Abstract
Recent advances now permit resection of many pharyngeal tumors through the open mouth, an approach that can greatly reduce the morbidity of surgical exposure. These transoral techniques are being rapidly adopted by the surgical community and hold considerable promise. On November 6–7, 2011, the National Cancer Institute sponsored a Clinical Trials Planning Meeting to address how to further investigate the use of transoral surgery, both in the good prognosis human papillomavirus (HPV)–initiated oropharyngeal cancers, and in those with HPV-unrelated disease. The proceedings of this meeting are summarized.
INTRODUCTION
The last 20 years have seen many advances in the management of squamous cancer of the head and neck. With the use of chemotherapy and radiation, locoregional control of many advanced cancers can be achieved without a highly morbid operation, and without compromising the chance for cure.1 Increasingly intensive combined modality treatment schedules have been developed, and new agents have been incorporated into the therapeutic armamentarium. This has been facilitated by the development of a robust clinical trials mechanism that has marshaled doctors and patients for clinical research in this uncommon disease. Although notable success has been achieved and new standards of care defined without the morbidity and functional sequelae once associated with extensive surgical procedures, these apparent therapeutic gains have been accompanied by significant early and late toxicity due to chemoradiation.2,3
Recently, a new disease—human papillomavirus (HPV)–initiated head and neck cancer, largely presenting in the oropharynx—has been identified and appears to be rapidly increasing in incidence.4 In the United States today, more than 2 of 3 patients with oropharynx cancer have HPV-initiated tumors. Such cancers typically present with smaller primary tumors than those caused by tobacco abuse. Because these HPV-initiated head and neck cancers more frequently present in a younger population and seem particularly responsive to treatment with a better overall survival, attention has begun to shift toward amelioration of the late toxicity of radiation-based treatment.5
Improved instrumentation now permits resection of many pharyngeal and laryngeal tumors through the open mouth. This limits dramatically the morbidity of surgical exposure and may substantially reduce the acute and late effects of resection. The frequent presentation of HPV-initiated cancers with small primary tumors makes these lesions particularly amenable to such an approach, because the functional deficit resulting from their resection is low.
Transoral laser microsurgery (TLM) for resection of oropharyngeal cancers has been practiced for decades at a few North American institutions, and both case series and cohort follow-up studies suggest that excellent long-term function may be anticipated after resection of appropriate lesions of the pharynx.6–8 More recent reports of transoral robotic resection of oropharyngeal lesions have been encouraging as well.9–16 The publications describing both approaches, however, reflect patients from a small number of institutions. Transoral robotic surgery (TORS) has been carefully investigated, primarily in prospective studies focused on approval of the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) by the Food and Drug Administration (FDA) for treatment of oropharynx cancer. However, neither TORS nor TLM, although increasingly used, has been compared with primary radiotherapy-based treatment through the rigorous standards of clinical research to which the profession has become accustomed. Indications, contraindications, standards of practice, and outcome reporting must be defined.
Surgical resection of oropharynx cancer can be curative when used as a single modality for patients with stage I–III tumors. Even stage IV patients, who usually require adjuvant radiation or chemoradiation after resection, are likely to receive lower doses of radiation than would be needed in definitive radiotherapeutic treatment with curative intent, and concurrent chemotherapy might be avoided altogether. Surgical therapy allows more appropriate use of postoperative adjuvant therapy based on pathologic staging and has the potential to diminish substantially the need for high-dose radiation or concurrent chemoradiation in patients who are expected to do well. Such benefits are increased if the resection can be accomplished with low morbidity.
For those patients with more advanced disease, or with poor-prognosis cancers unrelated to viral infection, surgical resection in combination with postoperative chemoradiation has the potential to increase locoregional control and improve survival. Thus, reintegration of modern low-morbidity resection into the multidisciplinary management of stage III–IV oropharynx cancer merits investigation.
It is of considerable importance to determine if patient quality of life and function after transoral resection is superior to that of patients treated without surgery. Can the use of transoral resection as a primary modality reduce late side effects by diminishing the aggregate toxicity of multiple treatment modalities in patients with a good long-term prognosis? Should transoral resection as a primary modality be used to intensify local therapy for patients with poor-prognosis tumors? Novel clinical trial designs and endpoint definitions are urgently needed to address the use of transoral surgery, both as a single-treatment modality and as part of multimodality approaches.
There can be significant collateral benefit as well. The last 2 decades’ emphasis on nonsurgical management of head and neck cancer has impeded efforts to collect and store fresh, untreated tumor for scientific investigation. Biopsy material has been collected, but is inevitably limited in quantity. Early surgical resection of disease encourages development of protocols for tissue collection, processing, and storage, and should facilitate efforts to elucidate the biology of disease.
The Previously Untreated Locally Advanced Disease Task Force of the National Cancer Institute Head and Neck Steering Committee identified this as an important priority for clinical investigation. As such, the National Cancer Institute sponsored a Clinical Trials Planning Meeting on the Transoral Resection of Pharynx Cancer in Arlington, Virginia on November 6–7, 2011. More than 60 investigators including surgical, radiation, and medical oncologists participated. Additional input was obtained from the diagnostic radiology and basic science communities, and from individuals conversant with functional and quality of life (QOL) assessments, comparative effectiveness research, and cost/benefit analysis. The objectives of the meeting were to design and initiate the conduct of one or several multicenter trials defining the role of transoral surgery in oropharynx cancer, to enlist investigators from these multiple disciplines, to identify appropriate endpoints for such trials, and to address obstacles to their successful conduct. In view of the breadth of expertise and divergent academic interests of the participants, considerable attention was directed toward establishing current standards in treatment and evaluation of quality of life, outcomes, and toxicity.
This monograph summarizes the proceedings of that meeting.
CURRENT TREATMENT EXPECTATIONS
1. State of the art for nonsurgical treatment of oropharynx cancer
Surgery and radiotherapy (RT) are both highly effective and equivalent modalities for the management of early-stage disease (T1–2N0). Planned multimodality treatment is not considered to be more effective than single-modality therapy. Therefore, the fundamental management principle is to use a single modality. Avoiding treatment strategies that will likely require multimodality therapy minimizes the risk, severity, and duration of treatment-induced toxicity. Conventional and altered fractionation RT regimens have roles in early-stage disease, depending on the clinical presentation.
Several standards of care exist for intermediate to advanced-stage presentations (T1N1–T4N3). Early-primary tumor Stage III disease (T1N1, T2N1) can be treated with radiotherapy alone. Randomized trials have demonstrated that accelerated fractionation and hyperfractionation are superior to conventional daily fractionation primarily due to improvements in locoregional disease control. A meta-analysis has shown that modified fractionation is associated with an 8% improvement in 5-year survival.17
The addition of epidermal growth factor receptor (EGFR) inhibition to radiotherapy constitutes a treatment option for presentations where radiation alone might not be the optimal treatment. A pivotal trial in which the majority of patients had oropharynx cancer demonstrated that RT plus the antibody cetuximab resulted in a 9% improvement in 5-year survival compared with RT alone.18 The improvement in survival again was the result of an improvement in locoregional control without any change in the incidence of distant metastasis.
The most commonly used nonsurgical strategy for the management of locally advanced head and neck cancer is radiotherapy and concurrent chemotherapy (CRT). A meta-analysis of 93 randomized clinical trials that enrolled more than 17,000 patients (mixed populations of oropharynx and nonoropharynx cancer) between 1965 and 2000 showed that CRT led to a 21% reduction in the risk of recurrence or death compared with RT alone.1 This improvement corresponded to a 6.5% absolute improvement in 5-year survival. Neither induction chemotherapy followed by RT nor RT followed by adjuvant chemotherapy was superior to RT alone. Single-agent cisplatin was determined to be the optimal concurrent chemotherapy agent. The survival benefit of CRT was predominantly due to a reduction in locoregional failure; there was a significant but much smaller reduction in distant metastases from concurrent therapy.
Modified fractionation, cetuximab with RT, and CRT all increase efficacy compared with RT alone, but they also increase both the acute and late toxicity of therapy. Mucositis represents the most prominent acute toxicity.
Cisplatin chemotherapy can also cause significant ototoxicity, nephrotoxicity, and peripheral neuropathy. Several studies have shown an approximate doubling in the incidence of severe mucositis from CRT compared with RT alone, whereas others have demonstrated that the recovery time from mucositis is as much as 50% longer after concurrent therapy.19–21 A French randomized trial in oropharynx cancer has also suggested that severe late toxicity was more frequent after CRT than after RT alone (56% vs 30%, p = .12),22 underscoring the importance of avoiding overtreatment in advanced-stage disease (in a fashion analogous to the use of single modality therapy in early-stage cancers).
Sequential chemoradiation represents another strategy for the management of advanced disease. It consists of multiagent induction chemotherapy followed by CRT. Induction chemotherapy is used to reduce the risk of distant metastases and to shrink locoregional disease. Sequential therapy constitutes a therapeutic intensification beyond CRT, but it has not to date been demonstrated to be more effective than CRT. However, toxicity considerations are certainly relevant to sequential therapy programs. Compliance rates with an entire course of treatment with these regimens are approximately 70% in the most experienced hands.23,24
Overall, as treatment is escalated along the continuum from conventional once daily RT alone to the most intensive combined modality regimens, more treatment engenders more toxicity. This highlights the need to identify prospectively patients with favorable and unfavorable prognoses, permitting focus on therapeutic deintensification strategies aimed at reducing toxicity without sacrificing efficacy in the former, and therapeutic intensification strategies designed to improve efficacy in the latter.
2. Transoral robotic surgery (TORS)
For years, head and neck surgery was synonymous with “radical” operations, performed through large incisions and often with significant postoperative functional deficits. Perhaps as a result, multidisciplinary care for cancers of the larynx and pharynx gradually shifted predominantly toward a radiation-based approach,25 and a robust literature, including multiple prospective clinical trials, documents the efficacy of radiation-based treatment, with or without chemotherapy.1,17,26,27 Although this nonsurgical approach has become the standard of care in many centers in the United States and worldwide, there remain serious concerns about both short- and long-term toxicity. In Machtay’s review of 3 prospective RTOG clinical trials of radiation and concurrent cisplatin-based chemotherapy, 40% of patients experienced severe long-term toxicity (grades 3–5, by NCI common toxicity criteria), with a reported gastrostomy-tube dependence rate of up to 29% at 2 years.2
Compared with “open” head and neck surgery, transoral “endoscopic” head and neck surgery (eHNS) of the oropharynx is performed without any external incisions and does not require mandibulotomy or transpharyngeal access. Using the laser and microscope (TLM) or the da Vinci Surgical System, a complete resection of the oropharyngeal primary tumor is performed with appropriate oncologic margins.
Transoral otolaryngology procedures with the da Vinci Surgical System were approved for use by the FDA in December 2009. After placing a suitable oral retractor, a binocular camera is introduced into the pharynx followed by 2 other arms carrying interchangeable 5- or 8-mm working instruments. These 3 tools within the patient’s mouth are then controlled by a surgeon sitting at a remote console.28 The surgeon is provided with an endoscopically derived 3D visual display that allows precise movements of instruments inside the oral cavity, including varieties of tissue forceps, scissors, an electrocautery spatula, CO2, and thulium lasers,29,30 which make it possible to perform an en bloc resection of oropharyngeal tumors.
McLeod and Melder31 performed the first transoral robotic head and neck procedure in 2003 at the Walter Reed Army Medical Center, but Hockstein et al,32 from the University of Pennsylvania, first demonstrated the preclinical feasibility of using the current robotic platform for more extensive laryngopharyngeal surgery. Rather than placing the instruments through a laryngoscope, as is done with TLM (as well as by McLeod and Melder in their first case report), Hockstein and colleagues determined that oral and oropharyngeal retractors more reliably facilitated surgery without external collisions.32,33 Coining the term “transoral robotic surgery” (TORS), they reported its application for the resection of oropharyngeal carcinoma of the tongue-base9 and tonsil.10 During the ensuing years, several centers adopted the technique which was associated with a pronounced “learning curve.”11
Moore et al12 reported a prospective study of 45 patients undergoing TORS and found that no patients required long-term feeding tubes or tracheostomy. However, for patients with T3–T4 cancers or location outside the oropharynx, TORS was associated with a higher risk for enterogastric feeding and poor swallowing outcomes.13 Adverse events following TORS appear to be limited. Most patients complain of 1 or 2 weeks of postoperative odynophagia and most return to normal swallowing within 30 days. Postoperative hemorrhage following transoral surgery is always a concern,14 but catastrophic hemorrhage has not been reported thus far. In 2010, Holsinger et al34 presented the first results of a multicenter experience detailing the feasibility, safety, and adequacy of surgical margins of 177 patients undergoing TORS. There was no intraoperative mortality or death in the immediate postoperative period. There were no cases of catastrophic hemorrhage or emergent airway compromise. The average blood loss was 83 mL and no patients required transfusion. Understanding the transoral surgical anatomy of the lateral oropharynx,15 which was well described prior to the advent of TORS, is critical for adequate hemostasis and good outcomes.
Although the safety and feasibility of TORS appears to be gaining acceptance, its role within the multidisciplinary treatment paradigm for this disease is less certain. In several of the initial reports, many of the patients who underwent TORS received postoperative radiation therapy (PORT) with or without chemotherapy. In view of the low rate of positive margins, the indication for PORT (often with chemotherapy) was most often the pathologic findings in the neck. Skeptics suggest that TORS results in overtreatment, since most of the tumors amenable to TORS would be effectively treated with definitive chemoradiation.35 Advocates point to the improved functional results in almost all of these reports. In the largest experience published to date, 89 patients were treated with TORS; 63% received PORT and 49% also received concurrent chemoradiation. Progression-free survival at 2 years was 86.5% and no patient required a feeding tube for nutrition.16 In the multicenter 177 patient report, for patients undergoing TORS without previous therapy, the percutaneous endoscopic gastrostomy tube dependence rate was 5.0%.34 Advocates also suggest that TORS permits treatment “deintensification.” If OPSCC patients treated initially with TORS, followed by PORT alone or chemoradiation, can achieve similar oncologic outcomes to patients treated with curative chemoradiation alone, the functional outcomes may improve because the overall radiation dose to the pharynx can be reduced through resection. This hypothesis requires further testing in a prospective clinical trial.
