Abstract
Purpose:
Cetuximab, which modulates immune responses, may affect the efficacy of subsequent immunotherapy. Here, we assessed outcomes with nivolumab, by prior cetuximab exposure, in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) who had experienced progression within 6 months of platinum-containing chemotherapy.
Patients and Methods:
In the randomized, open-label, phase III CheckMate 141 trial, patients were randomized 2:1 to nivolumab 3 mg/kg every 2 weeks or investigator's choice (IC) of single-agent chemotherapy, with stratification by prior cetuximab exposure. The primary endpoint was overall survival (OS); additional endpoints were progression-free survival, objective response rate, and safety.
Results:
In patients with prior cetuximab exposure, the median OS was 7.1 months with nivolumab versus 5.1 months with IC (HR, 0.84; 95% CI, 0.62–1.15); OS benefit with nivolumab was maintained across most demographic subgroups. In patients without prior cetuximab exposure, the median OS was 8.2 months with nivolumab versus 4.9 months with IC (HR, 0.52; 95% CI, 0.35–0.77); OS benefit with nivolumab was maintained across patient baseline subgroups including tumor programmed death ligand 1 (PD-L1) expression (<1% or ≥1%). Grade 3–4 treatment-related adverse event rates favored nivolumab versus IC in both subgroups.
Conclusions:
Nivolumab appeared to improve efficacy versus IC regardless of prior cetuximab use, supporting its use in patients with R/M SCCHN with or without prior cetuximab exposure. The reduction in risk of death with nivolumab compared with IC was greater in patients without prior cetuximab exposure versus with prior cetuximab exposure.
Introduction
Until recently, patients with platinum-refractory recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) had poor prognosis and limited options besides cetuximab monotherapy(1). In 2016, two programmed death-1(PD-1) inhibitors, nivolumab and pembrolizumab, were approved for the treatment of patients with R/M SCCHN who experienced disease progression after platinum-based therapy (2, 3).
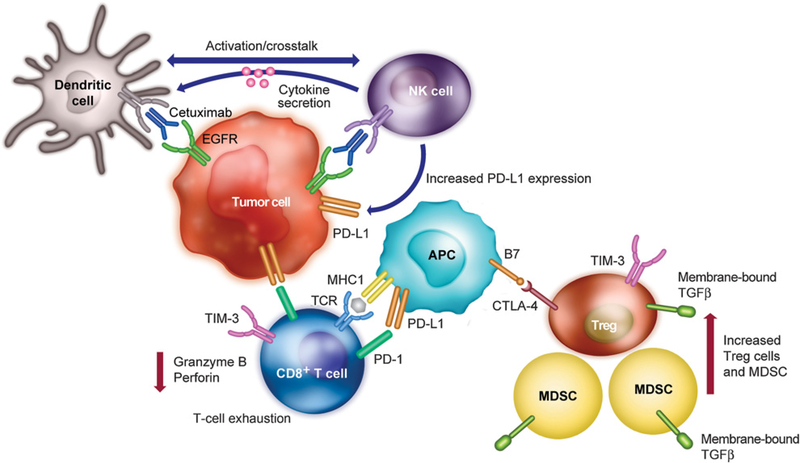
Cetuximab targets the EGFR and may interrupt oncogene signaling in tumors that have become oncogene-addicted; it can also result in induction of innate and adaptive immune responses and downregulation of immunosuppressive mechanisms (4–7). Cetuximab-mediated EGFR blockade has been shown to downregulate IFNγ-induced programmed death ligand 1 (PD-L1) expression in SCCHN, which may signify restoration of the antitumor immune response (8, 9). Cetuximab drives antibody-dependent cellular cytotoxicity of natural killer (NK) cells as well as maturation and crosstalk between NK and dendritic cells. However, cetuximab has also been shown to promote expansion of immunosuppressive regulatory T cells in the tumor microenvironment (6). In addition, it has been shown that after cetuximab monotherapy, the cytolytic activity of activated CD8+ T cells is suppressed through the increase and coexpression of PD-1 and TIM-3 in the tumor microenvironment (10). Cetuximab-activated NK cells also secrete cytokines, which enhance antigen presentation (11). Theresulting chronic antigen stimulation leads to upregulation of immune checkpoint receptors associated with T-cell exhaustion (such as CTLA-4, TIM-3, and TGFβ), creating a negative feedback loop (12). Thus, those patients who progress after cetuximab therapy have likely been selected for expansion of suppressive cell types (regulatory T cells, myeloid-derived suppressor cells) and might be less likely to respond to immunotherapy (6, 13). A schematic summarizing stimulatory and suppressive changes that may occur in the microenvironment in patients treated with cetuximab is shown in Fig. 1.
Figure 1.
Immune activity mediated by cetuximab in the SCCHN tumor microenvironment. Binding of cetuximab to EGFR recruits CD8+ T cells, which are activated through MHC complex/TCR and B7/CTLA-4 binding. In responders to treatment, cetuximab-mediated activation of NK cells induces dendritic cell maturation via crosstalk to promote antigen presentation and lyse tumor cells through ADCC. However, cetuximab binding also recruits and expands the Treg population in the tumor microenvironment. These Treg cells inhibit cetuximab-mediated cytotoxicity via expression of immune checkpoint molecules such as PD-1, PD-L1, CTLA-4, and TIM-3. Upregulation of these immune checkpoint molecules is associated with the exhausted T-cell phenotype, as seen in nonresponders to cetuximab treatment. Immunosuppressive TGFβ is also expressed on Treg cells as well as accumulating MDSCs, leading to inhibition of cytolytic activity via reduced levels of granzyme B and perforin. ADCC, antibody-dependent cellular cytotoxicity; APC, antigen presenting cell; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; EGFR, epidermal growth factor receptor; MDSC, myeloid-derived suppressor cell; NK, natural killer; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; SCCHN, squamous cell carcinoma of the head and neck; TCR, T-cell receptor; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; Treg, regulatory T cell.
