LACCASE8 from the model system Cleome hassleriana possesses the unusual property of oxidizing caffeyl alcohol but not coniferyl alcohol and plays a critical role in initiating C-lignin polymerization.
Abstract
Catechyl lignin (C-lignin) is a linear homopolymer of caffeyl alcohol found in the seed coats of diverse plant species. Its properties make it a natural source of carbon fibers and high-value chemicals, but the mechanism of in planta polymerization of caffeyl alcohol remains unclear. In the ornamental plant Cleome hassleriana, lignin biosynthesis in the seed coat switches from guaiacyl lignin to C-lignin at ∼12 d after pollination. Here we found that the transcript profile of the laccase gene ChLAC8 parallels the accumulation of C-lignin during seed coat development. Recombinant ChLAC8 oxidizes caffeyl and sinapyl alcohols, generating their corresponding dimers or trimers in vitro, but cannot oxidize coniferyl alcohol. We propose a basis for this substrate preference based on molecular modeling/docking experiments. Suppression of ChLAC8 expression led to significantly reduced C-lignin content in the seed coats of transgenic Cleome plants. Feeding of 13C-caffeyl alcohol to the Arabidopsis (Arabidopsis thaliana) caffeic acid o-methyltransferase mutant resulted in no incorporation of 13C into C-lignin, but expressing ChLAC8 in this genetic background led to appearance of C-lignin with >40% label incorporation. These results indicate that ChLAC8 is required for C-lignin polymerization and determines lignin composition when caffeyl alcohol is available.
INTRODUCTION
Lignins are complex aromatic polymers that are mainly deposited in the secondary cell walls of vascular plants. For a long time, it was believed that lignins are only composed of p-hydroxyphenyl (H-), guaiacyl (G-), and syringyl (S-) units derived from the polymerization of the corresponding monolignols p-coumaryl, coniferyl, and sinapyl alcohols, respectively (Vanholme et al., 2019). Increasing evidence has shown that the three classical hydroxycinnamyl alcohols are not the only compounds that can be incorporated into natural lignin, and additional monomers have been found to exist in genetically engineered plants with modifications to the monolignol biosynthetic pathway (Dixon and Barros, 2019). For example, 5-hydroxyguaiacyl units can be present in the lignin of transgenic plants with loss of function of caffeic acid/5-hydroxyconiferaldehyde 3/5-O-methyltransferase (COMT; Ralph et al., 2001; Weng et al., 2010). Moreover, examination of lignin structures from a broader range of plant species has led to the discovery of several new natural lignin building blocks (Annunziata, 2019), such as tricin and resveratrol (Lan et al., 2015; del Rio et al., 2017).

We previously discovered a novel type of lignin (named C-lignin), which is wholly derived from caffeyl alcohol, in seed coats of vanilla orchid (Vanilla planifolia), the ornamental plant Cleome (Cleome hassleriana), and many members of the Cactaceae (Chen et al., 2012, 2013; Tobimatsu et al., 2013). The linear linkages of benzodioxane units in C-lignin and its homopolymeric nature make it an ideal substrate for the production of carbon fibers and lignin valorization through depolymerization to uniform catechyl-type monomers (Nar et al., 2016; Li et al., 2018; Stone et al., 2018).
In C. hassleriana, G-lignin is biosynthesized in the seed coat during the first 6 to 12 d after pollination (DAP); after that time, G-lignin deposition stops and there is a switch to C-lignin formation (Tobimatsu et al., 2013). Due to this unique pattern of lignin accumulation during seed maturation, we have developed Cleome as a model system to investigate C-lignin biosynthesis and polymerization. In previous studies, we identified a complete set of C-lignin monomer biosynthesis genes from V. planifolia and Cleome transcriptomes through bioinformatic approaches (Rao et al., 2014; Zhuo et al., 2019). Biochemical characterization of several key enzymes, including caffeoyl CoA- and caffeic acid 3-O-methyltransferases (CCoAOMT and COMT) and cinnamyl alcohol dehydrogenase (ChCAD), provided a basis for understanding the mechanism of C-lignin monomer biosynthesis (Zhuo et al., 2019). However, knowledge about C-lignin polymerization is lacking.
The polymerization of caffeyl alcohol in planta, the final step in C-lignin biosynthesis, is likely to occur via an oxidative enzyme reaction followed by free-radical cross-coupling under simple chemical control, in the same manner as the in planta polymerization of the traditional monolignols (Chen et al., 2012). Oxidative polymerization of monolignols is catalyzed in vitro by two groups of enzyme systems: laccases (EC 1.10.3.2) and class-III peroxidases (EC 1.11.17; Sterjiades et al., 1992; Bao et al., 1993; Barros et al., 2015; Tobimatsu and Schuetz, 2019). However, many aspects of the mechanisms underlying this process remain unclear, as the possession of large gene families for both enzymes makes it generally difficult to interrogate their roles in planta (Duroux and Welinder, 2003; Turlapati et al., 2011).
In recent years, peroxidases were genetically proven to be involved in plant cell wall lignification (Shigeto and Tsutsumi, 2016). In Arabidopsis (Arabidopsis thaliana), cell-specific downregulation of PEROXIDASE64 significantly delayed the formation of the Casparian strip, a layer of lignified cells in the root endodermis (Lee et al., 2013). Like peroxidases, the essential functions of laccases in lignification have been revealed by loss-of-function approaches, using Arabidopsis, poplar (Populus trichocarpa), and Brachypodium distachyon plants (Berthet et al., 2011; Wang et al., 2015b; Le Bris et al., 2019). The laccase triple mutant (lac4 lac11 lac17) of Arabidopsis showed severe growth defects and lack of lignin in vascular tissues and fibers, but its Casparian strip structure was not affected, suggesting that laccases are essential for lignin polymerization and have non-redundant roles with peroxidases in lignification in vascular tissues (Zhao et al., 2013). Whether laccases can control lignin composition is currently unclear. Studies to date suggest that cell wall laccases are relatively promiscuous with respect to monolignol specificity in vitro, and the impact on lignin composition of their modified expression in planta cannot necessarily be predicted (He et al., 2019). Identification of laccases and/or peroxidases specifically involved in C-lignin polymerization would facilitate the introduction of C-lignin into non-seed–coat tissues of bioenergy crop plants as a co-product for bioprocessing (Ragauskas et al., 2014).
In this study, we describe a seed-coat–specific laccase from Cleome (ChLAC8), which exhibits an expression profile that parallels the accumulation pattern of C-lignin during seed maturation. The substrate specificity of the enzyme is consistent with a specialized role in C-lignin polymerization, and RNA interference (RNAi)-mediated knockdown experiments indicate that ChLAC8 is involved in C-lignin polymerization in the Cleome seed coat. The appearance of C-lignin upon expression of ChLAC8 in comt mutants of Medicago truncatula and Arabidopsis indicates that this enzyme can facilitate caffeyl alcohol polymerization in planta, making ChLAC8 an important component of a gene toolkit for engineering C-lignin.
RESULTS
The Expression Pattern of ChLAC8 Correlates with C-Lignin Accumulation during Seed Development
In Cleome, C-lignin is only deposited after 12 DAP in the seed coat and is not found in vegetative tissues (Tobimatsu et al., 2013). To identify candidate genes involved in C-lignin biosynthesis, we previously performed a comprehensive transcriptome analysis of RNA samples from different Cleome tissues (seed coat, stem, bark, fiber, and pith) and different stages of seed development (Zhuo et al., 2019). To determine whether specific enzymes might contribute to the polymerization of caffeyl alcohol, we interrogated this transcriptome database. Monolignol oxidation/polymerization is catalyzed by both peroxidases and laccases (Sterjiades et al., 1992; Bao et al., 1993; Barros et al., 2015; Tobimatsu and Schuetz, 2019), with laccase appearing to be essential for lignification in vascular tissues of Arabidopsis (Zhao et al., 2013). Seventy-two putative peroxidase transcripts were identified in our transcriptome database, but none of their transcript expression patterns correlated with C-lignin accumulation during seed development (Supplemental Figure 1; Supplemental Data Set 1). By contrast, 24 putative laccase (ChLAC) genes, resulting in 32 transcripts, were found in the transcriptome. The transcript profiles of ChLAC8X1, ChLAC8X2, and ChLAC8X3 exhibited good correlations with C-lignin content during seed maturation. Specifically, these transcripts, like those encoding ChCAD5 (a form of cinnamyl alcohol dehydrogenase with preference for caffeyl alcohol and functionally linked to C-lignin biosynthesis; Zhuo et al., 2019), were not expressed in vegetative tissues or during the period of G-lignin biosynthesis in the seed coat (Figure 1A). The expression pattern of ChLAC8s was the inverse of the expression patterns of ChCOMTs and CCoAOMTs (Figure 1A); these monolignol pathway O-methyltransferase genes are downregulated to block the 3-O-methylation reaction to allow for the formation of caffeyl alcohol.
Figure 1.
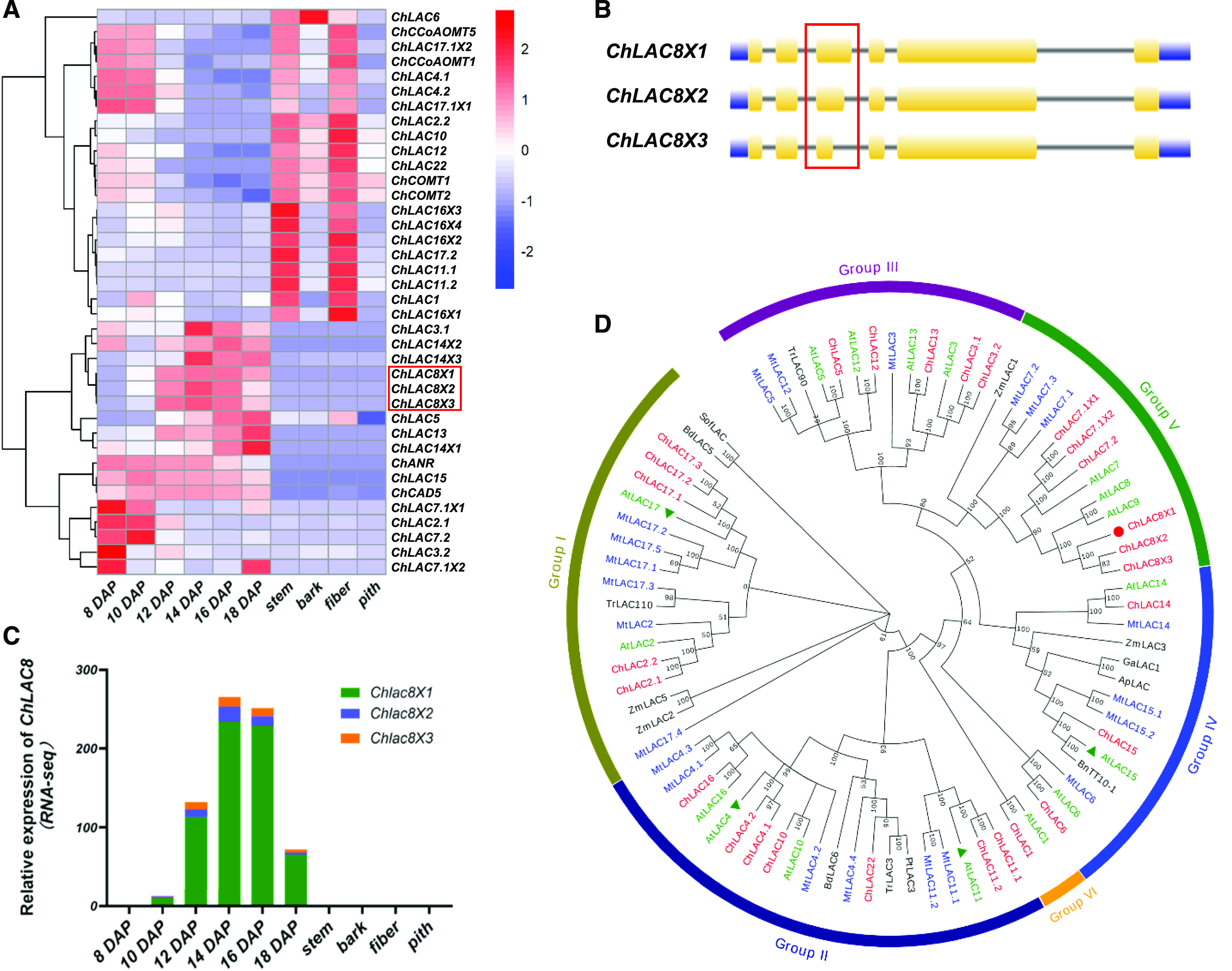
Transcript Level Profiles and Phylogenetic Analysis of Laccases Derived from C. hassleriana.
(A) Hierarchical clustering analysis of transcript levels of 32 ChLAC Cleome genes in different tissues and different stages of seed development. FPKM values (transcript levels) were transformed to log2(FPKM+1) for color scaling. The heatmap was drawn using the “pheatmap” package in the software R. The heatmap also shows the transcript levels of ChCAD5 (involved in C-lignin biosynthesis), ChCOMT1/2 and ChCoAOMT1/5 (encoding enzymes of G-lignin biosynthesis downregulated at the onset of C-lignin biosynthesis), and ChANR (associated with CT biosynthesis).
(B) Gene structure analysis of ChLAC8. ChLAC8X1, ChLAC8X2, and ChLAC8X3 arise from the same gene locus (LOC104823484) as a result of alternative splicing. The red box shows the third intron where the alternative splicing event takes place.
(C) Transcript levels of the three ChLAC8 transcript variants.
(D) Phylogenetic tree of laccases from C. hassleriana and other plants. The tree was constructed using the software MEGA 7.0 with neighboring-joining phylogeny testing and 1,000 bootstrap replicates. The Cleome ChLACs are in red, AtLACs from Arabidopsis are in green, and the Medicago laccases are in blue. The functionally characterized AtLACs (AtLAC4, 11, 15, and 17) are indicated by green triangles. The ChLAC8 characterized in this study is marked by a red circle. The accession numbers of the laccases are given in Supplemental Table 2. All laccase protein sequences are given in Supplemental Data Set 2.