It has been noted that to practice true multidisciplinary care, surgeons must be actively engaged in clinical research.36 Before transoral surgery can be compared with radiation therapy as a primary treatment modality, normative baseline prospective data should be collected. A national registry would provide an opportunity for surgeons to begin working across institutions in an orchestrated clinical research effort in anticipation of these cooperative group trials. More important will be the participation of the head and neck surgical community in prospective clinical trials focused on defining the role of this “new agent” in our current therapeutic armamentarium. Eligible patients should include patients with T1–2 oropharyngeal carcinomas and selected T3 tumors amenable to endoscopic resection, via TLM or TORS. Current cooperative group infrastructure exists, and should fully exploit current surgical expertise. Phase II studies can be rapidly initiated with the ultimate goal of a definitive phase III comparison.
3. Transoral surgery–transoral laser microsurgery (TLM)
Goals of transoral laser microsurgery.
TLM accomplishes precise, tumor-targeted treatment with excellent disease control, efficiency, and minimum morbidity. Metastatic neck disease is removed simultaneously. The technique is associated with minimal blood loss, rapid wound healing, short hospital stays, rapid rehabilitation, and it preserves function. Tracheostomy is seldom needed, except for extensive resections with flap reconstruction. The approach permits risk-based adjuvant therapy based on pathology specimens.
What is TLM? Technique and clinical application.
Using either laryngoscopes or spatulate retraction devices positioned in the mouth and pharynx, the tumor is transected at its most proximal portion with the CO2 laser to estimate depth of invasion. The primary tumor is then completely resected in 2 or more blocs to achieve tumor-free surgical margins. At the deep margin of each bloc, the surgeon applies ink and requests that a pathologist confirm tumor clearance by frozen section, providing an immediate assessment of tumor clearance at multiple loci around the invasive front. Unlike the principle of “getting around the tumor” as in open or robotic surgery, TLM is customized to facilitate complete tumor resection in accord with size and location, with histologically verified clear margins. This technique preserves normal surrounding tissue, vital anatomic structures, and eschews rote incision-making which might excise too much or too little. The intense illumination, magnification, and resolution provided by an operating microscope enable a clear distinction between healthy versus tumor tissue at the deep extent of the resection bed. This has resulted in the very high local control rates reported in published studies,6–8,37 dispelling theoretical concerns about local recurrence due to tumor seeding.
Apart from improved image resolution and use of laser versus electrocautery as a cutting tool, distinctions between TLM and robotic resection chiefly involve haptic (tactile) feedback. Contemporary robot arms and telescopes require sufficient access to limit collisions, whereas TLM may be accomplished through narrow bore laryngoscopes, a common requirement when tumors are anterolateral in location or extension.
The ease and versatility of TLM allows its application to multiple clinical scenarios in OPSCC, which include: (1) primary resectable, untreated tumors (T1–T4a), (2) recurrent tumors (post CRT, RT, or surgical failure), and (3) unknown primary detection and treatment, a very common mode of presentation for OPSCC.
Outcomes in OPSCC managed by TLM: disease control and survival.
The 3-year locoregional control for TLM in advanced OPSCC varies from 80% to 96% with a distant metastatic rate of approximately 6%.6–8,37 In a recent United States–based multicenter study,8 the largest published series for transoral (or TLM) approaches to OPSCC available to date, the 3-year overall survival, disease-specific survival, and disease-free survival were, respectively, 86%, 88%, and 82%. Local tumor control at the primary oropharynx site was 97%, encompassing T1 through T4 lesions. Significant indicators of survival in this study were p16 positivity, T classification, and margins. Adjuvant therapy was administered in 74% of patients (58% RT, 16% CRT). Another study6 of 59 untreated patients managed by TLM with and without adjuvant therapy reported 5-year locoregional control of 88%; 5-year local control estimates by T-classification were: T1, 100%; T2, 87%; T3, 100%; and T4, 69%. Five-year recurrence-free survival was 84%.
Functional outcomes.
Excellent swallowing and speech outcomes have been observed for patients with OPSCC treated with TLM with and without adjuvant therapy. Steiner et al37 in a study of 48 patients with tongue base cancer with a T classification distribution of T1 and T2, 27%; T3 and T4, 73%, reported a normal diet in 92% (range, 40%–100%), and understandability of speech at 88%. For patients with T2 tumors, the score for both functions was 92%; and for patients with T3 or T4 lesions, these scores were 92% and 86%, respectively. Eighty-seven percent of 204 patients in the multicenter report had normal swallowing or only episodic dysphagia.8 Long-term (>24 months) feeding tube rate varies across different studies from 0% to 8%.6,8,37 Permanent tracheostomy rates vary from 0% to 2%.6–8,37
Hospital days, morbidity, and complications.
Median durations of hospitalization vary from 4.0 to 4.4 days.6–8,37 The incidence of complications reported after TLM range from 0.4% to 2.8% for airway-related problems and 1.4% to 10% for bleeding, which was controlled without catastrophic hemorrhage.6–8,37 Conversion to an open procedure is described in 3%.8 No direct, treatment-related deaths have been observed in these published studies, although a few cases seem to have occurred.
TLM and adjuvant therapy.
HPV biology may mitigate the importance of “traditional” pathological prognosticators such as node size, number, and (perhaps especially) extracapsular spread (ECS), that have triggered chemotherapy-intensified adjuvant treatment. This has been observed in studies of both predominantly p16+,7,8,38 and exclusively p16+ cohorts.39
Conclusions.
TLM is currently a safe, efficient, and effective technique that has the potential to achieve the goals of optimizing disease control while minimizing toxicity and functional loss. When indicated, it combines seamlessly with adjuvant therapy, preserves salvage options for recurrent tumors and efficiently manages unknown primary OPSCC. TLM will partner well with robotic resections in the surgical arm of trials that compare surgery with chemoradiation in management of OPSCC.
4. Management of the neck in OPSCC with definitive nonoperative treatment
Radiotherapy is the predominant component of nonoperative management of the neck and is generally used to avoid neck dissection altogether, particularly if it is already being used to treat the primary site. The principles of RT of the neck should include targeting of appropriate levels at risk using techniques that permit sparing of vulnerable anatomy, especially parotid glands, mandible, and pharyngeal musculature.
Eradication of disease in the neck is accomplished by adjusting the intensity of the applied radiotherapy dose-fractionation prescription to control both “overt“ grossly involved nodal disease, and subclinical cancer in areas considered at high risk. It is acknowledged that the contribution of treatment to some regions may be small but might have significant implications for the rare patient where progression may not be amenable to salvage. For example, different situations and decision making may apply to the elective irradiation of retropharyngeal lymph nodes, where recurrence without irradiation is likely to be rare but difficult to manage successfully with surgery. This contrasts with withholding elective RT of the contralateral neck (where surgical salvage of neck recurrence is not uncommon).
Guiding principles concern the balance between achieving disease control in the neck and avoiding injury to normal tissue. This largely relates to damage to salivary tissue with consequent permanent xerostomia. Pharyngeal dysfunction with swallowing impairment, predominantly reflecting the dose relationship associated with treatment of the superior and other constrictor muscles of the pharynx, is an additional concern following high-dose RT to this region.
Radiotherapy intensity and delayed surgery.
In treatment of the uninvolved neck, a moderate dose of 50 Gy in 25 fractions or an equivalent dose/fraction/time schedule in the nonoperated neck should eradicate subclinical disease in 95% of cases, which represents a general feature of RT in almost all anatomic sites.40
For the grossly involved neck the RT regimen must be augmented to 70 Gy in 35 fractions or equivalent doses delivered to “gross” target areas identified by imaging, with “elective” moderate doses prescribed to remaining neck regions also judged to be at risk. In addition, the RT doses needed to eliminate gross disease are often intensified. Strategies include the use of concurrent chemotherapy, or hyperfractionation with dose intensification. Irrespective of the approach undertaken, a standard addition to the nonoperative neck paradigm is the effective use of elective neck surgery if there is doubt about disease eradication, based on clinical and imaging reassessment 8–12 weeks after the completion of radiotherapy.
Defining the anatomic targets for neck irradiation.
The risk of involvement by neck node levels in oropharyngeal cancer has been studied by numerous authors, and recently by Sanguinetti et al.41 Ipsilateral levels II and III nodes have an approximately 75% and 20% risk of involvement, respectively, with either overt or subclinical tumor, and should always be considered at risk irrespective of the radiographic or clinical findings. Level IV should also be included in the lowest dose/risk level clinical target volume (CTV) to eradicate subclinical disease (risk 6% to 10%). The chance of involvement of levels IB and V is very low (<5%) even with ipsilateral pathologically proven neck disease at other levels, questioning their routine inclusion in any target volume.
The retropharyngeal space is difficult to access and there are only limited data about the incidence and clinical significance of disease in this area. These lymph nodes are not routinely removed in classical neck dissections.42 Estimates of malignant involvement approximate 10% to 20%, depending on several features including the primary site within the oropharynx and the method of clinical assessment (eg, CT, MRI, or tissue examination).42 However, an adverse outcome can be expected from untreated tumor in these nodes43 and they have been considered in IMRT planning to minimize recurrence in the region of the skull base.44
In the presence of grossly involved lymph nodes in level II or elsewhere on that side contemporary practice customarily treats levels II–IV bilaterally, IB ipsilaterally, and level V and the ipsilateral retropharyngeal nodes on either side of neck. This is exemplified by the outcome of the recent RTOG multicenter trial of IMRT for early-stage oropharynx cancer (RTOG 00-22) in which these principles of lymph node coverage were used.45 This single arm phase II study reported a 2-year estimated locoregional failure (LRF) rate of 9% with an excellent overall and disease-free survival. Grade >2 xerostomia was observed in 55% at 6 months but fell to 25% and 16% at 12 and 24 months, respectively, thereby validating one of the major reasons to use IMRT in this setting: the reliable and important ability to protect salivary function.
In addition, a number of groups have examined the problem of pharyngeal function following radiotherapy to the neck. Graphical representations of candidate prognostic targets for pharyngeal dysfunction after IMRT of oropharyngeal cancer have been studied that include dosimetric goals for avoidance of damage to these structures. Recommendations are emerging regarding the optimal design of radiotherapy target volumes to reduce dysphagia.46,47
“Neck” and primary tumor factors guiding radiotherapy targeting.
There are several additional factors that influence radiotherapy targeting of the neck. In N0–N1 disease, unilateral neck radiotherapy may be considered assuming that the primary tumor conditions permit this, as discussed in the following text. However, with N2a–b, and unilateral N3 disease it is generally considered prudent to treat the opposite side of the neck electively in addition to the targets needed for the grossly apparent tumor.
From the standpoint of the location of the primary, bilateral elective radiotherapy is usual for cancers originating near the midline of the oropharynx. These include lesions of posterior pharyngeal wall (where the retropharyngeal nodes should also be treated) and posterior tonsillar pillar cancers that should be treated in the same way as posterior pharyngeal wall. In addition, carcinomas arising in the base of tongue or soft palate/uvula have usually been treated with elective irradiation of both sides of the neck.
In contrast, ipsilateral elective radiotherapy is possible (provided only modest nodal tumor is present) in lateralized tonsillar cancers (including those originating in the fossa and anterior pillar) and in very small palate and lateral pharyngeal wall (ie, lateralized T1 tumors).
In addition to the actual primary site of origin, lateralized lesions with medial extension (within 1 cm of midline on palate and/or base of tongue) will also generally receive bilateral elective radiation.48 This principle is best supported for tonsillar cancer. Data for base of tongue lesions are sparse.
Management of overt neck disease following “nonoperative” treatment.
Following the completion of RT, observation without neck dissection is frequently possible, but must be undertaken carefully. Patients achieving a complete response after CRT have a high probability of regional control, and may be safely observed without planned neck dissection.49 This has also been demonstrated by the group at Memorial Sloan-Kettering, which presented long-term results of the largest experience of neck observation in patients (n = 283) with node positive OPSCC and a PET/CT-confirmed complete response after CRT. Their cumulative rate of regional failure at 5 years was only 2.2% with this approach.50
5. Management of the neck after transoral approaches
The options for management of the neck following surgical treatment of an oropharyngeal cancer include observation, neck dissection, or RT. Observation is typically reserved for those patients with early T-stage disease with no evidence of node metastases and a risk of occult neck disease that is less than 20%. When the risk of occult neck disease is greater than 20%, the neck may be treated with either neck dissection or RT.
Both neck dissection and RT are associated with acute and chronic morbidity. Prior to the introduction of the selective neck dissection, radical neck dissection was associated with significant morbidity largely related to shoulder dysfunction reflecting the functional impairment and cosmetic deformity associated with sacrifice of the sternocleidomastoid muscle and injury to the spinal accessory nerve. Since the introduction of the selective neck dissection, the morbidity of this procedure has been greatly reduced, influencing less than 5% of patients.51 Similarly, prior to the introduction of IMRT, radiation was associated with a high rate of acute and chronic morbidity.52 Even IMRT is still associated with some measurable toxicity and dysfunction.
The lymphatic drainage characteristics of oropharyngeal cancer have been established53,54 and most data have demonstrated that lateralized tonsil cancer can be managed appropriately by treating the ipsilateral neck.55 An ipsilateral neck dissection should include removal of the lymph nodes in levels II, III, and IV. Unlike RT, the neck dissection is not designed to remove the retropharyngeal lymph nodes, although a careful review of the data demonstrates that in lateralized tumors this basin is rarely involved.56–58 Tumors involving the soft palate, nasopharynx, or the posterior pharyngeal wall are at the highest risk for retropharyngeal lymph node disease.56–58 With the introduction of high-resolution imaging and PET/CT scans, most data suggest that retropharyngeal disease can be identified on imaging, therefore obviating the need for empiric treatment of this basin.58 In contrast to neck dissection, RT may address the retropharyngeal basin in addition to the lymph nodes in levels II, III, and IV.56 However, the morbidity associated with radiotherapy of the superior constrictors has been credited with the debilitating dysphagia associated with this treatment modality.
Neck dissection offers unique advantages. In addition to reducing the cost of therapy, neck dissection provides pathological information including the number of pathological lymph nodes, the level of disease, and the presence of extracapsular spread (ECS). This information is crucial for tailoring the therapy to suit the disease. Information surrounding ECS may be used to intensify therapy in the high-risk population and deescalate therapy in the low-risk population. Pathologic information gleaned from the neck dissection could allow for the opportunity to reduce RT dose, and avoid systemic chemotherapy in appropriate patients. Both decreasing the need for systemic chemotherapy and reducing the dose of RT appear to have a significant impact on treatment morbidity and function.
