Abstract
Directional cell migration is a critical process underlying morphogenesis and post‐natal tissue regeneration. During embryonic myogenesis, migration of skeletal myogenic progenitors is essential to generate the anlagen of limbs, diaphragm and tongue, whereas in post‐natal skeletal muscles, migration of muscle satellite (stem) cells towards regions of injury is necessary for repair and regeneration of muscle fibres. Additionally, safe and efficient migration of transplanted cells is critical in cell therapies, both allogeneic and autologous. Although various myogenic cell types have been administered intramuscularly or intravascularly, functional restoration has not been achieved yet in patients with degenerative diseases affecting multiple large muscles. One of the key reasons for this negative outcome is the limited migration of donor cells, which hinders the overall cell engraftment potential. Here, we review mechanisms of myogenic stem/progenitor cell migration during skeletal muscle development and post‐natal regeneration. Furthermore, strategies utilised to improve migratory capacity of myogenic cells are examined in order to identify potential treatments that may be applied to future transplantation protocols.
Keywords: cell migration, cell therapy, muscle regeneration, muscle stem cells, muscular dystrophy
Subject Categories: Musculoskeletal System, Regenerative Medicine
This comprehensive review discusses our knowledge on dynamics and mechanisms of myogenic progenitor cell migration during morphogenesis and post‐natal tissue regeneration and provides insights on the possible strategies for the development of safe and efficient cell therapies.
Glossary
- Amoeboid migration
Amoeboid migration is a type of cell motility (often faster than mesenchymal migration) characterised by cycles of expansion and contraction, which allow cells to squeeze through gaps in the extracellular matrix adopting round or irregular shapes. Leucocytes and cancer cells are two examples of cells which are capable of amoeboid migration
- Angiopellosis
An alternative mechanism to leucocyte diapedesis proposed for cells that are not native to the blood circulation to extravasate. During angiopellosis, endothelial cells play a key role in enabling the extravasation of multiple cells during a single event
- Cell migration
The process by which translocation of the cell occurs. At the molecular level, cell migration is an orchestrated process and it is performed in a sequential manner
- Cell therapy
Treatment strategy based upon delivery of cells as medicine. Can be autologous (i.e. patient's own cells) or allogenic (i.e. from donors); if autologous, cells can be genetically corrected prior to delivery (i.e. gene and cell therapy). The transplanted cells can be stem cells, committed progenitors or differentiated cells. Cells are usually delivered to regenerate a diseased tissue but can also be delivered to kill a specific target (e.g. tumour)
- Endomysium
Thin layer of connective tissue ensheathing each individual skeletal muscle fibre
- Extracellular matrix
Three‐dimensional structure that comprises part of interstitial spaces of tissues, derived from secreted macromolecules. The extracellular matrix plays crucial roles in providing biophysical and biochemical cues as well as structural support to nearby cells
- Extravasation (transmigration)
The process by which (white blood) cells migrate through endothelial cell layers to exit the circulatory system towards inflamed tissues in which they are required
- Fibrosis
Excessive accumulation of extracellular matrix components, found upon abnormal wound healing, often resulting in tissue dysfunction
- Filopodia
Slim cellular protrusions containing 10–30 actin filaments in parallel arrays, often found at the leading edge of lamellipodia during cell migration
- Focal adhesion
Relatively stable sites of interaction between the cell and the surrounding extracellular matrix. Focal adhesions are multiprotein assemblies essential for functions such as generation of tension/traction forces for cell migration and mechanotransduction
- Lamellipodia
Broad, “fan‐shaped”, actin‐based, protrusions generated at the leading edge of cells undergoing mesenchymal migration
- Mesenchymal migration
A mode of motility in which polarisation of the cell results in generation of actin‐based structures such as lamellipodia. This allows the formation of adhesions generating traction forces. Actomyosin contractions at the rear of the cell subsequently propel the cell in a directional manner
- Mesoangioblasts
In vitro progeny of perivascular cells able to give rise to blood vessel lineages (mostly smooth muscle) and mesodermal lineages of the surrounding tissue. Skeletal muscle pericyte‐derived mesoangioblasts have been delivered intra‐arterially in pre‐clinical animal models of muscle diseases and in patients with Duchenne muscular dystrophy
- Muscular dystrophy
A heterogeneous group of primary genetic diseases of skeletal muscle, characterised by progressive muscle degeneration, wasting and premature death in the most severe forms
- Myoblasts (adult skeletal myoblasts)
Committed progeny of satellite stem cells most of which expand and fuse with nearby muscle fibres
- Pseudopodia
Temporary cytoplasmic processes of eukaryotic cells
- Sarcopenia
A pathological condition characterised by age‐related loss of skeletal muscle mass, strength and function
- Satellite cell
Skeletal muscle stem cells (also known as MuSCs) residing between the myofibre's plasmalemma and the surrounding endomysium. Upon activation, satellite cells give rise to committed progenitors called myoblasts (see above) most of which fuse with surrounding fibres (for repair or regeneration); a minority return to quiescence to replenish the self‐renewing stem cell pool of satellite cells
- Somite
Sphere of paraxial mesoderm paired bilaterally along the neural tube during embryonic development. Somites give rise to cells which in turn will generate different mesodermal derivatives such as cartilage, bone, muscle and tendons
Introduction
Cell migration is a fundamental process for embryogenesis, repair and regeneration of skeletal muscle, the most abundant human tissue. During development, skeletal myogenic progenitors migrate towards prospective skeletal muscles of the trunk and limbs, where they also give rise to stem cells responsible for post‐natal repair and regeneration of skeletal muscles: the muscle satellite cells (MuSCs). MuSCs represent the key skeletal muscle stem cell population, residing between the sarcolemma and the endomysium of muscle fibres (Mauro, 1961). Upon activation, MuSCs give rise to committed proliferating progenitors termed myoblasts, which migrate and fuse either amongst themselves or with pre‐existing myofibres to (re)generate and repair skeletal muscle (Watt et al, 1987; Morgan et al, 1987; Phillips et al, 1990; Siegel et al, 2009; Ishido & Kasuga, 2011; Baghdadi et al, 2018). Although processes that drive activation, proliferation and differentiation of muscle stem cells are well‐studied, the molecular mechanisms responsible for the migratory properties of myogenic cells have not been the focus of extensive investigation.
Migration has implications in cell therapies of muscle diseases such as muscular dystrophies, heterogenous myopathies characterised by progressive muscle wasting (Mercuri et al, 2019). Duchenne muscular dystrophy (DMD), the most common form of paediatric muscular dystrophy occurring in 1/3,500–5,000 boys, is caused by mutations in the DMD gene encoding dystrophin (Hoffman et al, 1987). Dystrophin functions as a shock absorber to stabilise the sarcolemma and DMD mutations lead to contraction‐induced degeneration of skeletal myofibres and impaired muscle function (Muntoni et al, 2003). DMD patients experience early loss of ambulation and mortality as a result of cardiorespiratory complications, often within the first three decades of life (Mercuri et al, 2019). Standardised pharmacological interventions for DMD include administration of corticosteroids (Matthews et al, 2016) and in some cases mutation‐specific drugs such as Ataluren and Eteplirsen which have recently been approved in Europe and the USA, respectively (Mendell et al, 2013; McDonald et al, 2017). Several experimental therapeutic strategies have been investigated for DMD and other muscular dystrophies, including gene therapy and stem cell transplantation (Benedetti et al, 2013) .
MuSCs possess extensive self‐renewal capacity and efficiently engraft into mouse muscles upon transplantation (Sacco et al, 2008). Therefore, a promising strategy to restore dystrophin expression is cell therapy: a procedure based upon transplantation of healthy donor or autologous (genetically corrected) cells which then fuse with existing multinucleated myofibres or form new fibres, re‐establishing tissue function. Cell‐based approaches to treat DMD have been explored since the 1980s, when transplantation studies of healthy donor myoblasts in the muscles of mdx mice, a mouse model of DMD, displayed robust engraftment and rescue of dystrophin expression (Partridge et al, 1989). However, the subsequent early phase clinical trials in DMD patients exhibited limited dystrophin restoration and functional amelioration (reviewed in (Tedesco et al, 2010)). Major efforts have since been made to circumvent some of the main issues associated with muscle cell therapy such as the host immune response, poor cell survival and limited cell migration post‐injection (Gussoni et al, 1992; Karpati et al, 1993; Vilquin et al, 1994; Bouchentouf et al, 2007). Myoblast transplantations were carried out under immunosuppressive regimens (Vilquin et al, 1994), and protocols entailing high‐density injections were also implemented (Skuk et al, 2007); nonetheless, results remain suboptimal (Skuk & Tremblay, 2015).
Efforts have since been made to overcome the loss of self‐renewal and engraftment potential during MuSC in vitro expansion, leading to protocols capable of preserving their regenerative capacity ex vivo (i.e. via pharmacological modulation or by application of biomimetic platforms; reviewed in (Judson & Rossi, 2020)). However, the field still looks for highly migratory myogenic cells or methods that mediate improved cell dispersal and dissemination in vivo, thereby facilitating the development of treatments for degenerative diseases affecting multiple large muscles or for severe volumetric muscle loss.
There are two predominant methods of cell transplantation into skeletal muscles: the intramuscular route and the systemic/intravascular route. Both modalities are constrained by insufficient migration of donor cells which limits the efficacy of treatments. Intramuscular injections have mostly been performed with skeletal myoblasts. A common observation of this mode of delivery is the formation of chimeric myofibres limited to the trajectory of injection, as opposed to dispersal throughout muscle tissue. This issue limits intramuscular administration to highly localised myopathies such as oculopharyngeal muscular dystrophy (OPMD), for which clinical improvement has been achieved (Périé et al, 2014). On the other hand, intra‐arterial delivery of donor cells into major arteries can simultaneously target multiple muscle groups downstream of the injection site and may be better suited for muscular dystrophies with widespread muscle involvement, particularly when affecting skeletal muscles otherwise difficult to access (e.g. diaphragm). However, MuSCs do not efficiently cross endothelial cell layers, and therefore, investigations on systemic delivery have largely focused on alternative myogenic cell types. Nonetheless, rare reports describe intra‐arterial infusion of unpurified myoblasts in rats and non‐human primates resulting in the occasional incorporation of some donor cells into host myofibres (Neumeyer et al, 1992; Skuk & Tremblay, 2014). Mesoangioblasts, myogenic cells derived from expansion of a subpopulation of skeletal muscle perivascular cells, exhibit a higher migratory capacity than MuSCs towards skeletal muscles upon intra‐arterial delivery and have proved efficacious in animal models of muscular dystrophy (reviewed in (Benedetti et al, 2013)) as well as relatively safe in a first‐in‐human clinical trial (Cossu et al, 2016). Nonetheless, myogenic differentiation and overall homing/engraftment of human mesoangioblasts require significant enhancement to reach clinical efficacy.
More recently, significant progress has been made to generate skeletal myogenic cells from induced pluripotent stem cells (iPSCs), which could provide an unlimited source of myogenic cells for cell therapy. However, no defined protocols are currently available for the generation of highly migratory human iPSC myogenic derivatives. Although the rapidly expanding iPSC field is now deriving potent myogenic progenitors increasingly comparable to native MuSCs (e.g. (Chal et al, 2015)), the latter are not capable of extravasation upon intravascular delivery.
It is, therefore, critical to develop strategies to enhance the migratory capacity of skeletal myogenic cells. To this aim, we review key studies on dynamics and mechanisms of skeletal myogenic cell migration in the embryo, adult and upon transplantation. Approaches previously applied to boost the transplantation efficacy of MuSCs have been reviewed, as well as mechanisms of trans‐endothelial migration such as those utilised by leucocytes (diapedesis) and cancer cells. We believe that understanding these mechanisms will be critical to engineer or derive populations of myogenic progenitors with enhanced migration capacity for efficacious skeletal muscle cell therapies.
Essential molecular machinery for myogenic cell motility and migration
Directional migration requires integration and coordination of various molecular and mechanical stimuli. The general dynamics of directional migration can be reduced to repetition of four basic steps: (1) generation of cellular protrusions, (2) adhesion to substrate, (3) contraction and (4) retraction of the cell rear (Vicente‐Manzanares et al, 2005). Specific mechanisms vary depending on cell type and context: limited substrate adhesion is required for amoeboid‐based migration, and shifts between modes of migration can occur rapidly in 3D environments in response to changes in levels of confinement and adhesion (Lautscham et al, 2015; Winkler et al, 2019). Additionally, spatiotemporal regulation is crucial for execution of the aforementioned steps in a sequential manner. This role is often mediated by Rho GTPases, which modulate migration in response to biochemical and biophysical cues by reorganising the actin cytoskeleton (Binamé et al, 2010; Ridley, 2015). The two primary modalities of migration are classified as mesenchymal or amoeboid migration. Mesenchymal migration of myoblasts has been relatively well‐studied in comparison with amoeboid migration, as the latter is difficult to observe on bidimensional surfaces (Yamada & Sixt, 2019).