Like other ChLACs, the putative protein sequences of all three ChLAC8 variants contained three typical characteristic laccase cupredoxin-like domains, CuRO_1_LCC (cd13849), CuRO_2_LCC (cd13875), and CuRO_3_LCC (cd13897), which are responsible for the binding of copper ions (Supplemental Figure 2). These three transcripts were produced by the same gene locus (ChLAC8, LOC104823484) due to alternative splicing at the 5′-end of the third intron (Figure 1B). However, ChLAC8X3 lacked a significant proportion of the copper binding domain, including two amino acid residues (His128 and His130 of ChLAC8X1) that coordinate to Cu atoms, and therefore likely encodes an inactive enzyme. Analysis by the online tool SignalP5.0 (Almagro Armenteros et al., 2019) indicated that ChLAC8 contains a typical N-terminal signal peptide with a cleavage site between amino acids 25 and 26. The subcellular localization of ChLAC8 was predicted to be extracellular with a certainty value of 0.92 by the online tool MultiLoc2 (Blum et al., 2009). Like other laccases, ChLAC8 is a glycoprotein, with 10 putative n-glycosylation sites (Supplemental Table) predicted using the online web servers NtetNGlyc 1.0 (http://www.cbs.dtu.dk/services/NetNGlyc) and N-GlyDE (http://bioapp.iis.sinica.edu.tw/N-GlyDE; Pitti et al., 2019).
Although the three ChLAC8 transcript variants showed similar expression patterns in our transcriptome database, ChLAC8X1 had more than 10-fold higher transcript level than the other two forms (Figure 1C), making it a strong candidate for involvement in C-lignin polymerization in the seed coat of Cleome. We further analyzed ChLAC8 transcript levels by qRT-PCR, revealing a similar expression pattern to that obtained from the RNA sequencing (RNA-seq) results (Supplemental Figure 3).
ChLAC8 is Phylogenetically Distinct from Functionally Characterized Laccases Involved in Lignin Biosynthesis
To investigate the evolutionary relationship between ChLAC8 and other plant laccases, we constructed a neighbor-joining tree with Cleome ChLACs, Arabidopsis AtLACs, Medicago MtLACs, and laccases that were previously characterized to be involved in lignification (Figure 1D; Supplemental Table 2; Caparrós-Ruiz et al., 2006; Wang et al., 2015a, 2015b; Bryan et al., 2016). Similar to Arabidopsis laccases, ChLAC members are divided into six subgroups (group I to VI; Turlapati et al., 2011). ChLAC8 is a homolog of AtLAC8 and AtLAC9 in group V, the exact functions of which are not known. In addition, ChLAC8 is phylogenetically distinct from AtLACs 4, 11, and 17, the three laccases that have been functionally ascribed roles in lignification in vascular tissues. It is also distinct from AtLAC15, more commonly known as TT10, which has been linked genetically to the oxidation of flavan-3-ols during the biosynthesis of condensed tannins (CTs) in the seed coat (Pourcel et al., 2005). Loss of function of AtLAC15 results in a transparent testa (TT) phenotype due to a lack of tannin oxidation, but AtLAC15 has also been ascribed a role in lignin biosynthesis (Liang et al., 2006). The Cleome homolog of AtLAC15, ChLAC15, is also exclusively expressed in the seed coat, but mostly at early stages of development (Figure 1A; Supplemental Figure 4A). This expression pattern is similar to that of ChANR, encoding anthocyanidin reductase, the key enzyme of CT biosynthesis (Figure 1A; Supplemental Figure 4A; Xie et al., 2003), and is consistent with the staining of CTs in the seed coat before the appearance of C-lignin (Supplemental Figure 4B). ChLAC5 is also expressed in the seed coat at a similar transcript level to CHLAC8 (Supplemental Figure 4A) and expressed in vegetative tissues, and was not pursued further.
Recombinant ChLAC8 Oxidizes Caffeyl and Sinapyl Alcohols but not Coniferyl Alcohol
As ChLAC8X1 was the full-length transcript with the highest expression, we expressed it in Escherichia coli and further purified the protein from bacterial extracts by His-Tag affinity chromatography (Supplemental Figure 5). To test the catalytic properties of recombinant ChLAC8 in vitro, we analyzed the oxidation of different monolignols by calculating the decrease in substrates by HPLC in the presence of this enzyme (Figures 2A to 2F). ChLAC8 could oxidize caffeyl alcohol (C-unit) and sinapyl alcohol (S-unit), but, surprisingly, not coniferyl alcohol (G-unit).
Figure 2.
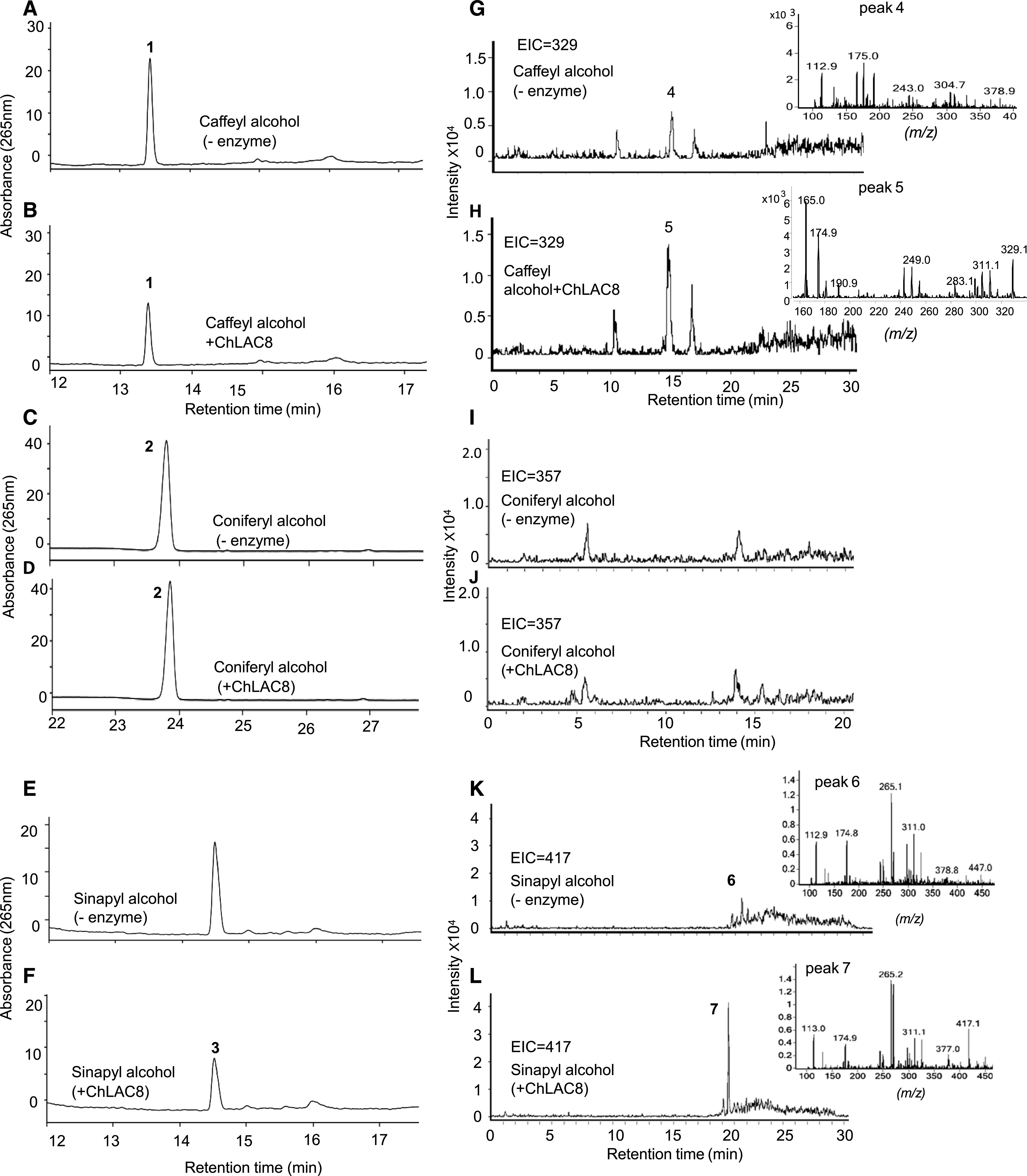
HPLC and LC-MS/MS Analysis of Reaction Products of ChLAC8 with Different Monolignols.
(A) to (F) HPLC chromatograms of reactions of ChLAC8 with caffeyl alcohol ([A] and [B], peak1), coniferyl alcohol ([C] and [D], peak 2), and sinapyl alcohol ([E] and [F], peak 3). (A), (C), and (E) show reactions incubated without recombinant enzyme. (B), (D), and (F) show reactions 30 min after incubation with recombinant ChLAC8.
(G) to (L) EICs, scanned at m/z = 329 for the dimer of caffeyl alcohol ([H], peak 5), m/z = 357 for the dimer of coniferyl alcohol ([I] and [J]), or m/z = 417 for the dimer of sinapyl alcohol (L, peak 7). (—) Indicates control assays without ChLAC8. No dimers were detected in the corresponding control reactions ([G], peak 4; [I] and [K], peak 6) or the reaction of ChLAC8 toward coniferyl alcohol (J).
We analyzed the products generated from caffeyl and sinapyl alcohols by liquid chromatography-tandem mass spectrometry (LC-MS/MS; Figures 2G to 2L). Analysis of the extracted ion chromatograms (EICs) and MS spectra of product profiles showed that the primary products (peaks 5 and 7) were consistent with the masses of dimers of the corresponding substrates (peak 5, m/z = 329 for dimer of caffeyl alcohol; peak 7, m/z = 417 for dimer of sinapyl alcohol). No dimers were detected in the reaction products from coniferyl alcohol (Figure 2J) or the control reactions (Figures 2G, 2I, and 2K). This finding confirms that ChLAC8 can oxidize caffeyl and sinapyl alcohols to generate their corresponding radicals in vitro. In addition, trimers were observed in the products generated from caffeyl alcohol (Supplemental Figure 6; peak 2, m/z = 493 for trimer of caffeyl alcohol). No trimers were detected in the reaction products from sinapyl alcohol or controls without ChLAC8.
The m/z values of the extracted ions of C-dimers for all three potential bonding modes (benzodioxane, phenylcoumaran, and resinol) are 329. The mass spectra of the dimers generated from caffeyl alcohol also showed a major peak ion at m/z = 165 (inset, Figure 2H). This can only arise from the breakdown of a benzodioxane-linked dimer, according to the scheme shown in Supplemental Figure 7 (Morreel et al., 2010). The m/z values for dimers of sinapyl alcohol will differ between resinols and β-aryl ethers (417 and 435, respectively). The data in Figure 2L showing the EICs at 417 indicate the formation of S-dimers as resinols joined via 8-8 linkage, but an ion was not detected at m/z = 435.
Next, we determined the kinetic parameters of ChLAC8 by measuring reaction rates over a range of different substrate concentrations. The Km value of ChLAC8 for caffeyl alcohol was ∼3.5-fold higher than that for sinapyl alcohol (Table 1). As a result, the catalytic efficiency (Kcat/Km) of ChLAC8 for caffeyl alcohol (92.53 M−1 s−1) was lower than that for sinapyl alcohol (218.0 M−1 s−1). However, sinapyl alcohol is not a natural substrate for laccase activity in the Cleome seed coat, which does not accumulate S-lignin due to the lack of expression of ferulate/coniferaldehyde 5-hydroxylase (Zhuo et al., 2019).
Table 1. Enzymatic Activity and Kinetic Parameters of Recombinant ChLAC8 Toward Different Monolignols.
| Substrate | Vmax (pKAT mg−1) | Km (μM) | Kcat (s−1) | Kcat/Km (M−1 s−1) |
|---|---|---|---|---|
| Caffeyl alcohol | 284.4 ± 50.4a | 210.3 ± 90.6a | 0.019 ± 0.003a | 92.53 |
| Coniferyl alcohol | N.D. | N.D. | N.D. | N.D. |
| Sinapyl alcohol | 192.0 ± 12.8 | 60.2 ± 16.7 | 0.013 ± 0.001 | 218.0 |
Purified recombinant ChLAC8 (10 to 20 μg) was incubated with 25 to 800 μM substrates at 30°C for 30 min. Three enzyme assays were performed at each substrate concentration, and the Vmax and Km values were calculated by nonlinear regression analysis. The significance of difference in kinetic parameters between caffeyl alcohol and sinapyl alcohol was estimated with the unpaired two-tailed Student’s t test, and P-values < 0.05 were deemed significant. N.D., not detected.
Significant difference found.
Molecular Modeling of ChLAC8 Catalysis
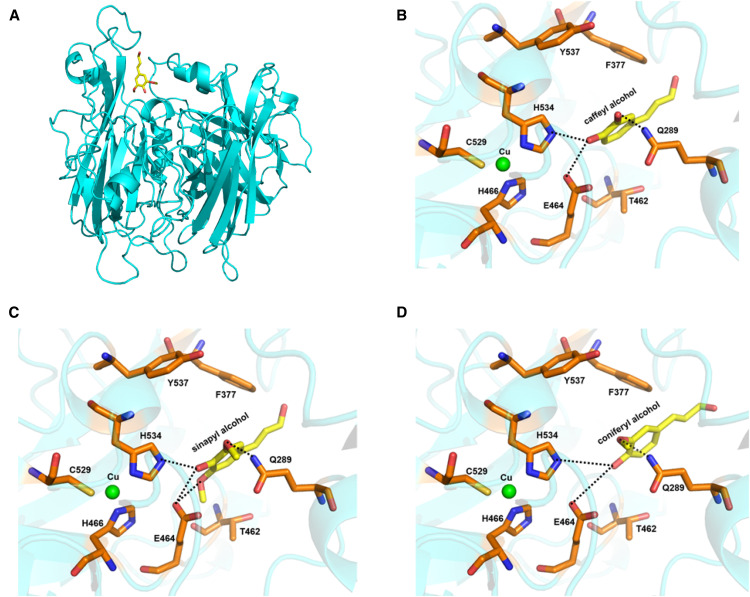
To understand the substrate specificity of ChLAC8, we performed molecular modeling and docking studies. Using the structure of ZmLAC3 (Protein Data Bank [PDB] ID: 6KLG; Xie et al., 2020) as a template, the three-dimensional structural model of ChLAC8 was obtained (Figure 3A). ZmLAC3 is in a different but adjacent group from ChLAC8 in the cladogram, closer to ChLAC15 (Figure 1D). However, similar to ZmLAC3, ChLAC8 consists of three cupredoxin-like domains, with one mononuclear copper (T1 Cu) site and one trinuclear copper site. The T1 Cu is coordinated by His466, His534, and Cys529. In the trinuclear copper site, each of the two T3 Cu atoms is coordinated to two sets of three His residues (His85/His128/His530 and His130/His471/His528, respectively), and the T2 Cu is coordinated to two His residues (His83 and His249).
Figure 3.
Examination of the Binding of Monolignols in the Active Site of ChLAC8 by Molecular Modeling.
