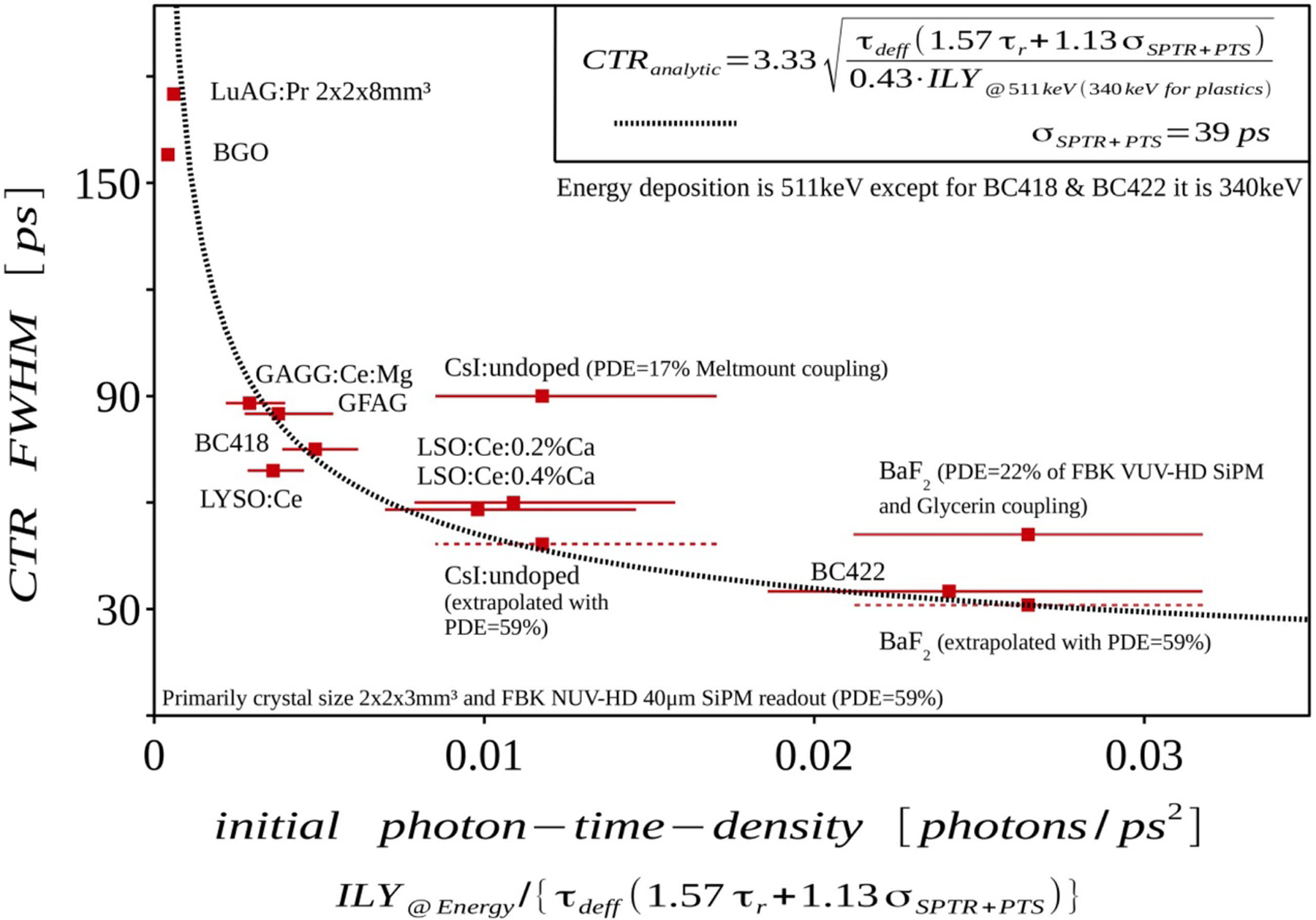
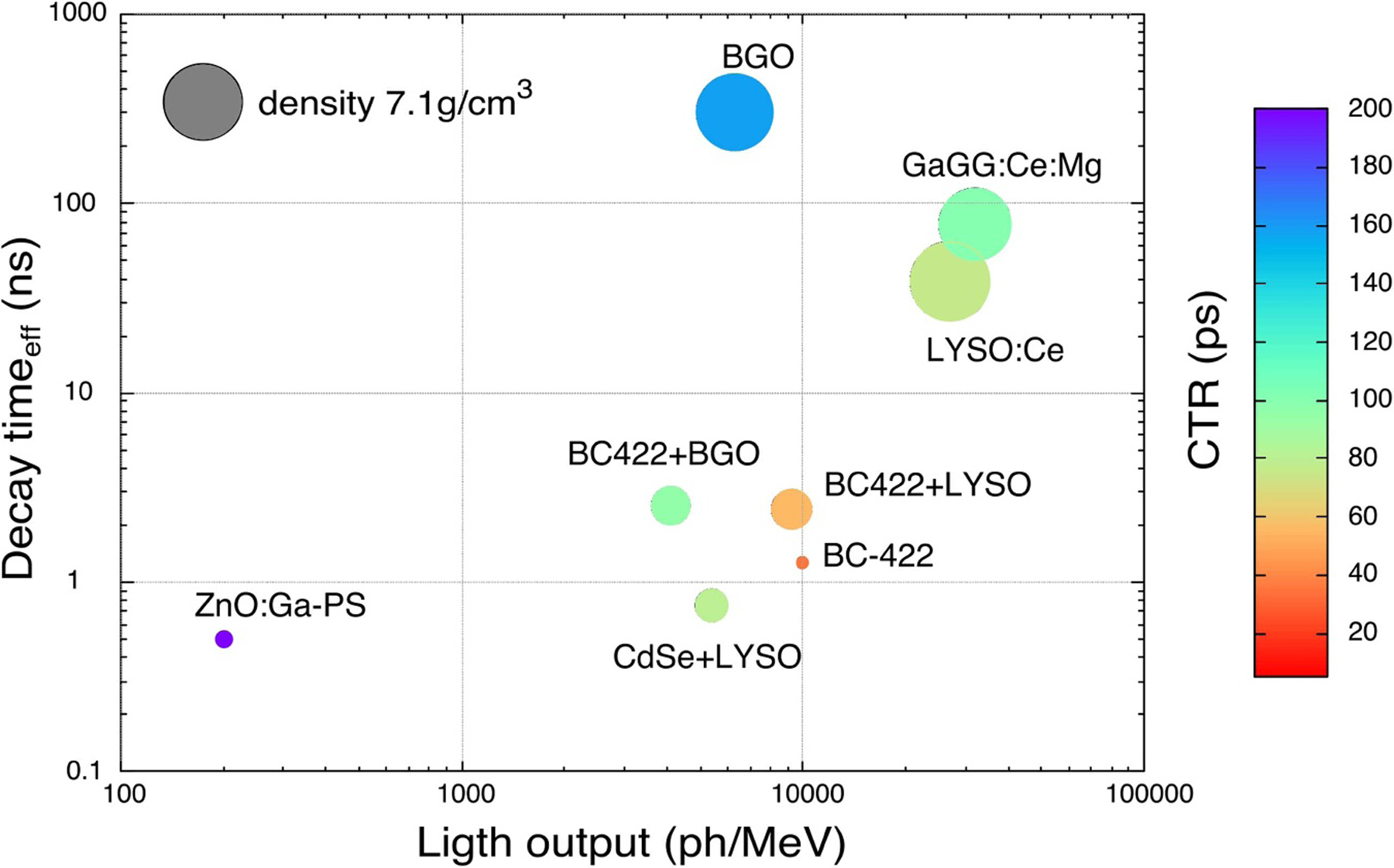
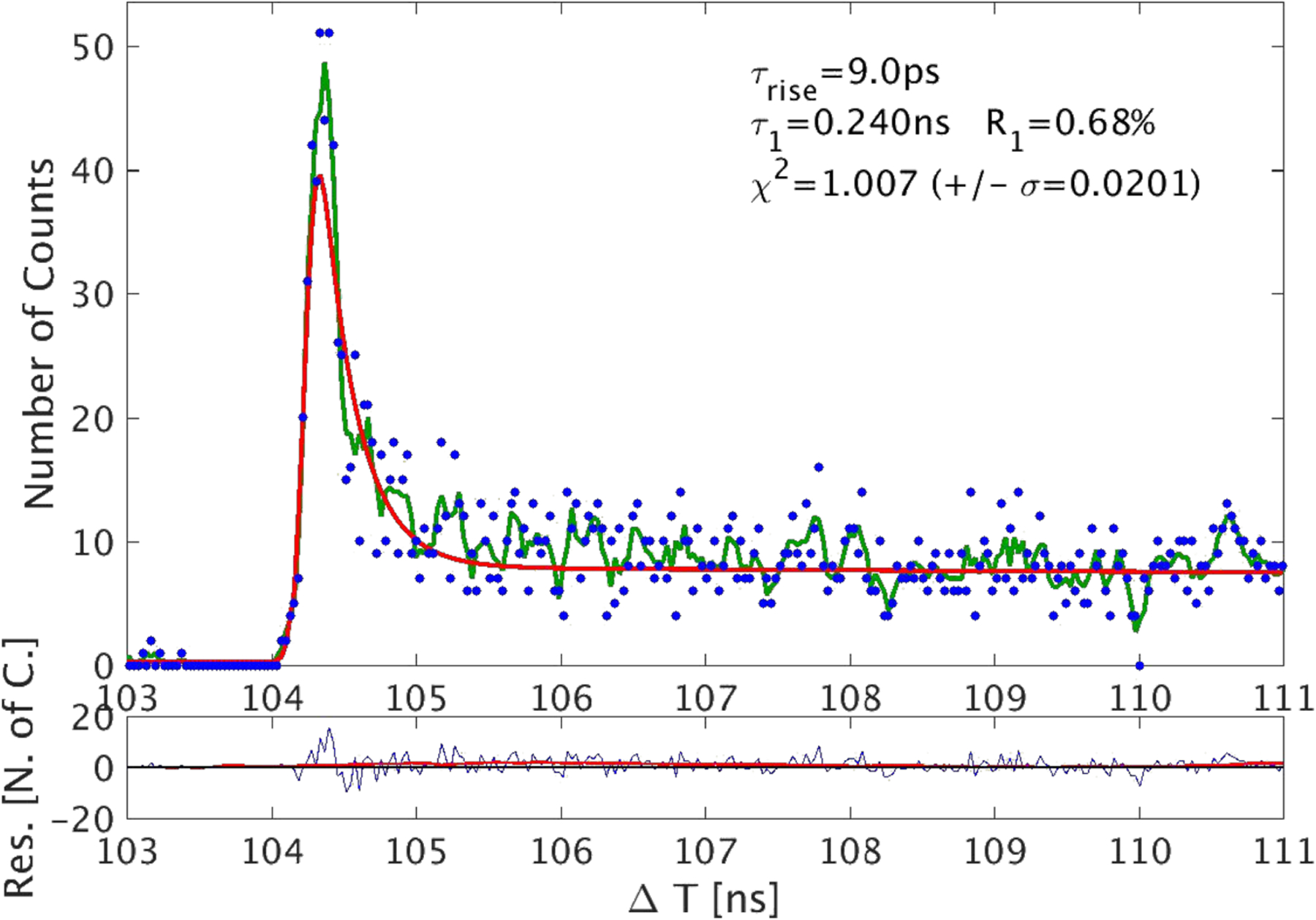
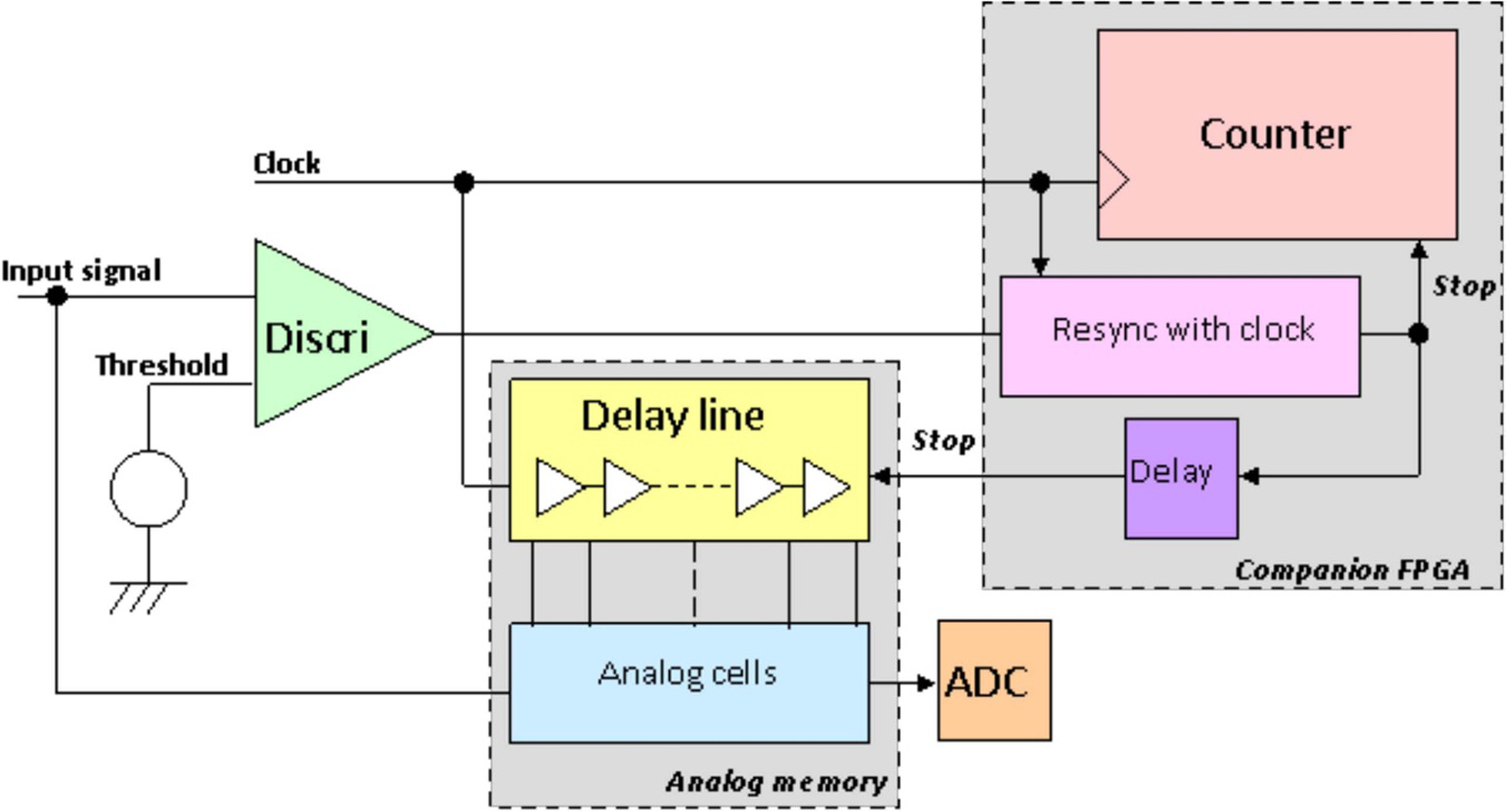
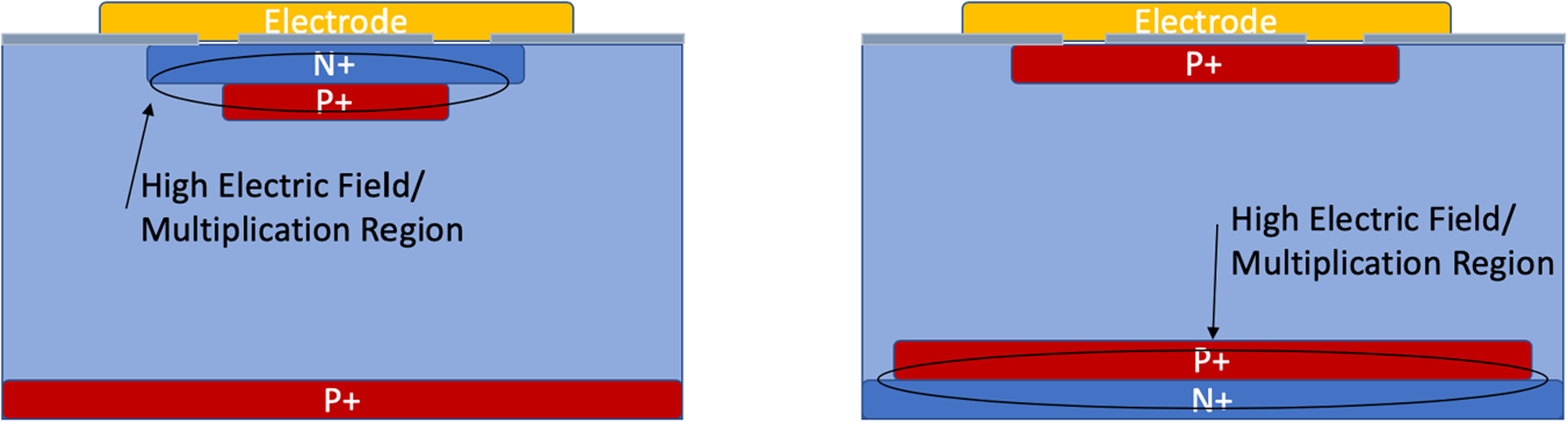
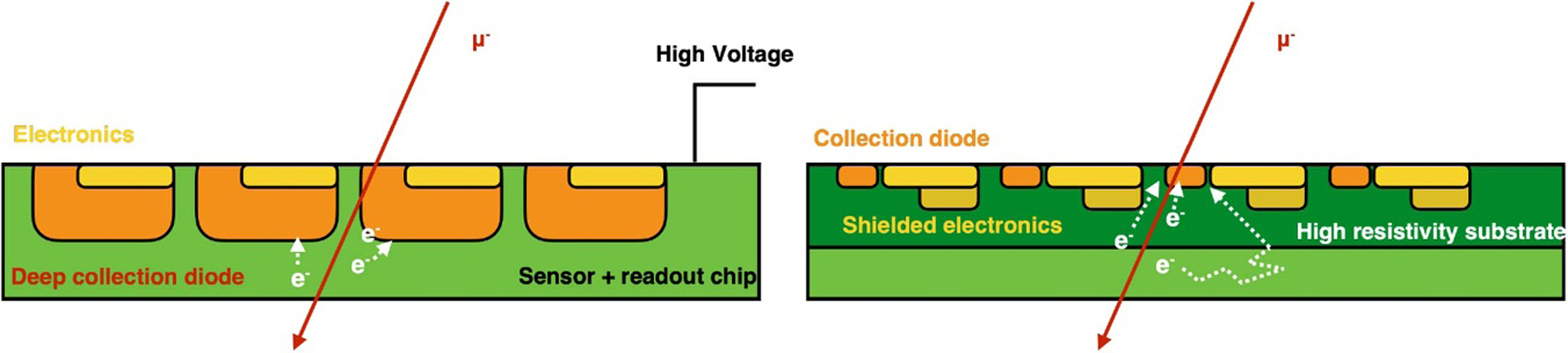
Abstract
Since the seventies, positron emission tomography (PET) has become an invaluable medical molecular imaging modality with an unprecedented sensitivity at the picomolar level, especially for cancer diagnosis and the monitoring of its response to therapy. More recently, its combination with x-ray computed tomography (CT) or magnetic resonance (MR) has added high precision anatomic information in fused PET/CT and PET/MR images, thus compensating for the modest intrinsic spatial resolution of PET. Nevertheless, a number of medical challenges call for further improvements in PET sensitivity. These concern in particular new treatment opportunities in the context personalized (also called precision) medicine, such as the need to dynamically track a small number of cells in cancer immunotherapy or stem cells for tissue repair procedures. A better signal-to-noise ratio (SNR) in the image would allow detecting smaller size tumours together with a better staging of the patients, thus increasing the chances of putting cancer in complete remission. Moreover, there is an increasing demand for reducing the radioactive doses injected to the patients without impairing image quality.
