Abstract
BACKGROUND
Transcatheter aortic valve implantation (TAVI) is a minimally invasive procedure that is considered a good alternative to surgical aortic valve replacement (sAVR) in selected patients. Our aim is to determine the baseline, procedural characteristics and one-year clinical outcomes of our TAVI registry.
METHODS
This study is a retrospective observational analysis of a prospectively designed cohort comprising 81 consecutive patients treated at Mohammed bin Khalifa Cardiac Centre (MKCC) who were enrolled in Bahrain TAVI registry from February 2014 to February 2019. The clinical endpoints were defined according to the updated Valve Academic Research Consortium-2 (VARC-2) consensus document.
RESULTS
Out of the 81 patients included in our study, there were 37 (45.7%) males. The mean age was 76.4 ± 8.9 years with a mean Logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE II) of 4.1 ± 2.5 and a mean Society of Thoracic Surgery (STS) Risk Score of 4.2 ± 3.5. Evolute-R valve was used for 36 (44.4%) patients, Edward Sapien for 26 patients (32.1%), and Core valve for 19 patients (23.5%). At one year follow up, all-cause death was reported in three (3.7%) patients; none of them was cardiovascular mortality. As per VARC-II criteria, no cases fulfilled the criteria of valve dysfunction but TAVI-related complications (i.e., TAV-in-TAV deployment) were reported in four (4.9%) cases. One (1.2%) case of major bleeding was encountered but no patient experienced life-threatening bleeding. Major vascular complications were documented in two patients (2.5%) only. Significant Acute Kidney Injury (AKI) occurred in two (2.5%) patients, both classified as stage-2 but no one deteriorated to stage-3 or hemodialysis. Seven (8.6%) patients required permanent pacemakers, and all were implanted during the index admission for TAVI. One patient (1.2%) had stroke and all survivors completed one-year follow up.
CONCLUSION
The TAVI program in Bahrain is encouraging and corresponds to the finest international centers outcomes in terms of procedural success and complications rate.
Keywords: Aortic stenosis, Aortic valve replacement, Corevalve, Evolute-R, Edward Sapien, Transcatheter aortic valve implantation
1. Introduction
The Kingdom of Bahrain has experienced a steady advancement in socio-economic status and healthcare systems over the past decades [1]. As a consequence, life expectancy above 65 years of age has increased and the population is aging swiftly [2]. Many chronic medical conditions, like diabetes mellitus, systemic and pulmonary hypertension, renal and liver failure, coronary and peripheral artery disease, lung disease and cancer frequently coexist in geriatrics, which constitute a high surgical risk, compared to the younger population.
Aortic stenosis is the most commonly encountered valvular heart disease and a substantial surge in its incidence is anticipated in the coming decades because of the progressive aging of mankind [3]. In symptomatic sever aortic stenosis (AS), surgical aortic valve replacement improves survival, in the absence of major comorbidities, with relatively low operative mortality [4]. However, in clinical practice, surgeons turn down 20%–40% of patients with severe symptomatic AS owing to prohibitive surgical risk [5]. For these patients, a less invasive option will be extremely valuable.
Transcatheter aortic valve implantation (TAVI) is a rapidly evolving procedure, in which a bioprosthetic valve is inserted through a catheter and implanted within the diseased native aortic valve. Since 2002, when Alain Cribier performed the first in-man implantation, TAVI has popped up as a practical, substitute treatment modality of stenotic aortic valves and exhibited outcomes as good as sAVR in patients who are considered inoperable or at intermediate to high surgical risk [6,7]. Recently, PARTNER-3 and Evolute Low-risk trial determined that TAVI is non-inferior to sAVR in low-risk patients [8,9]. The tremendous government financial support and the strong conviction in TAVI of Bahraini interventional cardiologists were behind the success of TAVI in our kingdom but, in spite of the wide acceptance of the TAVI procedure, there have been hardly any publications on TAVI from our county and hence, we thought to describe our experience, which was launched at MKCC early in 2014. This study is unique in that it has captured every TAVI performed in the kingdom of Bahrain and includes the entire learning curve and early experience without any publication bias as it represents individuals undergoing TAVI in the real world. Furthermore, treatment strategy that works very well in one centre may not be appropriate in another one therefore, it is felt that sharing local experience and outcomes would be crucial to provide roadmap to centers planning to start TAVI program.