(A) A modeled structure of ChLAC8 with sinapyl alcohol docked into the active site.
(B) Active site residues showing binding of caffeyl alcohol.
(C) Active site residues showing binding of sinapyl alcohol.
(D) Active site residues showing binding of coniferyl alcohol.
Caffeyl alcohol, sinapyl alcohol, and coniferyl alcohol are shown as a stick model in yellow. Some key protein residues in the active site and binding pocket are labeled and shown as stick models in orange. T1 Cu is shown as a sphere model in green. The predicted hydrogen bonds are indicated by dashed lines.
The putative substrate binding pocket contains residues Ala286, Gln289, Leu358, Phe377, Thr462, Ile461, Glu464, Ala533, His534, and Tyr537 (Figures 3B to 3D). Both caffeyl and sinapyl alcohols, as substrates, were docked with their 4-OH groups close to His534, which serves as the primary electron acceptor. In the model docked with caffeyl alcohol, the 3-OH of the substrate would form a strong hydrogen bond with Gln289, allowing stable maintenance of caffeyl alcohol in the active site for catalysis (Figure 3B). In the model with docked sinapyl alcohol, its 3-OMe forms a weak hydrogen bond with Gln289, but its 5-OMe forms a hydrogen bond with Glu464 to maintain its position near His534 for catalysis (Figure 3C). However, coniferyl alcohol lacks a 5-OMe; its 3-OMe alone may not form a strong enough interaction with Gln289 to maintain the compound in the active site for efficient catalysis (Figure 3D). Based on this model, Gln289 appears to be critical for the binding of caffeyl alcohol in the active site of ChLAC8.
Multiple sequence alignment of all laccases in Arabidopsis, Medicago, and Cleome showed that the regions around Gln289 are divergent, whereas Glu464 and His534 are conserved among the laccases from these species (Supplemental Figure 8). Finally, multiple sequence alignment of ChLAC8 with its Arabidopsis homolog and Arabidopsis and Cleome homologs of two laccases shown to be critical for lignin biosynthesis (LAC4 and LAC17) indicated that Gln289 is found only in ChLAC8 (Supplemental Figure 9).
RNAi-Mediated Downregulation of ChLAC8 Decreases the C-Lignin Content in Cleome Seed Coats
To test the role of ChLAC8 in C-lignin polymerization in planta, we generated 14 independent T1 transgenic RNAi lines of Cleome in which ChLAC8 was targeted for downregulation by RNA interference. To screen the T1 plants, we determined the transcript levels of ChLAC8 and the lignin composition in the seed coats by qRT-PCR and thioacidolysis, respectively. ChLAC8 transcript levels displayed a strong positive correlation (r = 0.737) with C-unit content for different T1 transgenic lines, while exhibiting a weaker positive relationship with G-unit content (r = 0.593), C/G ratio (r = 0.668), and the total content of C- and G-units (r = 0.669; Supplemental Figure 10).
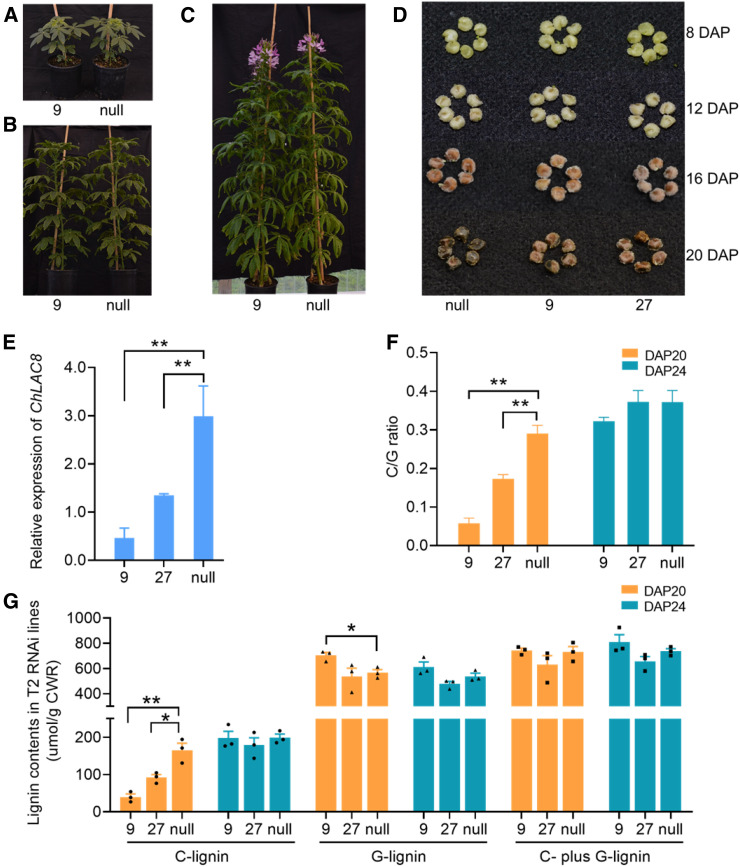
We selected two independent T2 transgenic lines (RNAi-9 and RNAi-27) with substantial downregulation of ChLAC8 for further analysis. Knock-down of ChLAC8 expression had no effects on plant growth rate, leaf size, or flowering time (Figures 4A to 4C), and the seed coats were morphologically similar, although the color of the coats was slightly lighter in the two independent RNAi lines (Figure 4D). At 20 DAP, the C-units levels and C/G ratios, as determined by thioacidolysis, were significantly reduced in the seed coats of the two RNAi lines compared with null segregant plants, and appeared to be proportional to ChLAC8 transcript levels among these plants (Figures 4E to 4G). By contrast, neither the G-unit nor total lignin (C- plus G-units) contents were consistently altered in the ChLAC8 downregulated plants, although the G-unit contents did appear to increase slightly in line RNAi-9 at 20 DAP, which might have compensated for the reduced levels of C-units at this time point (Figure 4G). When the seed coats were examined at 24 DAP, there was no significant reduction in C-unit levels or C/G ratios (Figures 4F and 4G). These data are consistent with the role of ChLAC8 in the initiation, or at least the early stages, of C-lignin polymerization.
Figure 4.
Downregulation of ChLAC8 by RNA Interference in Transgenic Cleome Plants.
(A) to (C) Visible phenotypes of ChLAC8 downregulated and null-segregant plants. Plants grown in the greenhouse for 2 months (A), 3 months (B), and 4 months (mature flowering plants (C).
(D) Seed coat phenotypes at 8, 12, 16, and 20 DAP.
(E) Transcript levels of ChLAC8 in the seed coats at 16 DAP.
(F) The C/G ratio in the seed coat at 20 and 24 DAP as determined by thioacidolysis.
(G) Lignin content in the seed coat at 20 and 24 DAP as determined by thioacidolysis.
Two independent T2 transgenic lines (9 and 27) were analyzed and compared with null segregant plants. Approximately 2 mg of the dry weight of seed coats were used for thioacidolysis analysis per replicate. Data are means ± se derived from three biological replicates. Solid circles, triangles, and squares represent individual data points. *P ≤ 0.05 or **P ≤ 0.01 level indicates significant differences by unpaired two-tailed Student’s t test.
We examined the levels of monolignol pathway intermediates in the seed coats of the RNAi-9 and null lines at 20 DAP by LC-MS/MS (Supplemental Table 3). The levels of ferulic acid and coniferaldehyde were significantly increased in the ChLAC8-RNAi line. Coniferaldehyde is a direct precursor of both ferulic acid via aldehyde dehydrogenase (Nair et al., 2004) and coniferyl alcohol via CAD. Although there appeared to be slightly more coniferyl alcohol and slightly less caffeyl alcohol in the RNAi line, none of the differences was significant, and it is not clear why precursors of coniferyl alcohol should accumulate in this line. The reduction of C-units in the lignin in the knockdown lines was therefore likely caused by reduced initiation or polymerization rather than by reduced substrate supply.
Synthesis of Labeled Monolignols and Determination of Their Incorporation into Lignin
We next addressed the in vivo activity of ChLAC8 through gain-of-function approaches. To better follow changes in lignin composition in response to altered expression of ChLAC8, we decided to perform labeling experiments with 13C-caffeyl alcohol and 13C-coniferyl alcohol. After testing several approaches to the synthesis of these labeled compounds, we finally used the schemes outlined in Figure 5. It was not possible to use the same synthetic approach for both compounds because of the additional reactivity of the ortho-dihydroxy substitution on the aromatic ring of caffeyl alcohol and its synthetic precursors. Full details of the syntheses are given in the “Methods”; the compounds were confirmed by LC-MS, and purities of the intermediates and final products are shown in Figures 5B to 5D, 5F, and 5G.
Figure 5.
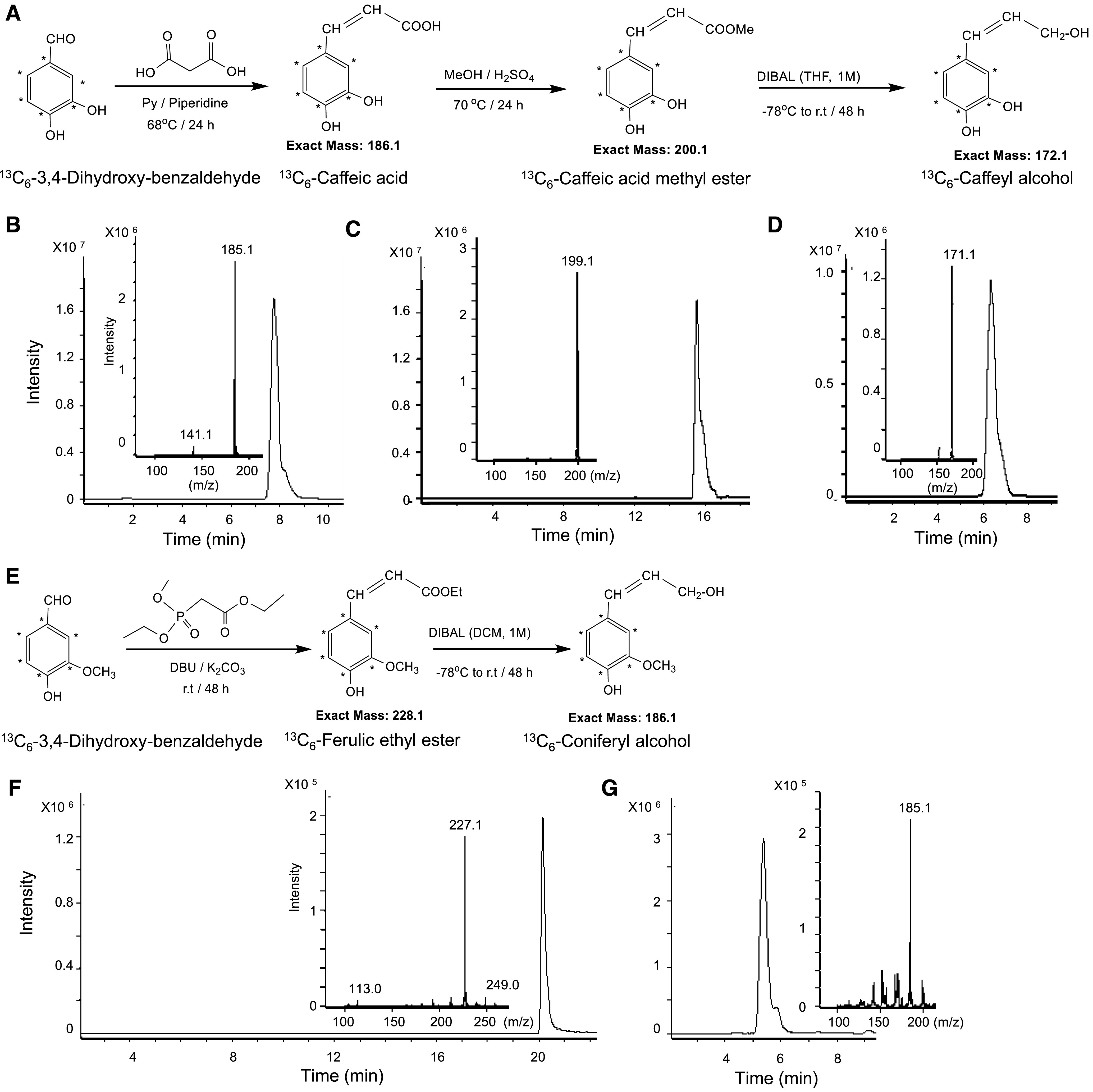
Scheme for the Synthesis of 13C6-Caffeyl and 13C6-Coniferyl Alcohols.
(A) Scheme for the synthesis of 13C6-caffeyl alcohol.
(B) to (D) LC-MS/MS analysis of 13C6-caffeic acid (B), 13C6-caffeic acid methyl ester (C) and 13C6-caffeyl alcohol (D).
(E) Scheme for the synthesis of 13C6-coniferyl alcohol.
(F) and (G) LC-MS/MS analysis of 13C6-ferulic acid ethyl ester (F) and 13C6-coniferyl alcohol (G).
Thioacidolysis products derived from incorporation of 13C-labeled monolignols into lignin have m/z values with six extra mass units (Figure 6A), and these can be readily distinguished from the unlabeled monolignol thioethethyl derivatives at the same retention time during gas chromatography-mass spectrometry analysis (Figures 6B to 6I).
Figure 6.