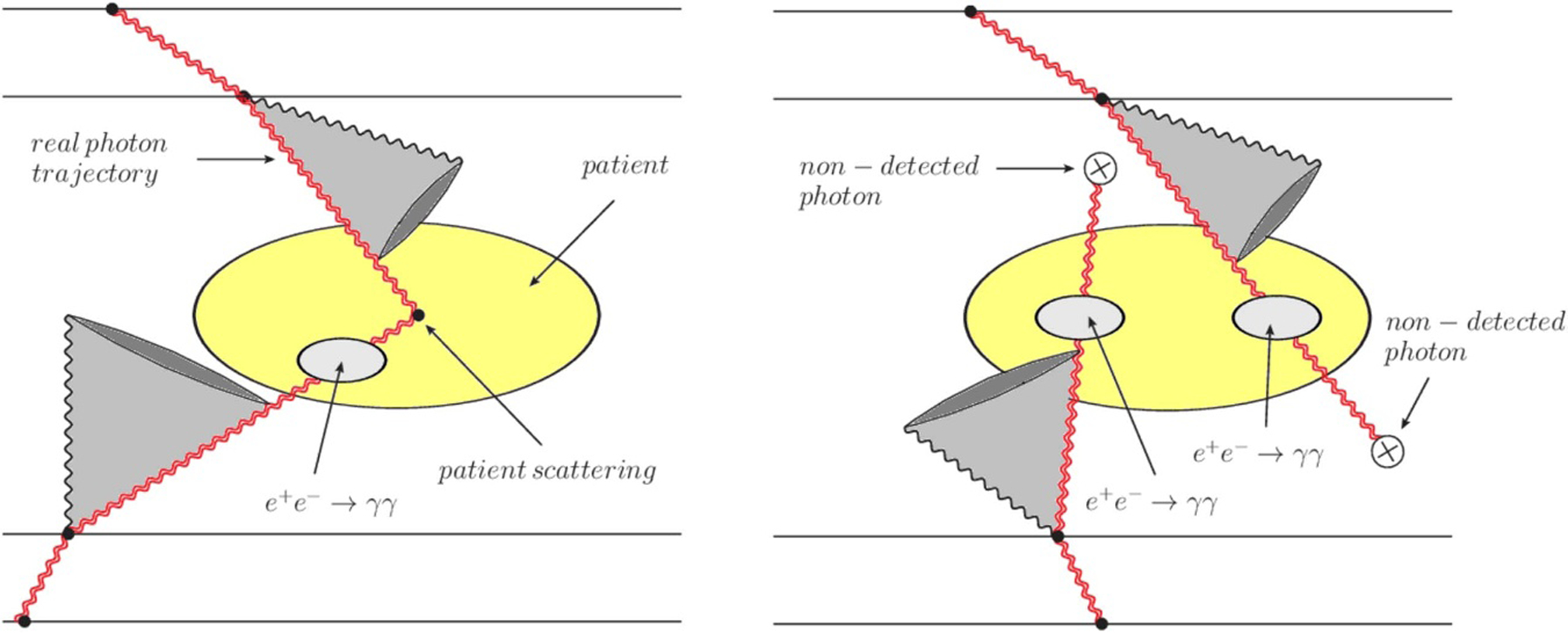
There are three ways to improve PET scanner sensitivity: improving detector efficiency, increasing geometrical acceptance of the imaging device and pushing the timing performance of the detectors. Currently, some pre-localization of the electron-positron annihilation along a line-of-response (LOR) given by the detection of a pair of annihilation photons is provided by the detection of the time difference between the two photons, also known as the time-of-flight (TOF) difference of the photons, whose accuracy is given by the coincidence time resolution (CTR). A CTR of about 10 picoseconds FWHM will ultimately allow to obtain a direct 3D volume representation of the activity distribution of a positron emitting radiopharmaceutical, at the millimetre level, thus introducing a quantum leap in PET imaging and quantification and fostering more frequent use of 11C radiopharmaceuticals.
The present roadmap article toward the advent of 10 ps TOF-PET addresses the status and current/future challenges along the development of TOF-PET with the objective to reach this mythic 10 ps frontier that will open the door to real-time volume imaging virtually without tomographic inversion. The medical impact and prospects to achieve this technological revolution from the detection and image reconstruction point-of-views, together with a few perspectives beyond the TOF-PET application are discussed.
Keywords: positron emission tomography, detection of annihilation photons, photo-detectors, image reconstruction, 10 ps TOF-PET
References
- Acerbi F et al. 2017. Cryogenic characterization of FBK HD near-UV sensitive SiPMs IEEE Trans. Electron Devices 64 521–6 [Google Scholar]
- Acerbi F and Gundacker S 2019. Understanding and simulating SiPMs Nucl. Instrum. Methods Phys. Res. A 926 16–35 [Google Scholar]
- Aglieri Rinella G et al. 2019. The NA62 GigaTracKer: a low mass high intensity beam 4D tracker with 65 ps time resolution on tracks (arXiv:1904.12837) [Google Scholar]
- Ahn S. et al. Joint estimation of activity and attenuation for PET using pragmatic MR-based prior: application to clinical TOF PET/MR whole-body data for FDG and non-FDG tracers. Phys. Med. Biol. 2018;63:045006. doi: 10.1088/1361-6560/aaa8a6. [DOI] [PubMed] [Google Scholar]
- Amaudruz P et al. 2009. Simultaneous reconstruction of scintillation light and ionization charge produced by 511 keV photons in liquid xenon: potential application to PET Nucl. Instrum. Methods Phys. Res. A 607 668–76 [Google Scholar]
- Aprile E, Curioni A, Giboni KL, Kobayashi M, Oberlack UG and Zhang S 2008. Compton imaging of MeV gamma-rays with the liquid xenon gamma-ray imaging telescope (LXeGRIT) Nucl. Instrum. Methods Phys. Res. A 593 414–25 [Google Scholar]
- Aprile E and Doke T 2010. Liquid xenon detectors for particle physics and astrophysics Rev. Mod. Phys 82 2053–97 [Google Scholar]
- Ariño-Estrada G, Mitchell GS, Kwon SI, Du J, Kim H, Cirignano LJ, Shah KS and Cherry SR 2018. Towards time-of-flight PET with a semiconductor detector Phys. Med. Biol 63 04LT01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASCIMAT 2020. Increasing the scientific excellence and innovation capacity in Advanced Scintillation Materials of the Institute of Physics from the Czech Academy of Sciences, European TWIN project grant agreement 690599 (http://www.h2020-ascimat.com) (Accessed: 14 June 2020)
- Badawi RD et al. 2019. First human imaging studies with the EXPLORER total-body PET scanner J. Nucl. Med 60 299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten HD and Flake AW 2019. Fetal surgery Pediatr. Clin. North Am 66 295–308 [DOI] [PubMed] [Google Scholar]
- Benoit M. Pixel detector R&D for the compact linear collider. J. Instrum. 2019;14:C06003. [Google Scholar]
- Berdalovic I. et al. Monolithic pixel development in TowerJazz 180 nm CMOS for the outer pixel layers in the ATLAS experiment. J. Instrum. 2018;13:C01023. [Google Scholar]
- Berg E and Cherry SR 2018. Using convolutional neural networks to estimate time-of-flight from PET detector waveforms Phys. Med. Biol 63 02LT01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berker Y and Li Y 2016. Attenuation correction in emission tomography—a review Med. Phys 43 807–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biassoni L and Easty M 2017. Paediatric nuclear medicine imaging Br. Med. Bull 123 127–48 [DOI] [PubMed] [Google Scholar]
- Bornheim A et al. 2017. Precision timing calorimetry with the upgraded CMS crystal ECAL Proc. Int. Conf. on Technology and Instrumentation in Particle Physics 2017 ed Z A Liu vol 213 (Berlin: Springer) ( 10.1007/978-981-13-1316-5_8) [DOI] [Google Scholar]
- Braga LHC et al. 2013. An 8 × 16-pixel 92kSPAD time-resolved sensor with on-pixel 64ps 12b TDC and 100MS/s real-time energy histogramming in 0.13microm CIS technology for PET/MRI applications Proc. IEEE Int. Solid-State Circuits Conf (San Francisco, CA) pp 486–7 [Google Scholar]
- Breton D, Delagnes E and Maalmi J 2009. Picosecond time measurement using ultra-fast analog memories Proc. of TWEPP-09 (Paris, France) pp 149–54 [Google Scholar]
- Breton D, De Cacqueray V, Delagnes E, Grabas H, Maalmi J, Minafra N, Royon C and Saimpert M 2016. Measurements of timing resolution of ultra-fast silicon detectors with the SAMPIC waveform digitizer Nucl. Instrum. Methods A 835 51–60 [Google Scholar]
- Breton D, Delagnes E, Maalmi J and Rusquart P 2014. The WaveCatcher family of SCA-based 12-bit 3.2-GS/s fast digitizers Conf. Rec. IEEE Real-Time pp 1–8 [Google Scholar]
- Brunner S and Schaart D 2017. Enabling cost-effective TOF-PET by exploiting the Cherenkov emission in BGO J. Nucl. Med 58 150 [Google Scholar]
- Budinger TF 1983. Time-of-flight positron emission tomography—status relative to conventional PET J. Nucl. Med 24 73–76 [PubMed] [Google Scholar]
- Buitenhuis HJT, Diblen F, Brzezinski KW, Brandenburg S and Dendooven P 2017. Beam-on imaging of short-lived positron emitters during proton therapy Phys. Med. Biol 62 4654–72 [DOI] [PubMed] [Google Scholar]
- Burgess AE 2011. Visual perception studies and observer models in medical imaging Semin. Nucl. Med 41 419–36 [DOI] [PubMed] [Google Scholar]
- Cai Z, Li S, Matuskey D, Nabulsi N and Huang Y 2019. PET imaging of synaptic density: a new tool for investigation of neuropsychiatric diseases Neurosci. Lett 691 44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. et al. Towards a new generation of pixel detector readout chips. J. Instrum. 2016;11:C01007. [Google Scholar]
- Carniti P. et al. CLARO-CMOS, a very low power ASIC for fast photon counting with pixellated photodetectors. J. Instrum. 2012;7:P11026. [Google Scholar]
- Carson RE and Kuo PH 2019. Brain-dedicated emission tomography systems: a perspective on requirements for clinical research and clinical needs in brain imaging IEEE Trans. Radiat. Plasma Med. Sci 3 254–61 [Google Scholar]
- Cartiglia N et al. 2017. Beam test results of a 16 ps timing system based on ultra-fast silicon detectors Nucl. Instrum. Methods A 850 83–88 [Google Scholar]
- Cates JW, Gundacker S, Auffray E, Lecoq P and Levin CS 2018. Improved single photon time resolution for analog SiPMs with front end readout that reduces influence of electronic noise Phys. Med. Biol 63 185022. [DOI] [PubMed] [Google Scholar]
- Chan KL et al. 2017. A 32.75-Gb/s voltage-mode transmitter with three-tap FFE in 16-nm CMOS IEEE J. Solid-State Circuits 52 2663–78 [Google Scholar]
- Chepel V, Lopes MI, Kuchenkov A, Ferreira Marques R and Policarpo AJPL 1997. Performance study of liquid xenon detector for PET Nucl. Instrum. Methods Phys. Res. A 392 427–32 [Google Scholar]
- Cherenkov PA 1934. Visible emission of clean liquids by action of radiation Doklady Akademii Nauk SSSR vol 2 p 451 [Google Scholar]
