KEY POINTS
Cardiovascular complications, including substantial myocardial necrosis, may occur after asymptomatic or mild infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Evaluation of myocardial injury related to SARS-CoV-2 infection may show transmural (ischemic-like injury) and subendocardial (eosinophilic-like myocarditis) infarct patterns, and involvement of both ventricles.
A 26-year-old man presented to the emergency department with a 1-month history of gradually progressive dyspnea on exertion. He had no history of fever, cough, chest pain, diarrhea or flu-like symptoms. He had no notable medical history and did not smoke. On examination, his pulse was 117 beats/min, blood pressure 135/100 mm Hg and oxygen saturation 98% on ambient air. Basal crepitations were heard in both lung fields.
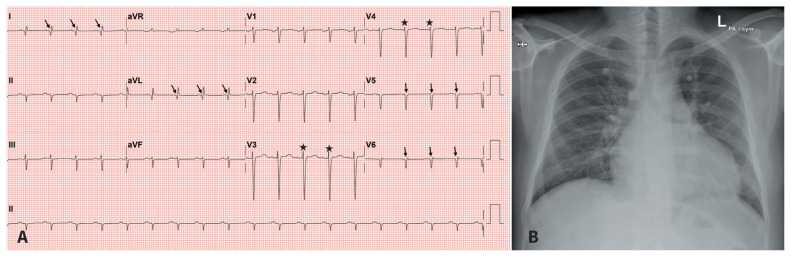
An electrocardiogram showed Q waves in I, aVL and V5–6, and loss of R waves in precordial leads (Figure 1A). A chest radiograph showed cardiomegaly (Figure 1B). We obtained the following blood test results: white blood cell count 12.8 (normal range 3.6–11) × 103/μL with 17% lymphocytes and 0% eosinophils, alanine aminotransferase 682 (normal range 0–41) U/L, mildly elevated coagulation parameters, random blood glucose 163 (normal range 80–140) mg/dL, glycated hemoglobin (HbA1C) 6.7% (normal range < 5.7%), troponin T level 1326 (normal range < 14) ng/L, NT-pro B-type natriuretic peptide (NT-proBNP) 5166 (normal range < 125) pg/mL and D-dimer 2.63 (normal range < 0.5) μg/mL. The patient’s platelet count, C-reactive protein, ferritin and renal function levels were normal.
Figure 1:
(A) Electrocardiogram of a 26-year-old man, showing Q waves in I, aVL, V5–6 (arrows), and loss of R waves in precordial leads (stars). (B) Chest radiograph showing cardiomegaly.
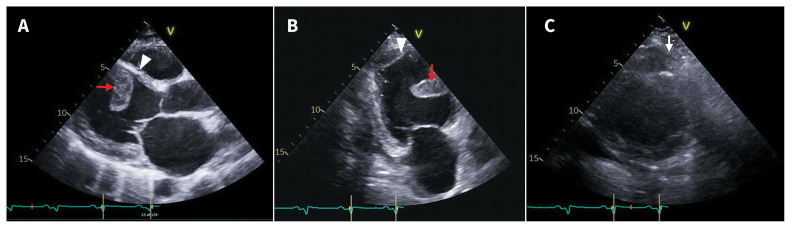
Echocardiography showed a severely dilated left ventricle (LV) and left atrium with LV ejection fraction of 15%–20% and grade 2/4 mitral regurgitation. There was akinesis and thinning of basal anteroseptal and inferoseptal; midcavity anterior, anteroseptal and inferoseptal; and all the apical LV segments. The patient’s right ventricle (RV) was dilated, with impaired systolic function and moderate tricuspid regurgitation. There was a large, mobile LV thrombus attached to the mid anteroseptal wall (Figure 2A), sessile thrombus at the LV apex (Figure 2B) and a thrombus at the RV apex (Figure 2C), along with a thin rim of pericardial effusion.
Figure 2:
Echocardiogram at time of admission. (A) Septal thrombus (arrow), thin septum (arrowhead) and dilated left ventricle. (B) Septal (arrow) and apical (arrowhead) left ventricular thrombi. (C) Thrombus at right ventricular apex (arrow).
We admitted the patient to the hospital with a working diagnosis of acute heart failure. Differential diagnoses included acute coronary syndrome, myocarditis, dilated cardiomyopathy and pulmonary thromboembolism. Coronary angiography showed normal coronary arteries and computed tomography pulmonary angiography was negative for pulmonary embolism. The patient’s brief history of symptoms and markedly elevated troponin values argued against a diagnosis of dilated cardiomyopathy.