Mesenchymal migration
Myoblasts display mesenchymal migration on bidimensional monolayers in vitro (Kawamura et al, 2004). Mesenchymal migration involves generation of protrusions, pseudopodia, at the leading front of the cell, such as the fan‐shaped lamellipodia consisting of branched actin filaments, as well as filopodia comprised of parallel, bundled filamentous actin (F‐actin) (Fletcher & Mullins, 2010). Distinct actin regulators are involved in generation of lamellipodia and filopodia. Lamellipodia formation is primarily dictated by polarised activity of Rho GTPase Rac1 and its downstream effectors such as actin‐related protein 2/3 (Arp2/3) and the WASP‐family verprolin‐homologous protein regulatory complex (WAVE complex) which increase the rate of actin nucleation and subsequently actin polymerisation (Fig 1) (Kawamura et al, 2004; Takenawa & Suetsugu, 2007). Filopodia generation, on the other hand, is canonically driven by the Rho GTPase CDC42 with diaphanous formins playing a role in nucleation of actin polymerisation at the migration front (Fig 1) (Mellor, 2010).
Figure 1. Schematic representation of cytoskeletal elements involved in mesenchymal migration.
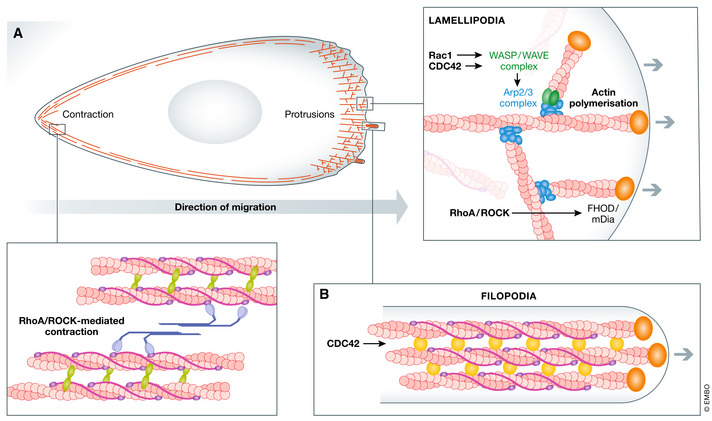
(A) Pathways involved in generation of lamellipodia are outlined. (B) Graphical presentation of a single filopodia, spike‐like protrusions at the leading edge of migration. These structures contain parallel F‐actin bundles crosslinked by fascin (yellow) with polymerisation of actin occurring at the + end of actin filaments, facilitated by diaphanous formins (orange).
Adhesion occurs simultaneously with the extension of cellular protrusions, which are crucial for the generation of traction forces. Binding of integrin receptors (transmembrane heterodimers capable of binding extracellular matrix (ECM) components) facilitates assembly of adhesion complexes which give rise to focal adhesions (FAs) (Harburger & Calderwood, 2009). FAs are multiprotein complexes involved in mechanotransduction as well as cell signalling and are found at the ends of stress fibres. Most FAs possess a laminar arrangement, with integrin cytoplasmic tails, focal adhesion kinase (FAK) and paxillin comprising the signalling module; talin and vinculin making up the intermediate force transduction layer and the innermost actin regulatory layer (Kanchanawong et al, 2010). FAs are frequent downstream targets of growth factor‐mediated migration of myogenic cells, and components of FAs are disrupted in some myopathies, resulting in impaired stem cell migration (Leloup et al, 2006; Bricceno et al, 2014).
Directional migration requires contractile forces for directed cell propulsion. Non‐muscle myosin II‐mediated contractions provide propulsive forces that facilitate directional movement and are crucial for maturation and disassembly of FAs (Schwartz & Horwitz, 2006; Parsons et al, 2010). Localisation of contractile activity is facilitated by RhoA, which accumulates at the rear of the cell. RhoA recruits its effector rho‐associated protein kinase (ROCK), capable of myosin light chain phosphorylation, to drive actomyosin contraction at stress fibres. Mechanical forces induced by rear‐end contraction are chiefly responsible for disassembly of FAs and, subsequently, retraction of the rear as the cell body translocates (Crowley & Horwitz, 1995; Chrzanowska‐Wodnicka & Burridge, 1996). Calcium‐dependent proteases such as calpains also play an important role in regulation of adhesion dynamics by cleavage of key FA proteins such as FAK and talin (Chan et al, 2010).
Amoeboid migration of MuSCs
Amoeboid migration involves generation of membrane protrusions (Charras & Paluch, 2008). In contrast to mesenchymal migration, amoeboid migration requires minimal cell‐substrate interactions and can occur under conditions of high confinement (Liu et al, 2015). Skeletal muscle injury stimulates activation and proliferation of MuSCs (Yin et al, 2013), which subsequently migrate towards sites of injury above the basal lamina (Siegel et al, 2009). Although precise mechanisms underlying migration of MuSCs in vivo have not been extensively studied, observations of MuSC migration on isolated myofibres indicate employment of an amoeboid mechanism dependent on nitric oxide (NO) and planar cell polarity (PCP) signalling (Otto et al, 2011). Other studies with similar experimental approaches indicated the presence of pseudopods on migrating MuSCs (Siegel et al, 2009). More recently, live intravital imaging of MuSC migration has similarly shown generation of long protrusions upon activation, suggesting that, in vivo, mesenchymal migration is primarily utilised (Baghdadi et al, 2018). However, the possibility that MuSCs could interchange between different modalities of migration in a context‐dependent manner remains, although this will require further investigation.
Mechanisms of skeletal myogenic cell migration during development
During embryonic development, myogenic precursors are required to undergo relatively long‐range migration to give rise to muscles of the developing limbs, tongue and diaphragm (Birchmeier & Brohmann, 2000). Skeletal muscles of the body (trunk and limbs) are derived from the somites: epithelial spheres of compacted paraxial mesoderm which form in pairs alongside the neural tube (Buckingham et al, 2003). The paraxial mesoderm segments into somites sequentially in a rostral‐caudal manner and is specified along the dorsoventral axis to form the epithelial dermomyotome dorsally and the mesenchymal sclerotome ventrally, eventually giving rise to cells of the cartilage, connective tissue, muscle, dermis and endothelial lineages (Thorsteinsdottir et al, 2011). The dorsomedial lip of the dermomyotome then gives rise to the myotome and subsequently to precursor cells which will generate skeletal myoblasts (Tajbakhsh, 2009). A highly orchestrated migratory event occurs when somitic muscle precursors undergo epithelial‐to‐mesenchymal transition, delaminate and migrate to generate the limb buds, diaphragm and tongue anlagen (Hollway & Currie, 2005; Parada et al, 2012; Merrell & Kardon, 2013). These migrating myogenic progenitors are directed by diffusible and cell surface signals, as well as by interactions with the surrounding ECM (Yin et al, 2013). As developmental programmes are partially activated during muscle regeneration, understanding migration and homing of myogenic precursors towards muscle anlagen is critical, as these mechanisms may be recapitulated during regeneration or could be exploited for cell transplantation (Fig 2).
Figure 2.
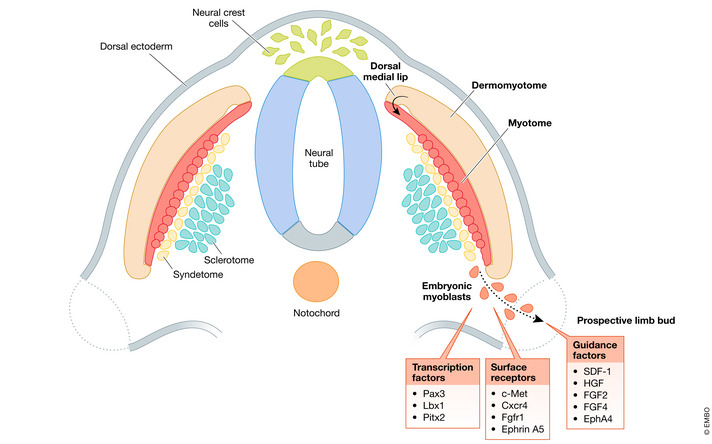
Schematic representation of key transcription factors, surface receptors and guidance factors involved in skeletal myogenic cell migration during development.
Specific transcription factors have been shown to be pivotal for migration of early myogenic precursors, in particular, Ladybird homeobox 1 (Lbx1) and paired box gene 3 (Pax3). Lbx1, a homeobox gene, is expressed in migrating myogenic progenitors and plays a role in directing migratory routes. Lbx1‐null mice demonstrated abnormal limb muscles due to defective migration of muscle precursors (Schäfer & Braun, 1999; Brohmann et al, 2000). However, muscle precursors lacking Lbx1 were still able to give rise to the tongue, diaphragm and some limb muscles, indicating that Lbx1 is necessary for the lateral, but not ventral, migration of embryonic muscle precursors (Gross et al, 2000). Another key player is Pax3, a homeodomain‐containing transcription factor and an early myogenic cell marker necessary for determining cell fate as well as muscle precursor migration (Williams & Ordahl, 1994; Daston et al, 1996; Kassar‐Duchossoy et al, 2005; Relaix et al, 2005). Several studies performed on Splotch mutant mice, carrying a mutation within the homeodomain of Pax3, demonstrated impaired development of limbs due to a loss of the migrating myogenic precursor population, which implies that Pax3 is vital for muscle precursor migration to distal regions of the embryo (Franz et al, 1993; Bober et al, 1994; Goulding et al, 1994; Tajbakhsh et al, 1997). Additionally, Splotch mutants display decreased expression of Lbx1 and c‐Met, which encodes the canonical hepatocyte growth factor receptor. It has been suggested that defects of Lbx1 and c‐Met may be due to direct Pax3‐mediated regulation of c‐Met expression (Epstein et al, 1996; Mennerich et al, 1998). Another homeobox gene, Pitx2, is expressed within prospective limb fields and skeletal muscle anlagen throughout all stages of myogenic development (Shih et al, 2007). Abrogation of Pitx2 expression resulted in anomalies of distal forelimbs. This has been attributed to impaired motility arising from defects in focal adhesions, as numerous regulators of the actin and microtubule cytoskeleton, as well as FA components, displayed changes in expression upon perturbation of Pitx2 (Campbell et al, 2012).
Certain chemokines and their respective receptors are also crucial for homing and maintenance of migratory capacity of muscle progenitors during development. Hepatocyte growth factor (HGF) is present in injured muscles and necessary for migration of myogenic precursors towards developing limb buds (Bladt et al, 1995; Tatsumi et al, 1998; Lee et al, 1999; Dietrich et al, 1999). Homozygous null mouse mutants for c‐Met show abrogation of myogenic precursor migration to limb buds, diaphragm and tongue and are embryonic lethal (Bladt et al, 1995; Amano et al, 2002). Additionally, knock‐out (KO) of GRB‐2‐associated binding protein 1 (Gab‐1), a HGF receptor adaptor protein, results in a similar phenotype with loss of extensor muscles, reduced flexor muscle migration and significant disorganisation of hindlimb muscles (Sachs et al, 2000; Vasyutina et al, 2005). HGF‐c‐Met‐mediated limb precursor migration may involve more than one mechanism. Limb myogenic precursor‐specific KO of B‐Raf, a serine/threonine kinase which acts downstream of c‐Met, has been shown to partially mimic the phenotype of c‐Met‐null mutants. B‐Raf has been suggested to promote migration of myogenic cells by direct phosphorylation of Pax3 at serine 205 (Shin et al, 2016). On the other hand, myogenic migration towards the developing tongue relies more on PI3K signalling and matrix metalloprotease‐9 (MMP9) (Bandow et al, 2004). However, myogenic precursors do not migrate towards ectopic sources of HGF within interlimb regions of the avian embryo, indicating that HGF may not necessarily play a role as a chemoattractant despite its expression along the route of delamination of the prospective limb field and branchial arches (Mennerich et al, 1998; Dietrich et al, 1999; Birchmeier & Brohmann, 2000). The stromal‐derived factor‐1 (SDF‐1) receptor, CXCR4, is also essential for myogenic progenitor migration, as CXCR4 −/− mice display lower numbers of muscle precursors migrating towards prospective limb buds (Vasyutina et al, 2005). However, loss of CXCR4 alone did not affect development of the tongue (Huang et al, 1999). In CXCR4− / − / Gab‐1− / − double KO mutant mice, in addition to a reduced number of migrating limb precursors, a small fragment of the tongue anlagen was present. Ectopic application of SDF‐1 was sufficient to direct migration of CXCR4 +/Pax3+ cells but endogenous migration patterns of myogenic precursors do not completely correlate with SDF‐1 expression patterns, suggesting that alternative factors are involved in the specification of migratory routes (Vasyutina et al, 2005). Fibroblast growth factors (FGFs) are also crucial in directing limb myogenic precursors. Avian embryonic myoblasts transfected with dominant‐negative isoforms of FGF receptor 1 (FGFR1) were unable to migrate towards the prospective limb (Itoh et al, 1996), whereas FGF2 (also known as basic FGF) and 4 have been shown to induce chemokinesis and chemotaxis of mouse embryonic myoblasts (Webb et al, 1997). Interestingly, novel microfluidic tools have revealed that within primary human myoblasts, chemokinesis, rather than chemotaxis, appears to be the main effect exerted by FGF2 (Ferreira et al, 2015). A candidate for further specification of migratory routes is ephrin A5 and its receptor EphA4. EphA4 is expressed within the Pax7+ population of lateral dermomyotome and ephrin A5 within the ventral dermomyotome. Ectopic application of ephrin A5 led to a reduction of migration towards the proximal limb bud as well as aberrant accumulation of muscle progenitors within the lateral dermomyotome indicating a role for Ephs and ephrins in migration during limb bud formation (Swartz et al, 2001; Stark et al, 2011).