CheckMate 141 was a phase III study that investigated nivolumab versus investigator's choice (IC) of therapy in patients with R/M SCCHN who had experienced tumor progression or recurrence within 6 months of platinum-based chemotherapy in the locally advanced (i.e., with radiation), recurrent, or metastatic setting. Patient randomization was stratified by prior cetuximab exposure to minimize imbalance in treatment arms due to the reported immune-modulatory effects of cetuximab (11). Nivolumab significantly improved survival versus IC in the overall study population at the primary analysis with a potential advantage noted among patients without prior cetuximab exposure (14). Efficacy at 1-year and 2-year follow-up were consistent with results from the primary analysis (15, 16). Nivolumab also stabilized quality of life compared with IC (17). Here, we analyzed the effects of prior cetuximab exposure, a prespecified stratification factor, on outcomes in CheckMate 141.
Patients and Methods
As described previously, CheckMate 141 was a randomized, open-label, phase III study in patients with histologically confirmed R/M stage III/IV SCCHN of the oral cavity, pharynx, or larynx that had progressed within 6 months of platinum-containing chemotherapy (14). Patients were randomized (2:1) to receive nivolumab (3 mg/kg i.v. every 2 weeks) or IC, consisting of methotrexate (40–60 mg/m2 i.v. weekly), docetaxel (30–40 mg/m2 i.v. weekly), or cetuximab (400 mg/m2 i.v. once, then 250 mg/m2 weekly), with stratification by prior cetuximab use. Patients continued treatment until disease progression, unacceptable toxicity, or withdrawal of consent.
The primary endpoint was overall survival (OS); secondary endpoints were progression-free survival and objective response rate (ORR; ref. 14). Tumor response was assessed per Response Evaluation Criteria In Solid Tumors v1.1 at baseline, week 9, and every 6 weeks thereafter (18). Patients were followed up for survival during treatment and every 3 months after discontinuation. Safety was monitored throughout treatment and for 100 days after administration of last dose. Assessment of tumor PD-L1 expression and human papillomavirus (HPV) status has been described previously (14).
The association of immune cell phenotypes with clinical response was assessed as an exploratory endpoint. Peripheral blood lymphocyte samples were collected at baseline and on day 43 of treatment and analyzed by flow cytometry. CD8+ effector T cells were defined as TCRα/β+CD8+CCR7−CD45RA+ and regulatory T cells as CD4+CD25hiCD127loFoxP3+. For this analysis, responders were defined as patients with complete or partial response and nonresponders as patients with stable or progressive disease.
CheckMate 141 was conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrollment. The study was approved by the institutional review board or independent ethics committee at each center and was conducted in accordance with Good Clinical Practice guidelines defined by the International Conference on Harmonisation.
Statistical analyses
Efficacy (in all randomized patients) and safety (in patients who received at least one dose of treatment) have been reported previously (14). This analysis of outcomes by cetuximab exposure is based on a September 2016 database lock, representing a minimum follow-up of 11.4 months.
Survival analyses were performed using the Kaplan-Meier method. HRs and confidence intervals (CIs) were estimated using a Cox proportional hazards model. Prespecified analyses were conducted to evaluate treatment effects by tumor PD-L1 expression and HPV status. A Cox regression was performed to investigate the association between OS and a set of predictor variables including age, Eastern Cooperative Oncology Group performance status (ECOG PS), prior radiotherapy, prior surgery, prior docetaxel/paclitaxel/taxane, number of prior lines of systemic therapy, region, tumor PD-L1 expression, HPV status, prior cetuximab, as well as the interaction of prior cetuximab exposure with ECOG PS, tumor PD-L1 expression, and HPV status (14).
A two-way ANOVA with Sidák multiple comparisons test correction was computed to descriptively analyze peripheral blood lymphocyte biomarker levels between responders and nonresponders.
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Results
Patients and treatment
Of 361 randomized patients, 147 of 240 patients in the nivolumab arm (61.3%) and 74 of 121 in the IC arm (61.2%) had previously received cetuximab (Supplementary Fig. S1). Among patients with prior cetuximab exposure randomized to the IC arm, 41 (55.4%), 32 (43.2%), and 1 (1.4%) received methotrexate, docetaxel, and cetuximab, respectively. Among patients without prior cetuximab exposure, the distribution was 11 (23.4%), 22 (46.8%), and 14 (29.8%) patients, respectively.
Baseline characteristics were similar between patients with and without prior cetuximab exposure, with a few exceptions (Table 1). Of note, patients with prior cetuximab exposure were heavily pretreated, with 69.7% in both treatment arms having received at least two prior lines of therapy. Among patients without prior cetuximab exposure, only 30.7% across both treatment arms had received at least two prior lines of therapy. A summary of treatments received by patients prior to enrollment in CheckMate 141 is included in Supplementary Tables S1 and S2. Patients with prior cetuximab had slightly higher exposure to taxanes and fluorouracil compared with patients without prior cetuximab exposure in both treatment arms. Details of cetuximab-containing regimens received by patients are summarized in Supplementary Table S3.
Table 1.
