Abstract
Vascular diseases are the most prevalent cause of ischemic necrosis of tissue and organ, which even result in dysfunction and death. Vascular regeneration or artificial vascular graft, as the conventional treatment modality, has received keen attentions. However, small-diameter (diameter < 4 mm) vascular grafts have a high risk of thrombosis and intimal hyperplasia (IH), which makes long-term lumen patency challengeable. Endothelial cells (ECs) form the inner endothelium layer, and are crucial for anti-coagulation and thrombogenesis. Thus, promoting in situ endothelialization in vascular graft remodeling takes top priority, which requires recruitment of endothelia progenitor cells (EPCs), migration, adhesion, proliferation and activation of EPCs and ECs. Chemotaxis aimed at ligands on EPC surface can be utilized for EPC homing, while nanofibrous structure, biocompatible surface and cell-capturing molecules on graft surface can be applied for cell adhesion. Moreover, cell orientation can be regulated by topography of scaffold, and cell bioactivity can be modulated by growth factors and therapeutic genes. Additionally, surface modification can also reduce thrombogenesis, and some drug release can inhibit IH. Considering the influence of macrophages on ECs and smooth muscle cells (SMCs), scaffolds loaded with drugs that can promote M2 polarization are alternative strategies. In conclusion, the advanced strategies for enhanced long-term lumen patency of vascular grafts are summarized in this review. Strategies for recruitment of EPCs, adhesion, proliferation and activation of EPCs and ECs, anti-thrombogenesis, anti-IH, and immunomodulation are discussed. Ideal vascular grafts with appropriate surface modification, loading and fabrication strategies are required in further studies.
Keywords: Vascular graft, In situ endothelialization, Thrombogenesis, Intimal hyperplasia, Immunomodulation
Graphical abstract
Highlights
-
•
This paper reviews challenges and strategies for enhanced in situ vascularization.
-
•
Strategies for homing, adhesion and activation of EPCs and ECs are summarized.
-
•
Approaches to prevent thrombogenesis and IH for long-term lumen patency are introduced.
-
•
Immune system plays a role in modulating biological behaviors of ECs and SMCs.
-
•
Perspectives for future development of vascular grafts are proposed.
1. Introduction
Vascular diseases are the most prevalent cause of ischemic necrosis of tissue and organ, which has attracted much attention [1]. Vascular defect caused by trauma or underlying diseases like diabetes can reduce oxygen and nutrients supply for tissues and organs, which may result in severe consequences, like claudication, sores, organ disfunctions, necrosis, or even death [2,3]. When long-segment defects occurred or the defects happened in vital organs like heart, artificial vascular grafts are required to restore blood supply for tissues.
Synthetic vascular grafts have been widely utilized in clinics as conventional strategies for vascular impairment, like polyurethane, polyester, expanded polytetrafluoroethylene (ePFTE), and etc., with diameter greater than 6 mm [4]. However, these synthetic grafts have long-term risk since they are prone to intimal hyperplasia (IH) and thrombogenesis, and result in implantation failure [5], particularly for small diameter vascular grafts (diameters less than 4 mm) [6]. Hence, ideal vascular grafts are required to imitate the framework and constitution of native vessels, as well as inhibit protein deposition, blood coagulation, and immunological rejection [7,8]. To construct a biomimetic vascular graft, it is indispensable to figure out the critical factors and challenges in graft development.
It has been widely recognized that endothelialization is critical for blood contacting devices [9,10]. The endothelium, the inner tunica with monolayer endothelial cells (ECs) lining in vessel lumen, directly contacts with blood, and plays an important role in maintaining vascular hemostasis and patency by releasing regulatory molecules including nitric oxide (NO), heparins, and plasmin, etc. [9]. Losing endothelium layer may lead to a cascade of pathological reactions, like thrombogenesis, inflammation reactions, and smooth muscle cell (SMC) hyperplasia [11,12]. Thus, endothelium regeneration is crucial for vascular graft.
In conventional tissue engineered vascular grafts (TEVGs), ECs are cultured and seeded on scaffolds prior to implantation, which is called in vitro endothelialization [13]. The proliferation ability of in vitro cultured ECs is limited. And greater stemness stem cells are applied, like endothelial progenitor cell (EPC), induced Pluripotent Stem Cell (iPSC), and mesenchymal stem cell (MSC) [[14], [15], [16], [17]]. However, the viability, bioactivity and stability of seeded cells after implantation cannot guarantee, and the clinical application of this strategy is inhibited by its poor effectiveness and practicality [18,19]. Moreover, in vitro cell culture consumes more time and cost, and have greater risk of contamination. In situ endothelialization, commanding the regeneration of a healthy endothelium on the surface of vascular grafts directly after implantation, is more effective than in vitro endothelialization [20,21]. Early strategies pay attention to appealing cells from anastomotic regions, but poor EC proliferative ability hinders the long-term expectation. Thus, the mobilization, recruitment and homing of EPC from peripheral blood and bone marrow has appealed much attentions [22,23]. Furthermore, ideal in situ endothelialization needs more attention on biomaterial type, surface modification and releasing factors to regulate cell performance, in which enhanced adhesion, orientation, proliferation and activation of ECs and EPCs on graft surface are required.
For long-term patency, thrombogenesis is the key factor leading to vascular occlusion. Vascular grafts, as the foreigner directly exposed to blood flux in vasculature, are easy to cause protein deposition and provoke thrombogenesis [24,25]. The aggregation of insoluble fibrin, platelet, and red cells induces the coagulation cascades and results in thrombus formation [[26], [27], [28]]. ECs serve as the first line for thrombogenesis. ECs can release and control key molecules, including tissue plasminogen activator (tPA), anti-thrombins, and plasmin, etc., to modulate anti-thrombogenesis process for vascular grafts, and these molecules are also potential treating drugs for application [24,29]. Another high potential risk is IH. The platelets, inflammatory cells, and SMCs aggregate and release growth factors, resulting in SMCs proliferating and migrating to vascular intima uncontrolledly, which exerts adverse effects on lumen patency [30].
Furthermore, inflammatory responses, induced by vascular graft implantation, are crucial in modulating graft development [31,32]. Biomaterial degradations act as stimulus, activate toll-like receptors (TLRs), and thus induce initial inflammation responses, which then cause white blood cells including neutrophils, monocytes, and lymphocytes to infiltrate from blood flow to implantation scaffolds [[33], [34], [35]]. And then growth factors released by macrophages, like tumor necrosis factor-α (TNF-α), TNF-β, and interleukin-1 (IL-1), etc., may play a role in influencing the biological behavior of ECs and SMCs, and thus modulating in situ endothelialization and lumen patency of vascular graft [36,37].
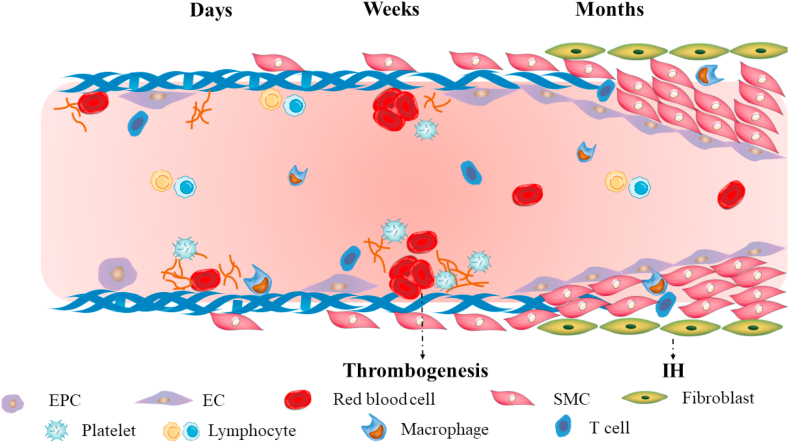
Maintaining long-term lumen patency for small-diameter vascular graft is still challenging. Days after graft implantation, the proliferation of ECs happens, and simultaneously blood cells, platelets and fibrin deposit onto foreign graft. Weeks after implantation, more ECs proliferate and adhere onto graft surface, but no intact EC layer forms, and coagulation cascades may be activated, then resulting in thrombogenesis. Months after implantation, SMCs migrate from anastomotic sites and proliferate uncontrollably, thus leading to IH. Meanwhile, inflammatory cells, especially macrophages, can regulate EC and SMC behavior via inflammatory factors. The process is shown in Fig. 1.
Fig. 1.
The challenges after vascular graft implantation. Days and weeks after implantation, insufficient endothelialization and thrombogenesis may happen. Months after implantation, uncontrollable proliferation of SMCs may lead to IH. Inflammatory cells play a role in regulating EC and SMC behavior.
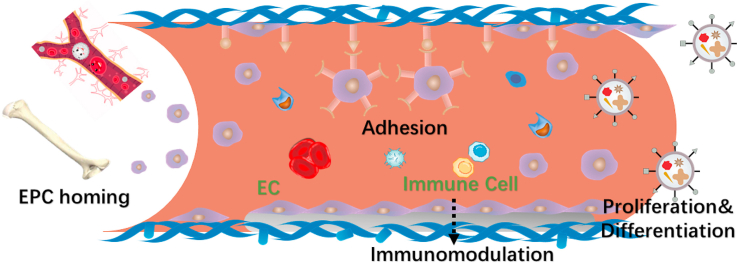
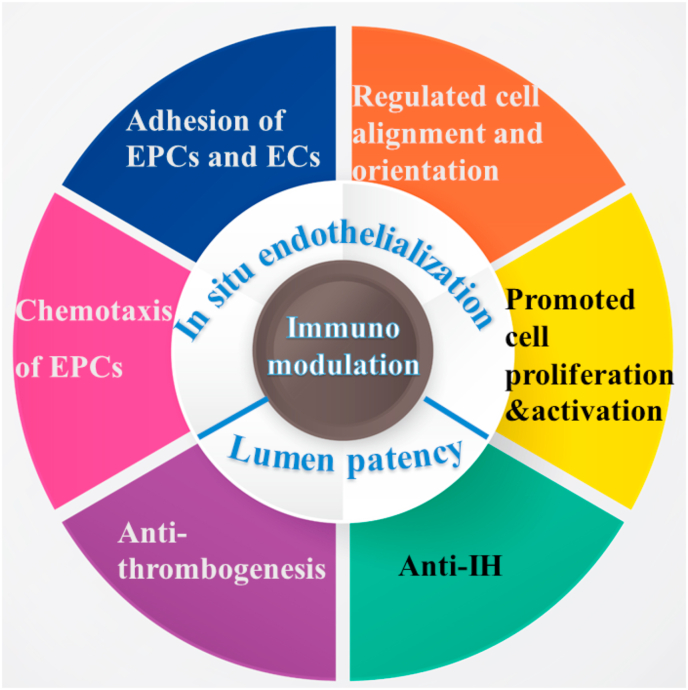
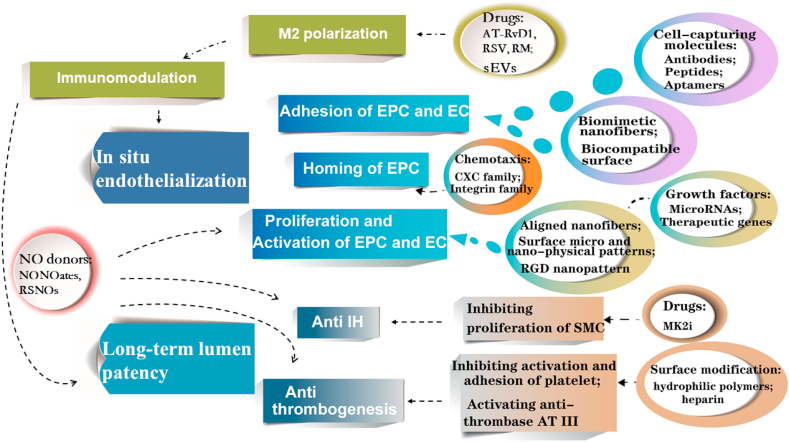
Multiple strategies can be adopted to respond to the challenges described above for enhanced long-term lumen patency of vascular grafts (Fig. 2). ECs form the inner endothelium layer, and are crucial in preventing coagulation and thrombogenesis. Thus, promoting in situ endothelialization in vascular graft remodeling takes top priority, which requires recruitment of EPCs, migration, adhesion, proliferation and activation of EPCs and ECs. Surface modification with heparin or hydrophilic polymers can reduce thrombogenesis, and some drug release can inhibit IH. Additionally, NO and macrophages also play a crucial role in regulating the biological behavior of ECs and SMCs. Thus, in this paper we will review and summarize different strategies in promoting in situ endothelialization and inhibiting thrombogenesis and IH for long-term lumen patency of vascular grafts.
Fig. 2.
Schematic illustration for in situ endothelialization and lumen patency strategies.
2. Homing and adhesion of EPCs and ECs for enhanced in situ endothelialization
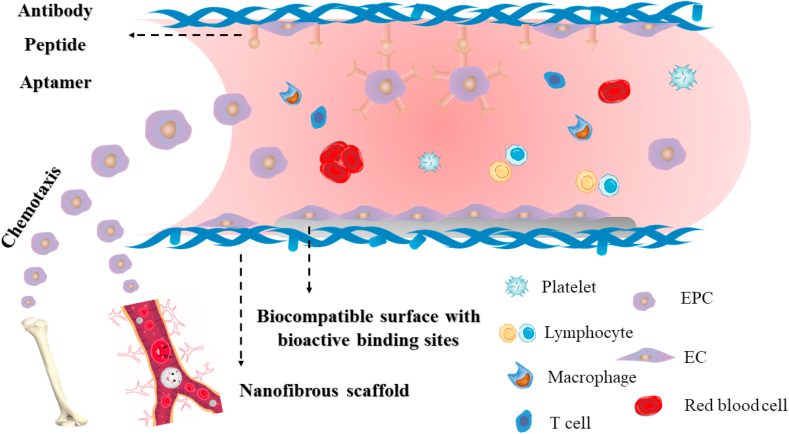
EPCs, circulating cells located in bone marrow and low quantities in peripheral blood, can differentiate into ECs [22,38]. EPCs has also been applied in cell therapy for the treatment of critical limb ischemia [39]. It has been recognized that EPCs plays a critical role in endothelialization of vascular grafts [40]. However, the quantity of EPC homing to the neovascularization sites is limited. For enhanced in situ endothelialization, the homing of EPCs and recruitment of ECs is vital, which includes chemotactic effects, capture and adhesion of cells on graft surface [41]. Multiple chemokines can be utilized for EPC chemotaxis, and strategies like nanofibrous structure, biocompatible surface with bioactive binding sites and specific molecules modification can be applied for cell adhesion (Fig. 3).
Fig. 3.
Recruitment and adhesion of EPCs and ECs. Chemokines can be utilized for EPC chemotaxis. Nanofibrous structure, biocompatible surface with bioactive binding sites and specific molecules modification can be applied for EPC and EC adhesion.
2.1. Homing of EPCs by chemokines
EPCs have multiple sub-populations and display different markers on their surface. Early EPCs display markers including CD34, CD133 and VEGFR2, etc., which are reduced as cell maturity increases [39,42]. These EPCs in different subpopulations exert synergistic effects on endothelialization [43,44]. Multiple chemokines that can be utilized for EPC homing are summarized in Table 1.
Table 1.
Chemokines for stem cell chemotaxis to enhance in situ endothelialization.
| Targeting Receptors | Chemokines | Loading approach | Targeting cells | Activated Signaling Pathway | Effects | Ref | ||
|---|---|---|---|---|---|---|---|---|
| CXC Family | CXCR4 | SDF-1α | / | CD117+ stem cell | SDF-1α/CXCR4 axis | CD117+ cell homing to injured sites | [47,48] | |
| CXCR4 | SDF-1α | Coating on synthetic polyester grafts | CD117+/CD34+ stem cell | SDF-1α/CXCR4 axis | CXCR4+ cell homing for in situ endothelialization | [50] | ||
| CXCR7 | SDF-1α | Immobilized onto heparin | CD34+ EPC, SMPC |
SDF-1α/CXCR7 axis | Both EPC and SMPC recruitment for in situ endothelialization | [51] | ||
| CXCR7 | Dkk3 | Co-electrospinning technology | Sca-1+ cells | Dkk3/CXCR7/ERK1/2; PI3K/AKT axis | EPC recruitment and differentiation | [52] | ||
| Integrin family | α4-integrin-VCAM1 | Fibronectin (Fn) | Coating on synthetic polyester grafts | CD117+/CD34+ stem cell | Fn/VCAM1 axis | VCAM+ cell homing | [50] | |
| Integrin | 8-pCPT-2′-O-Me-cAMP | / | EPC | GTPase Rap1 | EPC recruitment | [59] | ||
| Others | RAGE | HMGB1 | / | EPC | integrin-dependent adhesion of EPCs | EPC homing to injured sites | [60] | |
| Rac1 | MCP1 | / | SMC | p115 RhoGEF/Rac1 GTPase pathway | SMC migration and proliferation for vascular remodeling | [61] | ||
| VEGFR1, VEGFR2 | VEGF | Surface‐fixed on styrenated gelatin gel | EPC | VEGF/VEGFR | Graft for in situ EPC homing and capture | |||
The quantity of EPCs in circulation blood flow is low, and EPCs from bone marrow can mobilize into peripheral blood for enhanced endothelialization. Multiple growth factors play a role in the chemotaxis of EPC homing to the region of neovascularization, but the underlying mechanism of signaling pathways in EPC homing has not been elaborated.
2.1.1. Chemokines targeting CXC families on EPC surface
Stromal cell-derived factor-1α (SDF-1α), which can act as the chemoattract for CXC family, has potential in EPCs chemotaxis and recruitment [[45], [46], [47]]. SDF-1α can bind to CXCR4 on hematopoietic stem cell (HSC) surface for stem cell homing, and it has also been reported that SDF-1α can bind to CXCR4 expressed on EPC surface [[48], [49], [50]]. Yu et al. [51] immobilized SDF-1α on vascular graft and found that SDF-1α immobilization could recruit EPCs and smooth muscle progenitor cells (SMPCs) simultaneously for enhanced in situ endothelialization. The in vivo results indicated that the lumen patency 12 weeks after implantation for naked graft was 44%, heparin coated graft was 67%, and SDF-1α/heparin modified graft was 89%. Issa et al. [52] studied the performance of dickkopf-3 (Dkk3) and results indicated that Dkk3 could interact with cell surface ligand CXCR7, activate ERK1/2 and PI3K/AKT signaling pathway, and thus enhance the recruitment and differentiation of EPCs.
Chemokines can directly immobilize on graft surface, for example, Wang et al. [53] immobilized SDF-1α onto PLLA/PLGA/PLCL vascular scaffolds for EPC homing. Nanoparticles (NPs) are also ideal strategy for bioactive molecules carrier. He et al. [54] constructed chitosan/fucoidan NPs to load SDF-1α to induce EC migration for promoted in situ endothelialization.