- Cherry SR. et al. Total-body imaging: transforming the role of positron emission tomography. Sci. Transl. Med. 2017;9:eaaf6169. doi: 10.1126/scitranslmed.aaf6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry SR, Jones T, Karp JS, Qi J, Moses WW and Badawi RD 2018. Total-body PET: maximizing sensitivity to create new opportunities for clinical research and patient care J. Nucl. Med 59 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry SR, Sorenson JA and Phelps ME 2012. Physics in Nuclear Medicine e-Book (Amsterdam: Elsevier; ) [Google Scholar]
- Collazuol G. The SiPM physics and technology–a review. Proc. of the Int. Workshop on New Photon-Detectors (PhotoDet 2012) (Orsay, France) 2012 [Google Scholar]
- Consuegra D, Dolenec R, Korpar S, Križan P, Pestotnik R and Razdevšek G 2020. Simulation study to improve the performance of a whole-body PbF2 Cherenkov TOF-PE0T scanner Phys. Med. Biol 65 055013. [DOI] [PubMed] [Google Scholar]
- Conti M and Bendriem B 2019. The new opportunities for high time resolution clinical TOF PET Clin. Transl. Imaging 7 139–47 [Google Scholar]
- Crespo P, Shakirin G, Fiedler F, Enghardt W and Wagner A 2007. Direct time-of-flight for quantitative, real-time in-beam PET: a concept and feasibility study Phys. Med. Biol 52 6795–811 [DOI] [PubMed] [Google Scholar]
- Crystal Clear Collaboration RD18, CERN/DRDC/P27/91–15 (https://cds.cern.ch/record/291227)
- de La Taille C. et al. ALTIROC0, a 20 pico-second time resolution ASIC for the ATLAS high granularity timing detector (HGTD) PoS (TWEPP-17) 2018;313:006. [Google Scholar]
- Defrise M, Rezaei A and Nuyts J 2012. Time-of-flight PET data determine the attenuation sinogram up to a constant Phys. Med. Biol 57 885–99 [DOI] [PubMed] [Google Scholar]
- Defrise M, Rezaei A and Nuyts J 2014. Transmission-less attenuation correction in time-of-flight PET: analysis of a discrete iterative algorithm Phys. Med. Biol 59 1073–95 [DOI] [PubMed] [Google Scholar]
- Delagnes E 2016. What is the theoretical time precision achievable using a CFD algorithm? (arXiv:1606.05541) [Google Scholar]
- Delagnes E, Breton D, Grabas H, Maalmi J and Rusquart P 2015. Reaching a few picosecond timing precision with the 16-channel digitizer and time stamper SAMPIC ASIC Nucl. Instrum. Methods A 787 245–9 [Google Scholar]
- Delso G et al. 2017. Effect of time-of-flight information on PET/MR reconstruction artifacts: comparison of free-breathing versus breath-hold MR-based attenuation correction Radiology 282 229–35 [DOI] [PubMed] [Google Scholar]
- Dey S, Rudell JC, Lewellen TK and Miyaoka RS 2017. A CMOS front-end interface ASIC for SiPM-based positron emission tomography imaging systems Conf. Rec. IEEE BioCAS ( 10.1109/BIOCAS.2017.8325059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco A. et al. TOFPET2: a high-performance ASIC for time and amplitude measurements of SiPM signals in time-of-flight applications. J. Instrum. 2016;11:C03042. [Google Scholar]
- Doke T, Kikuchi J and Nishikido F 2006. Time-of-flight positron emission tomography using liquid xenon scintillation Nucl. Instrum. Methods Phys. Res. A 569 863–71 [Google Scholar]
- Dolenec R, Korpar S, Križan P and Pestotnik R 2020. Efficiency of a Cherenkov based PET module with an array of SiPMs Nucl. Instrum. Methods Phys. Res 952 162327 [Google Scholar]
- Dolenec R, Korpar S, Križan P, Pestotnik R and Verdel N 2016. The performance of silicon photomultipliers in Cherenkov TOF PET IEEE Trans. Nucl. Sci 63 2478–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgoshein B 2003. Silicon photomultipliers in particle physics: possibilities and limitations Proc. of the 42nd Workshop of the INFN ELOISATRON Project (Erice, Italy) pp 442–56 [Google Scholar]
- Dorenbos P et al. 2010. Fundamental limitations in the performance of Ce3+−, Pr3+−, and Eu2+−activated scintillators IEEE Trans. Nucl. Sci 57 1162–7 [Google Scholar]
- Dosovitskiy GA, Karpyuk PV, Evdokimov PV, Kuznetsova DE, Mechinsky VA, Borisevich AE, Fedorov AA, Putlayev VI, Dosovitskiy AE and Korjik MV 2017. First 3D-printed complex 3 inorganic polycrystalline scintillator CrystEngComm 19 4260–4 [Google Scholar]
- FAST: Fast Advanced Scintillator Timing, COST Action TD1401 (http://fast-cost.web.cern.ch/fast-cost/) (Accessed: 14 June 2020)
- Fayad H, Gilles M, Pan T and Visvikis D 2018. A 4D global respiratory motion model of the thorax based on CT images: a proof of concept Med. Phys 45 3043–51 [DOI] [PubMed] [Google Scholar]
- Fayad H, Lamare F, Merlin T and Visvikis D 2016. Motion correction using anatomical information in PET/CT and PET/MR hybrid imaging Q. J. Nucl. Med. Mol. Imaging 60 12–24 [PubMed] [Google Scholar]
- Feldmann J, Peter G, Göbel EO, Dawson P, Moore K, Foxon C and Elliott RJ 1987. Linewidth dependence of radiative exciton lifetimes in quantum wells Phys. Rev. Lett 59 2337–40 [DOI] [PubMed] [Google Scholar]
- Fernandez-Martinez P et al. 2016. Design and fabrication of an optimum peripheral region for low gain avalanche detectors Nucl. Instrum. Methods Phys. Res. A 821 93–100 [Google Scholar]
- Ferrero V. et al. Online proton therapy monitoring: clinical test of a silicon-photodetector-based in-beam PET. Sci. Rep. 2018;8:4100. doi: 10.1038/s41598-018-22325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini C, Gola A, Zanchi M, Longoni A, Lechner P, Soltau H and Struder L 2006. Gamma-ray spectroscopy with LaBr: cescintillator readout by a silicon drift detector IEEE Trans. Nucl. Sci 53 2392–7 [Google Scholar]
- Fishburn MW and Charbon E 2010. System tradeoffs in gamma-ray detection utilizing SPAD arrays and scintillator IEEE Trans. Nucl. Sci 57 2549–57 [Google Scholar]
- Fleury J. et al. Petiroc and Citiroc: front-end ASICs for SiPM read-out and ToF applications. J. Instrum. 2014;9:C01049. [Google Scholar]
- FONAR Corp 2011. Major diagnostic breakthrough in multiple sclerosis achieved with advanced UPRIGHT® MRI (http://www.fonar.com/news/100511.htm) (Accessed: 14 June 2020)
- Foss CA, Kulik L, Ordonez AA, Jain SK, Michael Holers V, Thurman JM and Pomper MG 2019. SPECT/CT imaging of mycobacterium tuberculosis infection with [125I]anti-C3d mAb Mol. Imaging Biol 21 473–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frach T, Prescher G, Degenhardt C, de Gruyter R, Schmitz A and Ballizany R 2009. The digital silicon photomultiplier—principle of operation and intrinsic detector performance Conf. Rec. IEEE Nucl. Sci. Symp pp 1959–65 [Google Scholar]
- Fülöp L and Biró T 1992. Cherenkov radiation spectrum Int. J. Theor. Phys 31 61–74 [Google Scholar]
- Gallego Manzano L et al. 2015. XEMIS: a liquid xenon detector for medical imaging Nucl. Instrum. Methods Phys. Res. A 787 89–93 [Google Scholar]
- Gallezot JD, Lu Y, Naganawa M and Carson RE 2019. Parametric Imaging with PET and SPECT IEEE Trans. Radiat. Plasma Med. Sci 4 1–23 [Google Scholar]
- Genat J-F, Varner GS, Tang F and Frisch HJ 2009. Signal processing for pico-second resolution timing measurements Nucl. Instrum. Methods A 607 387–93 [Google Scholar]
- Geoffroy C, Michaud J-B, Tetrault M-A, Clerk-Lamalice J, Brunet C-A, Lecomte R and Fontaine R 2015. Real time artificial neural network FPGA implementation for triple coincidences recovery in PET IEEE Trans. Nucl. Sci 62 824–31 [Google Scholar]
- Gomez-Cadenas JJ, Benlloch-Rodriguez JM and Ferrario P 2016. Application of scintillating properties of liquid xenon and silicon photomultiplier technology to medical imaging Spectrochim. Acta B 118 6–13 [Google Scholar]
- Gomez-Cadenas JJ, Benlloch-Rodríguez JM and Ferrario P 2017. Monte Carlo study of the coincidence resolving time of a liquid xenon PET scanner, using Cherenkov radiation J. Instrum 12 P08023 [Google Scholar]
- González AJ, Sánchez F and Benlloch JM 2018. Organ-dedicated molecular imaging systems IEEE Trans. Radiat. Plasma Med. Sci 2 388–403 [Google Scholar]
- Graves CE, Harrison MR and Padilla BE 2017. Minimally invasive fetal surgery Clin. Perinatol 44 729–51 [DOI] [PubMed] [Google Scholar]