2. Materials and methods
2.1. Setting
This is a single-center retrospective study including patients who underwent TAVI procedure between February 2014 and February 2019 at Mohammed bin Khalifa Cardiac Centre (MKCC) in the kingdom of Bahrain; which is the only tertiary cardiac center in Bahrain where all TAVI procedures are performed.
2.2. Study population and heart team
All patients have been selected for TAVI after multidisciplinary team review by a dedicated heart team of invasive and non-invasive cardiologists, cardiac surgeons and cardiac anesthetists at our institution. Heart Team members work closely together and meet regularly to discuss the most appropriate treatment options based on the clinical data, technical aspects, local experience and recommendations from the guidelines to determine the most appropriate individualized therapy and consequently set-up a center of excellence where innovative therapies can be further developed and the future generation of heart valve specialists effectively trained.
2.3. Eligibility
Inclusion criteria were patients with symptomatic severe aortic stenosis, deemed at high risk for conventional surgical aortic valve replacement. Exclusion criteria were patient’s refusal of informed consent to participate in the registry or lack of capacity to consent and/or high probability of non-adherence to follow-up requirements (e.g. visitors from abroad).
2.4. TAVI procedure
Baseline investigations included routine blood tests, cardiac markers, chest radiography, electrocardiogram (ECG), transthoracic echocardiography (TTE) and if indicated we proceed with transesophageal echocardiography (TEE), selective coronary angiography and computed tomography (CT) of the aortic valve, aorta and iliofemoral access.
Transthoracic echocardiography was used to measure aortic valve pressure gradient, aortic regurgitation grade and left ventricular ejection fraction (LVEF) before and after TAVI. Mean aortic gradient was further confirmed during cardiac catheterization with invasive measurement before and after the procedure.
Tortuosities, as well as the amount of calcifications were analyzed in CT to assert the feasibility for a transfemoral approach and to evaluate the aortic root anatomy including aortic annulus measurements, the valve morphology, commissural fusion, calcification distribution and coronary artery height from the hinge points.
AVI was performed via either transfemoral or subclavian routes with implantation of self-expanding Medtronic CoreValve and Evolute-R valves from February 2014 to January 2018 then we switched to balloon-expandable Edwards Sapien valves from January 2018 onward. A transfemoral first policy was applied, if non-applicable due to any reason then alternate access routes (mainly subclavian) were considered. All procedures conducted with sedation and local anesthesia.
Patients stayed in the intensive care unit during the first 24–48 h after the procedure and were then transferred to the cardiology ward, if there were no complications. Follow up was considered complete one-year after the TAVI procedure.
2.5. Outcomes
Endpoints at one-year follow up comprised all-cause death, cardiovascular death, valve dysfunction and the need for permanent pacemakers (PPM). Other studied outcomes were procedural related myocardial infarction, cerebrovascular accidents, major bleeding, major vascular access complications and acute kidney injury. The widely accepted Valve Academic Research Consortium (VARC-2) Criteria were used to define and assess procedural success, safety end points and clinical outcomes [10].
2.6. Statistical analysis
Categorical data were expressed as numbers and percentages. Continuous variables were reported as mean and standard deviation for normal distributions, or as the median with minimum and maximum values for skewed distributions. We used the statistical software package SPSS-24 for windows to perform the statistical analysis and for producing graphs and plots.
3. Results
Out of the 81 patients included in our study, there were 37 (45.7%) males. The mean age was 76.4 ± 8.9 years (See Fig. 1). In terms of preoperative predicted risk of surgical mortality, the mean Logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE II) was 4.1 ± 2.5 and the mean Society of Thoracic Surgery (STS) Risk Score was 4.2 ± 3.5.
Fig. 1.
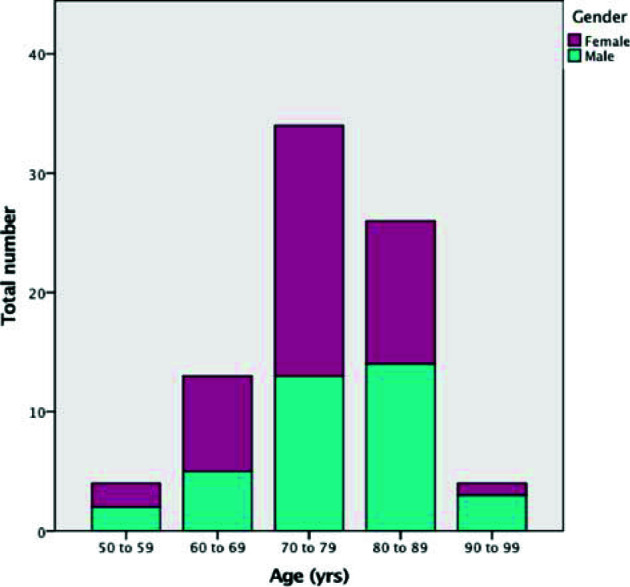
Age and gender distribution among TAVI patients in Bahrain.