2.1.2. Chemokines targeting integrin families on EPC surface
It has been reported that the integrins play a role in homing of EPCs. De Visscher et al. [50] constructed a synthetic graft coated with fibronectin (FN) and SDF-1α, and proved that FN could activate α4-integrin-VCAM1/FN axis for EPC homing. Particularly, integrinβ2 have potential in regulating EPC recruitment to ischemic sites [55,56]. Chavakis et al. [55] found that integrinβ2 not only induced the adherence of EPCs to ECs and ECM proteins, but also regulated the chemotaxis of EPCs to the neovascularization sites. Furthermore, integrinβ2 activated by specific anti-β2-integrin antibody can effectively enhance the homing of EPCs for in situ endothelialization. Integrin mediated migration of EPCs can be promoted by enhancing the activity of GTPase Rap1, and Rap1 can be activated by Epac1 [57,58]. Carmona et al. [59] utilized 8-pCPT-2′-O-Me-cAMP to directly activate Rap1 for recruitment of EPCs, providing a new strategy for promoted homing of EPCs. .
Moreover, some other molecules have also been explored and found to be able to promote homing of EPCs, like ephrine-B2-Fc chimera [62] and HMGB-1 [60], which target to specific receptors on EPC surface.
The markers like CD34 and CD133 on EPC surface are not specific, which may also display on hematopoietic stem cell (HSC) surface. Lacking specific makers on EPC surface for chemokines reduces homing efficiency. Moreover, the mechanism under chemokines induced stem cell homing has not been clearly figured out. Deep explorations are needed to uncover the mechanism, and more specific chemokines are required to effectively promote homing and recruitment of EPCs for endothelialization.
2.2. Adhesion of EPCs and ECs on graft surface
After homing of EPCs, cell adhesion on graft surface utilizing nanofibrous structure, biocompatible surface with bioactive binding sites or specific molecules modification takes prior consideration (Fig. 3).
2.2.1. Biomimetic nanofibrous scaffolds for enhanced cell adhesion
Extracellular matrix (ECM), with nanoscale construction, is the micro-environment for cell adhesion, proliferation and differentiation, and is essential for the maintenance of cell biological activity [[63], [64], [65]]. Biomimetic nanofibrous scaffolds, with greater surface to volume ratio, provide more binding ligands for cell adhesion and biomolecules adsorption [66,67]. Multiple approaches can be applied to obtain nanoscale vascular grafts. Generally, there are three approaches for nanofiber fabrication in vascular graft construction, including self-assembly, phase separation, and electrospinning, etc.
Self-assembly is a fabrication process by which individual components are arranged into hierarchical organized structures spontaneously supported by non-covalent interactions [68]. This process is ubiquitous in various molecular bio-behavior [3,69]. Peptide amphiphiles (PAs) have been widely applied in fabricating collagen-like adaptable nanofibrous biomaterials utilizing self-assembly strategy [70,71]. Nanofibrous structure developed by self-assembly can simulate the ECM with the lowest scale at 5–8 nm, but the synthesis process is difficult to be regulated and the efficiency of productivity is relatively low. Thus, the application of this strategy in vascular graft construction is limited [72]. Phase separation can also fabricate nanofibers at the size similar to native ECM collagens, with different porous structures at macroscale [73], which can also effectively enhance cellular adhesion onto the scaffold surfaces [74]. Various biodegradable aliphatic polyesters can be developed into nanofibers using phase separation, with fiber diameter ranging between 50 and 500 nm [72,75,76]. The porosity and pore sizes can be tuned by modulating parameters, like polymer concentrations, porogen morphology utilized, gel temperatures and frozen temperatures [73]. Compared to self-assembly strategy, phase separation is a relatively simpler strategy that does not need professional techniques. However, its application is restricted because of low yield efficiency, a finite number of selected polymers for fabrication and consequently inappropriate for industrialized scale manufacture [75].
Electrospinning has been considered as an appealing strategy to simulate native ECM, for its simplicity and scalability. To fabricate optimal electrospun scaffolds, construction parameters including voltage, solution concentration, interval to collector, and collection approaches, can be tailored to acquire ideal fibrous orientation, diameters, porosity and mechanic characteristics [[77], [78], [79], [80]]. Compared to self-assembly and phase separation, a broader spectrum of biomaterials can be produced into nanofibers using electrospinning. Furthermore, convenient preparation methods, abundant biomaterials with electrospin-ability and high yield efficiency make the electrospinning strategy accessible for scaffold fabrication both on laboratory and industrial scales [75,81].
Multiple biomaterials have been utilized in electrospinning, including synthetic polymers, natural polymers and hybrid biomaterials. Non-degradable synthetic polymers like poly (ethylene terephthalate) (PET) [82,83], expanded poly (tetrafluoroethene) (ePTFE) [[84], [85], [86], [87]], polyurethane (PU) [5,88,89] have ideal electrospinnability and outstanding mechanical characteristics. Non-degradable biomaterials can be implanted for long term application, but excellent tissue engineered grafts should possess proper biodegradability for minimized inflammatory reactions. Degradable biomaterials are favorable for adhesion and proliferation of ECs [90,91]. Biodegradable synthetic polymers like poly (ε-caprolactone) (PCL) [92,93], PGA [94], PLGA [95], and natural polymers like collagen [96,97], elastin [98], silk fibroin [99], gelatin [100], are also applied for vascular graft fabrication. Natural polymers have ideal biocompatibility, but insufficient mechanical properties. It is an appealing approach to blend the natural polymers possessing outstanding biocompatibility with the synthetic polymers possessing adequate mechanical properties to overcome these disadvantages, for example, collagen and PEG [96], elastin and PCL [98], gelatin and PVA [100], etc. For construction of biomimetic vessel structure, layer by layer (LBL) strategy can be applied in vascular graft fabrication, for example, silk fibroin as inner layer, hydrogels as the medial, and TPU nanofibers as outer layer, to simultaneously obtain mechanical strength and biological activity, and simulate natural vessel structure [101].
2.2.2. Biocompatible surface with bioactive binding sites for enhanced cell adhesion
Synthetic polymers can provide sufficient mechanical strength in vascular graft construction, but inadequate bioactivity since lacking sufficient cellular recognition sites for cell adhesion [102]. Natural polymers with outstanding biocompatibility can provide enough cellular ligands [103]. Thus, surface modification with natural polymer for synthetic polymers is an effective strategy for enhanced cell adhesion in vascular graft fabrication.
Gelatin possesses cell surface binding ligands RGD which are favorable for cell adhesion. Gelatin has attracted much attention for surface modification because of its biocompatibility, biodegradability, and editability. Cationized gelatin can be covalently grafted on electrospun PLLA nanofibers for better surface bioactivity [104]. Merkle et al. [105] applied co-axial electrospinning to construct core-shell structure, with PVA as core to provide mechanical strength, and with gelatin as shell to display biocompatible surface. The Young's modulus of core-shell structure was about 169 MPa and the tensile strength was about 5.4 MPa, and the mechanical properties was greatly enhanced compared with single PVA or gelatin [105]. Blended gelatin and heparin can also be an alternative strategy. Wang et al. [106] fabricated gelatin, heparin NPs, and polylysine nano-coating using self-assembly to construct a biomimetic vascular structure.
Collagen, component of natural ECM, possesses excellent biocompatibility and bioactivity [107]. Grus et al. [108] fabricated polyester vascular scaffold coated with collagen to enhance biocompatibility and improve lumen long-term patency. Furthermore, vascular graft PolyMaille (Perouse Medical, France) with collagen coating is already available. Some other natural polymers, like elastin [109], silk fibrin [110,111], are also alternative for vascular graft application. These polymeric coatings can provide non-specific binding sites not only for EC adhesion, but also for other blood cells like platelets, white cells, and SMCs, which may induce thrombogenesis and IH. Thus, more specific binding coatings are required for in situ endothelialization.
2.2.3. Cell-capturing molecules on surface for enhanced cell adhesion
EPCs can differentiate into ECs and generate the inner endothelium layer on graft surface. Hence, it is important to targetedly capture the circulating EPCs and ECs onto graft surfaces for enhanced in situ endothelialization. To promote the migration and adhesion of cells, antibodies, cell adhesive peptides and cell-specific aptamers have been extensively investigated [23,112,113].
CD34 and VEGFR-2 are the surface markers on EPC surface [39]. Anti CD34 antibodies (Ab) have been the most widely utilized for EPC target and capture [114,115]. But there are some disadvantages concerning about anti CD34 Abs, since anti CD34 Abs are not only specific for EPCs, they can also capture other cells, some of which can even differentiate into SMCs and lead to thrombosis [116,117]. Clinical investigations indicated that anti CD34 Abs coated graft cannot reduce risk of vascular occlusions than conventional graft surface, especially of IH [118,119]. To overcome this problem, it was proposed that anti CD34 Abs can combine with drugs like sirolimus to reduce IH [120,121]. Anti VEGFR-2 Abs are also alternative for EPC capture. Anti VEGFR-2 Abs can effectively target and capture EPCs and ECs from blood flowing [122,123]. It has been reported that the specificity of VEGFR is superior than that of CD34 and CD31. Although the superior specificity, the VEGFR-2 also expresses on surface of monocytes and macrophages, and the recruitment of immune cells will induce undesirable inflammatory responses. The specific markers for stem cell recognition remains unclear and needs more explorations.
Cell adhesive peptides are also critical for biological identification between cell membrane and relevant ligand for cell capture and adherence. Integrins on cell surface mediate the adhesion of cell onto ECM in a dominated manner [124]. Multiple peptide sequences have been applied for surface modification for enhanced adhesion of EPCs and ECs, including RGD [[125], [126], [127], [128], [129]], CAG [130,131], REDV [132,133], and YIGSR [134,135], etc. These peptide sequences modified on graft surface display specific affinity with ECs, enhancing the adhesion of ECs and inhibiting the adherence of platelets [[136], [137], [138], [139]].
Aptamer, the short oligonucleotide sequences, exhibits affinity to specific targeted molecules. Aptamer sequences can be obtained through cell-SELEX technology [140]. It was reported that aptamer could capture porcine EPCs for enhanced in situ endothelialization [141]. But the application of aptamers in EPC capture has not been widely used. The effects of aptamers on the in vitro and in vivo cell performance and vascular patency still require more studies to verify. The stability of aptamers in vivo is unclear. Moreover, some aptamers have risks of causing inflammation responses, and the relative immunomodulation is poor known [142].
The cell-adhesive peptides, originating from ECM, bind to integrins on EC surface for cell capture. The ECs possess stronger specificity to peptides like YIGSR and REDV than SMCs. Peptides can promote cell adhesion, but with poor targeting. Antibodies can target to relevant markers on cell surface, but the targeting markers are not specific on EPC or EC surface. Aptamers, the oligonucleotide sequence, have high affinity with target cells. However, the immune responses of aptamers in vivo remain unclear.
3. Cell behavior regulation for enhanced in situ endothelialization
Ideal vascular graft is also required for enhanced cell elongation, proliferation, activation and differentiation of EPCs and ECs.
Circulating EPCs can be subdivided into two main categories, hematopoietic lineage EPCs and nonhematopoietic lineage EPCs. The hematopoietic EPCs originate from bone marrow and represent a provasculogenic subpopulation of hematopoietic stem cells (HSCs), which play an indispensable role in vascular repair. Different stem cells have different stemness. Embryonic stem cells (ESCs) possess totipotent differentiation potential, and mesenchymal stem cells (MSCs) are also pleuripotent in osteogenic, chondrogenic and adipogenic differentiation. But the stemness and differentiation potential of EPCs, the somatic stem cell, is limited, without multiple differentiation potential. Under normal physiological conditions in adults, the stem cells are in quiescent conditions, and maintain a dynamic balance in growth and decay of tissues. But under pathological conditions or external inductions, the ability to differentiate, regenerate and re-new can be activated. Thus, after the circulating EPCs are recruited, the defected vascular environment will release the molecules, and initiate the oriented differentiation of EPCs into ECs. To further enhance the proliferation and oriented differentiation of EPC to EC for promoted endothelialization, topography to regulate cell orientation, bioactive molecules and therapeutic genes can be applied.
3.1. Regulated cell alignment and orientation
Topography cues for vascular graft surface can exert significant effects on modulating biological behaviors of ECs and endothelialization [143,144]. Micro- and nano-scale topography factors including aligned nanofibers and surface patterns can induce the formation of uniformly aligned ECs for intima construction [145,146].
3.1.1. Aligned nanofibers
Electrospinning technology has been widely applied in nanofibrous vascular graft fabrication, and fiber orientation can be tuned by regulating spinning parameters. The aligned nanofibers can induce cell orientation and modulate cell morphologies and biological performances [93]. Multiple studies have reported that the axially oriented fibers can arrange the morphology and alignment of ECs or MSCs for intima reconstruction [147,148]. Furthermore, the aligned fibers can also provide tensile mechanical strength, enhance SMC alignment in outer layer [149], and higher lumen patency rate [150].
3.1.2. Surface micro- and nano-patterns
Cell-surface interactions play a crucial role in enhancing endothelialization. Graft surface with micro-/nano-scale grove/ridge patterns can enhance the construction of an intact EC layer spontaneously aligned along a prior orientation, which then effectively simulates the elongated endothelium layer structure [151,152]. The aligned ECs have been reported to inhibit leukocyte invasion and consistent with elongated morphology of native ECs under blood flow [153,154].
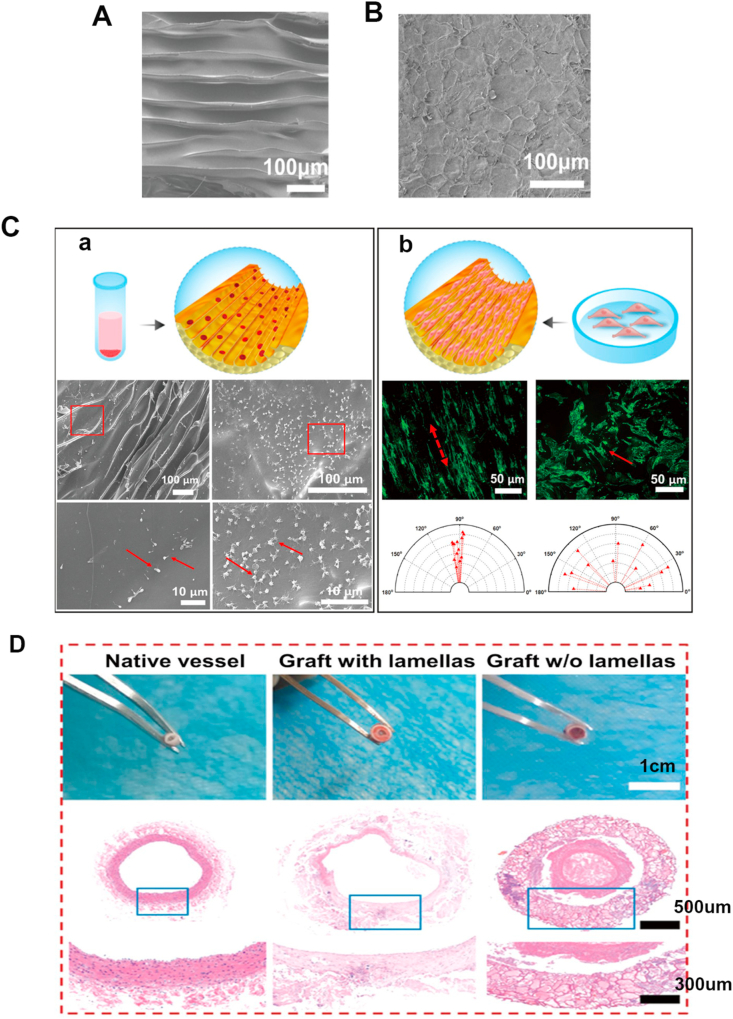
Surface patterning has potential in promoting spontaneous in situ endothelialization [146]. Photolithography, electron beam lithography, and soft lithography can be utilized to develop micro-/nano-scale tube/groove/pillar patterns in different sizes [151,155,156]. The patterns can enhance EC elongation and endothelialization, as well as inhibit platelet adhesion and maintain long-term patency rate [144,[157], [158], [159]]. Wang et al. [160] constructed a biomimetic vascular graft modified with nano-topographic lamellar structure utilizing freeze-cast technique, and the lamella was 10 μm high, 200 nm thick (Fig. 4A). The results indicated that the nano-lamellar framework could prevent platelet activation and enhance EC orientation (Fig. 4C). The number of platelet adhesion on lamellar surface was about 3 times fewer than that on non-lamellar surface. The HE staining images indicated that lamellar structure could promote in situ endothelialization, while distinct thrombus formed in non-lamellar structure group (Fig. 4D). Zorlutuna et al. [161] constructed a vascular graft with channel nanopatterns at the periodicity of 650 nm. The nanopatterns were on both sides of surface to induce orientation and proliferation of ECs and SMCs simultaneously. Different surface nanopatterns are effective strategies to tune cell orientation and vascular remodeling in a pattern-dependent way.
Fig. 4.
The influence of surface topography on cell morphology and biological behavior. (A–B): Scanning electron microscopy (SEM) for inner lamellar structure of vascular graft; A: Inner lamellar structure fabricated by freeze-cast, with the lamellar 10 μm high, 200 nm thick, and the interval between lamellas was 20 μm; B: Inner non-lamellar structure fabricated by direct freeze-drying. (C): Cell behavior on graft surface; (a): Platelets adhesion. SEM figures showed that less platelets adhered on lamellar structure, and they were not activated; b: ECs elongation. ECs displayed elongated adherence along aligned surface of vascular graft and enhanced proliferation. (D): Optical figures and HE staining 3 months after implantation. Reproduced from Ref. [160], ACS NANO, ACS Publication @ 2019.
The arrangements of adhesive peptides also influence the migration, morphology and proliferation of ECs [162]. Wang et al. [163] explored the effects of RGD nano-spacing in different nanoscale size (37–124 nm) on MSC performance, and the results indicated that RGD nano-spacing might have a role in modulating differentiation of MSCs. Saux et al. [164] found that micro-scale pyramids could impede cell migration, while RGD spacing with density of 6✕108 mm2 could enhance cell spreading and adhesion. Karimi et al. [165] compared random and nano-clustered RGD spacing on vascular graft surface, and demonstrated that the nano-island pattern on surface could promote the migration and adhesion of ECs for enhanced in situ endothelialization. RGD is specific for EC capture, and designing RGD pattern on surface can further promote cell migration and proliferation.