- Grim JQ, Christodoulou S, Stasio FD, Krahne R, Cingolani R, Manna L and Moreels I 2014. Continuous-wave biexciton lasing at room temperature using solution-processed quantum wells Nat. Nanotechnol 9 891–5 [DOI] [PubMed] [Google Scholar]
- Gundacker S. et al. Time of flight positron emission tomography towards 100 ps resolution with L(Y)SO: an experimental and theoretical analysis. J. Instrum. 2013;8:P07014. [Google Scholar]
- Gundacker S, Auffray E, Jarron P, Meyer T and Lecoq P 2015. On the comparison of analog and digital SiPM readout in terms of expected timing performance Nucl. Instrum. Methods Phys. Res. A 787 6–11 [Google Scholar]
- Gundacker S, Auffray E, Pauwels K and Lecoq P 2016. Measurement of intrinsic rise times for various L(Y)SO and LuAG scintillators with a general study of prompt photons to achieve 10 ps in TOF-PET Phys. Med. Biol 61 2802–37 [DOI] [PubMed] [Google Scholar]
- Gundacker S, Turtos RM, Auffray E and Lecoq P 2018. Precise rise and decay time measurements of inorganic scintillators bymeans of X-ray and 511 keV excitation Nucl. Instrum. Methods Phys. Res. A 891 42–52 [Google Scholar]
- Gundacker S, Turtos RM, Auffray E, Paganoni M and Lecoq P 2019. High-frequency SiPM readout advances measured coincidence time resolution limits in TOF-PET Phys. Med. Biol 64 055012. [DOI] [PubMed] [Google Scholar]
- Gundacker S, Turtos RM, Kratochwil N, Pots RH, Paganoni M, Lecoq P and Auffray E 2020. Experimental time resolution limits of modern SiPMs and TOF-PET detectors exploring different scintillators and Cherenkov emission Phys. Med. Biol 65 025001. [DOI] [PubMed] [Google Scholar]
- Harion T. et al. STiC—a mixed mode silicon photomultiplier readout ASIC for time-of-flight applications. J. Instrum. 2014;9:C02003. [Google Scholar]
- Hatt M, Tixier F, Pierce L, Kinahan PE, Le Rest CC and Visvikis D 2017. Characterization of PET/CT images using texture analysis: the past, the present… any future? Eur. J. Nucl. Med. Mol. Imaging 44 151–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Vaccaro DH, Haznedar MM and Goldstein KE 2019. F-18Fluorodeoxyglucose positron emission tomography studies of the schizophrenia spectrum: the legacy of Monte S. Buchsbaum, M.D. Psychiatry Res. 271 535–40 [DOI] [PubMed] [Google Scholar]
- Hutton BF, Yamaya T and Furenlid LR 2019. Dedicated molecular imaging systems for human neurological studies IEEE Trans. Radiat. Plasma Med. Sci 3 252–3 [Google Scholar]
- Ilisie V. et al. Improving PET sensitivity with a Compton algorithm. J. Phys.: Conf. Ser. 2017;931:012012. [Google Scholar]
- Ilisie V, Giménez-Alventosa V, Moliner L, Sánchez F, González AJ, Rodríguez-Álvarez MJ and Benlloch JM 2018. Building blocks of a multi-layer PET with time sequence photon interaction discrimination and double Compton camera Nucl. Instrum. Methods A 895 74–83 [Google Scholar]
- Intelum: European RISE project grant agreement 644260 (https://intelum.web.cern.ch) (Accessed: 14 June 2020)
- Jacobson O and Chen X 2013. Interrogating tumor metabolism and tumor microenvironments using molecular positron emission tomography imaging. Theranostic approaches to improve therapeutics Pharmacol. Rev 65 1214–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK 2017. The promise of molecular imaging in the study and treatment of infectious diseases Mol. Imaging Biol 19 341–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpar S, Dolenec R, Križan P, Pestotnik R and Stanovnik A 2011. Study of TOF PET using Cherenkov light Nucl. Instrum. Methods Phys. Res. A 654 532–8 [Google Scholar]
- Korpar S, Dolenec R, Križan P, Pestotnik R and Stanovnik A 2012. Study of TOF PET using Cherenkov light Phys. Procedia 37 1531–6 [Google Scholar]
- Kovalenko MV, Protesescu L and Bodnarchuk MI 2017. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals Science 358 745–50 [DOI] [PubMed] [Google Scholar]
- Kuo F-W et al. 2018. An all-digital PLL for cellular mobile phones in 28-nm CMOS with −55 dBc fractional and −91 dBc reference spurs IEEE Trans. Circ. Syst. I 65 3756–68 [Google Scholar]
- Kwon SI, Gola A, Ferri A, Piemonte C and Cherry SR 2016. Bismuth germanate coupled to near ultraviolet silicon photomultipliers for time-of-flight PET Phys. Med. Biol 61 L38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahorte CMM, Vanderheyden J-L, Steinmetz N, Van de Wiele C, Dierckx RA and Slegers G 2004. Apoptosis-detecting radioligands: current state of the art and future perspectives Eur. J. Nucl. Med. Mol. Imaging 31 887–919 [DOI] [PubMed] [Google Scholar]
- Lange J et al. 2017. Gain and time resolution of 45 μm thin low gain avalanche detectors before and after irradiation up to 1015 neq/cm2 (arXiv:1703.09004) [Google Scholar]
- Lavoie L 1976. Liquid xenon scintillators for imaging of positron emitters Med. Phys 3 283–93 [DOI] [PubMed] [Google Scholar]
- Lecoq P 2017. Pushing the limits in time-of-flight PET imaging IEEE Trans. Radiat. Plasma Med. Sci 1 473–85 [Google Scholar]
- Lecoq P, Auffray E, Brunner S, Hillemanns H, Jarron P, Knapitsch A, Meyer T and Powolny F 2010. Factors influencing time resolution of scintillators and ways to improve them IEEE Trans. Nucl. Sci 57 2411–6 [Google Scholar]
- Lecoq P, Korzhik M and Vasiliev A 2014. Can transient phenomena help improving time resolution in scintillators? IEEE Trans. Nucl. Sci 61 229–34 [Google Scholar]
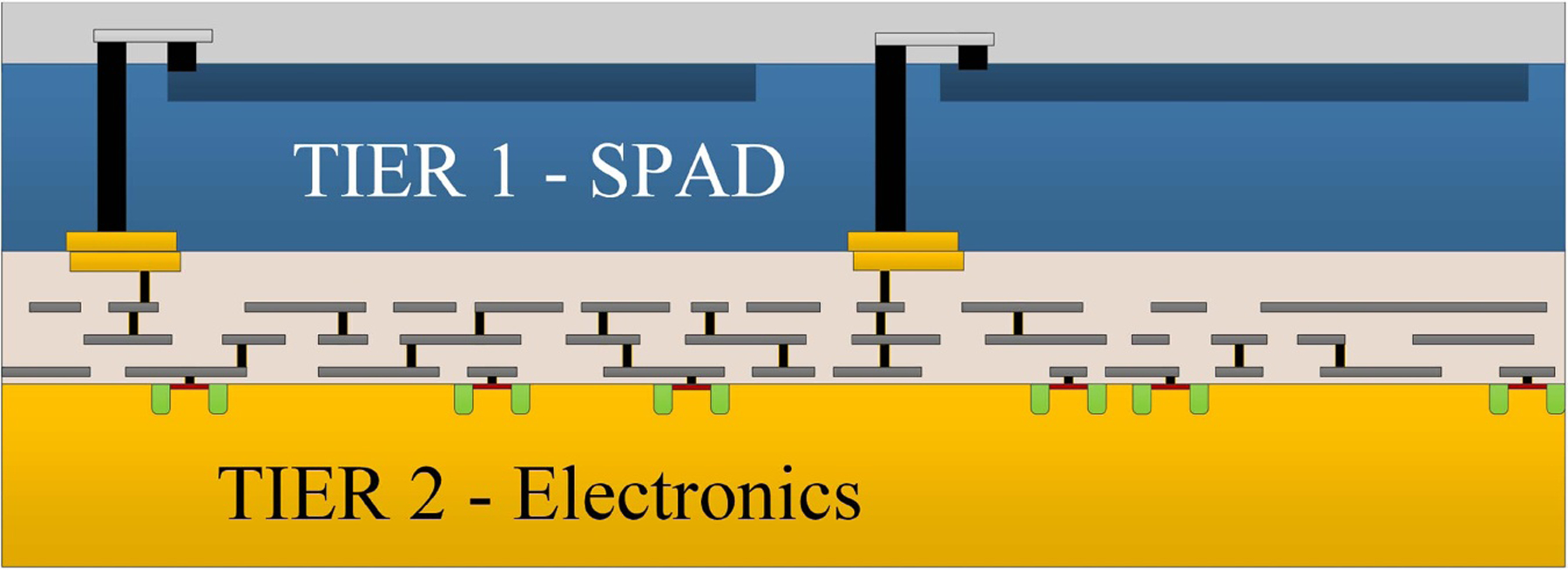
- Lee M-J, Ximenes AR, Padmanabhan P, Wang T-J, Huang K-C, Yamashita Y, Yaung D-N and Charbon E 2018. High-performance back-illuminated three-dimensional stacked single-photon avalanche diode implemented in 45-nm CMOS technology IEEE J. Sel. Top. Quantum Electron 24 3801809 [Google Scholar]
- Lemaire W, Therrien AC, Pratte J-F and Fontaine R 2020. Dark count resilient time estimators for time-of-flight PET IEEE Trans. Radiat. Plasma Med. Sci 4 24–9 [Google Scholar]
- Levin CS 2012. Promising new photon detection concepts for high-resolution clinical and preclinical PET J. Nucl. Med 53 167–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin CS, Maramraju SH, Khalighi MM, Deller TW, Delso G and Jansen F 2016. Design features and mutual compatibility studies of the time-of-flight PET capable GE SIGNA PET/MR system IEEE Trans. Med. Imaging 35 1907–14 [DOI] [PubMed] [Google Scholar]
- Li Y. Consistency equations in native detector coordinates and timing calibration for time-of-flight PET. Biomed. Phys. Eng. Express. 2019;5:025010. [Google Scholar]
- Liu C, Li Z, Hajagos TJ, Kishpaugh D, Chen DY and Pei Q 2017. Transparent ultra-high-loading quantum dot/polymer nanocomposite monolith for gamma scintillation ACS Nano 11 6422–30 [DOI] [PubMed] [Google Scholar]
- Liu Z, Gundacker S, Pizzichemi M, Ghezzi A, Auffray E, Lecoq P and Paganoni M 2016. In-depth study of single photon time resolution for the Philips digital silicon photomultiplier J. Instrum 11 P06006 [Google Scholar]
- Liu C-C and Qi J 2019. Higher SNR PET image prediction using a deep learning model and MRI image Phys. Med. Biol 64 115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llacer J et al. 1984. Imaging by injection of accelerated radioactive particle beams IEEE Trans. Med. Imaging 3 80–90 [DOI] [PubMed] [Google Scholar]
- Lopez-Mora DA et al. 2019. Comparison of image quality and lesion detection between digital and analog PET/CT Eur. J. Nucl. Med. Mol. Imaging 46 1383–90 [DOI] [PubMed] [Google Scholar]
- Lovinfosse P, Visvikis D, Hustinx R and Hatt M 2018. FDG PET radiomics: a review of the methodological aspects Clin. Trans. Imaging 6 379–91 [Google Scholar]
- Lucchini M et al. 2016. Effect of Mg2+ ions co-doping on timing performance and radiation tolerance of Cerium doped Gd3Al2Ga3O12 crystals Nucl. Instrum. Methods Phys. Res. A 816 176–83 [Google Scholar]
- Mabuchi K. Physical quantity detection device with pixel array column-aligned terminals and method of driving same. 2011 US Patent 7,868,283.
