study, the self-perceived cough-intensity correlated better with objectively measured cough rate (Figure 3B).
Figure 3.
Correlation between the self-reported intensity of cough at first (A) and last (B) study day with the average cough rate over the study period. *: statistically significant between groups.
There were significant correlations between the measured cough rate and the subjective cough severity of the same night, the preceding day and the following day (Tables s3-s5). After 33 min, this correlation exceeds the lower 95%CI boundary of the entire-night correlation, meaning that at least measuring this time is necessary to estimate subjective cough severity.
There was a significant correlation between nocturnal cough rate and FEV1 (Table 3), whereas we found no correlations between nocturnal cough rate and sex, asthma severity at baseline, rhinitis or FeNO.
Table 3.
Pearson Correlations Between Nocturnal Cough and FEV1% Pred
| Transformation of Nocturnal Cough | r | 95%CI | t | df | p |
|---|---|---|---|---|---|
| Count of the preceding night | −0.132 | −0.343; 0.092 | −1.167 | 77 | 0.247 |
| Mean of the preceding week | −0.223* | −0.424; −0.002 | −2.011 | 77 | 0.048 |
| Mean of the preceding month | −0.28* | −0.472; −0.062 | −2.556 | 77 | 0.013 |
Note: *p<0.05.
Discussion
In this longitudinal observational study, we investigated the prevalence and characteristics of nocturnal cough in 79 asthma patients over the course of four weeks. The study population was characterized by rather mild, well-controlled asthma. Interestingly, only 3% of participants were active smokers in comparison to approximately 25% in the general Swiss population.
Nocturnal cough in asthmatics occurred in more than half of all nights. There was a substantial variance between patients. Nocturnal cough was persistent within patients over the whole study period. In the first half hour in bed, cough rate was much higher (approximately 4.5-fold) than in the rest of the night. Most coughs were not isolated, but part of cough clusters. There was a significant correlation between objectively measured cough rate and subjective cough perception as well as baseline FEV1.
Past studies regarding nocturnal respiratory symptoms had different limitations, eg were restricted to cross-sectional data,5,12 considered limited data sources,13 relied exclusively on survey data14–16 or collected data in artificial settings, eg by using polysomnography.17–19 Our study setting with the smartphone lying near the bed, like many people do routinely, aimed to achieve a low patient disturbance and a high external validity. This was further supported by real-life eligibility criteria, which made inclusion possible for the vast majority of patients, which is often not the case in asthma-related trials.20
Hirai et al detected nocturnal cough in children with an asthma exacerbation by means of microphone and accelerometer and found that nocturnal cough rate was highest in severe exacerbations, followed by moderate exacerbations and no exacerbation. Cough counts were significantly increased at the beginning and the end of the night.21 The former could be observed in our study, too. Other studies described reduced cough frequencies during nighttime with increase at the time of waking.22,23 One hypothesis for the explanation of these findings could be that the change of body position from upright to supine or vice versa could influence lung physics and act as a trigger for asthma activity. Further, a central suppressive effect of sleep on cough has been described.22 Gastroesophageal reflux disease (GERD), which could be a trigger for coughing after changing the body position, seems not to have played a role in our cohort, because none of the participants suffered from GERD. In a recent trial, Radine et al used a microphone-based monitoring system to count nightly coughs in healthy subjects and in stable patients with cystic fibrosis (CF, n=25) and primary ciliary dyskinesia (PCD, n=25) over two consecutive nights. Nightly cough was absent in healthy subjects and higher in CF than in PCD. Further, cough rate in these conditions was associated with disease severity.24 Krönig et al used the same monitoring system, again over two consecutive nights in patients with chronic obstructive pulmonary disease (COPD) and detected cough in 42 out of 48 patients with a range of 1–326 events per night.25
Post hoc, we did not find significant differences regarding nocturnal cough counts depending on seasons. This finding has to be interpreted with caution, because robustness is questionable due to the study design, which was not intended to investigate seasonality effects. To answer this question properly, future research is necessary.
Marsden et al described a weak to moderate association between subjective measures of cough and objective cough frequency in asthma.26 In the current study, the correlation between objectively measured cough rate and subjective cough perception improved when comparing first and last study day, indicating that patients trained their ability to estimate their cough intensity over the study period. The correlation between cough rate and FEV1 could be a hint towards a possible correlation with asthma control.
To the best of our knowledge, this is the first study, which evaluated objectively measured nocturnal cough frequencies in adult asthmatic patients longitudinally (here for one month). Our findings of relevant nocturnal cough frequencies with a huge variance between patients (and within patients at different time points) are new and in line with the abovementioned results from smaller and shorter trials regarding other respiratory diseases like COPD, CF, and PCD. Our finding concerning the trends of nocturnal cough, eg demonstrating that it was a persistent symptom over up to 28 nights has also not been described in the literature until now.