Characteristics at baseline by prior cetuximab exposure
| Patients with prior exposure to cetuximab |
Patients without prior exposure to cetuximab |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Nivolumab (n = 147) |
IC (n = 74) |
Total (n = 221) |
Nivolumab (n = 93) |
IC (n = 47) |
Total (n = 140) |
| Age, median (range), y | 60 (31–83) | 62 (32–78) | 60 (31–83) | 59 (29–79) | 59 (28–78) | 59 (28–79) |
| ≥65 y, n (%) | 39 (26.5) | 28 (37.8) | 67 (30.3) | 29 (31.2) | 17 (36.2) | 46 (32.9) |
| ECOG PS, n (%) | ||||||
| 0 | 29 (19.7) | 12 (16.2) | 41 (18.6) | 20 (21.5) | 11 (23.4) | 31 (22.1) |
| 1 | 116 (78.9) | 59 (79.7) | 175 (79.2) | 73 (78.5) | 35 (74.5) | 108 (77.1) |
| 2 | 1 (0.7) | 2 (2.7) | 3 (1.4) | 0 | 1 (2.1) | 1 (0.7) |
| Not reported | 1 (0.7) | 1 (1.4) | 2 (0.9) | 0 | 0 | 0 |
| Site of primary tumor, n (%) | ||||||
| Oral cavity | 62 (42.2) | 42 (56.8) | 104 (47.1) | 46 (49.5) | 25 (53.2) | 71 (50.7) |
| Pharynx | 59 (40.1) | 22 (29.7) | 81 (36.7) | 33 (35.5) | 15 (31.9) | 48 (34.3) |
| Larynx | 24 (16.3) | 9 (12.2) | 33 (14.9) | 10 (10.8) | 5 (10.6) | 15 (10.7) |
| Other | 2 (1.4) | 1 (1.4) | 3 (1.4) | 4 (4.3) | 2 (4.3) | 6 (4.3) |
| Region, n (%) | ||||||
| North America | 57 (38.8) | 26 (35.1) | 83 (37.6) | 44 (47.3) | 18 (38.3) | 62 (44.3) |
| Europe | 75 (51.0) | 39 (52.7) | 114 (51.6) | 34 (36.6) | 23 (48.9) | 57 (40.7) |
| Rest of world | 15 (10.2) | 9 (12.2) | 24 (10.9) | 15 (16.1) | 6 (12.8) | 21 (15.0) |
| Tobacco use, n (%) | ||||||
| Current/former | 118 (80.3) | 53 (71.6) | 171 (77.4) | 73 (78.5) | 33 (70.2) | 106 (75.7) |
| Never | 22 (15.0) | 18 (24.3) | 40 (18.1) | 17 (18.3) | 13 (27.7) | 30 (21.4) |
| Unknown | 7 (4.8) | 3 (4.1) | 10 (4.5) | 3 (3.2) | 1 (2.1) | 4 (2.9) |
| HPV status, n (%) | ||||||
| Positive | 36 (24.5) | 18 (24.3) | 54 (24.4) | 27 (29.0) | 11 (23.4) | 38 (27.1) |
| Negative | 33 (22.4) | 20 (27.0) | 53 (24.0) | 22 (23.7) | 17 (36.2) | 39 (27.9) |
| Unknown | 1 (0.7) | 2 (2.7) | 3 (1.4) | 1 (1.1) | 0 | 1 (0.7) |
| Not reported | 77 (52.4) | 34 (45.9) | 111 (50.2) | 43 (46.2) | 19 (40.4) | 62 (44.3) |
| Tumor PD-L1 expression, n (%) | ||||||
| ≥1% (PD-L1 expressors) | 52 (35.4) | 40 (54.1) | 92 (41.6) | 36 (38.7) | 21 (44.7) | 57 (40.7) |
| <1% (PD-L1 non-expressors) | 50 (34.0) | 20 (27.0) | 70 (31.7) | 23 (24.7) | 18 (38.3) | 41 (29.3) |
| Not quantifiable | 45 (30.6) | 14 (18.9) | 59 (26.7) | 34 (36.6) | 8 (17.0) | 42 (30.0) |
| Lines of prior systemic cancer therapy, n (%) | ||||||
| 1 | 44 (29.9) | 23 (31.1) | 67 (30.3) | 62 (66.7) | 35 (74.5) | 97 (69.3) |
| 2 | 57 (38.8) | 32 (43.2) | 89 (40.3) | 23 (24.7) | 12 (25.5) | 35 (25.0) |
| ≥3 | 46 (31.3) | 19 (25.7) | 65 (29.4) | 8 (8.6) | 0 | 8 (5.7) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus; IC, investigator’s choice; PD-L1, programmed death ligand 1.
Survival
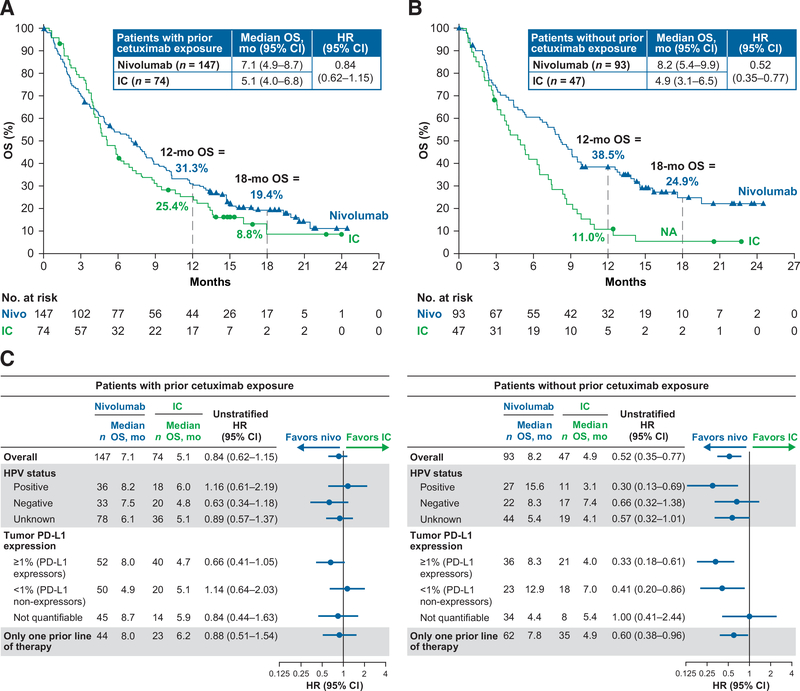
In patients with prior cetuximab exposure, the median OS was 7.1 months with nivolumab versus 5.1 months with IC (HR = 0.84; 95% CI, 0.62–1.15). In patients without prior cetuximab exposure, the median OS was 8.2 months versus 4.9 months, respectively (HR = 0.52; 95% CI, 0.35–0.77; Fig. 2A and B). Estimated 12-month OS rates were higher with nivolumab versus IC in both groups: 31.3% (95% CI, 23.9–38.9) versus 25.4% (95% CI, 16.0–35.8) in patients with prior cetuximab exposure and38.5%(95%CI,28.6–48.3) and 11.0%(95%CI,4.0–21.9)in patients without prior cetuximab exposure.