3.2. Promoted cell proliferation and activation
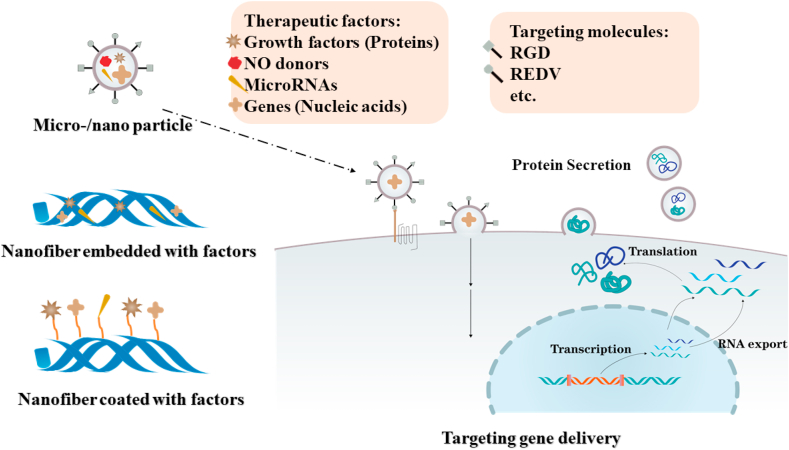
To promote the proliferation and activation of ECs and EPCs after cell adhesion, bioactive molecules and therapeutic genes are promising approaches (Fig. 5).
Fig. 5.
Bioactive molecules and therapeutic genes for enhanced in situ endothelialization. Strategies including micro/nano particle loading, nanofibers embedment or graft surface coating can be utilized to deliver therapeutic factors for promoted cell proliferation and activation. Furthermore, targeting molecules are used for more efficient gene delivery to targeted cells.
3.2.1. Microenvironment regulation for enhanced cell performance
Multiple molecules possess capability to motivate the proliferation, differentiation and activation of ECs and EPCs, including growth factors, gas and microRNAs (Fig. 5). Vascular endothelial growth factor (VEGF) is vital in vascularization, and plays a key role in regulating EC behavior [38,166,167]. Sustained VEGF releasing in vascular graft can promote endothelialization via facilitating differentiation of EPCs and enhancing the proliferation and activation of ECs [168,169]. VEGF can be loaded on vascular grafts through multiple strategies, like NPs [170], coaxial electrospinning [171], direct blending electrospinning [172], and emulsion electrospinning [173], etc. Remarkably, VEGF can also serve as chemotaxis for EPC homing via target of VEGFR1 and VEGFR2 receptors on cell surface [174,175]. Fibroblast growth factor-2 (FGF-2), with potential in directing stem cell differentiation, is also applied in vascular graft [176,177]. Rajangam et al. [178] combined heparin and PAs, and constructed a self-assembly nanofibrous gel to capture VEGFs and FGF-2 for enhanced angiogenesis. Furthermore, platelet-derived growth factor (PDGF) can promote the migration and proliferation of SMCs [179]. Han et al. [180] constructed a double-layer electrospun nanofibrous scaffold, with inner layer loaded with VEGF for EC proliferation, and outer layer with PDGF for SMC proliferation, thus inducing an intact vascular blood vessel formation. Growth factors can directly play a role in vascular construction, but for protein delivery, protein bioactivity and concentration maintenance in vivo are still concerned. To enhance delivery efficiency and maintain protein activity and concentration in vivo, multiple nanoscale and microscale carriers are utilized, which are summarized in Table 2.
Table 2.
Nanoscale and microscale carriers for growth factor delivery.
| Carriers | Cargos | Sizes | Dosages | Results | Ref. | |
|---|---|---|---|---|---|---|
| Nanoparticle | Chitosan/heparin NP | VEGF | 67–132 nm | 43, 113, or 237 ng/mL | Enhancing regeneration of decellularized tissue-engineered scaffolds | [170] |
| Microparticle | Alginate microbeads | FGF-1 | 140 μm | 600 ng | Rapid and persistent vascular response | [181] |
| Gelatin microspheres | FGF-2 | 40 μm | 30 mg | Improving mechanical properties and releasing FGF-2 | [182] | |
| Gelatin microparticles | VEGF | 75–125 μm | 0–100 ng/mL | Prolonging VEGF activity and increasing endothelialization | [183] | |
| Nanofibers | Electrospun PELCL/gelatin and PLGA/gelatin nanofibers | VEGF, PDGF | VEGF 0.59 ng/mg; PDGF 0.53 ng/mg |
PELCLC 568 nm; PLGA 940 nm | VEGF for EC proliferation, and PDGF for SMC proliferation | [180] |
| Self-assembly nanofibrous gel | VEGF, FGF-2 | 25 ng | / | Promoting the tube formation of ECs | [178] | |
Note: Poly (ethylene glycol)-b-poly (l-lactide-co-ε-caprolactone): PELCL; poly (l-lactide-co-glycolide): PLGA.
VEGF is efficient in promoting proliferation and activation of ECs, but the immunogenicity and high expense make the application of VEGF in clinic difficult. MicroRNAs (miRNAs), the non-coding RNAs, have been reported to play a role in regulating vascularization by bonding with promotor region of target genes in recent studies [184,185]. It has been showed that miRNA-126 can regulate the vascular development via modulating the responding of ECs to VEGF, and inhibiting Spred-1 expression, which restrains angiogenic signal pathways [186,187]. Electrospun nanofibers are viable and convenient to load biomolecules, and have been widely utilized to carry miRNAs. Zhou et al. [173] utilized REDV modified PEG-trimethyl chitosan to load miRNA-126, and delivered the miRNA to the targeted ECs. The miRNA complex was incorporated into electrospun polymers utilizing emulsion electrospinning to construct vascular scaffold, and miRNA was released sustainedly for enhanced EC performance. Cui et al. [188] loaded miRNA-126 in inner electrospun fibers, and miRNA-145 in outer fibers, respectively to regulate biological behavior of ECs and SMCs. Moreover, some other miRNAs are also found and explored their effects on vascularization. MiRNA-22 could prevent the apoptosis of SMCs via targeting p38-MAPK pathway during vascular remodeling [189]. Suppression of miRNA-21 restrains EC growing through PTEN dependent-PI3K pathway [190]. Nevertheless, effects of these miRNAs are still at primary research stage, and has not been widely recognized.
Moreover, NO can also stimulate proliferation and activation of ECs, as well as homing of EPCs [191,192], which makes NO donors attractive for vascularization. The biological functions and multiple NO donors applied in vascular grafts will be introduced in the following contents.
3.2.2. Therapeutic gene delivery for enhanced cell performance
Delivery of proteins like VEGF and FGF can directly bind to receptors on target cell surface, and modulate the expression of angiogenesis related genes via signaling pathways, but have risk of protein degradation, inactivation and gradual consumption. Gene therapy is a favorable approach for promoting endothelialization through transfecting ECs, since genes can be transfected into nucleus and translated for protein releasing, potential in maintaining a relative high protein concentration in vivo (Fig. 5) [193]. VEGF, FGF and ZNF580 genes are promising genes for therapeutic gene delivery in vascular graft applications.
To guarantee the transfection efficiency, gene carriers are vital for delivery. Nanoparticles (NPs) can act as carriers, preventing DNA from degradation by enzymes, targeting to the specific cells, entering cell membrane, translocating to the nucleus, and finally integrating to host genome. Various biomaterials have been widely utilized for gene delivery, including lipid NPs [194,195], polymeric NPs like cationic ester polymers [196], co-polymers [[197], [198], [199]], poly (ethylenimine) (PEI) [200], PEI based co-polymers [201,202], inorganic NPs like calcium phosphate [203], and peptide based NPs [204]. Furthermore, electrospinning technology is also attractive in gene loading. Plasmid-DNA (pDNA) can be directly mixed in solvent for electrospinning [203,205,206], or modify nanofibers with gene loaded microparticles [207].
The cell adhesive peptide like RGD [208,209], REDV [210] can be utilized for target gene delivery to facilitate peptide modified pDNA complex bind to integrins on EC surface. Wang et al. [210] constructed a REDV modified pZNF580 NP complexes utilizing self-assembling strategy. The REDV mediated NPs could prevent the pZNF580 from DNase degradation, display better hemocompatibility and enhance delivery efficiency for enhanced in situ endothelialization. Kibria et al. [211] utilized RGD and PEG as dual-ligand modification to promote target gene delivery efficiency.
Delivered genes can integrate into target cell genome, and stably expressing transfected proteins in a long period time, compared with direct protein delivery. But the transfection efficiency is a potential risk, and the transferred genes cannot function in vivo immediately like proteins.
4. Preventing vascular incidents for long-term lumen patency
Thrombogenesis, IH and calcification can reduce lumen diameter, and are major risks in maintaining long-term lumen patency after vascular implantation. Preventing thrombus formation, IH and calcification is crucial for survival of vascular graft. Hydrophilic surface and heparin coating are effective in anti-thrombogenesis, and some drugs can inhibit IH. Moreover, NO can play a role in inhibiting coagulation and IH.
4.1. Anti-thrombogenesis for enhanced long-term lumen patency
It is easy to provoke thrombogenesis if lacking ECs on graft surfaces, but during early healing process, there have not been adequate ECs lining on surface to release molecules for thrombus prevention. Thus, at the beginning of implantation, vascular grafts may direct contact with blood cells in vasculature. The vascular grafts are transplanted as foreign matters, and easy lead to the absorption of plasma proteins and blood cells, and then activate the coagulation cascades [212]. The graft surface with minimized protein adsorption, drugs or gases for anti-coagulation are effective strategies for thrombogenesis prevention.
To inhibit thrombogenesis, minimizing the absorption of plasma proteins on graft surfaces is crucial. Thus, a biocompatible surface with minimized protein adsorption is required. The hydrophilic surface can effectively prevent the protein adsorption. Some biocompatible and hydrophilic biomaterials have been utilized for the surface modification of vascular grafts, for example, PEG [213], zwitterionic polymers [214,215]. PEG and zwitterionic polymers or groups can be directly coated [216], blended [217,218] or covalently grafted [213,219] on the scaffold surfaces, and the hydrophilic surfaces can effectively inhibit the protein adsorption and platelet adherence [214,220].
Furthermore, heparin, known as an anti-coagulative drug, can be coated or immobilized on vascular graft surface, which plays a role in anti-thrombogenesis [[221], [222], [223]]. Heparin can interact with anti-thrombase AT III, to restrain the function of thrombase and coagulation factor Xa. Heparin, with carboxyl groups, can also be blended with gelatin to provide a biocompatible and anti-coagulative surface [88,224]. Liu et al. [225] constructed heparin/poly-l-lysine mixed NPs, and immobilized these NPs on a dopamine modified surface. They found that heparin modified surface could enhance biocompatibility, inhibit fibrin induced platelet adherence, and prolong thrombin time (TT) to 23.7–27.9s. Moreover, LBL technology can be utilized to graft heparin on the graft surfaces [226]. Easton et al. [227] constructed a LBL coating, utilizing ploy (acrylic acid) and polyethyleneimine to immobilized heparin on an electrospun nanofibrous scaffold, and the results indicated that the hemocompatibility of scaffold was enhanced.
Some other anti-coagulation drugs can also be utilized, for example, clopidogrel, warfarin, t-PA [228]. The t-PA is the enzyme converting plasminogen to plasmin, inducing the fibrinolytic process [229]. Liu et al. [228] immobilized t-PA on electrospun PVA nanofibrous mats, aiming to mimic natural fibrinolysis function and prevent thrombus formation. Furthermore, Gastrodin, which is applied in cardiovascular diseases, can lower blood viscosity and regulate inflammation reactions. Zheng et al. [25] fabricated Gastrodin-loaded PU scaffolds, and found that the Gastrodin modified grafts showed greater potential in preventing thrombogenesis and inflammations.
Apart from surface modification and drugs, one novel nanocomposite has been indicated to possess the ability to prevent thrombogenesis and display ideal mechanical characteristics [[230], [231], [232]]. To inhibit thrombogenesis of PU vascular graft scaffolds and improve bistability of PU in vivo, Kannan et al. [233] attached polyhedral oligomeric silsesquioxane (POSS) nanoparticles into poly (carbonate urethane) (PCU) scaffold with covalent bonding and developed a new POSS-PCU nanocomposite biomaterial. This POSS-PCU nanocomposite can be developed into small-diameter vascular grafts with enhanced mechanical properties and hemocompatibility, free from adverse events like IH and calcification.
For hydrophilic surface, it can reduce fibrin adherence and coagulation cascades, but can also decrease EC adhesion. For heparin modification, the bioactivity and coating density are concerned. Hence, more studies are required to obtain ideal graft surface with reduced platelet and fibrin adherence and enhanced EC adhesion.
4.2. Anti-IH for enhanced long-term lumen patency
IH has high incidence in causing long-term vascular occlusion several months after implantation, which is caused by uncontrollable SMC pathological proliferation [234].ECs, serving as the paramount defender in vascularization, can release molecules for IH inhibition. Apart from promoting EC adhesion and activation, some drugs like E2F and MK2i also have potential in preventing IH by controlling SMC proliferation in a more direct way [235]. In clinic, E2F transcription factor was utilized to keep a 30 min lumen patency for the vascular tissue in vitro. But the clinical trial results indicated that E2F treated grafts in vitro failed to preventing IH via inhibiting proliferation of SMCs after implantation [236,237]. The p38 MAPK signaling pathway can play a role in activating proliferation of SMCs, and then induce the downstream inflammatory and fibrotic cascades in IH [[238], [239], [240]]. To prevent IH, Evans et al. [241] utilized MAKP inhibitory peptide (MK2i) and constructed MK2i NPs for graft modification to prevent uncontrollable proliferation of SMCs.
4.3. Role of NO in enhancing long-term lumen patency
NO can play an important protective role in vasculature, and also play an effective role in influencing relevant physiological functions (Fig. 6A) [191]. In physiological environment, NO is secreted by endothelial cells, which is synthesized through catalytic reactions in the presence of nitric oxide synthase (NOS) [242]. NO can inhibit the aggregation and activation of platelets, to avoid thrombogenesis [243], restrain proliferation of SMCs, to avoid IH [242,244], as well as prohibit the recruitment and activation of inflammatory cells, to avoid inflammation (Fig. 6A) [245]. Furthermore, NO plays a critical role in promoting the growth of endothelial cells (Fig. 6A) [246]. Insufficient ECs lead to deficient NO release, which may then trigger pathological process [247]. Hence, abundant NO release is required to enhance ECs growth, inhibit SMCs proliferation, and provide an appropriate environment for endothelialization of vascular graft [248].
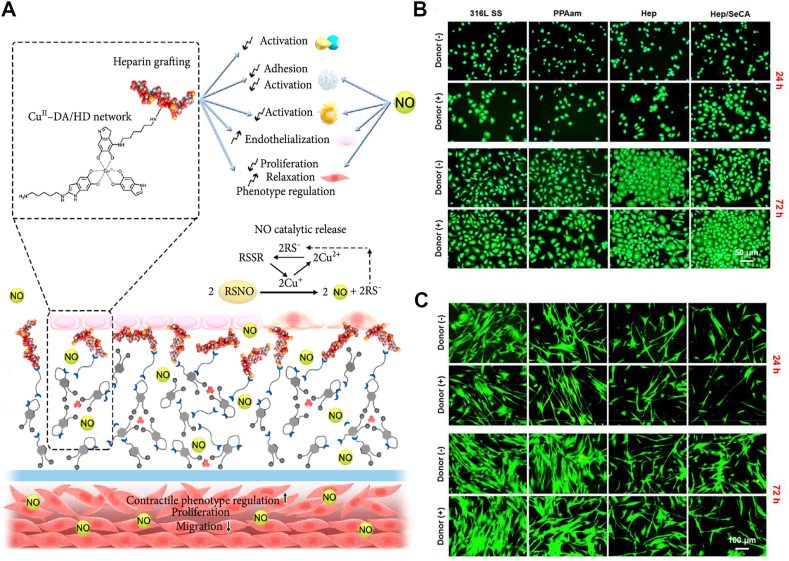
Fig. 6.
NO plays a crucial role in modulating endothelialization, thrombogenesis and IH. (A): The biological performance of NO. NO can be liberated by catalyzing NO donors, and play a role in vascularization, including inhibiting activation of thrombin, platelets, immune cells and proliferation of SMCs, as well as promoting proliferation and activation of ECs, relaxation and phenotype regulation of SMCs. (B–C): Fluorescence staining of ECs and SMCs 24h and 72h after in vitro culture. NO can promote EC proliferation (B) and inhibit SMC growth (C). (A) reproduced from Ref. [242], Research, CAST@ 2020. (B–C) reproduced from Ref. [249], Biomaterials, Elsevier @ 2019.
N-diazeniumdiolates (NONOates) and S-nitrosothiol (RSNOs) can serve as the potential NO donors to be applied in biomaterials. One mole of NONOate [−N(O) = NO−] can be catalyzed to release 2 mol NO via the hydrolysis reactions in the physiological environment (temperature at 37 °C, pH at 7.4) [250]. In RSNOs, thiols (-SH) can react with nitrous acid (HNO2) to release NO, generally under the catalysis of copper ion (Cu2+) [251,252]. Selenocystamine (SeCA), having glutathione peroxidase (GPX)-like functions, is also a potential catalysts for NO synthesis by decomposition of RSNOs [249,253,254].
Multiple strategies have been utilized in vascular devices to catalyze NO generation, like selenocystamine (SeCA) [249,254], metal-phenolic surface [253], and copper ion (Cu2+) [242,255]. Qiu et al. [249] utilized SeCA coating to catalyze NO generation, and the results indicated that NO can promote EC proliferation and inhibit SMC growth (Fig. 6B and C). The amount of ECs on SeCA/heparin surface was about 1.5–2.0 folds more than that on naked surface, and EC migration distance and density was enhanced by 26% and 23%, respectively on SeCA/heparin compared with the naked one [249]. Nanoparticles (NPs) can serve as effective delivery vehicles for NO release. The better surface to volume rate can offer more chance for optimized quantity of NO donors. Targeted NPs can deliver NO via altering surface chemical characteristic to regulate the localized areas [256]. Various NPs have been studied to serve as carriers for NO donors, including silica nanoparticles (SiNPs) [[257], [258], [259]], liposome nanoparticles [260,261], and metallic nanoparticles [255].
SiNPs are easily compounded in the nanoscale, and contain functional groups like amine groups (-NH2) on the surface [262]. The amine-functionalized SiNPs were synthesized, with the size ranging from 20 to 500 nm, to serve as NO carriers via converting the –NH2 groups into NONOates donors under high pressure of NO [262]. To promote the storage and releasing characteristics of NO, the synthesis of SiNPs following the preparation of NONOates modified aminosilanes was proposed by the same team [263]. Fumed silica (FS) particles can also serve as NO carrier, and can be embedded into polymers to control NO releasing [258]. Zhang et al. [259] synthesized FS particles (200–300 nm), with NONOates formed on the surface, and embedded within PU films as anti-thrombotic coating.