In conclusion, neck dissection for management of the neck following transoral resection of the oropharyngeal primary tumor can be accomplished with low morbidity. It provides an important source of pathological information that can be used to personalize therapy and may play an important role in reducing treatment morbidity without compromising survival.
6. Postsurgical adjuvant treatment
Prior to the era of definitive chemoradiation for oropharyngeal cancer, surgery, often followed by postoperative radiation therapy (PORT), was the standard of care. In selected patients it remains a good option. Indications for PORT to the primary tumor site continue to include “close” or positive surgical margins, T4 cancers, and lymphovascular or perineural invasion. The indications for postoperative radiation to the neck include more than 1 positive lymph node and the presence of extracapsular extension (ECE).59–61
The addition of cisplatin-based chemotherapy to PORT has been demonstrated to improve local control, disease-free survival,62,63 and overall survival63 when compared with radiation alone. Chemoradiation reduced local recurrence from approximately 30% to 15%, but there was no significant change in the development of distant metastasis. Grade 3 or greater toxicity rose with the addition of chemotherapy. A pooled analysis of the 2 largest randomized trials demonstrated that the strongest indications for the addition of chemotherapy to postoperative radiation were involved surgical margins and ECE of lymphatic disease.64 Thus, concurrent chemotherapy and radiation are regularly recommended for patients with such high-risk features. RTOG 0920 is currently evaluating the utility of adding cetuximab to PORT for patients with intermediate risk disease (such as large or multiple involved nodes, close margins, and perineural or lymphovascular invasion).
The anatomic volumes to be treated with radiation are dictated by the surgical findings. Generally, the surgical bed is treated to 60 Gy if margins are clear and 65–66 Gy if surgical margins are involved. Regions of the neck where ECE was present receive at least 63 Gy to optimize local regional control.65 The dose to the uninvolved neck (eg, an otherwise untreated contralateral neck) can range from 44 to 64 Gy. A nodal level where disease was present without ECE should receive 60 Gy. Additionally, when delivering PORT for a primary oropharynx lesion, treatment of the lateral retropharyngeal lymph nodes should also be considered, because recurrence in this region is not conventionally salvageable. IMRT is generally used for parotid sparing, for sparing of other normal tissues, and for better targeting of regions at risk. IMRT can be used with dose-painting (Simultaneous Integrated Boost) or with the primary volume and boost volume delivered sequentially.
If local control can be adequately addressed by the surgeon, then patients with T1–T2 tonsil and base of tongue cancer who have pN0 or possibly pN1 neck disease should be able to avoid both chemotherapy and radiation and still enjoy a 90% cure rate. However, TORS data documenting long-term freedom from disease for patients with T1–T2, pathologically N0 disease with margins ≥3 mm are sparse, and important questions about patients with early-stage oropharyngeal cancers abound. Is transoral surgery good enough, by itself, to control the primary disease? If not, is 60–66 Gy given postoperatively less morbid than 70–72 Gy given definitively? In patients with more advanced resectable cancers, will transoral surgery with neck dissection, and a pathologically determined risk-based approach to PORT produce similar outcomes? How will these alternative approaches impact function and QOL?
FUNCTIONAL AND QUALITY OF LIFE OUTCOMES
7. Functional and quality of life outcomes following transoral surgery
Transoral techniques for resection of oropharyngeal cancer have evolved in tandem with technologic advances and depend on the use of FDA-cleared devices to complete the resection. These include (1) handheld surgical scalpels, scissors, and lasers; (2) handheld electrocautery; (3) transoral laser microsurgery (TLM); and (4) transoral robotic surgery (TORS). Protocol planning requires an analysis of the existing evidence concerning functional and QOL outcomes following the transoral surgical approaches. This analysis was limited to transoral surgical approaches that have been previously studied in prospective Phase I or Phase II clinical trials for treatment of oropharyngeal carcinoma.
Although both TLM and TORS are transoral procedures, they represent fundamentally different oncologic approaches.8,9 TLM is generally performed with transtumoral transection, tailoring the extent of the operation to the interface between the tumor and the normal tissue. The amount of normal tissue spared or resected varies from operation to operation since the extent of the resection is determined by attempting to achieve 5-mm margins beyond the cancer, rather than by predefined en bloc resection margins. In contrast, TORS entails intended en bloc resections, the result of which is a predictable surgical defect. Therefore, it is not the technology used in TORS or surgical exposure that limits the extent of resection, but rather the predefined en bloc resection margins of the standardized procedures.
QOL and functional outcomes following TORS.
There have been 5 prospective cohort studies evaluating functional outcomes and QOL following TORS (Tables 1 and 2).12,66–69 Six different QOL and functional assessment instruments were used in these studies (Table 3). The numbers of patients in these series ranged from 30 to 54. Three of 5 series considered only patients with oropharynx cancer, and over 90% of the patients in the remaining 2 reports had oropharynx cancers. Only 2 series were limited to previously untreated patients, 2 included previously irradiated patients, and 1 did not clarify previous treatment. A weakness of all of the reports was that minimum follow-up was less than 1 year. The most common postoperative therapy was radiation alone, which was administered to between 30% and 61% of patients. There was no reported tracheotomy tube dependence and in 3 of the 5 series, there was no gastrostomy tube dependence. The two other series reported less than 3% gastrostomy tube dependence.
TABLE 1.
Comparative view of transoral robotic surgery study parameters.
| TORS study | Oropharynx only | No. of patients | Included previously treated patients | Follow-up, mo (mean) |
|---|---|---|---|---|
| Sinclair69 | Yes | 54 | Yes 12/54 (22%) | 13 (range, 2–?) |
| Moore12 | Yes | 45 | No | 12.3 (range, 1–16) |
| Genden66 | No (90% oropharynx) | 30 | No | 20.4 (range, 12.8–39.6) |
| Hurtuk67 | No (90.6% oropharynx) | 64 | N/A | 11.8 (range, 2–29) |
| Leonhardt68 | Yes | 38 | Yes 2/38 (5.3%) | 15.2 (range, 10–21) |
Abbreviations: TORS, transoral robotic surgery; N/A, not available.
TABLE 2.
Comparative view of transoral robotic surgery therapy and outcomes.
| TORS study | Use of free flaps | Tracheostomy dependence |
PEG tube dependence | No. (%) postop radiation | No. (%) postop chemoradiation | TORS alone (%) without adjuvant therapy |
|---|---|---|---|---|---|---|
| Sinclair69 | No | 0 | 0 | 19/42 (45.2%) | 13/42 (31%) | 10/42 (23.8%) |
| Moore12 | No | 0 | 0/45 (0%) | 25/45 (55.6%) | 8/45 (17.8%) | 12/45 (26.7%) |
| Genden66 | Yes* | 0 | 0/30 (0%) | 14/30 (46.7%) | 11/30 (36.7%) | 5/30 (16.7%) |
| Hurtuk67 | No | 0 | 1/54 (1.9%) | 16/54 (29.6%)† | 33/54 (61.1%) | 7/54 (12.9%) |
| Leonhardt68 | No | 0 | 1/38 (2.6%) | 22/36 (61.1%)‡ | 7/36 (19.4%) | 7/35 (19.4%) |
Abbreviations: TORS, transoral robotic surgery; PEG tube, percutaneous endoscopic gastrostomy tube.
1 patient;
54 patients had squamous cell carcinoma;
2 patients had prior radiation.
TABLE 3.
Instruments used for quality of life and functional outcomes assessment.
| Instrument | Description | Studies that used this instrument |
|---|---|---|
| MD Anderson Dysphagia Inventory (MDADI) | This scale is the first validated and reliable self-administered questionnaire designed specifically for evaluating the impact of dysphagia on the QOL of patients with head and neck cancer. | Sinclair69 |
| Functional Outcome of Swallowing Scale (FOSS) | This 5-point scale is rated by health care professionals, with 0 being normal and 4 or 5 being gastrostomy dependent. | Moore12; Grant6; Rich70 |
| Functional Oral Intake Scale (FOIS) | This scale was developed to document the functional level of oral intake of food and liquid in stroke patients. This scale is rated by health professionals. Not validated for patients with head and neck cancer. | Genden66 |
| Performance Status Scale for Patients with Head and Neck Cancer (PSS-HN) | This scale was designed to evaluate performance in areas of functioning most likely affected by head and neck cancer and its treatment, specifically eating, speaking, and eating in public. This scale is rated by health professionals. | Genden66; Leonhardt68 |
| Head and Neck Cancer Inventory (HNCI) | This scale is a self-administered health status assessment instrument with a small number of multiple-item domains that capture patients’ ratings of functional status and attitude about that function. | Hurtuk67 |
| SF-8 | This scale (using a standard format version with a 4-week recall) is divided in 8 domains: Physical Functioning, Role Physical (role limitations attributed to physical Health), Bodily Pain, Global Health, Mental Health, Vitality, Social Functioning, and Role Emotional (role limitations attributed to emotional problems). | Leonhardt68 |
Abbreviation: QOL, quality of life.
Sinclair et al69 using the MD Anderson Dysphagia Inventory (MDADI), noted that the only factors that significantly predicted worse dysphagia were nodal status, follow-up time of less than 12 months, and lower preoperative physical scores. Moore et al12 noted that patients with tonsil cancers had no decrease in Functional Outcome Swallowing Scale (FOSS) scores after TORS, and that patients with tongue base cancers had only minimal change. The Mount Sinai series noted that by 9 months following treatment the Performance Status Scale for Head and Neck Cancer Patients (PSS-HN) had returned to preoperative baseline.66 Hurtuk et al67 compared previously published historical Head and Neck Cancer Inventory (HNCI) outcomes for chemoradiation, to TORS followed by radiation therapy. Overall, there was a trend toward higher outcome scores in each domain for the TORS group. Leonhardt et al68 noted that results at 12 months for the PSS-HN Eating and Diet domains were not significantly different from preoperatively.
QOL and functional outcomes following TLM.
There have been 2 trials evaluating functional outcomes and QOL following TLM (Tables 4 and 5).6,70 Grant et al6 studied 93 patients who were followed prospectively after TLM for tongue base carcinoma from the Mayo Clinics in Scottsdale and Jacksonville. Twenty-two patients (37%) required a temporary tracheotomy but only 1 patient (2%) required a permanent tracheotomy after treatment. However, 5 patients (8%) remained dependent on long-term tube feeding after treatment. Grant et al6 noted that the FOSS scale trended toward worsening of swallowing outcomes with the addition of postoperative radiation following TLM.
TABLE 4.
Comparative view of transoral laser microsurgery study parameters.
| TLM study | Oropharynx only | No. of patients | Included previously treated patients | Follow-up, mo (mean) |
|---|---|---|---|---|
| Rich70 | Yes | 118 | No | 53.9 (range, 2–138) |
| Grant6 | Yes | 59 | No | 31 |
Abbreviation: TLM, transoral laser microsurgery.
TABLE 5.
Comparative view of transoral laser microsurgery therapy and outcomes.
| Study | Use of free flaps | Tracheostomy dependence | PEG tube dependence | No. (%) postop radiation | No. (%) postop chemoradiation | TLM alone (%) without adjuvant therapy |
|---|---|---|---|---|---|---|
| Rich70 | Yes* | Not reported | Not reported | 55/118 (46.6%) | 48/118 (40.7%) | 15/118 (12.7%) |
| Grant6 | No | 1 (2%) | 5 (8%) | 28/59 (47%) | 0 | 31/59 (53%) |
Abbreviations: PEG tube, percutaneous endoscopic gastrostomy tube; TLM, transoral laser microsurgery.
2 patients.
Rich et al70 retrospectively evaluated swallowing function from 118 patients with advanced oropharynx cancer undergoing TLM. At 1 year posttreatment, approximately 50% of patients with T4 cancers had what the authors considered to be poor swallowing outcomes (FOSS score 3–5) and that this did not change over the full 5-year follow-up. Approximately 20% of patients with T3 cancers had poor swallowing (FOSS score 3–5) between years 1 and 4, with improvement in the fifth year of follow-up (see Figure 1). Neither tracheotomy nor gastrostomy tube dependence were reported in this series, although 89 of these 118 patients were analyzed for gastrostomy dependence in an earlier study.8 One-year gastrostomy dependence was 18.8%, which dropped to 9.3% at 2 years and 3.8% at 5 years.8
FIGURE 1.
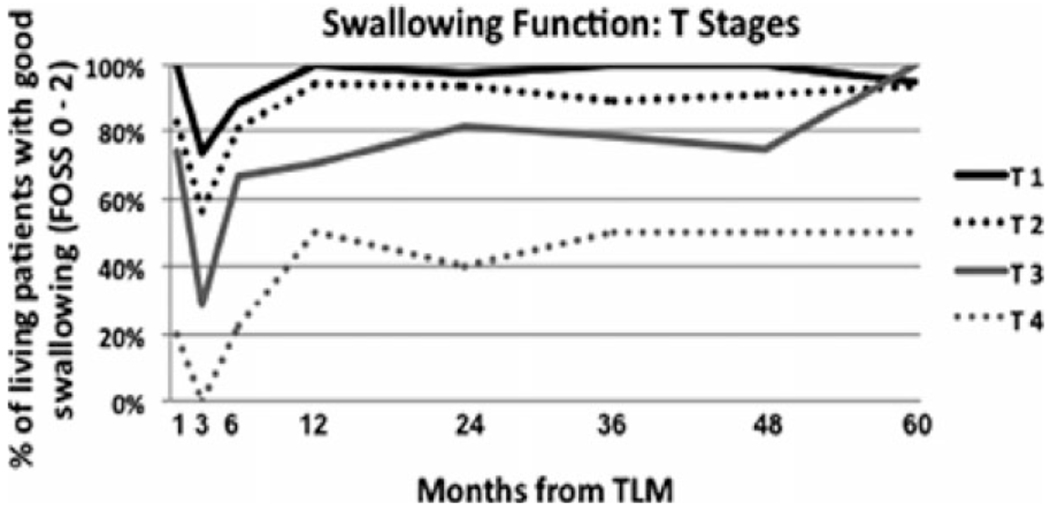
Swallowing function following transoral laser microsurgery for advanced oropharyngeal squamous carcinoma stratified by T stage. (Reprinted with permission from Laryngoscope 2011 (©John Wiley & Sons, Hoboken, NJ.70)
Comparative analysis TORS and TLM QOL studies.