Figure 2.
A, OS in patients with prior cetuximab exposure; B, OS in patients without prior cetuximab exposure; C, Treatment effect on OS by baseline subgroups. NA, not available, minimum follow-up not reached; nivo, nivolumab.
In patients without prior cetuximab exposure, HR estimates for death among patient baseline subgroups were consistent with the overall treatment effect (Fig. 2C). In this patient population, median OS was longer for nivolumab versus IC regardless of HPV status, with the greatest benefit observed in patients with HPV-positive tumors (median OS: 15.6 vs. 3.1 months). Median OS was also longer for nivolumab versus IC in patients without prior cetuximab exposure and tumor PD-L1expression ≥1%(PD-L1 expressors) and <1% (PD-L1 nonexpressors), and those with only one line of prior therapy. Among patients with prior cetuximab exposure, nivolumab extended median OS versus IC across most demographic subgroups.
In the Cox regression analysis for OS, adjusted 95% CIs for HRs did not include 1 for prior radiotherapy, region (Europe vs. North America), ECOG PS with prior cetuximab, PD-L1 expression with prior cetuximab exposure, HPV (negative vs. positive) without prior cetuximab exposure, and HPV (unknown vs. positive) without prior cetuximab exposure (Table 2). For all other variables listed in Table 2, including number of prior lines of systemic therapy, the adjusted 95% CIs for HRs included 1.
Table 2.
Cox regression analysis for overall survival in the nivolumab arm
| Effect | HR (95% CI) |
|---|---|
| Age (≥65 y vs. <65 y) | 1.196 (0.844–1.695) |
| Prior radiotherapy (yes vs. no) | 1.747 (1.022–2.988) |
| Prior surgery (yes vs. no) | 1.295 (0.780–2.149) |
| Prior docetaxel/paclitaxel/taxane (yes vs. no) | 1.278 (0.915–1.784) |
| Number of prior lines of systemic therapy (1 vs. ≥2) | 1.238 (0.887–1.728) |
| Region (Europe vs. North America) | 1.562 (1.093–2.231) |
| Region (rest of world vs. North America) | 0.831 (0.474–1.460) |
| ECOG PS (≥1 vs. 0) (prior cetuximab = yes) | 3.715 (2.047–6.742) |
| ECOG PS (≥1 vs. 0) (prior cetuximab = no) | 0.859 (0.445–1.658) |
| Tumor PD-L1 expression (≥1% vs. <1%) (prior cetuximab = yes) | 0.592 (0.375–0.935) |
| Tumor PD-L1 expression (≥1% vs. <1%) (prior cetuximab = no) | 1.112 (0.567–2.180) |
| HPV status (negative vs. positive) (prior cetuximab = yes) | 0.671 (0.383–1.176) |
| HPV status (negative vs. positive) (prior cetuximab = no) | 2.304 (1.076–4.931) |
| HPV status (unknown vs. positive) (prior cetuximab = yes) | 0.762 (0.479–1.211) |
| HPV status (unknown vs. positive) (prior cetuximab = no) | 2.885 (1.445–5.761) |
NOTE: Variables for which the adjusted 95% CI for HR did not include 1 are shown in bold.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus; PD-L1, programmed death ligand 1.
Consistent with the overall study population, median progression-free survival was similar in both treatment arms in patients with (nivolumab = 2.0 months; IC = 2.1 months; HR = 0.86; 95% CI, 0.63–1.18) and without (nivolumab = 2.2 months; IC = 2.6 months; HR = 0.89; 95% CI, 0.60–1.31) prior cetuximab exposure.
Best overall response
Nivolumab resulted in higher ORR versus IC in patients with and without prior cetuximab exposure, with odds ratios of 1.69 (0.59–4.80) and 4.68 (1.03–21.28), respectively (Table 3). In the nivolumab and IC arms, ORRs were 10.9% and 6.8% (prior cetuximab) and 17.2% and 4.3% (no prior cetuximab), respectively. In the nivolumab arm, the median duration of response was 9.7 months (prior cetuximab) and not reached (no prior cetuximab).
Table 3.