With this study, we addressed the largely unknown nature of nocturnal cough in asthma by objectively and extensively measuring its prevalence and describing its different characteristics. For future research, these findings have to be put in context with other clinical parameters, eg like asthma control and exacerbations to develop concrete practical implications. In a related second paper, which is currently under review, we focus on the interaction of nocturnal cough and asthma control.
Strengths and Limitations
As mentioned before, we aimed to achieve the best possible external validity and generalizability by using conventional smartphones in the bedrooms of the patients in their homes, with an app-based system and by keeping exclusion criteria at a minimum. This approach could result in a limitation of internal validity, because the study relies considerably on patient-reported outcomes and a purely sound-based cough annotation approach. Our measures to mitigate these aspects can be found in detail in the published protocol.7 This study constituted an invasion of patients’ privacy and therefore required participants to be open for such an intervention, possibly leading to a self-selection bias. However, it seems not plausible to assume that patients’ attitudes are associated with clinical aspects of their asthma, so validity should not be affected by this. Another limitation is due to the fact that asthma symptoms vary over time.1 With duration of 29 days, our study does not allow for the investigation of long-term within-person symptom trends and patterns.
Conclusions
In summary, our study is the first to describe prevalence and characteristics of nocturnal cough in asthma over a period of one month. Nocturnal cough was a prevalent symptom with high variance between patients and high persistence within patients. Cough events were 4.5 times more frequent within the first 30 min in bed indicating a potential role of positional change. Nocturnal cough characteristics could serve to generate hypotheses regarding pathophysiological aspects of cough. An important next step will investigate the association between nocturnal cough and asthma control. Based on the upcoming results, future research should aim at developing evidence-based automated intervention algorithms for adapting asthma treatment.
Acknowledgments
We thank all patients who participated in this study. Further we thank the study teams of all participating institutions for their support.
Frank Rassouli and Peter Tinschert are equally contributing first authors. Furthermore, Martin H Brutsche and Tobias Kowatsch should be considered joint senior authors.
Funding Statement
This study is funded by CSS Insurance, Switzerland. The CSS insurance supported the recruitment of participants but had no role in study design, app design, data management plans, or in reviewing and approving the manuscript for publication.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Peter Tinschert, Filipe Barata, Elgar Fleisch and Tobias Kowatsch are affiliated with the Center for Digital Health Interventions, a joint initiative of the Department of Management, Technology and Economics at ETH Zurich and the Institute of Technology Management at the University of St Gallen, which is funded in part by the Swiss health insurer CSS. Peter Tinschert reports grants from CSS Insurance, during the conduct of the study; and the foundation of a company, which is partly based on the results of the paper, is planned. Claudia Steurer-Stey reports personal fees from AstraZeneca, GlaxoSmithKline, and Novartis, outside the submitted work. Elgar Fleisch reports grants and nonfinancial support from CSS Health Insurance, and is also cofounder of Pathmate Technologies, a university spin-off company that creates and delivers digital clinical pathways and has used the open source MobileCoach platform for that purpose, too (however, Pathmate Technologies is not involved in the study app described in this paper; neither is CSS Health Insurance), during the conduct of the study. Tobias Kowatsch reports grants from CSS AG, during the conduct of the study; and is co-founder of Pathmate Technologies, a university spin-off company that creates and delivers digital clinical pathways and has used the open source MobileCoach platform for that purpose, too (however, Pathmate Technologies is not involved in the study app described in this paper), outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Asthma, G.I.f. Global Strategy for Asthma Management and Prevention, 2020. 2020: www.ginasthma.org. Accessed November19, 2020.