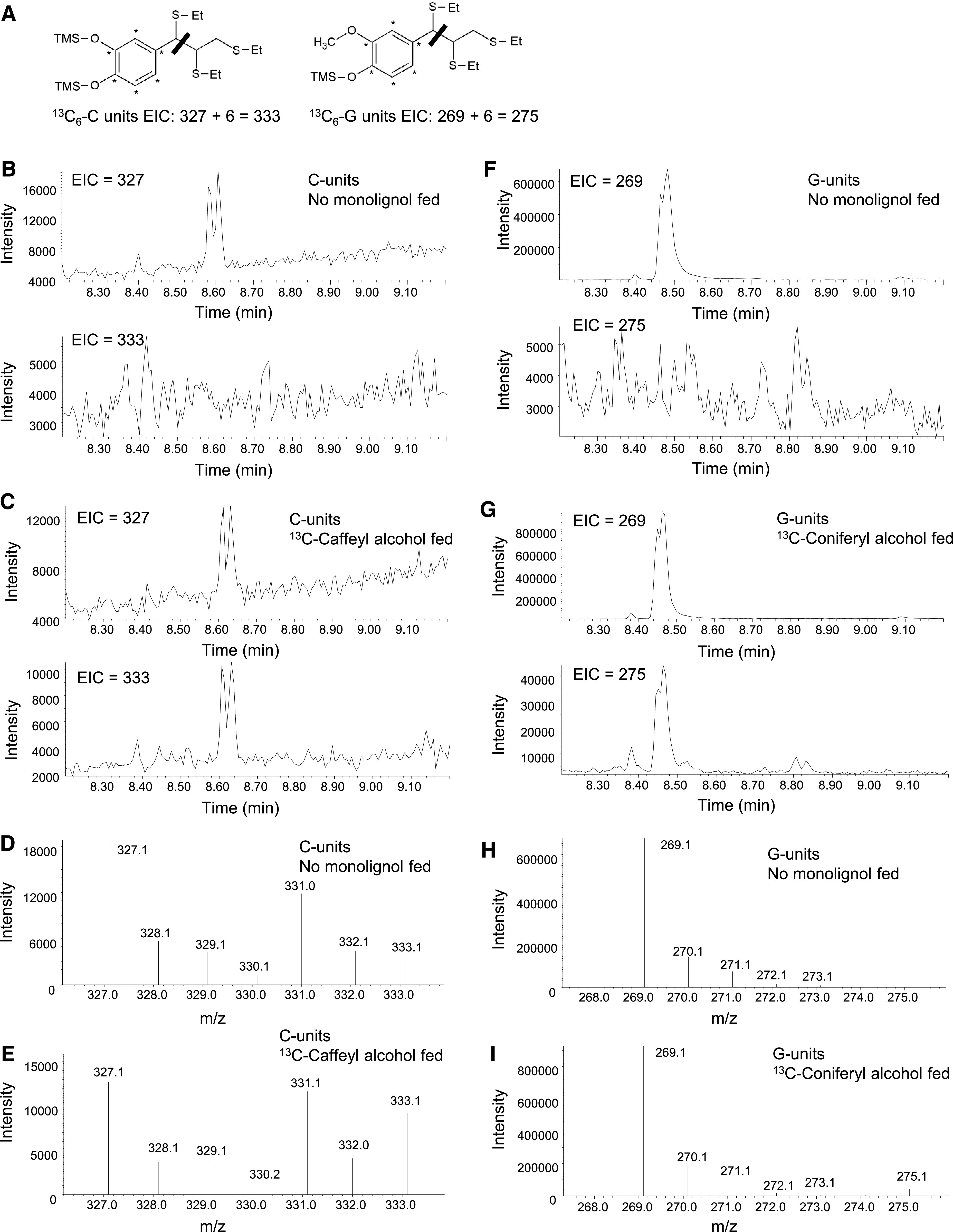
Use of Thioacidolysis to Reveal Lignin Labeling in the 13C Monolignol Feeding Experiments.
(A) Labeling pattern, structures, and masses of monolignol fragments obtained after thioacidolysis from lignin extracted from tissues fed with labeled 13C6-caffeyl alcohol or 13C6-coniferyl alcohol. The thiol group (−SEt) displaces the α-hydroxyl, α-ether, and β-aryl groups during thioacidolysis. The bar indicates the cleavage at the C7-C8 bond during the MS analysis, resulting in the primary fragments at m/z 327 and 269 from the benzylic cation of the trimethylsilylated (TMS) derivatives of unlabeled monolignols and at m/z 333 and 275 of the TMS derivatives of 13C6-labeled monolignols.
(B), (C), (F), and (G) EICs showing 13C-labeled and unlabeled monolignol thioacidolysis products after separation during GC. Double peaks in the EICs represent the presence of threo- and erythro-isomers of the tri-thioetherates shown in (A). EICs of transgenic Medicago hairy root line L6 are deployed here as an example. The no-monolignol–fed sample ([B] and [F]) showed a clear doublet corresponding to the m/z of the unlabeled thioacidolysis products, with no doublet at the m/z of the labeled products. Lignin from samples that were fed with 13C6-caffeyl (C) or 13C6-coniferyl (G) alcohol and could incorporate labeled precursors exhibited both M+6 and unlabeled monolignol-derived products.
(D), (E), (H), and (I) Traces showing the relative abundance of 13C-labeled and unlabeled monolignol thioacidolysis products after fragmentation during MS. Traces correspond to the monolignol thioacidolysis product peaks shown in (B), (C), (F), and (G). Samples that were fed with 13C6-caffeyl (E) or 13C6-coniferyl (I) alcohols showed an increased ratio of M+6 monolignol-derived fragmentation products to the unlabeled ions.
Expression of ChLAC8 Increases S- and C-Lignin Content in Medicago and Arabidopsis
To examine ChLAC8 by a gain-of-function approach, we first expressed the ChLAC8 open reading frame (ORF) in hairy roots of the M. truncatula comt mutant (Ha et al., 2019). In the Cleome seed coat, both COMT and CCoAOMT genes are downregulated at the time of C-lignin biosynthesis, but Arabidopsis and Medicago comt ccoaomt double mutant plants are severely compromised in their growth (Do et al., 2007; Zhou et al., 2010; Ha et al., 2019). If caffeyl alcohol can be produced in Medicago hairy roots, loss of function of COMT might prevent its conversion to coniferyl alcohol, as caffeyl alcohol is one of the best substrates for Medicago COMT (Parvathi et al., 2001).
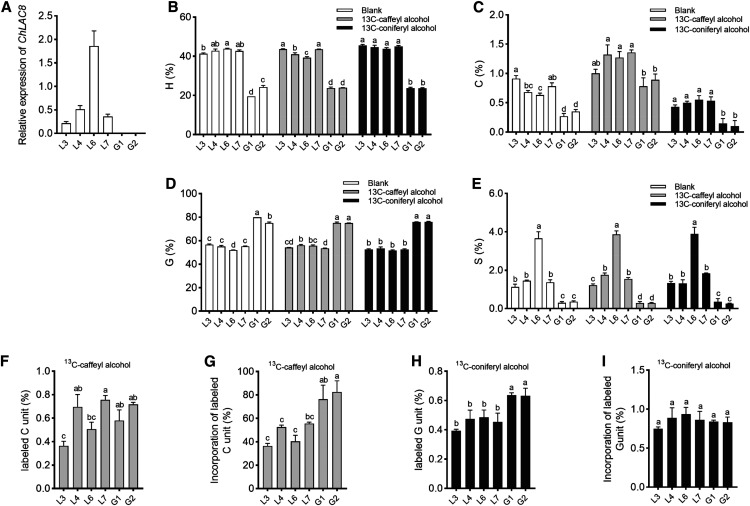
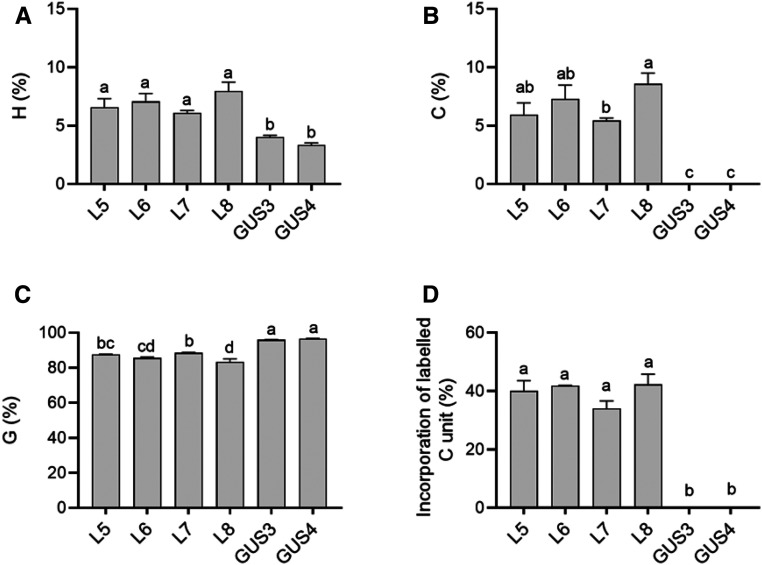
We selected four independent Medicago hairy root lines with differing ChLAC8 transcript levels (Figure 7A) and analyzed two controls expressing GUS in place of ChLAC8. In the GUS controls, the relative levels of lignin monomers were ∼80% G-units and 20% H-units, with ∼0.3% C-units and very low levels of S-units (due to the loss of function of COMT; Figures 7B to 7E). In all four lines overexpressing ChLAC8, there was a striking increase in the percentage of H-units and a corresponding decrease in the percentage of G-units. Furthermore, lignin from all lines expressing ChLAC8 now possessed significant levels of S-units as high as over 4% in line L6, with the highest ChLAC8 transcript levels (Figure 7E). The increase in S-units and decrease in G-units are consistent with the substrate preference of ChLAC8 for sinapyl rather than coniferyl alcohol, although an increase in S-units in the comt mutant background was surprising. C-unit levels were also significantly increased in all four lines expressing ChLAC8 (Figure 7C).
Figure 7.
Incorporation of Exogenous Monolignols into Lignin of M. truncatula comt Mutant Hairy Root Cultures Expressing ChLAC8.
(A) ChLAC8 transcript levels as determined by qRT-PCR, expressed relative to Tubulin as a housekeeping gene.
(B) to (E) Lignin monomer thioacidolysis yields for hydroxyphenyl (A), caffeyl (B), guaiacyl (C),and syringyl (D) lignin units in the hairy roots. The yields for 5HG were under the limit of quantification. Values shown are percentages of total lignin units in hairy root tissues.
(F) and (H) Lignin monomer thioacidolysis yields for labeled caffeyl (F) and guaiacyl (H) units in the hairy roots when 13C6-caffeyl (F) or 13C6-coniferyl (G) alcohols were applied, respectively. No labeled guaiacyl (F) or syringyl (H) units were detected. Values shown are percentages of total lignin units in hairy root tissues.
(G) and (I) Percentage incorporation of 13C6-caffeyl (G) or 13C6-coniferyl (I) alcohols into caffeyl (G) and guaiacyl (I) units of lignin.
Hairy root sections were incubated with 100 μM of 13C6-caffeyl alcohol or 13C6-coniferyl alcohol for 2 d before extraction of lignin and analysis by thioacidolysis. Four independent lines expressing ChLAC8 (L) were analyzed and compared with two lines expressing GUS (G), all in the comt mutant background. For each biological replicate, ∼100 mg of hairy root cultures harvested from one tissue culture dish was put into one well. Data are means of three biological replicates (after averaging two analytical replicates). Error bars indicate ses. The different letters above the bars represent statistically significant differences determined by one-way ANOVA (Duncan, P ≤ 0.05) with the software SPSS Statistics (v.22; IBM).
We labeled hairy root cultures with 100 μM of 13C-monolignols for 2 d, harvested and processed the cultures to give alcohol insoluble cell wall residues, and analyzed the alcohol insoluble cell wall residues for lignin composition and label incorporation by thioacidolysis (Lapierre et al., 1985; Chen et al., 2006). Percentage incorporation of a 13C-labeled monolignol into lignin was calculated based on the relative ratios of the M and M+6 ions at the retention time of the corresponding thioacidolysis product (Figure 6).
Feeding of 13C-caffeyl or coniferyl alcohols to Medicago hairy roots had virtually no effect on the overall % composition of H-, G-, or S-units (Figures 7B, 7D, and 7E). However, although the total incorporation of labeled monolignols was low as a percentage of total thioacidolysis yield (Figures 7F and 7H), feeding of 13C-caffeyl alcohol increased the % of C-units in both the ChLAC8-expressing and GUS control lines (Figure 7C). This suggests that M. truncatula possesses an endogenous enzyme that can facilitate incorporation of caffeyl alcohol into C-lignin. The percentage of C-units that were labeled was lower in the ChLAC8-expressing lines than in the GUS controls, presumably because these lines contained nearly twice as much C-lignin before application of the label (Figures 7C and 7G). Consistent with the in vitro substrate preference of ChLAC8, feeding 13C-coniferyl alcohol did not increase in the proportion of G-units or the percentage of labeled G-units in ChLAC8-expressing roots (Figures 7D and 7H).
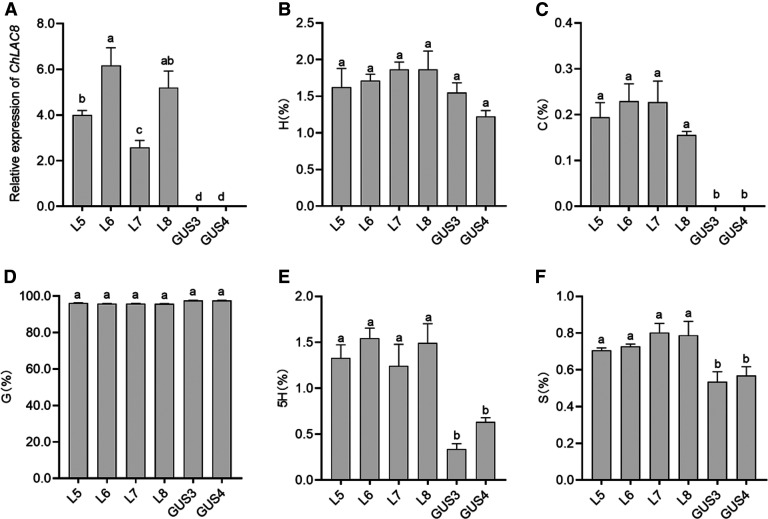
We then expressed ChLAC8 in the Arabidopsis comt mutant (Nakatsubo et al., 2008), with corresponding GUS-expressing plants used as controls (Figure 8A). We analyzed lignin composition in inflorescence stems of homozygous T3 transgenic lines by thioacidolysis. In contrast to the Medicago hairy roots, the lignin of the control lines contained >95% G-units, with <2% H-units and smaller amounts of S-units, but no C-units were present (Figures 8B to 8D and 8F). All lines expressing ChLAC8 now possessed detectable but low levels of C-units (∼0.2%), with a small but significant increase in S-units (Figures 8C and 8F). 5-Hydroxy-G-units, characteristic of the comt mutant (Ralph et al., 2001; Goujon et al., 2003), were also present at low levels and increased in these lines (Figure 8E). These results suggest that ChLAC8 can also initiate polymerization of 5-hydroxyconiferyl alcohol, which will accumulate in the comt mutant background.
Figure 8.
Lignin Composition in Stems of Arabidopsis comt Mutant Expressing ChLAC8.
(A) ChLAC8 transcript levels in inflorescence stems as determined by qRT-PCR, expressed relative to Tubulin as a housekeeping gene.
(B) to (F) Lignin monomer thioacidolysis yields for hydroxyphenyl (B), caffeyl (C), guaiacyl (D), 5-hydroxyguaiacyl (E), and syringyl (F) units in inflorescence stems.