Post‐natal migration of myogenic progenitors
The regenerative capacity of adult skeletal muscle can be observed in both acute injuries and (to some extent) in chronic myopathies (Hardy et al, 2016). The endogenous regenerative response is mediated primarily by MuSCs (Relaix & Zammit, 2012). Upon injury, MuSCs are activated and undergo a process of asymmetric division to generate committed progenitors and self‐renewing stem cells (reviewed in (Tedesco et al, 2010)). Upon activation in post‐natal skeletal muscles, myoblasts emerge above the basal lamina to migrate towards regions in which they are required to differentiate and fuse with damaged myofibres or with other differentiating myoblasts to generate new fibres. Myoblast migration requires precise modulation of the cytoskeleton, with signalling pathways such as PI3K/Akt and MAPK/ERK pathways chiefly responsible for regulating migration in vitro (Kowalski et al, 2017; González et al, 2017). Additionally, FA regulation is involved in muscle regeneration and disease (Bricceno et al, 2014). Several approaches have been explored to enhance the migratory capacity of myogenic cells upon intramuscular transplantation, most of which involve activation of specific signalling pathways and modulation of cell–ECM interactions.
Signalling pathways in adult myoblast migration
SDF‐1 drives an important signalling pathway in adult myoblast migration. Treating myoblasts with SDF‐1 leads to upregulation of the Rho GTPases CDC42 and Rac‐1, followed by formation of stress fibres and filopodia, whereas silencing of its receptor Cxcr4, but not Cxcr7, did not increase Rho GTPase expression or cell migration, suggesting direct actin regulation by the SDF‐1/Cxcr4 axis (Kowalski et al, 2017). Furthermore, SDF‐1 was shown to regulate the expression of several migration‐associated transcripts, including MMP9, α‐actinin and CAPSN1 (Kowalski et al, 2017).
Many other studies assessing migration of myogenic progenitors indicate that promotion of motility occurs frequently via upregulation of MAPK/ERK and PI3K/Akt signalling pathways. HGF‐associated migration is stunted when cells are treated with a MAPK inhibitor, and lamellipodia formation, a crucial step for motility on a monolayer, is abrogated upon PI3K inhibition in C2C12 myoblasts (Kawamura et al, 2004; Ishido & Kasuga, 2011; González et al, 2017). This decrease in migration is associated with downstream disruption of CDC42‐ and Rac‐1‐mediated actin polymerisation via N‐WASP and WAVE2, as LY294002 treatment also disrupts N‐WASP/WAVE2 localisation at the leading edge of lamellipodia (Kawamura et al, 2004; Takenawa & Suetsugu, 2007). Moreover, bisperoxovanadium (BpV), an inhibitor of phosphatase and tensin homolog (PTEN) which negatively regulates PI3K signalling, enhanced myoblast migration (Dimchev et al, 2013), whereas pharmacological inhibition of PI3K and MEK reduced myoblast migration (Al‐Shanti et al, 2011). Platelet lysates have also shown to enhance motility of C2C12 myoblasts, likely mediated by MAPK and PI3K signalling (Ranzato et al, 2009).
Although PI3K/Akt and MAPK/ERK signalling pathways are key mediators of myoblast migration, alternative pro‐migratory signalling pathways also exist. C2C12 myoblasts subject to dominant‐negative Ras‐related protein Ral‐A (dnRalA), an alternative pathway to MAPK/ERK downstream of Ras, showed significantly reduced chemotaxis induced by bFGF, IGF‐1 and HGF (Suzuki et al, 2000). Additionally, myogenic progenitors derived from FGF6− / − mice displayed decreased migration upon intramuscular injection, similarly to myoblasts expressing dominant‐negative forms of Ras and Ral (Neuhaus et al, 2003).
The TGF‐β superfamily represents another important signalling pathway involved in myoblast migration. Although canonical TGF‐β signalling involves the Smad signalling pathway, myogenic progenitor migration driven by members of the TGF‐β superfamily likely occurs in a non‐canonical manner, via MAPK/ERK or PI3K/Akt signalling. KO of Smad4, using Myf5‐Cre;Smad4flox/flox transgenic mice specifically targeting Smad4 in myogenic progenitors, has no effect on cell migration of myogenic progenitors during tongue morphogenesis (Massagué, 2012; Han et al, 2012). TGF‐β1‐mediated migration of myoblasts has been shown to occur via activation of the MAPK/ERK pathway which facilitates retraction of the trailing end of the cell via upregulation of CAPN2, the catalytic subunit of the ubiquitous calcium‐dependent protease m‐calpain, capable of disassembling FAs by proteolysis of FAK and talin (Franco et al, 2004; Leloup et al, 2007; Chan et al, 2010). Furthermore, bone morphogenetic protein 2 (BMP2), which canonically acts via Smad signalling, has also been suggested to act in a non‐canonical manner. BMP2 regulates cortical actin remodelling at the leading edge via PI3K‐mediated activation of PH‐like domain family B member 2 (LL5β), which recruits the actin crosslinker filamin and, subsequently, promotes cell protrusion (Takabayashi et al, 2010; Hiepen et al, 2014).
Although significant progress has been made in identifying the aforementioned pathways, mechanisms by which these pathways are coordinated in a spatiotemporal manner to sequentially regulate stages of directional migration of myogenic progenitors require further investigation. Additionally, different signalling pathways, such as those driven by Wnt and nitric oxide (NO), may play context‐dependent roles in alternative modes of migration. One such important external determinant of myogenic migration is the influence of the ECM which is discussed in the following section.
Influence of the extracellular environment on muscle stem/progenitor cell migration
Interactions between cell surface receptors, primarily integrins, with components of the ECM are crucial to generate traction forces for directional propulsion. Furthermore, timely disassembly and turnover of adhesion complexes are a prerequisite for maximal migration velocity, with dysfunctional FA activity implicated in impaired regeneration and disease. As the ECM is a dynamic entity with a tissue‐specific composition that has been suggested to be altered with age and pathology, changes in ECM composition or stiffness in myopathies may negatively affect migratory capacity of myogenic cells.
Turnover of FAs in muscle regeneration and pathology
Modulation of FA proteins affects myogenic cell migration. Tensins are FA‐associated proteins capable of binding cytoplasmic tails of integrins in addition to tyrosine‐phosphorylated proteins by their Src homology 2 (SH2) domains (Chen & Lo, 2003; Lo, 2004). Tensin‐1‐null mice display delayed skeletal muscle regeneration and inhibition of tensin‐3 reduces migration of MuSCs (Ishii & Lo, 2001; Chen & Lo, 2003; Baghdadi et al, 2018). Additionally, overexpression of microRNA‐708 recapitulates the tensin‐3 inhibitory phenotype, with reduction of phosphorylated FA kinase (p‐FAK), suggesting a role for microRNAs in regulating migration by acting on FAs (Baghdadi et al, 2018). Conversely, upregulation of FAK and paxillin, as well as increased proportions of p‐FAK and p‐paxillin, has been suggested to enhance migration. Swine myogenic progenitors with high p‐FAK and p‐paxillin have faster wound closure rates (Wang et al, 2016). Similarly, overexpression of platelet and endothelial aggregation receptor‐1 (PEAR‐1; involved in aggregation of platelets and neoangiogenesis) in MuSCs increases p‐FAK, p‐paxillin and vinculin expression, via upregulation and interaction with integrin β1 (Vandenbriele et al, 2015; Pang et al, 2019). Platelet‐rich plasma was also shown to increase spreading and migration of muscle progenitors by upregulation of FAK and paxillin, whereas deprivation of lysine, an essential amino acid for protein synthesis, resulted in decreased p‐FAK, p‐paxillin as well as decreased cell migration (Tsai et al, 2017; Jin et al, 2019).
Spinal muscular atrophy (SMA), caused by a deficiency of the survival motor neuron‐1 (SMN‐1) protein, is a severe neuromuscular disorder characterised by muscle atrophy and loss of motor function (D'Amico et al, 2011); interestingly, SMN‐1‐deficient myoblasts display reduced motility. This has been attributed to abnormal turnover of FAs, as FA‐associated proteins vinculin, talin‐1 and talin‐2 persist for extended periods of time resulting in prolonged adhesion and, subsequently, in a reduction of motility (Bricceno et al, 2014). The downstream effector of Rho GTPase, ROCK‐2, may also be necessary for maturation of FAs as ROCK‐2 pharmacological inhibition resulted in an increased number of vinculin‐positive FAs in myoblasts, which correlated with an increase in migratory velocity (Goetsch et al, 2014). Muscle biopsies from SMA patients do not show signs of regeneration, and although this is likely to be caused by several other reasons, the aforementioned observations suggest that reduced regeneration may be partially attributed to decreased migratory capacity of endogenous muscle progenitors as a result of abnormal FA activity.
Fibrotic microenvironment and muscle cell migration
Various pathological conditions, including muscular dystrophies and age‐related sarcopenia, are associated with fibrosis: an accumulation of ECM which reduces or impedes tissue function and regeneration (Gillies & Lieber, 2011). In muscular dystrophies, endomysial fibrosis is associated with poor motor control, possibly due to alterations in the load‐bearing, biomechanical role of the ECM (Desguerre et al, 2009; Gillies & Lieber, 2011). Fibrotic scar tissue has been postulated to be a limiting factor for the migratory capacity of transplanted cells, as reduction of fibrosis in these tissues via MMP‐1,‐2 or ‐9, has shown to be effective at improving cell migration and engraftment (Gargioli et al, 2008; Morgan et al, 2010; Pan et al, 2015).
An alternative method to reduce fibrotic tissue is by targeting pro‐fibrogenic cytokines, which are released as a result of chronic inflammation in response to recurrent muscle degeneration (Zhou & Lu, 2010). Targeting pro‐fibrogenic signalling pathways in fibroblasts has been shown to reduce skeletal and cardiac muscle fibrosis by inhibition of TGF‐β, which induces fibroblast‐mediated fibrogenesis (Bernasconi et al, 1995; McGaha et al, 2002; Li et al, 2004; Andreetta et al, 2006; Cohn et al, 2007; Taniguti et al, 2011). Furthermore, scid/mdx mice, which lack T and B lymphocytes, have been reported to have reduced TGF‐β1 activity and muscle fibrosis (Farini et al, 2007). These treatments, however, have not been systematically integrated into cell transplantation protocols; future preclinical and clinical studies should therefore focus also on reducing the pre‐existing fibrotic scars to enhance engraftment of intramuscularly or systemically delivered cells.
Modulating migration: intramuscular delivery
Understanding the migratory properties of myogenic progenitors has significant implications for muscle cell therapies, as lack of migration post‐transplantation remains a key issue that limits therapeutic efficacy. Several approaches have been explored to overcome the limited migratory capacity of transplanted myogenic cells, with varying success. The following section will discuss progress and future implications of these findings.
Pretreatment or co‐injection of myoblasts with chemokines
An approach to improve the migratory capacity of myogenic cells is by treating them with factors that stimulate activation and migration during development or regeneration. Extracts from crushed muscles have been shown to activate quiescent MuSCs and to stimulate myoblast migration (Bischoff, 1986, 1990; Allen et al, 1995; Corti et al, 2001). In vitro studies performed using growth factor treatment in transwell or wound healing assays identified several factors able to improve migration of myoblasts (Table 1 ). Studies attempting to understand underlying mechanisms driven by these factors may be useful for discovery of conserved pathways or mechanisms that could be targeted and modified for application in cell therapy protocols.
Table 1.
Examples of chemokines and their impact on myoblast migratory capacity in vitro.
| Chemokine | Concentration | ‐fold increase in migration | Cell type | Assay | ECM component | Reference |
|---|---|---|---|---|---|---|
| HGF | 10 ng/ml | 5.43‐; 5‐ | Primary myoblasts (rat; human) | Transwell | Fibronectin | Bischoff (1997), González et al (2017) |
| SDF‐1 | 10 ng/µl | 2.5‐ | Primary myoblasts (mouse) | Wound healing | Uncoated | Kowalski et al (2017) |
| TGF‐β | 5 ng/ml; 20 ng/ml | 4.42‐; 0.73‐ | Primary myoblasts (rat); C2C12 myoblasts | Transwell; wound healing | Fibronectin; uncoated | Bischoff (1997), Leloup et al (2006) |
| IGF‐1 | 40 ng/ml; 100 ng/ml | 0.66‐; 3.4‐ | C2C12 myoblasts; primary myoblast (mouse) | Wound healing; transwell | Uncoated | Leloup et al (2006), Yanagiuchi et al (2009) |
| Insulin | 15 µg/ml | 0.97‐ | C2C12 myoblasts | Wound healing | Uncoated | Leloup et al (2006) |
| FGF‐2 | 1 ng/ml; 10 ng/ml; 10 ngml; 100 ng/ml; 3.8–7.0 ng/ml | N/A; 7.8‐; 6.4‐ ; 3.4‐; N/A | Primary myoblasts (rat; mouse‐embryonic; mouse; mouse; human) | Transwell; Blind well chemotaxis chamber; Chemotaxis chamber; Chemotaxis chamber; Microfluidics device | Fibronectin; Uncoated; Fibronectin; Uncoated; Uncoated; | Bischoff (1997), Webb et al (1997), Neuhaus et al (2003), Yanagiuchi et al (2009), Ferreira et al (2015) |
| FGF‐4 | 10 ng/ml | 6.7‐ | Embryonic myoblasts (mouse) | Blind well chemotaxis chamber | Uncoated | Webb et al (1997) |
| FGF6 | 10 ng/ml | ~5‐ | Primary myoblasts (mouse) | Chemotaxis chamber | Fibronectin | Neuhaus et al (2003) |
| PDGF‐BB | 50 ng/ml | 3.3‐ | Primary myoblasts (human) | Transwell | Uncoated | Piñol‐Jurado et al (2017) |
| 5% Chick embryo extract | N/A | 6.7‐ | Primary myoblasts (rat) | Transwell | Fibronectin | Bischoff (1997) |
In cases where multiple concentrations were assessed, the concentration that facilitated the greatest fold change of migration was taken.