- Maccabee HD, Madhvanath U and Raju MR 1969. Tissue activation studies with alpha-particle beams Phys. Med. Biol 14 213–24 [DOI] [PubMed] [Google Scholar]
- Mandai S, Jain V and Charbon E 2014. A 780 × 800 μm2 multichannel digital silicon photomultiplier with column-parallel time-to-digital converter and basic characterization IEEE Trans. Nucl. Sci 61 44–52 [Google Scholar]
- Mao R and Zhang L 2010. Search for scintillation in doped cubic lead fluoride crystals IEEE Trans. Nucl. Sci 57 3841–5 [Google Scholar]
- Martin E et al. 2009. The 5ns peaking time transimpedance front end amplifier for the silicon pixel detector in the NA62 Gigatracker Conf. Rec. IEEE Nucl. Sci. Symp pp 381–8 [Google Scholar]
- Martinez O, Sosabowski J, Maher J and Papa S 2019. New developments in imaging cell-based therapy J. Nucl. Med 60 730–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata Pavia J, Scandini M, Lindner S, Wolf M and Charbon E 2015. A 1 × 400 backside-illuminated SPAD sensor with 49.7 ps resolution, 30 pJ/sample TDCs fabricated in 3D CMOS technology for near-infrared optical tomography IEEE J. Solid-State Circuits 50 2406–18 [Google Scholar]
- Matej S, Li Y, Panetta J, Karp JS and Surti S 2016. Image-based modeling of PSF deformation with application to limited angle PET data IEEE Trans. Nucl. Sci 63 2599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirelles GS, Erdi YE, Nehmeh SA, Squire OD, Larson SM, Humm JL and Schöder H 2007. Deep-inspiration breath-hold PET/CT: clinical findings with a new technique for detection and characterization of thoracic lesions J. Nucl. Med 48 712–9 [DOI] [PubMed] [Google Scholar]
- Miceli A et al. 2012. Simulations of a micro-PET system based on liquid xenon Phys. Med. Biol 57 1685–700 [DOI] [PubMed] [Google Scholar]
- Miyata M, Tomita H, Watanabe K, Kawarabayashi J and Iguchi T 2006. Development of TOF-PET using Cherenkov radiation J. Nucl. Sci. Technol 43 339–43 [Google Scholar]
- Mohammadi A, Tashima H, Iwao Y, Takyu S, Akamatsu G, Nishikido F, Yoshida E, Kitagawa A, Parodi K and Yamaya T 2019. Range verification of radioactive ion beams of 11C and 15O using in-beam PET imaging Phys. Med. Biol 64 145014. [DOI] [PubMed] [Google Scholar]
- Moore GE 1998. Cramming more components onto integrated circuits Proc. IEEE 86 82–85 [Google Scholar]
- Moreels I 2015. Energy transfer is speeded up in 2D Nat. Mater 14 464–5 [DOI] [PubMed] [Google Scholar]
- Moskal P. et al. Feasibility study of the positronium imaging with the J-PET tomograph. Phys. Med. Biol. 2019;64:055017. doi: 10.1088/1361-6560/aafe20. [DOI] [PubMed] [Google Scholar]
- Munker M. et al. Simulations of CMOS pixel sensors with a small collection electrode, improved for a faster charge collection and increased radiation tolerance. J. Instrum. 2019;14:C05013. [Google Scholar]
- Munker RM et al. 2017. Study of the ALICE investigator chip in view of the requirements at CLIC CERN Document Server (https://cds.cern.ch/record/2284145) [Google Scholar]
- Muñoz E, Barrio J, Etxebeste A, Garcia Ortega P, Lacasta C, Oliver J, Solaz C and Llosé G 2017. Performance evaluation of MACACO: a multilayer Compton camera Phys. Med. Biol 62 7321–41 [DOI] [PubMed] [Google Scholar]
- Muntean A, Venialgo E, Gnecchi S, Jackson C and Charbon E 2017. Toward a fully digital state-of-the-art analog SiPM Conf. Rec. IEEE Nucl. Sci. Symp ( 10.1109/NSSMIC.2017.8533036) [DOI] [Google Scholar]
- Niessl J, Baxter AE and Kaufmann DE 2018. Tools for visualizing HIV in cure research Curr. HIV/AIDS Rep 15 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikl M et al. 2014. Defect engineering in Ce-doped aluminum garnet single crystal scintillators Cryst. Growth Des 14 4827–33 [Google Scholar]
- Nolet F et al. 2016. A 2D proof of principle towards a 3D digital SiPM in HV CMOS with low output capacitance IEEE Trans. Nucl. Sci 6 2293–9 [Google Scholar]
- Nolet F, Dubois F, Roy N, Parent S, Lemaire W, Massie-Godon A, Charlebois SA, Fontaine R and Pratte J-F 2018a. Digital SiPM channel integrated in CMOS 65 nm with 17.5 ps FWHM single photon timing resolution Nucl. Instrum. Methods Phys. Res. A 912 29–32 [Google Scholar]
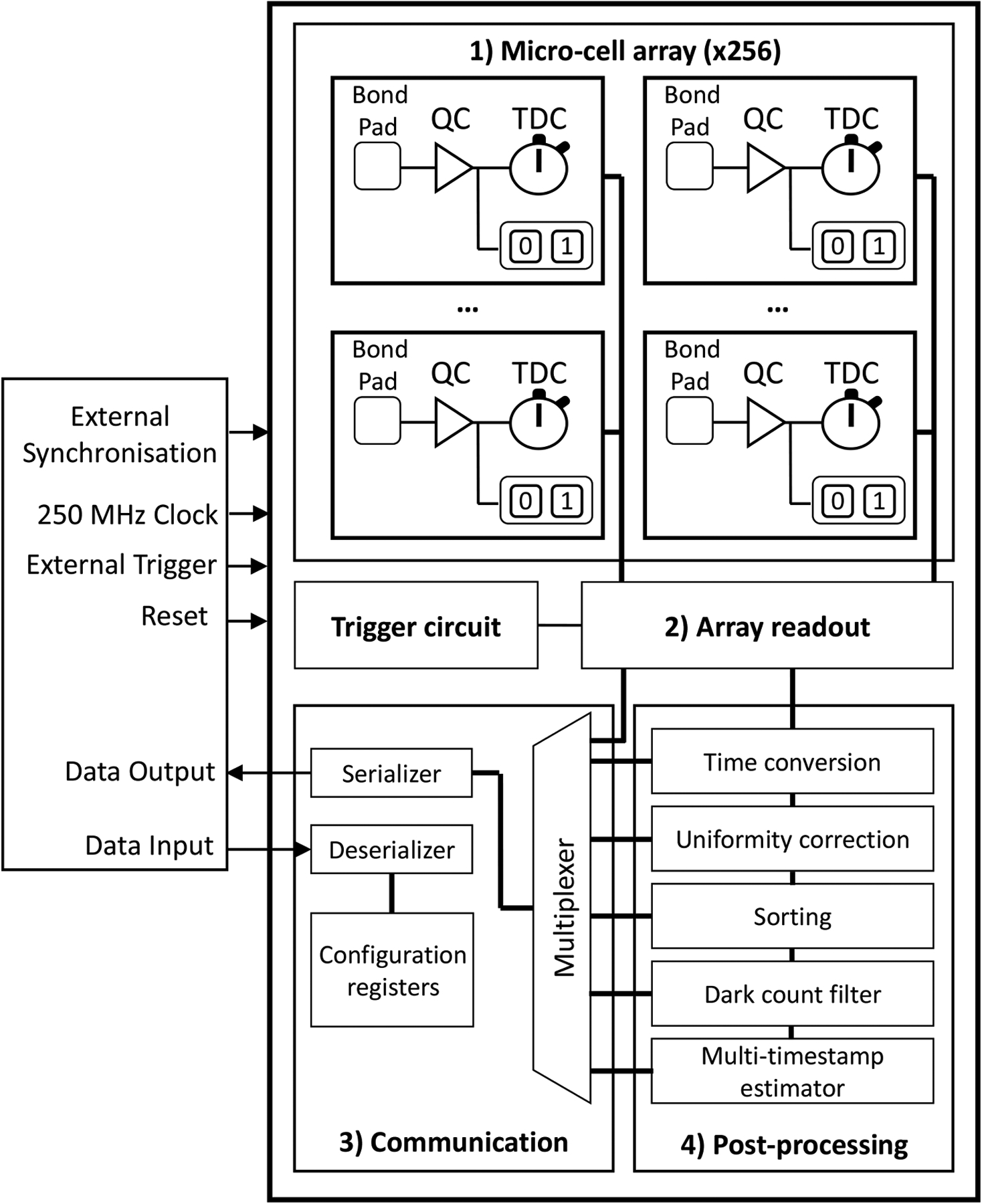
- Nolet F, Lemaire W, Dubois F, Roy SG, Samson A, Charlebois SA, Fontaine R and Pratte J-F 2020. A 256 pixelated SPAD readout ASIC with in-pixel TDC and embedded digital signal processing for uniformity and skew correction Nucl. Instrum. Methods A 949 162891 [Google Scholar]
- Nolet F, Parent S, Roy N, Mercier M-O, Charlebois SA, Fontaine R and Pratte J-F 2018b. Quenching circuit and SPAD integrated in CMOS 65 nm with 7.8 ps FWHM single photon timing resolution Instruments 2 19 [Google Scholar]
- Ogawa S 2018. Liquid xenon detector with VUV-sensitive MPPCs for MEG II experiment Springer Proc. Phys 213 76–79 [Google Scholar]
- Oliveira LB, Leitao CM and Silva MM 2012. Noise performance of regulated cascade trans impedance amplifiers for radiation detectors IEEE Trans. Circ. Syst. I 59 1841–8 [Google Scholar]
- Omelkov S, Nagirnyi V, Gundacker S, Spassky D, Auffray E, Lecoq P and Kirm M 2018. Scintillation yield of hot intraband luminescence J. Lumin 198 260–71 [Google Scholar]
- Ooba T, Fukushima T, Kawai H, Konishi M, Nakayama H, Tabata M, Adachi I, Nishida S, Kishimoto H and Yokogawa H 2004. Proposal of Cherenkov TOFPET with silica aerogel Conf. Rec. IEEE Nucl. Sci. Symp vol 6 pp 3781–4 [Google Scholar]
- Ota R, Nakajima K, Hasegawa T, Ogawa I and Tamagawa Y 2019a. Timing-performance evaluation of Cherenkov-based radiation detectors Nucl. Instrum. Methods Phys. Res. A 923 1–4 [Google Scholar]
- Ota R, Nakajima K, Ogawa I, Tamagawa Y, Shimoi H, Suyama M and Hasegawa T 2019b. Coincidence time resolution of 30 ps FWHM using a pair of Cherenkov-radiator-integrated MCP-PMTs Phys. Med. Biol 64 07LT01. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Bhatt NB, Almaguel F, Rideout-Danner S, Gage HD, Solingapuram Sai K K and Wadas TJ 2019. 89Zr-chloride can be used for immuno-PET radiochemistry without loss of antigen reactivity in vivo J. Nucl. Med 60 696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolozzi L. et al. Characterization of the demonstrator of the fast silicon monolithic ASIC for the TT-PET project. J. Instrum. 2019;14:P02009. [Google Scholar]