Response evaluation by prior cetuximab exposure
| Patients with prior exposure to cetuximab |
Patients without prior exposure to cetuximab |
|||
|---|---|---|---|---|
| Nivolumab (n = 147) |
IC (n = 74) |
Nivolumab (n = 93) |
IC (n = 47) |
|
| Best overall response, n (%) | ||||
| Complete response | 2 (1.4) | 1 (1.4) | 4 (4.3) | 0 |
| Partial response | 14 (9.5) | 4 (5.4) | 12 (12.9) | 2 (4.3) |
| Stable disease | 30 (20.4) | 22 (29.7) | 25 (26.9) | 21 (44.7) |
| Progressive disease | 65 (44.2) | 29 (39.2) | 35 (37.6) | 13 (27.7) |
| Unable to determine | 36 (24.5) | 18 (24.3) | 17 (18.3) | 11 (23.4) |
| ORR, n (%) | 16 (10.9) | 5 (6.8) | 16 (17.2) | 2 (4.3) |
| [95% CI] | [6.4–17.1] | [2.2–15.1] | [10.2–26.4] | [0.5–14.5] |
| OR (95% CI) | 1.69 (0.59–4.80) | 4.68 (1.03–21.28) | ||
| ORR by HPV status, n (%) | ||||
| Positive | 2 (5.6) | 1 (5.6) | 8 (29.6) | 0 |
| Negative | 3 (9.1) | 2 (10.0) | 5 (22.7) | 2 (11.8) |
| Unknown | 11 (14.1) | 2 (5.6) | 3 (7.0) | 0 |
| ORR by tumor PD-L1 expression, n (%) | ||||
| ≥1% (PD-L1 expressors) | 8 (15.4) | 1 (2.5) | 7 (19.4) | 0 |
| <1% (PD-L1 nonexpressors) | 4 (8.0) | 3 (15.0) | 5 (21.7) | 1 (5.6) |
| Not quantifiable | 4 (8.9) | 1 (7.1) | 4 (11.8) | 1 (12.5) |
| Duration of response, median, months | 9.7 | 3.0 | NR | NR |
| Range | 2.8+ to 16.5+ | 1.5+ to 3.0 | 2.8 to 20.3+ | 4.9 to 8.5+ |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; IC, investigator’s choice; NR, not reached; ORR, objective response rate; PD-L1, programmed death ligand 1.
Among patients with prior cetuximab exposure, ORR was higher with nivolumab versus IC in PD-L1 expressors (15.4% vs. 2.5%) but not in PD-L1 nonexpressors (8.0% vs. 15.0%). Among patients without prior cetuximab exposure, nivolumab improved ORR versus IC irrespective of tumor PD-L1 expression: 19.4% versus 0% (PD-L1 expressors) and 21.7% versus 5.6% (PD-L1 nonexpressors). In the nivolumab arm, 16 patients in each of the groups (with prior cetuximab, 10.9%; without prior cetuximab, 17.2%) had >30% reduction in target lesions (Supplementary Fig. S2).
Safety
Among patients with prior cetuximab exposure, any grade and grade 3–4 treatment-related adverse events were reported in 57.9% and 13.1% of patients (nivolumab) and 80.3% and 42.4% of patients (IC), respectively (Supplementary Table S4). Among patients without prior cetuximab exposure, the respective rates were 68.1% and 18.7% (nivolumab) and 77.8% and 26.7% (IC). The only grade 3–4 select treatment-related adverse events reported in more than one patient were pulmonary-related events in 2 of 145 (1.4%) patients with prior cetuximab exposure in the nivolumab arm (Supplementary Table S5).
Circulating immune cell phenotypes
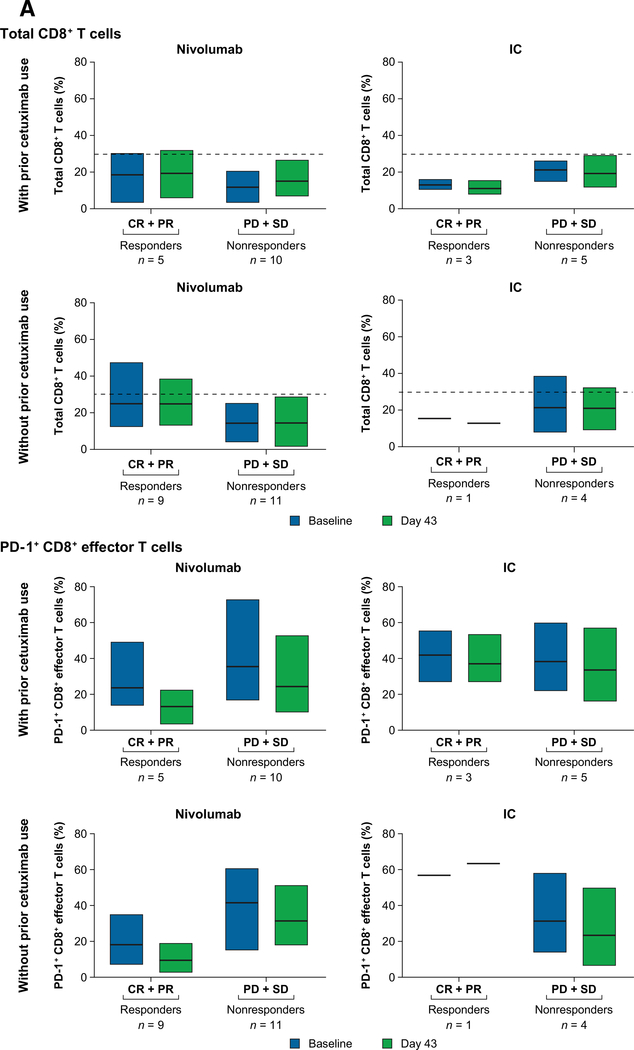
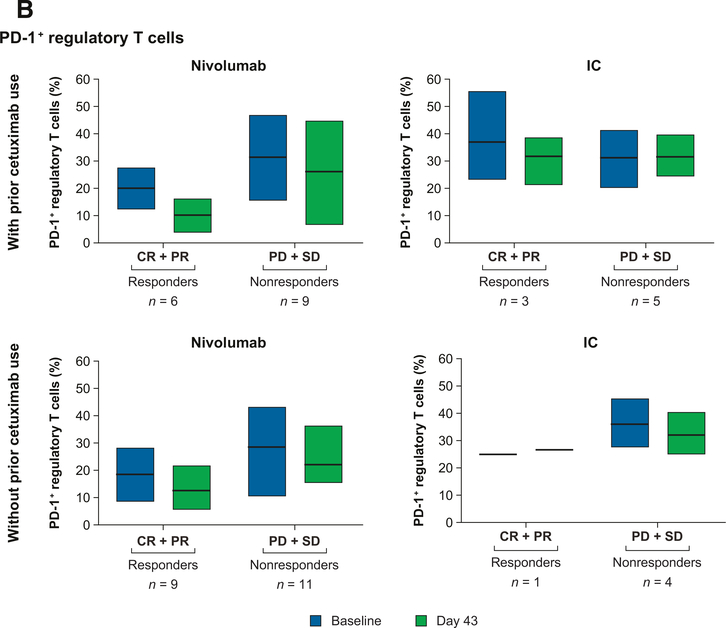
Among patients without prior cetuximab exposure who received nivolumab, responders (n = 9) had higher levels of total CD8+ T cells and lower levels of PD-1− CD8+ effector T cells than nonresponders (n = 11) at baseline and on day 43 (Fig. 3A). In this group, levels of PD-1+ regulatory T cells were lower in responders (n = 9) than nonresponders (n = 11) at both time points (Fig. 3B). Similar trends were observed in patients with prior cetuximab exposure receiving nivolumab.