The femoral artery was the most common access site and was utilized in 97.5% of the cases. Regarding valve types, Evolute-R valve was used in 36 (44.4%) patients, Edward Sapien in 26 patients (32.1%) and Corevalve in 19 patients (23.5%) (See Fig. 2). The full analysis of baseline characteristics of the study population is depicted in Table 1.
Fig. 2.
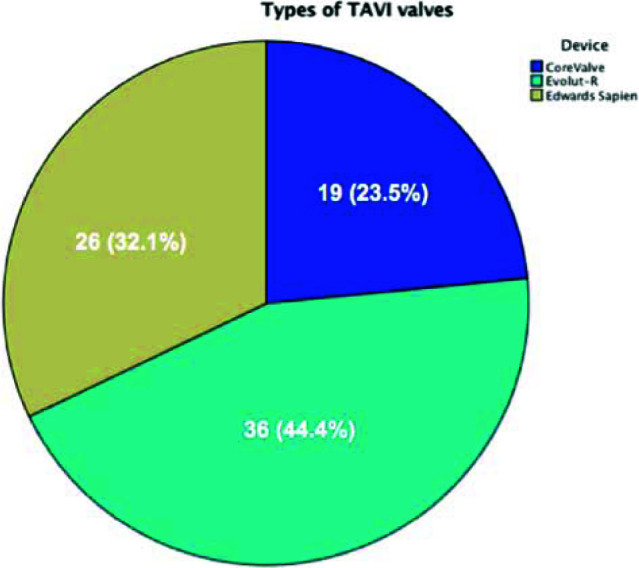
Types of valves in Bahrain TAVI registry.
Table 1.
Baseline and procedural characteristics (81 patient).
| Demographic variables | |
| Age (years), mean ± SD | 76.4 ± 8.9 |
| Male, no. (%) | 37 (45.7%) |
| BMI (kg/m2) mean ± SD | 31.6 ± 6.6 |
| Body surface area (m2) mean ± SD | 1.8 ± 0.21 |
| Clinical variables | |
| Diabetes mellitus, no. (%) | 52 (64.2%) |
| Systemic Hypertension, no. (%) | 71 (87.6%) |
| Dyslipidaemia, no. (%) | 63 (77.8%) |
| Coronary artery disease, no. (%) | 63 (77.8%) |
| Cerebrovascular accident, no. (%) | 8 (9.9%) |
| Haemodialysis, no. (%) | 3 (3.7%) |
| Peripheral vascular disease, no. (%) | 8 (9.9%) |
| Chronic obstructive airway disease, no. (%) | 5 (6.2%) |
| Pre-procedural investigations | |
| Aortic valve area (cm2), mean ± SD | 0.67 ± 0.19 |
| Non-invasive gradient (mmHg), mean ± SD | 44.3 ± 15.0 |
| Ejection fraction (%), mean ± SD | 53.1 ± 13.3 |
| Creatinine (umol/L), mean ± SD | 99.7 ± 84 |
| Glomerular filtration rate (mL/min) mean ± SD | 53.5 ± 11.5 |
| Haemoglobin (g/dL) mean ± SD | 11.6 ± 1.6 |
| Risk assessment of surgical mortality | |
| Euroscore II score (%), mean ± SD | 4.1 ± 2.5 |
| STS score (%), mean ± SD | 4.2 ± 3.5 |
| Procedural characteristics | |
| Access site | |
| Femoral, no. (%) | 79 (97.5%) |
| Subclavian, no. (%) | 2 (2.5%) |
| Device | |
| CoreValve, no. (%) | 19 (23.5%) |
| Evolute-R, no. (%) | 36 (44.4%) |
| Edward Sapien, no. (%) | 26 (32.1%) |
| Invasive mean gradient | |
| Pre-TAVI (mmHg), mean ± SD | 43.7 ± 19.1 |
| Post-TAVI (mmHg), mean ± SD | 1.9 ± 3.3 |
| Aortic regurgitation index, mean ± SD | 28.0 ± 7.8 |
During one-year follow up, three (3.7%) cases of all-cause death were reported, both were non-cardiovascular mortality. As per VARC-II criteria, no cases fulfilling the definition of valve dysfunction were encountered but TAVI-related complications (i.e. TAV-in-TAV deployment) were reported in four (4.9%) cases. One (1.2%) event of major bleeding was documented but no patient experienced procedural related life-threatening bleeding. Eight (9.8%) patients had vascular complications; the majority were minor and observed in six (7.4%) patients, while major vascular complications were documented in two patients (2.5%) only.