Values shown are percentages of total lignin units in stem tissues. Four independent homozygous T3 transgenic lines expressing ChLAC8 (L) were analyzed and compared with two lines expressing GUS (G), all in the comt mutant background. For each biological replicate, the inflorescence stems were harvested from three plants. Data are means of three biological replicates (after averaging two analytical replicates). Error bars indicate ses. The different letters above the bars represent statistically significant differences determined by one-way ANOVA (Duncan, P ≤ 0.05) with the software SPSS Statistics (v22; IBM).
Finally, we fed 13C6-caffeyl alcohol to the top portions of cut inflorescence stems of the Arabidopsis comt mutants expressing ChLAC8 and the corresponding GUS-expressing controls (Figure 9). After just 2 d of feeding with 100 µM of 13C6-caffeyl alcohol, the incorporation of labeled C-units reached 34% to 42% in the ChLAC8-expressing lines, and C-units now accounted for ∼5% to 9% of the total lignin thioacidolysis yield (Figures 9B and 9D). No labeled G-units were detected after feeding, and the levels of 5H- and S-units were under the limit of quantification in the top portion of the inflorescence stem. In contrast to the whole inflorescence stem, the top portions of the stems of all lines expressing ChLAC8 showed a significant decrease in G-units and a slight increase in H-units (Figure 9A and 9C). Importantly, no C-units were detected in the GUS control lines after feeding with caffeyl alcohol (Figure 9B), indicating that, in the presence of an exogenous supply of caffeyl alcohol precursor, the expression of ChLAC8 was essential for C-lignin biosynthesis in the Arabidopsis inflorescence stem.
Figure 9.
Incorporation of Exogenous Caffeyl Alcohol into Lignin in Arabidopsis-Expressing ChLAC8.
(A) to (C) Lignin monomer thioacidolysis yields for hydroxyphenyl (A), caffeyl (B), and guaiacyl (C) units. The yields for 5-hydroxyguaiacyl and syringyl units were under the limit of quantification. Values shown are percentages of total lignin units in stem tissues.
(D) Percentage incorporation of 13C6-caffeyl alcohol into caffeyl units. No labeled guaiacyl, 5-hydroxyguaiacyl, or syringyl units were detected.
Cut stems were incubated with 100 μM of 13C6-caffeyl alcohol for 2 d before extraction of lignin and analysis by thioacidolysis. Four independent lines expressing ChLAC8 (L) were analyzed and compared with two lines expressing GUS (G), all in the comt mutant background. For each biological replicate, five stem fragments were harvested. Data for lignin composition are means of three biological replicates (after averaging two analytical replicates). Error bars indicate ses. The different letters above the bars represent statistically significant differences determined by one-way ANOVA (Duncan, P ≤ 0.05) with the software SPSS Statistics (v22; IBM).
DISCUSSION
ChLAC8 Expression Parallels C-Lignin Accumulation in the Cleome Seed Coat
Laccases are a large group of multicopper oxidases that are widely distributed in bacteria, fungi, animals, and plants. Recent advances in high-throughput sequencing technology and molecular biology have allowed several laccases that participate in lignin polymerization to be characterized in multiple plant species (Cheng et al., 2019; He et al., 2019; Le Bris et al., 2019; Wang et al., 2019; Simões et al., 2020). Nevertheless, little is known about whether laccases contribute in any way to lignin monomer composition. In this study, 24 putative LACCASE genes were identified in Cleome by analysis of our previous transcriptome database and available genome information (Cheng et al., 2013; Zhuo et al., 2019).
Based on phylogenetic analysis, ChLAC members are divided into six subgroups (group I to VI), as are Arabidopsis laccases. Three Arabidopsis laccases (AtLAC4, AtLAC11, and AtLAC17) within group I and II are necessary for monolignol (H, G, and S) polymerization (Berthet et al., 2011; Zhao et al., 2013), implying similar roles for Cleome ChLAC4.1 (most closely related to AtLAC4), ChLAC11.1 (most closely related to AtLAC11), and ChLAC17.1 (most closely related to ChLAC17). ChLAC11.1 is expressed mainly in the stem (mostly in the fiber), whereas ChLAC4.1 and ChLAC17.1 are expressed in both the stem and seed coat during the first 8 to 10 DAP. Whereas G-lignin is deposited in both the stem and seed coat in Cleome, S-lignin accumulates in the stem but is absent in the seed coat (Tobimatsu et al., 2013) due to a lack of expression of ferulate/coniferaldehyde 5-hydroxylase (Zhuo et al., 2019). Based on the correlation between the transcript profiles of these three ChLACs and the G-/S-lignin deposition patterns in developing Cleome seeds and stem tissues, it is likely that ChLAC11.1 is related to G- or S-lignin biosynthesis in the stem, whereas ChLAC4.1 and ChLAC17.1 might be associated with G-lignin polymerization in the stem and the early stages of seed development (before 12 DAP).
ChLAC15 is a homolog of Arabidopsis TT10, a laccase previously implicated in the oxidation of CTs in the seed coat (Pourcel et al., 2005). Its expression early in seed development is consistent with the early appearance of CTs in the seed coat of Cleome. However, the tt10 mutant of Arabidopsis has reduced lignin levels in the seed coat (Liang et al., 2006), so it is possible that ChLAC15 could also contribute to G-lignin biosynthesis in the Cleome seed coat. We also cannot rule out the involvement of ChLAC15, and the other ChLACs that are expressed in the seed coat but also elsewhere, in C-lignin biosynthesis during seed coat development.
Among all ChLAC members, ChLAC8X1, ChLAC8X2, and ChLAC8X3 (three ChLAC8 variants) are expressed in the seed coat after 12 DAP but not in the stem, exhibiting good correlations with C-lignin accumulation during development. ChLAC8X1, the full-length transcript with the highest expression, is the most likely candidate for a specific role in C-lignin biosynthesis. In the phylogenetic tree, ChLAC8 exhibits a close relationship with Arabidopsis AtLAC8. AtLAC8 is uniquely expressed in pollen grains as well as phloem, and knock-out of AtLAC8 resulted in early flowering and a reduced leaf number (Cai et al., 2006; Turlapati et al., 2011). However, in this study, ChLAC8 knockdown lines showed no growth phenotype when plants were grown under greenhouse conditions. These results suggest that AtLAC8 and ChLAC8 might have divergent functions in planta, despite their 77% protein sequence identity.
ChLAC8 Shows a Strong Preference for Caffeyl and Sinapyl Alcohols
ChLAC8 was readily expressed in E. coli. Because laccases are glycoproteins, they are usually expressed in organisms that can catalyze n-glycosylation. However, there are several reports of the successful expression of laccases (mainly fungal and bacterial) in E. coli (e.g., Salony et al., 2008; Ihssen et al., 2015). The glycosyl portions appear to be associated with the stability rather than catalytic efficiency of the proteins (Maestre-Reyna et al., 2015). In the crystal structure of maize (Zea mays) ZmLAC expressed in the yeast Pichia pastoris, most of the n-glycosylation sites are substituted with single n-acetyl-d-glucosamine units after de-glycosylation (Xie et al., 2020). None of these sites in ZnLAC3 or ChLAC8 are in the key catalytic region of the protein.
To the best of our knowledge, whether caffeyl alcohol could function as a substrate for laccases from plants or fungi has not previously been tested. However, the substrate preference of ChLAC8, particularly the apparent lack of activity with coniferyl alcohol, is indeed unusual. Laccases from sycamore maple (Acer pseudoplatanus), Miscanthus, and maize (ZmLAC3) exhibit a preference, but not an absolute selectivity, for sinapyl alcohol over coniferyl alcohol (Sterjiades et al., 1992; He et al., 2019; Xie et al., 2020). This is consistent with a proposed mechanism that favors the presence of methoxyl groups in the substrate to be oxidized (Ramalingam et al., 2017) and the importance of interactions between the 5-methoxyl group of sinapyl alcohol and ZmLAC3 (Xie et al., 2020). The single methoxyl group of coniferyl alcohol may not allow for stable substrate binding. Our molecular modeling studies showed that, in addition to these interactions, Gln289 in ChLAC8 may help to stabilize caffeyl alcohol in the active site through strong hydrogen bonding; this residue is lacking from AtLAC8 and from AtLAC4 and AtLAC17, the two Arabidopsis laccases with confirmed roles in lignification (Berthet et al., 2011), and their Cleome homologs. The in vitro substrate preference of ChLAC8 was reflected in the results of expression of ChLAC8 in Medicago and Arabidopsis comt mutants, where increases in the levels of C- and S-units were observed without any increase in the levels of G-units. However, the activity with sinapyl alcohol is of no biological significance in the Cleome seed coat, which does not accumulate S-units (Zhuo et al., 2019). Nonetheless, the lack of activity of ChLAC8 with coniferyl alcohol could play a role in preventing polymerization of residual G monomer during the period of C-lignin biosynthesis.
Laccase Specificity as a Determinant of Lignin Composition
Both laccases and peroxidases have been ascribed roles in the polymerization of monolignols into lignin polymers. Their relative importance, as suggested by genetic loss-of-function experiments, appears to be largely dependent on tissue type; laccases are thought to play no role in lignification in the Casparian strip, a barrier to solutes but not a tissue responsible for providing mechanical strength (Lee et al., 2013). It has, however, recently been suggested that LACCASE3 provides positional information for Casparian strip formation in Arabidopsis (Zhuang et al., 2020). The loss of function of three laccases in Arabidopsis resulted in the loss of lignin from vascular and supporting tissues, but not the Casparian strip (Zhao et al., 2013). As compelling genetic evidence now places at least a subset of peroxidases as functional in monolignol polymerization in planta, and the Arabidopsis lac4 lac11 lac17 triple maintains a full complement of expressed peroxidase genes, it is clear that laccases are essential for lignification in tissues other than the Casparian strip and may be particularly important during the initiation stages (Zhao et al., 2013).
It has generally been assumed that lignin monomer composition is determined by the synthesis of monolignols in the cytosol and/or transport of monolignols from the cytosol to apoplast. However, in view of the few reports of monolignol transporters (Miao and Liu, 2010; Alejandro et al., 2012) coupled with computational evidence that monolignol transport can occur via passive diffusion (Vermaas et al., 2019), lignin composition could theoretically be determined at the level of monolignol polymerization. These results suggest that, indeed, ChLAC8 can determine the composition of lignin, not only in the plant system of origin, but also when introduced into a heterologous host plant. It has recently been shown that heterologous expression of a laccase from Miscanthus can alter lignin composition in transgenic Arabidopsis (He et al., 2019), although in this case, the alteration did not reflect the monolignol preference of the laccase in vitro.
Although C-lignin is believed to be primarily a component of seed coats, the presence of low levels of C-lignin in Medicago hairy roots, which can be increased on feeding caffeyl alcohol, suggests that Medicago may possess a laccase with similar activity to ChLAC8. Such an enzyme appears to be absent from Arabidopsis inflorescence stems. MtLAC7 is the most closely related Medicago laccase to ChLAC8 but lacks the Gln residue equivalent to Gln289 in ChLAC8.
ChLAC8 as a Component of a Toolkit for Engineering C-Lignin
The physical and chemical properties of C-lignin make it an excellent material source for carbon fibers and high-value chemicals (Nar et al., 2016; Li et al., 2018; Stone et al., 2018; Wang et al., 2020). Despite its favorable properties, C-lignin has, to date, only been found in the seed coats of a limited number of non-crop plants, but has not yet been observed in vegetative tissues of any plant so far, which is a major hurdle for large-scale exploitation. Thus, genetic modification of suitable biomass crops to produce high amounts of C-lignin in tissues such as vessels and fibers is a yet-to-be achieved aspiration (Ralph et al., 2019). Engineering C-lignin will require systems for both the production and polymerization of caffeyl alcohol.
Suppression of CCoAOMT, a key enzyme required for G-lignin biosynthesis, led to the incorporation of low levels (<10%) of caffeyl alcohol into the G-lignin polymer of the gymnosperm Pinus radiata (Wagner et al., 2011). However, downregulation of CCoAOMT and/or COMT failed to generate C-lignin in vascular tissues of the angiosperm species Arabidopsis, alfalfa (Medicago sativa), and poplar (Meyermans et al., 2000; Marita et al., 2003; Do et al., 2007). It is possible that the accumulation of caffeyl alcohol in vascular tissues has detrimental effects on the growth of angiosperms due to its high reactivity, but is tolerated better in the seed coat and/or the vascular tissues of gymnosperms. It is also possible that the diversion of flux in the phenylpropanoid pathway away from coniferyl alcohol toward caffeyl alcohol is problematic because of additional functions for the former monolignol (Do et al. 2007; Zhou et al. 2010, Zhuo et al. 2019). In this study, introducing ChLAC8 into the comt mutants of Medicago or Arabidopsis affected the lignin composition and led to significantly enhanced, although still low, levels of C-lignin. However, when caffeyl alcohol precursor was fed to Arabidopsis inflorescence stems, the levels of C-lignin dramatically increased, but only when ChLAC8 was expressed. These results suggest that, given a successful strategy for engineering sufficient levels of caffeyl alcohol, ChLAC8 can be an important component of a gene toolkit for engineering of C-lignin into vegetative tissues of commercial biomass crops such as switchgrass (Panicum virgatum) and poplar.
The Cleome seed coat also expresses over 20 peroxidase genes based on our transcriptomic analyses. The finding that downregulating ChLAC8 in the Cleome seed coat resulted in a significant reduction in C-lignin levels at 20 DAP but not at 24 DAP is consistent with a model in which the relative involvement of ChLAC8 compared with other seed-coat–expressed peroxidases and laccases decreases during seed coat development. Correctly linked C-lignin is formed in vitro as a dehydrogenation polymer from caffeyl alcohol using horseradish peroxidase (Tobimatsu et al., 2013). Based on the results of these feeding studies with 13C6-caffeyl alcohol in Arabidopsis, it appears, perhaps paradoxically, that endogenous peroxidases are not sufficient for the polymerization of caffeyl alcohol in planta in the absence of a specific laccase.
METHODS
Plant Materials and Chemicals
Cleome (Cleome hassleriana) plants were grown in a greenhouse in Metro-Mix 830/Fafard 3B soil (Sun Gro Horticulture) at 25°C to 28°C with a 16-h/8-h day/night cycle of 150 μmol m−2 s−1 light intensity using high pressure sodium (red spectrum) and metal halide (blue spectrum) lamps as supplemental lighting if needed. Flowers were hand-pollinated, and seeds harvested periodically at 8 to 20 DAP at 2-d intervals. To prepare seed coats, fresh seeds were cut into two pieces with a surgical knife to remove the embryo. Seed coats and stem samples (bark, fiber, pith) were frozen immediately in liquid nitrogen and stored at −80°C. Each experiment was performed with three biological replicates (separate experiments), and samples from at least five plants were pooled for each replicate.