A comparison of the function and QOL studies following TORS and TLM reveals that there were differences in the oncologic indications for the 2 surgical approaches as well as differences in functional outcomes. Although the overall American Joint Committee on Cancer (AJCC) staging was similar for both the TLM and the TORS series, only 11% of the TORS cases were for T3/T4 cancers, whereas TLM was performed on approximately 3 times as many T3/T4 cancers. It is likely that the predefined limits of resection that are used in TORS resulted in fewer T3 and T4 cancers being included. These limits of resection do not exist within the TLM paradigm of transtumor incisions, permitting resection of tumors with a higher T classification. The differences in the 1-year gastrostomy tube dependence rates of up to 18.8% for TLM versus 2.6% for TORS, and the higher FOSS scores for T3 and T4 cancers following TLM, may reflect the standardized resection of normal tissue in the TORS groups but the variability in normal tissue resection in TLM group due to inclusion of larger cancers. It is probable that the similarity between functional outcomes for TORS and TLM for T1/T2 oropharyngeal cancers results from comparable amounts of normal tissue resection following either approach. Additional study is needed.
Conclusions.
(1) The only transoral surgical approaches for oropharyngeal cancer that have been studied prospectively are TORS and TLM. Reliable data for outcomes following other techniques are limited. QOL and functional analysis data suggest that any proposed multi-institutional trial should be limited to the TORS and TLM approaches. (2) Functional outcomes appear similar for T1 and T2 cancers regardless of whether the transoral approach uses TLM or TORS. Functional outcomes are diminished following TLM for T3/T4 cancers when compared with T1/T2. The best current QOL and functional analysis data support the recommendation that the proposed multi-institutional trial be limited to T1 and T2 cancers. (3) Assessment should include validated QOL instruments as well as objective functional data including tracheotomy and gastrostomy tube dependence at 1 and 2 years after treatment.
8. Quality of life impact of nonsurgical management of head and neck carcinomas
Nonsurgical management of OPSCC is associated with a well-delineated spectrum of acute, subacute, and potential long-term toxicities that include risks of late swallowing dysfunction, necessitating enteral support for nutrition (RTOG grade 3/4). To date, there exists little in the way of large prospective longitudinal cohort studies to define the QOL after current nonsurgical treatment approaches, which were the product of an era of concerted treatment intensification efforts seeking to increase locoregional control rates.71
Early longitudinal functional and QOL studies in patients in head and neck squamous cell carcinomas (HNSCC) demonstrated a consistent and significant impairment of various QOL and functional domains following a course of radiation therapy (RT).72–76 Most studies identified this impairment after 3–6 months of follow-up. By 12 months, there was evidence of recovery of the QOL impairment in many domains such that the global measures demonstrated no significant difference from baseline.71–76 Reasons advanced for these observations have included patient recovery, adaptation to treatment toxicities, and changes in patient attitudes toward their toxicities. As a result, early interpretation of treatment effects on QOL measures suggested that treatment could be intensified to improve locoregional control and survival rates without undue toxicity. However, more recent longitudinal studies with larger cohorts demonstrate long-term impairment in global QOL measures, especially when late severe swallowing complications occur.75 ,77 Several retrospective cross-sectional QOL reports show a similar significant impact of late swallowing complications. That more severe swallowing complications correlated with greater impairment in various QOL domains and global QOL further supports a causal relationship.77
Several studies now confirm that both RT dose intensification and the use of chemoradiotherapy (CRT) are significant independent risk factors in the development of late RTOG grade 3/4 swallowing complications.78,79 More significantly, increasing RT dose to the pharyngeal constrictor muscles has been correlated with both the risk of late RTOG grade 3/4 swallowing complications and impaired QOL, as measured with the European Organization for Research and Treatment of Cancer (EORTC) H&N35 instrument.80 For these endpoints, the dose–effect relationships appear remarkably similar and may be characterized as exponential. A mean threshold dose of 55 Gy to the constrictor muscles is associated with a significantly increased risk of late RTOG grade 3/4 swallowing complications.80 Thus, the lower postoperative radiotherapy doses may be advantageous since doses of 57.6–63 Gy are typically used.
The proposition that either dose reductions to the constrictor muscles or the reduced use of CRT can diminish the rates of late swallowing complications or patient-reported QOL impairments (while maintaining locoregional control rates) remains unproven. However, it offers a rational foundation for future investigation of function and QOL in OPSCC.
9. Functional and quality of life outcomes after neck dissection
Critical to any comparison of surgical and nonsurgical treatment approaches is the impact of the neck dissection on overall patient function and QOL. Neck dissection–related QOL has been correlated with employment status, leisure, and recreational activities. Because the order of treatment and the number of different modalities used to treat the neck result in different patient-reported QOL results, it is important to understand the impact of the different treatment approaches on the neck.81
Neck dissection–related QOL can be assessed by single questions in general head and neck QOL scales such as the University of Washington Quality of Life (UWQOL), the University of Michigan Head and Neck Quality of Life (UM H&NQOL), the Quality of Life 30 (QLQ C30), and the SF 36.82,83 These measures lack specificity but show sensitivity for those patients who have undergone neck dissection resulting in pain and stiffness.84
Neck dissection–related QOL is more accurately assessed with instruments that were intended for assessment of the neck and shoulder. There are at least 5 instruments that were designed for the assessment of shoulder function for medical and surgical conditions in the field of orthopedics that have been used to assess patients after neck dissection. The instrument that has been used the most in patients with head and neck cancer is the Shoulder Disability Questionnaire (SDQ).85 This is one of the simplest available but is limited because the responses are dichotomous (yes/no), which may affect the ability to discriminate more subtle changes in clinical outcome. The Constant’s Assessment of Shoulder function is widely used in a variety of disease types and incorporates self-report, range of motion, and strength testing. The advantage of this approach is the varied measures that are used, although this is also an important limitation, because it takes more time and expertise to perform range-of-motion testing with a goniometer and strength testing with weights.86,87 The Disabilities of the Shoulder, Arm, and Hand (DASH) scale is an excellent self-report instrument that has shown good content validity and sensitivity in patients undergoing neck dissection in unpublished cohorts.88
The Neck Dissection Impairment Index (NDII) is a short, self-report instrument that has been used by a variety of different authors, is specific for neck dissection, and is sensitive to neck dissection type, radiation, chemotherapy, and order of therapy in patients with head and neck cancer.89 It has been used in cross-sectional studies but not in longitudinal ones. The NDII was developed because there was no neck dissection–specific QOL instrument.
A problematic area for the design of a phase III trial is the lack of information on neck and shoulder QOL in patients who have undergone CRT but have not undergone a neck dissection. At present there is a single report that assesses range of motion and neck-related QOL (using the SDQ) in a cross-sectional design. This study was done by the group at the Free University Hospital in the Netherlands and showed that there were statistically significant differences in the SDQ and range of motion in patients who have undergone chemoradiation versus untreated patients.90
There is also the question of when to administer the instrument(s). The measurement of the neck-related QOL should be performed prior to treatment to establish baseline. If only 1 other assessment is made, then the optimal point for assessment would be 12 months after the completion of treatment. This allows sufficient time for the regeneration of nerves and the completion of healing of the wound.
RESEARCH OPPORTUNITIES: CLINICAL TRIALS OF TRANSORAL RESECTION
10. What should be the study objectives?
Despite a decline in tobacco use, over the past decade the incidence of OPSCC in the United States has actually increased because of HPV infection.4 Serendipitously, the introduction of the surgical robot and the CO2 laser optical fiber has permitted more precise transoral resections for select oropharyngeal cancers, thus lessening the morbidity of surgical exposure and treatment. These factors have led to renewed interest in exploring the role of modern, low-morbidity surgery as a primary modality for treating patients with early- to intermediate-stage oropharyngeal cancer.
HPV-associated head and neck cancer: analysis of RTOG 0129.
The importance of tumor HPV status to therapeutic response and survival has been evaluated in several settings, including prospectively in a phase II clinical trial (E2399) conducted by the Eastern Cooperative Oncology Group (ECOG)91 and in retrospective analyses of 2 phase III trials, RTOG 0129 and TROG 02.02.92,93 The results from RTOG 0129, a phase III trial that compared standard and accelerated boost radiotherapy regimens, both with concurrent cisplatin, provide important clues about how best to study this disease further.91 When compared with the patients with HPV-associated disease and less than a 20-pack-year smoking history, the HPV-associated patients with a greater than 20-pack-year smoking history had a hazard ratio (HR) for overall survival of 1.91 (95% confidence interval [CI], 1.20–3.05) Patients with HPV-unrelated disease who had smoked less than 20 pack-years had a HR of 2.25 (1.44-3.50), and patients who were HPV-unrelated but smoked 20 pack-years or more had HR of 4.30 (95% CI, 2.40–7.71). The 2-year progression-free survivals were 95%, 80%, 71%, and 63%, respectively, and patients with HPV-unrelated oropharynx cancer had, in aggregate, an essentially identical progression-free survival as those with hypopharynx and larynx primaries, approximately 60%. In summary, results suggest that patients with an HPV-associated OPSCC have a more favorable prognosis, in part due to the natural biology of the cancer, and in part because it is potentially more radiosensitive. These very good prognosis patients are appropriate for less intensive treatment approaches if equivalent treatment outcomes and a reduction in acute and late toxicity can be achieved. Whether transoral surgery has a role in the treatment algorithm merits careful investigation.
RTOG 0129 also demonstrated that HPV-unrelated patients with a significant smoking history have worse locoregional disease control and survival, even with aggressive nonsurgical therapy. In these patients it seems unlikely that further treatment intensification with CRT will improve disease outcomes, nor could this approach be justified given the significant acute and late toxicities associated with concurrent CRT schedules. Treatment intensification with surgery might improve locoregional control in these patients by removing the disease, permitting a risk-based use of postoperative adjuvant therapy, with observation for patients with favorable pathology, radiation alone for intermediate-risk patients, and CRT for high-risk individuals.
Postoperative radiotherapy.
It is now clear that many of the late effects after RT that can significantly impact swallowing function are related to the dose, to the amount of normal tissue receiving radiation, and the use of concurrent chemotherapy.78,79,94 Current postoperative radiotherapy doses derive from a series of seminal investigations conducted at MD Anderson Cancer Center.65,95 The cumulative experience from these randomized trials demonstrated that for squamous cell carcinomas of all anatomic sites in the head and neck, the recommended postoperative radiotherapy dose can be pathologically guided and result in high rates of locoregional disease control. Although various risk stratification paradigms have been evaluated, the presence of a positive margin and nodal ECE warrant a dose of at least 63 Gy.96 In the presence of any pathologic risk factors identified in the tumor specimen, an increased risk of local relapse was statistically identified if <54 Gy was administered, compared with 57.6 Gy (63% vs 92%, p = .02).65 Although the optimal postoperative dose for ECE and positive margins has not been established, several cooperative group trials have used doses up to 66 Gy.62,63 These data should be reevaluated for HPV-initiated disease, where traditional pathologic risk features may not be as meaningful in the selection of adjuvant therapy regimens and doses. This must be addressed in well-designed prospective surgical trials where clinical–pathologic correlation carefully examines the relationship between ECE and treatment efficacy from the perspective of regional and distant disease control.
Following surgical resection, patients should receive protocol-defined risk-based adjuvant therapy based on established criteria, including adequacy of the surgical resection, margin status, the presence or absence of nodal metastasis, the number of lymph nodes involved, and the presence or absence of quantifiable nodal ECE. Such protocols will seek to differentiate risk-stratified posttransoral resection patients defined by the absence of high-risk features that include HPV status, a positive margin (defined as carcinoma within 5 mm of the cut specimen edge), ECE, smoking status, and the presence of multiple lymph nodes.
We propose in the low-risk HPV-initiated group that the primary tumor bed with an indication for irradiation (such as perineural invasion or lymphovascular invasion, but negative margins) can be effectively treated with 50 Gy. This represents an experimental reduction of approximately 10% from the lowest dose range that has been administered for the low-risk postoperative cohort of patients, but a dose potentially sufficient for such a good prognosis, HPV-initiated surgically resected cohort.
Patients with HPV-unrelated tumors who have indications for postoperative radiation would receive 56 Gy. The necessity for postoperative adjuvant radiation to a tumor bed that demonstrates no adverse pathologic features is a controversial practice, rooted historically in the use of transcervical neck exposures and the early and seminal observations of Fletcher97 that were also intended to address the risk of tumor surgical seeding. In the setting of a transoral approach, where there is no communication with cervical fascial planes, the evidence to date with both TLM and TORS10,98–100 suggests that the risk of local relapse after appropriate resection is less than 5%,101–105 with very acceptable functional outcomes.
A primary surgical role will therefore provide the opportunity to stage disease accurately so that adjuvant therapy and dose can be applied in a judicious manner. Surgical staging identifies patients with more aggressive or advanced disease where intensification of treatment with CRT seems justified, and allows for resection alone as treatment for patients with pathologically confirmed stage I and stage II disease.
Outstanding issues to be addressed by prospective surgical trials.
The ECOG 1308 trial, which has completed accrual, consisted of induction chemotherapy followed by reduced dose (54 Gy) IMRT with cetuximab or standard dose (69 Gy) IMRT with cetuximab in patients with HPV-associated oropharyngeal cancer. RTOG 1016 is comparing radiation and concurrent cisplatin with the less toxic radiation and cetuximab combination in patients with p16 positive (HPV-initiated) oropharynx cancer. Thus, the profession has entered an era of protocol-based deintensification for HPV-initiated OPSCC. There is an evolving standard of surgical care in OPSCC in light of the new surgical techniques, and establishing feasibility should be an important objective of trials under development. Furthermore, prospective, homogeneously treated cohorts would permit evaluation of whether N2 disease and ECE are appropriate determinants of adjuvant therapy, as shown in RTOG 9501,62 which was undertaken before the recent dramatic increase in incidence of HPV-initiated cancers. In the following text we summarize some of the specific objectives to be addressed by collaborative design of prospective surgical trials of transoral resection of OPSCC.
What should be the study objectives?