Liposomes are competent carriers, owing to their efficient cell encapsulation ability [264]. The influence of hydrophobic liposomes and surface micelles on the NO releasing were studied. Dinh et al. [261] found that anionic 1,2-dipalmitoyl-sn-glycero-3-[phospho-(1-glycerol)] sodium salt (DPPG) liposomes possessed greater NO releasing catalysis efficiency than sodium dodecylsulfate (SDS) micelles. Dinh et al. [265] further explored the influence of unilamellar (anionic and cationic) phospholipid vesicles on dissociating NO from NONOates, and found that anionic liposome NPs showed an enhanced NO releasing. Thermosensitive liposome NPs [266] and photo-sensitive NO donors [267] were also explored.
Silica and liposome NPs described above can effectively load and liberate NO, but cannot deliver the gas to targeted tissues. Some metallic NPs are potential strategies to serve as loading vehicles for NO delivery to targeted tissues, including gold (Au) NPs [[268], [269], [270]], platinum (Pt) NP [271], silver (Ag) NPs [[272], [273], [274]]. Furthermore, metal organic frameworks (MOFs), composed of metal ions as nodes and organic ligands as linkers, have been utilized to embed and release NO to promote re-endothelialization for vascular grafts [275]. Fan et al. [255] constructed nanoscale copper-based MOFs (Cu-MOFs), and proved that Cu-MOFs performed as heterogeneous catalysts for NO regeneration synthesized by endogenous RSNOs. Simultaneous delivery of NO and Cu2+ could restrain restenosis and enhance endothelialization synergistically [275].
Moreover, NO gas can be combined with growth factor VEGF, and spontaneously released to promote endothelialization and inhibit thrombogenesis and IH [276]. Although multiple studies have proved the influence of NO in in vitro and in vivo experiments, no clinical trials in therapeutic effects of NO donors have been conducted. Furthermore, the therapeutic effects on diabetic wounds still need more concerns [193].
5. Immunomodulation in vascular graft development
Inflammatory responses, induced by graft implantation, are crucial in modulating graft development. Molecules released from immune cells influence the biological behavior of ECs and SMCs, and thus modulating in situ endothelialization and lumen patency of vascular graft.
5.1. Immunomodulation and in situ endothelialization
Macrophages, as the key cells in innate immunity, can release multiple molecules modulating in situ endothelialization process [277,278]. It has been reported that TNF-α secreted by macrophages plays a role in regulating migration and differentiation of EPCs and graft development. TNF-α can induce the differentiation of EPCs through activating TNF-α receptor 1 and NF-κB signaling pathway [279]. Moreover, TNF-β1 can promote platelet mediated-EPC homing via integrin β3 on cell surface [280].
The polarization of macrophages can be controlled to regulate the inflammatory microenvironment. Classical macrophages (M1) may secret inflammation cytokines, while alternatively induced macrophages (M2) tend to activate anti-inflammation molecules and enhance tissue repairment [281]. The acute inflammation responses can be modulated by lipid mediators which possess potential in anti-inflammation, like Resolvins [282,283]. Shi et al. [284] incorporated Aspirin-Triggered resolvin D1 (AT-RvD1) into electrospun PCL vascular graft, and demonstrated that the incorporation of AT-RvD1 enhanced vessel tube formation in vitro through M2 macrophage polarization.
Inflammatory cells paly a complex role in regulating angiogenesis. VEGF-A is an important modulator in vascularization, and recently one subset type of neutrophils (which was shown as CD49d+VEGFR1highCXCR4high) were identified [285]. Massena et al. [285] demonstrated that homing this subset neutrophils to hypoxia neovascularization site could promote angiogenesis. The interaction between angiogenic cells and inflammatory cells is still not completely clarified, which requires more further explorations.
5.2. Immunomodulation and long-term lumen patency
Inflammatory cells also play a role in modulating the long-term lumen patency after graft implantation, like IH and intima calcification. Molecules secreted by macrophages and platelets, like TGF-β, cause enhanced migration and proliferation of SMC, and the unregulated SMC proliferation and the inrush of macrophages are dominating factor leading to IH [286]. SMCs from anastomosed sites migrate towards intima, and SMCs transform from the quiescent phenotype into the dedifferentiated, proliferative type, thus resulting in IH [[287], [288], [289]].
Macrophages are the predominant inflammatory cells targeting SMCs, and lead to the pathological proliferation of SMCs [290,291]. Thus, reducing IH via regulating macrophage behavior has attracted great attention. To attenuate chronic inflammation induced IH, Ding et al. [292] constructed resveratrol (RSV) modified carbon nanotube (CNT) coating for tissue engineered blood vessels. RSV has been reported to inhibit inflammation process via inducing M2 macrophage polarization [293]. The RSV modified CNT can be utilized for macrophage intake, and released intracellularly, which effectively promoted transformation of M1 into M2 and prevented IH [292]. Yang et al. [294] modified a decellularized vascular graft with the rapamycin (RM)-blended electrospun PCL coating, and the results indicated that the RM-loaded graft could enhance M2 polarization, inhibit IH, and promote endothelialization.
Inflammation is also the leading factor contributing to atherosclerotic calcification [295]. Wei et al. [296] extracted small extracellular vesicles (sEVs) containing VEGF, miRNA126, and miRNA145 from MSC, and loaded sEVs on a heparinized electrospun PCL scaffold. Wei et al. [296] found that sEVs loaded graft exhibited immunomodulatory function, and induced the transition of M1 into M2, which effectively reduced graft calcification and enhance lumen patency for hyperlipidemia patients.
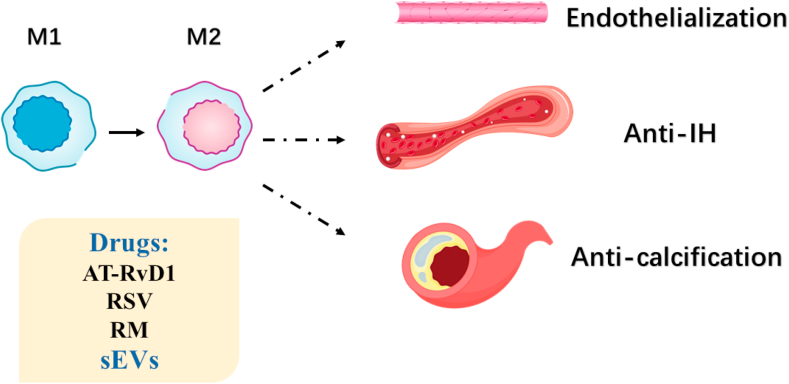
Macrophages, the key cell in innate immune system, can polarize from M1 type to M2 type, which is induced by biomaterials, drugs or sEVs, to regulate behavior of ECs and SMCs, thus promote endothelialization and inhibit IH and calcification (Fig. 7). NK cells also play a role in vascular remodeling. BALB/c mice lack in NK functions display distinctly less IH occurrence [297], but approaches to regulate NK cell functions using bioactive scaffolds have not been available. Generally, the role of complements and cytokines in innate immune system are understood. However, the performance of adaptive immune system, including T cells, B cells and mast cells, is not that clear. For better modulation of immune functions, more studies on regulation of adaptive immune cell behavior are required.
Fig. 7.
Macrophage performance in vascularization. Drugs or sEVs are utilized to promote the transition of M1 into M2 and regulate inflammation reactions for endothelialization enhancement, anti-IH and anti-calcification.
6. Conclusions and further perspectives
The challenges for in situ endothelailization and long-term patency of small-diameter vascular grafts still exist. ECs form the inner endothelium layer, which play a crucial role in maintaining vascular hemostasis and lumen patency, but homing and capture of EPCs and ECs for conventional graft is poor. Naked graft surface without lined EC layer is easy to be deposited with blood cells, fibronectin, and platelets, thus inducing coagulation cascades and thrombogenesis. Furthermore, uncontrollable proliferation of SMCs migrating to intimal layer may result in IH, and inflammatory cells also play a role in regulating biological behavior of ECs, EPCs, and SMCs.
Multiple strategies can be adopted for enhanced in situ endothelialization and long-term patency of vascular grafts (Fig. 8). Strategies for in situ endothelialization promotion, thrombogenesis and IH prevention, and immunomodulation in vascular graft remodeling are summarized as following.
-
(1)
Strategies for enhanced in situ endothelialization:
-
1)
Homing of EPCs: Chemokines aimed at ligands like CXC family and integrin family on EPC surface can be utilized for EPC homing.
-
2)
Migration and adhesion of EPCs and ECs: Nanofibrous structure, biocompatible surface for more binding sites like gelatin and cell-capturing molecules on graft surface including antibodies, specific peptides and aptamers can be applied for better cell adhesion.
-
3)
Proliferation and activation of EPCs and ECs: Topography of scaffold can regulate cell orientation, like aligned nanofibers, surface micro-/nano-patterns, and RGD patterns on surface. Growth factors, microRNAs and therapeutic genes can modulate cell bioactivity.
-
(2)
Strategies for long-term patency:
-
1)
Preventing thrombogenesis: Surface modification with heparin or hydrophilic polymers can inhibit activation and adhesion of platelets, as well as activate AT III, thus reduce thrombogenesis.
-
2)
Preventing IH: Some drugs releasing like MK2i can inhibit IH via inhibiting proliferation of SMCs.
-
3)
The role of NO: NO also plays a crucial role in vascular graft remodeling. NO is potential in inhibiting activation of thrombin, platelets, immune cells and proliferation of SMCs, as well as promoting proliferation and activation of ECs.
-
(3)
Strategies for modulating immunomodulation:
Fig. 8.
Multiple strategies for enhanced in situ endothelialization and long-term lumen patency.
Immunomodulation: Some drugs like AT-RvD1, RSV and RM promote M2 polarization, and exert influences on behavior of ECs and SMCs. sEVs can be utilized for M2 polarization and prevent calcification.
Multiple strategies have been explored to promote in situ endothelialization, inhibit thrombogenesis and IH, but the approaches are limited to experimental researches. More researches concerning toxicity, mechanical properties, degradation rate and delivery efficiency should be considered and conducted for further application in clinics.
Author contributions
Y.Z., C.Z., and M.C. collected references, summarized perspectives and wrote the manuscript. J.H. and Q.L. collected references. G.Y. gave the suggestion and help on the revision of this review. K.L. and Y.H. conceived the concept of this review. All authors discussed and commented on the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (82072396, 81871490, 81571022), Shanghai Collaborative Innovation Center for Translational Medicine (TM202010), Program of Shanghai Academic/Technology Research Leader (19XD1434500), Double Hundred Plan (20191819), and the Research Fund of Medicine and Engineering of Shanghai Jiao Tong University (YG2017MS06).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Kaili Lin, Email: lklecnu@aliyun.com.
Hongbo Yu, Email: yhb3508@163.com.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M., Adams R.J., Berry J.D., Brown T.M., Carnethon M.R., Dai S., de Simone G., Ford E.S., Fox C.S., Fullerton H.J., Gillespie C., Greenlund K.J., Hailpern S.M., Heit J.A., Ho P.M., Howard V.J., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Makuc D.M., Marcus G.M., Marelli A., Matchar D.B., McDermott M.M., Meigs J.B., Moy C.S., Mozaffarian D., Mussolino M.E., Nichol G., Paynter N.P., Rosamond W.D., Sorlie P.D., Stafford R.S., Turan T.N., Turner M.B., Wong N.D., Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 3.Tu C., Das S., Baker A.B., Zoldan J., Suggs L.J. Nanoscale strategies: treatment for peripheral vascular disease and critical limb ischemia. ACS Nano. 2015;9(4):3436–3452. doi: 10.1021/nn507269g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szilagyi D.E., Smith R.F., Elliott J.P., Allen H.M. Long-term behavior of a dacron arterial substitute: clinical, roentgenologic and histologic correlations. Ann. Surg. 1965;162(3):453–477. doi: 10.1097/00000658-196509000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilla P., Bezuidenhout D., Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials. 2007;28(34):5009–5027. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Rocco K.A., Maxfield M.W., Best C.A., Dean E.W., Breuer C.K. In vivo applications of electrospun tissue-engineered vascular grafts: a review. Tissue Eng. B Rev. 2014;20(6):628–640. doi: 10.1089/ten.TEB.2014.0123. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg C.B., Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736):397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 8.Seifu D.G., Purnama A., Mequanint K., Mantovani D. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol. 2013;10(7):410–421. doi: 10.1038/nrcardio.2013.77. [DOI] [PubMed] [Google Scholar]
- 9.Otsuka F., Finn A.V., Yazdani S.K., Nakano M., Kolodgie F.D., Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat. Rev. Cardiol. 2012;9(8):439–453. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 10.Jana S. Endothelialization of cardiovascular devices. Acta Biomater. 2019;99:53–71. doi: 10.1016/j.actbio.2019.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Deanfield J.E., Halcox J.P., Rabelink T.J. Endothelial function and dysfunction. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz B.G., Economides C., Mayeda G.S., Burstein S., Kloner R.A. The endothelial cell in health and disease: its function, dysfunction, measurement and therapy. Int. J. Impot. Res. 2010;22(2):77–90. doi: 10.1038/ijir.2009.59. [DOI] [PubMed] [Google Scholar]
- 13.Deutsch M., Meinhart J., Fischlein T., Preiss P., Zilla P. Clinical autologous in vitro endothelialization of infrainguinal ePTFE grafts in 100 patients: a 9-year experience. Surgery. 1999;126(5):847–855. doi: 10.1016/S0039-6060(99)70025-5. [DOI] [PubMed] [Google Scholar]
- 14.Shirota T., Yasui H., Shimokawa H., Matsuda T. Fabrication of endothelial progenitor cell (EPC)-seeded intravascular stent devices and in vitro endothelialization on hybrid vascular tissue. Biomaterials. 2003;24(13):2295–2302. doi: 10.1016/S0142-9612(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 15.Mirza A., Hyvelin J.M., Rochefort G.Y., Lermusiaux P., Antier D., Awede B., Bonnet P., Domenech J., Eder V. Undifferentiated mesenchymal stem cells seeded on a vascular prosthesis contribute to the restoration of a physiologic vascular wall. J. Vasc. Surg. 2008;47(6):1313–1321. doi: 10.1016/j.jvs.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 16.McIlhenny S.E., Hager E.S., Grabo D.J., DiMatteo C., Shapiro I.M., Tulenko T.N., DiMuzio P.J. Linear shear conditioning improves vascular graft retention of adipose-derived stem cells by upregulation of the alpha5beta1 integrin. Tissue Eng. 2010;16(1):245–255. doi: 10.1089/ten.TEA.2009.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., Chen C., Hellwarth P.B., Bao X. Biomaterials for stem cell engineering and biomanufacturing. Bioactive Materials. 2019;4:366–379. doi: 10.1016/j.bioactmat.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifalian A.M., Tiwari A., Hamilton G., Salacinski H.J. Improving the clinical patency of prosthetic vascular and coronary bypass grafts: the role of seeding and tissue engineering. Artif. Organs. 2002;26(4):307–320. doi: 10.1046/j.1525-1594.2002.06841.x. [DOI] [PubMed] [Google Scholar]
- 19.Bordenave L., Fernandez P., Rémy-Zolghadri M., Villars S., Daculsi R., Midy D. In vitro endothelialized ePTFE prostheses: clinical update 20 years after the first realization. Clin. Hemorheol. Microcirc. 2005;33:227–234. [PubMed] [Google Scholar]
- 20.Avci-Adali M., Ziemer G., Wendel H.P. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization — a review of current strategies. Biotechnol. Adv. 2010;28(1):119–129. doi: 10.1016/j.biotechadv.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Motwani M.S., Rafiei Y., Tzifa A., Seifalian A.M. In situ endothelialization of intravascular stents from progenitor stem cells coated with nanocomposite and functionalized biomolecules. Biotechnol. Appl. Biochem. 2011;58(1):2–13. doi: 10.1002/bab.10. [DOI] [PubMed] [Google Scholar]
- 22.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 23.Pang J.H., Farhatnia Y., Godarzi F., Tan A., Rajadas J., Cousins B.G., Seifalian A.M. In situ endothelialization: bioengineering considerations to translation. Small. 2015;11(47):6248–6264. doi: 10.1002/smll.201402579. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar S., Sales K.M., Hamilton G., Seifalian A.M. Addressing thrombogenicity in vascular graft construction. J. Biomed. Mater. Res. B Appl. Biomater. 2007;82B(1):100–108. doi: 10.1002/jbm.b.30710. [DOI] [PubMed] [Google Scholar]
- 25.Zheng M., Guo J., Li Q., Yang J., Han Y., Yang H., Yu M., Zhong L., Lu D., Li L., Sun L. Syntheses and characterization of anti-thrombotic and anti-oxidative Gastrodin-modified polyurethane for vascular tissue engineering. Bioactive Materials. 2021;6(2):404–419. doi: 10.1016/j.bioactmat.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver H., Shukla N., Ellinsworth D., Jeremy J.Y. Oxidative stress and vein graft failure: a focus on NADH oxidase, nitric oxide and eicosanoids. Curr. Opin. Pharmacol. 2012;12(2):160–165. doi: 10.1016/j.coph.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Depta J.P., Bhatt D.L. New approaches to inhibiting platelets and coagulation. Annu. Rev. Pharmacol. Toxicol. 2015;55:373–397. doi: 10.1146/annurev-pharmtox-010814-124438. [DOI] [PubMed] [Google Scholar]