Several factors displayed in Table 1, including HGF and SDF‐1, have been tested in vivo by either treating donor cells prior to administration or by co‐injection. HGF injection into the soleus muscle of mice was sufficient to increase MuSC migration velocity (Ishido & Kasuga, 2011), whereas injection of Sdf‐1‐treated myoblasts into Sdf‐1‐treated, injured gastrocnemius muscles led to an increase in donor‐derived fibres in comparison to controls (Kowalski et al, 2017).
Modulation of MMPs to facilitate cell migration within skeletal muscles
Extensive fibrosis of skeletal muscles negatively impacts on cell transplantation efficacy, as cells are required to travel through dense connective meshwork for effective dispersion. Similar to the concept of pretreatment irradiation in haematopoietic stem cell transplantation, which “clears space” for donor cells, MMPs may be able to play an analogous role by digesting fibrotic tissues.
MMPs have been previously identified to play a significant role for enhancement of myogenic progenitor migration. Pharmacological inhibition of MMP activity decreased myoblast migration in vivo (El Fahime et al, 2000). The role of specific MMPs in myogenic progenitors has also been studied. MMP13, an interstitial collagenase, displays increased expression in skeletal muscle of mdx mice as well as in muscles acutely injured with cardiotoxin. Overexpression of MMP‐13 in C2C12 myoblasts enhanced migration, which was abrogated upon MMP‐13 inhibition (Lei et al, 2013). Pretreatment of host muscles with collagenase and MMP‐7, a matrilysin capable of degrading collagen IV, laminin‐1 and fibronectin, was shown to increase cell engraftment in host muscles (Torrente et al, 2000). Furthermore, MMP‐14 has been demonstrated to increase invasive capacity of human but not murine myoblasts, suggesting disparities in the relative importance of MMPs between species (Lund et al, 2014). Enhanced growth factor‐mediated migration and motility may also be attributed to increased expression and activity of MMPs. The most likely candidates for this role are MMP‐1, ‐2 and ‐9, which are upregulated by IGF‐1, bFGF and TNF‐α (Allen et al, 2003; Lafreniere et al, 2004). Intramuscular transplantation of C2C12 myoblasts in scid/mdx mice with concomitant MMP‐1 administration resulted in a 3‐fold increase in cell engraftment (Wang et al, 2009). Additionally, a gene therapy approach to induce MMP‐1 expression in C2C12 myoblasts enhanced engraftment in scid/mdx mice (Pan et al, 2015).
Although MMP‐2 and ‐9 are generally upregulated upon growth factor treatment, MMP‐9 upregulation may be more efficacious in combinatorial therapies (Yanagiuchi et al, 2009). Tendon fibroblasts expressing MMP‐9 and the angiogenic factor placenta growth factor (PIGF) were able to restore a microvascular network and reduce fibrosis, enhancing cell therapy efficiency in aged dystrophic mice (Gargioli et al, 2008). Similarly, C2C12 myoblasts overexpressing MMP‐9 had superior migratory capacity over C2C12 cells overexpressing MMP‐2 (Morgan et al, 2010). Overall, these studies indicate that MMP‐9 expression is advantageous for remodelling of fibrotic tissues found in dystrophic muscles into an environment more favourable for cellular motility.
Bioscaffolds for intramuscular cell delivery
Biomaterials have been used to improve dispersion and viability of intramuscularly delivered cells. The advantage of biosynthetic scaffolds is that biophysical and biochemical parameters can be adjusted to modulate cell behaviour (Cezar & Mooney, 2015). Bioscaffolds can also be functionalised with pro‐survival or pro‐migratory factors for myoblasts. Implantation of alginate (a biocompatible polysaccharide) seeded with myoblasts, HGF and bFGF into injured mouse tibialis anterior muscles led to an increase in myoblast migration and muscle mass (Hill et al, 2006).
A more practical approach may be to substitute the saline solution in traditional intramuscular injection protocols with hydrogels to improve dispersion and viability of donor cells. A recent study performed by replacing saline with a hydrogel comprised of hyaluronan and methylcellulose resulted in increased donor cell dispersion (Davoudi et al, 2018). The increase in engraftment area could also be attributed to the bioactivity of hyaluronan, which has been shown to inhibit myogenic differentiation (Elson & Ingwall, 1980): delays in differentiation may facilitate proliferation and migration/dispersion of donor cells prior to fusion or differentiation. Similarly, a semisynthetic polyethylene glycol and fibrinogen hydrogel increased engraftment of intramuscularly transplanted mesoangioblasts in acute and chronic muscle injury mouse models (Fuoco et al, 2012). Therefore, cell–matrix interactions can be exploited to generate an environment advantageous for enhanced cell engraftment.
Trans‐endothelial migration of myogenic progenitors: state‐of‐the‐art and lessons from “professional” trans migrating cells
Viability of systemic delivery rests on the ability of injected cells to migrate across blood vessel walls while maintaining the capacity to disperse through complex ECM to reach damaged myofibres. Although mesoangioblasts and CD133+ cells can be systemically delivered and reach skeletal muscles (reviewed in (Benedetti et al, 2013)), precise molecular mechanisms underlying their extravasation process have not yet been established. Greater understanding of mechanisms dictating transmigration in cell types in which extravasation occurs normally (e.g. leucocytes) or pathologically (e.g. metastatic cancer cells) could facilitate development of efficient protocols for the intra‐vascular delivery of myogenic cells.
Leucocyte diapedesis
Leucocyte extravasation out of the blood vasculature into target tissues can be summarised into 4 major steps involving different surface protein interactions between leucocytes and endothelium: rolling adhesion, firm adhesion, crawling and diapedesis (Fig 3). As some myogenic progenitors may utilise a similar mechanism when delivered intra‐vascularly (Giannotta et al, 2014), targeting components that regulate leucocyte extravasation could improve transmigration of systemically deliverable myogenic cells.
Figure 3.
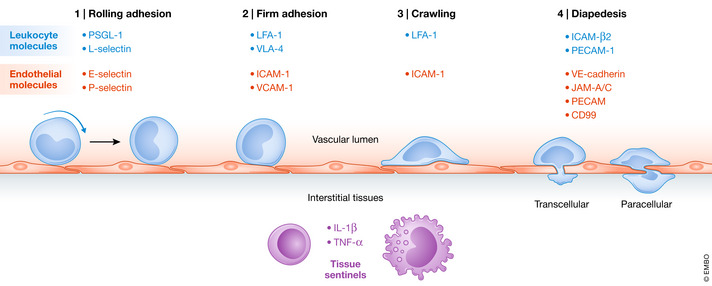
Schematic representation of the sequential events that occur during leucocyte diapedesis with key surface molecules involved at each step.
Rolling adhesion requires formation of transient bonds between leucocytes and endothelial cells. Adhesive molecules such as endothelial‐selectin (E‐selectin) and platelet‐selectin (P‐selectin) on endothelium are necessary for interaction with leucocyte surface proteins p‐selectin glycoprotein ligand‐1 (PSGL‐1) and leucocyte‐selectin (L‐selectin) (Kunkel & Ley, 1996; Hickey et al, 1999; Stein et al, 1999; Zarbock et al, 2008). Firm adhesion between leucocytes and endothelial cells requires activation of high affinity integrins on leucocytes. Subsequent generation of dense clusters of intercellular cell adhesion molecule‐1 (ICAM‐1), vascular cell adhesion molecule‐1 (VCAM‐1), lymphocyte function associated‐1 (LFA‐1), very late antigen‐4 (VLA‐4) and galectin‐3 at the leucocyte–endothelium surface, also called focal contacts, enables near‐complete arrest of leucocytes (Dustin & Springer, 1988; Elices et al, 1990; Nieminen et al, 2008; Muller, 2013). Following arrest, changes in leucocyte morphology occur, involving polarisation and formation of protrusions which promote “crawling” along the surface of endothelial cells (Schnoor et al, 2015). Once an exit signal is detected, migration through the endothelial barrier takes place. This can follow either the paracellular or the transcellular route: most transmigration events occur via the paracellular route, through endothelial cell–cell junctions, and require sequential activation of several adhesion proteins. Firstly, ICAM‐β2 integrin interactions are necessary for transient downregulation of vascular endothelial cadherin (VE‐cadherin). This process is pivotal to loosen adherens junctions (Filippi, 2016). Subsequently, sequential interactions of leucocytes with junction adhesion molecule‐A/C (JAM‐A/C), platelet endothelial cell adhesion molecule (PECAM) and CD99 mediate translocation through the intercellular gap (Schenkel et al, 2002; Woodfin et al, 2009). Alternatively, leucocytes can migrate through the cell body of endothelial cells via transcellular pores, although this is mostly found in regions which necessitate strict regulation of permeability, such as the blood–brain barrier (Lossinsky et al, 1989; Wolburg et al, 2005). Finally, entry into peripheral tissues requires migration through associated pericyte and perivascular basement membrane layers (Voisin & Nourshargh, 2013).
Metastatic cancer cells, angiopellosis and other unorthodox extravasation strategies
Pathological extravasation: cancer metastasis
Cancer metastasis involves formation of new tumours within tissues and organs distal to the primary mass. This process usually requires cancer cells to intravasate into proximal lymph or blood vessels, circulate, extravasate and, subsequently, proliferate to give rise to secondary tumours (Valastyan & Weinberg, 2011). The efficiency of cancer cell extravasation is a key factor that determines metastatic potential (Leong et al, 2014). Extravasation of cancer cells involves the same major steps as leucocytes but displays disparities at the molecular level. Additionally, a key difference between cancer cell and leucocyte extravasation is that cancer extravasation results in disruption of vascular integrity, whereas leucocyte extravasation induces transient, reversible modifications of the endothelium (Strell & Entschladen, 2008). Studying molecules involved in cancer metastasis may reveal general conserved mechanisms necessary for trans‐endothelial migration of non‐leucocyte cells, thus exposing key molecules that may be modulated to enhance the efficiency of myogenic cell trans‐endothelial migration.
Rolling of tumour cells has been shown to require several ligands of E‐ and P‐selectins. Metastases within bone, lymph node and the brain derived from human prostate tumours display increased PSGL‐1 expression, involved in leucocyte rolling (Dimitroff et al, 2005). Within PSGL‐1‐negative breast carcinoma cells, CD24 was shown to be responsible for rolling, suggesting the existence of alternative mechanisms for tumour cell rolling (Aigner et al, 1998). Isoforms of CD44 may also play crucial roles in cancer cell–endothelium interactions. Knockdown of CD44 glycoform haematopoietic cell E‐/L‐selectin ligand (HCELL) in colon carcinoma cells resulted in reduced binding to HUVECs and increased rolling speed (Burdick et al, 2006). CD44 variant (CD44v, a CD44 isoform) expression in colon carcinoma cell lines reduced rolling velocity and increased binding to P‐selectin (Napier et al, 2007). Upon knockdown of CD44, the glycoprotein carcinoembryonic antigen (CEA) acts as a compensatory mechanism to mediate colon cancer cell interactions with E‐selectin (Thomas et al, 2008). This suggests that although the process of rolling is conserved between tumour cells and leucocytes, disparities exist at the molecular level.
Firm adhesion of cancer cells to the vascular endothelium can occur in a leucocyte‐dependent or ‐independent manner. Tumour cells are capable of recruiting and binding to leucocytes, which subsequently interact with the endothelial cell layer as a proxy (Strell et al, 2007; Liang et al, 2007). Interestingly, it is currently unknown whether donor myogenic cells are able to interact with leucocytes intravascularly. Tumour cell firm adhesion also takes place independently of leucocytes. Similarly to leucocytes, VLA‐4 acts as the primary VCAM‐1 ligand during tumour cell extravasation. VLA‐4 was shown to be essential for adherence of melanoma cells to VCAM‐1‐expressing endothelial cells, with clones of melanoma cells expressing VLA‐4 displaying increased metastases in IL‐1‐treated mice (Garofalo et al, 1995). Additionally, treatment of mammary carcinoma cell lines, possessing high tropism for the brain, with anti‐VLA‐4 antibodies reduced incidence of tumour seeding (Soto et al, 2014). Firm adhesion of breast and prostate cancer cells has been shown to involve binding with galectin‐3 on the endothelial surface (Glinsky et al, 2000, 2003; Khaldoyanidi et al, 2003).