(1) Demonstrate feasibility/efficacy of multicenter surgical trials. (2) Develop/validate methods of toxicity assessment for primary surgical therapy. (3) Establish a prospectively collected, homogeneously treated biospecimen bank for molecular correlative/biomarker studies. (4) Establish a cadre of “credentialed” surgical investigators. (5) Assess, measure, and compare the toxicities and functional outcomes from surgical and nonsurgical therapies. (6) Assess cost of treatment package using surgical versus nonsurgical therapy, that is, does interventional staging and personalized treatment intensity offset costs added by operations?
Transoral surgery is feasible, safe, and FDA approved. Surgical staging allows for personalized postoperative adjuvant therapy. Trials to demonstrate equivalent outcomes and reduced toxicity after initial transoral surgery, with those achieved using conventional CRT are being designed for patients with HPV-initiated tumors. In HPV-unrelated disease, survival is unchanged and unacceptable. Transoral surgical complete response, combined with RT/CRT, may enhance oncologic outcomes with acceptable functional/QOL results.
11. What are the appropriate quality of life endpoints?
Understanding the true morbidity associated with a particular treatment regimen is an important issue. Classically, many cooperative group trials have used the “maximum grade” value as the measure of treatment toxicity. More recently, however, Trotti et al106 defined a more sensitive measure, the “T” score, which includes not only the maximum grade, but also the number of times adverse events occur over time. Thus, when comparing the morbidity of concurrent cisplatin with accelerated RT versus concurrent cetuximab with RT, the cisplatin and RT strategy is about 1.5-fold as “toxic” using the maximum grade score, but about 3-fold as intensive using the “T” score. The recently opened Radiation Therapy Oncology Group (RTOG) 1016 randomized study compares these 2 regimens in patients with HPV-associated oropharyngeal cancer. Importantly, this study has a dual endpoint. First, the survival on the cetuximab/RT arm cannot be inferior to the cisplatin/RT arm. The second objective is that the acute toxicity burden in the cetuximab/RT arm be reduced at least 50% compared with the cisplatin/RT arm, whereas the long-term swallowing function is similar to (or better than) the cisplatin/RT arm. Only if both objectives are met will concurrent cetuximab with RT be considered an effective and less toxic alternative to concurrent cisplatin with RT for locally advanced HPV-initiated oropharyngeal cancer.
A key challenge is to select the most relevant patient reported outcome (PRO) measures for a particular clinical setting. For a clinical trial, the PRO must be validated and specifically address the QOL endpoint of interest. In September 2011, the NCI sponsored a workshop with the goal of creating a core set of symptoms and QOL domains for prospective evaluation of PROs relevant to several disease sites including head and neck cancer. This process involved an evidence-based literature review, as well as expert discussion. The head and neck cancer working group recommendations will be published elsewhere. As might be expected, the list included widely recognized head and neck symptoms, such as swallowing, oral pain, and dry mouth. At the same time, the working group also included key chronic issues, such as dental health and mouth opening (trismus). The time course and trajectory of acute versus subacute versus chronic symptoms, needs to be studied more thoroughly. Head and neck symptoms and QOL domains are typically interrelated. For example, swallowing is affected by dry mouth, oral pain, excessive secretions, taste, and dental health. In addition, in the conduct of a clinical trial comparing a surgical with nonsurgical management, investigators must carefully incorporate measures covering the spectrum of expected side effects and symptoms from these differing modalities because surgical and nonsurgical approaches have different anticipated major side effects and toxicities.
12. What imaging questions should be considered?
The imaging techniques most commonly used for staging OPSCC include contrast-enhanced CT (CECT), magnetic resonance imaging (MRI), and fluorodeoxyglucose positron emission tomography (FDG-PET/CT). Each modality has advantages and limitations, suggesting that they may be best used in combination.107–109 For example, in the setting of tongue base cancer, MRI will best delineate extension of tumor into normal soft tissue, whereas CECT may more clearly identify small-volume nodal disease in the neck. FDG PET/CT can contribute to the interpretation of indeterminate (usually small volume) MRI or CECT findings both locoregionally and at potential distant metastatic sites.
Any imaging technique has the potential to over- or underestimate the extent of disease, particularly its superficial mucosal extent, such that their use must be combined with direct inspection and palpation at the time of physical examination. The integration of imaging tools into the overall management paradigm is an evolving process and is influenced by the individual patient presentation, cost, and/or availability of these examinations.
Imaging may influence the choice of primary locoregional treatment (surgery vs RT and/or concurrent systemic treatments). Furthermore, treatment details such as the specific surgical approach or radiation volumes and dose may be determined by pretreatment imaging. Adjuvant postoperative treatment decisions reflect baseline imaging results and pathologic findings at surgery. In addition, image-based response assessment criteria inform decisions regarding the need for neck dissection or surgical salvage of persistent primary disease following radiotherapy.
Contemporary imaging techniques can provide important functional metabolic data beyond anatomic tumor extent.110,111 Examples of functional imaging include dynamic contrast-enhanced MRI (DCE-MRI) and diffusion weighted MRI (DW-MRI), which can confer information regarding tumor stromal vascular function. PET can interrogate multiple parameters including glucose metabolism, cellular proliferation, and tumor hypoxia. Computed tomography perfusion (CTP) imaging is a noninvasive method of measuring regional blood perfusion through tissue. The performance of serial functional imaging, before, during, and/or after treatment may offer quantifiable markers for predicting outcome and influencing management. Functional imaging also has the potential to evaluate normal tissue, such as salivary gland, and play a role in risk assessment selection of treatment modalities and normal tissue consequences of treatment.112
Studying transoral resection of OPSCC within the context of a clinical trial affords a tremendous opportunity to investigate the impact of currently used and newer imaging techniques on management outcomes. Correlation of imaging performed pretreatment with the pathologic findings at surgery, tissue-derived mechanistic biomarkers, and subsequent clinical outcome should define and refine the interpretation of pretreatment imaging to guide treatment decision making.
13. Cost and comparative effectiveness considerations
Because medical care expenditures have increased at about 2.5% above real growth in gross domestic product for the past several decades, an increasing fraction of GDP has shifted to health. Policy makers must either find means to reduce this long-term growth trend or increase tax rates. Today, government policy efforts are directed toward reducing spending for products and services of uncertain or unproven clinical value. At the federal level, comparative effectiveness research (CER) has emerged as a prominent approach to find the most efficient and effective ways to treat common medical problems. The Institute of Medicine defines CER as the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition, or to improve the delivery of care. The purpose of CER is to assist consumers, clinicians, purchasers, and policy makers to make informed decisions that will improve health care at both the individual and population levels. CER has several unique features compared with typical medical research: (1) direct, head-to-head comparisons of competing treatments (vs placebo-controlled trials); (2) topics oriented to clinical decision making; (3) study design and endpoints relevant to a wide group of stakeholders (examples include: patients, clinicians, purchasers, and policy makers); and (4) study populations representative of clinical practice. By improving the knowledge base through direct comparisons, the goal of CER is to reduce variation in care and improve outcomes while at the same time reducing medical expenditures.
Cost-effectiveness analysis (CEA) is distinct from CER in that it is focused on assessing value, defined as the difference in cost divided by difference in outcomes for 2 competing approaches to treating individuals with the same health condition. Using this definition, when compared with an alternative, interventions can (1) cost more and provide worse health outcomes (dominated by the alternative); (2) cost less and provide better health outcomes (dominate the alternative); (3) cost less and provide worse health outcomes (uncommon); or (4) cost more and provide better health outcomes (the most common outcome of cost-effectiveness studies). Generally, as one increases spending health outcomes improve, but at a diminishing rate. At some point, increased spending on a particular condition (either through applying more of the same technology or by substituting a more costly and effective technology for a less costly and effective one) will produce diminishing returns—a point at which cost-effectiveness becomes unfavorable.
This issue of diminishing returns highlights a fundamental difference between comparative effectiveness and cost-effectiveness; one can identify comparatively effective interventions that are not cost-effective. The corollary is that cost-effectiveness does not always imply comparative effectiveness, because cost-effectiveness depends on what is being compared. For example, if the comparator treatment in a CEA is a less effective treatment than more commonly used alternatives, the CEA may not be meaningful to comparative effectiveness researchers.
The management of head and neck cancer provides many opportunities to find comparatively effective interventions as well as those that are cost-effective. Relevant endpoints for CER studies include but are not limited to: pain, quality of life, functional status, patient preferences for health states, adverse events, caregiver burden, survival, and costs of care. Economic evaluations typically consider survival, cost, global health state preferences (utilities), and productivity impact to be meaningful endpoints. Most experts in economic evaluation consider quality-adjusted life years (QALYs) as the preferable measure of outcomes for CEA studies. In clinical trials, it is generally possible to find a parsimonious set of outcomes that can be used to address both comparative and cost-effectiveness objectives. In general, both CER and CEA favor studies that are closer to “real” practice (as opposed to the carefully controlled setting of a clinical trial). This can be a problem for certain interventions, such as new surgical procedures, that require a degree of operator skill to perform well. In such situations, emerging treatments that are suitable for CER/CEA studies may require a period of operator credentialing prior to evaluation. Clinical trials ideally would seek to identify comparatively effective interventions that are also cost-effective.
14. Statistical issues in clinical trial design
We must entertain statistical issues arising in phase II studies that would subsequently lead to phase III trials in 2 groups of patients: those at high risk who need treatments that will lead to improved survival along with acceptable morbidity, and low-risk patients who might do well with less toxic therapy. For low-risk patients, the outcome is generally favorable and few progression or death events will be expected, making sample sizes large. Also, for these patients a noninferiority philosophy, if not design, will need to be considered. Several issues will arise.
Constrained populations.
There is evidence that the population of patients available for studies in head and neck cancer is somewhat constrained and therefore studies should be designed wisely. We need to plan trials carefully with respect to target differences and accrual rates. Expanding the available population by increasing percentage of patients accrued to trials may be a useful strategy. If the study design is easy to explain, if the calendar of required visits is patient-friendly, and if the outcome is important to patients, they will be most likely to consider enrollment. Having a systematic strategy for soliciting patients for protocol enrollment and having a full menu of trials available will also facilitate accrual.
Comorbidity and competing risks.
Rose et al113 recently showed that the presence of competing mortality can transform an otherwise positive study into a negative one due to deaths from competing causes. A tempting remedy is to exclude patients at high risk for deaths from other causes, although this reduces the study’s generalizability and limits accrual. Using statistical methods that account explicitly for the competing risks is possible.114 To do this, having good information about deaths from other causes in reports of head and neck cancer trials will be extremely useful. Another potential strategy is to use disease-specific survival as an outcome. This may be appropriate, but if the classification of causes of death could differ based on treatment, then results would be subject to ascertainment bias. The interpretation of a disease-specific survival outcome requires careful consideration of the necessary observed impact to make a conclusion about overall benefit.
Noninferiority trials.
For patients with low-risk cancer, we are interested in determining whether less toxic interventions can be used without adversely affecting disease control. This question calls for a noninferiority approach. A noninferiority trial tests whether a new treatment is not unacceptably inferior to standard therapy. The maximum amount by which the new intervention can be less effective than the standard is called the “noninferiority margin,” and must be specified in advance. The claim of “no statistically significant difference” from a trial designed to demonstrate superiority should not be considered evidence of noninferiority.115 Traditionally designed noninferiority trials require very large sample sizes. With constrained populations, such large trials are unlikely to represent an efficient allocation of subjects.
Even more than for superiority studies, the interpretation of noninferiority trials depends on good compliance with the protocol’s treatment and follow-up parameters. Missing data and nonadherence to study parameters dilute the treatment effect and make the arms appear more similar. Therefore, a per-protocol-treatment analysis may be more important in a noninferiority trial.116 Postrandomization events still have the potential to introduce bias, so the intent-to-treat population cannot be ignored completely. Ideally, extra safeguards ensure that protocol compliance is good. Analyses of both populations should be considered, with determinations a priori of how discordant data from the 2 populations would be interpreted.
It may be the case that a new treatment, in addition to being less toxic, can be expected to confer a modest benefit. Freidlin et al117 have proposed a hybrid design that might be useful in this circumstance.
Composite endpoints.
A composite endpoint is an aggregate of multiple single endpoints.118 For instance, in head and neck cancer, laryngectomy-free survival has been used. Composite endpoints can encompass efficacy or safety endpoints, or be combinations of both. Such endpoints are appealing because they can increase the number of events, thereby increasing precision and reducing sample size requirements. Use of composite endpoints has been sanctioned by regulatory agencies that generally view them as justified if the individual components are clinically meaningful.
If the individual components do not have similar importance, the distribution of outcomes may make it difficult to interpret the results. It is possible for treatment to alter the rate of occurrence of a single component of a composite endpoint without affecting its most important component. Competing risks between components can introduce bias. For example, if a composite endpoint is defined by nonfatal events without including death as a component, the risk of events may drop, whereas deaths increase. If the composite endpoint includes components that are insensitive to treatment, variability of the endpoint’s measurement will increase, reducing the study’s power.119
If composite endpoints are used, the components should have similar importance. They should be valid, biologically plausible, of importance to patients, balanced with respect to each other, and with respect to the treatments being studied. Results for each component of the outcome should be reported. Rules for censoring the endpoint for all possible combinations of outcomes should be described. There should be an explicit hierarchy of planned analyses. If the endpoint components are not completely objective and the trial is not blinded, it is wise to blind evaluation of the endpoints.
Patient-reported outcomes.
The most critical statistical issue is that of missing data. Unlike other types of missing data, a PRO can seldom, if ever, be considered missing at random, since adverse outcomes are likely to be highly correlated to “missingness.” Therefore, a key assumption for applying standard statistical methods is violated. Every effort must be made to obtain PRO assessments on all patients. In the absence of an assessment, good information about the reason the assessment was missing can be helpful. Another problem is related to our ability to capture PROs without bias on open-label studies.
The position of the QOL endpoint in the hierarchy of objectives should be clear. Although PRO collection may not be necessary for all patients in a study with another primary endpoint, it can be difficult to achieve good compliance if the QOL component is introduced midway through the study (or for only a fraction of patients), since it becomes difficult to keep track of which subjects are required to do the PRO measures. It is easier to start the PRO at the beginning of the study and discontinue midway through, if all participants are not required to participate in the QOL study.
If the symptom profile is expected to differ between arms, it may be more sensible to assess global quality of life. One method of doing so is by using a Q-TWiST analysis, which divides survival time into 3 states: on-treatment with toxicity, time without treatment or toxicity, and relapse. If relative value units are assigned to the health states, a quantitative comparison can be done, but even without these units a graphical depiction of these health states can be convincing.120
BREAKOUT SESSIONS
In keeping with the goals of the meeting, a series of smaller working groups were convened to address the specifics of clinical trial design. The recommendations from these breakout sessions are summarized.