- 28.Lievens D., Zernecke A., Seijkens T., Soehnlein O., Beckers L., Munnix I.C.A., Wijnands E., Goossens P., van Kruchten R., Thevissen L., Boon L., Flavell R.A., Noelle R.J., Gerdes N., Biessen E.A., Daemen M.J.A.P., Heemskerk J.W.M., Weber C., Lutgens E. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116(20):4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radke D., Jia W., Sharma D., Fena K., Wang G., Goldman J., Zhao F. Tissue engineering at the blood-contacting surface: a review of challenges and strategies in vascular graft development. Advanced Healthcare Materials. 2018;7(15) doi: 10.1002/adhm.201701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yahagi K., Kolodgie F.D., Otsuka F., Finn A.V., Davis H.R., Joner M., Virmani R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2016;13(2):79–98. doi: 10.1038/nrcardio.2015.164. [DOI] [PubMed] [Google Scholar]
- 31.Schepers A., Pires N.M.M., Eefting D., de Vries M.R., van Bockel J.H., Quax P.H.A. Short-term dexamethasone treatment inhibits vein graft thickening in hypercholesterolemic ApoE3Leiden transgenic mice. J. Vasc. Surg. 2006;43(4):809–815. doi: 10.1016/j.jvs.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Ozaki C.K. Cytokines and the early vein graft: strategies to enhance durability. J. Vasc. Surg. 2007;45(Suppl A):A92–A98. doi: 10.1016/j.jvs.2007.02.032. Suppl A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karper J.C., Vries MRd, Brand BTvd, Hoefer I.E., Fischer J.W., Jukema J.W., Niessen H.W.M., Quax P.H.A. Toll-like receptor 4 is involved in human and mouse vein graft remodeling, and local gene silencing reduces vein graft disease in hypercholesterolemic APOE*3Leiden mice. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):1033–1040. doi: 10.1161/ATVBAHA.111.223271. [DOI] [PubMed] [Google Scholar]
- 34.Piccinini A.M., Midwood K.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010 doi: 10.1155/2010/672395. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansson G.K., Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 36.Woollard K.J., Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat. Rev. Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickhout J.G., Basseri S., Austin R.C. Macrophage function and its impact on atherosclerotic lesion composition, progression, and stability. Arterioscler. Thromb. Vasc. Biol. 2008;28(8):1413–1415. doi: 10.1161/ATVBAHA.108.169144. [DOI] [PubMed] [Google Scholar]
- 38.Hristov M., Erl W., Weber P.C. Endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2003;23(7):1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 39.Asahara T., Kawamoto A., Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cell. 2011;29(11):1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 40.Hristov M., Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol. Res. 2008;58(2):148–151. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Langer H., May A.E., Daub K., Heinzmann U., Lang P., Schumm M., Vestweber D., Massberg S., Schönberger T., Pfisterer I., Hatzopoulos A.K., Gawaz M. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ. Res. 2006;98(2):e2–e10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- 42.Hirschi K.K., Ingram D.A., Yoder M.C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2008;28(9):1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoon C.-H., Hur J., Park K.-W., Kim J.-H., Lee C.-S., Oh I.-Y., Kim T.-Y., Cho H.-J., Kang H.-J., Chae I.-H., Yang H.-K., Oh B.-H., Park Y.-B., Kim H.-S. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 44.Hur J., Yang H.-M., Yoon C.-H., Lee C.-S., Park K.-W., Kim J.-H., Kim T.-Y., Kim J.-Y., Kang H.-J., Chae I.-H., Oh B.-H., Park Y.-B., Kim H.-S. Identification of a novel role of T cells in postnatal vasculogenesis. Circulation. 2007;116(15):1671–1682. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 45.De Visscher G., Lebacq A., Mesure L., Blockx H., Vranken I., Plusquin R., Meuris B., Herregods M.-C., Van Oosterwyck H., Flameng W. The remodeling of cardiovascular bioprostheses under influence of stem cell homing signal pathways. Biomaterials. 2010;31(1):20–28. doi: 10.1016/j.biomaterials.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Aiuti A., Webb I.J., Bleul C., Springer T., Gutierrez-Ramos J.C. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 1997;185(1):111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Askari A.T., Unzek S., Popovic Z.B., Goldman C.K., Forudi F., Kiedrowski M., Rovner A., Ellis S.G., Thomas J.D., DiCorleto P.E., Topol E.J., Penn M.S. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi J., Kusano K.F., Masuo O., Kawamoto A., Silver M., Murasawa S., Bosch-Marce M., Masuda H., Losordo D.W., Isner J.M., Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107(9):1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 49.Pelus L.M., Fukuda S. Chemokine-mobilized adult stem cells; defining a better hematopoietic graft. Leukemia. 2008;22(3):466–473. doi: 10.1038/sj.leu.2405021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Visscher G., Mesure L., Meuris B., Ivanova A., Flameng W. Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1α. Acta Biomater. 2012;8(3):1330–1338. doi: 10.1016/j.actbio.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Yu J., Wang A., Tang Z., Henry J., Li-Ping Lee B., Zhu Y., Yuan F., Huang F., Li S. The effect of stromal cell-derived factor-1α/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials. 2012;33(32):8062–8074. doi: 10.1016/j.biomaterials.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Issa Bhaloo S., Wu Y., Le Bras A., Yu B., Gu W., Xie Y., Deng J., Wang Z., Zhang Z., Kong D., Hu Y., Qu A., Zhao Q., Xu Q. Binding of dickkopf-3 to CXCR7 enhances vascular progenitor cell migration and degradable graft regeneration. Circ. Res. 2018;123(4):451–466. doi: 10.1161/circresaha.118.312945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W., Liu D., Li D., Du H., Zhang J., You Z., Li M., He C. Nanofibrous vascular scaffold prepared from miscible polymer blend with heparin/stromal cell-derived factor-1 alpha for enhancing anticoagulation and endothelialization. Colloids Surf. B Biointerfaces. 2019;181:963–972. doi: 10.1016/j.colsurfb.2019.06.065. [DOI] [PubMed] [Google Scholar]
- 54.He D., Zhao A.-S., Su H., Zhang Y., Wang Y.-N., Luo D., Gao Y., Li J.-A., Yang P. An injectable scaffold based on temperature-responsive hydrogel and factor-loaded nanoparticles for application in vascularization in tissue engineering. J. Biomed. Mater. Res. 2019;107(10):2123–2134. doi: 10.1002/jbm.a.36723. [DOI] [PubMed] [Google Scholar]
- 55.Chavakis E., Aicher A., Heeschen C., Sasaki K., Kaiser R., El Makhfi N., Urbich C., Peters T., Scharffetter-Kochanek K., Zeiher A.M., Chavakis T., Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J. Exp. Med. 2005;201(1):63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Y., Ip J.E., Huang J., Zhang L., Matsushita K., Liew C.C., Pratt R.E., Dzau V.J. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ. Res. 2006;99(3):315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 57.Bos J.L. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 2006;31(12):680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Enserink J.M., Price L.S., Methi T., Mahic M., Sonnenberg A., Bos J.L., Taskén K. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J. Biol. Chem. 2004;279(43):44889–44896. doi: 10.1074/jbc.M404599200. [DOI] [PubMed] [Google Scholar]
- 59.Carmona G., Chavakis E., Koehl U., Zeiher A.M., Dimmeler S. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood. 2008;111(5):2640–2646. doi: 10.1182/blood-2007-04-086231. [DOI] [PubMed] [Google Scholar]
- 60.Chavakis E., Hain A., Vinci M., Carmona G., Bianchi M.E., Vajkoczy P., Zeiher A.M., Chavakis T., Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ. Res. 2007;100(2):204–212. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 61.Singh N.K., Janjanam J., Rao G.N. p115 RhoGEF activates the Rac1 GTPase signaling cascade in MCP1 chemokine-induced vascular smooth muscle cell migration and proliferation. J. Biol. Chem. 2017;292(34):14080–14091. doi: 10.1074/jbc.M117.777896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foubert P., Silvestre J.-S., Souttou B., Barateau V., Martin C., Ebrahimian T.G., Leré-Déan C., Contreres J.O., Sulpice E., Levy B.I., Plouët J., Tobelem G., Le Ricousse-Roussanne S. PSGL-1–mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J. Clin. Invest. 2007;117(6):1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao C., Lin K., Wang X. Maintenance and modulation of stem cells stemness based on biomaterial designing via chemical and physical signals. Applied Materials Today. 2020;19 doi: 10.1016/j.apmt.2020.100614. [DOI] [Google Scholar]
- 64.Nikolova M.P., Chavali M.S. Recent advances in biomaterials for 3D scaffolds: a review. Bioactive Materials. 2019;4:271–292. doi: 10.1016/j.bioactmat.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jhala D., Rather H., Kedaria D., Shah J., Singh S., Vasita R. Biomimetic polycaprolactone-chitosan nanofibrous substrate influenced cell cycle and ECM secretion affect cellular uptake of nanoclusters. Bioactive Materials. 2019;4:79–86. doi: 10.1016/j.bioactmat.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhuang Y., Lin K., Yu H. Advance of nano-composite electrospun fibers in periodontal regeneration. Front Chem. 2019;7(495) doi: 10.3389/fchem.2019.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stevens M.M., George J.H. Exploring and engineering the cell surface interface. Science. 2005;310(5751):1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 68.Chen J., Zou X. Self-assemble peptide biomaterials and their biomedical applications. Bioactive Materials. 2019;4:120–131. doi: 10.1016/j.bioactmat.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Santis E., Ryadnov M.G. Peptide self-assembly for nanomaterials: the old new kid on the block. Chem. Soc. Rev. 2015;44(22):8288–8300. doi: 10.1039/c5cs00470e. [DOI] [PubMed] [Google Scholar]
- 70.Barnes C.P., Sell S.A., Boland E.D., Simpson D.G., Bowlin G.L. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007;59(14):1413–1433. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 71.Cui H., Webber M.J., Stupp S.I. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94(1):1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Z., Kotaki M., Inai R., Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11(1–2):101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 73.Smith L.A., Ma P.X. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces. 2004;39(3):125–131. doi: 10.1016/j.colsurfb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Stankus J.J., Soletti L., Fujimoto K., Hong Y., Vorp D.A., Wagner W.R. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28(17):2738–2746. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jayaraman K., Kotaki M., Zhang Y., Mo X., Ramakrishna S. Recent advances in polymer nanofibers. J. Nanosci. Nanotechnol. 2004;4(1–2):52–65. [PubMed] [Google Scholar]
- 76.Chen V.J., Ma P.X. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 77.Hasan A., Memic A., Annabi N., Hossain M., Paul A., Dokmeci M.R., Dehghani F., Khademhosseini A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014;10(1):11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson C.J., Chase G.G., Yarin A.L., Reneker D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer. 2007;48(23):6913–6922. doi: 10.1016/j.polymer.2007.09.017. [DOI] [Google Scholar]
- 79.Rnjak-Kovacina J., Weiss A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. B Rev. 2011;17(5):365–372. doi: 10.1089/ten.teb.2011.0235. [DOI] [PubMed] [Google Scholar]
- 80.Arras M.M., Grasl C., Bergmeister H., Schima H. Electrospinning of aligned fibers with adjustable orientation using auxiliary electrodes. Sci. Technol. Adv. Mater. 2012;13(3) doi: 10.1088/1468-6996/13/3/035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Venkatraman S., Boey F., Lao L.L. Implanted cardiovascular polymers: natural, synthetic and bio-inspired. Prog. Polym. Sci. 2008;33(9):853–874. doi: 10.1016/j.progpolymsci.2008.07.001. [DOI] [Google Scholar]
- 82.Ma Z., Kotaki M., Yong T., He W., Ramakrishna S. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials. 2005;26(15):2527–2536. doi: 10.1016/j.biomaterials.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 83.Burrows M.C., Zamarion V.M., Filippin-Monteiro F.B., Schuck D.C., Toma H.E., Campa A., Garcia C.R.S., Catalani L.H. Hybrid scaffolds built from PET and collagen as a model for vascular graft architecture. Macromol. Biosci. 2012;12(12):1660–1670. doi: 10.1002/mabi.201200154. [DOI] [PubMed] [Google Scholar]
- 84.Takagi H., Goto S-n, Matsui M., Manabe H., Umemoto T. A contemporary meta-analysis of Dacron versus polytetrafluoroethylene grafts for femoropopliteal bypass grafting. J. Vasc. Surg. 2010;52(1):232–236. doi: 10.1016/j.jvs.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Barshes N.R., Ozaki C.K., Kougias P., Belkin M. A cost-effectiveness analysis of infrainguinal bypass in the absence of great saphenous vein conduit. J. Vasc. Surg. 2013;57(6):1466–1470. doi: 10.1016/j.jvs.2012.11.115. [DOI] [PubMed] [Google Scholar]
- 86.Williams S.K., Kleinert L.B., Patula-Steinbrenner V. Accelerated neovascularization and endothelialization of vascular grafts promoted by covalently bound laminin type 1. J. Biomed. Mater. Res. 2011;99A(1):67–73. doi: 10.1002/jbm.a.33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho Y.K., Park D., Kim H., Lee H., Park H., Kim H.J., Jung D. Bioactive surface modifications on inner walls of poly-tetra-fluoro-ethylene tubes using dielectric barrier discharge. Appl. Surf. Sci. 2014;296:79–85. doi: 10.1016/j.apsusc.2014.01.048. [DOI] [Google Scholar]
- 88.Wang H., Feng Y., Behl M., Lendlein A., Zhao H., Xiao R., Lu J., Zhang L., Guo J. Hemocompatible polyurethane/gelatin-heparin nanofibrous scaffolds formed by a bi-layer electrospinning technique as potential artificial blood vessels. Front. Chem. Sci. Eng. 2011;5(3):392–400. doi: 10.1007/s11705-011-1202-0. [DOI] [Google Scholar]
- 89.Feng Y., Meng F., Xiao R., Zhao H., Guo J. Electrospinning of polycarbonate urethane biomaterials. Front. Chem. Sci. Eng. 2011;5(1):11–18. doi: 10.1007/s11705-010-1011-x. [DOI] [Google Scholar]
- 90.Ye X., Lu L., Kolewe M.E., Park H., Larson B.L., Kim E.S., Freed L.E. A biodegradable microvessel scaffold as a framework to enable vascular support of engineered tissues. Biomaterials. 2013;34(38):10007–10015. doi: 10.1016/j.biomaterials.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kong X., Han B., Li H., Liang Y., Shao K., Liu W. New biodegradable small-diameter artificial vascular prosthesis: a feasibility study. J. Biomed. Mater. Res. 2012;100A(6):1494–1504. doi: 10.1002/jbm.a.33298. [DOI] [PubMed] [Google Scholar]
- 92.Rahmati Nejad M., Yousefzadeh M., Solouk A. Electrospun PET/PCL small diameter nanofibrous conduit for biomedical application. Mater. Sci. Eng. C. 2020;110 doi: 10.1016/j.msec.2020.110692. [DOI] [PubMed] [Google Scholar]
- 93.Wu H., Fan J., Chu C.-C., Wu J. Electrospinning of small diameter 3-D nanofibrous tubular scaffolds with controllable nanofiber orientations for vascular grafts. J. Mater. Sci. Mater. Med. 2010;21(12):3207–3215. doi: 10.1007/s10856-010-4164-8. [DOI] [PubMed] [Google Scholar]
- 94.Iwasaki K., Kojima K., Kodama S., Paz A.C., Chambers M., Umezu M., Vacanti C.A. Bioengineered three-layered robust and elastic artery using hemodynamically-equivalent pulsatile bioreactor. Circulation. 2008;118(14 Suppl):S52–S57. doi: 10.1161/circulationaha.107.757369. [DOI] [PubMed] [Google Scholar]
- 95.Iwasaki Y., Sawada S-i, Ishihara K., Khang G., Lee H.B. Reduction of surface-induced inflammatory reaction on PLGA/MPC polymer blend. Biomaterials. 2002;23(18):3897–3903. doi: 10.1016/S0142-9612(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 96.Munoz-Pinto D.J., Jimenez-Vergara A.C., Gharat T.P., Hahn M.S. Characterization of sequential collagen-poly(ethylene glycol) diacrylate interpenetrating networks and initial assessment of their potential for vascular tissue engineering. Biomaterials. 2015;40:32–42. doi: 10.1016/j.biomaterials.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang F., Xie Y., Celik H., Akkus O., Bernacki S.H., King M.W. Engineering small-caliber vascular grafts from collagen filaments and nanofibers with comparable mechanical properties to native vessels. Biofabrication. 2019;11(3) doi: 10.1088/1758-5090/ab15ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wise S.G., Byrom M.J., Waterhouse A., Bannon P.G., Ng M.K.C., Weiss A.S. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011;7(1):295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 99.Elahi M.F., Guan G., Wang L., Zhao X., Wang F., King M.W. Surface modification of silk fibroin fabric using layer-by-layer polyelectrolyte deposition and heparin immobilization for small-diameter vascular prostheses. Langmuir. 2015;31(8):2517–2526. doi: 10.1021/la504503w. [DOI] [PubMed] [Google Scholar]
- 100.Merkle V.M., Martin D., Hutchinson M., Tran P.L., Behrens A., Hossainy S., Sheriff J., Bluestein D., Wu X., Slepian M.J. Hemocompatibility of poly(vinyl alcohol)–gelatin core–shell electrospun nanofibers: a scaffold for modulating platelet deposition and activation. ACS Appl. Mater. Interfaces. 2015;7(15):8302–8312. doi: 10.1021/acsami.5b01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mi H.-Y., Jiang Y., Jing X., Enriquez E., Li H., Li Q., Turng L.-S. Fabrication of triple-layered vascular grafts composed of silk fibers, polyacrylamide hydrogel, and polyurethane nanofibers with biomimetic mechanical properties. Mater. Sci. Eng. C. 2019;98:241–249. doi: 10.1016/j.msec.2018.12.126. [DOI] [PubMed] [Google Scholar]