Significant Acute Kidney Injury (AKI) occurred in two (2.5%) patients, both classified per VARC-II criteria as stage-2 but no one deteriorated to stage-3 or hemodialysis. Seven (8.6%) patients had high-degree atrioventricular block necessitating PPM implantation; all were implanted during the index admission for TAVI. One patient (1.2%) had cerebrovascular accident (CVA) two weeks after valve implantation. All survivors completed one year of follow up. The clinical outcomes are summarized in Table 2.
Table 2.
Clinical outcomes of Bahrain TAVI registry.
| Endpoints at one-year Follow up | |
| All-cause mortality, no. (%) | 3 (3.7%) |
| Cardiovascular mortality, no. (%) | 0 |
| Valve dysfunction, no. (%) | 0 |
| Pacemaker implantation, no. (%) | 7 (8.6%) |
| Periprocedural complications | |
| Bleeding, no. (%) | |
| Life threatening | 0 |
| Major | 1 (1.2%) |
| Vascular complications, no. (%) | |
| Major | 2 (2.5%) |
| Minor | 6 (7.4%) |
| Acute kidney injury, no. (%) | |
| Stage 1 | 5 (6.2%) |
| Stage 2 | 2 (2.5%) |
| Stage 3 | 0 |
| Myocardial infarction (<72 h), no. (%) | 0 |
| Cerebrovascular accident, no. (%) | 1 (1.2%) |
| TAV-in-TAV deployment, no. (%) | 4 (4.9%) |
4. Discussion
Our study describes the experience of TAVI in the one and only TAVI performing cardiac center in Bahrain. The Kingdom of Bahrain is an island in the Arabian Gulf and has approximately one and a half million inhabitants. Despite being one of the smallest countries in Asia, Bahrain has the entire health service expenditure per person comparable to leading European countries [1, 2 and 11].
According to the latest WHO age structure estimate (July 2018), only 3.0% of the population in Bahrain were above 65 years of age (this age category constitutes up to 18%–20% in many western countries), with comparable numbers of both genders in a ratio of 1.01 and estimated life expectancy of 76.9 years for males and 81.5 years for females [12]. The small number of inhabitants belonging to this age group clearly explains the low volume of TAVI cases in Bahrain.
The Placement of AoRTic TraNscathetER Valve (PARTNER) Trial is a very well-designed, meticulously executed, prospective randomized trial that demonstrated that TAVI with the Edwards Sapien valve was not inferior to standard surgical aortic valve replacement in patients with symptomatic sever aortic stenosis who are high risk for surgical therapy (cohort A), and was superior to medical therapy in the treatment of inoperable patients with aortic stenosis (cohort B) [13].
Our study population matched the same risk profile of PARTNER-II Trial (TAVI vs. sAVR in intermediate risk patients), with mean STS score of 5.8 ± 2.1 compared to 4.2 ± 3.5 in our cohort 13 (13a, b, 14). PARTNER-II showed that, aside from patients after TAVI resuming normal daily activities earlier than patients who underwent sAVR, TAVI is associated with lower incidence of the combined endpoint of mortality, all-stroke, and aortic regurgitation at one-year [14].
Comparing our baseline characteristics with PARTNER-II trial, we found that our patients were slightly younger (76.4 ± 8.9 vs. 81.5 ± 7.7 years) with less percentage of males (45.7% vs. 54.2%) [13,14].
In line with almost all TAVI trials, our invasive and echocardiography data demonstrated instant reduction in the pressure gradient across AV, as well as improvement of systolic pulmonary artery pressure [15].
In what could be a reflection of the successful evolution of TAVI procedure worldwide, PARTNER-II showed 12.3% all-cause mortality and 7.1% cardiovascular mortality after TAVI, compared to 3.7% and 0% in our study, respectively [14]. In addition, the experience of the operators should be considered and the acquisition of the cutting-edge evidence-based techniques and devices as well as relatively lower-risk patients.