1. Phase II trials: objectives, eligibility, and endpoints
There was consensus that the cooperative groups would proceed with the development of 2 separate protocol concepts. ECOG would be revising its E3311 phase II concept exploring transoral resection in HPV-initiated patients. RTOG would develop a phase II concept investigating transoral resection in HPV-unrelated patients. There was agreement in principle that both studies would be opened in both cooperative groups.
There was strong support for using a randomized phase II design. This would allow therapeutic questions to be addressed and identify a surgical comparative group to carry forward into a phase III randomized comparison with conventional concurrent chemoradiotherapy.
The exact design of these phase II trials was extensively discussed and will be finalized in the near future.
Eligibility will need to be carefully defined. Will studies be restricted to T1/T2 primary tumors of tonsil and lateralized base of tongue or will lateralized exophytic T3 tumors of the tonsil and or tongue base also be included?
It is not clear how best to include the “intermediate risk” HPV-initiated patients with a significant (≥10 pack year) smoking history, since they may have a more threatening disease.
Analysis will be based on intent to treat for all enrolled patients.
Early stopping rule(s) will must be incorporated in case toxicity or oncologic results are not as expected.
A biorepository will be developed for specimens and correlative studies, with logistics and operational aspects coordinated through existing NCI-funded infrastructure of each cooperative group sponsoring the trial (ECOG or RTOG).
Common acceptable definitions for negative, close, and positive surgical margins must be developed.
Common acceptable definitions for “high-risk” ECE/soft tissue metastasis must be developed.
Surgeon credentialing criteria must be established by each respective trial principal investigator and active participating surgeons, and will address hospital privileging, minimum number of cases, and interim quality assurance measures and mechanisms. They should be the same for each trial.
It will be necessary to poll participating institutions regarding their distribution of patients to determine if there are adequate subjects available to support a trial for patients with HPV-unrelated cancers.
The data elements to be captured as a basis for comparative effectiveness analysis need to be defined.
Guidelines will need to be incorporated into the therapeutic algorithm for the appropriate management of the contralateral neck with surgery, radiation, or both.
2. Imaging
A trial of transoral surgery for the management of oropharynx cancer presents an exciting opportunity to perform correlative research using both conventional anatomic and functional metabolic imaging modalities. First and foremost would be the opportunity for clinical–pathologic correlations between pretreatment imaging and histopathologic findings at resection. This information will improve the interpretation of pretreatment studies, to identify before treatment those patients with high-risk disease (eg, positive margins, perineural invasion, multiple nodal involvement, or extracapsular spread). Such data will prove valuable in decision making and in determining eligibility for future studies. Improving the pretreatment interpretation of neck involvement may inform decisions regarding the extent of neck dissection and subsequent adjuvant treatment. Capturing data from multiple imaging modalities such as contrast-enhanced CT scan (CECT), MRI, FDG PET/CT, and possibly ultrasound would permit intermodality comparisons and better define their individual roles in treatment planning and follow-up.
This trial will also provide opportunities to correlate functional imaging characteristics with tissue-derived tumor biomarkers. Functional imaging studies such as dynamic contrast-enhanced (DCE) and/or diffusion-weighted (DW)-MRI, SPECT, and PET with non-FDG tracers may be performed at a subset of participating centers with available imaging capabilities.
Any trial-associated imaging research will be influenced by the final study design and center by center availability of imaging resources. Key considerations include the following recommendations:
All study patients have pretreatment imaging studies performed in accord with trial-standardized imaging protocols. When possible, these would include CECT, MRI, and FDG PET/CT. Recognition must be given to the ability of participating centers to perform these studies and development of imaging requirements must take this into account. A survey of centers likely to be major trial participants (with respect to their ability to perform these studies) could provide some estimate of feasibility in this regard.
It will be desirable to create a common imaging repository for purposes of central review and subsequent image data analysis. The American College of Radiology Imaging Network (ACRIN) would be an ideal resource to facilitate this.
The central review of imaging by trial associated imaging specialists was deemed critical, given the known interobserver variability interpreting head and neck studies. The central review of images would be a vital component in subsequent analysis but can also play a role in a process of real-time review if deemed appropriate within the trial design. At least 1 surgeon and 1 radiation oncologist trial investigator should also participate in the imaging review.
Direct correlation of anatomic/functional imaging with prospectively collected, centrally banked whole tumor specimens will be a remarkable research opportunity. Pretreatment imaging studies must be annotated in conjunction with predefined clinical, surgical, and pathologic elements. These should include features of the physical exam.
Investigators with imaging expertise and interest in advancing imaging research for oropharyngeal cancer should be identified and engaged in the trial design. These individuals would contribute to the development of trial specific imaging and pathology standards. Participation from specialists in functional imaging will be of particular value.
3. Functional and quality of life (QOL) endpoints
Consensus emerged about the need to pursue the following categories of endpoints: (1) overall (global) QOL; (2) head and neck–specific QOL; (3) caregiver burden; and (4) symptom burden. Data should also be collected about societal/health system costs, patient utilities, and work productivity losses.
Overall (global) QOL. There was strong agreement that this construct is important. The group felt that a single question would be adequate, especially because patient utilities will also be pursued. The group felt that no generic QOL scale would be required if the disease-specific scale includes a single question about overall QOL (which they generally do).
Head and neck–specific QOL. The core symptoms and QOL domains recommended by the NCI Head and Neck working group (to be published separately) should be incorporated. Regarding QOL, 3 instruments, which are among the most widely used head and neck scales worldwide, received the most attention. These included the EORTC (including both QLQ and H&N35 modules), the UW-QOL scale, and the FACT (including both FACT-G and FACT-HN modules). The final instrument selection should be based primarily on the hypothesis of the study.
Caregiver burden. The patient advocates and health economists pointed out the importance of measuring this endpoint. The participants strongly endorsed this suggestion, and there are a number of validated scales that are caregiver-completed, and which are minimally burdensome.
- Symptom burden. There was agreement that some specific symptoms need to be assessed, and that complementary measures from different perspectives (patient-reported, observer-rated, and physiological tests) should be sought, if possible. The specific instruments will depend on the head and neck scale that is chosen, but the following discussion points were highlighted:
- Swallowing measurements will be critical. This will be relevant to both surgical and nonsurgical patients. Swallowing should be assessed with a combination of a patient-reported outcomes (eg, MDADI), an observer-rated assessment (eg, PSS-HN), and objective assessment (composite oral/pharyngeal score from a modified barium swallow, if possible). Alternative PROs were considered such as the SWAL-QOL but it may be too long for clinical use. Alternative observer-rated scales were also considered such as the Functional Outcome of Swallowing Scale (FOSS), but these may be less discriminating than the PSS-HN. Obtaining modified barium swallows may require additional grant funding because the studies may not be justified on clinical grounds.
- Shoulder function will also be important to measure if surgical treatment includes a neck dissection. If the UW-QOL (which includes this issue) is not used, the group agreed that the NDII or the DASH would be important measures to consider.
- Speech, skin toxicity, and fibrosis warrant attention as well.
4. Correlative science/tissue banking
The development of a surgical trial for oropharynx cancer was viewed as an unprecedented opportunity to obtain substantial clinical material for correlative scientific studies that would enrich the information gained from the outcomes of the clinical trial itself. Adequate tumor specimens from surgical resections will allow for not only the usual banking of tissue and blood by the cooperative trial mechanisms that already exist, but additional correlative studies could be carried out that are now impractical or impossible due to the limited material available from pretreatment biopsies, and the limitation that nearly all of that tissue is formalin-fixed, paraffin-embedded (FFPE).
Additional studies could include creation of new HPV-initiated cell lines from primary tumors that are representative of the highly responsive tumors that make up the majority of HPV-initiated OPSCC. Other studies that can be carried out on larger samples of fresh tumors include: characterization of lymphocyte populations in the tumor; fresh tumor xenografts directly from the patient; and isolation and analysis of cancer stem cells. Fresh specimens also permit isolation of high-quality RNA either by immediate extraction or extraction from flash-frozen tumor tissue. Fresh tumor has potential to provide information with respect to active signaling pathways.
The group agreed to a set of principles that include the following:
The correlative studies will provide opportunities to identify biomarkers that explain anomalies in response to therapy in different patients. Widespread cooperative studies should lead to biomarkers that will predict the best treatment for each individual based on the biology of the patient’s tumor. The availability of biomarkers that predict response can identify comparatively effective therapies that are also cost-effective, thereby leading to an increase in overall cost-effectiveness. These advances should improve therapeutic decision making, improve QOL by minimizing morbidity of treatment, and improve disease-specific survival and overall cure rates. Material must be preserved for future analyses.
It would be most cost-effective to make use of the experienced central cooperative group tissue repositories (such as the RTOG and ECOG pathology repositories) that have extensive experience receiving, storing, and distributing tumor and blood biospecimens.
Specimens must be linked to a robust relational database that includes detailed patient histories, pathology, adherence to trial protocols, the physical examination, detailed pathologic assessment using common pathology reporting elements, and complete radiologic assessment. Such information and powerful software for retrieval and analysis of such variables were considered to be critical to characterizing biomarkers.
An opportunity to partner with The Cancer Genome Atlas (TCGA) should not be missed. It is clear from the interim reports from TCGA that oropharyngeal cancers are underrepresented among the specimens submitted. This is directly related to the absence of fresh surgical material of adequate size for study.
Funding for biomarker studies that are not integral to the trial design is difficult to obtain. The BISQFP (Biomarkers, Imaging, Quality of Life, Cost Effectiveness, Analyses Funding Program) represent a potential source for competitive funding related to clinical trials.
5. Comparative effectiveness
Several elements of comparative effectiveness research (CER) are important in planning studies of transoral resection of pharynx cancer. First, CER emphasizes “effectiveness.” An intervention is “effective” when it provides benefit to patients in the context of routine practice. Although randomized clinical trials are considered the gold standard method by which to determine whether interventions work, the results often have limited relevance beyond the setting and subpopulation in which the study is conducted, limiting their use for patients and clinicians practicing in routine clinical settings. Limited applicability is of particular concern for trials of new or advanced techniques such as transoral resection or intensity-modulated radiotherapy for head and neck cancer, which may be undertaken by experienced clinicians at expert centers. Second, CER emphasizes “real outcomes” that are meaningful for patients, including length of life, quality of life, major clinical outcomes, and cost. Third, CER emphasizes “stakeholder engagement.” Patients, clinicians, vendors, payers (CMS and private payers), and federal agencies such as the National Cancer Institute, Patient-Centered Outcomes Research Institute (PCORI), or the Agency for Health Care Research and Quality (AHRQ) comprise stakeholder groups. Stakeholder engagement promotes the design and conduct of clinical trials that are responsive to the needs of patients, clinicians, payers, and policy makers.
There are three recommendations:
A stakeholder group should be established to advise the head and neck clinical trial research process. Best practice recommendations for developing stakeholder groups include early involvement in trial design, regular engagement in person and remotely via teleconference, and appropriate enfranchisement of important constituencies (like vendors and payers) that in the past did not participate in clinical trial design or conduct.
A prospective registry should be developed to capture patient outcomes in “real world” clinical practice. Patient registries are examples of prospective observational studies that have several beneficial attributes compared with randomized designs. Large, geographically diverse cohorts allow for the study of interventions across subgroups of patients defined by disease severity, comorbidity, or provider settings. Registry studies may also allow longer follow-up periods at lower cost than more conventional clinical trials, which is particularly important to examine late toxicities and possible second malignancies among patients with HPV-initiated cancer. Patient registries are increasingly feasible (given funding for their support and greater availability of electronic medical records and claims databases).
The surgical learning curve for transoral resection should be examined. The surgical learning curve may be steep, protracted or somewhere in between. In the experimental phase II and phase III settings, it is likely that surgery will be conducted by highly skilled practitioners. However, it may be possible (in the context of a phase II clinical trial with quality assurance) to conduct meaningful teaching, evaluation, and credentialing of individual surgeons, to promote adoption of the procedure by a larger group of providers. Such a program would develop the capabilities of additional specialists for a phase III study. Nonetheless, because expert surgeons generally participate in early trials of new technology, it will be challenging to assess the impact of the learning curve in a trial setting. The surgical learning curve may be more productively investigated as part of a prospective registry. Furthermore, transoral resection, whether robot-assisted or done with the laser, will likely be adopted in routine clinical care in advance of evidence supporting learning techniques, expertise thresholds, and appropriate clinical use. A registry will provide evidence to demonstrate whether and to what extent outcomes of transoral surgery are different in phase II/III settings versus real-world settings.
6. Cost-effectiveness
Four topics of interest were addressed:
Cost. The discussion regarding costs in these clinical trials focused on what costs to collect. Considerations such as caregiver cost and time missing from work will be important in these trials, so the group felt a societal perspective (as below) should be adopted in any analysis. Direct and indirect medical costs should be collected as well as those from time lost from work and out-of-pocket expenses should be included. These costs will include those of dental care since these can be significant and are not normally captured by insurance companies. One of the potentially more intensive endeavors will be to collect lifetime costs appropriately discounted, since some of these costs will occur years after the patient would have experienced any type of recurrence yet potentially could add significant expenses. It would be worthwhile to conduct a focus group of cancer survivors to determine what costs they incur out-of-pocket.
Perspective. A societal perspective was recommended for this analysis to incorporate costs such as time lost from work and care giver expenses.
Type of analyses. A cost–utility analysis would be the preferred type of economic analysis to incorporate a quality factor into survival. The trial would need to use an instrument to measure patient utility with either the EQ-5D (EuroQol) or Health Utilities Index III (HUI III) being the favored instruments. The EQ-5D has many translations and is free, whereas the HUI III has the benefit of incorporating a speech domain in addition to generating a utility value.
Modeling. A novel trial design approach would be to include modeling. The outcome of the trial could be modeled and an analysis performed to determine which costs would be the most important in determining the outcome and therefore the most important costs to collect.