- 102.Tan X., Gao P., Li Y., Qi P., Liu J., Shen R., Wang L., Huang N., Xiong K., Tian W., Tu Q. Poly-dopamine, poly-levodopa, and poly-norepinephrine coatings: comparison of physico-chemical and biological properties with focus on the application for blood-contacting devices. Bioactive Materials. 2021;6(1):285–296. doi: 10.1016/j.bioactmat.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ren X., Feng Y., Guo J., Wang H., Li Q., Yang J., Hao X., Lv J., Ma N., Li W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015;44(15):5680–5742. doi: 10.1039/C4CS00483C. [DOI] [PubMed] [Google Scholar]
- 104.Chen J.-P., Su C.-H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011;7(1):234–243. doi: 10.1016/j.actbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 105.Merkle V.M., Zeng L., Slepian M.J., Wu X. Core-shell nanofibers: integrating the bioactivity of gelatin and the mechanical property of polyvinyl alcohol. Biopolymers. 2014;101(4):336–346. doi: 10.1002/bip.22367. [DOI] [PubMed] [Google Scholar]
- 106.Wang D., Wang X., Li X., Jiang L., Chang Z., Li Q. Biologically responsive, long-term release nanocoating on an electrospun scaffold for vascular endothelialization and anticoagulation. Mater. Sci. Eng. C. 2020;107 doi: 10.1016/j.msec.2019.110212. [DOI] [PubMed] [Google Scholar]
- 107.Koens M.J.W., Krasznai A.G., Hanssen A.E.J., Hendriks T., Praster R., Daamen W.F., van der Vliet J.A., van Kuppevelt T.H. Vascular replacement using a layered elastin-collagen vascular graft in a porcine model: one week patency versus one month occlusion. Organogenesis. 2015;11(3):105–121. doi: 10.1080/15476278.2015.1038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grus T., Lambert L., Mlcek M., Chlup H., Honsova E., Spacek M., Burgetova A., Lindner J. In vivo evaluation of short-term performance of new three-layer collagen-based vascular graft designed for low-flow peripheral vascular reconstructions. BioMed Res. Int. 2018 doi: 10.1155/2018/3519596. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Z., Liu L., Mithieux S.M., Weiss A.S. Fabricating organized elastin in vascular grafts. Trends Biotechnol. 2020 doi: 10.1016/j.tibtech.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 110.Gupta P., Lorentz K.L., Haskett D.G., Cunnane E.M., Ramaswamy A.K., Weinbaum J.S., Vorp D.A., Mandal B.B. Bioresorbable silk grafts for small diameter vascular tissue engineering applications: in vitro and in vivo functional analysis. Acta Biomater. 2020;105:146–158. doi: 10.1016/j.actbio.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao L., Li X., Yang L., Sun L., Mu S., Zong H., Li Q., Wang F., Song S., Yang C., Zhao C., Chen H., Zhang R., Wang S., Dong Y., Zhang Q. Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo. Mater. Sci. Eng. C. 2021;118 doi: 10.1016/j.msec.2020.111441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu J., Wang A., Tang Z., Henry J., Li-Ping Lee B., Zhu Y., Yuan F., Huang F., Li S. The effect of stromal cell-derived factor-1α/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials. 2012;33(32):8062–8074. doi: 10.1016/j.biomaterials.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hersel U., Dahmen C., Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/S0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 114.Aoki J., Serruys P.W., van Beusekom H., Ong A.T.L., McFadden E.P., Sianos G., van der Giessen W.J., Regar E., de Feyter P.J., Davis H.R., Rowland S., Kutryk M.J.B. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (healthy endothelial accelerated lining inhibits neointimal growth-first in man) registry. J. Am. Coll. Cardiol. 2005;45(10):1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 115.Co M., Tay E., Lee C.H., Poh K.K., Low A., Lim J., Lim I.H., Lim Y.T., Tan H.C. Use of endothelial progenitor cell capture stent (Genous Bio-Engineered R Stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate- to long-term clinical follow-up. Am. Heart J. 2008;155(1):128–132. doi: 10.1016/j.ahj.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 116.Kaplan D., Husel W., Lewandowska K. CD34 expression on platelets. Platelets. 2003;14(2):83–87. doi: 10.1080/0953710031000080577. [DOI] [PubMed] [Google Scholar]
- 117.Yeh E.T.H., Zhang S., Wu H.D., Körbling M., Willerson J.T., Estrov Z. Transdifferentiation of human peripheral blood cd34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108(17):2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 118.Sethi R., Lee C.-H. Endothelial progenitor cell capture stent: safety and effectiveness. J. Intervent. Cardiol. 2012;25(5):493–500. doi: 10.1111/j.1540-8183.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 119.Rotmans J.I., Heyligers J.M.M., Verhagen H.J.M., Velema E., Nagtegaal M.M., Kleijn DPVd, Groot FGd, Stroes E.S.G., Pasterkamp G. In vivo cell seeding with anti-CD34 antibodies successfully Accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation. 2005;112(1):12–18. doi: 10.1161/CIRCULATIONAHA.104.504407. [DOI] [PubMed] [Google Scholar]
- 120.Nakazawa G., Granada J.F., Alviar C.L., Tellez A., Kaluza G.L., Guilhermier M.Y., Parker S., Rowland S.M., Kolodgie F.D., Leon M.B., Virmani R. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc. Interv. 2010;3(1):68–75. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 121.Haude M., Lee S.W.L., Worthley S.G., Silber S., Verheye S., Erbs S., Rosli M.A., Botelho R., Meredith I., Sim K.H., Stella P.R., Tan H.-C., Whitbourn R., Thambar S., Abizaid A., Koh T.H., Den Heijer P., Parise H., Cristea E., Maehara A., Mehran R. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC Cardiovasc. Interv. 2013;6(4):334–343. doi: 10.1016/j.jcin.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 122.Kawahara D., Matsuda T. Hydrodynamic shear-stress-dependent retention of endothelial and endothelial progenitor cells adhered to vascular endothelial growth factor-fixed surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100B(5):1218–1228. doi: 10.1002/jbm.b.32686. [DOI] [PubMed] [Google Scholar]
- 123.Capture of flowing endothelial cells using surface-immobilized anti-kinase insert Domain receptor antibody (2008). Tissue Eng. C Methods 14 (2):97-105. doi:10.1089/ten.tec.2007.0300. [DOI] [PubMed]
- 124.Liddington R.C., Ginsberg M.H. Integrin activation takes shape. J. Cell Biol. 2002;158(5):833–839. doi: 10.1083/jcb.200206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin H.-B., Sun W., Mosher D.F., García-Echeverría C., Schaufelberger K., Lelkes P.I., Cooper S.L. Synthesis, surface, and cell-adhesion properties of polyurethanes containing covalently grafted RGD-peptides. J. Biomed. Mater. Res. 1994;28(3):329–342. doi: 10.1002/jbm.820280307. [DOI] [PubMed] [Google Scholar]
- 126.Wang Z., Wang H., Zheng W., Zhang J., Zhao Q., Wang S., Yang Z., Kong D. Highly stable surface modifications of poly(3-caprolactone) (PCL) films by molecular self-assembly to promote cells adhesion and proliferation. Chem Commun. 2011;47(31):8901–8903. doi: 10.1039/C1CC11564B. [DOI] [PubMed] [Google Scholar]
- 127.Park J., Brust T.F., Lee H.J., Lee S.C., Watts V.J., Yeo Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS Nano. 2014;8(4):3347–3356. doi: 10.1021/nn405809c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang K., Lee J.S., Kim J., Lee Y.B., Shin H., Um S.H., Kim J.B., Park K.I., Lee H., Cho S.-W. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials. 2012;33(29):6952–6964. doi: 10.1016/j.biomaterials.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 129.Sun Y., Deng Y., Ye Z., Liang S., Tang Z., Wei S. Peptide decorated nano-hydroxyapatite with enhanced bioactivity and osteogenic differentiation via polydopamine coating. Colloids Surf. B Biointerfaces. 2013;111:107–116. doi: 10.1016/j.colsurfb.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 130.Kanie K., Narita Y., Zhao Y., Kuwabara F., Satake M., Honda S., Kaneko H., Yoshioka T., Okochi M., Honda H., Kato R. Collagen type IV-specific tripeptides for selective adhesion of endothelial and smooth muscle cells. Biotechnol. Bioeng. 2012;109(7):1808–1816. doi: 10.1002/bit.24459. [DOI] [PubMed] [Google Scholar]
- 131.Khan M., Yang J., Shi C., Lv J., Feng Y., Zhang W. Surface tailoring for selective endothelialization and platelet inhibition via a combination of SI-ATRP and click chemistry using Cys–Ala–Gly-peptide. Acta Biomater. 2015;20:69–81. doi: 10.1016/j.actbio.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 132.Seeto W.J., Tian Y., Lipke E.A. Peptide-grafted poly(ethylene glycol) hydrogels support dynamic adhesion of endothelial progenitor cells. Acta Biomater. 2013;9(9):8279–8289. doi: 10.1016/j.actbio.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 133.Lin Q.-K., Hou Y., Ren K.-F., Ji J. Selective endothelial cells adhesion to Arg-Glu-Asp-Val peptide functionalized polysaccharide multilayer. Thin Solid Films. 2012;520(15):4971–4978. doi: 10.1016/j.tsf.2012.03.041. [DOI] [Google Scholar]
- 134.Massia S.P., Hubbell J.A. Human endothelial cell interactions with surface-coupled adhesion peptides on a nonadhesive glass substrate and two polymeric biomaterials. J. Biomed. Mater. Res. 1991;25(2):223–242. doi: 10.1002/jbm.820250209. [DOI] [PubMed] [Google Scholar]
- 135.Wang P.-Y., Wu T.-H., Tsai W.-B., Kuo W.-H., Wang M.-J. Grooved PLGA films incorporated with RGD/YIGSR peptides for potential application on skeletal muscle tissue engineering. Colloids Surf. B Biointerfaces. 2013;110:88–95. doi: 10.1016/j.colsurfb.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 136.Wang W., Guo L., Yu Y., Chen Z., Zhou R., Yuan Z. Peptide REDV-modified polysaccharide hydrogel with endothelial cell selectivity for the promotion of angiogenesis. J. Biomed. Mater. Res. 2015;103(5):1703–1712. doi: 10.1002/jbm.a.35306. [DOI] [PubMed] [Google Scholar]
- 137.Wei Y., Ji Y., Xiao L.-L., Lin Q-k, Xu J-p, Ren K-f, Ji J. Surface engineering of cardiovascular stent with endothelial cell selectivity for in vivo re-endothelialisation. Biomaterials. 2013;34(11):2588–2599. doi: 10.1016/j.biomaterials.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 138.Plouffe B.D., Njoka D.N., Harris J., Liao J., Horick N.K., Radisic M., Murthy S.K. Peptide-mediated selective adhesion of smooth muscle and endothelial cells in microfluidic shear flow. Langmuir. 2007;23(9):5050–5055. doi: 10.1021/la0700220. [DOI] [PubMed] [Google Scholar]
- 139.Ceylan H., Tekinay A.B., Guler M.O. Selective adhesion and growth of vascular endothelial cells on bioactive peptide nanofiber functionalized stainless steel surface. Biomaterials. 2011;32(34):8797–8805. doi: 10.1016/j.biomaterials.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 140.Ohuchi S. Cell-SELEX technology. Biores Open Access. 2012;1(6):265–272. doi: 10.1089/biores.2012.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hoffmann J., Paul A., Harwardt M., Groll J., Reeswinkel T., Klee D., Moeller M., Fischer H., Walker T., Greiner T., Ziemer G., Wendel H.P. Immobilized DNA aptamers used as potent attractors for porcine endothelial precursor cells. J. Biomed. Mater. Res. 2008;84A(3):614–621. doi: 10.1002/jbm.a.31309. [DOI] [PubMed] [Google Scholar]
- 142.Avci-Adali M., Steinle H., Michel T., Schlensak C., Wendel H.P. Potential capacity of aptamers to trigger immune activation in human blood. PloS One. 2013;8(7) doi: 10.1371/journal.pone.0068810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dvir T., Timko B.P., Kohane D.S., Langer R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011;6(1):13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moffa M., Sciancalepore A.G., Passione L.G., Pisignano D. Combined nano- and micro-scale topographic cues for engineered vascular constructs by electrospinning and imprinted micro-patterns. Small. 2014;10(12):2439–2450. doi: 10.1002/smll.201303179. [DOI] [PubMed] [Google Scholar]
- 145.Miller D.C., Webster T.J., Haberstroh K.M. Technological advances in nanoscale biomaterials: the future of synthetic vascular graft design. Expet Rev. Med. Dev. 2004;1(2):259–268. doi: 10.1586/17434440.1.2.259. [DOI] [PubMed] [Google Scholar]
- 146.Chong D.S., Lindsey B., Dalby M.J., Gadegaard N., Seifalian A.M., Hamilton G. Luminal surface engineering, 'micro and nanopatterning': potential for self endothelialising vascular grafts? Eur. J. Vasc. Endovasc. Surg. 2014;47(5):566–576. doi: 10.1016/j.ejvs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 147.Xing Q., Qian Z., Tahtinen M., Yap A.H., Yates K., Zhao F. Aligned nanofibrous cell-derived extracellular matrix for Anisotropic vascular graft construction. Adv Healthc Mater. 2017;6(10) doi: 10.1002/adhm.201601333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu T., Zhang J., Wang Y., Li D., Sun B., El-Hamshary H., Yin M., Mo X. Fabrication and preliminary study of a biomimetic tri-layer tubular graft based on fibers and fiber yarns for vascular tissue engineering. Mater. Sci. Eng. C. 2018;82:121–129. doi: 10.1016/j.msec.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 149.Wong C.S., Liu X., Xu Z., Lin T., Wang X. Elastin and collagen enhances electrospun aligned polyurethane as scaffolds for vascular graft. J. Mater. Sci. Mater. Med. 2013;24(8):1865–1874. doi: 10.1007/s10856-013-4937-y. [DOI] [PubMed] [Google Scholar]
- 150.Liu R., Qin Y., Wang H., Zhao Y., Hu Z., Wang S. The in vivo blood compatibility of bio-inspired small diameter vascular graft: effect of submicron longitudinally aligned topography. BMC Cardiovasc. Disord. 2013;13:79. doi: 10.1186/1471-2261-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Biela S.A., Su Y., Spatz J.P., Kemkemer R. Different sensitivity of human endothelial cells, smooth muscle cells and fibroblasts to topography in the nano–micro range. Acta Biomater. 2009;5(7):2460–2466. doi: 10.1016/j.actbio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 152.Bettinger C.J., Orrick B., Misra A., Langer R., Borenstein J.T. Microfabrication of poly (glycerol–sebacate) for contact guidance applications. Biomaterials. 2006;27(12):2558–2565. doi: 10.1016/j.biomaterials.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 153.Cunningham K.S., Gotlieb A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 154.Wang B., Cai Q., Zhang S., Yang X., Deng X. The effect of poly (L-lactic acid) nanofiber orientation on osteogenic responses of human osteoblast-like MG63 cells. Journal of the Mechanical Behavior of Biomedical Materials. 2011;4(4):600–609. doi: 10.1016/j.jmbbm.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 155.Liliensiek S.J., Wood J.A., Yong J., Auerbach R., Nealey P.F., Murphy C.J. Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials. 2010;31(20):5418–5426. doi: 10.1016/j.biomaterials.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Morgan J.T., Wood J.A., Shah N.M., Hughbanks M.L., Russell P., Barakat A.I., Murphy C.J. Integration of basal topographic cues and apical shear stress in vascular endothelial cells. Biomaterials. 2012;33(16):4126–4135. doi: 10.1016/j.biomaterials.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding Y., Yang Z., Bi C.W.C., Yang M., Xu S.L., Lu X., Huang N., Huang P., Leng Y. Directing vascular cell selectivity and hemocompatibility on patterned platforms Featuring variable topographic Geometry and size. ACS Appl. Mater. Interfaces. 2014;6(15):12062–12070. doi: 10.1021/am502692k. [DOI] [PubMed] [Google Scholar]
- 158.Seunarine K., Meredith D.O., Riehle M.O., Wilkinson C.D.W., Gadegaard N. Biodegradable polymer tubes with lithographically controlled 3D micro- and nanotopography. Microelectron. Eng. 2008;85(5):1350–1354. doi: 10.1016/j.mee.2008.02.002. [DOI] [Google Scholar]
- 159.Zhou K., Li Y., Zhang L., Jin L., Yuan F., Tan J., Yuan G., Pei J. Nano-micrometer surface roughness gradients reveal topographical influences on differentiating responses of vascular cells on biodegradable magnesium. Bioactive Materials. 2021;6(1):262–272. doi: 10.1016/j.bioactmat.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Z., Liu C., Xiao Y., Gu X., Xu Y., Dong N., Zhang S., Qin Q., Wang J. Remodeling of a cell-free vascular graft with Nanolamellar intima into a Neovessel. ACS Nano. 2019;13(9):10576–10586. doi: 10.1021/acsnano.9b04704. [DOI] [PubMed] [Google Scholar]
- 161.Zorlutuna P., Vadgama P., Hasirci V. Both sides nanopatterned tubular collagen scaffolds as tissue-engineered vascular grafts. J Tissue Eng Regen Med. 2010;4(8):628–637. doi: 10.1002/term.278. [DOI] [PubMed] [Google Scholar]
- 162.Le Saux G., Magenau A., Gunaratnam K., Kilian K.A., Böcking T., Gooding J.J., Gaus K. Spacing of integrin ligands influences signal transduction in endothelial cells. Biophys. J. 2011;101(4):764–773. doi: 10.1016/j.bpj.2011.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang X., Yan C., Ye K., He Y., Li Z., Ding J. Effect of RGD nanospacing on differentiation of stem cells. Biomaterials. 2013;34(12):2865–2874. doi: 10.1016/j.biomaterials.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 164.Le Saux G., Magenau A., Böcking T., Gaus K., Gooding J.J. The relative importance of topography and RGD ligand density for endothelial cell adhesion. PloS One. 2011;6(7) doi: 10.1371/journal.pone.0021869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karimi F., McKenzie T.G., O'Connor A.J., Qiao G.G., Heath D.E. Nano-scale clustering of integrin-binding ligands regulates endothelial cell adhesion, migration, and endothelialization rate: novel materials for small diameter vascular graft applications. J. Mater. Chem. B. 2017;5(30):5942–5953. doi: 10.1039/C7TB01298E. [DOI] [PubMed] [Google Scholar]
- 166.Eichmann A., Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr. Opin. Cell Biol. 2012;24(2):188–193. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferrara N., Gerber H.-P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 168.Shin Y.M., Lee Y.B., Kim S.J., Kang J.K., Park J.-C., Jang W., Shin H. Mussel-Inspired immobilization of vascular endothelial growth factor (VEGF) for enhanced endothelialization of vascular grafts. Biomacromolecules. 2012;13(7):2020–2028. doi: 10.1021/bm300194b. [DOI] [PubMed] [Google Scholar]