Seeds of the Arabidopsis (Arabidopsis thaliana) comt mutant (Salk_135290) were obtained from the Arabidopsis Biological Resource Center at Ohio State University. T-DNA was inserted in the third exon of the COMT gene At5g54160. The phenotype of this comt mutant line was described by Nakatsubo et al. (2008). Seeds were sown in Sunshine no. 1/Fafard-1P soil (Sun Gro Horticulture) and vernalized at 4°C for 3 d in the dark before moving to a growth chamber set at 22°C with a 16-h/8-h day/night cycle of 150 μmol m−2 s−1 light intensity using both T8 fluorescent and halogen incandescent full-spectrum lamps. Primers used for genotyping are listed in Supplemental Table 4. After selecting homozygous T-DNA insertion mutants, plants were used for transformation.
Coniferyl alcohol and sinapyl alcohol were purchased from Sigma-Aldrich. Unlabeled caffeyl alcohol was a gift from Rui Katahira and Gregg Beckham (National Renewable Energy Laboratory, Golden, Colorado).
Synthesis of 13C-Labeled Caffeyl Alcohol
13C-caffeyl alcohol was synthesized in three steps with 66% overall yield (Figures 6A to 6D). First, 13C6-Caffeic acid was synthesized following a modification of the procedure described by Teixeira et al. (2013; Figure 6A). 13C6-3,4-Dihydroxy-benzaldehyde (75.0 mg, 0.52 mmol) and malonic acid (119.2 mg, 1.15 mmol) were dissolved in pyridine (3 mL) and piperidine (30 μL) added as a catalyst. The reaction took place at 68°C for 24 h. The mixtures were diluted with ethyl acetate (5 mL) and washed with 1 M HCl (3 mL) and water (3 × 5 mL). The organic layers were then dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica gel; n-hexane:ethyl acetate:methanol [2:1:0.1]) to obtain 62.8 mg (65% yield) of 13C6-caffeic acid.
13C6-Caffeic acid methyl ester was then synthesized by Fisher esterification according to Teixeira et al. (2013; Figure 6A). 13C6-Caffeic acid (60 mg, 0.32 mmol) was dissolved in methanol (4 mL), and 74 μL of H2SO4 was added. The reaction mixture was stirred at 70°C for 24 h. After cooling to room temperature, the solvent was removed in vacuo, and the residue dissolved in ethyl acetate (10 mL) and washed with NaHCO3 and water (3 × 5 mL). The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica gel; n-hexane/ethyl acetate/methanol [3:1:0.01]) to obtain 48.2 mg (75% yield) of 13C6-caffeic acid methyl ester.
13C6-Caffeyl alcohol was synthesized following a variation of a process described by Min-Kim et al. (2012; Figure 6A). 13C6-caffeic acid methyl ester (48.0 mg, 0.24 mmol) was dissolved in 6 mL of tetrahydrofuran under argon, cooled in a dry-ice bath to −78°C, and diisobutylaluminium hydride (2.5 mL, 1.0 M in CH2Cl2, 2.5 mmol) was slowly added via syringe over 5 min. After the addition was complete, stirring was continued for 48 h at room temperature. The reaction mixture was cooled to −78°C, carefully quenched with Rochelle salt (6 mL), and the reaction mixture stirred for 8 h and extracted with ethyl acetate (20 mL × 3). The combined organic layers were dried over Na2SO4, filtered, and concentrated under a vacuum. The residue was purified by flash chromatography (silica gel; n-hexane/ethyl acetate/methanol [1:1:0.01]) to obtain 24.5 mg (60% yield) of 13C6-caffeyl alcohol.
Synthesis of 13C6-Labeled Coniferyl Alcohol
13C6-4-hydroxy-3-methoxybenzaldehyde (200 mg, 1.27 mmol) was added to a mixture of triethyl phosphonoacetate (0.75 mL, 3.78 mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (0.4 mL, 2.53 mmol), and finely ground K2CO3 (522.4 mg, 3.78 mmol) and the resulting mixture stirred for 48 h at room temperature under argon. Ethyl acetate (10 mL) was added to the crude mixture and the solid was filtered off. The solid was rinsed with ethyl acetate (10 mL) and the combined filtrate was concentrated. The product, 13C6-ferulic acid ethyl ester, was isolated by flash chromatography (silica gel; n-hexane/ethyl acetate = 2:1) as a colorless oil (260 mg, 90% yield).
13C6-Coniferyl alcohol was synthesized from 13C6-ferulic acid ethyl ester following a variation of a process described by Min-Kim et al. (2012, their Scheme 7A).13C6-ferulic acid ethyl ester (250.0 mg, 1.10 mmol) was dissolved in 12 mL of dichloromethane, under argon, cooled in a dry-ice bath to −78°C, and diisobutylaluminium hydride (11.0 mL, 1.0 M in CH2Cl2, 11.0 mmol) was slowly added via syringe over 5 min. After the addition was complete, stirring was continued for 48 h at room temperature. The reaction mixture was cooled to −78°C and carefully quenched with Rochelle salt (12 mL). The reaction mixture was stirred for 8 h and extracted with ethyl acetate (50 mL × 3). The combined organic layers were dried over Na2SO4, filtered, and concentrated under a vacuum. The residue was purified by flash chromatography (silica gel; n-hexane/ethyl acetate/methanol = 2:1:0.01) to obtain 180.5 mg (88% yield) of 13C6-coniferyl alcohol.
Bioinformatics Analysis
To identify the putative peroxidase and laccase genes in Cleome, the protein sequences of Arabidopsis peroxidases and/or laccases were searched against our previously generated Cleome RNA-seq database (Zhuo et al., 2019) using the program TBLASTN (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=tblastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) with default parameters. All obtained laccase sequences were further confirmed by searching the Pfam database (http://pfam.janelia.org) for the existence of three Cu-oxidase domains (PF00394.19, PF07731.11, and PF07732.12). The Cleome laccases were named ChLAC1-ChLAC22 according to their annotations in the C. hassleriana genome (ASM46358v1) at the National Center for Biotechnology Information (Cheng et al., 2013) and their respective homologs in Arabidopsis. The exon–intron structures of the ChLAC genes were analyzed using the Gene Structure Display Server (v2.0; http://gsds.cbi.pku.edu.cn/). The fragments per kilobase of transcript per million mapped reads (FPKM) values for peroxidase and laccase were retrieved from the transcriptome data (Supplemental Data Set 1) and used for hierarchical clustering analysis.
The presence of signal peptides and the locations of their cleavage sites in the protein sequence of ChLAC8 were predicted using the online web server SignalP5.0 (http://www.cbs.dtu.dk/services/SignalP-5.0/index.php; Almagro Armenteros et al., 2019). Eukaryotes were chosen as the organism group. The subcellular localization of ChLAC8 was predicted using the online web server MultiLoc2 by the prediction method MultiLoc2-HighRes (plant) considering 10 localizations (https://abi- services.informatik.uni-tuebingen.de/multiloc2/webloc.cgi; Blum et al., 2009). n-Glycosylation sites in the protein sequence of ChLAC8 were predicted using the online web servers NtetNGlyc 1.0 (http://www.cbs.dtu.dk/services/NetNGlyc/) and N-GlyDE (http://bioapp.iis.sinica.edu.tw/N-GlyDE/; Pitti et al., 2019).
Phylogenetic Analysis
The amino acid sequences of ChLAC proteins and characterized laccases from Arabidopsis and other plants were retrieved from the National Center for Biotechnology Information website (Supplemental Data Set 2). Multiple alignment of these laccase sequences was performed using the Clustal W algorithm (Thompson et al., 2003) and visualized using the software BoxShade 3.21 (https://embnet.vital-it.ch/software/BOX_form.html) with the default setting. A phylogenetic tree was constructed using the software MEGA7.0 by the Neighbor-joining algorithm with 1,000 bootstrap replicates (Kumar et al., 2016). The tree was visualized and annotated with the program EvolView (https://evolgenius.info/evolview-v2). The alignments and machine-readable tree file for laccase are given in Supplemental Data Sets 3 and 4
RNA Isolation and qRT-PCR
Plant materials were ground to a fine powder in liquid nitrogen with a freezer mill (SPEX SamplePrep). Total RNA was isolated from the powdered samples using an RNeasy PowerPlant Kit (Qiagen) according to the manufacturer’s protocol. The RNA quality and concentration were measured with a model no. 2100 Bioanalyzer (Agilent Technologies). Approximately 2 μg of RNA per sample was treated with DNase I (Invitrogen) to remove residual genomic DNA and reverse-transcribed to first-strand cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen). qRT-PCR analysis was performed with three biological replicates using SYBR Green Master Mix (Applied Biosystems) on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). The Cleome ubiquitin-conjugating enzyme E2 11-like gene (XM_010555091.2) was used as an internal standard to normalize the amount of cDNA template (Zhuo et al., 2019). All primers used are listed in Supplemental Table 4. Relative transcript levels were calculated using the formula for comparative Ct value (Ranasinghe et al., 2008).
Expression and Purification of ChLAC8 from Escherichia coli
The ORF of ChLAC8 (ChLAC8X1, XM_010555066.1) was amplified with the primers listed in Supplemental Table 4, ligated into the pENTR/D-TOPO vector (Invitrogen), and subcloned into pDEST17 fused with a 6 × His tag at the N terminus via Gateway LR recombination reaction (Invitrogen). The resulting vector (pDEST17-ChLAC8) was transformed into E. coli Rosetta (DE3; Novagen) for protein expression. The transgenic E. coli strain was cultured in LB medium at 37°C until OD600 = 0.4 to 0.6 and then supplemented with 0.5 mM of isopropyl-β-d-thiogalactoside to induce the expression of ChLAC8 at 16°C for 20 h. After induction, cell cultures were harvested by centrifugation at 10,000g for 5 min at 4°C, and the resulting pellets were used for ChLAC8 protein purification via Ni-NTA resin (Thermo Fisher Scientific) according to the manufacturer’s manual. The eluted protein was further desalted into 50 mM of potassium phosphate buffer (pH 6.8) using a 30-kD cutoff Amicon Ultra centrifugal filter (Millipore). The purity of recombinant ChLAC8 was examined by SDS-PAGE and the protein concentration was quantified by Bradford assays.
Analysis of Enzyme Kinetics
Laccase activity assays were performed in a 100-μL reaction mixture consisting of 50 mM of potassium phosphate buffer (pH 6.8), 10 to 20 μg of recombinant ChLAC8, and 200 μM of monolignol substrate (caffeyl, coniferyl, or sinapyl alcohol). The reactions were incubated at 25°C for 30 min and terminated by adding 100 μL of methanol. The reaction products were injected into an HPLC or LC-MS/MS system for analysis as described below. The decrease in the level of the substrate was measured to calculate the enzyme activity. To determine kinetic parameters, ChLAC8 was incubated with different concentrations of caffeyl alcohol or sinapyl alcohol in a range from 25 to 800 μM. The enzyme assays were performed in triplicate at each substrate concentration, and the Vmax and Km values were calculated by the software GraphPad Prism 8 (GraphPad) with nonlinear regression analysis.
HPLC and LC-MS/MS Analysis of Reaction Products
The reaction products were analyzed on a model no. 1260 HPLC system (Agilent Technologies) equipped with a Luna C18(2) reverse-phase column (5 μm particle, 250 × 4.6 mm; Phenomenex) and separated in a mobile phase consisting of solvent A (1% [v/v] phosphoric acid in water) and solvent B (acetonitrile) with the following gradient: 5% B for 5 min, to 33% B in 25 min, to 45% B in 5 min, to 95% B in 5 min; keep at 95% B for 5 min, then back to 5% B in 5 min.
LC-MS/MS analysis was performed using a model no. 1290 Infinity II liquid chromatography system (Agilent Technologies) coupled to a model no. 6400 Series Triple Quadrupole System (Agilent Technologies) with an electrospray ionization source in negative ionization mode. A reverse-phase ZORBAX RR Eclipse Plus C18, 95 Å, 4.6 × 250 mm, 5 μm column (Agilent Technologies) was used for separation. The gradient for HPLC separation was 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B) with the following solvent gradient: 5% B for 5 min, to 40% B in 30 min, to 95% B in 5 min, 95% B for 5 min, to 5% B in 1 min. The total LC-MS/MS run was 46 min with a flow rate of 1.0 mL/min. Injection volume was 10 μL. MS data were recorded in the range of m/z 100 to 700 (Perna et al., 2018).
Quantification of monolignol intermediates was conducted using a model no. 1290 Infinity II liquid chromatography system (Agilent Technologies) coupled to a hybrid Triple Quadrupole 6500+ triple quadrupole (AB SCIEX). The metabolites were separated using a reverse phase C18 Symmetry column (4.6 × 75 mm; 3.5 μm) with a Symmetry C18 pre-column (3.9 × 20 mm; 5 μm; Waters). Details of the HPLC gradient and MS parameters were as described in Cocuron et al. (2019). Metabolites were simultaneously detected and quantified with multiple reaction monitoring determined using standards of each metabolite. Monolignols were identified and quantified using a mixture of known external standards run at the same time as the biological extracts.
Homology Modeling and Docking Studies of ChLAC8
Comparative modeling of ChLAC8 was performed using the SWISS-MODEL server (Schwede et al., 2003) with the structure of ZmLAC3 (PDB ID: 6KLG; Xie et al., 2020) as the template. The three-dimensional structural model of ChLAC8 was generated based on optimal sequence alignment of ChLAC8 and ZmLAC3 and the three-dimensional structure of ZmLAC3. Molecular docking studies of ChLAC8 with substrates (sinapyl, caffeyl, and coniferyl alcohols) were performed using the automated docking program AUTODOCK (Morris et al., 2009), and the sinapyl alcohol model in the ZmLAC3 structure (PDB ID: 6KLI) was used as a reference. Some minor manual adjustments of the modeling solution were made, and the structure model was analyzed using the graphics program COOT (Emsley and Cowtan, 2004). Figures were prepared with the program PyMOL (Schrödinger).