We also noted the low incidence of major vascular complications (2.5% vs. 7.9%) and major bleeding (1.25% vs. 10.4%) in comparison to PARTNER-II; this indicated that our team was very careful in patient selection and implementation of all possible precautions to avoid such complications, especially in that we learned from previous studies the negative impact of such complications on clinical outcomes of TAVI patients.
Furthermore, our registry showed 1.25% incidence of cerebrovascular accidents which is relatively low compared to PARTNER-II trial (5% stroke rate) [16], but comparable to recent studies including a report from the American College of Cardiology (ACC) Transcatheter Valve Therapies registry (n = 12,182) that reported less than 2.5% stroke rate [17].
While AKI was observed in seven (8.6%) of our patients, the true incidence may be underreported as the timing for the diagnosis of AKI is extended from 72 h to 7 days in the latest VARC criteria and many patients in our registry were discharged before completing seven days post TAVI. Two (2.5%) had significant AKI classified per VARC-II criteria as stage-2 (i.e. increase in serum creatinine to 200%–299% (2.0–2.99 × increase compared with baseline) [10], but no one progressed to stage-3 or required hemodialysis and both patients recovered completely during follow up.
The incidence of new PPM was almost similar to PARTNER-II (8.6% vs. 9.9%) [15]; our seven cases were all implanted during index admission for TAVI and distributed as follows: two (10.5%) in Corevalve group; three (8.3%) in Evolute-R group; and two (7.6%) in Edward Sapien group. Our study is definitely underpowered to determine any association between the need for PPM and the type of implanted device but the trend suggests that self-expandable Medtronic CoreValves are at higher risk [14,15]. These observations are supported by data from large registries in which Corevalve implantation was associated with more frequent development of conduction abnormalities, which is likely due to deep implantation that occurs more frequently in Corevalve than in Edward Sapien [18,19]. Lenders et al. showed that the use of a newer delivery system might help to solve this problem [20].
While there were four cases of TAVI-related complications in terms of severe aortic regurgitation discovered promptly after deployment of fist-valve, all managed successfully with implantation of second valve during the index procedure (TAV-in-TAV deployment), and we did not encounter any incidence of prosthetic valve dysfunction at one-year follow up using patient’s own initial post-implant study as a reference for serial comparison.
5. Conclusion
The TAVI program in Bahrain is encouraging and corresponding to the finest international centers outcomes in terms of procedural success and complications rate.
Acknowledgement
We acknowledge the efforts and great help of Mrs. Jawaher Mohamed and Mrs. Fatema Hamad in data collection and follow up of TAVI patients at Mohammed bin Khalifa Cardiac Centre (MKCC).
Abbreviations List
- AS
aortic stenosis
- CT
Computed tomography (CT)
- CVA
Cerebrovascular accident
- ECG
Electrocardiogram
- Euroscore
European system for cardiac operative risk evaluation
- LVEF
Left ventricular ejection fraction
- MKCC
Mohammed bin Khalifa Cardiac Centre
- PPM
Permanent pacemaker
- sAVR
Surgical aortic valve replacement
- STS
Society of Thoracic Surgery
- TAVI
Transcatheter aortic valve implantation
- TEE
Transesophageal echocardiography
- TTE
Transthoracic echocardiography
- VARC
Valve Academic Research Consortium-2
Author contribution
Conception and design of Study, Research investigation and analysis: Nooraldaem Yousif, Suddharsan Subbramaniyam. Literature review, Acquisition of data, Revising and editing the manuscript critically for important intellectual contents, Approval: Nooraldaem Yousif, Suddharsan Subbramaniyam, Babu Thevan, Mohammad amin, Leena Sulaibikh, Nazar Bukamal, Habib Tareif, Sadananda Shivappa, Haitham Amin, Husam A. Noor. Analysis and interpretation of data: Nooraldaem Yousif. Data collection: Nooraldaem Yousif, Suddharsan Subbramaniyam, Babu Thevan. Drafting of manuscript: Nooraldaem Yousif, Suddharsan Subbramaniyam, Husam A. Noor. Data preparation and presentation: Nooraldaem Yousif, Suddharsan Subbramaniyam, Babu Thevan, Husam A. Noor. Supervision of the research: Mohammad amin, Leena Sulaibikh, Nazar Bukamal, Habib Tareif, Sadananda Shivappa, Haitham Amin, Husam A. Noor. Research coordination and management: Sadananda Shivappa, Haitham Amin, Husam A. Noor.