- Parent S, Côté M, Vachon F, Groulx R, Martel S, Dautet H, Charlebois AS and Pratte J-F 2018. Single photon avalanche diodes and vertical integration process for a 3D digital SiPM using industrial semiconductor technologies Conf. Rec. IEEE Nucl. Sci. Symp ( 10.1109/NSSMIC.2018.8824571) [DOI] [Google Scholar]
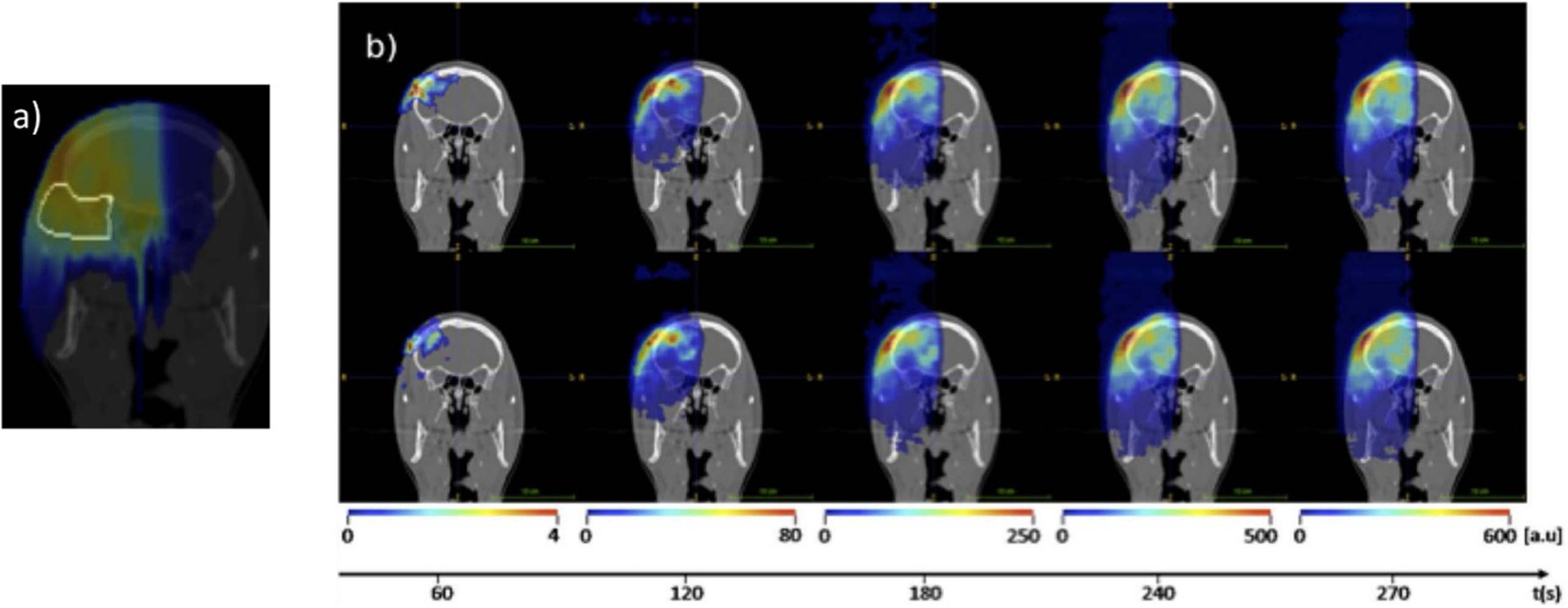
- Parodi K 2015. Vision 20/20: positron emission tomography in radiation therapy planning, delivery, and monitoring Med. Phys 42 7153–68 [DOI] [PubMed] [Google Scholar]
- Parodi K 2016. On- and off-line monitoring of ion beam treatment Nucl. Instrum. Methods A 809 113–9 [Google Scholar]
- Parodi K, Crespo P, Eickhoff H, Haberer T, Pawelke J, Schardt D and Enghardt W 2005. Random coincidences during in-beam PET measurements at microbunched therapeutic ion beams Nucl. Instrum. Methods A 545 446–58 [Google Scholar]
- Parodi K and Polf JC 2018. In vivo range verification in particle therapy Med. Phys 45 e1036–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra B et al. 2018. Cryo-CMOS circuits and systems for quantum computing applications IEEE J. Solid-State Circuits 53 309–21 [Google Scholar]
- Peric I et al. 2013. High-voltage pixel detectors in commercial CMOS technologies for ATLAS, CLIC and Mu3e experiments Nucl. Instrum. Methods Phys. Res. A 31 131–6 [Google Scholar]
- Perktold L and Christiansen J 2009. A multi-channel time-to-digital converter asic with better than 3 ps rms time resolution J. Instrum 9 C01060 [Google Scholar]
- PHOTOQUANT: Nano-photonics applied to ultrafast single photon quantum sensors, ATTRACT project (https://attract-eu.com/selected-projects/nano-photonics-applied-to-ultrafast-single-photon-quantum-sensors-photoquant/) (Accessed: 14 June 2020) [Google Scholar]
- Poikela T. et al. Timepix3: a 65K channel hybrid pixel readout chip with simultaneous ToA/ToT and sparse readout. J. Instrum. 2014;9:C05013. [Google Scholar]
- Rahmim A, Lodge MA, Karakatsanis NA, Panin VY, Zhou Y, Mcmillan A, Cho S, Zaidi H, Casey ME and Wahl RL 2019. Dynamic whole-body PET imaging: principles, potentials and applications Eur. J. Nucl. Med. Mol. Imaging 46 501–18 [DOI] [PubMed] [Google Scholar]
- Rezaei A, Defrise M, Bal G, Michel C, Conti AM, Watson C and Nuyts J 2012. Simultaneous reconstruction of activity and attenuation in time-of-flight PET IEEE Trans. Med. Imaging 31 2224–33 [DOI] [PubMed] [Google Scholar]
- Rezaei A, Schramm G, Willekens SM, Delso G, Van Laere K and Nuyts J 2019. A quantitative evaluation of joint activity and attenuation reconstruction in TOF-PET/MR brain imaging J. Nucl. Med 60 1649–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripiccini E et al. 2018. Expected performance of the TT-PET scanner (arXiv:1811.12381) [Google Scholar]
- Ritt S 2008. Design and performance of the 6 GHz waveform digitizing chip DRS4 Conf. Rec. IEEE Nucl. Sci. Symp pp 1512–5 [Google Scholar]
- Rowland CE, Fedin I, Zhang H, Gray SK, Govorov AO, Talapin DV and Schaller RD 2015. Picosecond energy transfer and multiexciton transfer outpaces auger recombination in binary CdsSe nanoplatelet solids Nat. Mater 14 484–9 [DOI] [PubMed] [Google Scholar]
- Roy N, Nolet F, Dubois F, Mercier M-O, Fontaine R and Pratte J-F 2017. Low power and small area, 6.9 ps RMS time-to-digital converter for 3D digital SiPM IEEE Trans. Radiat. Plasma Med. Sci 1 486–94 [Google Scholar]
- Salomoni M, Pots R, Auffray E and Lecoq P 2018. Enhancing light extraction of inorganic scintillators using photonic crystals Crystals 8 78 [Google Scholar]
- Salvadori J, Perrin M, Marie P-Y, Imbert L and Verger A 2019. High-resolution brain 18F-FDG images provided by fully digital PET Clin. Nucl. Med 44 301–2 [DOI] [PubMed] [Google Scholar]
- Schaart D, Charbon E, Frach T and Schulz V 2018. Advances in digital SiPMs and their application in biomedical imaging Nucl. Instrum. Methods A 809 31–52 [Google Scholar]
- Schellhammer C et al. 2018. OC-0605: First in-beam MR scanner for image-guided proton therapy: beam alignment and magnetic field effects Radiother. Oncol 127 S318–9 [Google Scholar]
- Schmall JP, Karp JS and Alavi A 2019. The potential role of total body PET imaging in assessment of atherosclerosis PET Clin. 14 245–50 [DOI] [PubMed] [Google Scholar]
- Shakirin G, Braess H, Fiedler F, Kunath D, Laube K, Parodi K, Priegnitz M and Enghardt W 2011. Implementation and workflow for PET monitoring of therapeutic ion irradiation: a comparison of in-beam, in-room, and off-line techniques Phys. Med. Biol 56 1281–98 [DOI] [PubMed] [Google Scholar]
- Shao Y 2007. A new timing model for calculating the intrinsic timing resolution of a scintillator detector Phys. Med. Biol 52 1103–17 [DOI] [PubMed] [Google Scholar]
- Sloan ME, Grant CW, Gowin JL, Ramchandani VA and Le Foll B 2019. Endocannabinoid signaling in psychiatric disorders: a review of positron emission tomography studies Acta Pharmacol. Sin 40 342–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DL, Thomas LJ and Terpogossian MM 1981. A mathematical-model for positron-emission tomography systems having time-of-flight measurements IEEE Trans. Nucl. Sci 28 3575–83 [Google Scholar]
- SONY Corp. 2012. SONY develops next-generation back-illuminated CMOS image sensor, which embodies the continuous evolution of the camera (www.sony.net/SonyInfo/News/Press/201201/12-009E/index.html) (Accessed: 9 August 2018)
- Sossi V, Cheng J-C and Klyuzhin IS 2019. Imaging in neurodegeneration: movement disorders IEEE Trans. Radiat. Plasma Med. Sci 3 262–74 [Google Scholar]
- Spurrier MA, Szupryczynski P, Yang K, Carey AA and Melcher CL 2008. Effects of Ca2+ Co-doping on the scintillation properties of LSO:Ce IEEE Trans. Nucl. Sci 55 1178–82 [Google Scholar]