RNAi Knockdown of ChLAC8 in the Cleome Seed Coat
An RNA interference construct targeting the ChLAC8 transcript was constructed by amplifying a nucleotide fragment from developing Cleome seed coat cDNA using the primers listed in Supplemental Table 4. The 162-bp ChLAC8 fragment was cloned into pENTR/D-TOPO (Invitrogen) and transferred to Gateway destination vector pH7GWIWG2(I) via LR reaction (Invitrogen). The resulting RNAi construct, which was driven by the constitutive cauliflower mosaic virus 35S promoter, was introduced into Agrobacterium tumefaciens strain AGL1. Transgenic plants harboring the ChLAC8 RNAi construct were generated by A. tumefaciens-mediated transformation of Cleome embryonic callus tissue as previously described by Zhuo et al. (2019). The T0 transgenic plants were checked by PCR using the hygromycin B phosphotransferase (HPH) gene as a marker with primers listed in Supplemental Table 4. The expression levels of ChLAC8 in different T1 transgenic lines were verified by qRT-PCR. Two independent transgenic lines in the subsequent generation (T2) exhibiting the highest downregulation of ChLAC8 transcripts were selected for further analysis. The lignin composition of the seed coat was determined by thioacidolysis methods (Lapierre et al., 1985; Chen et al., 2006) with docosane as the internal standard. The thioacidolysis monomer yields were calculated using the same response factor of 1.5 for all released lignin monomeric units (Lapierre and Monties, 1986). Approximately 2 mg of dry weight of seed coats were used for analysis per replicate, and three biological replicates were analyzed.
Expression of ChLAC8 in Medicago truncatula Hairy Roots
The complete ORF of ChLAC8, including the N-terminal signal peptide, in the pENTR/D-TOPO vector (Invitrogen) was cloned into the pB7WG2D binary vector by LR recombination reaction (Invitrogen). Primers are listed in Supplemental Table 4. The resulting vector pB7WG2D-ChLAC8 with the ChLAC8 ORF driven by the CaMV 35S promoter was transformed into Agrobacterium rhizogenes strain ARqual. Agrobacterium-mediated M. truncatula hairy root transformation was performed as described in Liu et al. (2014). The M. truncatula comt mutant (NF17882) was as described in Ha et al. (2019). The resulting hairy roots were checked by PCR using the phosphinothricin acetyl transferase (BAR) gene as a marker. Primers are listed in Supplemental Table 4. Transcript levels of ChLAC8 and lignin composition were determined as described above. For each biological replicate, hairy root cultures were harvested from one tissue culture dish. Three biological replicates were analyzed.
Expression of ChLAC8 in Arabidopsis
The vector pB7WG2D-ChLAC8 was transformed into A. tumefaciens strain GV3101. Agrobacterium-mediated Arabidopsis transformation was performed by floral dip (Clough and Bent, 1998). T1 to T3 transgenic plants were screened by spraying with 120 mg/L of BASTA (Finale) and verified by PCR using the BAR gene as a marker. Primers are listed in Supplemental Table 4. After selecting the homozygous transgenic plants, transcript levels of ChLAC8 and lignin composition in the inflorescence stems were determined as described above. For each biological replicate, the inflorescence stems were harvested from three plants. Three biological replicates were analyzed.
Feeding Medicago Hairy Roots with 13C6-Caffeyl and Coniferyl Alcohols
The portion 3 cm down from the root tip in Medicago hairy roots was cut under water, transferred into liquid Murashige and Skoog medium supplemented with 100 μM of 13C6-caffeyl and 13C6-coniferyl alcohols in 6-well plates, and vacuum-infiltrated for 10 min. For each biological replicate, ∼100 mg of hairy root cultures harvested from one tissue culture dish was put into one well. The samples were incubated for 2 d, harvested, and washed three times with water before isolation and analysis of lignin by thioacidolysis (Lapierre et al., 1985; Chen et al., 2006). 13C-incorporation was determined by measuring the m/z +6 ion peaks from the C- and G-unit thioacidolysis products. Three biological replicates were measured.
Feeding Arabidopsis Stems with 13C6-Caffeyl Alcohol
The top portions of inflorescence stems of 4-week–old Arabidopsis plants were cut under water and transferred into liquid Murashige and Skoog medium supplemented with 100 μM of 13C6-caffeyl alcohol in 2-mL tubes. The samples were incubated for 2 d, harvested, and analyzed as described above for hairy roots. Five stem fragments were harvested as one sample, and three biological replicates were measured.
Statistical Analysis
Unpaired two-tailed Student’s t test was used to test the significance of differences in the lignin composition between Cleome RNAi and null lines, as well the kinetics of ChLAC8 toward caffeyl alcohol and sinapyl alcohol. Multiple comparisons were done by one-way ANOVA Duncan grouping at the 0.05 probability level with the tool SPSS Statistics (v22; IBM). Student’s t test and ANOVA results, along with raw data, are provided in Supplemental Datasets 5 and 6.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL libraries under the following accession numbers: ChLAC1 (LOC104813919); ChLAC10 (LOC104816217); ChLAC11.1 (LOC104826904); ChLAC11.2 (LOC104816339); ChLAC12 (LOC104816528); ChLAC13 (LOC104810694); ChLAC14X1 (LOC104819341); ChLAC14X2 (LOC104819341); ChLAC14X3 (LOC104819341); ChLAC15 (LOC104800224); ChLAC16X1 (LOC104810729); ChLAC16X2 (LOC104810729); ChLAC16X3 (LOC104810729); ChLAC16X4 (LOC104810729); ChLAC17.1 (LOC104810824); ChLAC17.2 (LOC104814854); ChLAC17.3 (LOC104814851); ChLAC2.1 (LOC104800682); ChLAC2.2 (LOC104826315); ChLAC22 (LOC104816561); ChLAC3.1 (LOC104800769); ChLAC3.2 (LOC104815814); ChLAC4.1 (LOC104823387); ChLAC4.2 (LOC104806523); ChLAC5 (LOC104810126); ChLAC6 (LOC104817377); ChLAC7.1X1 (LOC104823274); ChLAC7.1X2 (LOC104823274); ChLAC7.2 (LOC104827034); ChLAC8X1 (LOC104823484); ChLAC8X2 (LOC104823484); ChLAC8X3 (LOC104823484); ChCAD5 (LOC104804389); ChCCoAOMT1 (LOC104819570); ChCCoAOMT5 (LOC104804378); ChCOMT1 (LOC104811887); ChCOMT2 (LOC104799941); ChANR (LOC104809521); AtLAC1 (AT1G18140); AtLAC2 (AT2G29130); AtLAC3 (AT2G30210); AtLAC4 (AT2G38080); AtLAC5 (AT2G40370); AtLAC6 (AT2G46570); AtLAC7 (AT3G09220); AtLAC8 (AT5G01040); AtLAC9 (AT5G01050); AtLAC10 (AT5G01190); AtLAC11 (AT5G03260); AtLAC12 (AT5G05390); AtLAC13 (AT5G07130); AtLAC14 (AT5G09360); AtLAC15 (AT5G48100); AtLAC16 (AT5G58910); AtLAC17 (AT5G60020); MtLAC2 (Medtr4g064530); MtLAC3 (Medtr5g073210); MtLAC4.1 (Medtr3g462760); MtLAC4.2 (Medtr4g015120); MtLAC4.3 (Medtr5g069680); MtLAC4.4 (Medtr5g081810); MtLAC5 (Medtr5g083360); MtLAC6 (Medtr8g027375); MtLAC7.1 (Medtr4g019225); MtLAC7.2 (Medtr7g065970); MtLAC7.3 (Medtr7g065980); MtLAC11.1 (Medtr5g020600); MtLAC11.2 (Medtr5g020620); MtLAC12 (Medtr3g071890); MtLAC14 (Medtr2g008330); MtLAC15.1 (Medtr3g101635); MtLAC15.2 (Medtr3g101640); MtLAC17.1 (Medtr7g058690); MtLAC17.2 (Medtr7g060460); MtLAC17.3 (Medtr7g062250); MtLAC17.4 (Medtr7g062310); MtLAC17.5 (Medtr7g458880); ZmLAC1 (Y897208); ZmLAC2 (AM086214); ZmLAC3 (AM086215); ZmLAC4 (AM086216); ZmLAC5 (AM086217); ApLAC (AAB09228.1); TrLAC3 (Q9ZQW3); TrLAC90 (Q9ZP47); TrLAC110 (Q9ZQW2); BdLAC5 (Bradi1g66720); BdLAC6 (Bradi1g74320); GaLAC1 (KX822020.1); SofLAC (SCUTST3084C11); and BnTT10-1 (HM805058).
Supplemental Data
Supplemental Figure 1. Hierarchical clustering analysis of the transcript levels of peroxidase genes in different tissues and different stages of seed development in C. hassleriana.
Supplemental Figure 2. Gene structure of C. hassleriana laccases.
Supplemental Figure 3. qRT-PCR analysis of ChLAC8 transcript profiles.
Supplemental Figure 4. Expression of laccase and anthocyanidin reductase genes in the Cleome seed coat and staining of CTs.
Supplemental Figure 5. SDS-PAGE analysis of purified recombinant ChLAC8.
Supplemental Figure 6. LC-MS/MS analysis of trimers in the reaction products of ChLAC8 with caffeyl alcohol.
Supplemental Figure 7. Dissociation pathways of the benzodioxane-linked C-dimer.
Supplemental Figure 8. Multiple sequence alignment of all laccase protein sequences from Cleome, Arabidopsis, and M. truncatula.
Supplemental Figure 9. Multiple sequence alignment of Cleome and Arabidopsis laccases involved in lignin biosynthesis.
Supplemental Figure 10. Correlation of ChLAC8 expression with lignin contents in different T1 RNA interference lines of C. hassleriana.
Supplemental Table 1. Prediction of n-glycosylation sites in ChLAC8.
Supplemental Table 2. The accession numbers of the laccases and other lignin-related genes examined in this study.
Supplemental Table 3. Impact of LAC8 downregulation on the levels of monolignol pathway intermediates during seed coat development in Cleome.
Supplemental Table 4. Primers used in this study.
Supplemental Dataset 1. The transcript levels of cleome peroxidase and laccase genes derived from RNA-seq analysis.
Supplemental Data Set 2. Protein sequences of all laccases used for alignment.
Supplemental Data Set 3. Alignment file of laccases used in this study.
Supplemental Data Set 4. Machine-readable tree file for laccases.
Supplemental Data Set 5. Data for bar graphs in this study.
Supplemental Data Set 6. Student’s t test and ANOVA results.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank the Bioanalytical facility of the BioDiscovery Institute, University of North Texas, for targeted metabolite profiling, and the Texas Academy of Mathematics and Science student Siqi Zhu for assisting in lignin composition analysis of hairy roots. The M. truncatula plants utilized in this research project are jointly owned by the Centre National de la Recherche Scientifique and the Samuel Roberts Noble Foundation, Ardmore, Oklahoma. This work was supported by the National Science Foundation (grant 703285); the National Science Foundation’s Integrated Organismal Systems program (grant 1456286); the Center for Bioenergy Innovation, Oak Ridge National Laboratory, a US Department of Energy’s Bioenergy Research Center in the Office of Biological and Environmental Research at the Department of Energy Office of Science; the University of North Texas; the National Natural Science Foundation of China (grant 31770339); and the China Scholarship Council (grant no. 201704910208 to Xin Wang).
AUTHOR CONTRIBUTIONS
R.A.D., Xin Wang, and C.L.Z. conceived and designed the research; Xin Wang, C.L.Z., X.R.X., Xiaoqiang Wang, M.D.-P., and F.C. carried out the experiments and performed the data analyses; R.A.D. and Xin Wang wrote the article.
Footnotes
Articles can be viewed without a subscription.