Several molecules involved in cancer cell extravasation and leucocyte extravasation are conserved, but there are also alternative mechanisms of tumour cell extravasation. Transcriptional profiles of circulating tumour cells from 5 different cancer types revealed conserved upregulation of PECAM‐1, JAM3 (JAM‐C) and F11R (JAM‐A), which are critical for leucocyte diapedesis (Yadavalli et al, 2017). Additionally, melanoma metastases are reduced in mice with an endothelial cell‐specific KO of JAM3 (Langer et al, 2011). Treatment of hepatoma and colon cancer cells with anti‐CXCR4 antibodies had no effect on adhesion but abrogated extravasation and, conversely, pretreatment of these cells with SDF‐1 enhanced extravasation (Gassmann et al, 2009). Homophilic CD146 interactions have also been shown to mediate melanoma extravasation, which is decreased upon cell delivery in CD146 KO mice (Jouve et al, 2015).
Cancer cell extravasation also involves vasculature modulation. Secretion of c‐terminal fibrinogen‐like domain of angiopoietin‐like 4 (cANGPTL4) by carcinomas and melanomas induces vascular leakiness by interacting with α5β1 integrin, VE‐cadherin and claudin‐5 (Huang et al, 2011). Similarly, the glycoprotein osteonectin interacts with VCAM‐1 and facilitates increased vascular permeability, resulting in enhanced melanoma cell extravasation (Tichet et al, 2015). Taken, overall this section highlights molecules and mechanisms that might be positively exploited to increase extravasation efficiency of systemically deliverable myogenic cells, although potential detrimental effects on vascular integrity and off‐target extravasation (i.e. into non‐skeletal muscle tissues) will need to be carefully assessed in future studies.
Angiopellosis
Recently, an alternative mechanism to diapedesis termed angiopellosis has been proposed for cells that are not native to the blood circulation, including tumour cells (Allen et al, 2019). Angiopellosis displays molecular and temporal disparities in comparison to canonical leucocyte extravasation and involves formation of endothelial protrusions which sequester and transport cells into the surrounding parenchyma (Cheng et al, 2012; Allen et al, 2017). In contrast to leucocyte diapedesis, angiopellosis allows for extravasation of multiple cells during a single event. Knockdown of CD11α, the α‐integrin subunit of LFA‐1 implicated in multiple stages of leucocyte extravasation, abolished diapedesis of leucocytes but not of mesenchymal stromal cells, indicating a difference of surface molecules involved in this process (Allen et al, 2017). Overall, angiopellosis appears relevant in the context of muscle cell therapies, as myogenic cells are by definition not native to the circulation. More research on this process, alongside cross‐validation with additional independent studies, will be necessary to exploit this modality of cell migration for the intravascular delivery of myogenic cells.
Modulating migration: systemic delivery
Treating myogenic cells with growth factors, inflammatory chemokines or other small molecules
Cells delivered intra‐arterially must perform two major tasks: (1) transmigration across the endothelial barrier of injured muscle tissues and (2) migration towards regions in which they are required. During embryonic myogenesis, myoblasts in close proximity to endothelial cells undergo a fate transition into pericyte‐like cells (Cappellari et al, 2013). This phenomenon can be mimicked in vitro by combined application of platelet‐derived growth factor‐BB (PDGF‐BB) (also expressed by regenerating and necrotic myofibres (Piñol‐Jurado et al, 2017)) and delta‐like 4 (DLL4; a Notch signalling ligand), inducing pericyte‐like features in both mouse and human myoblasts (Gerli et al, 2019). Importantly, treated MuSC‐derived myoblasts displayed enhanced endothelial transmigration capacity in vitro and in vivo (Gerli et al, 2019). Of note, a recent study also showed that PDGF‐BB promotes migration of various types of muscle interstitial cells via interaction with its putative receptor, platelet‐derived growth factor receptor‐β (PDGFR‐β) (Camps et al, 2019). As this phenomenon occurs during embryonic development (Cappellari et al, 2013), translating this approach to iPSC‐derived myogenic progenitors may result in greater cell transmigration than what observed with adult myoblasts, given the embryonic‐/foetal‐like identity of most currently available skeletal myogenic iPSC derivatives (Xi et al, 2020).
Cells delivered intra‐arterially require appropriate exit signals that regulate extravasation. It is therefore not surprising that pro‐inflammatory cytokines secreted by resident cells have previously shown to promote endothelial transmigration. Tumour necrosis factor‐α (TNF‐α), a cytokine secreted by macrophages, natural killer (NK) cells and lymphocytes, enhanced mesoangioblast migration in vitro and in vivo. Furthermore, highly mobility group box‐1 (HMGB‐1), a pro‐inflammatory cytokine released by necrotic cells or secreted by immune cells, has been shown to promote extravasation and homing of mesoangioblasts (Palumbo et al, 2004; Lotze & Tracey, 2005); a similar effect has been reported with nitric oxide (Sciorati et al, 2006). Interestingly, other pro‐inflammatory cytokines such as IL‐1, IL‐6 and IL‐10, had no significant effect on donor cell migration (Galvez et al, 2006); similarly, lipopolysaccharide (LPS)‐induced inflammation did not stimulate mesoangioblast homing or migration despite upregulation of IL‐1α, IL‐1β and IL‐6 in mouse and rat skeletal muscle (Frost et al, 2002; Lang et al, 2003; Palumbo et al, 2004). Therefore, specific growth factors, chemokines and other ligands can induce pro‐migratory properties to different classes of myogenic cells; translating these protocols to human myogenic cells will be a key step towards their preclinical validation.
Modification of cell–endothelial interactions to promote transmigration
Targeting proteins that dictate donor cell–endothelium interactions during extravasation is another promising strategy to promote cell engraftment in dystrophic muscles. Although surface proteins of candidate myogenic cell types differ from those of leucocytes, expression of key molecules involved in leucocyte diapedesis on myogenic cells has been shown to enhance their migration capacity (Tagliafico et al, 2004; Galvez et al, 2006).
Another strategy for increasing efficiency of transmigration is to target molecules that facilitate sequential migration through paracellular endothelial junctions. KO or inhibition of JAM‐A in mouse models of acute or chronic muscle injury significantly improved engraftment of intra‐arterially delivered mesoangioblasts (Giannotta et al, 2014). Downregulation of PW1, a direct transcriptional repressor of JAM‐A, inhibited transmigration of adult mouse mesoangioblasts when delivered via femoral arteries of scid/mdx mice preventing amelioration of the dystrophic phenotype (Bonfanti et al, 2015). Notably, a similar strategy in PECAM‐1‐null mice did not increase cell engraftment, indicating that not all endothelial junction molecules can be targeted to improve cell extravasation (Giannotta et al, 2014).
Future perspectives
Strategies to enhance migration and motility of myogenic progenitors have been investigated in previous studies; however, how these factors regulate the cellular cytoskeleton as downstream output is less studied and may be important to identify key processes or molecular components that may be perturbed in disease or augmented in cell therapies. Additionally, it may be advantageous for future studies to focus on migratory behaviours of myogenic cell types within 3D environments that recapitulate skeletal muscle architecture with higher fidelity with regards to ECM composition, stiffness and multicellular complexity (e.g. Bersini et al, 2018; Maffioletti et al, 2018). This could be achieved by observing migratory behaviour and cytoskeletal activity in synthetic 3D hydrogels that mimic interstitial ECM or by using decellularised matrices retaining the intrinsic architectural integrity of skeletal muscle tissue (Hughes et al, 2010; Webster et al, 2016; Yamada & Sixt, 2019). Furthermore, intravital imaging may reveal previously unidentified mechanisms or requirements for optimal migratory capacity of muscle stem cells upon intramuscular or intravascular delivery (Paul et al, 2015; Yan et al, 2019).
Identifying key genetic programmes and molecular machineries involved in myogenic cell migration could be instrumental to derive or engineer highly migratory cell populations for efficacious muscle cell therapies. For this purpose, several avenues could be explored:
Systematic assessment of the migratory properties of interstitial skeletal muscle cells may reveal distinct populations with migration capacity (Tedesco et al, 2017). Subsequently, pro‐migratory signals can be modulated to optimise migration and differentiation capacity for maximal engraftment in cell therapies (Gerli et al, 2019). Engineering myogenic derivatives from human iPSCs will become increasingly relevant as modifications of current transgene‐based (e.g. (Tedesco et al, 2012; Darabi et al, 2012)) or transgene‐free protocols (e.g. (Chal et al, 2016; Hicks et al, 2018)) could generate innovative advanced therapy medicinal products (ATMPs) with controllable proliferation, migration and differentiation capacity.
Phenotypic disparities between young and aged myogenic cell types have been investigated (Collins‐Hooper et al, 2012; Brown et al, 2017; Rotini et al, 2018). In addition to regenerative capacity, migration has been suggested to be altered with ageing: studying and understanding migration dynamics in young vs aged myogenic cells may highlight specific pathways to modulate to enhance migration of ATMPs.
Lastly, omics‐based comparative studies of myogenic cell populations treated with pro‐migratory factors or small molecules may unravel druggable pathways which could be further modulated to enhance cell motility. For intravascular delivery, similar studies focusing on cells with highly efficient transmigration capacity in health (e.g. leucocytes) or disease (e.g. metastatic cells) could provide insights on strategies to be deployed in next‐generation skeletal muscle cell therapies.
Author contributions
SWC wrote the manuscript's draft with supervision of GF and FST. FST coordinated the work, contributed to the draft, reviewed and finalised the manuscript and acquired funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Pending Issues.
Elucidating druggable pathways in human myogenic progenitors which can be targeted across different cell populations to enhance their migration without compromising safety, self‐renewal and differentiation potential.
Development of new models and screening platforms to assess migration potential of ATMPs for muscle diseases upon intravascular delivery (e.g. (Ferreira et al, 2015)).
Development of GMP‐GLP‐defined protocols to enhance migration human transplantable myogenic progenitors, particularly from iPSCs.
For more information.
Authors' website: www.tedescolab.org (Twitter: @lab_tedesco)
Cell migration resources & seminars: https://cellmigration.wixsite.com/seminars (Twitter: @CellMigration)
Muscle biology resources and seminars: @musclescitalks (Twitter)
-
Patients associations (examples):
Acknowledgements
We thank all members of the Tedesco lab for helpful discussions. This work was funded by the European Research Council (7591108‐HISTOID), Muscular Dystrophy UK (RA4/ 3023/1; 17GRO‐PS48‐0093–1; 19GRO‐PS48‐0188‐1), Duchenne Parent Project, AFM‐Telethon and the UK BBSRC, MRC and National Institute for Health Research (NIHR); the views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
EMBO Mol Med (2020) 12: e12357.
See the Glossary for abbreviations used in this article.