- Stricker-Shaver D, Ritt S and Pichler BJ 2014. Novel calibration method for switched capacitor arrays enables time measurements with sub-picosecond resolution IEEE Trans. Nucl. Sci 61 3607–17 [Google Scholar]
- Surti S and Karp JS 2008. Design considerations for a limited angle, dedicated breast, TOF PET scanner Phys. Med. Biol 53 2911–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surti S and Karp JS 2016. Advances in time-of-flight PET imaging Phys. Medica 32 12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surti S, Zou W, Daube-Witherspoon ME, Mcdonough J and Karp JS 2011. Design study of an in-situ PET scanner for use in proton beam therapy Phys. Med. Biol 56 2667–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashima H et al. 2016. Development of a small single-ring OpenPET prototype with a novel transformable architecture Phys. Med. Biol 61 1795–809 [DOI] [PubMed] [Google Scholar]
- The 10 ps challenge website (website under construction (https://the10ps-challenge.org/) (Accessed: 14 June 2020)
- TICAL: 4D total absorptionTime Imaging CALorimeter, ERC grant agreement 338953 (https://cordis.europa.eu/project/rcn/110957/factsheet/en) (Accessed: 14 June 2020)
- Tobias CA, Benton EV, Capp MP, Chatterjee A, Cruty MR and Henke RP 1977. Particle radiography and autoactivation Int. J. Radiat. Oncol 3 35–44 [DOI] [PubMed] [Google Scholar]
- Tobias CA, Chatterjee A and Smith AR 1971. Radioactive fragmentation of N7+ ion beam observed in a beryllium target Phys. Lett. A 37 119–20 [Google Scholar]
- Toci G, Gizzi LA, Koester P, Baffigi F, Fulgentini L, Labate L, Hospodkova A, Jary V, Nikl M and Vannini M 2019. InGaN/GaN multiple quantum well for superfast scintillation application: photoluminescence measurements of the picosecond rise time and excitation density effect J. Lumin 208 119–24 [Google Scholar]
- Tomanová K, Čuba V, Brik MG, Mihóková E, Martinez Turtos R, Lecoq P, Auffray E and Nikl M 2019. On the structure, synthesis, and characterization of ultrafast blue-emitting CsPbBr3 nanoplatelets APL Mater. 7 011104 [Google Scholar]
- Tomitani T 1981. Image reconstruction and noise evaluation in photon time-of-flight assisted positron emission tomography IEEE Trans. Nucl. Sci 28 4582–9 [Google Scholar]
- Turtos RM, Gundacker S, Auffray E and Lecoq P 2019a. Towards a metamaterial approach for fast timing in PET: experimental proof-of-concept Phys. Med. Biol 64 185018. [DOI] [PubMed] [Google Scholar]
- Turtos RM. et al. On the use of CdSe scintillating nanoplatelets as time taggers for high-energy gamma detection. NPJ 2D Mater. Appl. 2019b;3:37. [Google Scholar]
- Turtos RM, Gundacker S, Polovitsyn A, Christodoulou S, Salomoni M, Auffray E, Moreels I, Lecoq P and Grim JQ 2016. Ultrafast emission from colloidal nanocrystals under pulsed X-ray excitation J. Instrum 11 P10015 [Google Scholar]
- UCDAVIS: EXPLORER total-body PET scanner (https://explorer.ucdavis.edu/) (Accessed: 14 June 2020)
- Van Eijk CWE 1994. Cross-luminescence J. Lumin 60 936–41 [Google Scholar]
- van Sluis JJ, de Jong J, Schaar J, Noordzij W, van Snick P, Dierckx R, Borra R, Willemsen A and Boellaard R 2019. Performance characteristics of the digital biograph vision PET/CT system J. Nucl. Med 60 1031–6 [DOI] [PubMed] [Google Scholar]
- Vandenberghe S, Daube-Witherspoon ME, Lewitt RM and Karp JS 2006. Fast reconstruction of 3D time-of-flight PET data by axial rebinning and transverse mashing Phys. Med. Biol 51 1603–21 [DOI] [PubMed] [Google Scholar]
- Vandenberghe S and Lemahieu I 2007. System characteristics of simulated limited angle TOF PET Nucl. Instrum. Methods A 571 480–3 [Google Scholar]
- Vandenberghe S, Mikhaylova E, D’Hoe E, Mollet P and Karp JS 2016. Recent developments in time-of-flight PET EJNMMI Phys. 3 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke J, Bechtol K, Funk S, Okumura A, Tajima H and Varner GS 2011. Development of an ASIC for dual mirror telescopes of the Cherenkov telescope array (arXiv:1110.4692) [Google Scholar]
- Vasil’ev A 1999. Relaxation of hot electronic excitations in scintillators: account for scattering, track effects, complicated electronic structure Proc. Scint99 (Moscow State University) pp 43–52 [Google Scholar]
- Venialgo E, Mandai S and Charbon E 2013. Time mark estimators for MD-SiPM and impact of system parameters Conf. Rec. IEEE Nucl. Sci. Symp (Seoul, 27 October–2 November 2013) (Piscataway, NJ: IEEE) ( 10.1109/NSSMIC.2013.6829083) [DOI] [Google Scholar]
- Vinogradov S 2018. Approximations of coincidence time resolution models of scintillator detectors with leading edge discriminator Nucl. Instrum. Methods Phys. Res. A 912 149–53 [Google Scholar]
- Wagadarikar AA, Ivan A, Dolinsky S and Mcdaniel DL 2014. Sensitivity improvement of time-of-flight (ToF)-PET detector through recovery of Compton scattered annihilation photons IEEE Trans. Nucl. Sci 61 121–5 [Google Scholar]
- Walrand S, Hesse M and Jamar F 2018. Update on novel trends in PET/CT technology and its clinical applications Br. J. Radiol 91 20160534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H and Dai FF 2017. A 14-Bit, 1-ps resolution, two-step ring and 2D Vernier TDC in 130nm CMOS technology Conf. Rec. IEEE ESSCIRC pp 143–6 [Google Scholar]
- Werner ME and Karp JS 2013. TOF PET offset calibration from clinical data Phys. Med. Biol 58 4031–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Washington IR, Bhatt AD and Knopp MV 2019. Emerging opportunities for digital PET/CT to advance locoregional therapy in head and neck cancer Semin. Radiat. Oncol 29 93–101 [DOI] [PubMed] [Google Scholar]
- Yang F and Mok PKT 2017. A 65nm inverter-based low-dropout regulator with rail-to-rail regulation and over −20dB PSR at 0.2V lowest supply voltage Conf. Rec. IEEE ISSCC pp 106–7 [Google Scholar]
- Yoshida E et al. 2017. Development of a whole-body dual ring openPET for in-beam PET IEEE Trans. Radiat. Plasma Med. Sci 1 293–300 [Google Scholar]
- Zhang J, Knopp MI and Knopp MV 2019. Sparse detector configuration in SiPM digital photon counting PET: a feasibility study Mol. Imaging Biol 21 447–53 [DOI] [PubMed] [Google Scholar]