- 169.Zhang J., Silva T., Yarovinsky T., Manes T.D., Tavakoli S., Nie L., Tellides G., Pober J.S., Bender J.R., Sadeghi M.M. VEGF blockade inhibits lymphocyte recruitment and Ameliorates immune-mediated vascular remodeling. Circ. Res. 2010;107(3):408–417. doi: 10.1161/CIRCRESAHA.109.210963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tan Q., Tang H., Hu J., Hu Y., Zhou X., Tao Y., Wu Z. Controlled release of chitosan/heparin nanoparticle-delivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. Int. J. Nanomed. 2011;6:929–942. doi: 10.2147/ijn.s18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hu Y.-T., Pan X.-D., Zheng J., Ma W.-G., Sun L.-Z. In vitro and in vivo evaluation of a small-caliber coaxial electrospun vascular graft loaded with heparin and VEGF. Int. J. Surg. 2017;44:244–249. doi: 10.1016/j.ijsu.2017.06.077. [DOI] [PubMed] [Google Scholar]
- 172.Yang B., Cao G., Cai K., Wang G., Li P., Zheng L., Cai H., Zhu Y., Li X., Wu Y. VEGF-modified PVA/silicone nanofibers enhance islet function transplanted in subcutaneous site followed by Device-less procedure. Int. J. Nanomed. 2020;15:587–599. doi: 10.2147/ijn.s232224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou F., Jia X., Yang Y., Yang Q., Gao C., Hu S., Zhao Y., Fan Y., Yuan X. Nanofiber-mediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration. Acta Biomater. 2016;43:303–313. doi: 10.1016/j.actbio.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 174.Miyazu K., Kawahara D., Ohtake H., Watanabe G., Matsuda T. Luminal surface design of electrospun small-diameter graft aiming at in situ capture of endothelial progenitor cell. J. Biomed. Mater. Res. B Appl. Biomater. 2010;94(1):53–63. doi: 10.1002/jbm.b.31623. [DOI] [PubMed] [Google Scholar]
- 175.Suzuki Y., Yamamoto K., Ando J., Matsumoto K., Matsuda T. Arterial shear stress augments the differentiation of endothelial progenitor cells adhered to VEGF-bound surfaces. Biochem. Biophys. Res. Commun. 2012;423(1):91–97. doi: 10.1016/j.bbrc.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 176.Cho Y., Lee J.B., Hong J. Tunable growth factor release from nano-assembled starch multilayers. Chem. Eng. J. 2013;221:32–36. doi: 10.1016/j.cej.2013.01.087. [DOI] [Google Scholar]
- 177.Park J.H., Hong J. Continuous release of bFGF from multilayer nanofilm to maintain undifferentiated human iPS cell cultures. Integr Biol (Camb) 2014;6(12):1196–1200. doi: 10.1039/c4ib00210e. [DOI] [PubMed] [Google Scholar]
- 178.Rajangam K., Arnold M.S., Rocco M.A., Stupp S.I. Peptide amphiphile nanostructure–heparin interactions and their relationship to bioactivity. Biomaterials. 2008;29(23):3298–3305. doi: 10.1016/j.biomaterials.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang H., Jia X., Han F., Zhao J., Zhao Y., Fan Y., Yuan X. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials. 2013;34(9):2202–2212. doi: 10.1016/j.biomaterials.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 180.Han F., Jia X., Dai D., Yang X., Zhao J., Zhao Y., Fan Y., Yuan X. Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials. 2013;34(30):7302–7313. doi: 10.1016/j.biomaterials.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 181.Khanna O., Huang J.-J., Moya M.L., Wu C.-W., Cheng M.-H., Opara E.C., Brey E.M. FGF-1 delivery from multilayer alginate microbeads stimulates a rapid and persistent increase in vascular density. Microvasc. Res. 2013;90:23–29. doi: 10.1016/j.mvr.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 182.Selcan Gungor-Ozkerim P., Balkan T., Kose G.T., Sezai Sarac A., Kok F.N. Incorporation of growth factor loaded microspheres into polymeric electrospun nanofibers for tissue engineering applications. J. Biomed. Mater. Res. 2014;102(6):1897–1908. doi: 10.1002/jbm.a.34857. [DOI] [PubMed] [Google Scholar]
- 183.Poldervaart M.T., Gremmels H., van Deventer K., Fledderus J.O., Öner F.C., Verhaar M.C., Dhert W.J.A., Alblas J. Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J. Contr. Release. 2014;184:58–66. doi: 10.1016/j.jconrel.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 184.Ev Rooij. The Art of MicroRNA research. Circ. Res. 2011;108(2):219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- 185.Feinberg M.W., Moore K.J. MicroRNA regulation of atherosclerosis. Circ. Res. 2016;118(4):703–720. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. The endothelial-specific MicroRNA miR-126 Governs vascular integrity and angiogenesis. Dev. Cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.-F., Wythe J.D., Ivey K.N., Bruneau B.G., Stainier D.Y.R., Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cui C., Wen M., Zhou F., Zhao Y., Yuan X. Target regulation of both VECs and VSMCs by dual-loading miRNA-126 and miRNA-145 in the bilayered electrospun membrane for small-diameter vascular regeneration. J. Biomed. Mater. Res. 2019;107(2):371–382. doi: 10.1002/jbm.a.36548. [DOI] [PubMed] [Google Scholar]
- 189.Xiao Y., Sun Y., Ma X., Wang C., Zhang L., Wang J., Wang G., Li Z., Tian W., Zhao Z., Jing Q., Zhou J., Jing Z. MicroRNA-22 inhibits the apoptosis of vascular smooth muscle cell by targeting p38MAPKα in vascular remodeling of aortic dissection. Mol. Ther. Nucleic Acids. 2020 doi: 10.1016/j.omtn.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lu J.-M., Zhang Z.-Z., Ma X., Fang S.-F., Qin X.-H. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Exp. Eye Res. 2020;190 doi: 10.1016/j.exer.2019.107886. [DOI] [PubMed] [Google Scholar]
- 191.de Mel A., Murad F., Seifalian A.M. Nitric oxide: a Guardian for vascular grafts? Chem. Rev. 2011;111(9):5742–5767. doi: 10.1021/cr200008n. [DOI] [PubMed] [Google Scholar]
- 192.Andukuri A., Sohn Y.D., Anakwenze C.P., Lim D.J., Brott B.C., Yoon Y.S., Jun H.W. Enhanced human endothelial progenitor cell adhesion and differentiation by a bioinspired multifunctional nanomatrix. Tissue Eng. C Methods. 2013;19(5):375–385. doi: 10.1089/ten.TEC.2012.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lou D., Luo Y., Pang Q., Tan W.-Q., Ma L. Gene-activated dermal equivalents to accelerate healing of diabetic chronic wounds by regulating inflammation and promoting angiogenesis. Bioactive Materials. 2020;5(3):667–679. doi: 10.1016/j.bioactmat.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lajunen T., Hisazumi K., Kanazawa T., Okada H., Seta Y., Yliperttula M., Urtti A., Takashima Y. Topical drug delivery to retinal pigment epithelium with microfluidizer produced small liposomes. Eur. J. Pharmaceut. Sci. 2014;62:23–32. doi: 10.1016/j.ejps.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 195.Negishi Y., Tsunoda Y., Hamano N., Omata D., Endo-Takahashi Y., Suzuki R., Maruyama K., Nomizu M., Aramaki Y. Ultrasound-mediated gene delivery systems by AG73-modified bubble liposomes. Peptide Science. 2013;100(4):402–407. doi: 10.1002/bip.22246. [DOI] [PubMed] [Google Scholar]
- 196.Carr L.R., Jiang S. Mediating high levels of gene transfer without cytotoxicity via hydrolytic cationic ester polymers. Biomaterials. 2010;31(14):4186–4193. doi: 10.1016/j.biomaterials.2010.01.110. [DOI] [PubMed] [Google Scholar]
- 197.Huang Z., Teng W., Liu L., Wang L., Wang Q., Dong Y. Efficient cytosolic delivery mediated by polymersomes facilely prepared from a degradable, amphiphilic, and amphoteric copolymer. Nanotechnology. 2013;24(26):265104. doi: 10.1088/0957-4484/24/26/265104. [DOI] [PubMed] [Google Scholar]
- 198.Teng W., Huang Z., Chen Y., Wang L., Wang Q., Huang H. pVEGF-loaded lipopolysaccharide-amine nanopolymersomes for therapeutic angiogenesis. Nanotechnology. 2014;25(6) doi: 10.1088/0957-4484/25/6/065702. [DOI] [PubMed] [Google Scholar]
- 199.Zhang J., Wang Z., Lin W., Chen S. Gene transfection in complex media using PCBMAEE-PCBMA copolymer with both hydrolytic and zwitterionic blocks. Biomaterials. 2014;35(27):7909–7918. doi: 10.1016/j.biomaterials.2014.05.066. [DOI] [PubMed] [Google Scholar]
- 200.Calarco A., Bosetti M., Margarucci S., Fusaro L., Nicolì E., Petillo O., Cannas M., Galderisi U., Peluso G. The genotoxicity of PEI-based nanoparticles is reduced by acetylation of polyethylenimine amines in human primary cells. Toxicol. Lett. 2013;218(1):10–17. doi: 10.1016/j.toxlet.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 201.Zheng M., Zhong Z., Zhou L., Meng F., Peng R., Zhong Z. Poly(ethylene oxide) grafted with short polyethylenimine Gives DNA polyplexes with superior colloidal stability, low cytotoxicity, and potent in vitro gene transfection under serum conditions. Biomacromolecules. 2012;13(3):881–888. doi: 10.1021/bm2017965. [DOI] [PubMed] [Google Scholar]
- 202.Li Q., Shi C., Zhang W., Behl M., Lendlein A., Feng Y. Nanoparticles complexed with gene vectors to promote proliferation of human vascular endothelial cells. Advanced Healthcare Materials. 2015;4(8):1225–1235. doi: 10.1002/adhm.201400817. [DOI] [PubMed] [Google Scholar]
- 203.Chen F., Wan H., Xia T., Guo X., Wang H., Liu Y., Li X. Promoted regeneration of mature blood vessels by electrospun fibers with loaded multiple pDNA-calcium phosphate nanoparticles. Eur. J. Pharm. Biopharm. 2013;85(3):699–710. doi: 10.1016/j.ejpb.2013.07.009. Part A) [DOI] [PubMed] [Google Scholar]
- 204.Qu W., Qin S.-Y., Ren S., Jiang X.-J., Zhuo R.-X., Zhang X.-Z. Peptide-based vector of VEGF plasmid for efficient gene delivery in vitro and vessel formation in vivo. Bioconjugate Chem. 2013;24(6):960–967. doi: 10.1021/bc300677n. [DOI] [PubMed] [Google Scholar]
- 205.He S., Xia T., Wang H., Wei L., Luo X., Li X. Multiple release of polyplexes of plasmids VEGF and bFGF from electrospun fibrous scaffolds towards regeneration of mature blood vessels. Acta Biomater. 2012;8(7):2659–2669. doi: 10.1016/j.actbio.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 206.Luu Y.K., Kim K., Hsiao B.S., Chu B., Hadjiargyrou M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Contr. Release. 2003;89(2):341–353. doi: 10.1016/S0168-3659(03)00097-X. [DOI] [PubMed] [Google Scholar]
- 207.Yu L., Feng Y., Li Q., Hao X., Liu W., Zhou W., Shi C., Ren X., Zhang W. PLGA/SF blend scaffolds modified with plasmid complexes for enhancing proliferation of endothelial cells. React. Funct. Polym. 2015;91–92:19–27. doi: 10.1016/j.reactfunctpolym.2015.04.003. [DOI] [Google Scholar]
- 208.Anwer K., Kao G., Rolland A., Driessen W.H.P., Sullivan S.M. Peptide-mediated gene transfer of cationic lipid/plasmid DNA complexes to endothelial cells. J. Drug Target. 2004;12(4):215–221. doi: 10.1080/10611860410001724468. [DOI] [PubMed] [Google Scholar]
- 209.Suh W., Han S.O., Yu L., Kim S.W. An angiogenic, endothelial-cell-targeted polymeric gene carrier. Mol. Ther. 2002;6(5):664–672. [PubMed] [Google Scholar]
- 210.Wang H., Feng Y., Yang J., Guo J., Zhang W. Targeting REDV peptide functionalized polycationic gene carrier for enhancing the transfection and migration capability of human endothelial cells. J. Mater. Chem. B. 2015;3(16):3379–3391. doi: 10.1039/C4TB02019G. [DOI] [PubMed] [Google Scholar]
- 211.Kibria G., Hatakeyama H., Ohga N., Hida K., Harashima H. Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J. Contr. Release. 2011;153(2):141–148. doi: 10.1016/j.jconrel.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 212.Chen J., Dai S., Liu L., Maitz M.F., Liao Y., Cui J., Zhao A., Yang P., Huang N., Wang Y. Photo-functionalized TiO2 nanotubes decorated with multifunctional Ag nanoparticles for enhanced vascular biocompatibility. Bioactive Materials. 2021;6(1):45–54. doi: 10.1016/j.bioactmat.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Neffe A.T., von Ruesten-Lange M., Braune S., Luetzow K., Roch T., Richau K., Jung F., Lendlein A. Poly(ethylene glycol) grafting to poly(ether imide) membranes: influence on protein adsorption and thrombocyte adhesion. Macromol. Biosci. 2013;13(12):1720–1729. doi: 10.1002/mabi.201300309. [DOI] [PubMed] [Google Scholar]
- 214.Cai X., Yuan J., Chen S., Li P., Li L., Shen J. Hemocompatibility improvement of poly(ethylene terephthalate) via self-polymerization of dopamine and covalent graft of zwitterions. Mater. Sci. Eng. C. 2014;36:42–48. doi: 10.1016/j.msec.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 215.Feng W., Brash J.L., Zhu S. Non-biofouling materials prepared by atom transfer radical polymerization grafting of 2-methacryloloxyethyl phosphorylcholine: separate effects of graft density and chain length on protein repulsion. Biomaterials. 2006;27(6):847–855. doi: 10.1016/j.biomaterials.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 216.Takahashi N., Iwasa F., Inoue Y., Morisaki H., Ishihara K., Baba K. Evaluation of the durability and antiadhesive action of 2-methacryloyloxyethyl phosphorylcholine grafting on an acrylic resin denture base material. J. Prosthet. Dent. 2014;112(2):194–203. doi: 10.1016/j.prosdent.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 217.Su Y., Li C., Zhao W., Shi Q., Wang H., Jiang Z., Zhu S. Modification of polyethersulfone ultrafiltration membranes with phosphorylcholine copolymer can remarkably improve the antifouling and permeation properties. J. Membr. Sci. 2008;322(1):171–177. doi: 10.1016/j.memsci.2008.05.047. [DOI] [Google Scholar]
- 218.Xiaofen H., Gongyan L., Jian J., Dezeng F., Xinhao Y. Lipid-like Diblock copolymer as an Additive for improving the blood compatibility of poly(lactide-co-glycolide) J. Bioact. Compat Polym. 2010;25(6):654–668. doi: 10.1177/0883911510384836. [DOI] [Google Scholar]
- 219.Feng Y., Tian H., Tan M., Zhang P., Chen Q., Liu J. Surface modification of polycarbonate urethane by covalent linkage of heparin with a PEG spacer. Trans. Tianjin Univ. 2013;19(1):58–65. doi: 10.1007/s12209-013-1894-y. [DOI] [Google Scholar]
- 220.Liu P., Chen Q., Li L., Lin S., Shen J. Anti-biofouling ability and cytocompatibility of the zwitterionic brushes-modified cellulose membrane. J. Mater. Chem. B. 2014;2(41):7222–7231. doi: 10.1039/C4TB01151A. [DOI] [PubMed] [Google Scholar]
- 221.Liu T., Zeng Z., Liu Y., Wang J., Maitz M.F., Wang Y., Liu S., Chen J., Huang N. Surface modification with dopamine and heparin/poly-l-lysine nanoparticles provides a favorable release behavior for the healing of vascular stent Lesions. ACS Appl. Mater. Interfaces. 2014;6(11):8729–8743. doi: 10.1021/am5015309. [DOI] [PubMed] [Google Scholar]
- 222.Tan M., Feng Y., Wang H., Zhang L., Khan M., Guo J., Chen Q., Liu J. Immobilized bioactive agents onto polyurethane surface with heparin and phosphorylcholine group. Macromol. Res. 2013;21(5):541–549. doi: 10.1007/s13233-013-1028-3. [DOI] [Google Scholar]
- 223.Cheng Q., Komvopoulos K., Li S. Plasma-assisted heparin conjugation on electrospun poly(L-lactide) fibrous scaffolds. J. Biomed. Mater. Res. 2014;102(5):1408–1414. doi: 10.1002/jbm.a.34802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang H., Feng Y., Zhao H., Xiao R., Lu J., Zhang L., Guo J. Electrospun hemocompatible PU/gelatin-heparin nanofibrous bilayer scaffolds as potential artificial blood vessels. Macromol. Res. 2012;20(4):347–350. doi: 10.1007/s13233-012-0012-7. [DOI] [Google Scholar]
- 225.Liu T., Liu Y., Chen Y., Liu S., Maitz M.F., Wang X., Zhang K., Wang J., Wang Y., Chen J., Huang N. Immobilization of heparin/poly-l-lysine nanoparticles on dopamine-coated surface to create a heparin density gradient for selective direction of platelet and vascular cells behavior. Acta Biomater. 2014;10(5):1940–1954. doi: 10.1016/j.actbio.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 226.Elahi M.F., Guan G., Wang L., King M.W. Improved hemocompatibility of silk fibroin fabric using layer-by-layer polyelectrolyte deposition and heparin immobilization. J. Appl. Polym. Sci. 2014;131(18) doi: 10.1002/app.40772. [DOI] [Google Scholar]
- 227.Easton C.D., Bullock A.J., Gigliobianco G., McArthur S.L., MacNeil S. Application of layer-by-layer coatings to tissue scaffolds – development of an angiogenic biomaterial. J. Mater. Chem. B. 2014;2(34):5558–5568. doi: 10.1039/C4TB00448E. [DOI] [PubMed] [Google Scholar]
- 228.Liu W., Wu Z., Wang Y., Tang Z., Du J., Yuan L., Li D., Chen H. Controlling the biointerface of electrospun mats for clot lysis: an engineered tissue plasminogen activator link to a lysine-functionalized surface. J. Mater. Chem. B. 2014;2(27):4272–4279. doi: 10.1039/C4TB00488D. [DOI] [PubMed] [Google Scholar]
- 229.Absar S., Choi S., Yang V.C., Kwon Y.M. Heparin-triggered release of camouflaged tissue plasminogen activator for targeted thrombolysis. J. Contr. Release. 2012;157(1):46–54. doi: 10.1016/j.jconrel.2011.09.060. [DOI] [PubMed] [Google Scholar]
- 230.Ahmed M., Ghanbari H., Cousins B.G., Hamilton G., Seifalian A.M. Small calibre polyhedral oligomeric silsesquioxane nanocomposite cardiovascular grafts: influence of porosity on the structure, haemocompatibility and mechanical properties. Acta Biomater. 2011;7(11):3857–3867. doi: 10.1016/j.actbio.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 231.Ahmed M., Hamilton G., Seifalian A.M. The performance of a small-calibre graft for vascular reconstructions in a senescent sheep model. Biomaterials. 2014;35(33):9033–9040. doi: 10.1016/j.biomaterials.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 232.Chong D.S.T., Turner L.A., Gadegaard N., Seifalian A.M., Dalby M.J., Hamilton G. Nanotopography and plasma treatment: redesigning the surface for vascular graft endothelialisation. Eur. J. Vasc. Endovasc. Surg. 2015;49(3):335–343. doi: 10.1016/j.ejvs.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 233.Kannan R.Y., Salacinski H.J., Odlyha M., Butler P.E., Seifalian A.M. The degradative resistance of polyhedral oligomeric silsesquioxane nanocore integrated polyurethanes: an in vitro study. Biomaterials. 2006;27(9):1971–1979. doi: 10.1016/j.biomaterials.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 234.Kent K.C., Liu B. Intimal hyperplasia—still Here after All these years! Ann. Vasc. Surg. 2004;18(2):135–137. doi: 10.1007/s10016-004-0019-4. [DOI] [PubMed] [Google Scholar]