7. Surgical quality assurance
Definition of transoral resection. Transoral resection will include any modality deemed appropriate by the surgeon to remove oropharyngeal squamous cell carcinoma completely with a goal of obtaining sound oncologic margins. Transoral resection will predominantly consist of transoral robotic surgery (TORS) or laser microsurgery (TLMS). The techniques might include use of electrocautery, carbon dioxide laser, thallium-YAG laser, or other tissue-ablation techniques. The technique may include en bloc or “piecemeal” removal of tissue, but the goal must be complete margin-clearing resection of the tumor.
Primary site tumor characteristics. Eligible patients will include those with T1–T3 (select T3 deemed completely resectable transorally by surgeon), previously untreated, tonsil or base of tongue, histopathologically confirmed squamous cell carcinomas. HPV analysis will be required.
Margin analysis. Margin analysis will include use of frozen section pathology to guide resection. Margins will be taken if surgically feasible until they are tumor-free circumferentially around the tumor. Ultimately, the final pathology report will determine whether a complete “negative margin” resection was achieved; margins are either “clear” (negative) or “involved” (positive) as evidenced by the final histopathology report. “Close” margins can be recorded, but will not influence the “risk” status of the tumor and subsequent treatment. Dysplasia at the edge should not be considered a positive margin, but carcinoma in situ at the edge will be considered a positive margin.
-
Neck dissection. Selective neck dissection will include levels II–IV (including IIB) on the ipsilateral side of the neck. In a selective node dissection level I may be removed, but this is not mandatory. The retropharyngeal nodes will be resected either during transcervical neck dissection or through the transoral resection if imaging or clinical exam suggests metastasis. In the absence of clinical or radiographic evidence of metastasis, the retropharyngeal lymph nodes will not generally be removed.
The contralateral clinically N0 neck at risk of metastasis (base of tongue primary or tonsillar primary with substantial palate involvement) may be addressed surgically with a level II–IV neck dissection or prophylactic radiotherapy.
Reconstruction. Patient with an oropharyngeal primary site resection requiring major (pedicle flap or free flap) reconstruction of the primary site defect will be excluded from the study.
Center/surgeon inclusion criteria. Adequate experience with transoral surgery will be considered necessary for surgeons entering patients in the trial. This will be determined by review of adequate training. What would constitute adequate training was hotly debated. Possible criteria include: (1) Minimum number of transoral resections (perhaps 20) as primary surgeon. (2) Performance of at least 5 transoral resections of oropharyngeal squamous cell cancer in the past 12 months. (3) Review of submitted video of transoral surgery on patient may be helpful.
Contribution of single center. No single individual or center will be allowed to submit more than 25% of the patients into the trial.
Ongoing surgical quality assurance. Cases will be assessed during study period by surgical review panel for complications deemed uncommon or dire, and for departure from commonly accepted tenets/techniques of transoral surgery.
Incompletely resolved questions. (1) Definition and implications of “close margins.” (2) Necessity of standardization of resection technique. (3) Extent of neck dissection (ipsilateral/contralateral). (4) Minimal criteria to establish transoral surgical competency. (5) Pre-/postoperative swallowing and physiotherapy standardization. (6) In-patient hospitalization/care.
8. Radiation oncology considerations
HPV-unrelated disease.
The overall consensus was that the poor prognosis for this group of patients, when treated with curative intent using concurrent chemoradiation, mandates a trial using transoral surgical resection as a component of an overall therapeutic intensification strategy. The schema for such trial(s) would be resection of the primary site (± neck dissection) followed by postoperative irradiation with doses and treatment volumes being similar to those used in the published RTOG and EORTC postop trials. Two or 3 cycles of concurrent cisplatin would be the most likely candidates for chemotherapy, although a taxane-based regimen should also be considered based on preliminary data of its efficacy in a postoperative setting in RTOG 0234.
HPV-initiated disease.
The final recommendation was to adopt a risk-based strategy for a phase II trial. The theme would be to incorporate transoral resection into an overall program designed to diminish treatment-induced toxicity without sacrificing therapeutic efficacy. Concern was expressed that the initiation of such a trial might compete with RTOG 1016 for enrollment. It was pointed out, however, that RTOG 1016 was already open and rapidly accruing patients. It would probably be close to the attainment of its enrollment target by the time that the risk-based phase II trial under discussion would be ready to open. Considered more likely was that interim findings from RTOG 1016 might influence some of the aspects of the final phase II risk-based trial design.
Most HPV-initiated oropharynx cancers originate in the tonsil, and overall comfort was expressed with allowing eligibility for T1–T3 presentations including scenarios where the primary disease extended into the glossopharyngeal sulcus and lateral aspect of the base of tongue. Lesions extending close (not precisely defined) to the midline, deeply infiltrative lesions originating in the base of tongue (≥T2), and T4 lesions originating in any oropharynx subsite were not believed to be appropriate for enrollment onto this trial because the likelihood that significant functional morbidity arising from the surgery itself would contravene the overall philosophy of reducing treatment-induced functional morbidity.
The group envisioned 3 risk categories after transoral resection. The low-risk group would be those patients in whom negative margins had been attained at the primary site without evidence of perineural invasion and who had pathologic N0–N1 necks with no evidence of gross extracapsular extension (ECE). These patients would not receive any additional adjuvant therapy and would undergo observation only. The high-risk patients would be those with positive margins, gross extracapsular nodal disease, and/or ≥2 positive lymph nodes with microscopic or macroscopic ECE. These patients would be assigned to receive postoperative concurrent chemoradiation with doses equivalent to those prescribed in the published RTOG and EORTC postoperative adjuvant trials. Treatment volumes would be defined based on the surgical pathologic findings.
The remaining patients would be classified as intermediate risk. Pathologic features underpinning this classification might include close surgical margins (to be defined) perineural invasion at the primary site, and/or ≥2 positive lymph nodes without ECE or with focal/microscopic ECE. The overall theme for the treatment of the intermediate risk patients would be to generate data supporting the use of this treatment scheme as the experimental arm of a future phase III trial that would randomize patients with HPV-initiated cancers between transoral surgery/postoperative radiation and a control arm of concurrent chemoradiation.
Intermediate risk patients on the envisioned phase II trial might be assigned to receive radiation therapy alone without any systemic chemotherapy or biologic therapy. Consensus was not reached on exact treatment volumes. There was recognition that the pathologic status of the neck (pN0 vs pN+) would guide this matter to a considerable extent. The latter may include 2 dose levels, 1 for the high-risk region where gross disease had been present prior to resection, and another dose level targeting elective regions. However, unanimous strong interest was expressed conceptually for the delivery of a reduced total dose of radiation to these volumes. One idea advanced was to only treat a single target volume to 50–54 Gy (with stopping rules in place should a high locoregional recurrence rate be observed).
A final issue that received attention concerned the need to avoid bilateral neck dissection in patients where it was apparent that radiotherapy would be needed regardless of the result of the dissection (eg, primary approaching the midline or N2b disease). Thus, elective irradiation of the clinically uninvolved neck would have a high probability of disease eradication in such patients rather than requiring adjuvant radiotherapy to both necks following bilateral neck dissection if adverse disease is detected pathologically.
MEETING SUMMARY
The meeting closed with consensus that further investigation of transoral surgery for oropharynx cancer was a priority for the cooperative groups. Given the novelty of testing and integrating new surgical techniques in multimodality clinical trials, several phase II trials will initially be considered. One of these trials will be designed to explore the potential of transoral surgery to reduce the aggregate toxicity in patients with good prognosis HPV-initiated disease, whereas another will look at the possibility that transoral surgery can magnify the locoregional control benefit in poor prognosis HPV-unrelated cancers.
Similar endpoints will be used in both trials including conventional survival outcomes, but also exploring function, quality of life, toxicity, and cost. Surgical credentialing will be carefully outlined, and tissue banking will be integral. Although the difficulties of randomizing patients between surgical and nonsurgical treatment were acknowledged, there was agreement, among surgeons and nonsurgeons alike, that this work should ultimately lead to the development and conduct of phase III trials of this approach.
REFERENCES
- 1.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
- 2.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008;26:3582–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol 2007;25:4096–4103. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, DC. Head Neck 2009;31:1393–1422. [DOI] [PubMed] [Google Scholar]
- 6.Grant DG, Salassa JR, Hinni ML, Pearson BW, Perry WC. Carcinoma of the tongue base treated by transoral laser microsurgery, part one: untreated tumors, a prospective analysis of oncologic and functional outcomes. Laryngoscope 2006;116:2150–2155. [DOI] [PubMed] [Google Scholar]
- 7.Rich JT, Milov S, Lewis JS Jr, Thorstad WL, Adkins D, Haughey BH. Transoral laser microsurgery (TLM) ± adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope 2009;119:1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck 2011;33:1683–1694. [DOI] [PubMed] [Google Scholar]
- 9.O’Malley BW Jr, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 2006;116:1465–1472. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein GS, O’Malley BW Jr, Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg 2007;133:1220–1226. [DOI] [PubMed] [Google Scholar]
- 11.Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck 2009;31:283–289. [DOI] [PubMed] [Google Scholar]
- 12.Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope 2009;119:2156–2164. [DOI] [PubMed] [Google Scholar]
- 13.Iseli TA, Kulbersh BD, Iseli CE, Carroll WR, Rosenthal EL, Magnuson JS. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg 2009;141:166–171. [DOI] [PubMed] [Google Scholar]
- 14.Salassa JR, Hinni ML, Grant DG, Hayden RE. Postoperative bleeding in transoral laser microsurgery for upper aerodigestive tract tumors. Otolaryngol Head Neck Surg 2008;139:453–459. [DOI] [PubMed] [Google Scholar]
- 15.Holsinger FC, McWhorter AJ, Menard M, Garcia D, Laccourreye O. Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region: I. Technique, complications, and functional results. Arch Otolaryngol Head Neck Surg 2005;131:583–591. [DOI] [PubMed] [Google Scholar]
- 16.White HN, Moore EJ, Rosenthal EL, et al. Transoral robotic-assisted surgery for head and neck squamous cell carcinoma: one- and 2-year survival analysis. Arch Otolaryngol Head Neck Surg 2010;136:1248–1252. [DOI] [PubMed] [Google Scholar]
- 17.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006;368:843–854. [DOI] [PubMed] [Google Scholar]
- 18.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–28. [DOI] [PubMed] [Google Scholar]
- 19.Dobrowsky W, Naude J. Continuous hyperfractionated accelerated radiotherapy with/without mitomycin C in head and neck cancers. Radiother Oncol 2000;57:119–124. [DOI] [PubMed] [Google Scholar]
- 20.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 1998;338:1798–1804. [DOI] [PubMed] [Google Scholar]
- 21.Wendt TG, Grabenbauer GG, Rodel CM, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol 1998;16:1318–1324. [DOI] [PubMed] [Google Scholar]
- 22.Denis F, Garaud P, Bardet E, et al. Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004;22:69–76. [DOI] [PubMed] [Google Scholar]
- 23.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007;357:1705–1715. [DOI] [PubMed] [Google Scholar]
- 24.Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 2005;23:8636–8645. [DOI] [PubMed] [Google Scholar]
- 25.Holsinger FC. Swing of the pendulum: optimizing functional outcomes in larynx cancer. Curr Oncol Rep 2008;10:170–175. [DOI] [PubMed] [Google Scholar]
- 26.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med 1991;324:1685–1690. [DOI] [PubMed] [Google Scholar]
- 27.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091–2098. [DOI] [PubMed] [Google Scholar]
- 28.Cadiere GB, Himpens J, Germay O, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg 2001;25:1467–1477. [DOI] [PubMed] [Google Scholar]
- 29.Desai SC, Sung CK, Jang DW, Genden EM. Transoral robotic surgery using a carbon dioxide flexible laser for tumors of the upper aerodigestive tract. Laryngoscope 2008;118:2187–2189. [DOI] [PubMed] [Google Scholar]
- 30.Solares CA, Strome M. Transoral robot-assisted CO2 laser supraglottic laryngectomy: experimental and clinical data. Laryngoscope 2007;117:817–820. [DOI] [PubMed] [Google Scholar]
- 31.McLeod IK, Melder PC. Da Vinci robot-assisted excision of a vallecular cyst: a case report. Ear Nose Throat J 2005;84:170–172. [PubMed] [Google Scholar]
- 32.Hockstein NG, Nolan JP, O’Malley BW Jr, Woo YJ. Robotic microlaryngeal surgery: a technical feasibility study using the daVinci surgical robot and an airway mannequin. Laryngoscope 2005;115:780–785. [DOI] [PubMed] [Google Scholar]
- 33.Hockstein NG, Nolan JP, O’Malley BW Jr, Woo YJ. Robot-assisted pharyngeal and laryngeal microsurgery: results of robotic cadaver dissections. Laryngoscope 2005;115:1003–1008. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein GS, Magnuson JS, Moore EJ, et al. Transoral robotic surgery: interim analysis from a multicenter trial. Las Vegas, NV: American Head and Neck Society; 2010. Abstract #S002. [Google Scholar]