References
- Aigner S, Ramos CL, Hafezi‐Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K (1998) CD24 mediates rolling of breast carcinoma cells on P‐selectin. FASEB J 12: 1241–1251 [DOI] [PubMed] [Google Scholar]
- Al‐Shanti N, Faulkner SH, Saini A, Loram I, Stewart CE (2011) A semi‐automated programme for tracking myoblast migration following mechanical damage: manipulation by chemical inhibitors. Cell Physiol Biochem 27: 625–636 [DOI] [PubMed] [Google Scholar]
- Allen DL, Teitelbaum DH, Kurachi K (2003) Growth factor stimulation of matrix metalloproteinase expression and myoblast migration and invasion in vitro . Am J Physiol Cell Physiol 284: C805–C815 [DOI] [PubMed] [Google Scholar]
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM (1995) Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro . J Cell Physiol 165: 307–312 [DOI] [PubMed] [Google Scholar]
- Allen TA, Asad D, Amu E, Hensley MT, Cores J, Vandergriff A, Tang J, Dinh PU, Shen D, Qiao L et al (2019) Circulating tumor cells exit circulation while maintaining multicellularity, augmenting metastatic potential. J Cell Sci 132: jcs231563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Gracieux D, Talib M, Tokarz DA, Hensley MT, Cores J, Vandergriff A, Tang J, de Andrade JBM, Dinh PU et al (2017) Angiopellosis as an alternative mechanism of cell extravasation. Stem Cells 35: 170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano O, Yamane A, Shimada M, Koshimizu U, Nakamura T, Iseki S (2002) Hepatocyte growth factor is essential for migration of myogenic cells and promotes their proliferation during the early periods of tongue morphogenesis in mouse embryos. Dev Dyn 223: 169–179 [DOI] [PubMed] [Google Scholar]
- Andreetta F, Bernasconi P, Baggi F, Ferro P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R, Confalonieri P (2006) Immunomodulation of TGF‐beta1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: Implications for antifibrotic therapy. J Neuroimmunol 175: 77–86 [DOI] [PubMed] [Google Scholar]
- Baghdadi MB, Firmino J, Soni K, Evano B, Di Girolamo D, Mourikis P, Castel D, Tajbakhsh S (2018) Notch‐induced miR‐708 antagonizes satellite cell migration and maintains quiescence. Cell Stem Cell 23: 859–868. [DOI] [PubMed] [Google Scholar]
- Bandow K, Ohnishi T, Tamura M, Semba I, Daikuhara Y (2004) Hepatocyte growth factor/scatter factor stimulates migration of muscle precursors in developing mouse tongue. J Cell Physiol 201: 236–243 [DOI] [PubMed] [Google Scholar]
- Benedetti S, Hoshiya H, Tedesco FS (2013) Repair or replace? Exploiting novel gene and cell therapy strategies for muscular dystrophies. FEBS J 280: 4263–4280 [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R (1995) Expression of transforming growth factor‐β1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest 96: 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersini S, Gilardi M, Ugolini GS, Sansoni V, Talò G, Perego S, Zanotti S, Ostano P, Mora M, Soncini M et al (2018) Engineering an environment for the study of fibrosis: a 3D human muscle model with endothelium specificity and endomysium. Cell Rep 25: 3858–3868. [DOI] [PubMed] [Google Scholar]
- Binamé F, Pawlak G, Roux P, Hibner U (2010) What makes cells move: requirements and obstacles for spontaneous cell motility. Mol Biosyst 6: 648–661 [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Brohmann H (2000) Genes that control the development of migrating muscle precursor cells. Curr Opin Cell Biol 12: 725–730 [DOI] [PubMed] [Google Scholar]
- Bischoff R (1986) Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol 115: 129–139 [DOI] [PubMed] [Google Scholar]
- Bischoff R (1990) Interaction between satellite cells and skeletal muscle fibers. Development 109: 943–952 [DOI] [PubMed] [Google Scholar]
- Bischoff R (1997) Chemotaxis of skeletal muscle satellite cells. Dev Dyn 208: 505–515 [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C (1995) Essential role for the c‐met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376: 768–771 [DOI] [PubMed] [Google Scholar]
- Bober E, Franz T, Arnold HH, Gruss P, Tremblay P (1994) Pax‐3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development 120: 603–612 [DOI] [PubMed] [Google Scholar]
- Bonfanti C, Rossi G, Tedesco FS, Giannotta M, Benedetti S, Tonlorenzi R, Antonini S, Marazzi G, Dejana E, Sassoon D et al (2015) PW1/Peg3 expression regulates key properties that determine mesoangioblast stem cell competence. Nat Commun 6: 6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchentouf M, Skuk D, Tremblay JP (2007) Early and massive death of myoblasts transplanted into skeletal muscle: Responsible factors and potential solutions. Curr Opin Organ Transplant 12: 664–667 [Google Scholar]
- Bricceno KV, Martinez T, Leikina E, Duguez S, Partridge TA, Chernomordik LV, Fischbeck KH, Sumner CJ, Burnett BG (2014) Survival motor neuron protein deficiency impairs myotube formation by altering myogenic gene expression and focal adhesion dynamics. Hum Mol Genet 23: 4745–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohmann H, Jagla K, Birchmeier C (2000) The role of Lbx1 in migration of muscle precursor cells. Development 127: 437–445 [DOI] [PubMed] [Google Scholar]
- Brown AD, Close GL, Sharples AP, Stewart CE (2017) Murine myoblast migration: influence of replicative ageing and nutrition. Biogerontology 18: 947–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F (2003) The formation of skeletal muscle: from somite to limb. J Anat 202: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick MM, Chu JT, Godar S, Sackstein R (2006) HCELL is the major E‐ and L‐selectin ligand expressed on LS174T colon carcinoma cells. J Biol Chem 281: 13899–13905 [DOI] [PubMed] [Google Scholar]
- Campbell AL, Shih HP, Xu J, Gross MK, Kioussi C (2012) Regulation of motility of myogenic cells in filling limb muscle anlagen by Pitx2. PLoS One 7: e35822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps J, Grosemans H, Gijsbers R, Maes C, Sampaolesi M (2019) Growth factor screening in dystrophic muscles reveals PDGFB/PDGFRB‐mediated migration of interstitial stem cells. Int J Mol Sci 20: 1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellari O, Benedetti S, Innocenzi A, Tedesco FS, Moreno‐Fortuny A, Ugarte G, Lampugnani MG, Messina G, Cossu G (2013) Dll4 and PDGF‐BB convert committed skeletal myoblasts to pericytes without erasing their myogenic memory. Dev Cell 24: 586–599 [DOI] [PubMed] [Google Scholar]
- Cezar CA, Mooney DJ (2015) Biomaterial‐based delivery for skeletal muscle repair. Adv Drug Deliv Rev 84: 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chal J, Oginuma M, Al Tanoury Z, Gobert B, Sumara O, Hick A, Bousson F, Zidouni Y, Mursch C, Moncuquet P et al (2015) Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat Biotechnol 33: 962–969 [DOI] [PubMed] [Google Scholar]
- Chal J, Al Tanoury Z, Hestin M, Gobert B, Aivio S, Hick A, Cherrier T, Nesmith AP, Parker KK, Pourquié O (2016) Generation of human muscle fibers and satellite‐like cells from human pluripotent stem cells in vitro . Nat Protoc 11: 1833–1850 [DOI] [PubMed] [Google Scholar]
- Chan KT, Bennin DA, Huttenlocher A (2010) Regulation of adhesion dynamics by calpain‐mediated proteolysis of focal adhesion kinase (FAK). J Biol Chem 285: 11418–11426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras G, Paluch E (2008) Blebs lead the way: How to migrate without lamellipodia. Nat Rev Mol Cell Biol 9: 730–736 [DOI] [PubMed] [Google Scholar]
- Chen H, Lo SH (2003) Regulation of tensin‐promoted cell migration by its focal adhesion binding and Src homology domain 2. Biochem J 370: 1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Shen D, Xie Y, Cingolani E, Malliaras K, Marbán E (2012) Brief report: mechanism of extravasation of infused stem cells. Stem Cells 30: 2835–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska‐Wodnicka M, Burridge K (1996) Rho‐stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 133: 1403–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn RD, Van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, Ap Rhys CM, Holm TM, Loeys BL et al (2007) Angiotensin II type 1 receptor blockade attenuates TGF‐β‐induced failure of muscle regeneration in multiple myopathic states. Nat Med 13: 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins‐Hooper H, Woolley TE, Dyson L, Patel A, Potter P, Baker RE, Gaffney EA, Maini PK, Dash PR, Patel K (2012) Age‐related changes in speed and mechanism of adult skeletal muscle stem cell migration. Stem Cells 30: 1182–1195 [DOI] [PubMed] [Google Scholar]
- Corti S, Salani S, Del Bo R, Sironi M, Strazzer S, D'Angelo MG, Comi GP, Bresolin N, Scarlato G (2001) Chemotactic factors enhance myogenic cell migration across an endothelial monolayer. Exp Cell Res 268: 36–44 [DOI] [PubMed] [Google Scholar]
- Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, Noviello M, Roostalu U, Natali Sora MG, Scarlato M et al (2016) Intra‐arterial transplantation of HLA‐matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol Med 8: 1470–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley E, Horwitz AF (1995) Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol 131: 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico A, Mercuri E, Tiziano FD, Bertini E (2011) Spinal muscular atrophy. Orphanet J Rare Dis 6: 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RCR (2012) Human ES‐ and iPS‐derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10: 610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daston G, Lamar E, Olivier M, Goulding M (1996) Pax‐3 is necessary for migration but not differentiation of limb muscle precursors in the mouse. Development 122: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Davoudi S, Chin CY, Cooke MJ, Tam RY, Shoichet MS, Gilbert PM (2018) Muscle stem cell intramuscular delivery within hyaluronan methylcellulose improves engraftment efficiency and dispersion. Biomaterials 173: 34–46 [DOI] [PubMed] [Google Scholar]
- Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C (2009) Endomysial fibrosis in duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol 68: 762–773 [DOI] [PubMed] [Google Scholar]
- Dietrich S, Abou‐Rebyeh F, Brohmann H, Bladt F, Sonnenberg‐Riethmacher E, Yamaai T, Lumsden A, Brand‐Saberi B, Birchmeier C (1999) The role of SF/HGF and c‐Met in the development of skeletal muscle. Development 126: 1621–1629 [DOI] [PubMed] [Google Scholar]
- Dimchev GA, Al‐Shanti N, Stewart CE (2013) Phospho‐tyrosine phosphatase inhibitor Bpv(Hopic) enhances C2C12 myoblast migration in vitro. Requirement of PI3K/AKT and MAPK/ERK pathways. J Muscle Res Cell Motil 34: 125–136 [DOI] [PubMed] [Google Scholar]
- Dimitroff CJ, Descheny L, Trujillo N, Kim R, Nguyen V, Huang W, Pienta KJ, Kutok JL, Rubin MA (2005) Identification of leukocyte E‐selectin ligands, P‐selectin glycoprotein ligand‐1 and E‐selectin ligand‐1, on human metastatic prostate tumor cells. Cancer Res 65: 5750–5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Springer TA (1988) Lymphocyte function‐associated antigen‐1 (LFA‐1) interaction with intercellular adhesion molecule‐1 (ICAM‐1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol 107: 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR (1990) VCAM‐1 on activated endothelium interacts with the leukocyte integrin VLA‐4 at a site distinct from the VLA‐4/Fibronectin binding site. Cell 60: 577–584 [DOI] [PubMed] [Google Scholar]
- Elson HF, Ingwall JS (1980) The cell substratum modulated skeletal muscle differentiation. J Supramol Cell Biochem 14: 313–328 [DOI] [PubMed] [Google Scholar]
- Epstein JA, Shapiro DN, Cheng J, Lam PYP, Maas RL (1996) Pax3 modulates expression of the c‐met receptor during limb muscle development. Proc Natl Acad Sci USA 93: 4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP (2000) In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res 258: 279–287 [DOI] [PubMed] [Google Scholar]
- Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D'Antona G, Gavina M, Ottoboni L, Constantin G, Bottinelli R et al (2007) T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol 213: 229–238 [DOI] [PubMed] [Google Scholar]
- Ferreira MM, Dewi RE, Heilshorn SC (2015) Microfluidic analysis of extracellular matrix‐bFGF crosstalk on primary human myoblast chemoproliferation, chemokinesis, and chemotaxis. Integr Biol 7: 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi MD (2016) Mechanism of diapedesis: importance of the transcellular route. Adv Immunol 129: 25–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD (2010) Cell mechanics and the cytoskeleton. Nature 463: 485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A (2004) Calpain‐mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol 6: 977–983 [DOI] [PubMed] [Google Scholar]
- Franz T, Kothary R, Surani MAH, Halata Z, Grim M (1993) The Splotch mutation interferes with muscle development in the limbs. Anat Embryol 187: 153–160 [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH (2002) Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol 283: R698–R709 [DOI] [PubMed] [Google Scholar]
- Fuoco C, Salvatori M, Biondo A, Shapira‐Schweitzer K, Santoleri S, Antonini S, Bernardini S, Tedesco FS, Cannata S, Seliktar D et al (2012) Injectable polyethylene glycol‐fibrinogen hydrogel adjuvant improves survival and differentiation of transplanted mesoangioblasts in acute and chronic skeletal‐muscle degeneration. Skelet Muscle 2: 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Costantin G, Torrente Y, Cossu G (2006) Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol 174: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G (2008) PlGF‐MMP‐9‐expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med 14: 973–978 [DOI] [PubMed] [Google Scholar]
- Garofalo A, Chirivi RGS, Foglieni C, Pigott R, Mortarini R, Martin‐Padura I, Anichini A, Gearing AJ, Sanchez‐Madrid F, Dejana E et al (1995) Involvement of the very late antigen 4 integrin on melanoma in interleukin 1‐augmented experimental metastases. Cancer Res 55: 414–419 [PubMed] [Google Scholar]