Figure 3.
Changes in the levels of circulating immune cell phenotypes in patients with and without prior cetuximab exposure in the nivolumab arm. A, CD8+ effector T cells. CD8+ effector T cells were defined as TCRα/β+CD8+CCR7−CD45RA+. B, Regulatory T cells. Regulatory T cells were defined as CD4+CD25hiCD127loFoxP3+. Abbreviations: CR, complete response; IC, investigator's choice; PD, progressive disease; PR, partial response; SD, stable disease.
Frequencies of CD4+, TIM-3+, CTLA-4+, LAG-3+, CD39+, or Nrp-1+ regulatory T cells were similar between responders and nonresponders in the nivolumab arm, irrespective of prior cetuximab exposure. Immune cell subtype levels were also similar in patients with or without prior cetuximab exposure receiving IC. Owing to insufficient specimens, analyses by HPV status or other subgroup analyses could not be performed.
Discussion
In this analysis of CheckMate141, nivolumab appeared to improve clinical outcomes versus IC regardless of prior cetuximab exposure. The OS benefit with nivolumab versus IC was maintained at 2-year follow-up, with HR (95% CI) of 0.79 (0.59–1.06) in patients with prior cetuximab exposure and 0.52 (0.36–0.76) in patients without prior cetuximab exposure (15). Nivolumab was well tolerated versus IC, regardless of prior cetuximab use, and its safety profile in both groups of patients was similar to that of the overall population.
Cetuximab modulates the PD-1 axis, and prior cetuximab exposure could potentially affect outcomes with nivolumab (4–6, 9). Cetuximab has been shown to significantly downregulate IFNγ-induced PD-L1 expression in head and neck tumor cell lines (9). In CheckMate 141, tumor PD-L1 expression (<1% and ≥1%) was similar in patients with and without prior cetuximab exposure, indicating that differences in response to nivolumab between these patient groups may not be related to the effect of cetuximab on tumor PD-L1 expression. Cetuximab may also induce regulatory T cells, particularly in nonresponders (6). While further studies are needed, one hypothesis is that the above effect could potentially predispose patients who experienced recurrence after prior cetuximab exposure to exhibit lower clinical benefitto immunotherapeutic strategies than those not previously exposed to cetuximab.
Owing to small sample sizes, statistical significance is not reported for the exploratory immune cell biomarker analysis. Nonetheless, differences in levels of total CD8+ T cells and PD-1+ CD8+ effector T cells, and PD-1+ regulatory T cells were noted among responders and nonresponders, primarily in patients without prior cetuximab exposure. In particular, higher levels of total CD8+ T cells at baseline were associated with better response, as were lower levels of CD8+ PD-1+ effector T cells, the latter associated with T-cell exhaustion. These findings were more pronounced in patients without prior cetuximab exposure, raising the possibility that cetuximab modulates the CD8 T-cell compartment, as previously suggested (6, 8, 9). While these results have potential prognostic value, the analysis was exploratory and additional research is warranted.
To our knowledge, this is the first detailed published report on the effect of prior cetuximab exposure on response to a PD-1 inhibitor. A post hoc analysis of the phase III KEYNOTE-040 evaluating pembrolizumab in R/M SCCHM was recently published (19). Our analysis provides insights on the potential impact of prior cetuximab exposure on efficacy of subsequent nivolumab treatment; however, CheckMate 141 was not powered to detect significant differences between patients with and without cetuximab exposure. Another limitation of the current analysis is that data on timing of the prior cetuximab treatment relative to on-treatment study were not available. In addition, information on whether prior cetuximab was administered in combination with radiation, and consequently, the context for treatment, was also not available. Prospective randomized phase III clinical trials could help assess the impact of prior cetuximab exposure on the efficacy of subsequent immunotherapy. For example, comparison of efficacy among patients with prior cetuximab exposure randomized to treatment with nivolumab versus IC and stratified by prior cisplatin exposure (to standardize prior lines of therapy) could yield useful results. Alternatively, efficacy could be compared among patients with prior exposure to the EXTREME regimen who are randomized to receive treatment with nivolumab versus IC.
Recently, data have been published on the utility of cetuximab plus radiation in the treatment of certain patient populations (e.g., HPV-positive oropharyngeal cancer, elderly) with locally advanced SCCHN (20–22). In addition, results on the first-line treatment of recurrent/metastatic SCCHN with pembrolizumab have been published (23). These emerging data underscore the need to optimize the treatment approach for SCCHN based on patient and disease characteristics with the goal of maximizing options for patients. To that end, the data presented in this article may be relevant in informing decisions with regard to sequencing of therapy in patients with SCCHN.
In this analysis, reduction in risk of death with nivolumab was 16% in patients with prior cetuximab exposure and 48% in patients without prior cetuximab use. In the first-line setting for R/M disease, cetuximab as part of the EXTREME regimen has been the preferred option for patients with ECOG PS of 0–1 (24). Therefore, patients without prior cetuximab exposure in CheckMate 141 may not yet have received treatment for R/M disease. Indeed, among patients without prior cetuximab exposure, 69% had only one prior line of therapy, whereas patients with prior cetuximab were heavily pretreated with 70% having undergone two or more prior lines of therapy. However, a Cox regression analysis identified that the number of prior lines of systemic therapy was a nonsignificant predictor of OS in the nivolumab arm.