- 35.Garden AS, Kies MS, Weber RS. To TORS or not to TORS: but is that the question? Arch Otolaryngol Head Neck Surg 2010;136:1085–1087. [DOI] [PubMed] [Google Scholar]
- 36.Ridge JA. We show pictures, they show curves. Arch Otolaryngol Head Neck Surg 2010;136:1170–1175. [DOI] [PubMed] [Google Scholar]
- 37.Steiner W, Fierek O, Ambrosch P, Hommerich CP, Kron M. Transoral laser microsurgery for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg 2003;129:36–43. [DOI] [PubMed] [Google Scholar]
- 38.Lewis JS Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol 2011;24:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha P, Lewis JS Jr, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer 2012;118:3519–3530. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher GH. Elective irradiation of subclinical disease in cancers of the head and neck. Cancer 1972;29:1450–1454. [DOI] [PubMed] [Google Scholar]
- 41.Sanguineti G, Califano J, Stafford E, et al. Defining the risk of involvement for each neck nodal level in patients with early T-stage node-positive oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2009;74:1356–1364. [DOI] [PubMed] [Google Scholar]
- 42.Coskun HH, Ferlito A, Medina JE, et al. Retropharyngeal lymph node metastases in head and neck malignancies. Head Neck 2011. ;33:1520–1529. [DOI] [PubMed] [Google Scholar]
- 43.Dirix P, Nuyts S, Bussels B, Hermans R, Van den Bogaert W. Prognostic influence of retropharyngeal lymph node metastasis in squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys 2006;65:739–744. [DOI] [PubMed] [Google Scholar]
- 44.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys 2004;59:28–42. [DOI] [PubMed] [Google Scholar]
- 45.Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00–22). Int J Radiat Oncol Biol Phys 2010;76:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 2004;60:1425–1439. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz DL, Hutcheson K, Barringer D, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Sullivan B, Warde P, Grice B, et al. The benefits and pitfalls of ipsilateral radiotherapy in carcinoma of the tonsillar region. Int J Radiat Oncol Biol Phys 2001;51:332–343. [DOI] [PubMed] [Google Scholar]
- 49.Porceddu SV, Pryor DI, Burmeister E, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck 2011;33:1675–1682. [DOI] [PubMed] [Google Scholar]
- 50.Morris L, Goenka A, Rao S, et al. Long-term regional control in the observed neck following definitive chemoradiation for node-positive oropharyngeal squamous cell cancer. Int J Radiat Oncol Biol Phys 2011;81 (Suppl 2):S16 (abstract). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murer K, Huber GF, Haile SR, Stoeckli SJ. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the N0 neck in patients with oral squamous cell carcinoma. Head Neck 2011;33:1260–1264. [DOI] [PubMed] [Google Scholar]
- 52.Bentzen SM, Saunders MI, Dische S, Bond SJ. Radiotherapy-related early morbidity in head and neck cancer: quantitative clinical radiobiology as deduced from the CHART trial. Radiother Oncol 2001;60:123–135. [DOI] [PubMed] [Google Scholar]
- 53.Lindberg R Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972;29:1446–1449. [DOI] [PubMed] [Google Scholar]
- 54.Mukherji SK, Armao D, Joshi VM. Cervical nodal metastases in squamous cell carcinoma of the head and neck: what to expect. Head Neck 2001;23:995–1005. [DOI] [PubMed] [Google Scholar]
- 55.Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg 1988;10:160–167. [DOI] [PubMed] [Google Scholar]
- 56.Bussels B, Hermans R, Reijnders A, Dirix P, Nuyts S, Van den Bogaert W. Retropharyngeal nodes in squamous cell carcinoma of oropharynx: incidence, localization, and implications for target volume. Int J Radiat Oncol Biol Phys 2006;65:733–738. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu K, Inoue H, Saitoh M, et al. Distribution and impact of lymph node metastases in oropharyngeal cancer. Acta Otolaryngol 2006;126:872–877. [DOI] [PubMed] [Google Scholar]
- 58.Coskun HH, Ferlito A, Medina JE, et al. Retropharyngeal lymph node metastases in head and neck malignancies. Head Neck 2011;33:1520–1529. [DOI] [PubMed] [Google Scholar]
- 59.Vikram B, Strong EW, Shah JP, Spiro R. Failure in the neck following multimodality treatment for advanced head and neck cancer. Head Neck Surg 1984;6:724–729. [DOI] [PubMed] [Google Scholar]
- 60.Amdur RJ, Parsons JT, Mendenhall WM, et al. Postoperative irradiation for squamous cell carcinoma of the head and neck: an analysis of treatment results and complications. Int J Radiat Oncol Biol Phys 1989;16:25–36. [DOI] [PubMed] [Google Scholar]
- 61.Richard JM, Sancho-Garnier H, Micheau C, et al. Prognostic factors in cervical lymph node metastasis in upper respiratory and digestive tract carcinomas: study of 1,713 cases during a 15-year period. Laryngoscope 1987;97:97–101. [DOI] [PubMed] [Google Scholar]
- 62.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–1944. [DOI] [PubMed] [Google Scholar]
- 63.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–1952. [DOI] [PubMed] [Google Scholar]
- 64.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005;27:843–850. [DOI] [PubMed] [Google Scholar]
- 65.Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys 1993;26:3–11. [DOI] [PubMed] [Google Scholar]
- 66.Genden EM, Kotz T, Tong CC, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope 2011;121:1668–1674. [DOI] [PubMed] [Google Scholar]
- 67.Hurtuk AM, Marcinow A, Agrawal A, et al. Quality-of-life outcomes in transoral robotic surgery. Otolaryngol Head Neck Surg 2012;146:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leonhardt FD, Quon H, Abrahao M, et al. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck 2012;34:146–154. [DOI] [PubMed] [Google Scholar]
- 69.Sinclair CF, McColloch NL, Carroll WR, et al. Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2011;137:1112–1116. [DOI] [PubMed] [Google Scholar]
- 70.Rich JT, Liu J, Haughey BH. Swallowing function after transoral laser microsurgery (TLM) ± adjuvant therapy for advanced-stage oropharyngeal cancer. Laryngoscope 2011;121:2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol 2010;28:5294–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.List MA, Siston A, Haraf D, et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol 1999;17:1020–1028. [DOI] [PubMed] [Google Scholar]
- 73.Maguire PD, Papagikos M, Hamann S, et al. Phase II trial of hyperfractionated intensity-modulated radiation therapy and concurrent weekly cisplatin for Stage III and IVa head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011;79:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bjordal K, Ahlner-Elmqvist M, Hammerlid E, et al. A prospective study of quality of life in head and neck cancer patients. Part II: longitudinal data. Laryngoscope 2001;111:1440–1452. [DOI] [PubMed] [Google Scholar]
- 75.Ringash J, Lockwood G, O’Sullivan B, et al. Hyperfractionated, accelerated radiotherapy for locally advanced head and neck cancer: quality of life in a prospective phase I/II trial. Radiother Oncol 2008;87:181–187. [DOI] [PubMed] [Google Scholar]
- 76.Curran D, Giralt J, Harari PM, et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol 2007;25:2191–2197. [DOI] [PubMed] [Google Scholar]
- 77.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 2008;26:3770–3776. [DOI] [PubMed] [Google Scholar]
- 78.Langendijk JA, Doornaert P, Rietveld DH, et al. A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiother Oncol 2009;90:189–195. [DOI] [PubMed] [Google Scholar]
- 79.Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head- and-neck cancer. Int J Radiat Oncol Biol Phys 2009;73:410–415. [DOI] [PubMed] [Google Scholar]
- 80.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle:a dose–effect relationship. Radiother Oncol 2007;85:64–73. [DOI] [PubMed] [Google Scholar]
- 81.Guldiken Y, Orhan KS, Demirel T, et al. Assessment of shoulder impairment after functional neck dissection: long term results. Anris Nasns Larynx 2005;32:387–391. [DOI] [PubMed] [Google Scholar]
- 82.Kuntz AL, Weymuller EA. Impact of neck dissection on quality of life. Laryngoscope 1999;109:1334–1338. [DOI] [PubMed] [Google Scholar]
- 83.Schiefke F, Akdemir M, Weber A, et al. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck 2009;31:503–512. [DOI] [PubMed] [Google Scholar]
- 84.Rogers SN, Scott B, Lowe D. An evaluation of the shoulder domain of the University of Washington quality of life scale. Br J Oral Maxillofac Surg 2007;45:5–10. [DOI] [PubMed] [Google Scholar]
- 85.Stuiver MM, van Wilgen CP, de Boer EM, et al. Impact of shoulder complaints after neck dissection on shoulder disability and quality of life. Otolaryngol Head Neck Surg 2008;139:32–39. [DOI] [PubMed] [Google Scholar]
- 86.Chepeha DB, Taylor RJ, Chepeha JC, et al. Functional assessment using Constant’s Shoulder Scale after modified radical and selective neck dissection. Head Neck 2002;24:432–436. [DOI] [PubMed] [Google Scholar]
- 87.Salerno G, Cavaliere M, Foglia A, et al. The 11th nerve syndrome in functional neck dissection. Laryngoscope 2002;112:1299–1307. [DOI] [PubMed] [Google Scholar]
- 88.Desai AS, Dramis A, Hearnden AJ. Critical appraisal of subjective outcome measures used in the assessment of shoulder disability. Ann R Coll Surg Engl 2010;92:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor RJ, Chepeha JC, Teknos TN, et al. Development and validation of the neck dissection impairment index: a quality of life measure. Arch Otolaryngol Head Neck Surg 2002;128:44–49. [DOI] [PubMed] [Google Scholar]
- 90.van Wouwe M, de Bree R, Kuik DJ, et al. Shoulder morbidity after nonsurgical treatment of the neck. Radiother Oncol 2009;90:196–201. [DOI] [PubMed] [Google Scholar]
- 91.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–269. [DOI] [PubMed] [Google Scholar]
- 92.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 2010;28:4142–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2010;76:403–409. [DOI] [PubMed] [Google Scholar]
- 95.Ang KK, Byers RM, Peters LJ, et al. Regional radiotherapy as adjuvant treatment for head and neck malignant melanoma. Preliminary results. Arch Otolaryngol Head Neck Surg 1990;116:169–172. [DOI] [PubMed] [Google Scholar]
- 96.Bernier J, Vermorken JB, Koch WM. Adjuvant therapy in patients with resected poor-risk head and neck cancer. J Clin Oncol 2006;24:2629–2635. [DOI] [PubMed] [Google Scholar]
- 97.Fletcher GH, Lindberg RD, Jesse RH Jr. The combination of radiation and surgery in oropharynx and laryngopharynx squamous cell carcinomas. Aktuelle Probl Chir 1970;14:347–366. [PubMed] [Google Scholar]
- 98.Cohen MA, Weinstein GS, O’Malley BW Jr, et al. Transoral robotic surgery and human papillomavirus status: oncologic results. Head Neck 2011;33:573–580. [DOI] [PubMed] [Google Scholar]
- 99.Quon H, O’Malley BW Jr, Weinstein GS. Postoperative adjuvant therapy after transoral robotic resection for oropharyngeal carcinomas: rationale and current treatment approach. ORL J Otorhinolaryngol Relat Spec 2011;73:121–130. [DOI] [PubMed] [Google Scholar]
- 100.Weinstein GS, O’Malley BW Jr, Hockstein NG. Transoral robotic surgery: supraglottic laryngectomy in a canine model. Laryngoscope 2005;115:1315–1319. [DOI] [PubMed] [Google Scholar]
- 101.Grant DG, Salassa JR, Hinni ML, et al. Carcinoma of the tongue base treated by transoral laser microsurgery, part two: persistent, recurrent and second primary tumors. Laryngoscope 2006;116:2156–2161. [DOI] [PubMed] [Google Scholar]
- 102.Camp AA, Fundakowski C, Petruzzelli GJ, Emami B. Functional and oncologic results following transoral laser microsurgical excision of base of tongue carcinoma. Otolaryngol Head Neck Surg 2009;141:66–69. [DOI] [PubMed] [Google Scholar]
- 103.Petruzzelli GJ. Transoral laser microsurgery: applications in head and neck oncology. Expert Rev Med Devices 2009;6:599–602. [DOI] [PubMed] [Google Scholar]
- 104.Galati LT, Myers EN, Johnson JT. Primary surgery as treatment for early squamous cell carcinoma of the tonsil. Head Neck 2000;22:294–296. [DOI] [PubMed] [Google Scholar]
- 105.Walvekar RR, Li RJ, Gooding WE, et al. Role of surgery in limited (T1–2, N0–1) cancers of the oropharynx. Laryngoscope 2008;118:2129–2134. [DOI] [PubMed] [Google Scholar]
- 106.Trotti A, Pajak TF, Gwede CK, et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol 2007;8:613–624. [DOI] [PubMed] [Google Scholar]
- 107.Leslie A, Fyfe E, Guest P, et al. Staging of squamous cell carcinoma of the oral cavity and oropharynx: a comparison of MRI and CT in T- and N-staging. J Comput Assist Tomogr 1999;23:43–49. [DOI] [PubMed] [Google Scholar]
- 108.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst 2008;100:712–720. [DOI] [PubMed] [Google Scholar]
- 109.Trotta BM, Pease CS, Rasamny JJ, Raghavan P, Mukherjee S. Oral cavity and oropharyngeal squamous cell cancer: key imaging findings for staging and treatment planning. Radiographics 2011;31:339–354. [DOI] [PubMed] [Google Scholar]
- 110.Emonts P, Bourgeois P, Lemort M, Flamen P. Functional imaging of head and neck cancers. Curr Opin Oncol 2009;21:212–217. [DOI] [PubMed] [Google Scholar]
- 111.Brizel DM. Head and neck cancer as a model for advances in imaging prognosis, early assessment, and posttherapy evaluation. Cancer J 2011;17:159–165. [DOI] [PubMed] [Google Scholar]
- 112.Roach MC, Turkington TG, Higgins KA, Hawk TC, Hoang JK, Brizel DM. FDG-PET assessment of the effect of head and neck radiotherapy on parotid gland glucose metabolism. Int J Radiat Oncol Biol Phys 2012;82:321–326. [DOI] [PubMed] [Google Scholar]
- 113.Rose BS, Jeong J-H, Nath SK, et al. Population-based study of competing mortality in head and neck cancer. J Clin Oncol 2011;29:3503–3509. [DOI] [PubMed] [Google Scholar]
- 114.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics 1986;42:507–519. [PubMed] [Google Scholar]
- 115.Dahlberg SE, Gray RJ, Johnson BE. Gefitinib for recurrent non–small-cell lung cancer: all things are not created equal. J Clin Oncol 2008;26:4233–4235. [DOI] [PubMed] [Google Scholar]
- 116.Sanchez MM, Chen X. Choosing the analysis population in non-inferiority studies: per protocol or intent-to-treat. Statist Med 2006;25:1169–1181. [DOI] [PubMed] [Google Scholar]
- 117.Freidlin B, Korn EL, George SL, Gray R. Randomized clinical trial design for assessing noninferiority when superiority is expected. J Clin Oncol 2007;25:5019–5023. [DOI] [PubMed] [Google Scholar]
- 118.Kleist P Composite endpoints: proceed with caution. Appl Clin Trials Online 2006. May be accessed at http://appliedclinicaltrialsonline.find-pharma.com/appliedclinicaltrials/article/articleDetail.jsp?id=324331
- 119.Tomlinson G, Detsky AS. Composite end points in randomized trials: there is no free lunch. JAMA 2010;303:267–268. [DOI] [PubMed] [Google Scholar]
- 120.Wang J, Zhao Z, Barber B, Sherrill B, Peeters M, Wiezorek J. A Q-TWiST analysis comparing panitumumab plus best supportive care (BSC) with BSC alone in patients with wild-type KRAS metastatic colorectal cancer. Br J Cancer 2011;104:1848–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]