- Gassmann P, Haier J, Schlüter K, Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B et al (2009) CXCR4 regulates the early extravasation of metastatic tumor cells in vivo . Neoplasia 11: 651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerli MFM, Moyle LA, Benedetti S, Ferrari G, Ucuncu E, Ragazzi M, Constantinou C, Louca I, Sakai H, Ala P et al (2019) Combined notch and PDGF signaling enhances migration and expression of stem cell markers while inducing perivascular cell features in muscle satellite cells. Stem Cell Reports 12: 461–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotta M, Benedetti S, Tedesco FS, Corada M, Trani M, D'Antuono R, Millet Q, Orsenigo F, Gálvez BG, Cossu G et al (2014) Targeting endothelial junctional adhesion molecule‐A/ EPAC/ Rap‐1 axis as a novel strategy to increase stem cell engraftment in dystrophic muscles. EMBO Mol Med 6: 239–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies AR, Lieber RL (2011) Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP (2003) Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res 63: 3805–3811 [PubMed] [Google Scholar]
- Glinsky VV, Huflejt ME, Glinsky GV, Deutscher SL, Quinn TP (2000) Effects of Thomsen‐Friedenreich antigen‐specific peptide P‐30 on β‐ galactoside‐mediated homotypic aggregation and adhesion to the endothelium of MDA‐MB‐435 human breast carcinoma cells. Cancer Res 60: 2584–2588 [PubMed] [Google Scholar]
- Goetsch KP, Snyman C, Myburgh KH, Niesler CU (2014) ROCK‐2 is associated with focal adhesion maturation during myoblast migration. J Cell Biochem 115: 1299–1307 [DOI] [PubMed] [Google Scholar]
- González MN, de Mello W, Butler‐Browne GS, Silva‐Barbosa SD, Mouly V, Savino W, Riederer I (2017) HGF potentiates extracellular matrix‐driven migration of human myoblasts: Involvement of matrix metalloproteinases and MAPK/ERK pathway. Skelet Muscle 7: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ (1994) Regulation of Pax‐3 expression in the dermomyotome and its role in muscle development. Development 120: 957–971 [DOI] [PubMed] [Google Scholar]
- Gross MK, Moran‐Rivard L, Velasquez T, Nakatsu MN, Jagla K, Goulding M (2000) Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development 127: 413–424 [DOI] [PubMed] [Google Scholar]
- Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, Blau HM (1992) Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature 356: 435–438 [DOI] [PubMed] [Google Scholar]
- Han D, Zhao H, Parada C, Hacia JG, Bringas P, Chai Y (2012) A TGFβ‐Smad4‐Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development 139: 1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger DS, Calderwood DA (2009) Integrin signalling at a glance. J Cell Sci 122: 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, Pascal Q, Guguin A, Gayraud‐Morel B, Cavaillon JM et al (2016) Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11: e0147198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MJ, Kanwar S, McCafferty DM, Granger DN, Eppihimer MJ, Kubes P (1999) Varying roles of E‐selectin and P‐selectin in different microvascular beds in response to antigen. J Immunol 162: 1137–1143 [PubMed] [Google Scholar]
- Hicks MR, Hiserodt J, Paras K, Fujiwara W, Eskin A, Jan M, Xi H, Young CS, Evseenko D, Nelson SF et al (2018) ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat Cell Biol 20: 46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiepen C, Benn A, Denkis A, Lukonin I, Weise C, Boergermann JH, Knaus P (2014) BMP2‐induced chemotaxis requires PI3K p55γ/p110α‐dependent phosphatidylinositol (3,4,5)‐triphosphate production and LL5 recruitment at the cytocortex. BMC Biol 12: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Boontheekul T, Mooney DJ (2006) Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng 12: 1295–1304 [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Kunkel LM (1987) Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell 51: 919–928 [DOI] [PubMed] [Google Scholar]
- Hollway G, Currie P (2005) Vertebrate myotome development. Birth Defects Res C Embryo Today 75: 172–179 [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Izpisua‐Belmonte JC, Christ B, Patel K (1999) Origin and development of the avian tongue muscles. Anat Embryol 200: 137–152 [DOI] [PubMed] [Google Scholar]
- Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, Lam CRI, Sng MK, Leong DTW, Tan SM et al (2011) ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE‐cadherin and claudin‐5 clusters. Blood 118: 3990–4002 [DOI] [PubMed] [Google Scholar]
- Hughes CS, Postovit LM, Lajoie GA (2010) Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10: 1886–1890 [DOI] [PubMed] [Google Scholar]
- Ishido M, Kasuga N (2011) In situ real‐time imaging of the satellite cells in rat intact and injured soleus muscles using quantum dots. Histochem Cell Biol 135: 21–26 [DOI] [PubMed] [Google Scholar]
- Ishii A, Lo SH (2001) A role of tensin in skeletal‐muscle regeneration. Biochem J 356: 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Mima T, Mikawa T (1996) Loss of fibroblast growth factor receptors is necessary for terminal differentiation of embryonic limb muscle. Development 122: 291–300 [DOI] [PubMed] [Google Scholar]
- Jin CL, Zhang ZM, Ye JL, Gao CQ, Yan HC, Li HC, Yang JZ, Wang XQ (2019) Lysine‐induced swine satellite cell migration is mediated by the FAK pathway. Food Funct 10: 583–591 [DOI] [PubMed] [Google Scholar]
- Jouve N, Bachelier R, Despoix N, Blin MG, Matinzadeh MK, Poitevin S, Aurrand‐Lions M, Fallague K, Bardin N, Blot‐Chabaud M et al (2015) CD146 mediates VEGF‐induced melanoma cell extravasation through FAK activation. Int J Cancer 137: 50–60 [DOI] [PubMed] [Google Scholar]
- Judson RN, Rossi FMV (2020) Towards stem cell therapies for skeletal muscle repair. NPJ Regen Med 5: 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM (2010) Nanoscale architecture of integrin‐based cell adhesions. Nature 468: 580–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G, Ajdukovic D, Arnold D, Gledhill RB, Guttmann R, Holland P, Koch PA, Shoubridge E, Spence D, Vanasse M et al (1993) Myoblast transfer in duchenne muscular dystrophy. Ann Neurol 34: 8–17 [DOI] [PubMed] [Google Scholar]
- Kassar‐Duchossoy L, Giacone E, Gayraud‐Morel B, Jory A, Gomès D, Tajbakhsh S (2005) Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev 19: 1426–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Takano K, Suetsugu S, Kurisu S, Yamazaki D, Miki H, Takenawa T, Endo T (2004) N‐WASP and WAVE2 acting downstream of phosphatidylinositol 3‐kinase are required for myogenic cell migration induced by hepatocyte growth factor. J Biol Chem 279: 54862–54871 [DOI] [PubMed] [Google Scholar]
- Khaldoyanidi SK, Glinsky VV, Sikora L, Glinskii AB, Mossine VV, Quinn TP, Glinsky GV, Sriramarao P (2003) MDA‐MB‐435 human breast carcinoma cell homo‐ and heterotypic adhesion under flow conditions is mediated in part by Thomsen‐Friedenreich antigen‐galectin‐3 interactions. J Biol Chem 278: 4127–4134 [DOI] [PubMed] [Google Scholar]
- Kowalski K, Kołodziejczyk A, Sikorska M, Płaczkiewicz J, Cichosz P, Kowalewska M, Stremińska W, Jańczyk‐Ilach K, Koblowska M, Fogtman A et al (2017) Stem cells migration during skeletal muscle regeneration ‐ the role of Sdf‐1/Cxcr4 and Sdf‐1/Cxcr7 axis. Cell Adhes Migr 11: 384–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel EJ, Ley K (1996) Distinct phenotype of E‐selectin‐deficient mice: E‐selectin is required for slow leukocyte rolling in vivo . Circ Res 79: 1196–1204 [DOI] [PubMed] [Google Scholar]
- Lafreniere JF, Mills P, Tremblay JP, El Fahime E (2004) Growth factors improve the in vivo migration of human skeletal myoblasts by modulating their endogenous proteolytic activity. Transplantation 77: 1741–1747 [DOI] [PubMed] [Google Scholar]
- Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA (2003) Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin‐1beta, ‐6, and high‐mobility‐group protein‐1 in skeletal muscle. Shock 19: 538–546 [DOI] [PubMed] [Google Scholar]
- Langer HF, Orlova VV, Xie C, Kaul S, Schneider D, Lonsdorf AS, Fahrleitner M, Choi EY, Dutoit V, Pellegrini M et al (2011) A novel function of junctional adhesion molecule‐C in mediating melanoma cell metastasis. Cancer Res 71: 4096–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautscham LA, Kämmerer C, Lange JR, Kolb T, Mark C, Schilling A, Strissel PL, Strick R, Gluth C, Rowat AC et al (2015) Migration in confined 3D environments is determined by a combination of adhesiveness, nuclear volume, contractility, and cell stiffness. Biophys J 109: 900–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KKH, Wong CC, Webb SE, Tang MK, Leung AKC, Kwok PF, Cai DQ, Chan KM (1999) Hepatocyte growth factor stimulates chemotactic response in mouse embryonic limb myogenic cells in vitro . J Exp Zool 283: 170–180 [DOI] [PubMed] [Google Scholar]
- Lei H, Leong D, Smith LR, Barton ER (2013) Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration. Am J Physiol Cell Physiol 305: C529–C538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup L, Daury L, Mazères G, Cottin P, Brustis JJ (2007) Involvement of the ERK/MAP kinase signalling pathway in milli‐calpain activation and myogenic cell migration. Int J Biochem Cell Biol 39: 1177–1189 [DOI] [PubMed] [Google Scholar]
- Leloup L, Mazères G, Daury L, Cottin P, Brustis JJ (2006) Involvement of calpains in growth factor‐mediated migration. Int J Biochem Cell Biol 38: 2049–2063 [DOI] [PubMed] [Google Scholar]
- Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine‐Simmen K, McPherson VA et al (2014) Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep 8: 1558–1570 [DOI] [PubMed] [Google Scholar]
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J (2004) Transforming growth factor‐β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164: 1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Sharma A, Peng HH, Robertson G, Dong C (2007) Targeting mutant (V600E) B‐Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Res 67: 5814–5820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Le Berre M, Lautenschlaeger F, Maiuri P, Callan‐Jones A, Heuzé M, Takaki T, Voituriez R, Piel M (2015) Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160: 659–672 [DOI] [PubMed] [Google Scholar]
- Lo SH (2004) Tensin. Int J Biochem Cell Biol 36: 31–34 [DOI] [PubMed] [Google Scholar]
- Lossinsky AS, Badmajew V, Robson JA, Moretz RC, Wisniewski HM (1989) Sites of egress of inflammatory cells and horseradish peroxidase transport across the blood‐brain barrier in a murine model of chronic relapsing experimental allergic encephalomyelitis. Acta Neuropathol 78: 359–371 [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ (2005) High‐mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331–342 [DOI] [PubMed] [Google Scholar]
- Lund DK, Mouly V, Cornelison DDW (2014) MMP‐14 is necessary but not sufficient for invasion of three‐dimensional collagen by human muscle satellite cells. Am J Physiol Cell Physiol 307: C140–C149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffioletti SM, Sarcar S, Henderson ABH, Mannhardt I, Pinton L, Moyle LA, Steele‐Stallard H, Cappellari O, Wells KE, Ferrari G et al (2018) Three‐dimensional human iPSC‐derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep 23: 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J (2012) TGFβ signalling in context. Nat Rev Mol Cell Biol 13: 616–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY (2016) Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev 5: CD003725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CM, Campbell C, Torricelli RE, Finkel RS, Flanigan KM, Goemans N, Heydemann P, Kaminska A, Kirschner J, Muntoni F et al (2017) Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 390: 1489–1498 [DOI] [PubMed] [Google Scholar]
- McGaha TL, Phelps RG, Spiera H, Bona C (2002) Halofuginone, an inhibitor of type‐I collagen synthesis and skin sclerosis, blocks transforming‐growth‐factor‐β‐mediated Smad3 activation in fibroblasts. J Invest Dermatol 118: 461–470 [DOI] [PubMed] [Google Scholar]
- Mellor H (2010) The role of formins in filopodia formation. Biochim Biophys Acta Mol Cell Res 1803: 191–200 [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino‐Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, Alfano L, Gomez AM, Lewis S, Kota J et al (2013) Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol 74: 637–647 [DOI] [PubMed] [Google Scholar]
- Mennerich D, Schäfer K, Braun T (1998) Pax‐3 is necessary but not sufficient for lbx1 expression in myogenic precursor cells of the limb. Mech Dev 73: 147–158 [DOI] [PubMed] [Google Scholar]
- Mercuri E, Bönnemann CG, Muntoni F (2019) Muscular dystrophies. Lancet 394: 2025–2038 [DOI] [PubMed] [Google Scholar]
- Merrell AJ, Kardon G (2013) Development of the diaphragm ‐ a skeletal muscle essential for mammalian respiration. FEBS J 280: 4026–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J, Rouche A, Bausero P, Houssaïni A, Gross J, Fiszman MY, Alameddine HS (2010) MMP‐9 overexpression improves myogenic cell migration and engraftment. Muscle Nerve 42: 584–595 [DOI] [PubMed] [Google Scholar]
- Morgan JE, Coulton GR, Partridge TA (1987) Muscle precursor cells invade and repopulate freeze‐killed muscles. J Muscle Res Cell Motil 8: 386–396 [DOI] [PubMed] [Google Scholar]
- Muller WA (2013) Getting leukocytes to the site of inflammation. Vet Pathol 50: 7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni F, Torelli S, Ferlini A (2003) Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol 2: 731–740 [DOI] [PubMed] [Google Scholar]
- Napier SL, Healy ZR, Schnaar RL, Konstantopoulos K (2007) Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44V and alternative sialofucosylated selectin ligands. J Biol Chem 282: 3433–3441 [DOI] [PubMed] [Google Scholar]
- Neuhaus P, Oustanina S, Loch T, Krüger M, Bober E, Dono R, Zeller R, Braun T (2003) Reduced mobility of fibroblast growth factor (FGF)‐deficient myoblasts might contribute to dystrophic changes in the musculature of FGF2/FGF6/mdx triple‐mutant mice. Mol Cell Biol 23: 6037–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeyer AM, DiGregorio DM, Brown RH (1992) Arterial delivery of myoblasts to skeletal muscle. Neurology 42: 2258–2262 [DOI] [PubMed] [Google Scholar]
- Nieminen J, St‐Pierre C, Bhaumik P, Poirier F, Sato S (2008) Role of Galectin‐3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae . J Immunol 180: 2466–2473 [DOI] [PubMed] [Google Scholar]
- Otto A, Collins‐Hooper H, Patel A, Dash PR, Patel K (2011) Adult skeletal muscle stem cell migration is mediated by a blebbing/amoeboid mechanism. Rejuvenation Res 14: 249–260 [DOI] [PubMed] [Google Scholar]
- Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME (2004) Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 164: 441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Vojnits K, Liu TT, Meng F, Yang L, Wang Y, Huard J, Cox CS, Lally KP, Li Y (2015) MMP1 gene expression enhances myoblast migration and engraftment following implanting into mdx/SCID mice. Cell Adh Migr 9: 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Zhang Z, Wang Z, Wang Y, Yan Y, Li S, Tong H (2019) Platelet endothelial aggregation receptor‐1 regulates bovine muscle satellite cell migration and differentiation via integrin beta‐1 and focal adhesion kinase. Cell Adhes Migr 13: 192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C, Han D, Chai Y (2012) Molecular and cellular regulatory mechanisms of tongue myogenesis. J Dent Res 91: 528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, Schwartz MA (2010) Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11: 633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM (1989) Conversion of mdx myofibres from dystrophin‐negative to ‐positive by injection of normal myoblasts. Nature 337: 176–179 [DOI] [PubMed] [Google Scholar]
- Paul NR, Allen JL, Chapman A, Morlan‐Mairal M, Zindy E, Jacquemet G, Fernandez del Ama L, Ferizovic N, Green DM, Howe JD et al (2015) α5β1 integrin recycling promotes Arp2/3‐independent cancer cell invasion via the formin FHOD3. J Cell Biol 210: 1013–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périé S, Trollet C, Mouly V, Vanneaux V, Mamchaoui K, Bouazza B, Marolleau JP, Laforêt P, Chapon F, Eymard B et al (2014) Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol Ther 22: 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GD, Hoffman JR, Knighton DR (1990) Migration of myogenic cells in the rat extensor digitorum longus muscle studied with a split autograft model. Cell Tissue Res 262: 81–88 [DOI] [PubMed] [Google Scholar]
- Piñol‐Jurado P, Gallardo E, de Luna N, Suárez‐Calvet X, Sánchez‐Riera C, Fernández‐Simón E, Gomis C, Illa I, Díaz‐Manera J (2017) Platelet‐derived growth factor bb influences muscle regeneration in duchenne muscle dystrophy. Am J Pathol 187: 1814–1827 [DOI] [PubMed] [Google Scholar]
- Ranzato E, Balbo V, Boccafoschi F, Mazzucco L, Burlando B (2009) Scratch wound closure of C2C12 mouse myoblasts is enhanced by human platelet lysate. Cell Biol Int 33: 911–917 [DOI] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M (2005) A Pax3/Pax7‐dependent population of skeletal muscle progenitor cells. Nature 435: 948–953 [DOI] [PubMed] [Google Scholar]
- Relaix F, Zammit PS (2012) Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Dev 139: 2845–2856 [DOI] [PubMed] [Google Scholar]
- Ridley AJ (2015) Rho GTPase signalling in cell migration. Curr Opin Cell Biol 36: 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotini A, Martínez‐Sarrà E, Duelen R, Costamagna D, Di Filippo ES, Giacomazzi G, Grosemans H, Fulle S, Sampaolesi M (2018) Aging affects the in vivo regenerative potential of human mesoangioblasts. Aging Cell 17: e12714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM (2008) Self‐renewal and expansion of single transplanted muscle stem cells. Nature 456: 502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M, Brohmann H, Zechner D, Müller T, Hülsken J, Walther I, Schaeper U, Birchmeier C, Birchmeier W (2000) Essential role of Gab1 for signaling by the c‐Met receptor in vivo . J Cell Biol 150: 1375–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer K, Braun T (1999) Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nat Genet 23: 213–216 [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA (2002) CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol 3: 143–150 [DOI] [PubMed] [Google Scholar]
- Schnoor M, Alcaide P, Voisin MB, Van Buul JD (2015) Crossing the vascular wall: common and unique mechanisms exploited by different leukocyte subsets during extravasation. Mediators Inflamm 2015: 946509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Horwitz AR (2006) Integrating adhesion, protrusion, and contraction during cell migration. Cell 125: 1223–1225 [DOI] [PubMed] [Google Scholar]
- Sciorati C, Galvez BG, Brunelli S, Tagliafico E, Ferrari S, Cossu G, Clementi E (2006) Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J Cell Sci 119: 5114–5123 [DOI] [PubMed] [Google Scholar]
- Shih HP, Gross MK, Kioussi C (2007) Expression pattern of the homeodomain transcription factor Pitx2 during muscle development. Gene Expr Patterns 7: 441–451 [DOI] [PubMed] [Google Scholar]
- Shin J, Watanabe S, Hoelper S, Krüger M, Kostin S, Pöling J, Kubin T, Braun T (2016) BRAF activates PAX3 to control muscle precursor cell migration during forelimb muscle development. Elife 5: e18351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DDW (2009) 3D timelapse analysis of muscle satellite cell motility. Stem Cells 27: 2527–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Piette V, Côté CH, Chapdelaine P, Hogrel JY, Paradis M, Bouchard JP, Sylvain M et al (2007) First test of a 'high‐density injection' protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow‐up. Neuromuscul Disord 17: 38–46 [DOI] [PubMed] [Google Scholar]
- Skuk D, Tremblay JP (2014) First study of intra‐arterial delivery of myogenic mononuclear cells to skeletal muscles in primates. Cell Transplant 23: 141–150 [DOI] [PubMed] [Google Scholar]
- Skuk D, Tremblay JP (2015) Cell therapy in muscular dystrophies: many promises in mice and dogs, few facts in patients. Expert Opin Biol Ther 15: 1307–1319 [DOI] [PubMed] [Google Scholar]
- Soto MS, Serres S, Anthony DC, Sibson NR (2014) Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro Oncol 16: 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DA, Karvas RM, Siege AL, Cornelison DDW (2011) Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development 138: 5279–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JV, Cheng G, Stockton BM, Fors BP, Butcher EC, Von Andrian UH (1999) L‐selectin‐mediated leukocyte adhesion in vivo: microvillous distribution determines tethering efficiency, but not rolling velocity. J Exp Med 189: 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strell C, Entschladen F (2008) Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal 6: 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F (2007) Surface molecules regulating rolling and adhesion to endothelium of neutrophil granulocytes and MDA‐MB‐468 breast carcinoma cells and their interaction. Cell Mol Life Sci 64: 3306–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Yamazaki Y, Li G, Kaziro Y, Koide H (2000) Involvement of Ras and Ral in chemotactic migration of skeletal myoblasts. Mol Cell Biol 20: 7049–7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz ME, Eberhart J, Pasquale EB, Krull CE (2001) EphA4/ephrin‐A5 interactions in muscle precursor cell migration in the avian forelimb. Development 128: 4669–4680 [DOI] [PubMed] [Google Scholar]
- Tagliafico E, Brunelli S, Bergamaschi A, De Angelis L, Scardigli R, Galli D, Battini R, Bianco P, Ferrari S, Cossu G et al (2004) TGFβ/BMP activate the smooth muscle/bone differentiation programs in mesoangioblasts. J Cell Sci 117: 4377–4388 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S (2009) Skeletal muscle stem cells in developmental versus regenerative myogenesis. J Intern Med 266: 372–389 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M (1997) Redefining the genetic hierarchies controlling skeletal myogenesis: Pax‐ 3 and Myf‐5 act upstream of MyoD. Cell 89: 127–138 [DOI] [PubMed] [Google Scholar]
- Takabayashi T, Xie MJ, Takeuchi S, Kawasaki M, Yagi H, Okamoto M, Rahman TM, Malik F, Kuroda K, Kubota C et al (2010) LL5β directs the translocation of Filamin A and SHIP2 to sites of phosphatidylinositol 3,4,5‐triphosphate (PtdIns(3,4,5)P3) accumulation, and PtdIns(3,4,5)P3 localization is mutually modified by co‐recruited SHIP2. J Biol Chem 285: 16155–16165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S (2007) The WASP‐WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 8: 37–48 [DOI] [PubMed] [Google Scholar]
- Taniguti APT, Pertille A, Matsumura CY, Neto HS, Marques MJ (2011) Prevention of muscle fibrosis and myonecrosis in mdxmice by suramin, a TGF‐β1 blocker. Muscle Nerve 43: 82–87 [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE (1998) HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 194: 114–128 [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Dellavalle A, Diaz‐Manera J, Messina G, Cossu G (2010) Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest 120: 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco FS, Gerli MFM, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E et al (2012) Transplantation of genetically corrected human iPSC‐derived progenitors in mice with limb‐girdle muscular dystrophy. Sci Transl Med 4: 140ra189 [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Moyle LA, Perdiguero E (2017) Muscle interstitial cells: a brief field guide to non‐satellite cell populations in skeletal muscle. Methods Mol Biol 1556: 129–147 [DOI] [PubMed] [Google Scholar]
- Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K (2008) Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E‐ and L‐selectin in shear flow. J Biol Chem 283: 15647–15655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir S, Deries M, Cachaço AS, Bajanca F (2011) The extracellular matrix dimension of skeletal muscle development. Dev Biol 354: 191–207 [DOI] [PubMed] [Google Scholar]
- Tichet M, Prodhomme V, Fenouille N, Ambrosetti D, Mallavialle A, Cerezo M, Ohanna M, Audebert S, Rocchi S, Giacchero D et al (2015) Tumour‐derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat Commun 6: 6993 [DOI] [PubMed] [Google Scholar]
- Torrente Y, El Fahime E, Caron NJ, Bresolin N, Tremblay JP (2000) Intramuscular migration of myoblasts transplanted after muscle pretreatment with metalloproteinases. Cell Transplant 9: 539–549 [PubMed] [Google Scholar]
- Tsai WC, Yu TY, Lin LP, Lin MS, Tsai TT, Pang JHS (2017) Platelet rich plasma promotes skeletal muscle cell migration in association with up‐regulation of FAK, paxillin, and F‐Actin formation. J Orthop Res 35: 2506–2512 [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA (2011) Tumor metastasis: Molecular insights and evolving paradigms. Cell 147: 275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbriele C, Kauskot A, Vandersmissen I, Criel M, Geenens R, Craps S, Luttun A, Janssens S, Hoylaerts MF, Verhamme P (2015) Platelet endothelial aggregation receptor‐1: a novel modifier of neoangiogenesis. Cardiovasc Res 108: 124–138 [DOI] [PubMed] [Google Scholar]
- Vasyutina E, Stebler J, Brand‐Saberi B, Schulz S, Raz E, Birchmeier C (2005) CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev 19: 2187–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente‐Manzanares M, Webb DJ, Horwitz AR (2005) Cell migration at a glance. J Cell Sci 118: 4917–4919 [DOI] [PubMed] [Google Scholar]
- Vilquin JT, Asselin I, Guerette B, Kinoshita I, Lille S, Roy R, Tremblay JP (1994) Myoblast allotransplantation in mice: degree of success varies depending on the efficacy of various immunosuppressive treatments. Transplant Proc 26: 3372–3373 [PubMed] [Google Scholar]
- Voisin MB, Nourshargh S (2013) Neutrophil transmigration: emergence of an adhesive cascade within venular walls. J Innate Immun 5: 336–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Gao CQ, Chen RQ, Jin CL, Li HC, Yan HC, Wang XQ (2016) Focal adhesion kinase and paxillin promote migration and adhesion to fibronectin by swine skeletal muscle satellite cells. Oncotarget 7: 30845–30854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pan H, Murray K, Jefferson BS, Li Y (2009) Matrix metalloproteinase‐1 promotes muscle cell migration and differentiation. Am J Pathol 174: 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt DJ, Morgan JE, Clifford MA, Partridge TA (1987) The movement of muscle precursor cells between adjacent regenerating muscles in the mouse. Anat Embryol (Berl) 175: 527–536 [DOI] [PubMed] [Google Scholar]
- Webb SE, Lee KKH, Tang MK, Ede DA (1997) Fibroblast growth factors 2 and 4 stimulate migration of mouse embryonic limb myogenic cells. Dev Dyn 209: 206–216 [DOI] [PubMed] [Google Scholar]
- Webster MT, Manor U, Lippincott‐Schwartz J, Fan CM (2016) Intravital imaging reveals ghost fibers as architectural units guiding myogenic progenitors during regeneration. Cell Stem Cell 18: 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA, Ordahl CP (1994) Pax‐3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development 120: 785–796 [DOI] [PubMed] [Google Scholar]
- Winkler B, Aranson IS, Ziebert F (2019) Confinement and substrate topography control cell migration in a 3D computational model. Commun Phys 2: 82 [Google Scholar]
- Wolburg H, Wolburg‐Buchholz K, Engelhardt B (2005) Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol 109: 181–190 [DOI] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S (2009) Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM‐2, JAM‐A, and PECAM‐1. Blood 113: 6246–6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi H, Langerman J, Sabri S, Chien P, Young CS, Younesi S, Hicks M, Gonzalez K, Fujiwara W, Marzi J et al (2020) A human skeletal muscle atlas identifies the trajectories of stem and progenitor cells across development and from human pluripotent stem cells. Cell Stem Cell 27: 158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadavalli S, Jayaram S, Manda SS, Madugundu AK, Nayakanti DS, Tan TZ, Bhat R, Rangarajan A, Chatterjee A, Gowda H et al (2017) Data‐driven discovery of extravasation pathway in circulating tumor cells. Sci Rep 7: 43710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Sixt M (2019) Mechanisms of 3D cell migration. Nat Rev Mol Cell Biol 20: 738–752 [DOI] [PubMed] [Google Scholar]
- Yan SLS, Hwang IY, Kamenyeva O, Kehrl JH (2019) In vivo F‐actin filament organization during lymphocyte transendothelial and interstitial migration revealed by intravital microscopy. iScience 16: 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagiuchi A, Miyake H, Nomi M, Takenaka A, Fujisawa M (2009) Modulation of the microenvironment by growth factors regulates the in vivo growth of skeletal myoblasts. BJU Int 103: 1569–1573 [DOI] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki MA (2013) Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K (2008) PSGL‐1 engagement by E‐selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcRγ to induce slow leukocyte rolling. J Exp Med 205: 2339–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lu H (2010) Targeting fibrosis in duchenne muscular dystrophy. J Neuropathol Exp Neurol 69: 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]