The lower efficacy in the IC arm among patients without prior cetuximab exposure could potentially be attributed to patient and/or disease characteristics, or choice of therapy. ECOG PS, however, was similar among patients with and without prior cetuximab exposure, with 16.2% and 23.4%, respectively, having a PS of 0. The proportions of patients receiving docetaxel as IC therapy were balanced between patients with (43%) and without (47%) prior cetuximab exposure. The use of methotrexate and cetuximab as IC therapy was more variable: among patients with prior cetuximab exposure, all but one of the remaining patients (55%) received methotrexate, whereas among patients without prior cetuximab exposure, 23% received methotrexate, and 30% received cetuximab. The design of the study precluded assessing efficacy of nivolumab versus the individual agents used in IC. Qualitatively, however, treatment with methotrexate had better outcomes than with cetuximab (14). This may have contributed to the reduced efficacy of the IC arm among patients without prior cetuximab exposure.
With regard to tumor PD-L1 expression and HPV status, among patients with prior cetuximab exposure, nivolumab improved ORR and OS versus IC in PD-L1 expressors only, and no consistent association was noted between HPV status and efficacy. Among patients without prior cetuximab exposure, response rates were higher with nivolumab versus IC regardless of PD-L1 expression or HPV status. These results may be more of a reflection of the overall better performance of patients without prior cetuximab exposure and the poor performance of the IC arm rather than any underlying biology.
Overall, findings from this post hoc analysis of clinical outcomes of the CheckMate 141 study are consistent with results from the primary analysis and support the use of nivolumab across a broad population of patients with R/M SCCHN post-platinum therapy. The reduction in the risk of death with nivolumab compared with IC was higher in patients without prior cetuximab exposure, and prognostic biomarker assessments were promising in this patient population. Further research is needed to optimize treatment sequence in SCCHN in order to maximize therapy options and to understand the impact of prior treatments on response to PD-1 inhibitors; studies are underway to assess nivolumab combinations, including with cetuximab and radiotherapy (25).
Supplementary Material
Translational Relevance.
Nivolumab is a programmed death-1 inhibitor approved for the treatment of recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN) post-platinum therapy. In the first-line setting for recurrent/metastatic SCCHN, cetuximab as part of the platinum-based EXTREME regimen is a common treatment option. Cetuximab modulates immune responses and may affect the efficacy of subsequent immunotherapy. In this post hoc analysis of the randomized phase III CheckMate 141 trial in recurrent/metastatic SCCHN post-platinum therapy, nivolumab appeared to prolong overall survival versus investigator's choice of therapy in patients with and without prior cetuximab exposure; reduction in risk of death with nivolumab was 16% and 48%, respectively. Safety in both subgroups was similar to the overall population. Prospective randomized clinical trials could help elucidate the impact of prior cetuximab treatment on the efficacy of subsequent immunotherapy.
Acknowledgments
The authors thank the patients and their families as well as the clinical study teams for making this study possible. This study was sponsored by Bristol-Myers Squibb (Princeton, New Jersey) and ONO Pharmaceutical Company, Ltd. (Osaka, Japan). Henry Kao,PhD,provided assistance with biomarker data analyses and Jennifer Schick served as the protocol manager. Professional medical writing assistance was provided by Beth Burke, PhD, CMPP, and Meenakshi Subramanian, PhD, CMPP, of Evidence Scientific Solutions, and was funded by Bristol-Myers Squibb. K.J. Harrington acknowledges support from the Royal Marsden/the Institute of Cancer Research, National Institute of Health Research Biomedical Research Centre, and Oracle Cancer Trust. Dr. Ferris and his laboratory research effort are supported by the NIH grants, P50 CA097190–14, P30 CA047904–28, and R01 CA206517. The University of Texas MD Anderson Cancer Center is supported by the NIH (grant P30 CA016672). This study was funded by Bristol-Myers Squibb.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Disclosure of Potential Conflicts of Interest
R.L. Ferris reports receiving commercial research grants from Astra-Zeneca/Medimmune, Bristol-Myers Squibb, Merck, Tesaro, and VentiRx Pharmaceuticals, and is a consultant/advisory board member for Aduro Biotech, Amgen, Astra-Zeneca/Medimmune, Bain Capital Life Sciences, Bristol-Myers Squibb, EMD Sorono, GlaxoSmithKline, Iovance Biotherapeutics, Inc, Lilly, Merck, Numab Therapeutics AG, Oncorus, Inc., Ono Pharmaceutical Co Ltd, Pfizer, PPD, Regeneron Pharmaceuticals, Inc, Tesaro, Torque Therapeutics Inc, and TTMS. L. Licitra is a consultant/advisory board member for AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, MSD, Merck Serono, Boehringer Ingelheim, Novartis, Roche, Debiopharm, Sobi, Incyte Biosciences Italy, Doxa Pharma, Amgen, Nanobiotics Sa, and GlaxoSmithKline. J. Fayette is a consultant/advisory board member for Bristol-Myers Squibb, AstraZeneca, MSD, and Merck Serono. C. Even is a consultant/ advisory board member for Bristol-Myers Squibb, MSD, and Innate Pharma. G. Blumenschein Jr reports receiving commercial research grants from Adaptimmune, Bristol-Myers Squibb, Exelixis, Immatics, Immunoc Incyte, KITE Pharma, Macrogenics, GlaxoSmithKline, Celgene, Genetech, Medimmune, Novartis, Merck, Roche, Xcovery, Torque, AstraZeneca, and Bayer, holds ownership interest (including patents) in Johnson & Johnson, and is a consultant/advisory board member for Abbvie, Adicet, Amgen, Bristol-Myers Squibb, Merck, Celgene, Genetech, Medimmune, Novartis, Roche, Xcovery, and