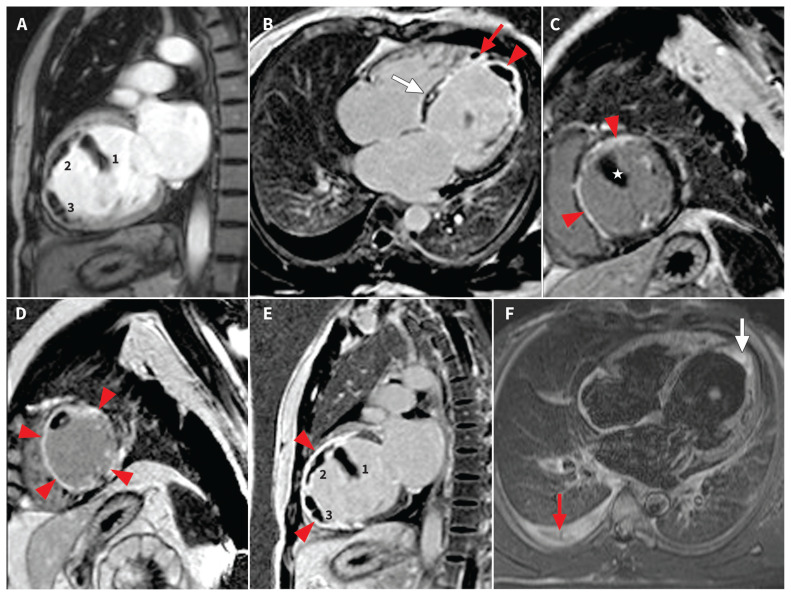
Cardiac magnetic resonance imaging (MRI) showed dilated LV with LV ejection fraction of 20%. T1 mapping and gadolinium images in the early phase showed 3 thrombi in the LV (Figure 3A). In the late phase, there was subtle mid-wall linear enhancement in the anterobasal segment (Figure 3B). Notably, there was endomyocardial enhancement of the basal septum extending to the anterior and septal segments at the midventricular level (Figure 3C), which was transmural and circumferential toward the apex (Figure 3D). There was fibrosis of enhanced segments, severe thinning of the septum and corresponding regional wall dysfunction.
Figure 3:
Cardiac magnetic resonance images (performed on a 1.5-T scanner [Siemens Aera]). Red arrowheads in all images show myocardial enhancement. (A)–(E) Inversion recovery T1 images. (A) Vertical longitudinal axis, early gadolinium image showing 3 thrombi (numbered) in left ventricle (LV). (B) Four-chamber view showing (1) LV apical thrombus and (2) a small thrombus at right ventricular apex (red arrow) with transmural enhancement at LV apex and subendocardial enhancement of septum. A subtle linear mid-myocardial enhancement in anterior septum is seen (white arrow). (C) Midventricular, short-axis view showing subendocardial enhancement in anterior and septal walls with a large LV thrombus (star). (D) Apical, short-axis view showing transmural enhancement of myocardium in all segments with intraluminal thrombus. (E) Two-chamber view showing 3 intraluminal thrombi (numbers 1, 2, 3) and transmural enhancement in septum and inferior myocardium. (F) Short τ inversion recovery T2 image showing apical endomyocardial edema (white arrow) and right-side pleural effusion (red arrow).
We tested for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a potential causative agent of his heart failure. We performed a test for SARS-CoV-2 on nasopharyngeal samples by real-time reverse transcriptase–polymerase chain reaction (Cepheid Inc. Sunnyvale, United States) at admission and 72 hours later; both were negative. A coronavirus disease 2019 (COVID-19) immunoglobulin G (IgG) antibody test (Abbott Architect SARS-CoV-2 IgG, Abbott, US), however, was positive. We advised hormonal tests because certain endocrine changes are associated with the convalescent phase of COVID-19. The patient’s early morning cortisol was 30 (normal range 133–537) nmol/L, and adrenocorticotropic hormone < 7.1 (normal range 10–46) pg/mL. Thyroid function test results were normal.
We treated the patient with furosemide, ramipril, bisoprolol, spironolactone, warfarin and therapeutic doses of enoxaparin for his heart failure. We did not administer antiviral therapy for COVID-19. His symptoms gradually improved over a period of 2 weeks, and troponin T values declined after a few days, consistent with acute myocardial injury. His predischarge echocardiogram, 12 days after initial presentation, showed no residual LV or RV thrombi (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202106/tab-related-content).
Discussion
Cardiovascular involvement during the acute phase of SARS-CoV-2 infection is common. In 1 US study of 2736 patients admitted to hospital with COVID-19, one-third showed troponin elevation, signifying myocardial injury, which was associated with a higher risk of death compared with those without raised troponins.1 Echocardiographic abnormalities have been reported in about half of patients with COVID-19,2 but little is known about persistent cardiac injury or chronic sequelae of COVID-19 after resolution of acute illness.