References
- Alejandro S., Lee Y., Tohge T., Sudre D., Osorio S., Park J., Bovet L., Lee Y., Geldner N., Fernie A.R., Martinoia E.(2012). AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr. Biol. 22: 1207–1212. [DOI] [PubMed] [Google Scholar]
- Almagro Armenteros J.J., Tsirigos K.D., Sønderby C.K., Petersen T.N., Winther O., Brunak S., von Heijne G., Nielsen H.(2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 37: 420–423. [DOI] [PubMed] [Google Scholar]
- Annunziata M.G.(2019). What is lignin made of? New components discovered!. Plant Physiol. 180: 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W., O’Malley D.M., Whetten R., Sederoff R.R.(1993). A laccase associated with lignification in loblolly pine xylem. Science 260: 672–674. [DOI] [PubMed] [Google Scholar]
- Barros J., Serk H., Granlund I., Pesquet E.(2015). The cell biology of lignification in higher plants. Ann. Bot. 115: 1053–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet S., Demont-Caulet N., Pollet B., Bidzinski P., Cézard L., Le Bris P., Borrega N., Hervé J., Blondet E., Balzergue S., Lapierre C., Jouanin L.(2011). Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 23: 1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum T., Briesemeister S., Kohlbacher O.(2009). MultiLoc2: Integrating phylogeny and gene ontology terms improves subcellular protein localization prediction. BMC Bioinformatics 10: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan A.C., et al. (2016). Knockdown of a laccase in Populus deltoides confers altered cell wall chemistry and increased sugar release. Plant Biotechnol. J. 14: 2010–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Davis E.J., Ballif J., Liang M., Bushman E., Haroldsen V., Torabinejad J., Wu Y.(2006). Mutant identification and characterization of the laccase gene family in Arabidopsis. J. Exp. Bot. 57: 2563–2569. [DOI] [PubMed] [Google Scholar]
- Caparrós-Ruiz D., Fornalé S., Civardi L., Puigdomènech P., Rigau J.(2006). Isolation and characterisation of a family of laccases in maize. Plant Sci. 171: 217–225. [Google Scholar]
- Chen F., Srinivasa Reddy M.S., Temple S., Jackson L., Shadle G., Dixon R.A.(2006). Multi-site genetic modulation of monolignol biosynthesis suggests new routes for formation of syringyl lignin and wall-bound ferulic acid in alfalfa (Medicago sativa L.). Plant J. 48: 113–124. [DOI] [PubMed] [Google Scholar]
- Chen F., Tobimatsu Y., Havkin-Frenkel D., Dixon R.A., Ralph J.(2012). A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. USA 109: 1772–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Tobimatsu Y., Jackson L., Nakashima J., Ralph J., Dixon R.A.(2013). Novel seed coat lignins in the Cactaceae: Structure, distribution and implications for the evolution of lignin diversity. Plant J. 73: 201–211. [DOI] [PubMed] [Google Scholar]
- Cheng S., et al. (2013). The Tarenaya hassleriana genome provides insight into reproductive trait and genome evolution of crucifers. Plant Cell 25: 2813–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Li G., Ma C., Abdullah M., Zhang J., Zhao H., Jin Q., Cai Y., Lin Y.(2019). Comprehensive genome-wide analysis of the pear (Pyrus bretschneideri) laccase gene (PbLAC) family and functional identification of PbLAC1 involved in lignin biosynthesis. PLoS One 14: e0210892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F.(1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Cocuron J.C., Casas M.I., Yang F., Grotewold E., Alonso A.P.(2019). Beyond the wall: High-throughput quantification of plant soluble and cell-wall bound phenolics by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 1589: 93–104. [DOI] [PubMed] [Google Scholar]
- Del Río J. C., Rencoret J., Gutiérrez A., Kim H., Ralph J.(2017). Hydroxystilbenes are monomers in palm fruit endocarp lignins. Plant Physiol. 174: 2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R.A., Barros J.(2019). Lignin biosynthesis: Old roads revisited and new roads explored. Open Biol. 9: 190215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do C.-T., Pollet B., Thévenin J., Sibout R., Denoue D., Barrière Y., Lapierre C., Jouanin L.(2007). Both caffeoyl coenzyme A 3-o-methyltransferase 1 and caffeic acid o-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta 226: 1117–1129. [DOI] [PubMed] [Google Scholar]
- Duroux L., Welinder K.G.(2003). The peroxidase gene family in plants: A phylogenetic overview. J. Mol. Evol. 57: 397–407. [DOI] [PubMed] [Google Scholar]
- Emsley P., Cowtan K.(2004). Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60: 2126–2132. [DOI] [PubMed] [Google Scholar]
- Goujon T., Sibout R., Pollet B., Maba B., Nussaume L., Bechtold N., Lu F., Ralph J., Mila I., Barrière Y., Lapierre C., Jouanin L.(2003). A new Arabidopsis thaliana mutant deficient in the expression of o-methyltransferase impacts lignins and sinapoyl esters. Plant Mol. Biol. 51: 973–989. [DOI] [PubMed] [Google Scholar]
- Ha C. M., Fine D., Bhatia A., Rao X., Martin M.Z., Engle N.L., Wherritt D.J., Tschaplinski T.J., Sumner L.W., Dixon R.A.(2019). Ectopic defense gene expression is associated with growth defects in Medicago truncatula lignin pathway mutants. Plant Physiol. 181: 63–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Machemer-Noonan K., Golfier P., Unda F., Dechert J., Zhang W., Hoffmann N., Samuels L., Mansfield S.D., Rausch T., Wolf S.(2019). The in vivo impact of MsLAC1, a Miscanthus laccase isoform, on lignification and lignin composition contrasts with its in vitro substrate preference. BMC Plant Biol. 19: 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen J., Reiss R., Luchsinger R., Thöny-Meyer L., Richteret M.(2015). Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci. Rep. 5: 10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K.(2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., Lu F., Regner M., Zhu Y., Rencoret J., Ralph S.A., Zakai U.I., Morreel K., Boerjan W., Ralph J.(2015). Tricin, a flavonoid monomer in monocot lignification. Plant Physiol. 167: 1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre C., Monties B.(1986). Thioacidolysis of poplar lignins: Identification of monomeric syringyl products and characterization of guaiacyl-syringyl lignin fractions. Holzforschung 40: 113–118. [Google Scholar]
- Lapierre C., Monties B., Rolando C.(1985). Thioacidolysis of lignin: Comparison with acidolysis. J. Wood Chem. Technol. 5: 277–292. [Google Scholar]
- Le Bris P., et al. (2019). Inactivation of LACCASE8 and LACCASE5 genes in Brachypodium distachyon leads to severe decrease in lignin content and high increase in saccharification yield without impacting plant integrity. Biotechnol. Biofuels 12: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Rubio M.C., Alassimone J., Geldner N.(2013). A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412. [DOI] [PubMed] [Google Scholar]
- Li Y., et al. (2018). An “ideal lignin” facilitates full biomass utilization. Sci. Adv. 4: eaau2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Davis E., Gardner D., Cai X., Wu Y.(2006). Involvement of AtLAC15 in lignin synthesis in seeds and in root elongation of Arabidopsis. Planta 224: 1185–1196. [DOI] [PubMed] [Google Scholar]
- Liu C., Jun J.H., Dixon R.A.(2014). MYB5 and MYB14 play pivotal roles in seed coat polymer biosynthesis in Medicago truncatula. Plant Physiol. 165: 1424–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre-Reyna M., Liu W.C., Jeng W.Y., Lee C.C., Hsu C.A., Wen T.N., Wang A.H., Shyur L.F.(2015). Structural and functional roles of glycosylation in fungal laccase from Lentinus sp. PLoS One 10: e0120601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marita J.M., Ralph J., Hatfield R.D., Guo D., Chen F., Dixon R.A.(2003). Structural and compositional modifications in lignin of transgenic alfalfa down-regulated in caffeic acid 3-o-methyltransferase and caffeoyl coenzyme A 3-o-methyltransferase. Phytochemistry 62: 53–65. [DOI] [PubMed] [Google Scholar]
- Meyermans H., et al. (2000). Modifications in lignin and accumulation of phenolic glucosides in poplar xylem upon down-regulation of caffeoyl-coenzyme A o-methyltransferase, an enzyme involved in lignin biosynthesis. J. Biol. Chem. 275: 36899–36909. [DOI] [PubMed] [Google Scholar]
- Miao Y.-C., Liu C.-J.(2010). ATP-binding cassette-like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proc. Natl. Acad. Sci. USA 107: 22728–22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min-Kim S., Sug-Kim Y., Wan-Kim D., Yang J.W.(2012). Transition metal-free, NaOtBu-O2-mediated one-pot cascade oxidation of allylic alcohols to α,β-unsaturated carboxylic acids. Green Chem. 14: 2996–2998. [Google Scholar]
- Morreel K., Kim H., Lu F., Dima O., Akiyama T., Vanholme R., Niculaes C., Goeminne G., Inzé D., Messens E., Ralph J., Boerjan W.(2010). Mass spectrometry-based fragmentation as an identification tool in lignomics. Anal. Chem. 82: 8095–8105. [DOI] [PubMed] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J.(2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30: 2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsubo T., Kitamura Y., Sakakibara N., Mizutani M., Hattori T., Sakurai N., Shibata D., Suzuki S., Umezawa T.(2008). At5g54160 gene encodes Arabidopsis thaliana 5-hydroxyconiferaldehyde o-methyltransferase. J. Wood Sci. 54: 312–317. [Google Scholar]
- Nar M., Rizvi H.R., Dixon R.A., Chen F., Kovalcik A., D’Souza N.(2016). Superior plant-based carbon fibers from electrospun poly-(caffeyl alcohol) lignin. Carbon 103: 372–383. [Google Scholar]
- Nair R.B., Bastress K.L., Ruegger M.O., Denault J.W., Chapple C.(2004). The Arabidopsis thaliana REDUCED EPIDERMAL FLUORESCENCE1 gene encodes an aldehyde dehydrogenase involved in ferulic acid and sinapic acid biosynthesis. Plant Cell 16: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna V., Agger J.W., Holck J., Meyer A.S.(2018). Multiple reaction monitoring for quantitative laccase kinetics by LC-MS. Sci. Rep. 8: 8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathi K., Chen F., Guo D., Blount J.W., Dixon R.A.(2001). Substrate preferences of o-methyltransferases in alfalfa suggest new pathways for 3-o-methylation of monolignols. Plant J. 25: 193–202. [DOI] [PubMed] [Google Scholar]
- Pitti T., Chen C.-T., Lin H.-N., Choong W.-K., Hsu W.-L., Sung T.-Y.(2019). N-GlyDE: A two-stage N-linked glycosylation site prediction incorporating gapped dipeptides and pattern-based encoding. Sci. Rep. 9: 15975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel L., Routaboul J.M., Kerhoas L., Caboche M., Lepiniec L., Debeaujon I.(2005). TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 17: 2966–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragauskas A.J., et al. (2014). Lignin valorization: Improving lignin processing in the biorefinery. Science 344: 1246843. [DOI] [PubMed] [Google Scholar]
- Ralph J., et al. (2001). Elucidation of new structures in lignins of CAD- and COMT-deficient plants by NMR. Phytochemistry 57: 993–1003. [DOI] [PubMed] [Google Scholar]
- Ralph J., Lapierre C., Boerjan W.(2019). Lignin structure and its engineering. Curr. Opin. Biotechnol. 56: 240–249. [DOI] [PubMed] [Google Scholar]
- Ramalingam B., Sana B., Seayad J., Ghadess F.J., Sullivan M.B.(2017). Towards understanding of laccase-catalysed oxidative oligomerisation of dimeric lignin model compounds. RSC Advances 7: 11951–11958. [Google Scholar]
- Ranasinghe S., Rogers M.E., Hamilton J.G.C., Bates P.A., Maingon R.D.C.(2008). A real-time PCR assay to estimate Leishmania chagasi load in its natural sand fly vector Lutzomyia longipalpis. Trans. R. Soc. Trop. Med. Hyg. 102: 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X., Krom N., Tang Y., Widiez T., Havkin-Frenkel D., Belanger F.C., Dixon R.A., Chen F.(2014). A deep transcriptomic analysis of pod development in the vanilla orchid (Vanilla planifolia). BMC Genomics 15: 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salony, Garg N., Baranwal R., Chhabra M., Mishra S., Chaudhuri T.K., Bisaria V.S.(2008). Laccase of Cyathus bulleri: Structural, catalytic characterization and expression in Escherichia coli. Biochim. Biophys. Acta. Proteins Proteomics 1784: 259–268. [DOI] [PubMed] [Google Scholar]
- Schwede T., Kopp J., Guex N., Peitsch M.C.(2003). SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 31: 3381–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeto J., Tsutsumi Y.(2016). Diverse functions and reactions of class III peroxidases. New Phytol. 209: 1395–1402. [DOI] [PubMed] [Google Scholar]
- Simões M.S., Carvalho G.G., Ferreira S.S., Hernandes-Lopes J., de Setta N., Cesarino I.(2020). Genome-wide characterization of the laccase gene family in Setaria viridis reveals members potentially involved in lignification. Planta 251: 46. [DOI] [PubMed] [Google Scholar]
- Sterjiades R., Dean J.F., Eriksson K.E.(1992). Laccase from sycamore maple (Acer pseudoplatanus) polymerizes monolignols. Plant Physiol. 99: 1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M.L., Anderson E.M., Meek K.M., Reed M., Katahira R., Chen F., Dixon R.A., Beckham G.T., Román-Leshkov Y.(2018). Reductive catalytic fractionation of C-lignin. ACS Sustain. Chem. Eng. 6: 11211–11218. [Google Scholar]
- Thompson J.D., Gibson T.J. and Higgins D.G (2003). Multiple sequence alignment using ClustalW and ClustalX. Curr. Protocols Bioinformatics Chapter 2:Unit 2.3. [DOI] [PubMed] [Google Scholar]
- Teixeira J., Silva T., Benfeito S., Gaspar A., Garrido E.M., Garrido J., Borges F.(2013). Exploring nature profits: Development of novel and potent lipophilic antioxidants based on galloyl-cinnamic hybrids. Eur. J. Med. Chem. 62: 289–296. [DOI] [PubMed] [Google Scholar]
- Tobimatsu Y., Chen F., Nakashima J., Escamilla-Treviño L.L., Jackson L., Dixon R.A., Ralph J.(2013). Coexistence but independent biosynthesis of catechyl and guaiacyl/syringyl lignin polymers in seed coats. Plant Cell 25: 2587–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu Y., Schuetz M.(2019). Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 56: 75–81. [DOI] [PubMed] [Google Scholar]
- Turlapati P.V., Kim K.W., Davin L.B., Lewis N.G.(2011). The laccase multigene family in Arabidopsis thaliana: Towards addressing the mystery of their gene function(s). Planta 233: 439–470. [DOI] [PubMed] [Google Scholar]
- Vanholme R., De Meester B., Ralph J., Boerjan W.(2019). Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 56: 230–239. [DOI] [PubMed] [Google Scholar]
- Vermaas J.V., Dixon R.A., Chen F., Mansfield S.D., Boerjan W., Ralph J., Crowley M.F., Beckham G.T.(2019). Passive membrane transport of lignin-related compounds. Proc. Natl. Acad. Sci. USA 116: 23117–23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A., Tobimatsu Y., Phillips L., Flint H., Torr K., Donaldson L., Pears L., Ralph J.(2011). CCoAOMT suppression modifies lignin composition in Pinus radiata. Plant J. 67: 119–129. [DOI] [PubMed] [Google Scholar]
- Wang J., Feng J., Jia W., Chang S., Li S., Li Y.(2015a). Lignin engineering through laccase modification: A promising field for energy plant improvement. Biotechnol. Biofuels 8: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Li G., Zheng K., Zhu X., Ma J., Wang D., Tang K., Feng X., Leng J., Yu H., Yang S., Feng X.(2019). The soybean laccase gene family: Evolution and possible roles in plant defense and stem strength selection. Genes (Basel) 10: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Su S., Xiao L.-P., Wang B., Sun R.-C., Song G.(2020). Catechyl lignin extracted from castor seed coats using deep eutectic solvents: Characterization and depolymerization. ACS Sustain. Chem. Eng. 8: 7031–7038. [Google Scholar]
- Wang Y., et al. (2015b). LACCASE5 is required for lignification of the Brachypodium distachyon Culm. Plant Physiol. 168: 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J.K., Mo H., Chapple C.(2010). Over-expression of F5H in COMT-deficient Arabidopsis leads to enrichment of an unusual lignin and disruption of pollen wall formation. Plant J. 64: 898–911. [DOI] [PubMed] [Google Scholar]
- Xie D.-Y., Sharma S.B., Paiva N.L., Ferreira D., Dixon R.A.(2003). Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 299: 396–399. [DOI] [PubMed] [Google Scholar]
- Xie T., Liu Z., Wang G.(2020). Structural basis for monolignol oxidation by a maize laccase. Nat. Plants 6: 231–237. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Nakashima J., Chen F., Yin Y., Fu C., Yun J., Shao H., Wang X., Wang Z.Y., Dixon R.A.(2013). Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 25: 3976–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Zuo D., Tao Y., Cai H., Li L.(2020). Laccase3-based extracellular domain provides possible positional information for directing Casparian strip formation in Arabidopsis. Proc. Natl. Acad. Sci. USA 117: 15400–15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Jackson L., Shadle G., Nakashima J., Temple S., Chen F., Dixon R.A.(2010). Distinct cinnamoyl CoA reductases involved in parallel routes to lignin in Medicago truncatula. Proc. Natl. Acad. Sci. USA 107: 17803–17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo C., Rao X., Azad R., Pandey R., Xiao X., Harkelroad A., Wang X., Chen F., Dixon R.A.(2019). Enzymatic basis for C-lignin monomer biosynthesis in the seed coat of Cleome hassleriana. Plant J. 99: 506–520. [DOI] [PubMed] [Google Scholar]