- 2.Osman LM, et al. Patient weighting of importance of asthma symptoms. Thorax. 2001;56(2):138–142. doi: 10.1136/thorax.56.2.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Marco R, Marcon A, Jarvis D, et al. Prognostic factors of asthma severity: a 9-year international prospective cohort study. J Allergy Clin Immunol. 2006;117(6):1249–1256. doi: 10.1016/j.jaci.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 4.Thomson NC, Chaudhuri R, Messow CM, et al. Chronic cough and sputum production are associated with worse clinical outcomes in stable asthma. Respir Med. 2013;107(10):1501–1508. doi: 10.1016/j.rmed.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 5.Marsden PA, Satia I, Ibrahim B, et al. Objective Cough Frequency, Airway Inflammation, and Disease Control in Asthma. Chest. 2016;149(6):1460–1466. doi: 10.1016/j.chest.2016.02.676 [DOI] [PubMed] [Google Scholar]
- 6.Fukuhara A, et al. Clinical Characteristics of Cough Frequency Patterns in Patients with and without Asthma. J Allergy Clin Immunol Pract. 2020;8(2):654–661. [DOI] [PubMed] [Google Scholar]
- 7.Tinschert P, Rassouli F, Barata F, et al. Prevalence of nocturnal cough in asthma and its potential as a marker for asthma control (MAC) in combination with sleep quality: protocol of a smartphone-based, multicentre, longitudinal observational study with two stages. BMJ Open. 2019;9(1):e026323. doi: 10.1136/bmjopen-2018-026323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29(6):1256–1276. doi: 10.1183/09031936.00101006 [DOI] [PubMed] [Google Scholar]
- 9.Spinou A, Birring SS. An update on measurement and monitoring of cough: what are the important study endpoints? J Thorac Dis. 2014;6(Suppl 7):S728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filler A, Haug S, Wahle F, Staake T, Fleisch E. “MobileCoach: A novel open source platform for the design of evidence-based, scalable and low-cost behavioral health interventions: Overview and preliminary evaluation in the public health context”, in Wireless Telecommunications Symposium (WTS). New York; 2015:1–6. [Google Scholar]
- 11.Kowatsch T, Shih I, Rüegger D. Design and Evaluation of a Mobile Chat App for the Open Source Behavioral Health Intervention Platform Mobilecoach, in Designing the Digital Transformation. Lecture Notes in Computer Science. Cham: Springer; 2017. [Google Scholar]
- 12.Luyster FS, Teodorescu M, Bleecker E, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath. 2012;16(4):1129–1137. doi: 10.1007/s11325-011-0616-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janson C, Gislason T, Boman G, et al. Sleep disturbances in patients with asthma. Respir Med. 1990;84(1):37–42. doi: 10.1016/S0954-6111(08)80092-3 [DOI] [PubMed] [Google Scholar]
- 14.Campos FL, de Bruin PFC, Pinto TF, et al. Depressive symptoms, quality of sleep, and disease control in women with asthma. Sleep Breath. 2017;21(2):361–367. doi: 10.1007/s11325-016-1422-0 [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick MF, et al. Snoring, asthma and sleep disturbance in Britain: a community-based survey. Eur Respir J. 1993;6(4):531–535. [PubMed] [Google Scholar]
- 16.Garden M, O’Callaghan M, Suresh S, et al. Asthma and sleep disturbance in adolescents and young adults: A cohort study. J Paediatr Child Health. 2016;52(11):1019–1025. doi: 10.1111/jpc.13234 [DOI] [PubMed] [Google Scholar]
- 17.Drugman T, Dutoit T, Assessment of audio features for automatic cough detection, 19th European Signal Processing Conference 2011. [Google Scholar]
- 18.McGuinness K. Validation of the VitaloJAK™ 24 Hour Ambulatory Cough Monitor. Thorax. 2012;67. [Google Scholar]
- 19.Stores G, Ellis AJ, Wiggs L, et al. Sleep and psychological disturbance in nocturnal asthma. Arch Dis Child. 1998;78(5):413–419. doi: 10.1136/adc.78.5.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 21.Hirai K, Enseki M, Tabata H, et al. Objective measurement of frequency and pattern of nocturnal cough in children with asthma exacerbation. Ann Allergy Asthma Immunol. 2016;117(2):169–174. doi: 10.1016/j.anai.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 22.Lodhi S, Smith JA, Satia I, et al. Cough rhythms in asthma: potential implication for management. J Allergy Clin Immunol Pract. 2019;7(6):2024–2027. doi: 10.1016/j.jaip.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousaf N, Monteiro W, Matos S, et al. Cough frequency in health and disease. Eur Respir J. 2013;41(1):241–243. doi: 10.1183/09031936.00089312 [DOI] [PubMed] [Google Scholar]
- 24.Radine A, Werner C, Raidt J, et al. Comparison of Nocturnal Cough Analysis in Healthy Subjects and in Patients with Cystic Fibrosis and Primary Ciliary Dyskinesia: A Prospective Observational Study. Respiration. 2019;97(1):60–69. doi: 10.1159/000493323 [DOI] [PubMed] [Google Scholar]
- 25.Kronig J, Hildebrandt O, Weissflog A, et al. Long-term Recording of Night-Time Respiratory Symptoms in Patients with Stable COPD II-IV. COPD. 2017;14(5):498–503. doi: 10.1080/15412555.2017.1338681 [DOI] [PubMed] [Google Scholar]
- 26.Marsden PA, Smith JA, Kelsall AA, et al. A comparison of objective and subjective measures of cough in asthma. J Allergy Clin Immunol. 2008;122(5):903–907. doi: 10.1016/j.jaci.2008.08.029 [DOI] [PubMed] [Google Scholar]