As evidenced by negative nasopharyngeal swabs and positive IgG antibodies, it is likely that this patient had SARS-CoV-2 infection. His symptoms of progressive dyspnea on exertion and heart failure may have been related to acute infection with SARS-CoV-2. The antibody assay used is reported to have specificity of 99.6% (95% confidence interval [CI] 99.1–99.9) and sensitivity of 93.9% (95% CI 86.3–98.0) for samples taken over 14 days after onset of symptoms.3,4 We considered 4 disease entities that may have been associated with our patient’s acute heart failure: acute coronary syndrome, myocarditis, dilated cardiomyopathy and myocardial infarction with nonobstructive coronary arteries. Of these, we believe myocardial infarction with nonobstructive coronary arteries is the most likely.
An absence of angina, ST-segment elevation and T-wave changes of ischemia on serial electrocardiograms, coupled with a normal coronary angiogram, made diagnoses of acute coronary syndrome, coronary spasm, dissection and thromboembolism less likely. The patient’s imaging scans were inconsistent with acute myocarditis and nonischemic dilated cardiomyopathy, either separately or together. He had subtle evidence of acute myocarditis only without associated myocardial edema on cardiac MRI, an observation that does not align with his severe LV dysfunction and acute heart failure. The pattern of late gadolinium enhancement, myocardial fibrosis and severe thinning of the interventricular septum on cardiac MRI pointed to an extensive infarct involving the left anterior descending and right coronary artery supply regions, which is inconsistent with nonischemic dilated cardiomyopathy. This led us to consider a diagnosis of myocardial infarction with nonobstructive coronary arteries, based on his age and combination of nonobstructive coronary arteries with a transmural pattern of infarction.5
Our patient’s cardiac troponin T and NT-proBNP were substantially elevated without concurrent elevation of inflammatory markers, a pattern that would be consistent with the timing of his hospital presentation in the convalescent phase of his SARS-CoV-2 infection.6 As indirect support for this association, our patient had new-onset diabetes mellitus and suppression of the hypothalamic–pituitary–adrenal axis, both of which have been reported after SARS-CoV-2 infection.7 Yet, he did not experience typical respiratory symptoms of COVID-19 before or during his hospital stay.
Nonobstructive coronary artery disease presenting with myocardial infarction has been described in patients admitted to hospital with COVID-19.8,9 An ischemic pattern of myocardial enhancement on cardiac MRI has been reported in 12% of patients after recovery from COVID-19.10 Even so, it is difficult to conclusively establish a causal link between SARS-CoV-2 infection and myocardial infarction with nonobstructive coronary arteries.
The mechanisms of cardiac injury are not well established in COVID-19. There is a possibility of extensive coronary microvascular thrombi leading to a predominant infarction pattern, although this may not be uniformly spread across all areas of the heart, as reported in the cardiac pathology of a patient with COVID-19.11 Microvascular thrombi may explain our patient’s biventricular dysfunction as well as his transmural infarct pattern with normal coronary arteries, which may have occurred in conjunction with direct viral infection to the heart.
SARS-CoV-2 infection has been associated with several cardiovascular manifestations. This case suggests that it is possible for SARS-CoV-2 infection to present with extensive myocardial injury. Careful follow-up of patients recovering from SARS-CoV-2 infection may corroborate this association, and aid in their early management.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: Shubham Agarwal and Sanjeev Kumar Agarwal contributed to the conception and design of the work; carried out data collection, analysis and interpretation; and drafted the manuscript. All the authors edited the manuscript and approved the final version. The authors agree to be accountable for all aspects of the work.
References
- 1.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial Injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020; 76:533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging 2020;21:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol 2020;58:e00941–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duggan J. Evaluation of the Abbott SARS-CoV-2 IgG for the detection of anti-SARS-CoV-2 antibodies. London (UK): Public Health England; 2020. June 8 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/890566/Evaluation_of_Abbott_SARS_CoV_2_IgG_PHE.pdf (accessed 2020 Oct. 1). [Google Scholar]
- 5.Agewall S, Beltrame JF, Reynolds HR, et al. on behalf of the WG on cardiovascular pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–53. [DOI] [PubMed] [Google Scholar]
- 6.Ma KL, Liu ZH, Cao CF, et al. COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv 2020. Mar. 23. 10.1101/2020.03.19.20034124v1 (accessed 2020 Oct. 29). [DOI] [Google Scholar]
- 7.Agarwal S, Agarwal SK. Endocrine changes in SARS-CoV-2 patients and lessons from SARS-CoV. Postgrad Med J 2020;96:412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with COVID-19: a case series. N Engl J Med 2020;382:2478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvillo-Argüelles O, Ross HJ. Cardiac considerations in patients with COVID-19. CMAJ 2020;192:E630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance Imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020. July 27 [Epub ahead of print]. 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guagliumi G, Sonzogni A, Pescetelli I, et al. Microthrombi and ST-segment elevation myocardial infarction in COVID-19. Circulation 2020;142:804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]