- 235.Zhao Y., Zang G., Yin T., Ma X., Zhou L., Wu L., Daniel R., Wang Y., Qiu J., Wang G. A novel mechanism of inhibiting in-stent restenosis with arsenic trioxide drug-eluting stent: enhancing contractile phenotype of vascular smooth muscle cells via YAP pathway. Bioactive Materials. 2021;6(2):375–385. doi: 10.1016/j.bioactmat.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Conte M.S., Bandyk D.F., Clowes A.W., Moneta G.L., Seely L., Lorenz T.J., Namini H., Hamdan A.D., Roddy S.P., Belkin M., Berceli S.A., DeMasi R.J., Samson R.H., Berman S.S. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J. Vasc. Surg. 2006;43(4):742–751. doi: 10.1016/j.jvs.2005.12.058. e741. [DOI] [PubMed] [Google Scholar]
- 237.Alexander J.H., Hafley G., Harrington R.A., Peterson E.D., Ferguson T.B., Jr., Lorenz T.J., Goyal A., Gibson M., Mack M.J., Gennevois D., Califf R.M., Kouchoukos N.T. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: prevent IV: a randomized controlled trial. J. Am. Med. Assoc. 2005;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 238.Bakin A.V., Rinehart C., Tomlinson A.K., Arteaga C.L. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 2002;115(Pt 15):3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 239.Hedges J.C., Dechert M.A., Yamboliev I.A., Martin J.L., Hickey E., Weber L.A., Gerthoffer W.T. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J. Biol. Chem. 1999;274(34):24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 240.Rousseau S., Morrice N., Peggie M., Campbell D.G., Gaestel M., Cohen P. Inhibition of SAPK2a/p38 prevents hnRNP A0 phosphorylation by MAPKAP-K2 and its interaction with cytokine mRNAs. EMBO J. 2002;21(23):6505–6514. doi: 10.1093/emboj/cdf639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Evans B.C., Hocking K.M., Osgood M.J., Voskresensky I., Dmowska J., Kilchrist K.V., Brophy C.M., Duvall C.L. MK2 inhibitory peptide delivered in nanopolyplexes prevents vascular graft intimal hyperplasia. Sci. Transl. Med. 2015;7(291) doi: 10.1126/scitranslmed.aaa4549. 291ra295-291ra295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yang Y., Gao P., Wang J., Tu Q., Bai L., Xiong K., Qiu H., Zhao X., Maitz M.F., Wang H., Li X., Zhao Q., Xiao Y., Huang N., Yang Z. Research; Washington, DC: 2020. Endothelium-Mimicking Multifunctional Coating Modified Cardiovascular Stents via a Stepwise Metal-Catechol-(Amine) Surface Engineering Strategy. 2020. 9203906-9203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Naghavi N., de Mel A., Alavijeh O.S., Cousins B.G., Seifalian A.M. Nitric oxide donors for cardiovascular implant applications. Small. 2013;9(1):22–35. doi: 10.1002/smll.201200458. [DOI] [PubMed] [Google Scholar]
- 244.Zhang F., Zhang Q., Li X., Huang N., Zhao X., Yang Z. Mussel-inspired dopamine-CuII coatings for sustained in situ generation of nitric oxide for prevention of stent thrombosis and restenosis. Biomaterials. 2019;194:117–129. doi: 10.1016/j.biomaterials.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 245.de Mel A., Jell G., Stevens M.M., Seifalian A.M. Biofunctionalization of biomaterials for accelerated in situ endothelialization: a review. Biomacromolecules. 2008;9(11):2969–2979. doi: 10.1021/bm800681k. [DOI] [PubMed] [Google Scholar]
- 246.Elnaggar M.A., Seo S.H., Gobaa S., Lim K.S., Bae I.-H., Jeong M.H., Han D.K., Joung Y.K. Nitric oxide releasing coronary stent: a new approach using layer-by-layer coating and liposomal encapsulation. Small. 2016;12(43):6012–6023. doi: 10.1002/smll.201600337. [DOI] [PubMed] [Google Scholar]
- 247.Tanner F.C., Meier P., Greutert H., Champion C., Nabel E.G., Lüscher T.F. Nitric oxide modulates expression of cell cycle regulatory proteins: a cytostatic strategy for inhibition of human vascular smooth muscle cell proliferation. Circulation. 2000;101(16):1982–1989. doi: 10.1161/01.cir.101.16.1982. [DOI] [PubMed] [Google Scholar]
- 248.Elnaggar M.A., Subbiah R., Han D.K., Joung Y.K. Lipid-based carriers for controlled delivery of nitric oxide. Expet Opin. Drug Deliv. 2017;14(12):1341–1353. doi: 10.1080/17425247.2017.1285904. [DOI] [PubMed] [Google Scholar]
- 249.Qiu H., Qi P., Liu J., Yang Y., Tan X., Xiao Y., Maitz M.F., Huang N., Yang Z. Biomimetic engineering endothelium-like coating on cardiovascular stent through heparin and nitric oxide-generating compound synergistic modification strategy. Biomaterials. 2019;207:10–22. doi: 10.1016/j.biomaterials.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 250.Hrabie J.A., Keefer L.K. Chemistry of the nitric oxide-releasing diazeniumdiolate (“Nitrosohydroxylamine”) functional group and its oxygen-substituted Derivatives. Chem. Rev. 2002;102(4):1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 251.Al-Sa'doni H., Ferro A. S-Nitrosothiols: a class of nitric oxide-donor drugs. Clin. Sci. (Lond.) 2000;98(5):507–520. [PubMed] [Google Scholar]
- 252.Al-Sa'doni H.H., Ferro A. S-nitrosothiols as nitric oxide-donors: chemistry, biology and possible future therapeutic applications. Curr. Med. Chem. 2004;11(20):2679–2690. doi: 10.2174/0929867043364397. [DOI] [PubMed] [Google Scholar]
- 253.Yang Z., Yang Y., Xiong K., Wang J., Lee H., Huang N. Metal-phenolic surfaces for generating therapeutic nitric oxide gas. Chem. Mater. 2018;30(15):5220–5226. doi: 10.1021/acs.chemmater.8b01876. [DOI] [Google Scholar]
- 254.Yang Z., Yang Y., Zhang L., Xiong K., Li X., Zhang F., Wang J., Zhao X., Huang N. Mussel-inspired catalytic selenocystamine-dopamine coatings for long-term generation of therapeutic gas on cardiovascular stents. Biomaterials. 2018;178:1–10. doi: 10.1016/j.biomaterials.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 255.Fan Y., Zhang Y., Zhao Q., Xie Y., Luo R., Yang P., Weng Y. Immobilization of nano Cu-MOFs with polydopamine coating for adaptable gasotransmitter generation and copper ion delivery on cardiovascular stents. Biomaterials. 2019;204:36–45. doi: 10.1016/j.biomaterials.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 256.Saraiva J., Marotta-Oliveira S.S., Cicillini S.A., Eloy Jde O., Marchetti J.M. Nanocarriers for nitric oxide delivery. Journal of drug delivery. 2011;2011 doi: 10.1155/2011/936438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Carpenter A.W., Slomberg D.L., Rao K.S., Schoenfisch M.H. Influence of scaffold size on bactericidal activity of nitric oxide-releasing silica nanoparticles. ACS Nano. 2011;5(9):7235–7244. doi: 10.1021/nn202054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Frost M.C., Meyerhoff M.E. Synthesis, characterization, and controlled nitric oxide release from S-nitrosothiol-derivatized fumed silica polymer filler particles. J. Biomed. Mater. Res. 2005;72(4):409–419. doi: 10.1002/jbm.a.30275. [DOI] [PubMed] [Google Scholar]
- 259.Zhang H., Annich G.M., Miskulin J., Stankiewicz K., Osterholzer K., Merz S.I., Bartlett R.H., Meyerhoff M.E. Nitric oxide-releasing fumed silica particles: synthesis, characterization, and biomedical application. J. Am. Chem. Soc. 2003;125(17):5015–5024. doi: 10.1021/ja0291538. [DOI] [PubMed] [Google Scholar]
- 260.Huang S.L., Kee P.H., Kim H., Moody M.R., Chrzanowski S.M., Macdonald R.C., McPherson D.D. Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J. Am. Coll. Cardiol. 2009;54(7):652–659. doi: 10.1016/j.jacc.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dinh B., Dove K., Jappar D., Hrabie J.A., Davies K.M. Effect of hydrophobic structure on the catalysis of nitric oxide release from zwitterionic diazeniumdiolates in surfactant and liposome media. Nitric Oxide. 2005;13(3):204–209. doi: 10.1016/j.niox.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 262.Shin J.H., Metzger S.K., Schoenfisch M.H. Synthesis of nitric oxide-releasing silica nanoparticles. J. Am. Chem. Soc. 2007;129(15):4612–4619. doi: 10.1021/ja0674338. [DOI] [PubMed] [Google Scholar]
- 263.Shin J.H., Schoenfisch M.H. Inorganic/organic hybrid silica nanoparticles as a nitric oxide delivery scaffold. Chem. Mater. 2008;20(1):239–249. doi: 10.1021/cm702526q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Malam Y., Loizidou M., Seifalian A.M. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 265.Dinh B.T., Price S.E., Majul A., El-Hajj M., Morozov V., Hrabie J.A., Davies K.M. Diazeniumdiolate reactivity in model membrane systems. Nitric Oxide. 2008;18(2):113–121. doi: 10.1016/j.niox.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tai L.A., Wang Y.C., Yang C.S. Heat-activated sustaining nitric oxide release from zwitterionic diazeniumdiolate loaded in thermo-sensitive liposomes. Nitric Oxide. 2010;23(1):60–64. doi: 10.1016/j.niox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 267.Koehler J.J., Zhao J., Jedlicka S.S., Porterfield D.M., Rickus J.L. Compartmentalized nanocomposite for dynamic nitric oxide release. J. Phys. Chem. B. 2008;112(47):15086–15093. doi: 10.1021/jp803276u. [DOI] [PubMed] [Google Scholar]
- 268.Templeton A.C., Wuelfing W.P., Murray R.W. Monolayer-protected cluster molecules. Acc. Chem. Res. 2000;33(1):27–36. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- 269.Jain S., Hirst D.G., O'Sullivan J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012;85(1010):101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mody V.V., Siwale R., Singh A., Mody H.R. Introduction to metallic nanoparticles. J. Pharm. BioAllied Sci. 2010;2(4):282–289. doi: 10.4103/0975-7406.72127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Caruso E.B., Petralia S., Conoci S., Giuffrida S., Sortino S. Photodelivery of nitric oxide from water-soluble platinum nanoparticles. J. Am. Chem. Soc. 2007;129(3):480–481. doi: 10.1021/ja067568d. [DOI] [PubMed] [Google Scholar]
- 272.de Mel A., Chaloupka K., Malam Y., Darbyshire A., Cousins B., Seifalian A.M. A silver nanocomposite biomaterial for blood-contacting implants. J. Biomed. Mater. Res. 2012;100(9):2348–2357. doi: 10.1002/jbm.a.34177. [DOI] [PubMed] [Google Scholar]
- 273.Chaloupka K., Malam Y., Seifalian A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 274.Vittorino E., Giancane G., Manno D., Serra A., Valli L., Sortino S. Photofunctional multilayer films by assembling naked silver nanoparticles and a tailored nitric oxide photodispenser at water/air interface. J. Colloid Interface Sci. 2012;368(1):191–196. doi: 10.1016/j.jcis.2011.09.084. [DOI] [PubMed] [Google Scholar]
- 275.Horcajada P., Gref R., Baati T., Allan P.K., Maurin G., Couvreur P., Férey G., Morris R.E., Serre C. Metal–organic frameworks in biomedicine. Chem. Rev. 2012;112(2):1232–1268. doi: 10.1021/cr200256v. [DOI] [PubMed] [Google Scholar]
- 276.Tu Q., Zhao X., Liu S., Li X., Zhang Q., Yu H., Xiong K., Huang N., Yang Z. Spatiotemporal dual-delivery of therapeutic gas and growth factor for prevention of vascular stent thrombosis and restenosis. Applied Materials Today. 2020;19 doi: 10.1016/j.apmt.2019.100546. [DOI] [Google Scholar]
- 277.de Vries M.R., Quax P.H.A. Inflammation in vein graft disease. Frontiers in cardiovascular medicine. 2018;5:3. doi: 10.3389/fcvm.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chen W., Jia S., Zhang X., Zhang S., Liu H., Yang X., Zhang C., Wu W. Dimeric thymosin β4 loaded nanofibrous interface enhanced regeneration of muscular artery in aging body through modulating perivascular adipose stem cell–macrophage interaction. Advanced Science. 2020;7(8) doi: 10.1002/advs.201903307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wong M.M., Chen Y., Margariti A., Winkler B., Campagnolo P., Potter C., Hu Y., Xu Q. Macrophages control vascular stem/progenitor cell plasticity through tumor necrosis factor-α-mediated nuclear factor-κB activation. Arterioscler. Thromb. Vasc. Biol. 2014;34(3):635–643. doi: 10.1161/atvbaha.113.302568. [DOI] [PubMed] [Google Scholar]
- 280.Liang M., Wang Y., Liang A., Dong J.F., Du J., Cheng J. Impaired integrin β3 delays endothelial cell regeneration and contributes to arteriovenous graft failure in mice. Arterioscler. Thromb. Vasc. Biol. 2015;35(3):607–615. doi: 10.1161/atvbaha.114.305089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Akahori H., Karmali V., Polavarapu R., Lyle A.N., Weiss D., Shin E., Husain A., Naqvi N., Van Dam R., Habib A., Choi C.U., King A.L., Pachura K., Taylor W.R., Lefer D.J., Finn A.V. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat. Commun. 2015;6(1):7792. doi: 10.1038/ncomms8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Vasconcelos D.P., Costa M., Amaral I.F., Barbosa M.A., Águas A.P., Barbosa J.N. Development of an immunomodulatory biomaterial: using resolvin D1 to modulate inflammation. Biomaterials. 2015;53:566–573. doi: 10.1016/j.biomaterials.2015.02.120. [DOI] [PubMed] [Google Scholar]
- 283.Sok M.C.P., Tria M.C., Olingy C.E., San Emeterio C.L., Botchwey E.A. Aspirin-Triggered Resolvin D1-modified materials promote the accumulation of pro-regenerative immune cell subsets and enhance vascular remodeling. Acta Biomater. 2017;53:109–122. doi: 10.1016/j.actbio.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shi J., Zhang X., Jiang L., Zhang L., Dong Y., Midgley A.C., Kong D., Wang S. Regulation of the inflammatory response by vascular grafts modified with Aspirin-Triggered Resolvin D1 promotes blood vessel regeneration. Acta Biomater. 2019;97:360–373. doi: 10.1016/j.actbio.2019.07.037. [DOI] [PubMed] [Google Scholar]
- 285.Massena S., Christoffersson G., Vågesjö E., Seignez C., Gustafsson K., Binet F., Herrera Hidalgo C., Giraud A., Lomei J., Weström S., Shibuya M., Claesson-Welsh L., Gerwins P., Welsh M., Kreuger J., Phillipson M. Identification and characterization of VEGF-A–responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126(17):2016–2026. doi: 10.1182/blood-2015-03-631572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sun D.X., Liu Z., Tan X.D., Cui D.X., Wang B.S., Dai X.W. Nanoparticle-mediated local delivery of an antisense TGF-β1 construct inhibits intimal hyperplasia in autogenous vein grafts in rats. PloS One. 2012;7(7) doi: 10.1371/journal.pone.0041857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kalra M., Miller V.M. Early remodeling of saphenous vein grafts: proliferation, migration and apoptosis of adventitial and medial cells occur simultaneously with changes in graft diameter and blood flow. J. Vasc. Res. 2000;37(6):576–584. doi: 10.1159/000054091. [DOI] [PubMed] [Google Scholar]
- 288.Mitra A.K., Gangahar D.M., Agrawal D.K. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol. Cell Biol. 2006;84(2):115–124. doi: 10.1111/j.1440-1711.2005.01407.x. [DOI] [PubMed] [Google Scholar]
- 289.Jiang Z., Tao M., Omalley K.A., Wang D., Ozaki C.K., Berceli S.A. Established neointimal hyperplasia in vein grafts expands via TGF-beta-mediated progressive fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2009;297(4):H1200–H1207. doi: 10.1152/ajpheart.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Packard R.R., Lichtman A.H., Libby P. Innate and adaptive immunity in atherosclerosis. Semin. Immunopathol. 2009;31(1):5–22. doi: 10.1007/s00281-009-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hibino N., Mejias D., Pietris N., Dean E., Yi T., Best C., Shinoka T., Breuer C. The innate immune system contributes to tissue-engineered vascular graft performance. Faseb. J. 2015;29(6):2431–2438. doi: 10.1096/fj.14-268334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ding N., Dou C., Wang Y., Liu F., Guan G., Huo D., Li Y., Yang J., Wei K., Yang M., Tan J., Zeng W., Zhu C. Antishear stress bionic carbon nanotube mesh coating with intracellular controlled drug delivery constructing small-diameter tissue-engineered vascular grafts. Adv Healthc Mater. 2018;7(11) doi: 10.1002/adhm.201800026. [DOI] [PubMed] [Google Scholar]
- 293.Buttari B., Profumo E., Segoni L., D'Arcangelo D., Rossi S., Facchiano F., Saso L., Businaro R., Iuliano L., Riganò R. Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7-oxo-cholesterol: potential therapeutic implications in atherosclerosis. Oxid Med Cell Longev. 2014 doi: 10.1155/2014/257543. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yang Y., Lei D., Zou H., Huang S., Yang Q., Li S., Qing F.-L., Ye X., You Z., Zhao Q. Hybrid electrospun rapamycin-loaded small-diameter decellularized vascular grafts effectively inhibit intimal hyperplasia. Acta Biomater. 2019;97:321–332. doi: 10.1016/j.actbio.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 295.Bessueille L., Magne D. Inflammation: a culprit for vascular calcification in atherosclerosis and diabetes. Cell. Mol. Life Sci. 2015;72(13):2475–2489. doi: 10.1007/s00018-015-1876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wei Y., Wu Y., Zhao R., Zhang K., Midgley A.C., Kong D., Li Z., Zhao Q. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials. 2019;204:13–24. doi: 10.1016/j.biomaterials.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 297.de Vries M.R., Seghers L., van Bergen J., Peters H.A.B., de Jong R.C.M., Hamming J.F., Toes R.E.M., van Hinsbergh V.W.M., Quax P.H.A. C57BL/6 NK cell gene complex is crucially involved in vascular remodeling. J. Mol. Cell. Cardiol. 2013;64:51–58. doi: 10.1016/j.yjmcc.2013.08.009. [DOI] [PubMed] [Google Scholar]