Limitations
The relatively small sample size and retrospective design precluded more extensive characterization of the cohort population.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Salam AA, Al-Khraif RM. Child mortality transition in the arabian Gulf: wealth, health system reforms, and development goals. Front Public Health. 2020;7:402. doi: 10.3389/fpubh.2019.00402. Published 2020 Jan 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AH, Jaber S, Aziz MIA, AlBuhairan F, AlGhaithi A, AlHamad NM, et al. The state of health in the Arab world, 1990–2010: an analysis of the burden of diseases, injuries, and risk factors. Lancet. 2014;383:309–20. doi: 10.1016/S0140-6736(13)62189-3. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010 Oct 21;363(17):1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 4.Eleid MF, Holmes Jr DR. Transcatheter aortic valve replacement: state of the art and future directions. Annu Rev Med. 2017;68:15–28. doi: 10.1146/annurev-med-101615-020427. [DOI] [PubMed] [Google Scholar]
- 5.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Eltchaninoff H, Durand E, Cribier A. TAVI durability beyond five years: no alarms, but stay alert. EuroIntervention. 2018 Jul 20;14(4):e380–2. doi: 10.4244/EIJV14I4A67. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen V, Michel M, Eltchaninoff H, Gilard M, Dindorf C, Iung B, et al. Implementation of transcatheter aortic valve replacement in France. J Am Coll Cardiol. 2018;71(15):1614–27. doi: 10.1016/j.jacc.2018.01.079. [DOI] [PubMed] [Google Scholar]
- 8.Polimeni A, Sorrentino S, De Rosa S, Spaccarotella C, Mongiardo A, Sabatino J, et al. Transcatheter versus surgical aortic valve replacement in low-risk patients for the treatment of severe aortic stenosis. J Clin Med. 2020;9(2):439. doi: 10.3390/jcm9020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voigtländer L, Seiffert M. Expanding TAVI to low and in termediate risk patients. Front Cardiovasc Med. 2018;5:92. doi: 10.3389/fcvm.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145(1):6–23. doi: 10.1016/j.jtcvs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Abuzeyad F, Alqasem L, Al Farras MI, Al Jawder SS, Al Qasim G, Alghanem S. Emergency medicine in the kingdom of Bahrain. Int J Emerg Med. 2018 Feb 8;11(1):4. doi: 10.1186/s12245-018-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. https://www.worldometers.info/demographics/lifeexpectancy/#countries-ranked-by-life-expectancy.
- 13.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017 Apr 6;376(14):1321–31. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 14.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016 Apr 28;374(17):1609–20. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 15.Fauvel C, Capoulade R, Durand E, Durand E, Beziau DM, Schott JJ, Le Tourneau T, et al. Durability of transcatheter aortic valve implantation: a translational review. Arch Cardiovasc Dis. 2020 Mar;113(3):209–21. doi: 10.1016/j.acvd.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Svensson LG, Tuzcu M, Kapadia S, Blackstone EH, Roselli EE, Gillinov AM, et al. A comprehensive review of the PARTNER trial. J Thorac Cardiovasc Surg. 2013 Mar;145(3 Suppl):S11–6. doi: 10.1016/j.jtcvs.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 17.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012 May 3;366(18):1686–95. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 18.Siontis GC, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014 Jul 15;64(2):129–40. doi: 10.1016/j.jacc.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Gaede L, Kim WK, Liebetrau C, Dörr O, Sperzel J, Blumenstein J, et al. Pacemaker implantation after TAVI: predictors of AV block persistence. Clin Res Cardiol. 2018 Jan;107(1):60–9. doi: 10.1007/s00392-017-1158-2. [DOI] [PubMed] [Google Scholar]
- 20.Lenders GD, Collas V, Hernandez JM, Legrand V, Danenberg HD, den Heijer P, et al. Depth of valve implantation, conduction disturbances and pacemaker implantation with CoreValve and CoreValve Accutrak system for Trans-catheter Aortic Valve Implantation, a multi-center study. Int J Cardiol. 2014 Oct 20;176(3):771–5. doi: 10.1016/j.ijcard.2014.07.092. [DOI] [PubMed] [Google Scholar]