Maverick. K.J. Harrington reports receiving commercial research grants from AstraZeneca, Boehringer-Ingelheim, MSD, and Replimune, speakers bureau honoraria from AstraZeneca, Amgen, Boehringer-Ingelheim, Merck-Serono, MSD, Pfizer, and Replimune, and is a consultant/ advisory board member for AstraZeneca, Amgen, Boehringer-Ingelheim, Merck-Serono,MSD,Oncolys,Pfizer, and Replimune. J. Guigay is a consultant/advisory board member for Bristol-Myers Squibb, Merck, AstraZeneca, Innate pharma, and Nanobiotix. E.E. Vokes is a consultant/advisory board member for Abbvie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, EMD Serono, Genentech, Merck, Novartis, and Rengeneron. N.F. Saba reports receiving commercial research grants from Bristol-Myers Squibb and Exelixis, and is a consultant/advisory board member for Merck, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, and Pfizer. R. Haddad is a consultant/ advisory board member for Bristol-Myers Squibb, Merck, Pfizer, Genentech, Loxo, Bayer, Immunomic, Nanobiotix, and GlaxoSmithKline. P. Brossart reports receiving speakers bureau honoraria from MSD, Bristol-Myers Squibb, and AstraZeneca, and is a consultant/advisory board member for MSD, Amgen, Roche, AstraZeneca, and Bristol-Myers Squibb. M. Tahara reports receiving speakers bureau honoraria from Merck Serono, Bristol-Myers Squibb, Eisai, Ono Pharmaceutical, MSD, and AstraZeneca, and is a consultant/advisory board member for Ono Pharmaceutical, MSD, Pfizer, Bristol-Myers Squibb, Rakuten Medical, and Amgen. A.D. Colevas reports receiving commercial research grants from Bristol-Myers Squibb, Regeneron, AstraZeneca, and Genentech, and other commercial research support from Merck. L. Li holds ownership interest (including patents) in Bristol-Myers Squibb. M.L. Gillison is an employee of Bristol-Myers Squibb, EMD Serono, Merck, Genocea, TRM Oncology, Celgene, Roche, NewLink Genetics, Amgen, AstraZeneca, Aspyrian, and Bayer. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.de Andrade DA, Machiels JP. Treatment options for patients with recurrent or metastatic squamous cell carcinoma of the head and neck, who progress after platinum-based chemotherapy. Curr Opin Oncol 2012;24:211–7. [DOI] [PubMed] [Google Scholar]
- 2.KEYTRUDA (pembrolizumab) for injection, for intravenous use [prescribing information]. Whitehouse Station, NJ: Merck & Co., Inc.; 2017. [Google Scholar]
- 3.OPDIVO (nivolumab) injection, for intravenous use [prescribing information]. Princeton, NJ: Bristol-Myers Squibb; 2017. [Google Scholar]
- 4.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 2003; 21:2787–99. [DOI] [PubMed] [Google Scholar]
- 5.Trivedi S, Concha-Benavente F, Srivastava RM, Jie HB, Gibson SP, Schmitt NC, et al. Immune biomarkers of anti-EGFR monoclonalantibodytherapy. Ann Oncol 2015;26:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S,et al. CTLA-4+ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res 2015;75:2200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez R, Crombet T, de Leon J, Moreno E. A view on EGFR-targeted therapies from the oncogene-addiction perspective. Front Pharmacol 2013;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Concha-Benavente F, Srivastava RM, Ferrone S, Ferris RL. EGFR-mediated tumor immunoescape: the imbalance between phosphorylated STAT1 and phosphorylated STAT3. Oncoimmunology 2013;2: e27215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the cell-intrinsic and -extrinsic pathways down-stream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res 2016;76:1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jie HB, Srivastava RM, Argiris A, Bauman JE, Kane LP, Ferris RL. Increased PD-1+ and TIM-3+ TILs during cetuximab therapy inversely correlate with response in head and neck cancer patients. Cancer Immunol Res 2017;5: 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res 2013;19:1858–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris RL, Lenz HJ, Trotta AM, Garcia-Foncillas J, Schulten J, Audhuy F, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev 2018;63:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer 2015;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillison ML, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. CheckMate 141: 1-Year Update and Subgroup Analysis of Nivolumab as First-Line Therapy in Patients with Recurrent/Metastatic Head and Neck Cancer. Oncologist 2018;23:1079–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington KJ, Ferris RL, Blumenschein G Jr, Colevas AD, Fayette J, Licitra L, et al. Nivolumab versus standard, single-agent therapy of investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol 2017;18:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 19.Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393: 156–67. [DOI] [PubMed] [Google Scholar]
- 20.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised multicentre, non-inferiority trial. Lancet 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zandberg DP, Cullen K, Bentzen SM, Goloubeva OG. Definitive radiation with concurrent cetuximab vs. radiation with or without concurrent cytotoxic chemotherapy in older patients with squamous cell carcinoma of the head and neck: Analysis of the SEER-medicare linked database. Oral Oncol 2018;86:132–40. [DOI] [PubMed] [Google Scholar]
- 22.Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 2019;393:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burtness B, Harrington KJ, Greil R, Soulierès D, Tahara M, DeCastro G Jr, et al. , editors. KEYNOTE-048: Phase 3 study of first-line pembrolizumab(P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) [abstract]. In: ESMO 2018 Congress; October 19–23, 2018; Munich, Germany. Lugano (Switzerland): ESMO; 2018. [Google Scholar]
- 24.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–27. [DOI] [PubMed] [Google Scholar]
- 25.Szturz P, Vermorken JB. Immunotherapy in head and neck cancer: aiming at EXTREME precision. BMC Med 2017;15:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.